- Department of Gynecological Oncology, Women's Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang, China
Background: Postpartum hemorrhage (PPH) is the most common cause of maternal morbidity and mortality worldwide. A reliable risk assessment tool for PPH could optimize available interventions to reduce adverse maternal outcomes.
Objective: The objective of this study was to explore a nomogram predicting the risk of postpartum hemorrhage after cesarean delivery for twin pregnancies.
Methods: This single-center retrospective cohort study conducted twin pregnancies who underwent cesarean delivery between January 2014 and July 2021. Propensity score matching at baseline was used to match PPH (blood loss ≥1000 mL) and non-PPH group (blood loss <1000 mL). A nomogram was developed to predict the risk of PPH in cesarean delivery for twin pregnancies. The receiver operating characteristic curve (ROC), calibration plot, and decision curve analysis (DCA) were, respectively, used to evaluate the discrimination, calibration, and clinical utility of the prediction models.
Results: After propensity score matching, 186 twin pregnancies in the PPH group were matched with 186 controls in the non-PPH group. Seven independent prognostic variables, including antepartum albumin, assisted reproductive technology, hypertensive disorders of pregnancy, placenta previa, placenta accrete spectrum, intrapartum cesarean delivered, and estimated weights of twins, were used to build the nomogram. Based on the performance of the model, it appears that a good calibration (Hosmer–Lemeshow χ2 = 4.84, P > 0.05), an excellent predictive ability (area under the curve: 0.778, 95% CI: 0.732–0.825), and a good positive net benefit in the predictive model have been achieved.
Conclusion: The nomogram was first generated to predict PPH in cesarean delivery for twin pregnancies, which could help clinicians to provide a reference for the preoperative surgical plan, choose optimal treatments, optimize healthcare resources, and thereby reduce the associated adverse maternal outcomes.
Introduction
Globally, postpartum hemorrhage is the leading cause of maternal mortality and morbidity, accounting for nearly one-third of all maternal deaths (1, 2). With the widespread availability of ART, the incidence of multiple pregnancies is increasing, accompanying with obstetric complications. Compared with singleton pregnancies, twin pregnancies have a higher risk of PPH. Hence, the perinatal management of twin pregnancies needs to receive high priority (3–7).
The purpose of this study was to develop a model that can predict the risk of postpartum hemorrhage after cesarean delivery in twin pregnancies using clinical data, identify women at risk of PPH, and improve the capability of PPH prediction. The model was incorporated into a specially designed nomogram that could be used as a tool to optimize the available interventions to reduce negative maternal outcomes.
Methods
Study population
Among the study population were all women who had cesarean deliveries at Women's Hospital School of Medicine Zhejiang University between January 2014 and July 2021. The exclusion criteria included vaginal delivery, gestational weeks at delivery < 28, core variable data missing, and intrauterine death of either fetus (including fetal reduction or natural death). Propensity score matching was used to match twins with PPH in a 1:1 ratio to the twins without PPH. The final dataset included 186 twin pregnancies with PPH and 186 propensity score-matched controls with non-PPH (Figure 1).
Data collection
– Primary outcome variable: According to the classifications of the American College of Obstetricians and Gynecologists, PPH is defined as estimated blood loss (EBL) ≥ 1000 mL within 24 h after delivery, regardless of delivery mode (8). To improve accuracy, a weighing method was used for hemorrhage evaluation during the 24 h following delivery. During the operation, the amount of blood loss was quantified by measuring the blood in gauze and the fluid collected via the suction tube. All women giving birth should be offered conventional IM/IV oxytocin (10 IU) after the delivery of the placenta to prevent PPH. If persistent bleeding, the use of additional oxytocin (10 IU, IV/IM) and other injectable uterotonics (i.e., carbetocin or carboprost trometamol) was recommended.
– Independent variables were as follows:
• Maternal: maternal age, gravidity, parity, pre-pregnancy body mass index (BMI), gestational age at delivery, history of intrauterine surgery, assisted reproductive technology (ART), antepartum hemoglobin, and antepartum albumin.
• Obstetric: premature rupture of membranes (PROM), diabetes mellitus (pre-gestational or gestational) (DM), hypertensive disorders of pregnancy (HDP, including chronic hypertension, gestational hypertension, preeclampsia, or eclampsia), placental abruption, placenta previa, placenta accreta spectrum (PAS), and intrapartum cesarean delivery.
• Fetal: chorionicity of twins; estimate the fetal weight (EFW) of twins.
Definitions for analysis
The continuous variables were categorized based on the clinical cutoff value. Parity was classified as primipara and multipara status. Maternal age is divided into two groups according to advanced age or not (<35 years or ≥ 35 years). Chorionicity of twins includes dichorionic-diamniotic (DCDA), monochorionic-diamniotic (MCDA), and monochorionic-monoamniotic (MCMA). Intrapartum cesarean delivery was referred to as a cesarean delivery performed after the onset of labor or induction of labor (9). Gestational age at delivery was divided into three groups: 28–33 + 6 weeks, 34–36 + 6 weeks, and >37 weeks. The EFW of twins was classified into three groups: <4000 g, 4000–4999g, and ≥5000g. Pre-pregnancy BMI was classified as follows: underweight (BMI <18.5 kg/m2), normal (18.5–23.99 kg/m2, reference), overweight (24–27.99 kg/m2), and obese (BMI ≥28 kg/m2) according to the WHO classification; (10). PAS introduced by FIGO in 2018 was defined abnormal adhesion and invasive placenta (11).
