- 1College of Clinical Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 2School of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
- 3Clinical Medicine College, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Colorectal and Anal Surgery, Chengdu Anorectal Hospital, Chengdu, Sichuan, China
- 5Colorectal and Anal Surgery, Shenzhen Traditional Chinese Medicine Hospital, Shenzhen, China
- 6Colorectal and Anal Surgery, The First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, China
Purpose: This meta-analysis was exerted in assessing the anticancer efficacy and safety of nab-paclitaxel (nab-P) when combined with platinum compound agents for therapy in patients with non-small cell lung cancer (NSCLC).
Method: We systematically searched the following seven electronic databases: PubMed, Cochrane Library, Web of Science, Embase, CNKI, Wan Fang, and China Science and Technology Journal Data. Randomized comparative clinical [randomized controlled clinical trial (RCT)] studies on nab-P plus platinum and carboplatin or cisplatin in combination with conventional chemotherapy agents or traditional paclitaxel were searched.
Results: A total of 19 RCT studies involving 6,011 patients were analyzed. The primary outcome includes the overall response rate (ORR), overall survival (OS), and progression-free survival (PFS). The secondary outcome includes adverse events (AEs). Nab-P combined with platinum (carboplatin/cisplatin) had a better ORR [odds ratio (OR) = 1.66, 95% confidence interval (CI) (1.34, 2.05), p < 0.001] and improved PFS [hazard ratio (HR) = 0.84, 95% CI: (0.74, 0.94), p = 0.01] and OS [HR = 0.86, 95% CI: (0.78, 0.96), p = 0.008] in NSCLC patients. ORR [OR = 2.18, 95% CI: (1.07, 4.43)], PFS [HR = 0.62, 95% CI: (0.40, 0.97)], and OS [HR = 0.63, 95% CI: (0.49, 0.81)] were significantly improved among patients aged >70 years, and ORR [OR = 1.80, 95% CI: (1.20, 2.70)] and PFS [HR = 0.74, 95% CI: (0.56, 0.97)] were significantly elevated with SCC rate ≥65% in NSCLC patients (all p > 0.05). Among the adverse effects, the prevalence of neutropenia, neuralgia, and arthralgia/myalgia (≥ grade 3) compared to that of the control group. On the other hand, the prevalence of anemia and thrombocytopenia was higher in the nab-P plus platinum (carboplatin/cisplatin) compared to that of controls. It is worth noting that fatigue did not show statistical significance.
Conclusion: Nab-P in combination with carboplatin/cisplatin regimen improves efficacy and tolerability in patients with NSCLC.
Systematic review registration: http://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022288499.
1. Introduction
Lung cancer, one of the most common cancers, ranked second in new cancer cases (11.4%, 2,206,771) and first in new deaths (18.0%, 1,796,144) in 2020 (1). Based on the cellular origin, lung cancer can be classified into two major categories, namely, small cell type and non-small cell type (2), with non-small cell lung cancer (NSCLC) constituting ~85% of the lung cancer (3). NSCLC is categorized into three subtypes, namely, lung adenocarcinoma, squamous cell lung cancer, and large cell carcinoma (4). NSCLC patients with a survival rate of 5 years are only ~15%, as the majority of patients with NSCLC are unresectable or metastatic at diagnosis (5) and are unsuitable for excision surgery, but they instead ought to undergo aggressive systemic therapy (consisting of chemotherapy, targeted therapy, or a combination of both) to achieve a good benefit. Nowadays, nab-P plus platinum compounds have been recommended as chemotherapy regimens to cure advanced NSCLC (6, 7).
Nab-P, a paclitaxel of solvent micelles-free, was revealed superior response rates and tolerability than solvent-based paclitaxel treatment regimens with patients in NSCLC and MBC (8). Albumin, as a natural transport carrier, is a general carrier for drug delivery into tumors due to features of abundance in blood and long half-life (9), with high accumulation in tumor tissues. It seems that promoting albumin binding to albumin-specific receptor mediated transport mechanisms to access tumors by enhanced permeation and retention (EPR) effect (10, 11). Thus, nab-P could reach the tumor microenvironment more effectively and accumulate in the tumor. In the study (10), nab-paclitaxel (nab-P) and sb-paclitaxel were radiolabeling then quantified the paclitaxel reaching tumors presenting that tumors have absorbed one-third more nab-P. This likewise illustrated that nab-P takes advantage of albumin mechanisms so that it could reach tumors more advantageously and consequently inhibit tumor growth.
The NCCN guidelines suggest that albumin-bound paclitaxel combined with platinum was used as standard treatment (6). A stage III/IV squamous NSCLC, clinical trial, indicated the beneficial effects of nab-P plus platinum compounds as the first-line chemotherapy for patients who have commonly used advanced NSCLC (12). In addition, in a clinical trial, among patients with NSCLC aged ≥60 years, nab-P plus carboplatin (nab-P + C) considerably increased overall response rate (ORR; 34 vs. 25.6%) and prolonged overall survival (OS; 13.8 vs. 11.0 months) compared with solvent-based paclitaxel in combination with carboplatin; however, it did not significantly show progression-free survival (PFS; 6.9 vs. 5.7 months). Among adverse effects, the maximum number of neutropenia incidence (≥ grade 3) was lower in nab-P + C than sb-paclitaxel plus carboplatin (134 vs. 152) and the incidence of anemia (74 vs. 16) or thrombocytopenia (45 vs. 20) is slightly higher in nab-P + C (13). However, single studies generally have heterogeneity and risk of bias, and the accuracy of study results may be limited. We, therefore, systematically assessed the therapeutic efficacy and tolerability of nab-P plus platinum using meta-analysis in accordance with published clinical trial studies and standard methods.
2. Materials and methods
2.1. Literature search
We searched, using the keywords “Carcinoma, Non-Small-Cell Lung” and “Albumin-Bound Paclitaxel,” PubMed, Cochrane Library, Web of Science, Embase, CNKI, Wan Fang, and China Science and Technology Journal Data databases from 1 January 2012 until the end of May 2022.
2.2. Eligibility criteria
The inclusion criteria were as follows: (1) the study category was a clinical trial or prospective study; (2) the study was a randomized controlled trial; (3) the study population was patients with histologically or pathologically confirmed NSCLC; (4) the study involved different intervention drugs, using albumin-bound paclitaxel combined with platinum chemotherapy in the trial group and platinum combined with other drugs in the control group or traditional paclitaxel; and (5) outcomes reported post-intervention as ORR, PFS, OS, adverse event (AE) outcomes grade ≥3.
The exclusion criteria were as follows: (1) articles in which the required information was not available and (2) article types, namely, retrospective study, case report, meta-analysis, review, and animal studies.
2.3. Research quality assessment
We checked the quality of each included study using Cochrane and evaluated the risk of bias using Review Manager 5.4.
