
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 12 April 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1147782
This article is part of the Research TopicUnsolved Challenges in Hepatitis B and Hepatitis C: From Prevention to TreatmentView all 20 articles
Purine adenosine pathway exists widely in the body metabolism, and is involved in regulating various physiological processes. It is one of the important pathways of environmental regulation in human body. CD73 is essentially a protease that catalyzes further dephosphorylation of extracellular adenine nucleotides, hydrolyzing extracellular AMP to adenosine and phosphate. CD73 is an important part of the adenosine signaling pathway. Studies have shown that CD73-mediated adenosine pathway can convert the inflammatory ATP into the immunosuppressant adenosine. This paper aims to summarize the relevant effects of CD73 in the occurrence, development and prognosis of liver diseases such as viral hepatitis, highlight the important role of CD73 in liver diseases, especially in viral hepatitis such as HBV and HCV, and explore new clinical ideas for future treatment targets of liver diseases.
As of late, the frequency of liver diseases has expanded decisively year by year, and liver diseases have turned into a significant clinical issue on the planet. It is estimated that 1.5 billion individuals worldwide suffer from the ill effects of persistent chronic liver diseases (1). The Asia-Pacific region accounted for 62.6 percent of liver disease deaths globally in 2015, as per the Lancet Commission on Gastroenterology and Hepatology. Hepatitis virus infection, particularly the transmission of hepatitis B virus (HBV), is the essential driver of death in more than half part of patients with cirrhosis (2). In 2017, the World Health Organization announced that there are 324 million people with viral hepatitis around the world, and 1.34 million individuals pass away from the infection every year. In this way, it is of extraordinary importance to investigate the inward physiological system and biomolecular focuses of liver diseases, especially for viral hepatitis, to work on the better quality of human life.
Purinergic signaling was first proposed and laid out by the notable scientist Jeffrey Bernstock (3). It is basically composed of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), various adenosine kinases and receptors. It is ubiquitous in the entire body metabolism (4) and has been widely studied and discussed in various diseases. As a major adenosine kinase, CD73 is a critical component of the extracellular adenosine pathway and can be expressed and labeled on an assortment of cell surfaces. CD73 is generally present in liver tissues and profoundly expressed in liver pathology, which indicates that CD73 assumes an important part in liver diseases. This paper aims to summarize the relationship between CD73 and liver physiological and pathological phenomena, highlight the significance of CD73 in liver diseases, in order to reveal potential biological links and provide new ideas for clinical treatment strategy innovation.
CD73, complete name extracellular-5′-nucleotide enzyme, is a 70-kD glycoylphosphatidylinositol (GPI), a multifunctional transmembrane glycoprotein anchored to the surface of cell membranes by 523 amino acids encoded by NT5E gene (located at 6q14-21). As an exonucleotide enzyme, CD73 has enzyme-induced and non-enzymatic functions in cells, which can catalyze further dephosphorylation of extracellular adenine nucleotides and hydrolyze extracellular AMP into adenosine and phosphate (5), acting an important role in purinergic signaling pathways (Table 1).
As an important downstream piece of the extracellular adenosine pathway, CD73 can convert AMP from upstream extracellular ATP and ADP hydrolyzed by cell surface enzymes such as CD39, ALP and NTPases into adenosine (ADO). Including the binding of four G-protein-coupled receptors (GPCR) subtypes A1, A2A, A2B, and A3 adenosine receptors (ARs) (6), activating a multi-step coordinated cascade of intracellular signaling pathways (7), and regulating the aggregation and dispersion of proteins all through cells by changing the CD73 enzyme activity in purine metabolism (8). Jointly play anti-inflammatory, dilated blood vessels and many other functions (Figure 1).
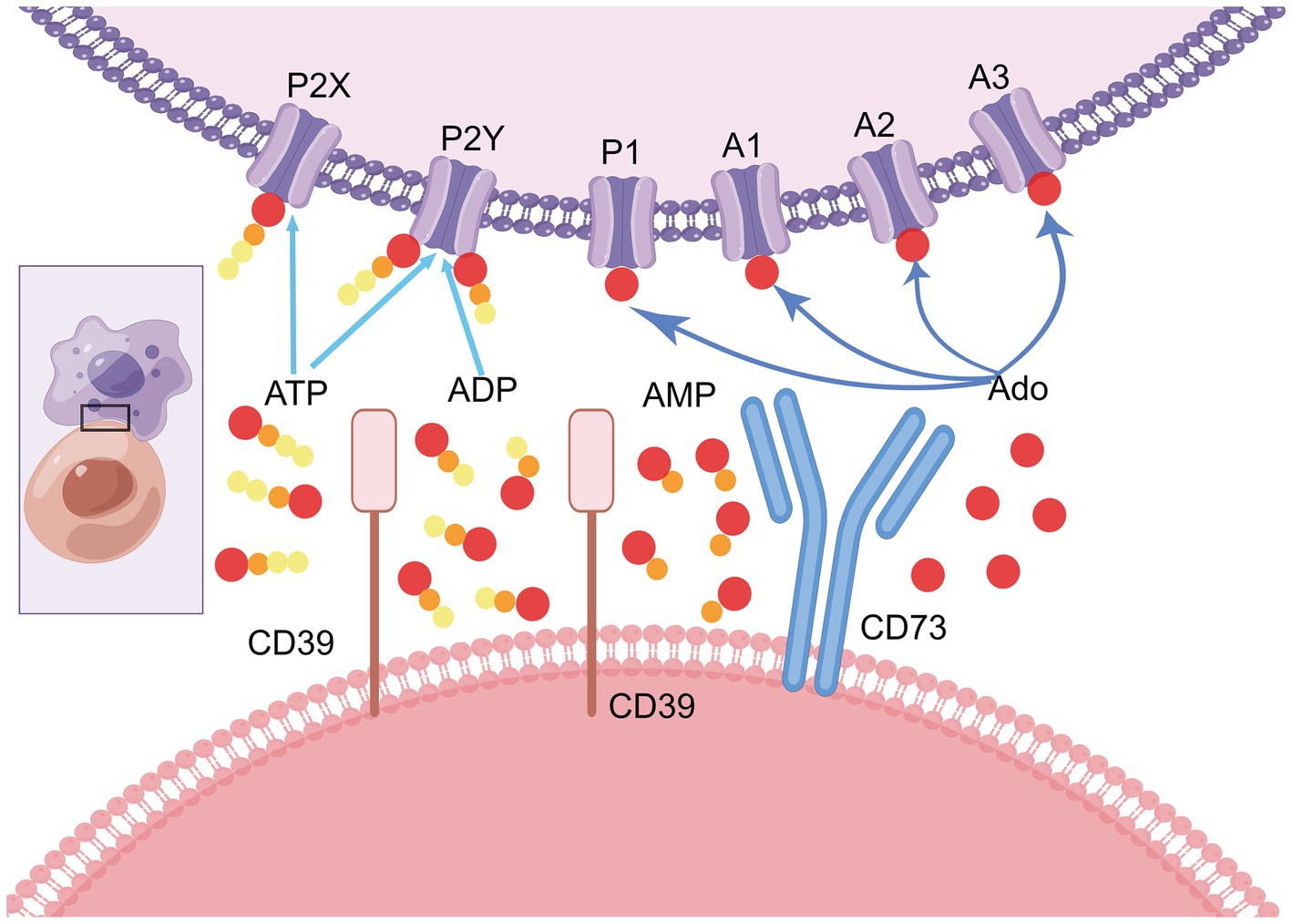
Figure 1. Adenosine signaling mediated by CD73. Affected by various environmental factors, extracellular pro-inflammatory risk factor ATP can bind to receptors such as P2X and P2Y to induce intense inflammation in the human body. Under the guidance of extracellular inflammation and energy balance mechanism, excessive pro-inflammatory ATP is gradually dephosphorylated to form AMP with the help of cell surface enzymes such as CD39, ALP and NTPases. AMP is further dephosphorylated by CD73 to produce adenosine which has immunosuppressive effects. Adenosine can tie to various adenosine receptors on cell surface like P1, A1, A2 and A3, and participate in various physiological and pathological reactions of the body, playing different functions and roles. CD73 is the absolute most significant hydrolytic protease for the conversion of AMP to adenosine. It is a critical step before adenosine binds to adenosine receptors and plays a vital role in the regulation and conduction of purine signaling pathways (by figdraw, export ID: YIYRA26629).