Statistical analysis
Propensity scores were calculated using logistic regression with PPH as the dependent variable. Controls were selected using propensity-matching methods based on multiple confounders simultaneously. With a caliper width of 0.3 of the standard deviation of the propensity score, propensity score matching was performed using a 1:1 matching protocol without replacement (greedy-matching algorithm).
Continuous variables were expressed as mean ± SD or interquartile range (IQR) depending on their normality. Categorical variables were expressed as numbers (percentages). The analysis was carried out by using Student's t-test or the ANOVA for continuous variates and the chi-square test/Fisher's exact test for categorical variates, respectively. The variates with p ≤ 0.1 in the univariate logistic regression analysis and a factor that did not meet the standards in the univariate analysis but was clinically significant were selected for the multivariable analysis, in which forward stepwise algorithms were conducted. The performance of the multivariable logistic regression-based nomogram was evaluated by the discrimination and calibration, using the ROC curve and its area under the curve (AUC) to illustrate its discriminating power, while a calibration curve and the Hosmer–Lemeshow (H-L) test was used to determine the adequacy of calibration. A bootstrap sampling method (Bootstrap) was used to internally validate the model. With bootstrapping, the calibration plot illustrated the association between predicted and actual probabilities, which reflected the nomogram's performance characteristics. Decision curve analyses (DCA) were employed to demonstrate the clinical usefulness of the predictive nomogram model by estimating the net benefits over a spectrum of probability thresholds. Statistical significance was defined as p < 0.05 with two-sided analysis. All data management and statistical analyses were accomplished by IBM SPSS Statistics (version 20.0) and R software (version 3.6.1).
Results
Patient characteristics
There was no statistically significant difference between the two groups in maternal age, gravidity, parity, gestational age at delivery, pre-pregnancy BMI, DM, placental abruption, chorionicity, history of intrauterine surgery, and scarred uterus. The median amount of PPH was 1350 ml and 300 ml in both groups, respectively (Table 1).
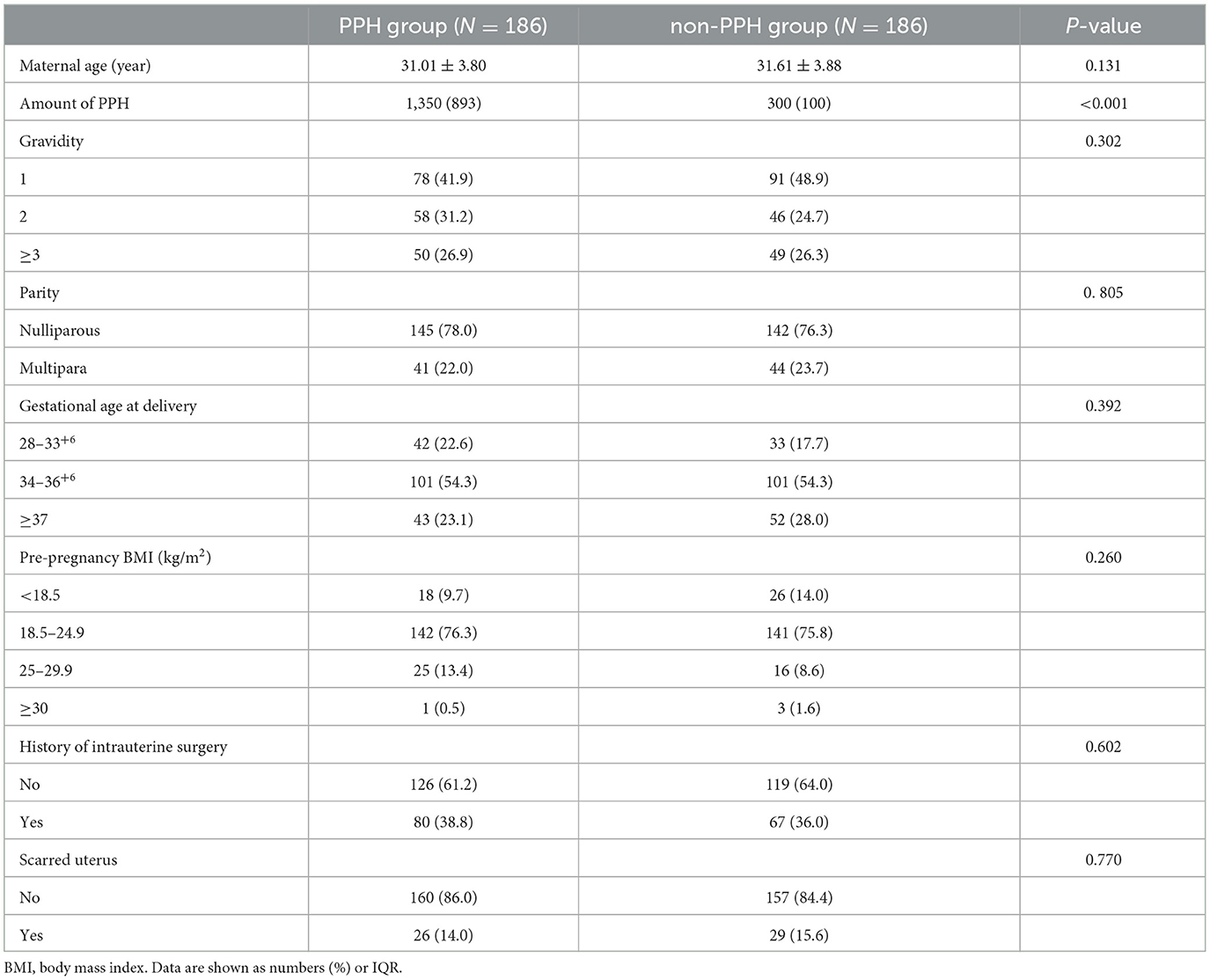
Table 1. Baseline characteristics of propensity score-matched PPH study cohort with and non-PPH control group.