2.3.1. Data extraction
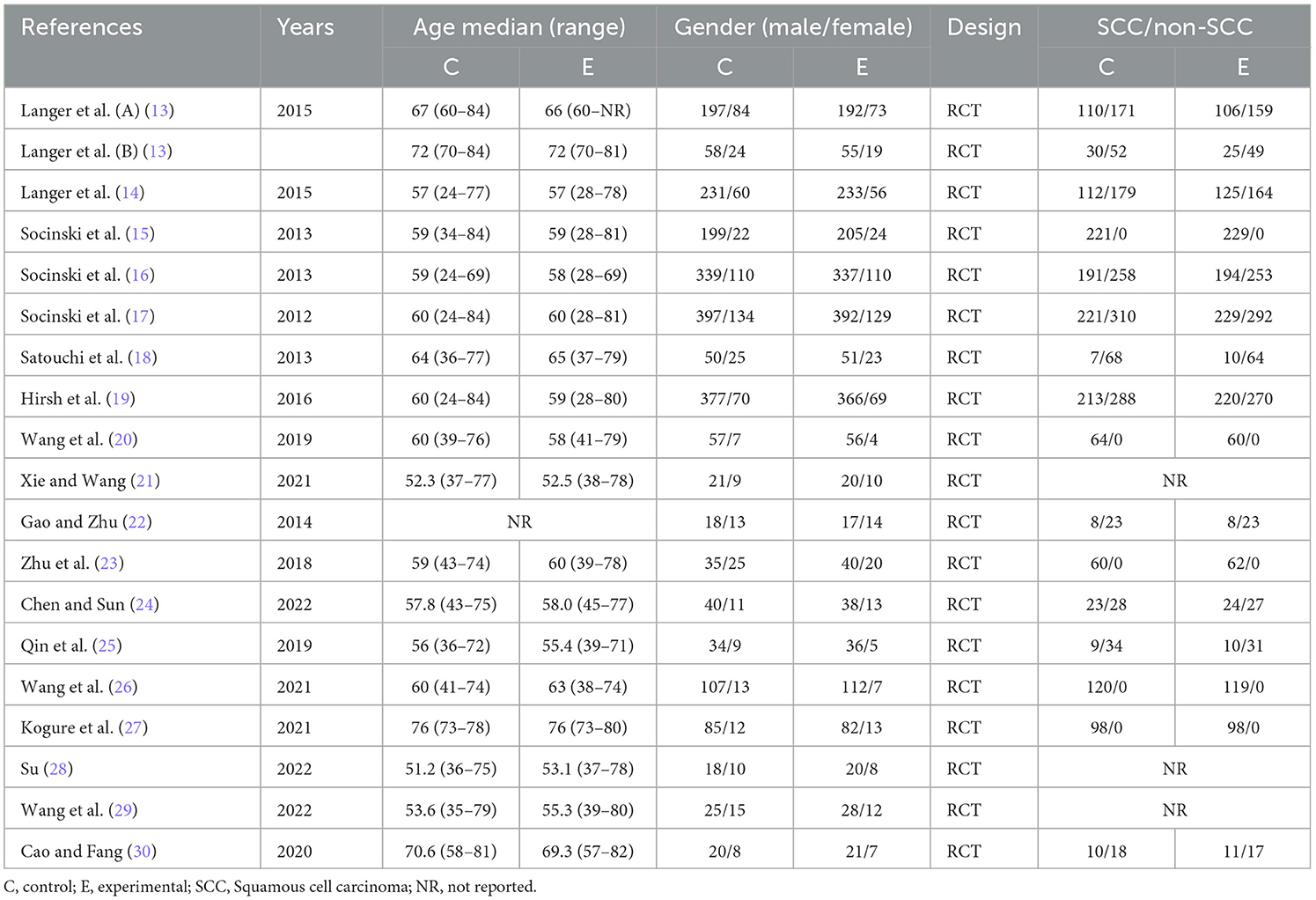
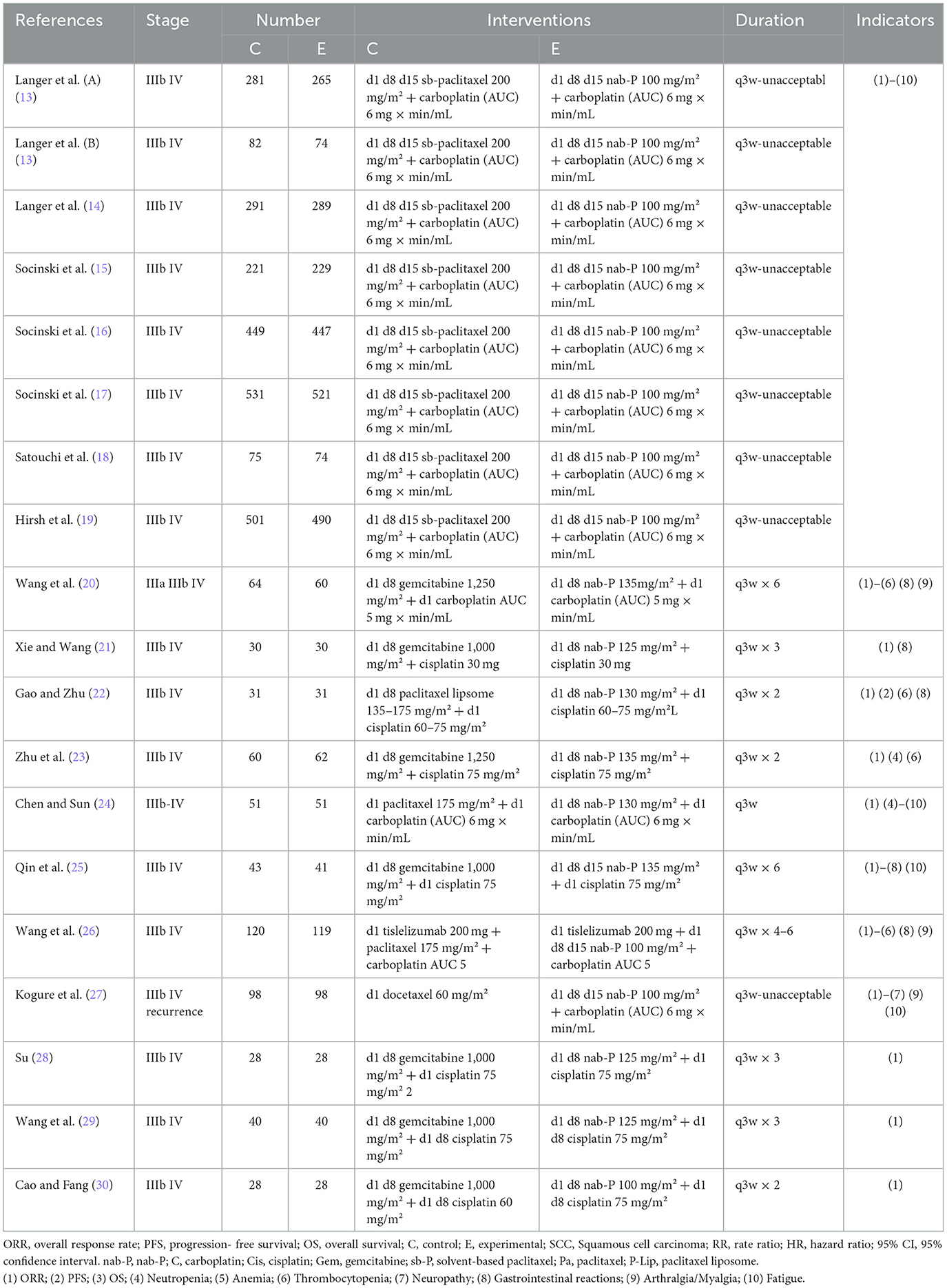
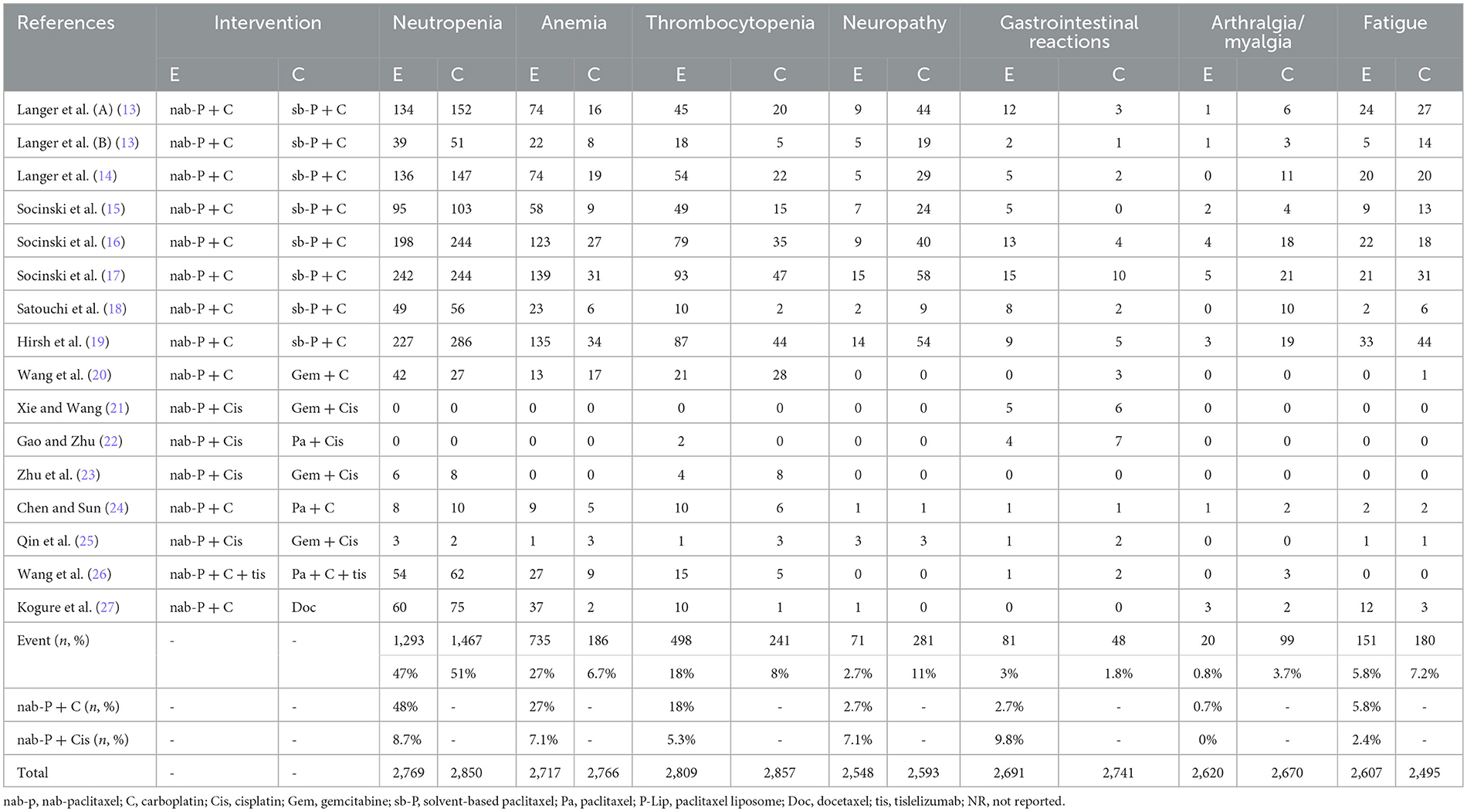
After screening by two authors, the information extracted for the selected articles included the first author's name and year of publication; patient number, gender, and age median; squamous cell carcinoma (SCC)/non-SCC; and style of study (Table 1). Table 2 includes treatment regimen per group; number of samples studied; treatment duration; primary outcome points including ORR, PFS, and OS; and subsidiary outcome points including the number of AEs ≥ grade 3 (in the case of a disagreement, a consensus was reached by conferring with the third author).
