- 1Medical College of Nanchang University, Nanchang, Jiangxi Province, China
- 2Department of Cardiology, Jiangxi Provincial People's Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, Jiangxi Province, China
Therapy for patients with ST-elevation myocardial infarction (STEMI) has been a controversial topic since the introduction of thrombolytic agents in the 1980s. The use of morphine, fentanyl and lidocaine has increased substantially during this period. However, there is still limited evidence on their advantages and limitations. In this review, the clinical application, as well as future considerations of morphine, fentanyl and lidocaine in patients with ST segment elevation myocardial infarction were discussed.
1. Introduction
Ischemic heart disease, such as coronary heart disease, is currently the most common cause of death globally, and its incidence is rising (1). Among them, ST-segment elevation myocardial infarction (STEMI) is one of the most dangerous subtypes of coronary heart disease. In the latest STEMI practice guidelines, a loading dose of dual antiplatelet therapy (DATP), an early start of reperfusion therapy, relief of ischemic chest pain and anxiety are emphasized (2). It is important to use Anti-platelet drugs and analgesics before hospitalization, in combination with percutaneous coronary intervention, to relieve chest pain. Commonly used analgesic drugs include morphine, fentanyl, and lidocaine, but using these drugs may reduce the anti-platelet aggregation effect of oral P2Y12 receptor antagonists (Table 1) (3), which may increase the risk of outcome adverse events.
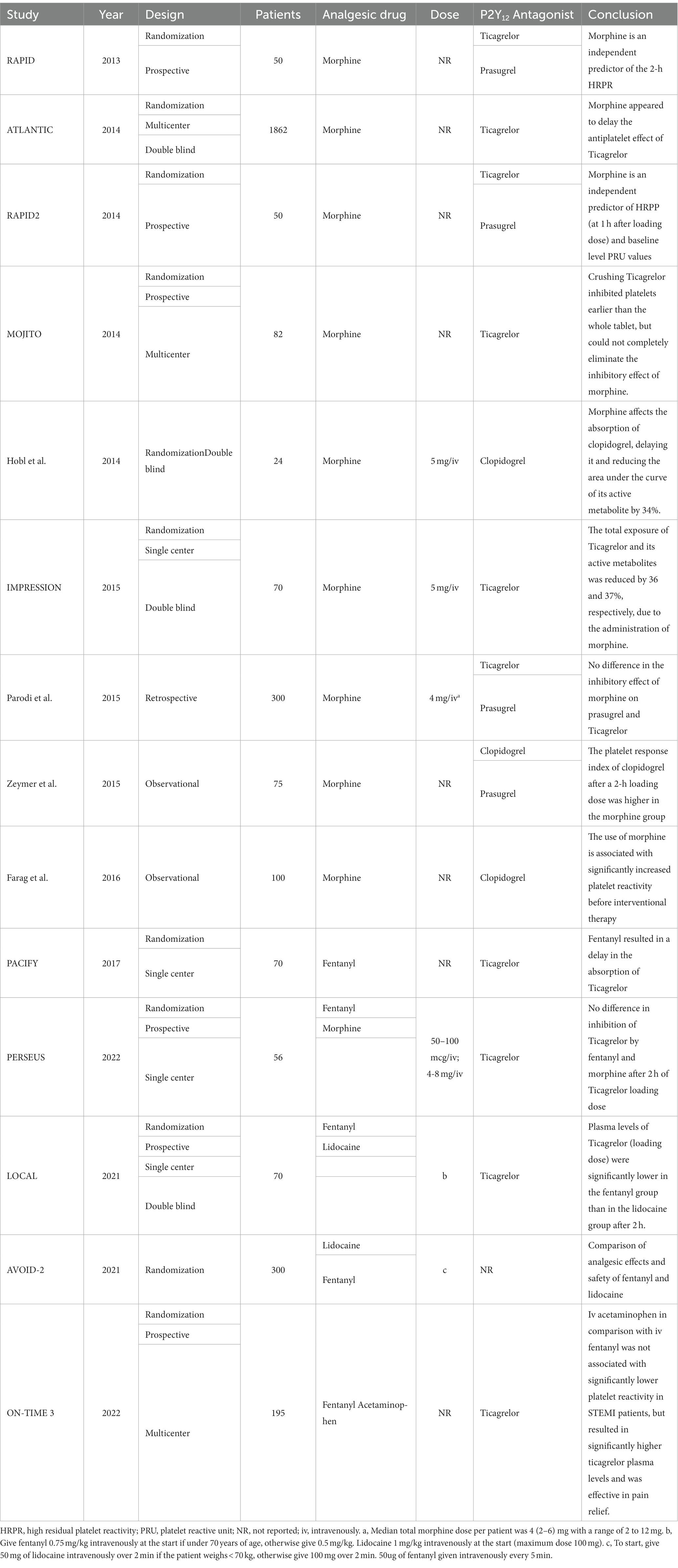
Table 1. Basic information for the studies of interactions between analgesics and P2Y12 receptor antagonists.
The use of relevant analgesic drugs in patients with STEMI can have a significant impact on prognosis and should be carefully evaluated. This article reviews the studies on the combination of P2Y12 receptor antagonists and analgesics in patients with STEMI. In the meantime, the clinical application and important points for attention of analgesics were discussed.