Analysis of risk factors for PPH between two groups
Univariate analysis showed that twin pregnancies with PPH were more likely to have HDP (24.7 vs. 14.0%), placenta previa (29.0 vs. 1.6%), PAS (21.5 vs. 3.2%), anemic (54.% vs. 71.5%), intrapartum CD (33.9 vs. 17.2%), and hypoproteinemia compared with those with non-PPH (Table 2).
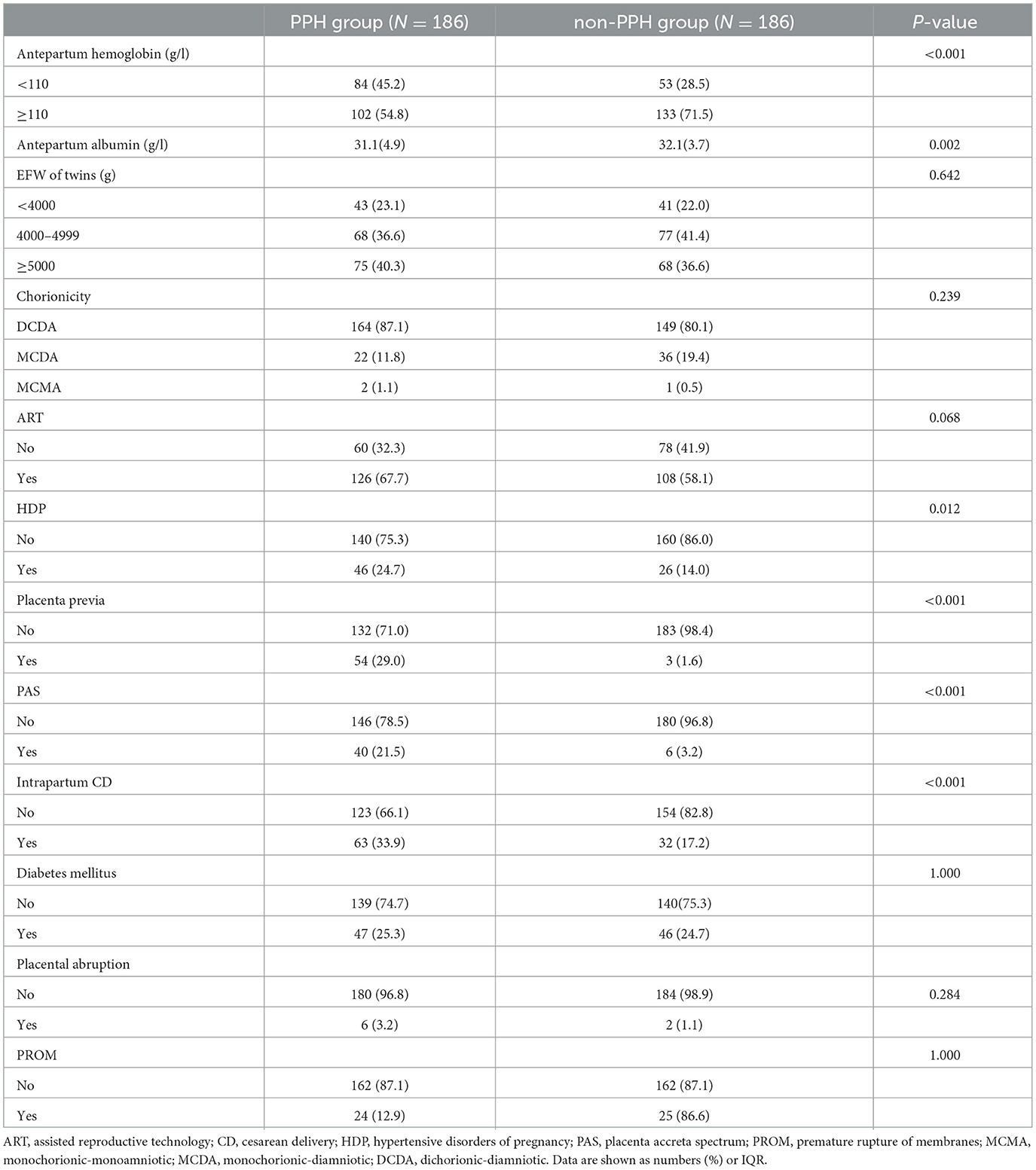
Table 2. Univariate logistic regression analysis of the risk factors for PPH after cesarean delivery of twin pregnancies.
