
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 16 March 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1140185
This article is part of the Research Topic Insights in Gastroenterology: 2022 View all 6 articles
Objective: This study was to explore the relationship between fibrinogen and advanced colorectal adenoma among inpatients.
Methods: From April 2015 to June 2022, 3738 participants (566 case subjects and 3172 control subjects) who underwent colonoscopies enrolled, and smooth curve fitting and logistic regression models were applied to explore the association between fibrinogen and advanced colorectal adenoma. In addition, sensitivity and subgroup analyses were performed to assess the stability of the results.
Results: Compared with lower fibrinogen quantile 1 (< 2.4 g/L), the adjusted OR values for fibrinogen and advanced colorectal adenoma in quantile 2 (2.4–2.75 g/L), quantile 3 (2.76–3.15 g/L), and quantile 4 (≥3.16 g/L) were 1.03 (95% confidence interval [CI]: 0.76–1.41), 1.37 (95% CI: 1.01–1.85), and 1.43 (95% CI: 1.06–1.94), respectively. A linear relationship between fibrinogen and advanced colorectal adenoma was observed. Sensitivity and subgroup analyses showed stable results.
Conclusion: Complements the evidence that fibrinogen was positively associated with advanced adenomas, suggesting that fibrinogen may play a role in the adenoma-carcinoma sequence.
A colorectal adenoma is the precursor to colorectal cancer (CRC) (1). The adenoma-carcinoma sequence is central to the pathogenesis of CRC (2). Although most CRCs originate from colorectal adenoma (3–5), most common adenomas cannot develop into cancer (5, 6), and advanced colorectal adenomas are instead more likely to become cancerous.
Some coagulation indicators have been recognized as potential biomarkers for CRC (7–10). However, research on risk factors for advanced adenomas, a critical stage in the adenoma-carcinoma pathway, is limited (11–13), especially for the components of the hemostatic system (14–16).
Fibrinogen is a glycoprotein produced in the liver (10). The interaction of fibrinogen with the perivascular environment influences the progression of the disease beyond its conventional role in the acute hemostatic cascade and is associated with the disease that has inflammatory components, particularly with pro-inflammatory effects in several types of cancer (17). The relationship between fibrinogen and CRC has been studied. Preoperative elevated fibrinogen levels were associated with poor prognosis/disease-free survival and worst response to treatment and tumor size (18–21). Based on the theory of adenoma-carcinoma sequence and the existing studies suggesting that fibrinogen may influence the progression from adenoma to CRC, we hypothesized that fibrinogen is associated with advanced adenoma.
Electronic medical records generate a wealth of clinical data that can describe conditions in detail and are the hallmark of modern healthcare systems. Clinical data can be extracted and integrated, cleaned and transformed, and converted into a data format to create a database that can be used for disease management, early diagnosis, or treatment decisions (22).
Studies targeting advanced colorectal adenoma are more critical to understand the adenoma-carcinoma sequence (12) and be beneficial for developing strategies to prevent CRC (12). Given that the association between fibrinogen plasma levels and advanced adenoma has not been studied, we conducted this case–control study to assess the association between fibrinogen plasma levels and advanced adenoma.
The present study was conducted among 3,738 consecutive inpatients who underwent colonoscopies from April 2015 to June 2022. Subjects with advanced colorectal adenoma who underwent pathological examination to confirm the diagnosis were considered eligible cases. Subjects with normal colonoscopies during the same period were considered controls. Each patient was analyzed only once.
Exclusion criteria: history of colorectal surgery, an incomplete colonoscopy (no cecum reached), inadequate bowel preparation, younger than 18, incomplete medical records, history of any cancer treatment within 3 years, combined with malignancies, and fibrinogen data missing. A total of 3,738 subjects (566 cases and 3,172 controls) were included. Shown in Figure 1.
For the current study, individuals with advanced colorectal adenoma, defined in another piece of literature (23), comprised the case group.