2. Methods
2.1. Literature search
Retrieval should be conducted using PubMed, EMBASE, and Sino Med databases. Search terms pre-defined in titles, abstracts, and keywords are used to identify pertinent studies. More information about the terms used in the search can be located in Supplementary material 1. The retrieval period spans from the inception of the databases up to February 2023.
2.2. Data extraction
After independently reviewing the literature, the two researchers gathered data manually, including the type, year, drug subjects, and results of the study. To ensure impartiality, a third researcher was selected to evaluate any differences of opinion.
3. Application of analgesics in patients with STEMI
3.1. Morphine
As a potent opioid drug, morphine produces significant analgesic and sedative effects by stimulating the action of the endogenous anti-pain substance enkephalin and activating opioid receptors in the central nervous system (4). In 1930, Moor et al. reported for the first time that intravenous morphine was used to relieve pain in patients with acute myocardial infarction, and the analgesic effect reached its peak in a few minutes (5). Yet, as research on the effects of morphine continues, more and more adverse effects are being discovered, such as gastrointestinal reactions, bradycardia, hypotension, and respiratory depression (6).
Intravenous morphine may reduce the efficacy of antiplatelet medications in patients with acute myocardial infarction, especially those with STEMI. In 2013, Parodi and colleagues compared the effect of prasugrel and ticagrelor in patients with STEMI undergoing percutaneous coronary intervention (RAPID study) (7). The findings indicated that only 50% of patients achieved effective platelet inhibition 2 h after the loading dose, whereas it took a minimum of 4 h for most patients to reach effective platelet inhibition. Intravenous morphine may cause delayed antiplatelet effects of Ticagrelor or prasugrel. Researchers believe that morphine use is a predictor of high residual platelet reactivity (HRPR). The results of the ATLANTIC study concluded that, in patients with STEMI, prehospital administration of Ticagrelor in the short term before PCI appears to be safe but does not improve coronary reperfusion before PCI. One potential reason for the delayed absorption of ticagrelor may be due to the drug interaction with morphine. However, this has not been further clarified in this experiment (8).
The results of the RAPID-2 study demonstrated that a greater load dose of ticagrelor could result in more efficient and quicker platelet inhibition. It was also proved that morphine was an independent predictor of HRPR at 1 h after loading dose (OR: 4.49 [1.19–16.88], p = 0.026) and platelet reactive unit (PRU) values (OR: 1.015 [1–1.03], p = 0.039) (9). The inhibitory effect of morphine on antiplatelet agents is most pronounced at the time of initial administration. To further determine the difference in the negative effects of morphine among antiplatelet drugs, Parodi et al., conducted a study in 300 patients with PCI who received loading doses of prasugrel or ticagrelor. Platelet reactivity was evaluated by Verify Now at 1, 2 and 4 h after loading. Patients who were treated with morphine were found to have higher rates of vomiting. There was no difference in the inhibitory effect of morphine on prasugrel and ticagrelor (10). In addition, the MOJITO study found that crushing ticagrelor inhibits platelets earlier than taking the drug whole. However, even with this faster reaction time, using morphine can increase the reactivity of residual platelets (11). The TASTER study, led by Guido Parodi et al., examined the effectiveness of a new Ticagrelor formulation, an oral dispersible tablet (ODT) that does not require water and quickly releases its components upon swallowing. Despite the lack of significant distinction between the new orally disintegrating tablet (ODT) and the conventional Ticagrelor tablets in terms of platelet inhibition, ODT has a superior safety and convenience advantage, particularly in ambulances (12).
However, most of the previous studies were retrospective, observational, or did not focus on morphine. The IMPRESSION trial is the first randomized trial to confirm the negative effects of morphine on the pharmacokinetics and antiplatelet effect of ticagrelor in patients with AMI (13). Patients were given an intravenous dose of either morphine (5 mg) or placebo, followed by a load dose of ticagrelor (180 mg). The results showed that morphine reduced the total exposure of ticagrelor and its active metabolites by approximately one-third (AUC (0–12): 6307 vs. 9,791 ng h/ml; p = 0.003) and (AUC (0–12): 1503 vs. 2,388 ng h/ml; p = 0.008).
Clopidogrel is another P2Y12 receptor antagonist that is frequently used in the clinic. According to Hobl et al., morphine has the potential to reduce the concentration of clopidogrel and its active metabolite, as well as delay their absorption (14). Morphine can delay the inhibitory effect of platelet occlusion at high shear rates, thereby prolonging the occlusion time caused by collagen diphosphate by 3 times with its active metabolite. Zeymer and Farag et al. also demonstrated that morphine can delay the platelet inhibition of clopidogrel (600 mg) (15, 16). In addition, Farag also found that the delayed effect of morphine on clopidogrel disappeared after 24 h, which may be related to the metabolism of morphine from the body. A meta-analysis of 207 STEMI patients from 5 studies found that morphine caused an approximately 40% increase in expected platelet reactivity (p < 0.001) (17).