The subsequent multivariable analysis revealed that antepartum albumin [OR = 0.92, 95% CI (0.86–0.99)], ART [OR = 1.81, 95% CI (1.08–3.03)], HDP [OR = 2.03, 95% CI (1.06–3.91)], placenta previa [OR = 28.15, 95% CI (7.95–99.76)], PAS [OR = 4.97, 95% CI (1.85–13.32)], intrapartum CD [OR = 2.48, 95% CI (1.40–4.37)], and EFW of twins were significantly related to PPH in the overall cohort. The detailed results are shown in Table 3.
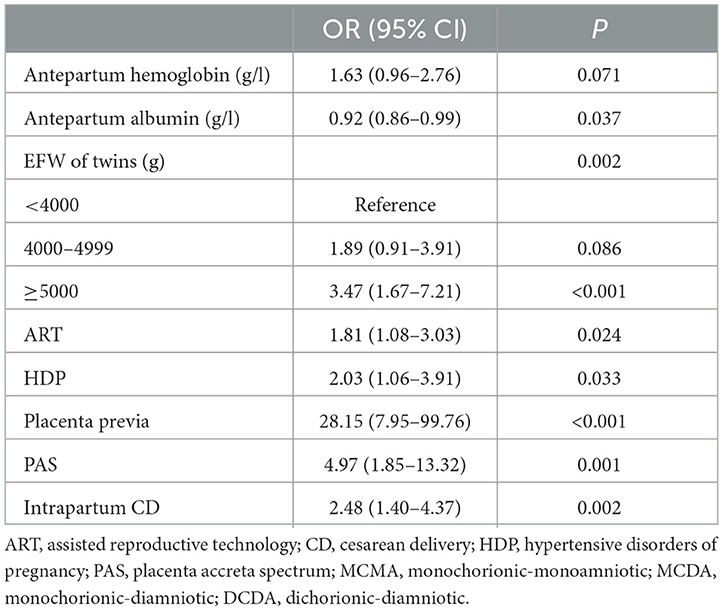
Table 3. Multivariable logistic regression analyses of the risk factors for PPH after cesarean delivery of twin pregnancies.
Development of an individualized prognostic model
A nomogram was developed to predict the risk of PPH based on the model generated by multi-factor logistic regression analysis (Figure 2). For each variable value, we can draw an upward vertical line along the point axis and then sum all those points, and then, we can draw a downward vertical line from the “Total Points” line to estimate the risk of PPH.
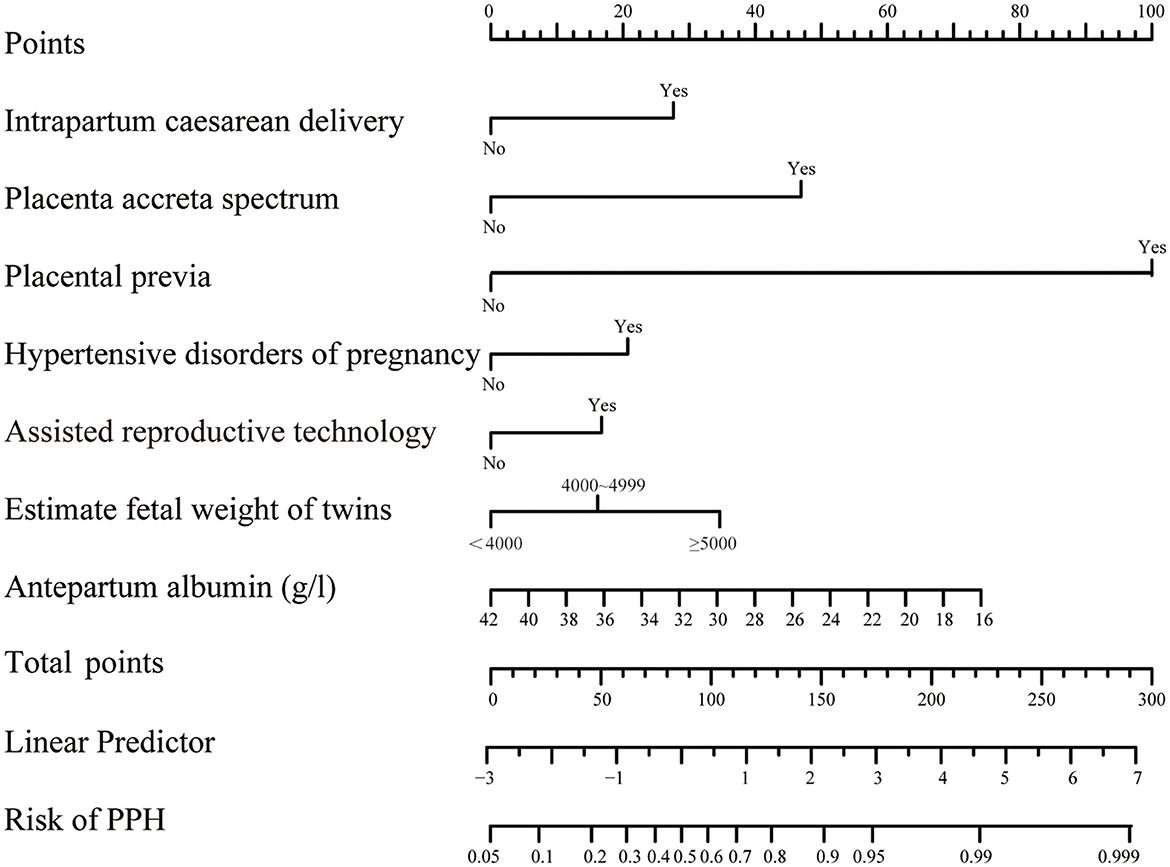
Figure 2. Nomograms for twin pregnancies in PPH probability estimate. The score of each variable was obtained by drawing a vertical line from the corresponding value to the top point axis separately, and then, the cumulative point score for all the variables was matched to the corresponding risk of postpartum hemorrhage.