Plasma fibrinogen was measured using a Mindray CX-9000 automatic coagulation analyzer (Shenzhen, China) and its associated reagents. As soon as possible after collection, all blood samples were processed within 4 h. In the present study, the median time between colonoscopy and fibrinogen testing is 2 (1, 5) days.
The baseline variables were extracted from electronic medical records, including age, sex, weight, marital status, past medical history, family history (including colorectal and digestive system malignancy), drinking status, smoking status, and co-morbidities. Marital status is divided into three categories: married, single/divorced, and other. The history of smoking and drinking were classified into four categories, respectively: never, former, current, and NA (not recorded). A current drinker is defined as an individual who consumes any type of alcohol per week (self-reported) (24). An ex-smoker or ex-drinker is defined as someone who has smoked or used alcohol in the previous period, although the smoking has now ceased. Co-morbidities included hypertension, ischemic cerebrovascular disease, coronary heart disease (CHD), hyperlipidemia (HLP), liver diseases (including liver cirrhosis, fatty liver, and hepatitis), and diabetes mellitus (DM). Individuals with DM were diagnosed with one of the following criteria: HbA1c ≥6.5%, fasting glucose ≥7.0 mmol/L (25, 26), or medical records. In addition, we extracted the data of participants from the laboratory information system and electronic medical records during their hospitalization, including fibrinogen, albumin (ALB), aspartate aminotransferase (AST), alanine aminotransferase (ALT), glucose (GLU), alkaline phosphatase (ALP), creatinine (CREA), urea, uric acid (UA), platelets (PLT), thrombin time (TT), activated partial thromboplastin time (APTT), the international normalized ratio of prothrombin time (PT-INR), prothrombin time (PT), activated partial thromboplastin time (APTT). If there are multiple eligible test reports for the same test during the hospitalization period, the first result within this period of hospitalization will prevail (before the colonoscopy).
Continuous variables are expressed as mean ± standard deviation or median (quartile 1–quartiles 3) values according to the normality of the distribution. Comparisons between groups were performed by the chi-squared test or Fisher exact test and Student’s t-test or the Mann–Whitney U-test for categorical variables or continuous variables, respectively, as appropriate.
We evaluated the relationship between fibrinogen and advanced colorectal adenoma using smooth curve fitting and logistic regression analysis. To evaluate the impact of fibrinogen, fibrinogen level was categorized into quartiles (quartile 1, <2.40 g/L; quartile 2, 2.40–2.75 g/L; quartile 3, 2.76–3.15 g/L; quartile 4, ≥3.16 g/L). We constructed three models: (i) crude model, no other covariates were adjusted; (ii) adjustment by age and sex; and (iii) adjustment by age, sex, hypertension, DM, APTT, PLT, ALP, and CREA. These potential confounders were chosen based on previous scientific literature, or a more than 10% change in effect estimates. Subgroup analyses were performed using a binary logistic regression model and then performed an interaction test. In the subgroup analyses, considering the small number of ex-smokers and ex-drinkers, we integrated ex-smokers and ex-drinkers with non-smokers and non-drinkers as a group, respectively. Multiple imputations (five replications), based on a chained equation approach method in the R mice procedure, were performed to maximize statistical power and reduce bias to account for missing data. In addition, sensitivity analysis was conducted using all complete cases.
All statistical analyses were conducted by packages R 3.3.2 (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.7.1, p < 0.05 (two-tailed test) was considered statistically significant.
The retrospective study was approved by the Ethics Review Board of Shijiazhuang Traditional Chinese Medicine Hospital (NO.20220919029). Requirements for informed consent were waived due to the retrospective nature.
A total of 3,738 subjects, including 566 subjects with advanced colorectal adenoma and 3,172 with normal colonoscopies, were included in this study. The subjects’ baseline characteristics were shown in Table 1. Some differences existed between groups concerning various covariates, including age, sex, weight, smoking status, drinking status, fibrinogen, ALB, ALP, UA, GLU, CREA, Urea, PLT, hypertension, ischemic cerebrovascular disease, CHD, DM (all p < 0.05).