CD73 is expressed in a grouping of cell types, including leukocytes, myofibroblasts, endothelial cells and epithelial cells, and so on (9), particularly in tumor, immune and other related cells (10), such as macrophages, myofibroblasts, dendritic cells and NK cells (11), etc. CD73 is also expressed in neutrophils to a certain extent, which can propel liver regeneration and regulate inflammation (12). In ongoing years, the mechanism of adenosine generating enzyme CD73 has been preliminarily researched in liver diseases, including viral hepatitis, hepatic steatosis, hepatic fibrosis and hepatocellular carcinoma. Continuous examinations have shown that under the influence of multiple factors, CD73-mediated adenosine metabolism is immovably connected with the liver and dynamically regulates various pathological manifestations such as liver steatosis, inflammation, fibrosis and primary tumor (13), prompting the occurrence of all kinds of liver diseases.
As a universally prevalent chronic liver disease, viral hepatitis is a typical common infectious disease caused by a variety of hepatotropic viruses. It can cause intense and persistent liver inflammation in people, and then develop into cirrhosis and liver cancer, which seriously endangers human wellbeing globally. Purinergic signaling is firmly related to the generation and progression of liver inflammation (14). As a perilous signal that promotes inflammation, ATP has an invigorating unstable quality (15). On the contrary, ADO has anti-inflammatory effects on immune cells and can safeguard tissue integrity (16). As a key component of the adenosine pathway, CD73 is one of the cell surface compounds that decompose extracellular ATP into ADO, promoting the transformation of the body from the pro-inflammatory environment stimulated by ATP to the anti-inflammatory environment directed by adenosine (13).
After the liver is hit by the virus infection and produces inflammation, the level of ATP which is a dangerous signal of extracellular inflammation increases significantly (17), stimulates and induces neutrophils to aggregate and produce chemokines (18), and recruit immune cells with high expression of CD73 (19), deplete extracellular ATP to produce adenosine, inhibit the activation of immune cells, and control the inflammatory response (10). Simultaneously, activation of adenosine receptors can increment intracellular AMP concentration, structure a partition hindrance, block intercellular substance trade, and avoid the abnormal accumulation of immune cells to stimulate inflammation (20). CD73-intervened adenosine pathway takes immune cells such as B cells and Tregs as the principal carriers to promote adenosine production and suppress immunity, which plays an important role in the pathogenic process of several common hepatitis viruses and is considered as a new possible therapeutic target for liver viral inflammatory diseases (21).
In patients with chronic hepatitis B (CHB), when liver inflammation occurs, CD73 on the surface of B cells is diminished, the amount of extracellular adenosine production is decreased, and the activation of B cells is enhanced, prompting the progression of inflammation (22). Blocking CD73 activity in CHB patients can lead to impaired IgG conversion of B cells, temporarily slowing down humoral immune inflammation, but further aggravating later inflammation in the long term (23). As of now, there is no clear report on the internal mechanism of CD73 increase in Treg cells of patients with hepatitis B, but some studies have shown that CD73 is an important regulator of Treg cells to restrain intracellular environmental inflammation, and the author conjectures that it may be related to the independent expression of CD73 in Treg cells under inflammatory environment and the immunosuppressant effect mediated by adenosine (24). In addition, studies have found that the expression level of CD73 decreases with the increase of HBV-DNA load and liver inflammatory response. In patients with complete antiviral response, the amount of CD73 can be gradually recovered with the transformation of serum HBeAg or the reduction of HBsAg, yet after effective antiviral treatment, the expression of CD73 does not increase (22).
In addition to hepatitis B, TOX+ HCV-specific CD8 + T cells in patients infected with hepatitis C virus (HCV) express a mass of CD73 characteristic memory phenotype (25), especially in activated Treg cells (26), playing an important role in inflammation in patients with chronic hepatitis C (CHC). ZhiqinLi believes that during antiviral treatment, the overall number of Treg cells expressing higher levels of CD73 in CHC patients show a downward trend, which also explain the reason why NatashaT. Snider found in the study that the liver CD73mRNA level of CHC patients is significantly reduced (9). This phenomenon is more obvious in liver fibrosis caused by HCV (10).