2.3.2. Statistical analysis
Meta-analysis was compared with the pooled results of hazard ratio (HR) with 95% confidence interval (CI) of the primary outcome (ORR, OS, and PFS) and the number of AEs (≥ grade 3), using the STATA14.0 software. The heterogeneity present in the studies was estimated by Cochrane's Q-test and I2 statistics test, and the data were analyzed to use a random-effects model if the I2 was >50% or heterogeneity p was < 0.1. In the opposite case, a fixed-effects model was used. Moreover, p-values > 0.05 were not recognized as statistically significant. A sensitivity analysis was generated using Comprehensive Meta-Analysis V3 to judge the stability of the pooled results. Publication bias was examined by contour-enhanced funnel plots and Begg's and Egger's tests. Publication bias existed when there was asymmetry in the funnel plot or p < 0.05 in Begg's and Egger's tests.
2.3.3. Evidence grading
The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) guideline suggested a tool that estimated the evidence quality for 10 outcomes (ORR, PFS, OS, neutropenia, anemia, thrombocytopenia, neuropathic pain, gastrointestinal reactions, joint myalgia, and fatigue).
3. Results
We searched 1,044 studies after screening the electronic databases. Excluding 1,026 studies based on the titles and abstracts, we finally obtained 18 studies for random trial studies that conformed to the inclusion and exclusion criteria (Figure 1). An included original study (13) grouped the data pooled according to age and was considered as two articles at inclusion. Therefore, the total number of included studies was 19. The total sample size of meta-analysis was 6,011 cases. The general characteristics of the patients are presented in Table 1. To enable research, we aggregated the drug interventions referred to in the included reports as shown in Table 2.
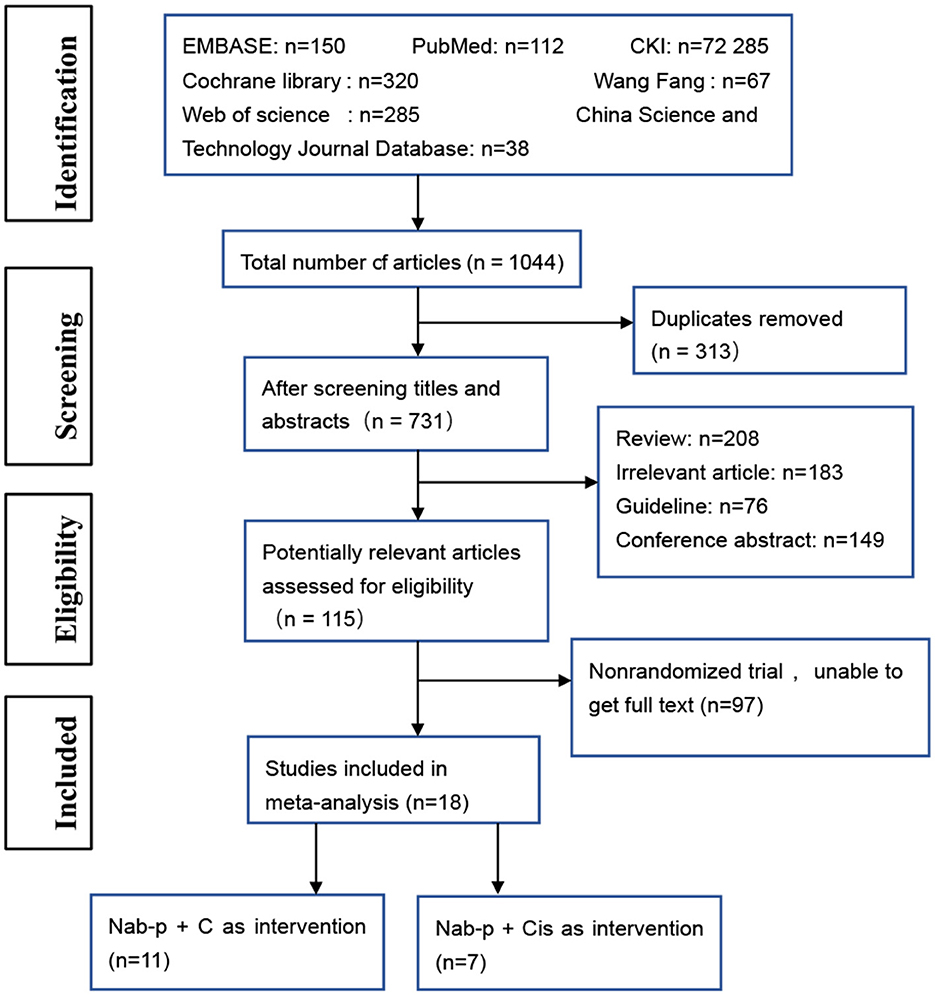
Figure 1. The flow diagram of the study selection process. nab-P + C, nab-p combined with carboplatin; nab-P + Cis, nab-p combined with cisplatin.
The methodological quality graph and summary of all included studies are shown in Figures 2A, B. The included articles do not address the allocation concealment. Furthermore, none of the studies had or illustrated double-blind procedures.
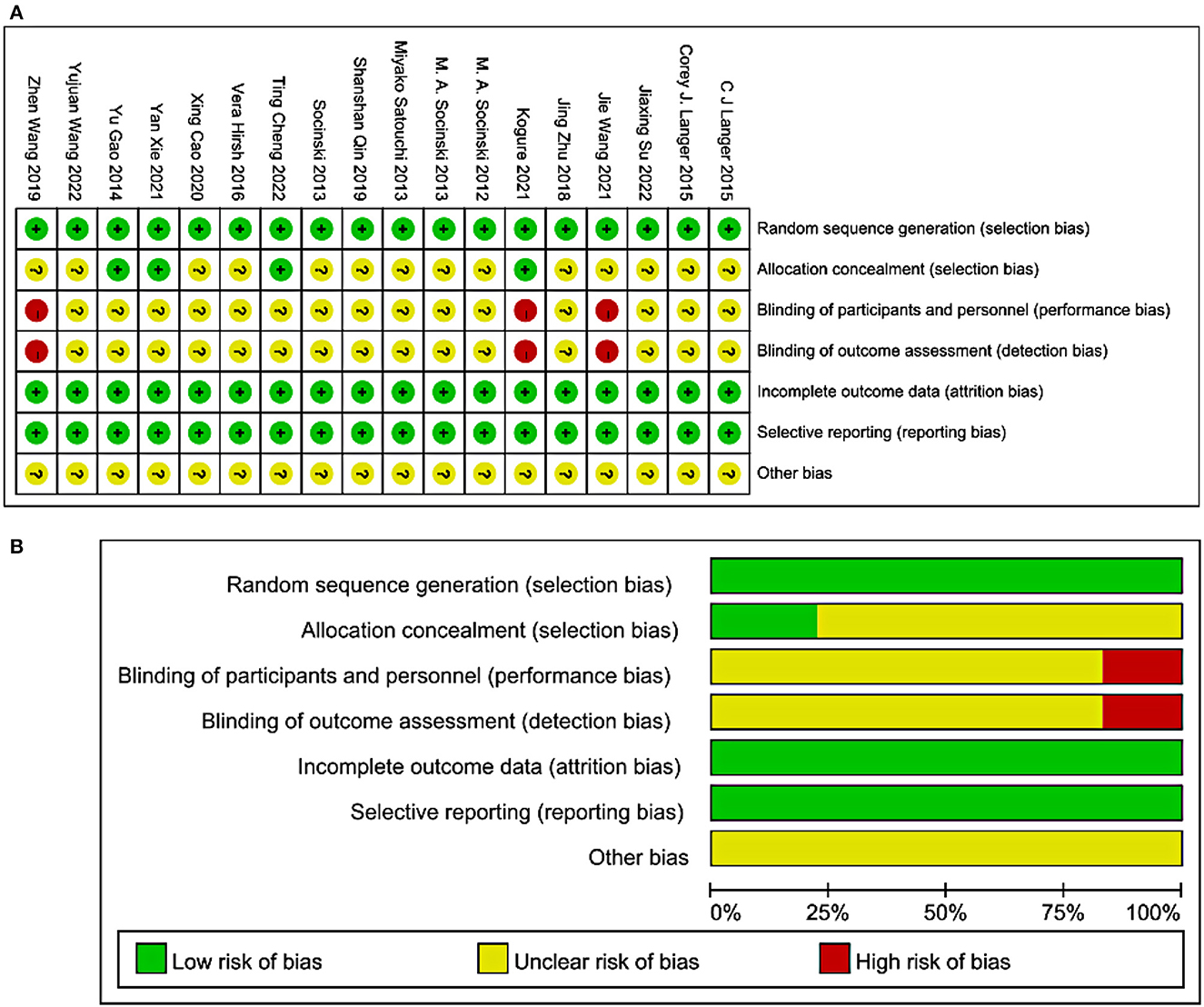
Figure 2. Methodological quality graph and summary of the included studies: (A) Risk of bias summary; +, low risk of bias; –, high risk of bias; ?, unclear risk of bias; (B) Risk of bias graph.