Validation of the nomogram and its clinical usefulness
A nomogram based on forward stepwise multiple logistic regression was developed to estimate the probability of PPH during cesarean delivery for twin pregnancies (Figure 2). According to the ROC curve analysis, this nomogram performed well as a diagnostic model with an AUC value of 0.778, with the sensitivity and specificity of 68.3 and 78.5%, respectively (Figure 3A). The prediction effect of the model was verified using bootstrap self-sampling with 1000 replicates, and the result was visualized in the calibration curve, which demonstrated a good agreement between prediction and observation in the dataset (χ2 = 4.843, p > 0.05), implying that the nomograms predicting were well-calibrated (Figure 3B). According to our decision curve analysis, our predictive model showed good benefits for different threshold probabilities, which demonstrated a favorable clinical effect in the predictive model (Figure 4).
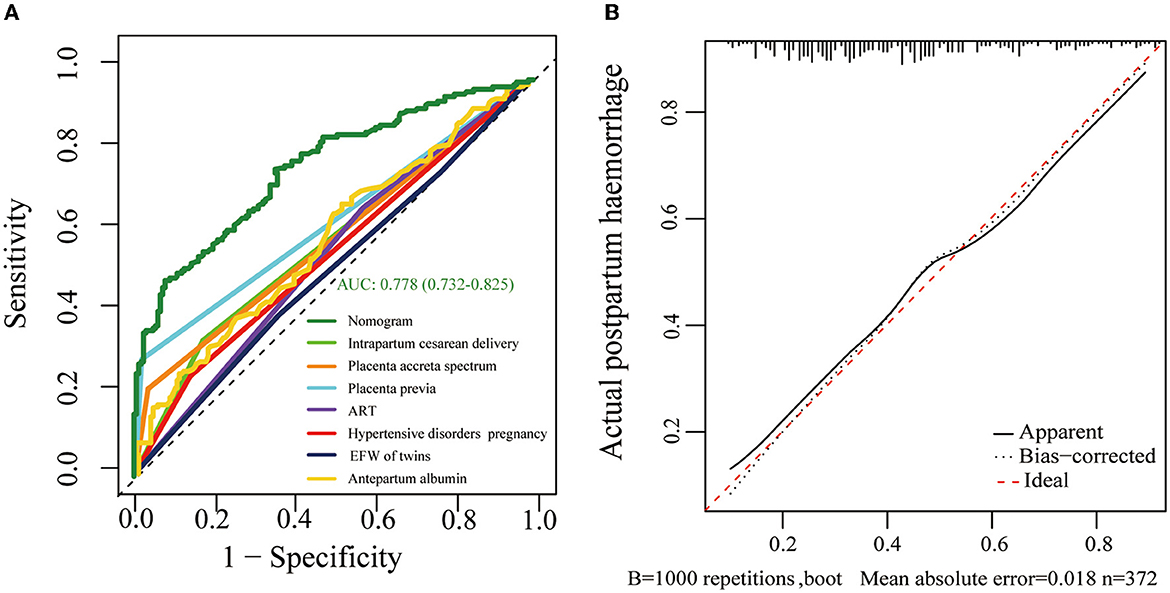
Figure 3. (A) Receiver operating characteristic (ROC) curves of the formulated nomogram model. The nomogram of the area under the receiver operating characteristic curve (AUC) was 0.778 (95% CI 0.732–0.825). (B) Calibration curve delineates the goodness of fit between the predicted probability and the actual probability. The horizontal axis represents the predicted risk of postpartum hemorrhage, and the vertical axis represents the observed outcome of the event. The diagonal dashed line signifies the ideal prediction, and the dotted line shows the predictive performance of the nomogram.
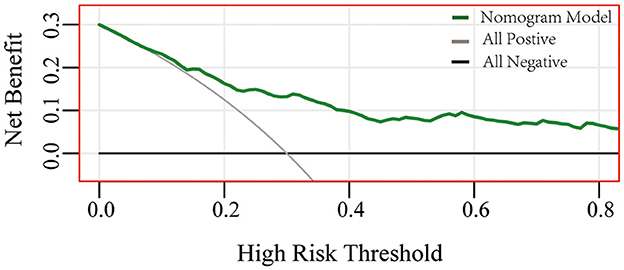
Figure 4. Performance of decision curve analysis. Decision curve analysis demonstrates the theoretical relationship between the net benefit of the model across different threshold probabilities (12). The horizontal axis represents the threshold risk of twin pregnancies patients who happened to have postpartum hemorrhage. The gray line assumes that all patients happened to postpartum hemorrhage, and the black thin line assumes no patients happened to postpartum hemorrhage. At the same threshold probability, the larger net benefit implies that more benefits patients can achieve using the model.