3.2. Fentanyl
Fentanyl is a potent, rapidly administered synthetic opioid that is injected intravenously (18). In recent years, an increasing number of studies have reported the effect of fentanyl in early analgesia in patients with acute myocardial infarction. The effect of fentanyl on the blood concentration of a P2Y12 receptor antagonist has not been well studied. It is possible that fentanyl could reduce the concentration and delay the antiplatelet effect. John and his colleagues first reported in the PACIFY trial the effect of intravenous fentanyl on ticagrelor absorption and platelet inhibition in patients receiving PCI (19, 20). The results showed that the incidence of high residual platelet reactivity and platelet aggregation was significantly higher in the intravenous fentanyl group than in the non-fentanyl group (20% vs. 6% and 33% vs. 5%).
The effects of fentanyl and morphine on the absorption of a loading dose of ticagrelor (180 mg) and on platelet inhibition were compared in patients with STEMI by Sophie et al. (21, 22). Fifty-six patients were randomly assigned to either the group receiving morphine or the group receiving fentanyl at a 1:1 ratio. Subsequently, the platelet reactivity to Ticagrelor was recorded 2 h after the loading dose and evaluated by P2Y12 response units (PRU). The results of the study showed that fentanyl can have an adverse effect on platelet inhibition after 2 h of ticagrelor loading dose comparable to morphine (173.3 ± 89.7 vs. 173.3 ± 89.7, p = 0.179). After 4 h, the patients who were treated with fentanyl had significantly lower PRU values than the patients who were treated with morphine (90.1 ± 97.4 vs. 168.0 ± 72.2; p = 0.011). This may indicate that the inhibition of ticagrelor by fentanyl is short-lived, compared to morphine. In the end, the results of this study are inconclusive, leaving it unclear whether fentanyl is a better option than morphine. Furthermore, the lack of assessment of pain outcomes between the fentanyl and morphine groups in this study is a limitation. Consequently, the actual clinical implications of this variation require additional investigation.
3.3. Lidocaine
The exploration of non-opioid analgesics has provoked comprehensive interest among scholars. Lidocaine is a sodium channel blocker that is usually used for local analgesia and local anesthesia, as well as for the treatment of ventricular arrhythmias. Prior investigations have demonstrated that lidocaine is efficacious in reducing ischemic pain, including among patients with coronary artery disease (23, 24). Lidocaine produces analgesia by interfering with the function of sodium channels and G proteins, which in turn prevents activation of N-methyl-D-aspartic acid receptor (NMDA). It can reduce circulating inflammatory cytokines, preventing secondary hyperalgesia and central hyperalgesia (25).
In the LOCAL trial, the study by Himawan et al., demonstrated the safety and efficacy of lidocaine for analgesia in patients with coronary artery disease. Moreover, it will not impair the bioavailability of ticagrelor or delay its antiplatelet effect, compared to fentanyl (26). In this study, it was found that intravenous lidocaine and fentanyl have comparable analgesic effects (pain assessment by 11-point numerical rating scale -NRS). Both treatments receive high marks from patients in terms of satisfaction. The study under discussion is a PK/PD study of a certain drug, rather than a clinical study of outcomes. Thus, it is difficult to ascertain the clinical significance of the findings of this study. In addition to this, patients with unstable angina pectoris or non-ST segment elevation myocardial infarction were the only patients included in this study. In the AOVID-2 trial, 300 patients who were suspected of having STEMI were randomly assigned to receive either intravenous fentanyl or lidocaine in the emergency ambulance in order to relieve chest pain (27). Patient analgesic effect (pain reduction on arrival at the hospital) and safety (e.g., adverse drug reactions) were the co-primary endpoints of this study. The findings of the recent study have revealed that lidocaine does not meet the criteria for non-inferior efficacy, and the effect of prehospital analgesia is inferior to that of fentanyl. However, lidocaine is safer and better tolerated than intravenous fentanyl (28). Interestingly, the study’s secondary endpoint also compared the relationship between the size of the infarct and the dose of analgesics in the two groups. A dose-dependent relationship between opioids and the size of myocardial infarction has been reported in previous studies (29). The administration of analgesic drugs may need to be more individualized in patients with myocardial infarction.
4. Discussion
Ischemic chest pain is a common symptom in patients with coronary artery disease. Painful stimuli are thought to increase sympathetic activation, which may lead to an increase in myocardial oxygen consumption. This, in turn, may elevate heart rate and systemic vascular resistance (30). Therefore, restoring the blood supply to ischemic myocardium as soon as possible is the most effective way to relieve pain (31). A large number of patients still do not have immediate access to interventional treatment because of the local medical service. Consequently, having access to effective and rapid pain medication is very important. However, the use of analgesics may lead to a decline in the efficacy of drugs used to treat coronary heart disease. How to use these drugs is worth discussing. Oral P2Y12 receptor inhibitor is a new type of anti-platelet aggregation inhibitor. Combination with aspirin is considered a cornerstone drug for the prevention of thrombotic events in STEMI patients undergoing percutaneous coronary intervention (32). However, among STEMI patients undergoing percutaneous coronary intervention (PCI), due to hemodynamic changes and delayed gastrointestinal absorption, the platelet inhibition induced by oral P2Y12 receptor antagonists will be delayed (33). Morphine or fentanyl will further delay the exposure of P2Y12 inhibitors and reduces their exposure by slowing gastrointestinal motility (Figure 1) (34). In addition, the inhibitory effect of morphine typically lasts for more than 4 h, which may contribute to the poor clinical outcome of patients with STEMI. Therefore, lidocaine can be considered a viable option for analgesia in elderly patients with gastrointestinal diseases. Lidocaine has been found to not diminish the antiplatelet effects of ticagrelor, making it a promising non-opioid pain relief option. Furthermore, the use of lidocaine in patients with STEMI did not uncover an increased likelihood of bradyarrhythmia, tachyarrhythmia or neurotoxicity (35). A clinical study comparing intravenous acetaminophen and fentanyl for pain relief in patients with STEMI found no difference between the two. Plasma Ticagrelor concentration in patients who received intravenous acetaminophen was significantly higher than that in those who received intravenous fentanyl, which further demonstrated the inhibitory effect of fentanyl on P2Y12 antagonists (36). In the meantime, there is no evidence that it is affected by opioid analgesics. Whether or not they can become mainstream pain relief programs remains to be determined.