In patients with chronic hepatitis E, the expression of CD73 in different cells is also significantly different. For example, studies found that the expression of CD73 on the surface of Tregs and effector T cells (Teff) in patients infected with hepatitis E virus (HEV) was increased. Moreover, the inhibitory ability of Treg cells in patients with acute hepatitis E is obviously higher than that in recovered individuals (27), which might be influenced by CD73-mediated adenosine pathway during the course of the disease. However, the activity and function of CD73 on B cell surface under HEV invasion need to be further investigated.
As for hepatitis A and hepatitis D, few similar articles have mentioned the effect of CD73 on hepatitis A. Undeniably, we found that the infection of hepatitis D depends on the replication of hepatitis B virus itself, and adenosine receptors are the necessary proteins for human hepatocytes to infect two viruses (28). However, no studies have discussed the effect of CD73 on hepatitis D through adenosine pathway, so the intervention of CD73 on the pathogenesis of hepatitis A and hepatitis D needs to be further explored.
With the improvement of modern living standards, coupled with unhealthy diet and hygiene habits, the incidence of various liver diseases increases year by year (29). Various chronic liver diseases persist and are prone to progress to irreversible end-stage liver disease, affecting normal metabolism of the body and eventually leading to death (30).
Studies have proven that CD73 is expressed at a high level in liver tissues (31), which is mainly distributed in the apical membrane of hepatocytes and endothelial cells of hepatic sinuses and bile ducts, and is expressed in bands in pericentral hepatocytes near the central vein of the liver (32). In CD73-deficient hepatocytes, AMP-dependent protein kinases are affected, resulting in the destruction of liver homeostasis and unexpected liver injury (33). What’s more, the expression of CD73 is highly regulated in chronic liver diseases (9). In terms of current research progress, CD73 can inhibit the progression of viral hepatitis to a certain extent, promote the formation of fatty liver, delay the progression of steatohepatitis, and promote the progression of liver fibrosis and liver cancer through the adenosine pathway.
Autoimmune liver disease (AILD) is a chronic, progressive immune-related liver disease caused by the activation of the body’s immune system function due to various unknown reasons. As the final rate-limiting enzyme of adenosine production, CD73 mediates purine signaling pathway, plays an important part in regulating adenosine and immune diseases (25). Tregs act an irreplaceable role in AILD (34). Studies indicate that autoimmune hepatitis (AIH) and primary biliary cirrhosis (PBC) are closely related to adenosine pathway mediated by CD73 on Treg surface.
AIH, as a chronic liver disease caused by abnormal activation of immune cells leading to interfacial hepatitis (35), is a common clinical AILD type (36). CD73-mediated adenosine pathway is immunosuppressive toward AIH, and Treg impairment and Teff subgroup activation are typical manifestations of AIH pathogenesis (13). CD73 around the surface of normal Treg cells mediates the production of immune-suppressing adenosine (24), blocks cell communication, inhibits overimmunity, and simultaneously upregates CD73 expression (10). However, the level of CD73 on the damaged Treg surface was down-regulated (37), the secretion of TGF-β and other anti-inflammatory factors was reduced (38), and the immunosuppressive function of Treg was defective (39), leading to the occurrence of AIH. On the other hand, IL-6 and TGF-β can induce the expression of CD39 and CD73 in helper T17 cells (Th17), stimulate the production of adenosine, inhibit the transformation of naive T cells into Th (40), along with reducing the production of pro-inflammatory cytokines (41), which can be charactered as immune deficiency. On the contrary, low level of CD73 stimulates the production of pro-inflammatory factors in liver cells, and the immune effect of Th17 cells continue (42), leading to the continuous progression of chronic liver inflammation.
PBC is an immune-related liver disease characterized by diffuse destruction of small bile ducts in the liver (43). The literature indicates that there may be significant individual differences in the mechanism of PBC effect (44). There are few studies on the progression of CD73 in PBC disease. Due to immune deficiency, CD73 expression in dnRIITreg from PBC mice is significantly reduced compared with WTTreg. Studies found that there are certain differences in the expression profiles of CD39 and CD73 on Tregs, which can form energy circle outside immune cells and mediate a series of immune responses, which may be close to the pathogenesis of PBC, and its internal mechanism is worthy of further lucubrating (37).
Unlike PBC, the lesions of primary sclerosing cholangitis (PSC) are mainly in the bold ducts inside and outside the liver. At present, relevant studies on the pathogenesis of CD73 in PSC are still lacking, but previous studies have shown that the loss of CD39 in the adenosine pathway can stimulate the increase of intestinal endocrine ATP, activate dendritic cells and CD8 + T cells and transport them to the liver. Damage to biliary epithelial cells induces PSC (45), which also proves that adenosine pathway is closely connected to the progression of PSC disease. Therefore, the role of CD73 in the course of PSC disease needs to be further analyzed.
Liver is an important organ for ethanol metabolism, and a large amount of ethanol metabolism tend to have toxic effects on the liver, resulting in hepatocyte damage (46), and then abnormal accumulation of metabolism-related fat, leading to hepatic steatosis. Studies have confirmed that CD73 activity is associated with ethanol-induced hepatic steatosis, mice lacking CD73 show less cell expansion and steatosis, significantly reducing the incidence of fatty liver (47). Wang, Ping et al. hypothesized that CD73-deficient mice may reduce adenosine-mediated extracellular matrix deposition through hepatic stellate cells, thereby protecting mice from ethanol induced fatty liver (10). Under chronic alcohol stimulation, AMP is released in the liver (48) and phosphorylated to adenosine catalyzed by high expression of CD73 on the cell surface, adenosine A1 and A2B receptors are activated and promote lipid depositional degeneration (47). At the same time, ethanol absorption also reduces the influx of nucleoside transporter (49), inhibits intracellular adenosine uptake and increases extracellular adenosine concentration (50), promoting the progression of fatty liver. Therefore, blocking the expression of CD73, A1 or A2B receptors in the liver can effectively reduce the accumulation of liver lipids caused by alcohol and delay the course of fatty liver disease (26).