3.1. Efficacy analysis
3.1.1. ORR
The included 19 studies reported ORR. The pooled ORR was 1.66 [95% CI: (1.34, 2.05), p < 0.001]. The results of the meta-analysis indicated evidence of higher ORR after nab-P + C/Cis therapy compared with carboplatin/cisplatin in combination with conventional chemotherapy agents or traditional paclitaxel (Figure 3), with significant heterogeneity (I2 = 62.8%, p = 0.469) than control. Therefore, a random-effects model was employed for analysis. The subgroup analysis forest plot of ORR is generated in Table 3. The nab-P + C group included 12 studies and the nab-P + Cis group included 7 studies, and significant heterogeneity appeared in nab-P + C (I2 = 51.5%, p = 0.019) and nab-P + Cis (I2 = 76.5%, p < 0.001) groups. No distinct difference was observed between nab-P + C [OR: 1.62, 95% CI: (1.34, 1.94), p < 0.001] ORR and overall ORR. Additionally, the nab-P + Cis [OR: 1.69, 95% CI: (0.73, 3.91), p = 0.220] subgroup did not arrive at statistical significance. Among the subgroup analyses by age median-based, ORR was significantly elevated in the subgroup of patients with NSCLC aged >70 years [OR: 2.18, 95% CI: (1.07, 4.43), p = 0.031] than the subgroup of patients aged ≤ 60 years [OR: 1.85, 95% CI: (1.39, 2.46), p < 0.001], and significant heterogeneity between subgroups of both age > 70 years (I2 = 79.7%, p = 0.007) and ≤ 60 years (I2 = 62.2%, p = 0.003) was showed. The SCC rate was ≥65% of ORR [OR: 1.80, 95% CI: (1.20, 2.70), p = 0.004] elevated even more significantly than 35–65% [OR: 1.43, 95% CI: (1.22, 1.67), p < 0.001]. Significant heterogeneity in SCC rate was ≥65% (I2 = 72.9%, p = 0.002).
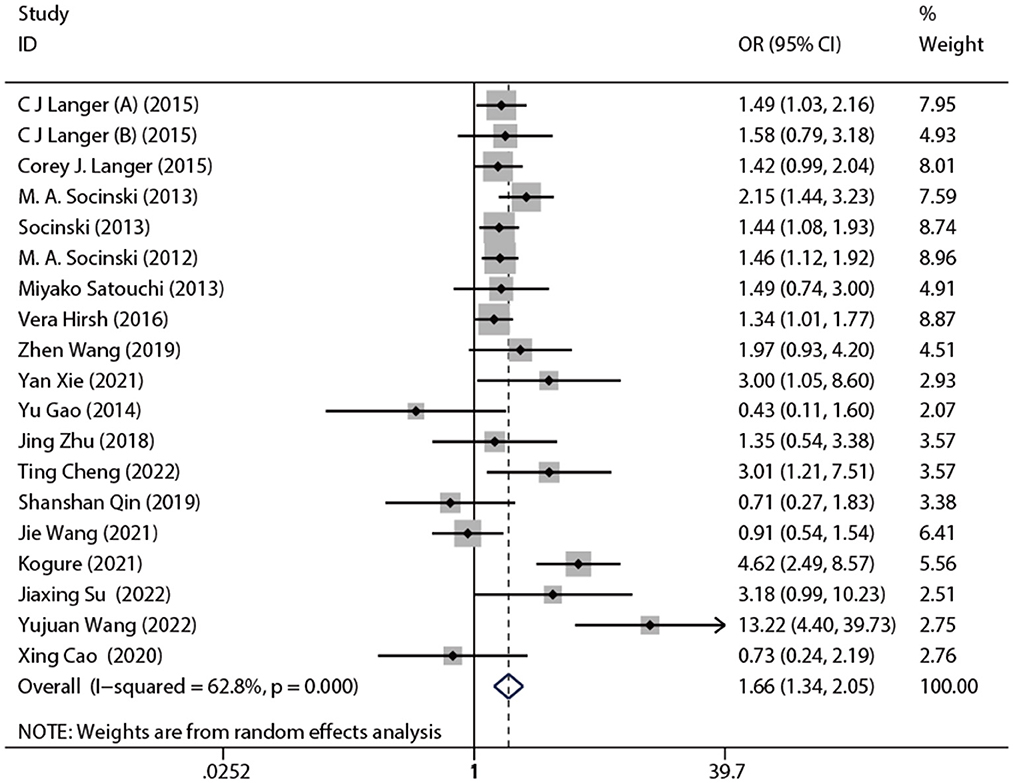
Figure 3. Forest plot of ORR of nab-P + C/Cis arm vs. control arm. The random model was used for ORR.
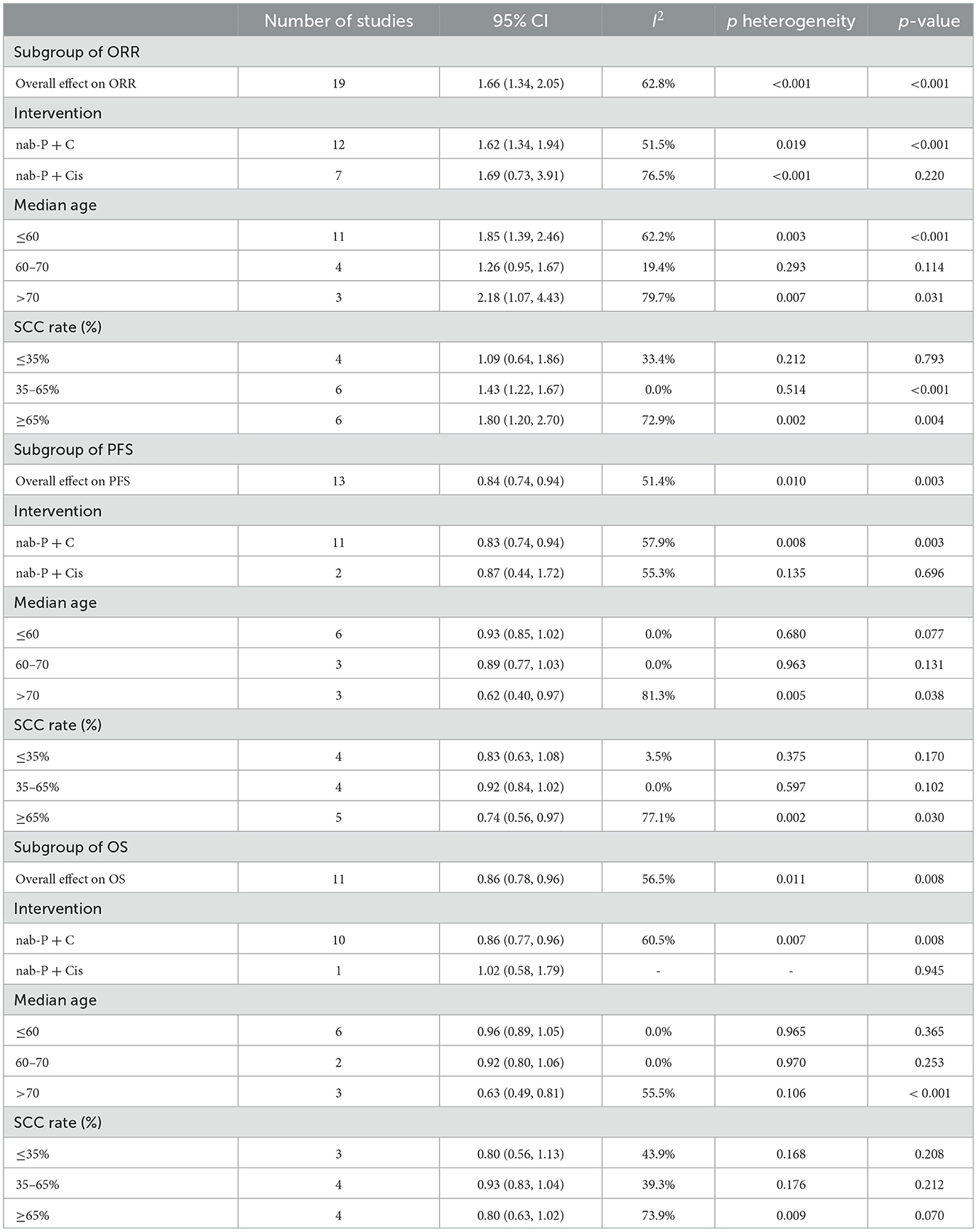
Table 3. Subgroup analysis of overall response rate, progression-free survival and overall survival.