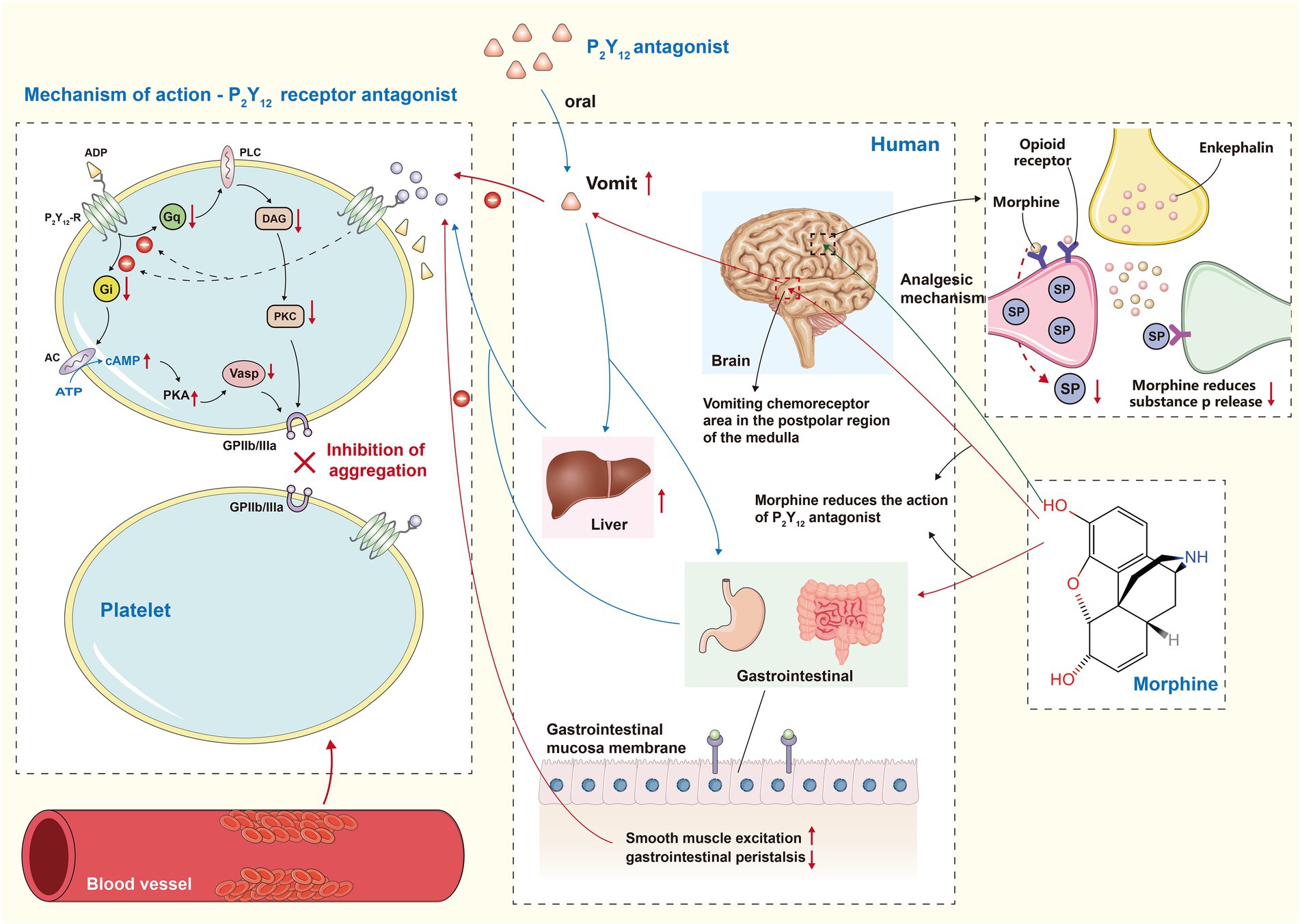
Figure 1. P2Y12 receptor antagonist and morphine interaction effect diagram. Gi, G inhibitory protein; AC, adenylate cyclase; PKA, protein kinase A; Vasp, Vaso dilator stimulatory protein; Gq, Gq protein; PLC, phospho lipase C; DAG, diacylglycerol; PKC, protein kinase C; The left part of the figure represents the mechanism of action of P2Y12 receptor antagonist. They ultimately reduce the stimulation of GPIIb/IIIa by inhibiting both Gi/AC/PKA and Gq/DAG/PKC pathways. The middle part of the figure shows that morphine slows down the absorption of P2Y12 receptor antagonist and reduces the final exposure by delaying gastrointestinal motility and stimulating vomiting. The green arrow in the right part of the figure illustrates the analgesic mechanism of morphine, which simulates endogenous enkephalins enabling a decrease in the release of substance P.