At the same time, non-alcoholic fatty liver disease (NAFLD) is another important cause of hepatic steatosis in modern society (51). Non-alcoholic steatohepatitis (NASH), as a typical inflammatory disease of NAFLD, is closely related to the adenosine pathway. As a hydrolytic product of CD73, adenosine can perform cell protective and immunosuppressive functions through P1 receptors, thus terminating liver inflammation and promoting liver regeneration. In addition, CD73 can block the TLR4/MyD88/NF-κB signaling pathway (52), reduce the secretion of IL-6 and IL-1β, and delay the inflammatory process. In liver biopsies of NAFLD patients, CD73mRNA levels were significantly reduced (9), so CD73 knockout mice rarely developed fatty liver disease, or even progressed to steatohepatitis (13). However, under chronic inflammatory stimulation, damaged inflammatory liver cells can induce the approach of extracellular immune cells with high expression of CD39 and CD73, clear extracellular ATP, generate adenosine negative feedback to regulate endothelial cells and immune cells, inhibit white blood cell recruitment, and reduce the inflammatory response. The study on the connection between CD73-related adenosine metabolism pathway and hepatic fatty lesions is worth further exploration.
Hepatic fibrosis is a reaction of repeated prolongation of various chronic liver lesions, causing liver self-limiting healing (53), which can lead to the formation of cirrhosis. CD73, as the final rate-limiting enzyme produced by adenosine (25), has an important place in the process of liver fibrosis. CD73 is weakly expressed in normal hepatic stellate cells and portal vein fibroblasts, while its activity is enhanced in hepatic fibrosis (54) and significantly increased in cirrhosis (55). Among them, hepatic stellate cell (HSC) activation is a pivotal feature of hepatic fibrosis, CD73 and HSC activation interact with each other to jointly promote the process of hepatic fibrosis (56). For example, the CD73-adenosine-A1R axis regulates HSC activation and apoptosis through the PLC-IP3-Ca2+/DAG-PKC signaling pathway (57). CD73-deficient mice are resistant to the development of liver fibrosis (58) and protect liver cells from the risk of CCl4 and thioacetamide (TAA) inducing liver fibrosis (59). After CD73 deletion, adenosine production is reduced, resulting in the suppression of HSC activation and proliferation mediated by p2 receptor and decreased collagen expression, inhibiting the production of liver fibrosis. Meanwhile, inhibition of CD73 can promote HSC apoptosis and alleviate alcohol-induced liver fibrosis (52). Similarly, A2A adenosine receptor deficient mice were also protected from the effects of liver fibrosis by blocking the adenosine pathway (60). Just the opposite, after alcohol intake, CD73 is activated in acetaldehyde induced HSC, and the expressions of pro-fibrotic cytokines TGF-β, α-SMA and type I and III collagen are increased (61), promoting the generation of liver fibrosis. Activated HSC can up-regulate CD73 expression through specific SP1 and SMAD promoter elements (58). These studies have verified the intrinsic influence of CD73 expression and HSC activation and their co-promoting effect on liver fibrogenesis. Therefore, blocking CD73 expression may be an important approach for the treatment of hepatic fibrosis.
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related death and is one of the most frequent malignant lesions of digestive tract in the world (62). A large number of literatures have elaborated that CD73 is an important regulatory protein in the progression of various malignant tumors and is highly expressed in cancer tissues (63). CD73 was significantly increased in HCC patients and negatively correlated with overall survival (64). Studies have stated that CD73 is highly expressed in about 50% of HCC samples (55), which promotes the progression and metastasis of tumors, and can be used as a reference indicator for poor prognosis of HCC clinical outcomes (65). Recent studies claimed that tumor microenvironment (TME), as the basis for tumor survival, provides the driving force for tumor proliferation and metastasis (64). Purinergic signaling pathway is the main immunosuppressive mechanism of TME (66). As one of the core enzymes of adenosine pathway, CD73 is expressed on the surface of tumor endothelial cells, regulatory T cells (Treg), NK cells, medullogenic suppressor cells (MDSC), tumor-associated macrophages and other cells of TME. Regulated by epidermal growth factor receptor (HER) and other molecules (67), TME can induce immune escape to improve intracellular AMP level and start downstream signaling pathways, such as promoting proliferation induction of Tregs (68), blocking Teff aggregation and invasion (69), reducing NK cytotoxicity (70), and stimulating MDSC and macrophage polarization (71). Inhibit the production of cytokines, reduce the antigen-presenting effect of tumor (72), and inhibit anti-tumor response. CD73 has also been verified to motivate the increase of tumor vascular endothelial growth factor (VEGF), promote angiogenesis (73) and help tumor cells survive (74). Additionally, CD73 may also be in relation to inflammatory cancer signal transduction in liver cancer. Through A1R, A2AR, A2BR, and A3 adenosine receptor signaling pathways, CD73 stimulates inflammation, provides a suitable pro-inflammatory environment for tumor cells to survive, and plays an important role in the occurrence, development and metastasis of hepatocellular carcinoma. As mentioned above, inflammatory cells generally have low expression of CD73, while HCC cells with high expression of CD73 release a large number of inflammatory factors, which may be due to the fact that adenosine pathway is not the main pathway for the generation of inflammation in tumor cells. The influence of CD73 on the relationship between inflammatory and carcinoma transformation needs to be further explored (Tables 2, 3).
The latest World Health Organization figures for 2020 show that 325 million people worldwide are living with viral hepatitis B and C. Each year there are 900,000 deaths due to hepatitis B virus infection. Back in 2016, the World Health Organization set a goal of eliminating hepatitis B as a public health threat by 2030. Hepatitis virus infection is a serious harm to human health, and it is still a global public health problem worthy of attention.
The incidence of liver virus infection is related to the number of virus replication and the strength of the body’s immunity. The progression of the disease is due to the continuous replication of the virus and the poor immunity of the body, resulting in progressive damage to the liver cells, causing a series of serious consequences. At present, antiviral therapy is difficult to achieve the ideal state of completely removing virus from the body. Therefore, the focus of treatment of viral hepatitis is to regulate the immune function of the body and weaken the damage of virus metabolism to the liver.