In multivariable logistic regression analyses, a positive relationship was found between fibrinogen and advanced colorectal adenoma. In the crude model, fibrinogen was found to be positively related to advanced colorectal adenoma [odds ratio (OR), 1.36; 95% confidence interval (CI), 1.21–1.53]. The results were similar after adjusting for age and gender (OR, 1.17; 95% CI, 1.03–1.33). After adjustment for sex, age, hypertension, DM, APTT, PLT, CREA, ALP, and ALB, the OR value was also stable (OR, 1.14; 95% CI, 0.99–1.3). When fibrinogen was performed as a quartile for analysis, a positive association between them was shown even after adjustment for potential confounders. Compared to participants with low fibrinogen in quartile 1 (<2.40 g/L), the adjusted OR values for fibrinogen and advanced colorectal adenoma in quartile 2 (2.40–2.75 g/L), quartile 3 (2.76–3.15 g/L), and quartile 4 (≥3.16 g/L) were 1.03 (95% CI: 0.76–1.41, p = 0.834), 1.37 (95% CI:1.01–1.85, p = 0.04), and 1.43 (95% CI: 1.06–1.96, p = 0.019; Table 2), respectively. The quartile 3 and quartile 4 groups (≥2.76 g/L) had the higher advanced colorectal adenoma incidence. To further explore the association between fibrinogen and advanced colorectal adenoma, smooth curve fitting was performed, and the results present a linear association between them (only 99% of the data was shown, P for non-linearity: 0.536, Figure 2).
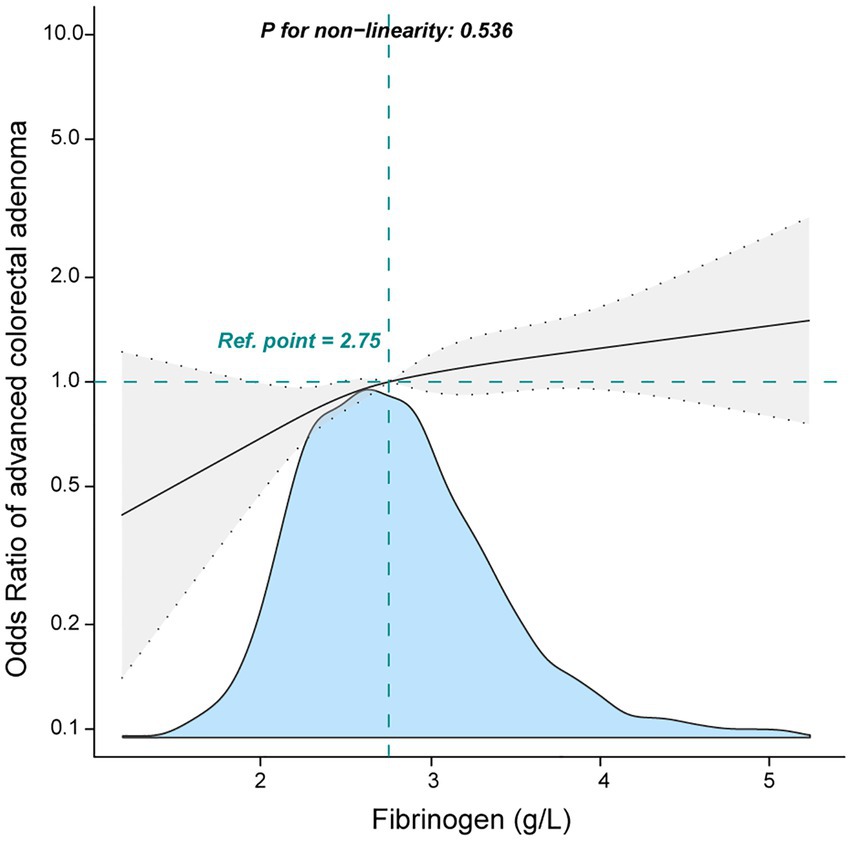
Figure 2. Association between fibrinogen and advanced colorectal adenoma among inpatients. They were adjusted for age, sex, albumin, alkaline phosphatase, creatinine, platelets, activated partial thromboplastin time, hypertension, and diabetes mellitus.