3.1.2. PFS and OS
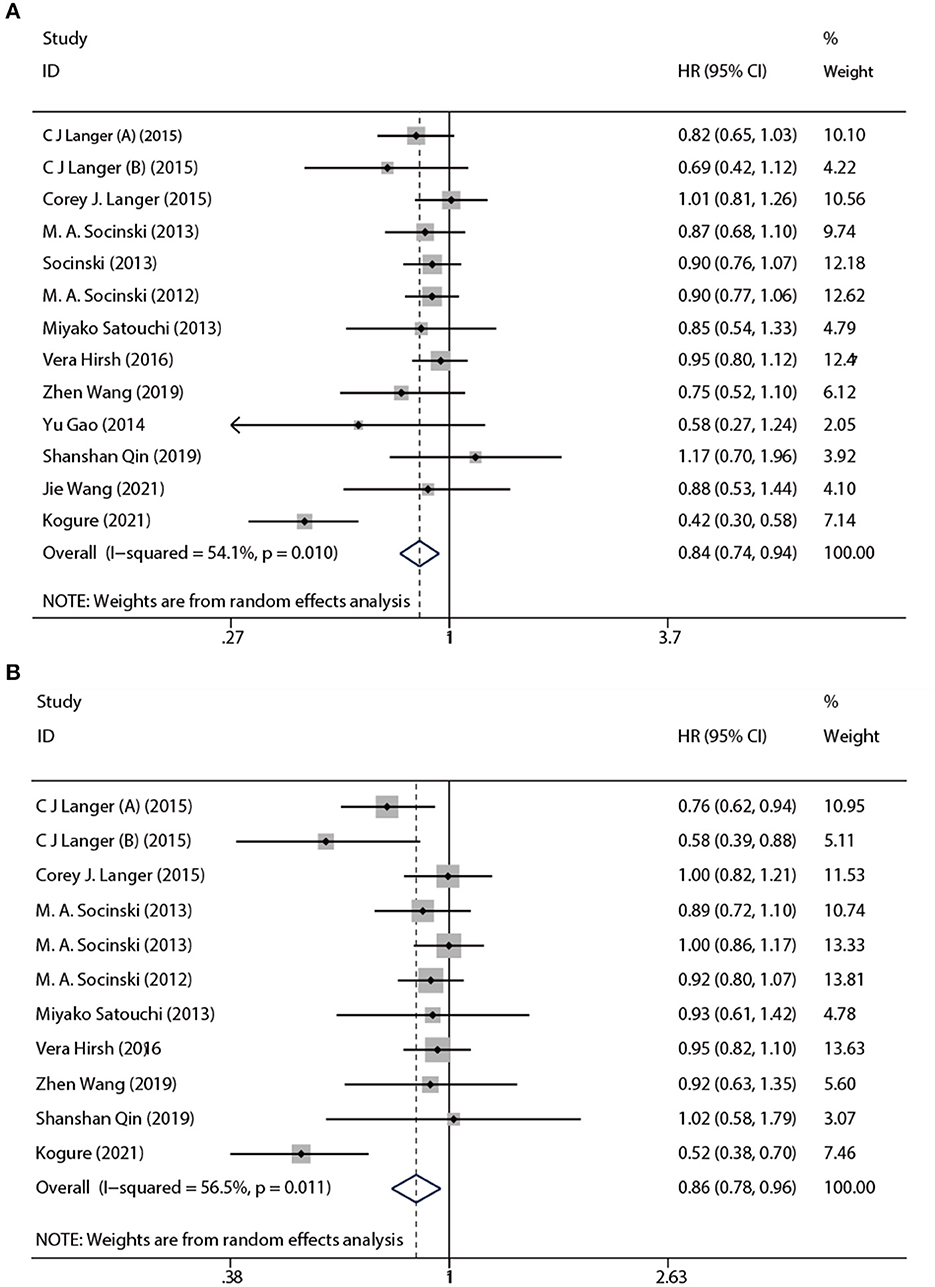
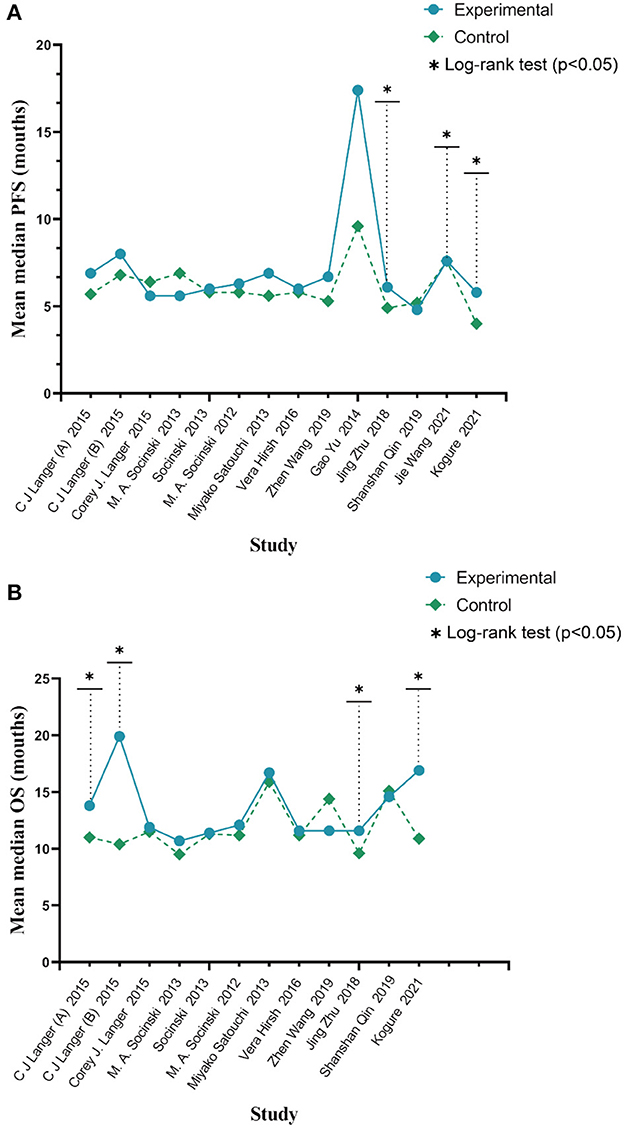
Based on a combined analysis of 13 studies, indicating that pooled PFS was of significant heterogeneity (I2 = 51.4%, p = 0.01), this study used a random-effects model. The results (Figure 4A) demonstrated that the experimental group had more profit than the control group [HR: 0.84, 95% CI: (0.83, 0.94), p = 0.003]. By analyzing subgroups (Table 3), nab-P + C [HR: 0.83, 95% CI: (0.74, 0.94), p = 0.003], the age >70 years [HR: 0.62, 95% CI: (0.40, 0.97), p = 0.038], and SCC rate ≥ 65% [HR: 0.74, 95% CI: (0.56, 0.97), p = 0.03] decreased HR of PFS among all of PFS. Among nab-P + C (I2 = 57.9%, p = 0.008), the age >70 years (I2 = 81.3%, p = 0.005), and SCC rate ≥ 65% (I2 = 77.1%, p = 0.002), the PFS results were analyzed by 13 trials. Of these 13 trials, three were reported to be statistically significant, improving median PFS probability in the experimental group (p < 0.05). The median PFS time in nab-P + C/Cis arm ranged from 5.6 to 17.4 (mean 7.12) months, and in the control arm, it ranged from 4.9 to 9.6 (mean 6.10) months (Figure 5A).
The data of OS was provided in 11 articles. The pooled OS was of significant heterogeneity (I2 = 56.5%, p = 0.011). Hence, a random-effects model was also used. A comparison of the experimental and the control groups (Figure 4B) indicated that nab-P + C had prolonged OS [HR: 0.86, 95% CI: (0.78, 0.96), p = 0.008]. During the subgroup analysis (Table 3), considering nab-P + C [HR: 0.86, 95% CI: (0.77, 0.96), p = 0.008] and the age >70 years [HR: 0.63, 95% CI: (0.49, 0.81), p < 0.001], significant heterogeneity existed in nab-P + C (I2 = 60.5%, p = 0.007) and the age > 70 years (I2 = 55.5%, p = 0.106). The results of median OS were calculated in 12 trials, with 4 studies showing prolonged OS in the experimental arm (p < 0.05). The median OS time in the nab-P + C/Cis group was 10.7–19.9 (mean, 13.57) months in comparison to 9.5–15.9 (mean, 11.83) months in the control group (Figure 5B).
3.1.3. Security analysis
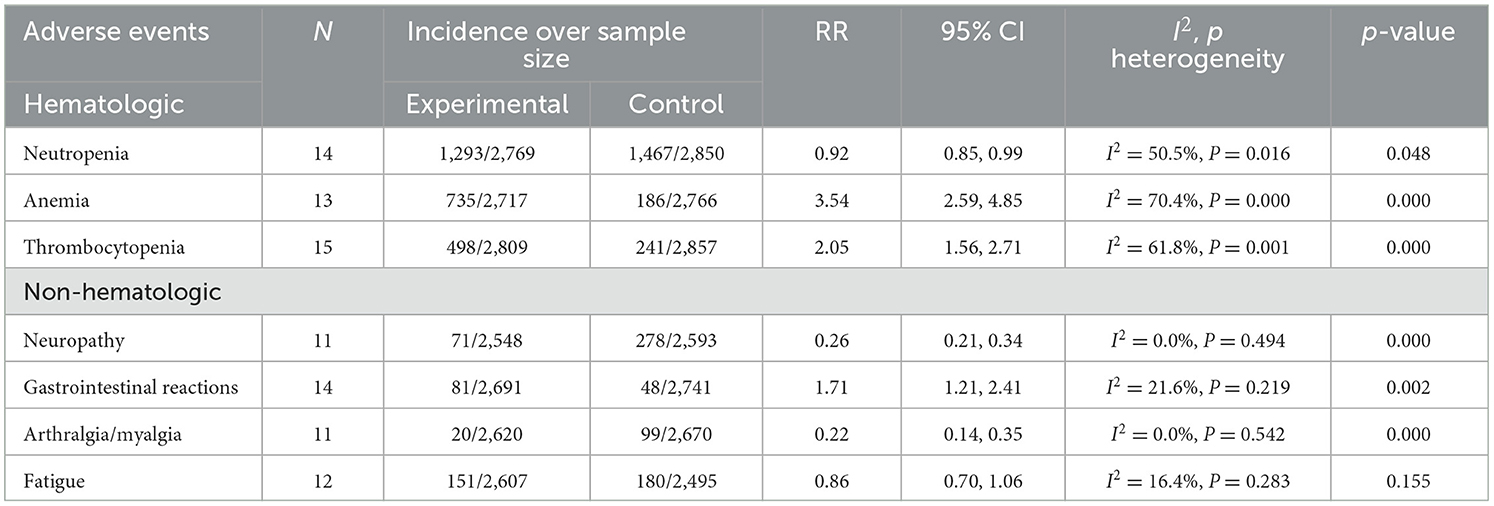
The results of treatment associated AEs grade ≥ 3 are shown in Table 4 and Figure 6. AEs can be divided into hematologic and non-hematologic AEs, with neutropenia [control arms vs. experiment arms (51%: 47%)] being the most common as shown in Table 5. The pooled result of fatigue [RR: 0.86, 95% CI: (0.70, 1.06), p = 0.155] showed no significant differences between the experimental and the control groups. When compared to the control group, the therapy with nab-P + C/Cis could abate the occurrence rate of neutropenia [RR: 0.92, 95% CI: (0.85, 0.99), p = 0.048], neuropathy [RR: 0.26, 95% CI: (0.21, 0.34), p < 0.001], and arthralgia/myalgia [RR: 0.22, 95% CI: (0.04, 0.19), p < 0.001]. Furthermore, the incidence of anemia [RR: 3.54, 95% CI: (2.59, 4.85), p < 0.001], thrombocytopenia [RR: 2.05, 95% CI: (1.56, 2.71), p < 0.001], and gastrointestinal reactions [RR: 1.71, 95% CI: (1.21, 2.41), p < 0.001] were higher in the therapy based on nab-P. Among AEs, the case of neutropenia (I2 = 50.5%, p = 0.016), anemia (I2 = 70.4%, p = 0.000), and thrombocytopenia (I2 = 61.8%, p = 0.001) showed significant heterogeneity. Meanwhile, this study showed that there was no significant heterogeneity in the event of neuropathy (I2 = 0.0%, p = 0.494), gastrointestinal reactions (I2 = 21.6%, p = 0.219), arthralgia/myalgia (I2 = 0.0%, p = 0.542), and fatigue (I2 = 16.4%, p = 0.283).