Platelet reactivity (PR) is considered to be a key pathophysiological factor in the development of ST segment elevation myocardial infarction (STEMI) (37). Previous research has highlighted the capacity of morphine to suppress the activity of P2Y12 receptor antagonists. Then, most studies ignore whether patients have high platelet reactivity (HPR) before treatment, which leads to a lack of understanding of the role that platelet reactivity plays in the outcome and prognosis of patients. Through the research of MarioE Canonico et al., we have been able to gain further insight into the prevalence, clinical characteristics, treatment response and results of STEMI patients with no high platelet reactivity prior to treatment (38). The research demonstrated that 20% of STEMI patients had NHPR, and it took hours for HPR patients to achieve effective platelet inhibition after P2Y12 antagonist load before treatment, leading to a detrimental prognosis in hospital. The wide range of clinical diversity observed in STEMI patients further underlines the importance of providing tailored medication options. For instance, individuals with HPR may benefit from a more advanced and intensive antiplatelet therapy, while avoiding the use of opioid analgesics.
It is uncertain whether drug interactions can have a direct impact on clinical results, and only a few observational studies have been conducted. Etienne Puymirat and his colleagues conducted a one-year assessment of in-hospital complications such as mortality, non-fatal re-myocardial infarction, stroke, stent thrombosis, and bleeding in 2438 patients with STEMI, as part of the 2010 FAST-MI study, in order to assess the impact of prehospital morphine use (39). The findings indicated that there was no statistically significant distinction between those who had pre-hospital morphine and those who had not in terms of 1-year mortality. It is possible that the beneficial effects of morphine on hemodynamics, such as a decrease in heart rate and no major changes in systolic blood pressure, may be counteracting its detrimental effect on the delayed clopidogrel effect, thus resulting in no effect on clinical outcome. Surprisingly, no substantial impact of morphine use on mortality in STEMI patients receiving fibrinolytic therapy was noticed in the research conducted by Cantor et al. (40). Nevertheless, there was a strong association between the ingestion of morphine and the danger of reinfarction at 7 and 30 days (OR = 4.45; p = 0.018) and (OR = 1.72; p = 0.041). It is not difficult to comprehend why morphine use can impede the antiplatelet effect, as well as why it may be associated with more intense chest pain and more serious conditions in those who require it, such as those with anterior wall infarction and larger infarcts. Remo HM et al. found that the use of morphine was linked to a greater likelihood of short-term cardiac ischemic events (adjusted OR = 1.40; p = 0.026), with the primary focus of the study being NSTEACS patients (41).
How to overcome the conflicting effects between analgesic drugs and oral P2Y12 receptor antagonists is of urgent concern. The following strategies are available: Pre-hospital emergency treatment with P2Y12 receptor antagonist (8); A gradually upgraded loading dose regimen for P2Y12 receptor inhibitors (42); The concomitant use of glycoprotein IIb/IIIa inhibitors may be advantageous (43); The simultaneous use of gastrointestinal motility promoting drugs and P2Y12 receptor inhibitors to hasten absorption (44). In addition, cangrelor (an intravenous, reversible platelet P2Y12 receptor antagonist) and selatogrel, a new subcutaneous P2Y12 inhibitor to be used in clinical practice, also need to be considered in the future (45–48). A new reticular meta-analysis found that there was no significant difference between cangrelor, clopidogrel, ticagrelor and prasugrel in reducing the risk of ischemic events (49). Selatogrel is more rapidly absorbed by the body when given intravenously or subcutaneously rather than orally. In the meantime, it is not affected by opioid analgesics.
In conclusion, the effects of morphine, fentanyl, and lidocaine on the antiplatelet effects of P2Y12 receptor antagonists were reviewed. Although pharmacodynamic studies have shown that opioid analgesics can lead to high residual platelet reactivity, however, it is still unclear how this phenomenon affects clinical outcomes. However, it is still advisable to avoid the routine use of opioid analgesics in STEMI patients, unless in specific condition. The advantages of lidocaine include its rapid analgesic effects and lack of inhibition of the antiplatelet effect. However, larger and prospective randomized clinical studies are needed to confirm whether this will become the new mainstream analgesic method.