The subgroup analyses were conducted to explore the relationship between fibrinogen and advanced colorectal adenoma among different layers. None of the variables, including age (<65 years, or ≥ 65 years), sex (male and female), smoking status, drinking status, history of cancer, DM, CHD, HLP, hypertension, ischemic cerebrovascular disease, significantly affected the relationship between fibrinogen and advanced colorectal adenoma (Figure 3). In subgroup analyses, we performed more indicators that have a significant difference in Table 1 (Supplementary Table S2). The results were stable as well.
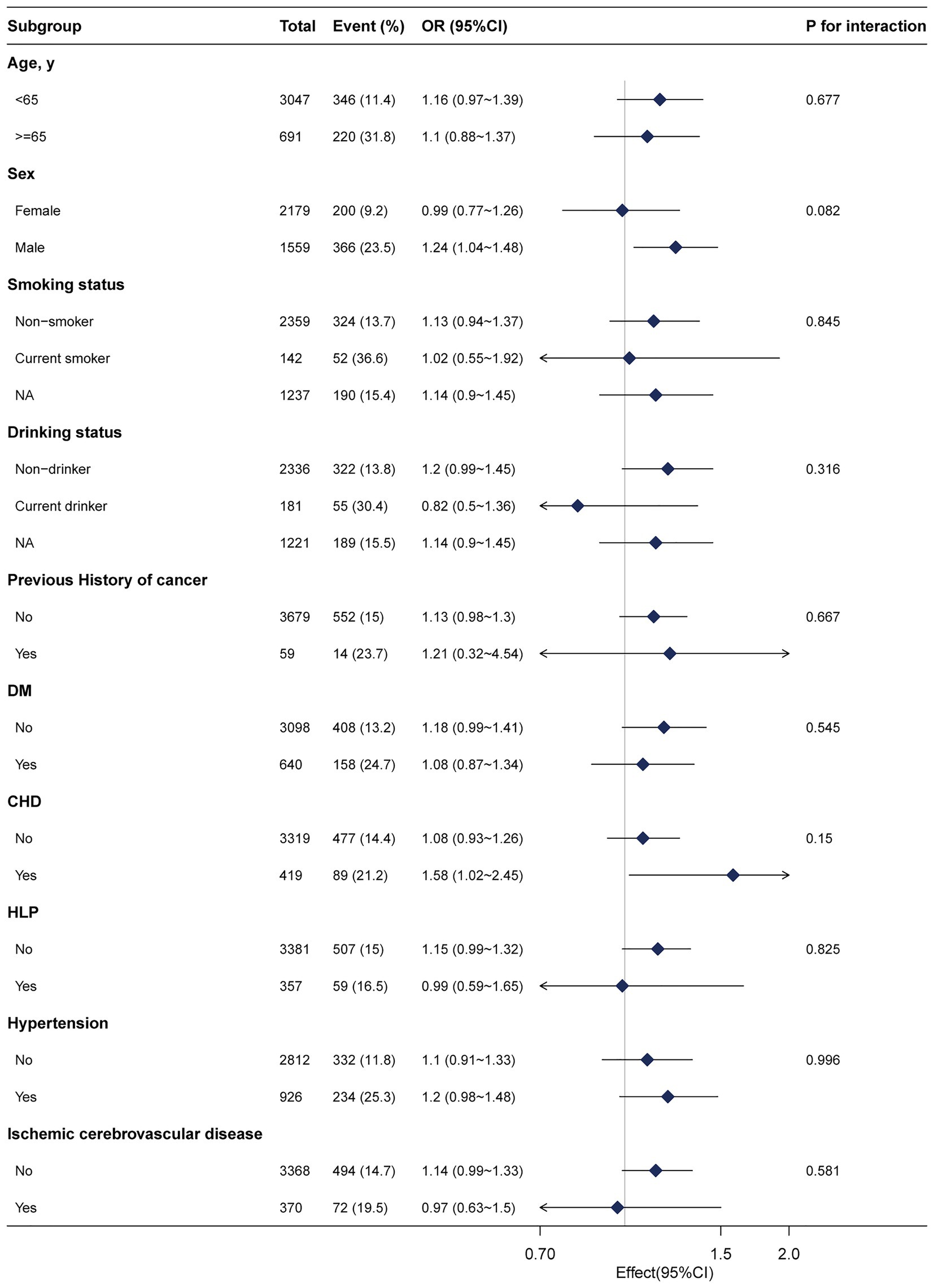
Figure 3. Subgroup analyses of the fibrinogen and advanced colorectal adenoma among inpatients. Except for the stratification component itself, each stratification factor was adjusted for age, sex, albumin, alkaline phosphatase, creatinine, platelets, activated partial thromboplastin time, hypertension, and diabetes mellitus. NA, not recorded.