Studies have confirmed that the CD73-mediated adenosine pathway has a profound effect on the activated immune response of B cells. The activation of B cells induced by low expression of CD73 is an essential part of the effective immune response against HBV infection and a reference idea for the future treatment of viral liver inflammation and restoration of liver immune homeostasis. On the other hand, CD73 content increases, inducing adenosine-mediated immunosuppression. Whether this is also influenced by liver pathological microenvironment such as hepatitis B viral load, liver inflammation and antiviral intervention, and the role of CD73 in cellular immunity needs to be further studied.
Similarly, under the stimulation of hepatitis C and hepatitis E virus, the expression of CD73 on the surface of activated Treg increased, but the number of Treg cells in patients with hepatitis C decreased, so the overall content of CD73 decreased, inducing immune inflammation in the liver. However, there is still a lack of experimental studies on the effect of CD73 on humoral immunity under hepatitis C and hepatitis E virus infection in the existing literature, which needs to be improved.
As an important link in the adenosine pathway, CD73 co-conducts with upstream ATP and AMP and downstream adenosine and adenosine receptors, and is involved in the occurrence and development of viral hepatitis and other liver diseases. In view of the important role of CD73 and its related metabolic pathways in liver diseases, Targeted intervention of CD73 can be one of the key breakthroughs in the future treatment of liver diseases and restoration of environmental homeostasis in the liver, which has a good application prospect. At present, there have been experimental or clinical reports on related drugs, such as metformin, which can inhibit CHB immune-related pathogenesis by regulating CD73 adenosine pathway (22). In addition, some studies have also found that CD73-related pathways in the field of traditional Chinese medicine. For example, cordycepin, as an adenosine analogue, can specifically activate adenosine receptors and improve chronic inflammation caused by liver fat accumulation in an immunosuppressive environment with high CD73 expression (75). Curcumin can inhibit the carcinogenic effect of aflatoxin on liver to a certain extent through CD73-mediated purine pathway (76), induce the differentiation of bone marrow mesenchymal stem cells, and promote liver regeneration.
Recent studies have proved that there are certain differences in the expression levels of CD73mRNA and protein in two different kinds of mice (9). This also means that the intrinsic influence of species genes on CD73 expression, including viral metabolism in the liver, needs to be further confirmed by further studies.
As an important part of the adenosine pathway, CD73 is the key to the transition from AMP to Ado, which implies partial intervention in energy metabolism and immune regulation. For CD73 itself, it exists as a protein widely spread in various cells of the body, is one of the components of human gene expression. For example, as for liver cancer, CD73 not only acts as an intermediary to help tumor cells evade immune monitoring and regulate internal environmental inflammation, but also provides nutritional support and metastasis pathway to tumor cells by promoting angiogenesis. Therefore, following studies should explore more value of CD73 affecting human metabolism and pathological changes from different new ideas and perspectives.
With the continuous development of more and more experimental studies based on protein CD73, many drugs targeting CD73 have been introduced into the clinic, which can be widely used in the field of liver disease in the future, especially providing new treatment options for patients with chronic hepatitis B and chronic hepatitis C, delaying the onset of cirrhosis and liver cancer, and hopefully improving the quality of life of patients with liver diseases.
YC conceived and guided the study. HS, HD and QS completed the main part of this work, SW is responsible for the submitting of manuscripts. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (NSFC) 81903974 to HS and 82070804 to YC, the Natural Science Foundation of Jiangsu Province BK20221421 to HS, and the Natural Science Foundation of Nanjing University of Chinese Medicine XZR2021022 to HS.
This study is related to the project National Natural Science Foundation of China (NSFC) 81903974 to HS and 82070804 to YC. We also appreciate the Natural Science Foundation of Jiangsu Province BK20221421 to HS, and the Natural Science Foundation of Nanjing University of Chinese Medicine XZR2021022 to HS for their financial support of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor MY declared a shared parent affiliation with the author YC at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Moon, AM, Singal, AG, and Tapper, EB. Contemporary epidemiology of chronic liver disease and cirrhosis. Clin Gastroenterol Hepatol. (2020) 18:2650–66. doi: 10.1016/j.cgh.2019.07.060