Author contributions
HC: conceptualization, writing – original draft, and methodology. HW and LH: writing – review and editing. BL and MK: data curation and validation. LY: supervision, conceptualization, and visualization. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Doctor Start-up fund of Jiangxi provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College (No. 19–236).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1148581/full#supplementary-material
References
1. Mensah, GA, Roth, GA, and Fuster, V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol. (2019) 74:2529–32. doi: 10.1016/j.jacc.2019.10.009
2. Collet, JP, Thiele, H, Barbato, E, Barthélémy, O, Bauersachs, J, Bhatt, DL, et al. ESC scientific document group. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
3. Farag, M, Spinthakis, N, Srinivasan, M, and Gorog, DA. Should STEMI patients receive opiate analgesia? The morphine paradox. Curr Vasc Pharmacol. (2018) 16:477–83. doi: 10.2174/1570161116666180117145704
4. Eidelberg, E, and Schwartz, AS. Possible mechanism of action of morphine on brain. Nature. (1970) 225:1152–3. doi: 10.1038/2251152a0
5. Moor, F . Intravenous use of morphine in acute myocardial infarction. Lancet. (1930) 216:959–60. doi: 10.1016/S0140-6736(01)09746-X
6. Cherny, N, Ripamonti, C, Pereira, J, Davis, C, Fallon, M, McQuay, H, et al. Strategies to manage the adverse effects of oral morphine: an evidence-based report. J Clin Oncol. (2001) 19:2542–54. doi: 10.1200/JCO.2001.19.9.2542
7. Parodi, G, Valenti, R, Bellandi, B, Migliorini, A, Marcucci, R, Comito, V, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid activity of platelet inhibitor drugs) primary PCI study. J Am Coll Cardiol. (2013) 61:1601–6. doi: 10.1016/j.jacc.2013.01.024
8. Montalescot, G, van Hof, AW, Lapostolle, F, Silvain, J, Lassen, JF, Bolognese, L, et al. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N Engl J Med. (2014) 371:1016–27. doi: 10.1056/NEJMoa1407024
9. Parodi, G, Bellandi, B, Valenti, R, Migliorini, A, Marcucci, R, Carrabba, N, et al. Comparison of double (360 mg) ticagrelor loading dose with standard (60 mg) prasugrel loading dose in ST-elevation myocardial infarction patients: the rapid activity of platelet inhibitor drugs (RAPID) primary PCI 2 study. Am Heart J. (2014) 167:909–14. doi: 10.1016/j.ahj.2014.03.011
10. Parodi, G, Bellandi, B, Xanthopoulou, I, Capranzano, P, Capodanno, D, Valenti, R, et al. Morphine is associated with a delayed activity of oral antiplatelet agents in patients with ST-elevation acute myocardial infarction undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. (2014) 8:e001593. doi: 10.1161/CIRCINTERVENTIONS.114.001593
11. Parodi, G, Xanthopoulou, I, Bellandi, B, Gkizas, V, Valenti, R, Karanikas, S, et al. Ticagrelor crushed tablets administration in STEMI patients: the MOJITO study. J Am Coll Cardiol. (2015) 65:511–2. doi: 10.1016/j.jacc.2014.08.056
12. Parodi, G, Talanas, G, Mura, E, Canonico, ME, Siciliano, R, Guarino, S, et al. Orodispersible Ticagrelor in acute coronary syndromes: the TASTER study. J Am Coll Cardiol. (2021) 78:292–4. doi: 10.1016/j.jacc.2021.05.015
13. Hobl, EL, Reiter, B, Schoergenhofer, C, Schwameis, M, Derhaschnig, U, Kubica, J, et al. Morphine decreases ticagrelor concentrations but not its antiplatelet effects: a randomized trial in healthy volunteers. Eur J Clin Investig. (2016) 46:7–14. doi: 10.1111/eci.12550
14. Hobl, EL, Stimpfl, T, Ebner, J, Schoergenhofer, C, Derhaschnig, U, Sunder-Plassmann, R, et al. Morphine decreases clopidogrel concentrations and effects: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol. (2014) 63:630–5. doi: 10.1016/j.jacc.2013.10.068
15. Zeymer, U, Mark, B, and Montalescot, G. Influence of morphine on the effect of clopidogrel and prasugrel in patients with ST elevation myocardial infarction. Results of the ETAMI trial. Eur Heart J. (2015) 117:227–8. doi: 10.1160/TH16-07-0569
16. Farag, M, Srinivasan, M, and Gorog, D. Morphine use impairs thrombotic status in patients with ST?Elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Am Coll Cardiol. (2016) 67:40. doi: 10.1016/S0735-1097(16)30041-9
17. Xanthopoulou, I, Davlouros, P, Tsigkas, G, Koutsogiannis, N, Patsilinakos, S, Deftereos, S, et al. Factors affecting platelet reactivity 2 hours after P2Y12 receptor antagonist loading in primary percutaneous coronary intervention for ST-elevation myocardial infarction – impact of pain-to-loading time. Circ J. (2016) 80:442–9. doi: 10.1253/circj.CJ-15-0495