In addition, sensitivity analysis was performed using the complete cases. All patients with missing data were excluded, and 3,738 individuals were left. And the association between fibrinogen and advanced colorectal adenoma remained stable [Model I: OR (95% CI), 1.17 (1.02–1.35); Model II: OR (95% CI), 1.13 (0.98–1.37)]. When serum GGT as a categorized variable, compared with individuals with lower fibrinogen in quartile 1 (<2.40 g/L), the adjusted OR values for fibrinogen and advanced colorectal adenoma in quartile 2 (2.40–2.74 g/L), quartile 3 (2.75–3.15 g/L), and quartile 4 (≥3.16 g/L) were 1.02 (95% CI: 0.73–1.42, p = 0.903), 1.39 (95% CI:1.01–1.9, p = 0.042), and 1.41 (95% CI: 1.02–1.94, p = 0.036; Model II, Supplementary Table SI), respectively. Furthermore, we used the fibrinogen-Z score in the multivariable logistic regression analyses (Supplementary Table S3) and found that with an elevated FIB-Z Score, the risk of advanced colorectal adenoma increased. These findings indicated the results were stable.
This study explored the association between fibrinogen and advanced colorectal adenoma among inpatients. The fibrinogen was independently associated with advanced colorectal adenoma with linear association curves among inpatients. The present study found that the incidence of advanced colorectal adenoma increased as fibrinogen increased. The relationship between fibrinogen and advanced colorectal adenoma was stable between layers.
To our knowledge, there are no clinical studies demonstrating the correlation between fibrinogen and advanced adenoma. However, some literature may substantiate the relationship between them. Several studies have analyzed the plasma proteome of patients with advanced adenoma or colorectal cancer and found elevated fibrinogen α and β chains in the adenoma-cancer sequence, suggesting that fibrinogen may play a critical role in tumor progression (27–29). In addition, only two studies have associated plasma fibrinogen levels with CRC. Kristine et al. found elevated fibrinogen to be associated with an increased risk of CRC in 84,000 subjects, and this relationship was greatest during the early follow-up years. In addition, the authors suggest that the possible role of fibrinogen in CRC may be interpreted through its part in the inflammatory reaction (30). This finding supports the chronic low-level inflammation as a latent contributor of cancer development (30, 31). A nested case-cohort study indicated that fibrinogen (≥400 mg/dl) was positively correlated with CRC, suggesting that it may be a latent biomarker of CRC and supporting the “common soil” hypothesis (32). Advanced adenoma, a precursor of CRC, also showed an increased risk associated with elevated fibrinogen levels in our study. Complements the evidence that fibrinogen was positively associated with advanced adenomas.
Fibrinogen in the adenoma-carcinoma progression can be interpreted in terms of both hemostatic and inflammatory factors, and several hypotheses have been proposed. (i) Fibrinogen can bind growth factors (33), and therefore, fibrinogen residing on the cell matrix may act as a reservoir to control the bioavailability and accessibility of growth factors, and affect cancer cell proliferation, inhibit apoptosis, angiogenesis, and metastasis (34). (ii) Fibrinogen can bind to several types of cells. Fibrinogen-mediated cell bridging can exert traction for adhesion, shape change, motility, and invasion potential of cancer cells (35). (iii) Fibrinogen contributes to the protection of neoplastic cells from natural killer cell cytotoxicity through the interaction of β3 integrin’s with platelets, thus allowing escape from host immune surveillance (36). (iv) Fibrinogen is a critical regulator of inflammation in diseases, including cancer (17), and may have a role in cellular signaling by interaction with cellular receptors (37). Such interactions may promote inflammation, angiogenesis, and cell proliferation (33). Overall, fibrinogen effects and favors the adenoma-carcinoma progression, whether as an inflammatory or hemostatic factor.