2. Sarin, SK, Kumar, M, Eslam, M, al Mahtab, M, Akbar, SMF, Jia, J, et al. Liver diseases in the asia-pacific region: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. (2020) 5:167–228. doi: 10.1016/S2468-1253(19)30342-5
3. Di Virgilio, F, Jacobson, KA, and Williams, M. Geoffrey Burnstock-An accidental pharmacologist. Biochem Pharmacol. (2021) 187:114421. doi: 10.1016/j.bcp.2021.114421
4. Patritti-Cram, J, Coover, RA, Jankowski, MP, and Ratner, N. Purinergic signaling in peripheral nervous system glial cells. Glia. (2021) 69:1837–51. doi: 10.1002/glia.23969
5. Bhattarai, S, Freundlieb, M, Pippel, J, Meyer, A, Abdelrahman, A, Fiene, A, et al. α,β-Methylene-ADP (AOPCP) derivatives and analogues: development of potent and selective ecto-5’-nucleotidase (CD73) inhibitors. J Med Chem. (2015) 58:6248–63. doi: 10.1021/acs.jmedchem.5b00802
6. Pasquini, S, Contri, C, Borea, PA, Vincenzi, F, and Varani, K. Adenosine and inflammation: here, there and everywhere. Int J Mol Sci. (2021) 22:7685. doi: 10.3390/ijms22147685
7. Yegutkin, GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. BBA-Mol Cell Res. (2008) 1783:673–94. doi: 10.1016/j.bbamcr.2008.01.024
8. Sauer, AV, Brigida, I, Carriglio, N, Jofra Hernandez, R, Scaramuzza, S, Clavenna, D, et al. Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood. (2012) 119:1428–39. doi: 10.1182/blood-2011-07-366781
9. Snider, NT, Griggs, NW, Singla, A, Moons, DS, Weerasinghe, SVW, Lok, AS, et al. CD73 (ecto-5’-nucleotidase) hepatocyte levels differ across mouse strains and contribute to mallory-denk body formation. Hepatology. (2013) 58:1790–800. doi: 10.1002/hep.26525
10. Wang, P, Jia, J, and Zhang, D. Purinergic signalling in liver diseases: pathological functions and therapeutic opportunities. JHEP Reports. (2020) 2:100165. doi: 10.1016/j.jhepr.2020.100165
11. Beldi, G, Wu, Y, Banz, Y, Nowak, M, Miller, L, Enjyoji, K, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. Hepatology. (2008) 48:841–52. doi: 10.1002/hep.22401
12. Pulte, ED, Broekman, MJ, Olson, KE, Drosopoulos, JHF, Kizer, JR, Islam, N, et al. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb Res. (2007) 121:309–17. doi: 10.1016/j.thromres.2007.04.008
13. Wang, S, Gao, S, Zhou, D, Qian, X, Luan, J, and Lv, X. The role of the CD39-CD73-adenosine pathway in liver disease. J Cell Physiol. (2021) 236:851–62. doi: 10.1002/jcp.29932
14. Bours, MJL, Dagnelie, PC, Giuliani, AL, Wesselius, A, and Di Virgilio, F. P2 receptors and extracellular ATP: a novel homeostatic pathway in inflammation. Front Biosci (Schol Ed). (2011) 3:1443–56. doi: 10.2741/235
15. Subauste, CS. The CD40-ATP-P2X(7) receptor pathway: cell to cell cross-talk to promote inflammation and programmed cell death of endothelial cells. Front Immunol. (2019) 10:10. doi: 10.3389/fimmu.2019.02958
16. Linden, J, Koch-Nolte, F, and Dahl, G. “Purine release, metabolism, and signaling in the inflammatory response,” in Annual Review of Immunology, Vol. 37. ed. WM Yokoyama Annual Reviews (2019). 325–47. doi: 10.1146/annurev-immunol-051116-052406
17. Zoetewij, JP, van de Water, B, de Bont, HJ, and Nagelkerke, JF. The role of a purinergic P2z receptor in calcium-dependent cell killing of isolated rat hepatocytes by extracellular adenosine triphosphate. Hepatology (Baltimore, MD). (1996) 23:858–65. doi: 10.1002/hep.510230429
18. McDonald, B, Pittman, K, Menezes, GB, Hirota, SA, Slaba, I, Waterhouse, CCM, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. (2010) 330:362–6. doi: 10.1126/science.1195491
19. Alvarenga, DM, Mattos, MS, Araujo, AM, Antunes, MM, and Menezes, GB. Neutrophil biology within hepatic environment. Cell Tissue Res. (2018) 371:589–98. doi: 10.1007/s00441-017-2722-9
20. Eltzschig, HK, Ibla, JC, Furuta, GT, Leonard, MO, Jacobson, KA, Enjyoji, K, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A(2B) receptors. J Exp Med. (2003) 198:783–96. doi: 10.1084/jem.20030891
21. Wang, X, Yuan, X, Su, Y, Hu, J, Ji, Q, Fu, S, et al. Targeting purinergic receptor P2RX1 modulates intestinal microbiota and alleviates inflammation in colitis. Front Immunol. (2021) 12:12. doi: 10.3389/fimmu.2021.696766
22. Zhou, S-N, Zhang, N, Liu, H-H, Xia, P, Zhang, C, Song, JW, et al. Skewed CD39/CD73/adenosine pathway contributes to B-cell hyperactivation and disease progression in patients with chronic hepatitis B. Gastroenterol Rep. (2021) 9:49–58. doi: 10.1093/gastro/goaa048
23. Schena, F, Volpi, S, Faliti, CE, Penco, F, Santi, S, Proietti, M, et al. Dependence of immunoglobulin class switch recombination in B cells on vesicular release of ATP and CD73 ectonucleotidase activity. Cell Rep. (2013) 3:1824–31. doi: 10.1016/j.celrep.2013.05.022
24. Deaglio, S, Dwyer, KM, Gao, W, Friedman, D, Usheva, A, Erat, A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. (2007) 204:1257–65. doi: 10.1084/jem.20062512
25. Allard, D, Chrobak, P, Allard, B, Messaoudi, N, and Stagg, J. Targeting the CD73-adenosine axis in immuno-oncology. Immunol Lett. (2019) 205:31–9. doi: 10.1016/j.imlet.2018.05.001