18. Schug, SA, and Ting, S. Fentanyl formulations in the management of pain: an update. Drugs. (2017) 77:747–63. doi: 10.1007/s40265-017-0727-z
19. McEvoy, J, Ibrahim, K, Kickler, T, Clarke, WA, Hasan, RK, Czarny, MJ, et al. Effect of intravenous fentanyl on ticagrelor absorption and platelet inhibition among patients undergoing percutaneous coronary intervention. Circulation. (2018) 137:307–9. doi: 10.1161/circulationa-ha.117.031678
20. Ibrahim, K, Shah, R, Goli, RR, Kickler, TS, Clarke, WA, Hasan, RK, et al. Fentanyl delays the platelet inhibition effects of oral ticagrelor: full report of the PACIFY randomized clinical trial. Thromb Haemost. (2018) 118:1409–18. doi: 10.1055/s-0038-1666862
21. Degrauwe, S, Roffi, M, Lauriers, N, Muller, O, Masci, PG, Valgimigli, M, et al. Influence of intravenous fentanyl compared with morphine on ticagrelor absorption and platelet inhibition in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: rationale and design of the PERSEUS randomized trial. Eur Heart J Cardiovasc Pharmacother. (2019) 5:158–63. doi: 10.1093/ehjcvp/pvy031
22. Iglesias, JF, Valgimigli, M, Carbone, F, Lauriers, N, Masci, PG, and Degrauwe, S. Comparative effects of fentanyl versus morphine on platelet inhibition induced by ticagrelor in patients with ST-segment elevation myocardial infarction: full results of the PERSEUS randomized trial. Cardiol J. (2022) 29:591–600. doi: 10.5603/CJ.a2022.0049
23. Vahidi, E, Shakoor, D, Aghaie Meybodi, M, and Saeedi, M. Comparison of intravenous lidocaine versus morphine in alleviating pain in patients with critical limb ischaemia. Emerg Med J. (2015) 32:516–9. doi: 10.1136/emermed-2014-203944
24. Frölich, MA, McKeown, JL, Worrell, MJ, and Ness, TJ. Intravenous lidocaine reduces ischemic pain in healthy volunteers. Reg Anesth Pain Med. (2010) 35:249–54. doi: 10.1097/AAP.0b013e3181d23386
25. Kandil, E, Melikman, E, and Adinoff, B. Lidocaine infusion: a promising therapeutic approach for chronic pain. J Anesth Clin Res. (2017) 08:697. doi: 10.4172/2155-6148.1000697
26. Fernando, H, Duong, T, Huynh, K, Noonan, J, Shaw, J, Duffy, SJ, et al. Effects of lignocaine vs. opioids on antiplatelet activity of ticagrelor: the LOCAL trial. Eur Heart J. (2021) 42:4025–36. doi: 10.1093/eurheartj/ehab557
27. Fernando, H, Nehme, Z, Milne, C, O’Brien, J, Bernard, S, Stephenson, M, et al. LidocAine versus opioids in MyocarDial infarction: the AVOID-2 randomized controlled trial. Eur Heart J Acute Cardiovasc Care. (2023) 12:2–11. doi: 10.1093/ehjacc/zuac154
28. Fernando, H, Milne, C, Nehme, Z, Ball, J, Bernard, S, Stephenson, M, et al. An open-label, non-inferiority randomized controlled trial of lidocAine versus opioids in MyocarDial infarction study (AVOID-2 study) methods paper. Contemp Clin Trials. (2021) 105:106411. doi: 10.1016/j.cct.2021.106411
29. Fernando, H, Nehme, Z, Peter, K, Bernard, S, Stephenson, M, Bray, J, et al. Prehospital opioid dose and myocardial injury in patients with ST elevation myocardial infarction. Open Heart. (2020) 7:e001307. doi: 10.1136/openhrt-2020-001307
30. Rouby, JJ, Eurin, B, Glaser, P, Guillosson, JJ, Nafziger, J, Guesde, R, et al. Hemodynamic and metabolic effects of morphine in the critically ill. Circulation. (1981) 64:53–9. doi: 10.1161/01.cir.64.1.53
31. Giannopoulos, G, Deftereos, S, Kolokathis, F, Xanthopoulou, I, Lekakis, J, and Alexopoulos, D. P2Y12 receptor antagonists and morphine: a dangerous liaison? Circ Cardiovasc Interv. (2016) 9:e004229. doi: 10.1161/CIRCINTERVENTIONS.116.004229
32. Franchi, F, Rollini, F, and Angiolillo, DJ. Antithrombotic therapy for patients with STEMI undergoing primary PCI. Nat Rev Cardiol. (2017) 14:361–79. doi: 10.1038/nrcardio.2017.18
33. Fernando, H, Shaw, JA, Myles, PS, Peter, K, and Stub, D. The opioid-P2Y12 inhibitor interaction: potential strategies to mitigate the interaction and consideration of alternative analgesic agents in myocardial infarction. Pharmacol Ther. (2021) 217:107665. doi: 10.1016/j.pharmthera.2020.107665
34. Holzer, P . Opioid receptors in the gastrointestinal tract. Regul Pept. (2009) 155:11–7. doi: 10.1016/j.regpep
35. Martí-Carvajal, AJ, Simancas-Racines, D, Anand, V, and Bangdiwala, S. Prophylactic lidocaine for myocardial infarction. Cochrane Database Syst Rev. (2015):CD008553. doi: 10.1002/14651858
36. Tavenier, AH, Hermanides, RS, Ottervanger, JP, Tolsma, R, van Beurden, A, Slingerland, RJ, et al. Impact of opioids on P2Y12 receptor inhibition in patients with ST-elevation myocardial infarction who are pre-treated with crushed ticagrelor: opioids aNd crushed Ticagrelor in myocardial infarction evaluation (ON-TIMsE 3) trial. Eur Heart J Cardiovasc Pharmacother. (2022) 8:4–12. doi: 10.1093/ehjcvp/pvaa095