Our study also had several limitations. First, the present research had limitations inherent to retrospective studies. We collected the participant’s data from the laboratory information system and electronic medical records, there were missing data for some indicators. However, we performed a sensitivity analysis and found that the results were stable with or without multiple imputations of the data. Second, in a present retrospective study, we did not collect repeated measurements for fibrinogen, which may not represent their long-term levels and their association with advanced colorectal adenoma. Third, even though multivariable logistic regression, subgroup analyses, and sensitivity analysis were performed, residual confounding effects from unmeasured or unknown factors could not be completely excluded. Finally, the findings were analyzed in a single-center database from China, and the generalizability may be limited for populations with different demographic characteristics.
Studying risk factors for advanced colorectal adenoma could help develop more comprehensive and non-invasive screening recommendations and possibly provide a clearer understanding of the mechanism of colon cancer. Studies on the epidemiology of advanced adenomas may be crucial in revealing why only a fraction of common adenomas progresses to CRC.
A linear association between fibrinogen and advanced colorectal adenoma was found among inpatients. With an increase in plasma fibrinogen, the risk of advanced adenoma increased. These findings provide evidence that fibrinogen may be a potential high-risk factor for colorectal screening.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Review Board of Shijiazhuang Traditional Chinese Medicine Hospital (NO.20220919029). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HW and HuZ conceived and designed the study and wrote and revised the manuscript. HW, XC, PM, JL, ZW, TZ, and HaZ collected and analyzed the data. All authors contributed to the article and approved the submitted version.
This work was supported by the Hebei Administration of Traditional Chinese Medicine (grant number 2023145).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1140185/full#supplementary-material
1. Chen, YX, Gao, QY, Zou, TH, Wang, BM, Liu, SD, Sheng, JQ, et al. Berberine versus placebo for the prevention of recurrence of colorectal adenoma: a multicentre, double-blinded, randomised controlled study. Lancet Gastroenterol Hepatol. (2020) 5:267–75. doi: 10.1016/s2468-1253(19)30409-1
2. Vogelstein, B, Fearon, ER, Hamilton, SR, Kern, SE, Preisinger, AC, Leppert, M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. (1988) 319:525–32. doi: 10.1056/NEJM198809013190901
3. Fearon, ER, and Vogelstein, B. A genetic model for colorectal tumorigenesis. Cells. (1990) 61:759–67. doi: 10.1016/0092-8674(90)90186-i
4. Hill, MJ, Morson, BC, and Bussey, HJ. Aetiology of adenoma--carcinoma sequence in large bowel. Lancet. (1978) 1:245–7. doi: 10.1016/s0140-6736(78)90487-7
5. Neugut, AI, Jacobson, JS, and De Vivo, I. Epidemiology of colorectal adenomatous polyps. Cancer Epidemiol Biomark Prev. (1993) 2:159–76. doi: 10.1016/j.bpg.2017.06.004
6. Peipins, LA, and Sandler, RS. Epidemiology of colorectal adenomas. Epidemiol Rev. (1994) 16:273–97. doi: 10.1093/oxfordjournals.epirev.a036154
7. Falanga, A, Santoro, A, Labianca, R, De Braud, F, Gasparini, G, D’alessio, A, et al. Hypercoagulation screening as an innovative tool for risk assessment, early diagnosis and prognosis in cancer: the hypercan study. Thromb Res. (2016) 140:S55–9. doi: 10.1016/s0049-3848(16)30099-8
8. Lima, LG, and Monteiro, RQ. Activation of blood coagulation in cancer: implications for tumour progression. Biosci Rep. (2013) 33:e00064. doi: 10.1042/bsr20130057
9. Mosesson, MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. (2005) 3:1894–904. doi: 10.1111/j.1538-7836.2005.01365.x
10. Vilar, R, Fish, RJ, Casini, A, and Neerman-Arbez, M. Fibrin(ogen) in human disease: both friend and foe. Haematologica. (2020) 105:284–96. doi: 10.3324/haematol.2019.236901
11. Stegeman, I, De Wijkerslooth, TR, Stoop, EM, Van Leerdam, ME, Dekker, E, Van Ballegooijen, M, et al. Colorectal cancer risk factors in the detection of advanced adenoma and colorectal cancer. Cancer Epidemiol. (2013) 37:278–83. doi: 10.1016/j.canep.2013.02.004
12. Terry, MB, Neugut, AI, Bostick, RM, Potter, JD, Haile, RW, and Fenoglio-Preiser, CM. Reliability in the classification of advanced colorectal adenomas. Cancer Epidemiol Biomark Prev. (2002) 11:660–3.