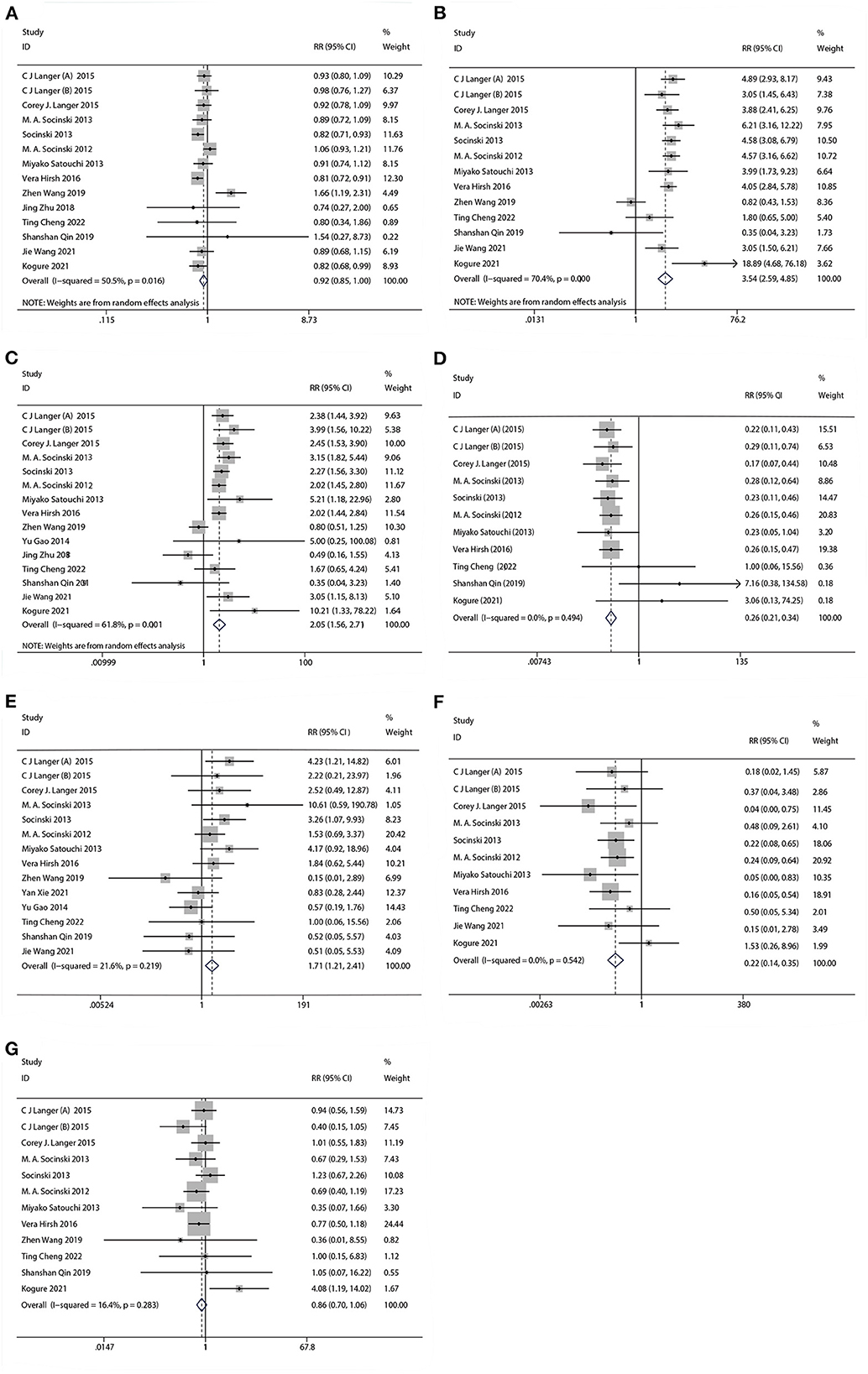
Figure 6. Forest plots of advance events for nab-P + C/Cis in the therapeutic of NSCLC. (A) Neutropenia, (B) anemia, (C) thrombocytopenia, (D) neuropathy, (E) gastrointestinal reactions, (F) arthralgia/myalgia, and (G) fatigue.
3.1.4. Meta-regression analysis
The aim of this analysis was to appraise the correlation by dose and duration of the intervention (months) of nab-P + C/Cis with ORR, PFS, and OS. According to the results, no linear correlation was observed for the absolute changes in these factors with the intervention dose and intervention duration (Figure 7). Based on the data of intervention, the duration of OS was consistent across the 11 studies, and thus no results were available in OS.
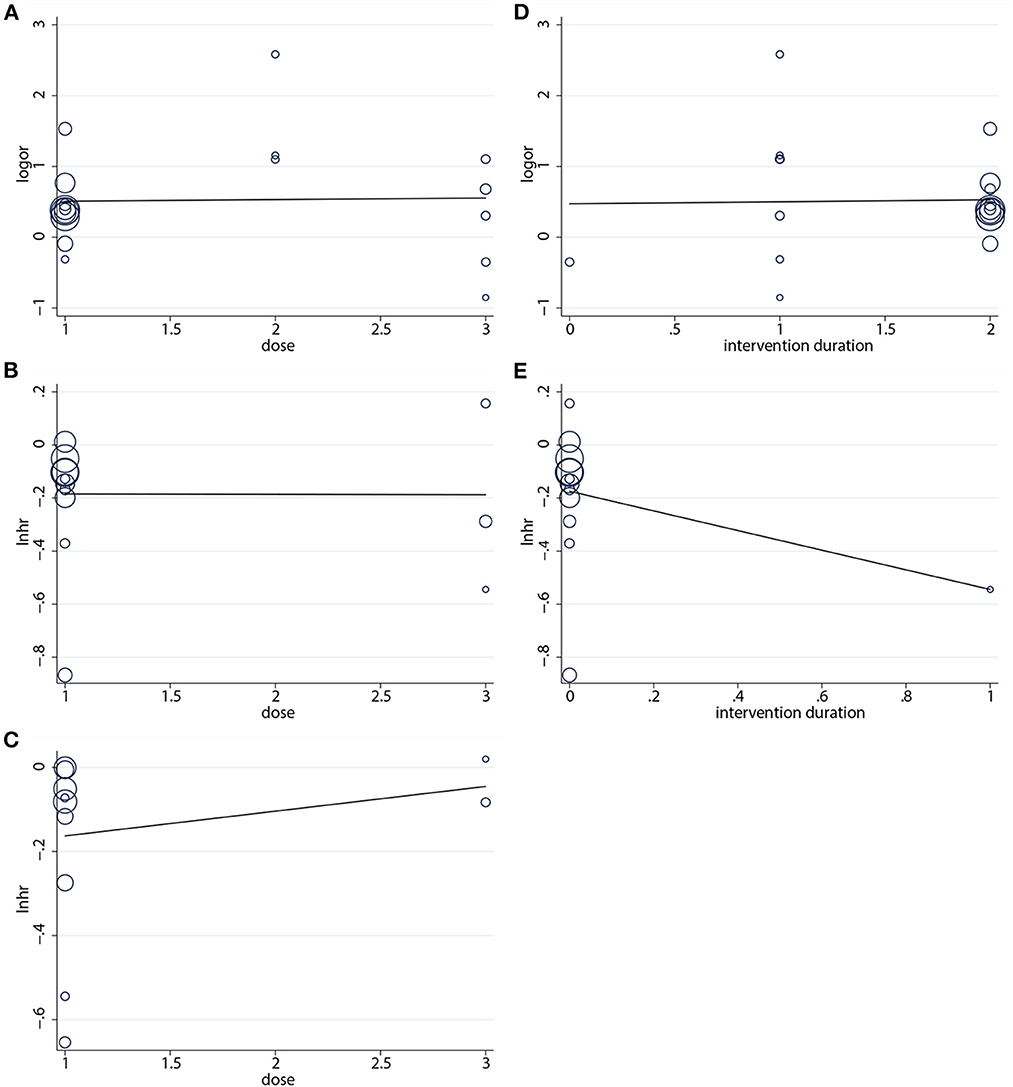
Figure 7. Regression analysis of (A) ORR, (B) PFS, (C) OS on intervention dose, (D) ORR, and (E) PFS on intervention duration.