37. Falk, E, Nakano, M, Bentzon, JF, Finn, AV, and Virmani, R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. (2013) 34:719–28. doi: 10.1093/eurheartj/ehs411
38. Canonico, ME, Sanna, GD, Siciliano, R, Guarino, S, Bellandi, B, Scudiero, F, et al. Not-high before-treatment platelet reactivity in patients with STEMI: prevalence, clinical characteristics, response to therapy and outcomes. Platelets. (2022) 33:390–7. doi: 10.1080/09537104.2021.1915973
39. Puymirat, E, Lamhaut, L, Bonnet, N, Aissaoui, N, Henry, P, Cayla, G, et al. Correlates of pre-hospital morphine use in ST-elevation myocardial infarction patients and its association with in-hospital outcomes and long-term mortality: the FAST-MI (French registry of acute ST-elevation and non-ST-elevation myocardial infarction) programme. Eur Heart J. (2016) 37:1063–71. doi: 10.1093/eurheartj/ehv567
40. Cantor, WJ, Tan, M, Berwanger, O, Lavi, S, White, HD, Nicolau, JC, et al. Morphine and clinical outcomes in patients with ST segment elevation myocardial infarction treated with fibrinolytic and antiplatelet therapy: insights from the TREAT trial. Am Heart J. (2022) 251:1–12. doi: 10.1016/j.ahj.2022.05.005
41. Furtado, RHM, Nicolau, JC, Guo, J, Im, K, White, JA, Sabatine, MS, et al. Morphine and cardiovascular outcomes among patients with non-ST-segment elevation acute coronary syndromes undergoing coronary angiography. J Am Coll Cardiol. (2020) 75:289–300. doi: 10.1016/j.jacc.2019.11.035
42. Franchi, F, Rollini, F, Cho, JR, Bhatti, M, DeGroat, C, Ferrante, E, et al. Impact of escalating loading dose regimens of ticagrelor in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of a prospective randomized pharmacokinetic and pharmacodynamic investigation. JACC Cardiovasc Interv. (2015) 8:1457–67. doi: 10.1016/j.jcin.2015.02.030
43. Valgimigli, M, Tebaldi, M, Campo, G, Gambetti, S, Bristot, L, Monti, M, et al. Prasugrel versus tirofiban bolus with or without short post-bolus infusion with or without concomitant prasugrel administration in patients with myocardial infarction undergoing coronary stenting: the FABOLUS PRO (facilitation through Aggrastat by drOpping or shortening infusion line in patients with ST-segment elevation myocardial infarction compared to or on top of PRasugrel given at loading dOse) trial. JACC Cardiovasc Interv. (2012) 5:268–77. doi: 10.1016/j.jcin.2012.01.006
44. Saad, M, Meyer-Saraei, R, de Waha-Thiele, S, Stiermaier, T, Graf, T, Fuernau, G, et al. Impact of morphine treatment with and without metoclopramide Coadministration on Ticagrelor-induced platelet inhibition in acute myocardial infarction: the randomized MonAMI trial. Circulation. (2020) 141:1354–6. doi: 10.1161/CIRCULATIONAHA.119.042816
45. Storey, RF, and Sinha, A. Cangrelor for the management and prevention of arterial thrombosis. Expert Rev Cardiovasc Ther. (2016) 14:991–9. doi: 10.1080/14779072.2016.1207528
46. Storey, RF . Antiplatelet therapy. Cangrelor succeeds, at last, in PCI. Nat Rev Cardiol. (2013) 10:302–4. doi: 10.1038/nrcardio.2013.61
47. Franchi, F, Rollini, F, Rivas, A, Wali, M, Briceno, M, Agarwal, M, et al. Platelet inhibition with cangrelor and crushed ticagrelor in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Circulation. (2019) 139:1661–70. doi: 10.1161/CIRCULATIONAHA.118.038317
48. Storey, RF, Gurbel, PA, ten Berg, J, Bernaud, C, Dangas, GD, Frenoux, JM, et al. Pharmacodynamics, pharmacokinetics,and safety of single-dose subcutaneous administration of selatogrel, a novel P2Y12 receptor antagonist, in patients with chronic coronary syndromes. Eur Heart J. (2020) 41:3132–40. doi: 10.1093/eurheartj/ehz807
Keywords: STEMI, P2y12 receptor antagonists, morphine, analgesic, lidocaine
Citation: Chen H, Wang H, Li B, Hong L, Kuang M and Yang L (2023) Analgesic drug use in patients with STEMI: Current perspectives and challenges. Front. Med. 10:1148581. doi: 10.3389/fmed.2023.1148581
Edited by:
Marcelo Arruda Nakazone, Faculdade de Medicina de São José do Rio Preto, BrazilReviewed by:
Remo Furtado, Brazilian Clinical Research Institute, BrazilMario Enrico Canonico, University of Colorado, United States
Copyright © 2023 Chen, Wang, Li, Hong, Kuang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Yang, Y2l3dWppYUBmb3htYWlsLmNvbQ==
 Huaigang Chen
Huaigang Chen Hong Wang2
Hong Wang2 Maobin Kuang
Maobin Kuang Liu Yang
Liu Yang