13. Wark, PA, Wu, K, van’t Veer, P, Fuchs, CF, and Giovannucci, EL. Family history of colorectal cancer: a determinant of advanced adenoma stage or adenoma multiplicity? Int J Cancer. (2009) 125:413–20. doi: 10.1002/ijc.24288
14. Niederseer, D, Stadlmayr, A, Huber-Schönauer, U, Plöderl, M, Schmied, C, Lederer, D, et al. Cardiovascular risk and known coronary artery disease are associated with colorectal adenoma and advanced neoplasia. J Am Coll Cardiol. (2017) 69:2348–50. doi: 10.1016/j.jacc.2017.02.065
15. Park, J, Han, JS, Jo, HJ, Kim, HY, Yoon, H, Shin, CM, et al. Resting heart rate is associated with colorectal advanced adenoma. PLoS One. (2021) 16:e0254505. doi: 10.1371/journal.pone.0254505
16. Yang, SY, Kim, YS, Chung, SJ, Song, JH, Choi, SY, Park, MJ, et al. Association between colorectal adenoma and coronary atherosclerosis detected by ct coronary angiography in korean men; a cross-sectional study. J Gastroenterol Hepatol. (2010) 25:1795–9. doi: 10.1111/j.1440-1746.2010.06330.x
17. Davalos, D, and Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. (2012) 34:43–62. doi: 10.1007/s00281-011-0290-8
18. Li, M, Wu, Y, Zhang, J, Huang, L, Wu, X, and Yuan, Y. Prognostic value of pretreatment plasma fibrinogen in patients with colorectal cancer: a systematic review and meta-analysis. Medicine. (2019) 98:e16974. doi: 10.1097/md.0000000000016974
19. Lin, Y, Liu, Z, Qiu, Y, Zhang, J, Wu, H, Liang, R, et al. Clinical significance of plasma d-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. (2018) 44:1494–503. doi: 10.1016/j.ejso.2018.07.052
20. Moik, F, Posch, F, Grilz, E, Scheithauer, W, Pabinger, I, Prager, G, et al. Haemostatic biomarkers for prognosis and prediction of therapy response in patients with metastatic colorectal cancer. Thromb Res. (2020) 187:9–17. doi: 10.1016/j.thromres.2020.01.002
21. Sun, Y, Han, W, Song, Y, Gao, P, Yang, Y, Yu, D, et al. Prognostic value of preoperative fibrinogen for predicting clinical outcome in patients with nonmetastatic colorectal cancer. Cancer Manag Res. (2020) 12:13301–9. doi: 10.2147/cmar.S275498
22. Wu, WT, Li, YJ, Feng, AZ, Li, L, Huang, T, Xu, AD, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. (2021) 8:44. doi: 10.1186/s40779-021-00338-z
23. Baron, JA, Cole, BF, Sandler, RS, Haile, RW, Ahnen, D, Bresalier, R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. (2003) 348:891–9. doi: 10.1056/NEJMoa021735
24. Chen, SB, Liu, DT, and Chen, YP. Prognostic value of body mass index stratified by alcohol drinking status in patients with esophageal squamous cell carcinoma. Front Oncol. (2022) 12:769824. doi: 10.3389/fonc.2022.769824
25. Alberti, KG, and Zimmet, PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. (1998) 15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
26. Kerner, W, and Brückel, J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. (2014) 122:384–6. doi: 10.1055/s-0034-1366278