26. Li, Z, Ping, Y, Yu, Z, Wang, M, Yue, D, Zhang, Z, et al. Dynamic changes in CD45RA Foxp3(high) regulatory T-cells in chronic hepatitis C patients during antiviral therapy. Int J Infect Dis. (2016) 45:5–12. doi: 10.1016/j.ijid.2016.02.006
27. Rathod, SB, Das, R, Thanapati, S, Arankalle, VA, and Tripathy, AS. Suppressive activity and altered conventional phenotype markers/mediators of regulatory T cells in patients with self-limiting hepatitis E. J Viral Hepat. (2014) 21:141–51. doi: 10.1111/jvh.12125
28. Taylor, JM, and Han, Z. Purinergic receptor functionality is necessary for infection of human hepatocytes by hepatitis delta virus and hepatitis B virus. PLoS One. (2010) 5:e15784. doi: 10.1371/journal.pone.0015784
29. Huang, DQ, El-Serag, HB, and Loomba, R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2021) 18:223–38. doi: 10.1038/s41575-020-00381-6
30. Xue, R, Yang, RX, and Fan, JG. Epidemiological trends and clinical characteristic of NAFLD/MAFLD in Asia. J Dig Dis. (2022) 23:354–7. doi: 10.1111/1751-2980.13117
31. Alcedo, KP, Bowser, JL, and Snider, NT. The elegant complexity of mammalian ecto-5’-nucleotidase (CD73). Trends Cell Biol. (2021) 31:829–42. doi: 10.1016/j.tcb.2021.05.008
32. Minor, M, Alcedo, KP, Battaglia, RA, and Snider, NT. Cell type- and tissue-specific functions of ecto-5’-nucleotidase (CD73). Am J Phys Cell Phys. (2019) 317:C1079–92. doi: 10.1152/ajpcell.00285.2019
33. Alcedo, KP, Rouse, MA, Jung, GS, Fu, D, Minor, M, Willcockson, HH, et al. CD73 maintains hepatocyte metabolic integrity and mouse liver homeostasis in a sex-dependent manner. Cell Mol Gastroenterol Hepatol. (2021) 12:141–57. doi: 10.1016/j.jcmgh.2021.01.016
34. Vuerich, M, Harshe, RP, Robson, SC, and Longhi, MS. Dysregulation of adenosinergic signaling in systemic and organ-specific autoimmunity. Int J Mol Sci. (2019) 20:528. doi: 10.3390/ijms20030528
35. Geller, SA. Autoimmune hepatitis: Histopathology. Clinical Liver Disease. (2014) 3:19–23. doi: 10.1002/cld.301
36. Lad, SG, Kolhe, K, Chauhan, S, Gattani, M, Sethiya, P, Singh, GK, et al. AIH in HIV: a very much possible entity. J Clin Exp Hepatol. (2022) 12:1388–92. doi: 10.1016/j.jceh.2022.05.003
37. Tanaka, H, Zhang, W, Yang, GX, Ando, Y, Tomiyama, T, Tsuneyama, K, et al. Successful immunotherapy of autoimmune cholangitis by adoptive transfer of forkhead box protein 3(+) regulatory T cells. Clin Exp Immunol. (2014) 178:253–61. doi: 10.1111/cei.12415
38. Regateiro, FS, Howie, D, Nolan, KF, Agorogiannis, EI, Greaves, DR, Cobbold, SP, et al. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur J Immunol. (2011) 41:2955–65. doi: 10.1002/eji.201141512
39. Huang, C, Shen, Y, Shen, M, Fan, X, Men, R, Ye, T, et al. Glucose metabolism reprogramming of regulatory t cells in concanavalin a-induced hepatitis. Front Pharmacol. (2021) 12:726128. doi: 10.3389/fphar.2021.726128
40. Csoka, B, Himer, L, Selmeczy, Z, Vizi, ES, Pacher, P, Ledent, C, et al. Adenosine A(2A) receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. (2008) 22:3491–9. doi: 10.1096/fj.08-107458
41. Hynes, TR, Yost, EA, Yost, SM, Hartle, CM, Ott, BJ, and Berlot, CH. Inhibition of Galphas/cAMP signaling decreases TCR-stimulated IL-2 transcription in CD4(+) T helper cells. J Mol Signal. (2015) 10:2. doi: 10.5334/1750-2187-10-2
42. Liberal, R, Grant, CR, Ma, Y, Csizmadia, E, Jiang, ZG, Heneghan, MA, et al. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease. J Autoimmun. (2016) 72:102–12. doi: 10.1016/j.jaut.2016.05.005
43. Ide, R, Oshita, A, Nishisaka, T, Nakahara, H, Aimitsu, S, and Itamoto, T. Primary biliary cholangitis metachronously complicated with combined hepatocellular carcinoma-cholangiocellular carcinoma and hepatocellular carcinoma. World J Hepatol. (2017) 9:1378–84. doi: 10.4254/wjh.v9.i36.1378
44. Kawata, K, Yang, G-X, Ando, Y, Tanaka, H, Zhang, W, Kobayashi, Y, et al. Clonality, activated antigen-specific CD8(+) T cells, and development of autoimmune cholangitis in dnTGF beta RII Mice. Hepatology. (2013) 58:1094–104. doi: 10.1002/hep.26418
45. Peng, Z-W, Rothweiler, S, Wei, G, Ikenaga, N, Liu, SB, Sverdlov, DY, et al. The ectonucleotidase ENTPD1/CD39 limits biliary injury and fibrosis in mouse models of sclerosing cholangitis. Hepatol Commun. (2017) 1:957–72. doi: 10.1002/hep4.1084
46. Rocco, A, Compare, D, Angrisani, D, Zamparelli, MS, and Nardone, G. Alcoholic disease: liver and beyond. World J Gastroenterol. (2014) 20:14652–9. doi: 10.3748/wjg.v20.i40.14652
47. Peng, Z, Borea, PA, Wilder, T, Wilder, T, Yee, H, Chiriboga, L, et al. Adenosine signaling contributes to ethanol-induced fatty liver in mice. J Clin Investig. (2009) 119:582–94. doi: 10.1172/JCI37409
48. Puig, JG, and Fox, IH. Ethanol-induced activation of adenine nucleotide turnover. Evidence for a role of acetate. J Clin Invest. (1984) 74:936–41. doi: 10.1172/JCI111512
49. Nagy, LE, Diamond, I, Casso, DJ, Franklin, C, and Gordon, AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. (1990) 265:1946–51. doi: 10.1016/S0021-9258(19)39923-5
50. Nagy, LE, Diamond, I, and Gordon, AS. cAMP-dependent protein kinase regulates inhibition of adenosine transport by ethanol. Mol Pharmacol. (1991) 40:812–7.