Discussion
In the world, postpartum hemorrhage is the leading cause of maternal mortality, accounting for 19.9 to 36.2% of all maternal deaths (1). The risk of PPH is significantly higher in twin pregnancies than in singleton pregnancies. Studies have reported that the incidence of PPH in multiple gestations is about 2.26 to 4 times higher than that in singleton pregnancies (13, 14). The high incidence of PPH in twin pregnancies makes predicting the risk of PPH extremely important from a clinical perspective. Therefore, early detection and appropriate interference of PPH without delay are crucial in preventing maternal death. In this study, we successfully developed a risk prediction model for predicting PPH in cesarean delivery for twin pregnancies, which enables prediction of the incidence of PPH before delivery to facilitate assessment and guide individualized treatment.
According to previous reports, numerous prenatal risk factors are associated with PPH (15–22). In this study, factors that could potentially affect PPH in twin pregnancies were statistically analyzed, and regression analysis revealed that HDP, placenta previa, intrapartum cesarean delivery, ART, and PAS were significantly associated with postpartum hemorrhage in twin pregnancies, which is consistent with the results of previous studies on risk factors for PPH in singleton pregnancies (9, 23–26). In addition, the results of this study suggest that antepartum albumin and EFW of twins were risk factors for postpartum hemorrhage in twin pregnancies. Antepartum hypoproteinemia may cause myometrial edema, which may be associated with uterine atony, increasing the risk of PPH (27, 28). Estimated fetal weight of twins, which has important clinical implications for PPH, is related to overdistention of the uterus, leading, in turn, to impair myometrial contractility retractability of the uterine muscles after delivery, which increases the incidence of PPH.
Although there are many studies evaluating factors that influence PPH, no appropriate predictive models could be available for clinical application for twin pregnancies. Neary et al. conducted a systematic review of 14 published prediction models for PPH, and the results showed that three of them had some clinical application potential. However, these three models were limited by their applicable population who experienced CS, placenta accreta spectrum disorders with MRI placental evaluation, or placenta previa patients (29). Blitz MJ et al. developed a model to predict PPH for twin pregnancies, which listed PPH requiring the administration of packed red blood cells (PPH + PRBC) as an outcome. However, there were some subjective factors in the decision to administer a PRBC transfusion to some extent (5). Compared to these prior models, this study first established a nomogram to predict the risk of PPH in cesarean delivery for twin pregnancies. The results show a good calibration (Hosmer–Lemeshow χ2 = 4.84, P > 0.05), an excellent predictive ability (AUC: 0.778, 95% CI: 0.732–0.825), and a good positive net benefit.
Placenta previa and placenta accrete spectrum, which acts as the strongest predictor for PPH, may result in severe PPH or even a hysterectomy (30, 31). In recent years, intravascular balloon occlusion has become an increasingly popular prophylactic measure, which includes intravascular balloon occlusion of the abdominal aorta or internal iliac arteries (32–36). During the surgery, the abdominal aorta/internal iliac artery can be temporarily blocked by the balloon, thus reducing the uterine artery's blood supply, and the hemostatic procedures can be simplified and shortened. However, intravascular balloon occlusion may also cause complications, such as thrombosis diseases, hematoma, rare artery rupture, and high healthcare costs. Given these, only for patients with very high-risk PPH, such as pernicious placenta previa complicated with placenta accreta, the technique is performed in our hospital. There were three patients were diagnosed with pernicious placenta previa complicating with placenta accreta after intravascular balloon occlusion performed in our hospital in 2015. Two of them were terminated at 28 weeks of gestation without intravascular balloon occlusion because of the low benefit of the intravascular balloon occlusion technique given the small gestational weeks. In the other case, the emergency CD termination was chosen without the intravascular balloon occlusion technique at 35 weeks because of high vaginal bleeding after contractions accompanied by unstable vital signs. We hope to introduce related data about intravascular balloon occlusion in twin pregnancies in the future.
When a high-risk patient with PPH is identified, reasonable preventive measures can be taken, such as ameliorating of patient's antepartum albumin, timeous operative delivery in cases of prolonged labor, meticulous surgical technique, intraoperative cell salvage, and the preparation of additional plasma or serum in advance. Extremely high-risk patients, such as pernicious placenta previa or placenta accrete spectrum, may be considered for prophylactic arterial balloon occlusion to reduce the rate of blood loss. However, many of the seven influencing factors could not be modified, and what we can do is make an adequate preoperative preparation and work up by an experienced multidisciplinary team.
Strengths and limitations of the study
By using nomograms, it is possible to implement complex logistic regression models in a convenient visual way, and nomograms are widely used in clinical events. As of now, predictive models for PPH have largely been developed for singleton pregnancies, and fewer have been developed for twin pregnancies. In our study, we first developed the prediction models for PPH in cesarean delivery for twin pregnancies, and the model can accurately quantify an individual's comprehensive risk of PPH with favorable discrimination, calibration, and positive net benefit.