27. Agatea, L, Crotti, S, Ragazzi, E, Bedin, C, Urso, E, Mammi, I, et al. Peptide patterns as discriminating biomarkers in plasma of patients with familial adenomatous polyposis. Clin Colorectal Cancer. (2016) 15:e75–92. doi: 10.1016/j.clcc.2015.12.002
28. Bedin, C, Crotti, S, Ragazzi, E, Pucciarelli, S, Agatea, L, Tasciotti, E, et al. Alterations of the plasma peptidome profiling in colorectal cancer progression. J Cell Physiol. (2016) 231:915–25. doi: 10.1002/jcp.25196
29. Fayazfar, S, Zali, H, Arefi Oskouie, A, Asadzadeh Aghdaei, H, Rezaei Tavirani, M, and Nazemalhosseini Mojarad, E. Early diagnosis of colorectal cancer via plasma proteomic analysis of crc and advanced adenomatous polyp. Gastroenterol Hepatol Bed Bench. (2019) 12:328–39.
30. Allin, KH, Bojesen, SE, and Nordestgaard, BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer. (2016) 139:1493–500. doi: 10.1002/ijc.30194
31. Grivennikov, SI, Greten, FR, and Karin, M. Immunity, inflammation, and cancer. Cells. (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
32. Parisi, R, Panzera, T, Russo, L, Gamba, S, De Curtis, A, Di Castelnuovo, A, et al. Fibrinogen levels in relation to colorectal cancer onset: a nested case-cohort study from the moli-sani cohort. Front Cardiovasc Med. (2022) 9:1009926. doi: 10.3389/fcvm.2022.1009926
33. Kabat, GC, Salazar, CR, Zaslavsky, O, Lane, DS, and Rohan, TE. Longitudinal association of hemostatic factors with risk for cancers of the breast, colorectum, and lung among postmenopausal women. Eur J Cancer Prev. (2016) 25:449–56. doi: 10.1097/CEJ.0000000000000193
34. Terry, MB, Neugut, AI, Bostick, RM, Sandler, RS, Haile, RW, Jacobson, JS, et al. Risk factors for advanced colorectal adenomas: a pooled analysis. Cancer Epidemiol Biomark Prev. (2002) 11:622–9.
35. Simpson-Haidaris, PJ, and Rybarczyk, B. Tumors and fibrinogen. The role of fibrinogen as an extracellular matrix protein. Ann N Y Acad Sci. (2001) 936:406–25. doi: 10.1111/j.1749-6632.2001.tb03525.x
36. Zheng, S, Shen, J, Jiao, Y, Liu, Y, Zhang, C, Wei, M, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. (2009) 100:859–65. doi: 10.1111/j.1349-7006.2009.01115.x
Keywords: association, fibrinogen, advanced colorectal adenoma, retrospective study, case-control study
Citation: Wang H, Zheng H, Cao X, Meng P, Liu J, Wang Z, Zhang T and Zuo H (2023) Relationship between fibrinogen level and advanced colorectal adenoma among inpatients: A retrospective case-control study. Front. Med. 10:1140185. doi: 10.3389/fmed.2023.1140185
Received: 08 January 2023; Accepted: 28 February 2023;
Published: 16 March 2023.
Edited by:
Angel Lanas, University of Zaragoza, SpainReviewed by:
Jun Lyu, First Affiliated Hospital of Jinan University, ChinaCopyright © 2023 Wang, Zheng, Cao, Meng, Liu, Wang, Zhang and Zuo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huanwei Zheng, MTMzMjMxMTkzMTdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.