3.1.5. Sensitivity analyses
We used the sensitivity analysis to evaluate the outcomes. These outcomes were presented with no significant modifications of ORR, PFS, OS, and AES (Figure 8) after deleting the studies one by one, suggesting that the valid results of therapeutic response were relatively stable in patients under nab-P + C/Cis treatment.
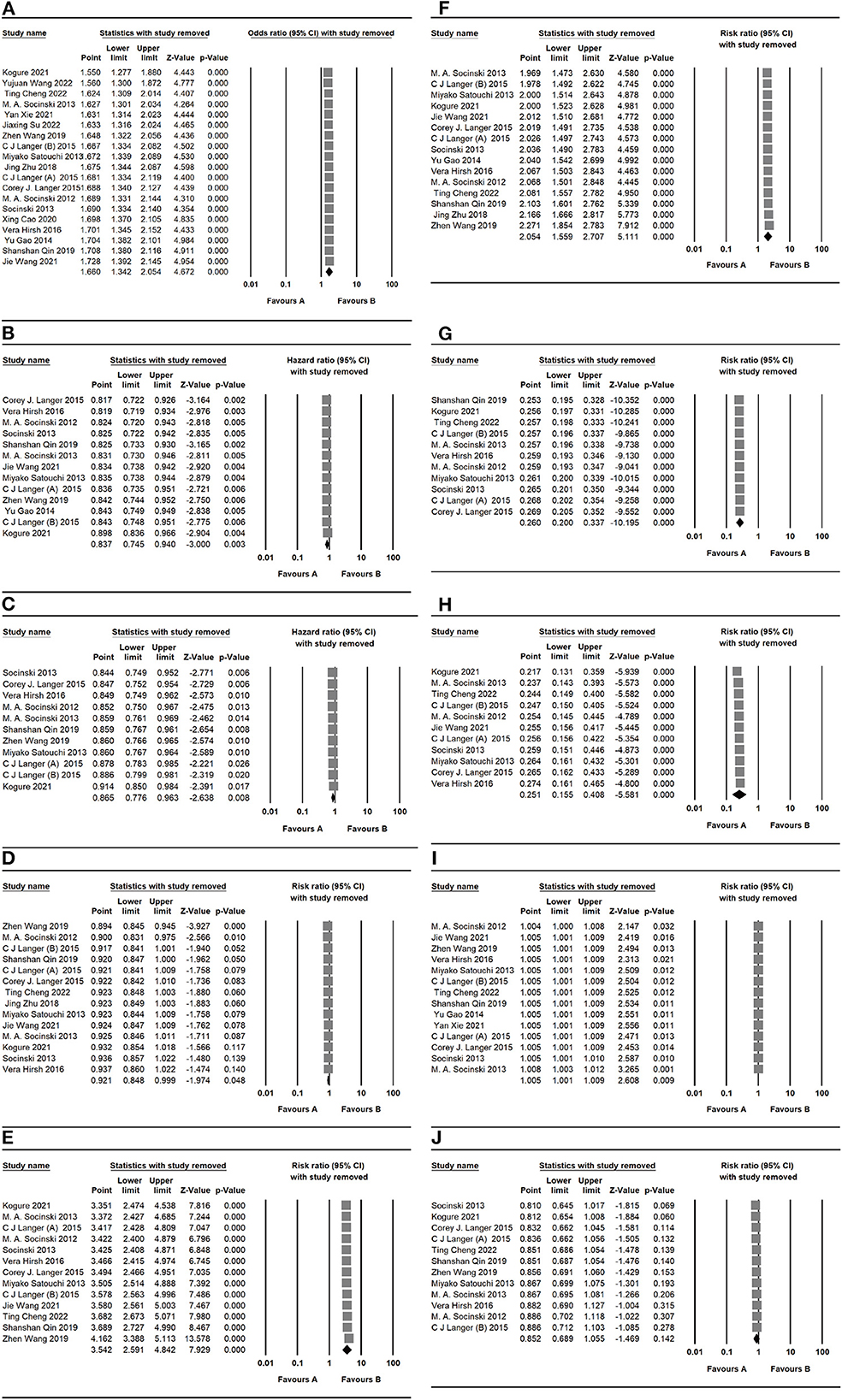
Figure 8. Sensitivity analysis of primary outcomes, including (A) ORR, (B) PFS, (C) OS, (D) neutropenia, (E) anemia, (F) thrombocytopenia, (G) neuropathy, (H) gastrointestinal reactions, (I) arthralgia/myalgia, and (J) fatigue for nab-P + C/Cis in the therapeutic of NSCLC. Favors A is the experimental and Favors B is the control.
3.1.6. Assessment of publication bias
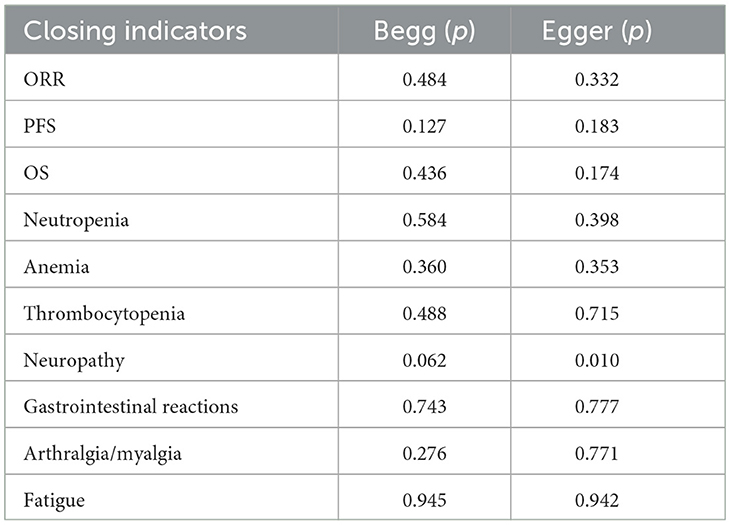
Contour-enhanced funnel plots were performed to appraise the results of potential publication bias. Conventional assignment criteria at the statistical significance level (p < 0.01, < 0.05, and < 0.1) were added to the funnel plot for distinguishing the detailed causes of publication bias. Figure 9 shows that there were asymmetrical, with many missing studies that fall in the areas of high statistical significance. Using Begg's and Egger's tests for further assessments, the results obtained were quantifiable, indicating the absence of potential publication bias. More details are presented in Table 6. In total, this study illustrated that the asymmetry may occur as a result of reasons other than publication bias.
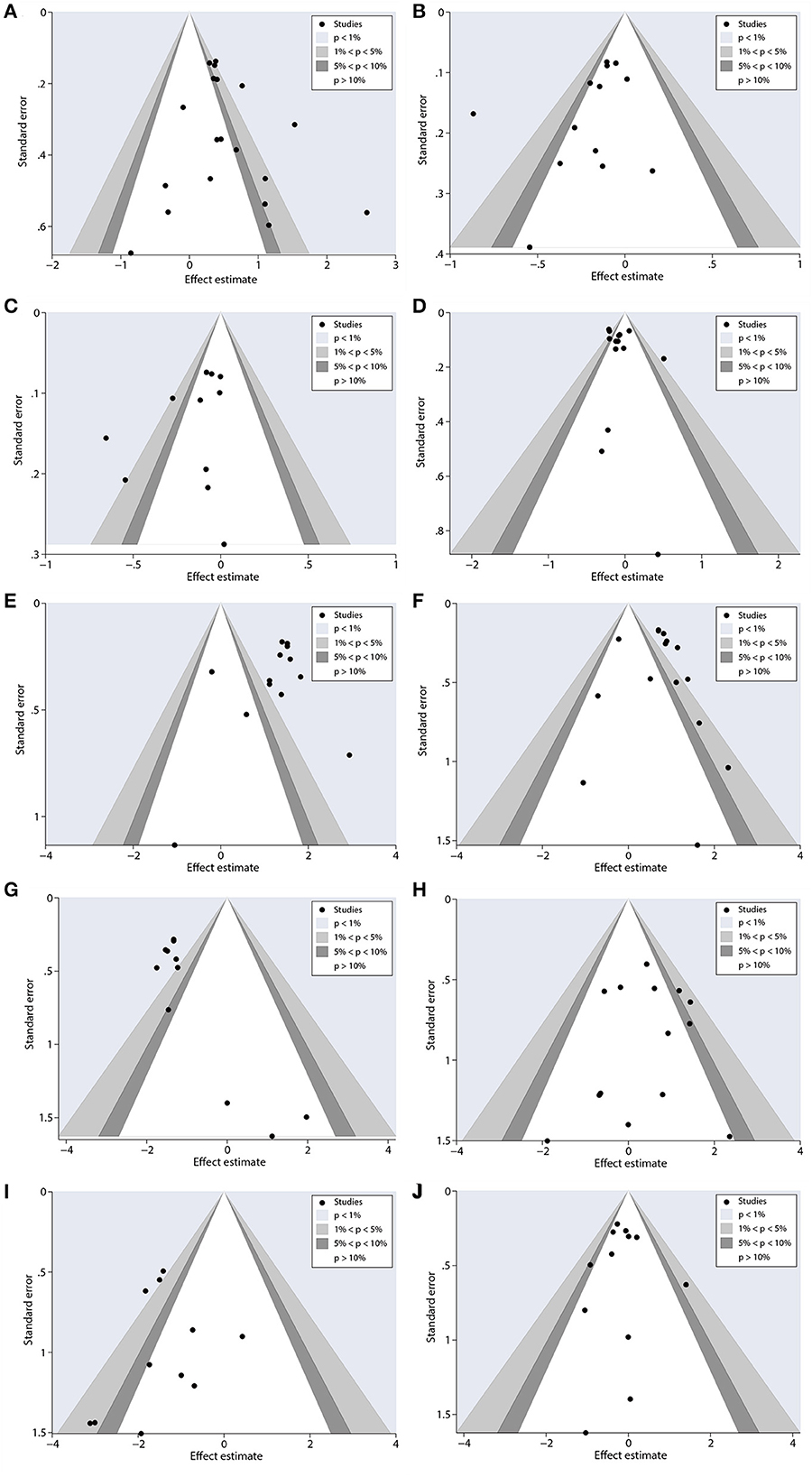
Figure 9. Contour-enhanced funnel plots to analyze potential publication bias. (A) ORR, (B) PFS, (C) OS, (D) neutropenia, (E) anemia, (F) thrombocytopenia, (G) neuropathy, (H) gastrointestinal reactions, (I) arthralgia/myalgia, and (J) fatigue.