Our study had some limitations. First, the main limitation in our study related directly to its retrospective nature, and limitation bias might be present. Second, this is a single-center study, and all subjects are limited to a single hospital, which does not reflect most women with twin pregnancies. Lastly, the study lacked external validation, more data on PPH should be included, and external validation should be performed to demonstrate the predictive ability of the nomogram in further studies.
Our study is the first to construct a nomogram for predicting postpartum hemorrhage in cesarean delivery for twin pregnancies, which exhibited a predictive performance. The nomogram can be applied in clinical practice, if the result of external verification is satisfactory, which may help accurately predict the risk of PPH and provide a reference for targeted intervention measures, thereby reducing the associated adverse maternal outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Women's Hospital School of Medicine Zhejiang University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YZ was responsible for study conceptualization and design, data curation and analysis, and writing the manuscript. WZ and LC were responsible for acquiring data, interpreting it, and determining the methodology. JL helped with the design of the study and critical revision of the manuscript. HW contributed to the design of the study, gathering, analyzing data, reviewing, and editing the manuscript. All authors have read and approved the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. The Lancet Global health. (2014) 2:e323–33. doi: 10.1016/S2214-109X(14)70227-X
2. Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. (2018) 218:75–87. doi: 10.1016/j.ajog.2017.05.067
3. Suzuki S, Hiraizumi Y, Miyake H. Risk factors for postpartum hemorrhage requiring transfusion in cesarean deliveries for Japanese twins: comparison with those for singletons. Arch Gynecol Obstet. (2012) 286:1363–7. doi: 10.1007/s00404-012-2461-9
4. Gao L, Lyu SP, Zhao XR, Wu Y, Hua RY, Wang S, et al. Systematic management of twin pregnancies to reduce pregnancy complications. Chin Med J. (2020) 133:1355–7. doi: 10.1097/CM9.0000000000000808
5. Blitz MJ, Yukhayev A, Pachtman SL, Reisner J, Moses D, Sison CP, et al. Twin pregnancy and risk of postpartum hemorrhage. J Matern Fetal Neonatal Med. (2020) 33:3740–5. doi: 10.1080/14767058.2019.1583736
6. Medicine FWGoGCPiM-F. Good clinical practice advice: Management of twin pregnancy. Int J Gynaecol Obstet. (2019) 144:330–7. doi: 10.1002/ijgo.12742
7. American college of obstetricians and gynecologists' committee on practice bulletins—obstetrics SfM-FM. Obstet Gynecol. (2021) 137:145–62. doi: 10.1097/AOG.0000000000004397
8. Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage: green-top guideline No. 52. BJOG. (2017) 124:e106–49. doi: 10.1111/1471-0528.14178
9. Butwick AJ, Ramachandran B, Hegde P, Riley ET, El-Sayed YY, Nelson LM. Risk factors for severe postpartum hemorrhage after cesarean delivery. Anesthes. Analg. (2017) 125:523–32. doi: 10.1213/ANE.0000000000001962
10. Consultation WH. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. WHO Tech Rep Series. (2000) 894: 1–253.
11. Cahill AG, Beigi R, Heine RP, Silver RM, Wax JR. Obstetric care consensus no. 7: placenta accreta spectrum. Obstet Gynecol. (2018) 132:e259-75. doi: 10.1097/AOG.0000000000002983
12. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. (2006) 26:565–74. doi: 10.1177/0272989X06295361
13. Tsakiridis I, Giouleka S, Mamopoulos A, Athanasiadis A, Dagklis T. Management of twin pregnancies: a comparative review of national and international guidelines. Obstet Gynecol Surv. (2020) 75:419–30. doi: 10.1097/OGX.0000000000000803
14. National Collaborating Centre for WS Children's H. National Institute for Health and Clinical Excellence: Guidance. Multiple Pregnancy: The Management of Twin and Triplet Pregnancies in the Antenatal Period. London: RCOG Press (2011).