51. Alonso, C, Fernandez-Ramos, D, Varela-Rey, M, Martínez-Arranz, I, Navasa, N, Van Liempd, SM, et al. Metabolomic Identification of Subtypes of Nonalcoholic Steatohepatitis. Gastroenterology. (2017) 152:1449–1461.e7. doi: 10.1053/j.gastro.2017.01.015
52. Liu, Z-N, Wu, X, Fang, Q, Li, ZX, Xia, GQ, Cai, JN, et al. CD73 attenuates alcohol-induced liver injury and inflammation via blocking TLR4/MyD88/NF-kappa B signaling pathway. J Inflamm Res. (2022) 15:53–70. doi: 10.2147/JIR.S341680
53. Bu, F-t, Jia, P-c, Zhu, Y, Yang, YR, Meng, HW, Bi, YH, et al. Emerging therapeutic potential of adeno-associated virus-mediated gene therapy in liver fibrosis. Mol Ther-Methods Clin Dev. (2022) 26:191–206. doi: 10.1016/j.omtm.2022.06.009
54. Vuerich, M, Robson, SC, and Longhi, MS. Ectonucleotidases in intestinal and hepatic inflammation. Front Immunol. (2019) 10:507. doi: 10.3389/fimmu.2019.00507
55. Jain, S, and Jacobson, KA. Purinergic signaling in liver pathophysiology. Front Endocrinol. (2021) 12:718429. doi: 10.3389/fendo.2021.718429
56. Hernandez-Gea, V, and Friedman, SL. “Pathogenesis of liver fibrosis” in Annual review of pathology: mechanisms of disease, Vol. 6. eds. AK Abbas, SJ Galli, and PM Howley. Annual Reviews. (2011) 425–56
57. Liu, Z, Wu, X, Wang, Q, Li, Z, Liu, X, Sheng, X, et al. CD73-adenosine A(1)R axis regulates the activation and apoptosis of hepatic stellate cells through the PLC-IP3-Ca2+/DAG-PKC signaling pathway. Front Pharmacol. (2022) 13:922885. doi: 10.3389/fphar.2022.922885
58. Fausther, M, Sheung, N, Saiman, Y, Bansal, MB, and Dranoff, JA. Activated hepatic stellate cells upregulate transcription of ecto-5’-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am J Physiol-Gastrointestinal Liver Physiol. (2012) 303:G904–14. doi: 10.1152/ajpgi.00015.2012
59. Peng, Z, Fernandez, P, Wilder, T, Yee, H, Chiriboga, L, Chan, ESL, et al. Ecto-5’-nucleotidase (Cd73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis. Nucleosides Nucleotides Nucleic Acids. (2008) 27:821–4. doi: 10.1080/15257770802146403
60. Chiang, DJ, Roychowdhury, S, Bush, K, McMullen, MR, Pisano, S, Niese, K, et al. Adenosine 2A receptor antagonist prevented and reversed liver fibrosis in a mouse model of ethanol-exacerbated liver fibrosis. PLoS One. (2013) 8:e69114. doi: 10.1371/journal.pone.0069114
61. Shuai, C, Xia, G-q, Yuan, F, Wang, S, and Lv, X-w. CD39-mediated ATP-adenosine signalling promotes hepatic stellate cell activation and alcoholic liver disease. Eur J Pharmacol. (2021) 905:174198. doi: 10.1016/j.ejphar.2021.174198
62. Testino, G, Leone, S, Patussi, V, Scafato, E, and Borro, P. Hepatocellular carcinoma: diagnosis and proposal of treatment. Minerva Med. (2016) 107:413–26.
63. Roh, M, Wainwright, DA, Wu, JD, Wan, Y, and Zhang, B. Targeting CD73 to augment cancer immunotherapy. Curr Opin Pharmacol. (2020) 53:66–76. doi: 10.1016/j.coph.2020.07.001
64. Snider, NT, Altshuler, PJ, Wan, S, Welling, TH, Cavalcoli, J, and Omary, MB. Alternative splicing of human NT5E in cirrhosis and hepatocellular carcinoma produces a negative regulator of ecto-5’-nucleotidase (CD73). Mol Biol Cell. (2014) 25:4024–33. doi: 10.1091/mbc.e14-06-1167
65. Ma, X-L, Hu, B, Tang, W-G, Xie, SH, Ren, N, Guo, L, et al. CD73 sustained cancer-stem-cell traits by promoting SOX9 expression and stability in hepatocellular carcinoma. J Hematol Oncol. (2020) 13:11. doi: 10.1186/s13045-020-0845-z
66. Young, A, Ngiow, SF, Gao, Y, Patch, AM, Barkauskas, DS, Messaoudene, M, et al. A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res. (2018) 78:1003–16. doi: 10.1158/0008-5472.CAN-17-2826
67. Beavis, PA, Stagg, J, Darcy, PK, and Smyth, MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. (2012) 33:231–7. doi: 10.1016/j.it.2012.02.009
68. Ohue, Y, and Nishikawa, H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. (2019) 110:2080–9. doi: 10.1111/cas.14069
69. Sundstrom, P, Stenstad, H, Langenes, V, Ahlmanner, F, Theander, L, Ndah, TG, et al. Regulatory T cells from colon cancer patients inhibit effector T-cell migration through an adenosine-dependent mechanism. Cancer Immunol Res. (2016) 4:183–93. doi: 10.1158/2326-6066.CIR-15-0050
70. Wang, J, and Matosevic, S. NT5E/CD73 as correlative factor of patient survival and natural killer cell infiltration in glioblastoma. J Clin Med. (2019) 8. doi: 10.3390/jcm8101526
71. del Barrio, IM, Penski, C, Schlahsa, L, Stein, RG, Diessner, J, Wöckel, A, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages – a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer. (2016) 4:4. doi: 10.1186/s40425-016-0154-9
72. Stagg, J, and Smyth, MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. (2010) 29:5346–58. doi: 10.1038/onc.2010.292
73. Allard, B, Turcotte, M, Spring, K, Pommey, S, Royal, I, and Stagg, J. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. (2014) 134:1466–73. doi: 10.1002/ijc.28456
74. van de Veen, W, Globinska, A, Jansen, K, Straumann, A, Kubo, T, Verschoor, D, et al. A novel proangiogenic B cell subset is increased in cancer and chronic inflammation. Sci Adv. (2020) 6:eaaz3559. doi: 10.1126/sciadv.aaz3559
75. Patil, S, Reda, R, Boreak, N, Taher, HA, Melha, AA, Albrakati, A, et al. Adipogenic stimulation and pyrrolidine dithiocarbamate induced osteogenic inhibition of dental pulp stem cells is countered by cordycepin. J Pers Med. (2021) 11:915. doi: 10.3390/jpm11090915
Keywords: CD73, adenosine pathway, liver diseases, HBV, HCV
Citation: Shi H, Dai H, Sun Q, Wang S and Chen Y (2023) CD73, a significant protein in liver diseases. Front. Med. 10:1147782. doi: 10.3389/fmed.2023.1147782
Received: 26 January 2023; Accepted: 17 March 2023;
Published: 12 April 2023.
Edited by:
Ming Yue, Nanjing Medical University, ChinaReviewed by:
Xiaolin Wang, Shanghai Jiao Tong University, ChinaCopyright © 2023 Shi, Dai, Sun, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huilian Shi, c2hpaHVpbGlhbjgyMEAxNjMuY29t; Yuanyuan Chen, eXVhbnl1YW5jaEBuam11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.