15. Cahill AG, Beigi R, Heine RP, Silver RM, Wax JR. Placenta accreta spectrum. Am J Obstet Gynecol. (2018) 219:B2–16. doi: 10.1016/j.ajog.2018.09.042
16. Kim JW, Lee YK, Chin JH, Kim SO, Lee MY, Won HS, et al. Development of a scoring system to predict massive postpartum transfusion in placenta previa totalis. J Anesth. (2017) 31:593–600. doi: 10.1007/s00540-017-2365-8
17. Diag FP, Jauniaux E, Bhide A, Kennedy A, Woodward P, Hubinont C, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. Int J Gynaecol Obstet. (2018) 140:274–80. doi: 10.1002/ijgo.12408
18. Jauniaux ER, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. BJOG. (2019) 126:e1–48. doi: 10.1111/1471-0528.15306
19. Gong J, Chen Z, Zhang Y, Liu YY, Pu JC, Xiong CY, et al. Risk-factor model for postpartum hemorrhage after cesarean delivery: a retrospective study based on 3498 patients. Sci Rep. (2022) 12:22100. doi: 10.1038/s41598-022-23636-5
20. DiSciullo A, Mokhtari N, Landy H, et al. Effect of mild preoperative thrombocytopenia on postpartum hemorrhage after cesarean deliveries. Am J Obstet Gynecol MFM. (2021) 3:100368. doi: 10.1016/j.ajogmf.2021.100368
21. Kawakita T, Mokhtari N, Huang JC, Landy HJ. Evaluation of risk-assessment tools for severe postpartum hemorrhage in women undergoing cesarean delivery. Obstetr Gynecol. (2019) 134:1308–16. doi: 10.1097/AOG.0000000000003574
22. Ende HB, Lozada MJ, Chestnut DH, Osmundson SS, Walden RL, et al. Risk factors for atonic postpartum hemorrhage: a systematic review and meta-analysis. Obstet Gynecol. (2021) 137:305–23. doi: 10.1097/AOG.0000000000004228
23. Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. (2008) 115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x
24. Nyfløt LT, Sandven I, Stray-Pedersen B. Risk factors for severe postpartum hemorrhage: a case-control study. BMC Preg Childbirth. (2017) 17:17. doi: 10.1186/s12884-016-1217-0
25. Healy DL, Breheny S, Halliday J, Jaques A, Rushford D, Garrett C, et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod. (2010) 25:265–74. doi: 10.1093/humrep/dep376
26. Nyfløt LT, Sandven I, Oldereid NB, Stray-Pedersen B, Vangen S, et al. Assisted reproductive technology and severe postpartum haemorrhage: a case-control study. BJOG. (2017) 124:1198–205. doi: 10.1111/1471-0528.14471
27. Chen H, Tao F, Fang X. Association of hypoproteinemia in preeclampsia with maternal and perinatal outcomes: a retrospective analysis of high-risk women. J Res Med Sci. (2016) 21:98. doi: 10.4103/1735-1995.193170
28. Takahashi H, Hisano M, Sago H, Murashima A, Yamaguchi K. Hypoproteinemia in the second trimester among patients with preeclampsia prior to the onset of clinical symptoms. Hypert Preg. (2014) 33:55–60. doi: 10.3109/10641955.2013.837172
29. Neary C, Naheed S, McLernon DJ, Black M. Predicting risk of postpartum haemorrhage: a systematic review. BJOG. (2021) 128:46–53. doi: 10.1111/1471-0528.16379
30. Chen M, Xie L. Clinical evaluation of balloon occlusion of the lower abdominal aorta in patients with placenta previa and previous cesarean section: a retrospective study on 43 cases. Int J Surg. (2016) 34:6–9. doi: 10.1016/j.ijsu.2016.08.016
31. Jain V, Bos H, Bujold E. Guideline No. 402: diagnosis and management of placenta previa. J Obstet Gynaecol Can. (2020) 42:906–17. doi: 10.1016/j.jogc.2019.07.019
32. Chen D, Xu J, Tian Y, Ye P, Zhao F, Liu X, et al. Effect of prophylactic balloon occlusion of internal iliac artery in pregnancies complicated by placenta previa and accreta. BMC Pregnancy Childbirth. (2021) 21:640. doi: 10.1186/s12884-021-04103-x
33. Tokue H, Tokue A, Tsushima Y, Kameda T. Safety and efficacy of aortic vs internal iliac balloon occlusion for cesarean delivery in coexisting placenta accreta and placenta previa. Cardiovasc Intervent Radiol. (2020) 43:1277–84. doi: 10.1007/s00270-020-02548-9
34. Kyozuka H, Sugeno M, Murata T, Jin T, Ito F, Nomura Y. Introduction and utility of resuscitative endovascular balloon occlusion of the aorta for cases with a potential high risk of postpartum hemorrhage: a single tertiary care center experience of two cases. Fukushima J Med Sci. (2022) 68:117–22. doi: 10.5387/fms.2022-01
35. Zhu B, Yang K, Cai L. Discussion on the timing of balloon occlusion of the abdominal aorta during a caesarean section in patients with pernicious placenta previa complicated with placenta accreta. Biomed Res Int. (2017) 2017:8604849. doi: 10.1155/2017/8604849
36. Kingdom JC, Hobson SR, Murji A, Allen L, Windrim RC, Lockhart E, et al. Minimizing surgical blood loss at cesarean hysterectomy for placenta previa with evidence of placenta increta or placenta percreta: the state of play in 2020. Am J Obstet Gynecol. (2020) 223:322–9. doi: 10.1016/j.ajog.2020.01.044
Keywords: postpartum hemorrhage, nomogram, cesarean delivery, twin pregnancies, prediction model
Citation: Zhang Y, Chen L, Zhou W, Lin J and Wen H (2023) Nomogram to predict postpartum hemorrhage in cesarean delivery for twin pregnancies: a retrospective cohort study in China. Front. Med. 10:1139430. doi: 10.3389/fmed.2023.1139430
Received: 07 January 2023; Accepted: 28 March 2023;
Published: 18 April 2023.
Edited by:
Amerigo Vitagliano, University of Padua, ItalyReviewed by:
Depeng Zhao, Shenzhen Maternity and Child Healthcare Hospital, ChinaRyo Kamidani, Gifu University Hospital, Japan
Copyright © 2023 Zhang, Chen, Zhou, Lin and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Wen, d2VuaG9uZ0B6anUuZWR1LmNu
 Yanhua Zhang
Yanhua Zhang Lu Chen
Lu Chen