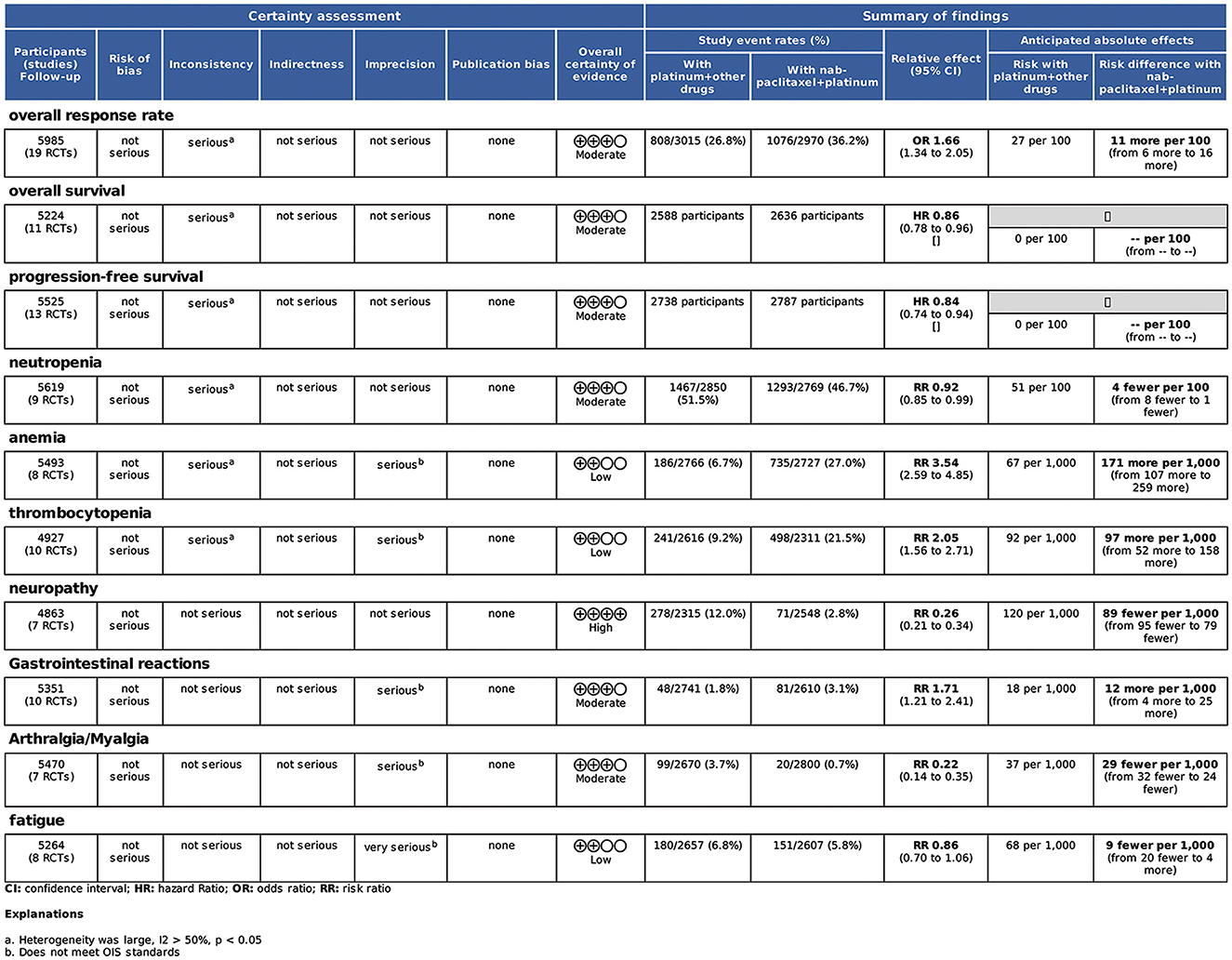
3.1.7. Quality of evidence
The quality of the evidence table (Figure 10) was assessed for each outcome. Neutropenia presented high evidence. ORR, PFS, OS, neuropathy, gastrointestinal reactions, and arthralgia/myalgia outcomes had moderate certainty. Anemia, thrombocytopenia, and fatigue presented with low certainty.
4. Discussion
The meta-analysis included 19 randomized clinical studies with 6,011 participants and presented the effectiveness of nab-P combined with carboplatin/cisplatin interventions in achieving improved ORR, prolonged PFS and OS, and declined AEs. These studies revealed that the combined regiments increase anticancer efficacy in patients with NSCLC and reasonable AEs occurrence which is generally acceptable.
Depending on the results, nab-P combined with platinum (carboplatin/cisplatin) elevated ORR [OR: 1.66, 95% CI: (1.34, 2.05)], and extended PFS [HR: 0.84, 95% CI: (0.74, 0.94)] and OS [HR: 0.86, 95% CI: (0.78, 0.96)], which indicated that nab-P + C/Cis was more effective than carboplatin/cisplatin in combination with conventional chemotherapeutic agents or traditional paclitaxel. For subgroup analysis based on interventions, first, only a few studies (22, 25) could obtain HR for PFS and OS from the original studies of nab-P + Cis interventions for treatment. Next, the nab-P + Cis intervention was not statistically significant in ORR [OR: 0.87, 95% CI: (0.44, 1.72)]. In this study, we did not obtain information about which combination chemotherapy regimen was more beneficial for patients with NSCLC between nab-P + C and nab-P + Cis. The lack of directly comparable clinical trials prevents us from determining which chemotherapy regimen is more effective. In an analysis of subgroups according to median age, the nab-P + C/Cis regimen significantly increased ORR and extended the duration of PFS and OS in the age > 70 years arm. Furthermore, by subgroup analysis depending on the SCC rate, the nab-P + C/Cis regimen was found to enhance ORR and growth in PFS greater in the SCC rate ≥ 65% arm. This implies that nab-P + C/Cis chemotherapy regimens may benefit more dramatically in older patients with NSCLC and patients with squamous cell carcinoma.
AEs can be separated into hematologic and non-hematologic events, based on the occurrence of neutropenia with the highest rate. Among the adverse results (grade ≥ 3) that showed in terms of hematological toxicities, when compared to carboplatin or cisplatin in combination with conventional chemotherapy agents or traditional paclitaxel, anemia [3.54 (2.59, 4.85)] and thrombocytopenia [2.05 (1.56, 2.71)] markedly increased in patients who were treated for nab-P + C/Cis, and neutropenia [0.92 (0.85, 0.99)] was relatively lower. In relation to non-hematological toxicities, neuropathy [0.26 (0.21, 0.34)] and arthralgia/myalgia [0.22 (0.14, 0.35)] occurred less in the nab-P + C group. However, significantly more incidence of gastrointestinal reactions [1.71 (1.21, 2.41)] were observed in nab-P + C/Cis compared with carboplatin/cisplatin in combination with conventional chemotherapy agents or traditional paclitaxel. Moreover, fatigue [0.86 (0.70, 1.06), p = 0.155] did now show a statistically significant difference.
In a present meta-analysis of the efficacy and safety of nab-P in combination with carboplatin for NSCLC, Tan et al. (31) demonstrated that, in comparison to control, nab-P + C improved ORR and extended PFS and OS. In the area of AEs, nab-P + C raised the incidence of anemia (grade ≥ 3) and diminished the risk of grade ≥ 3 neuropathy and arthralgia. There was no previous meta-analysis of nab-P + Cis intervention in NSCLC, but a review analysis (32) has reported an aggressive effect of nab-P + Cis intervention in NSCLC, with a tendency to obtain a higher ORR and improved PFS and OS. nab-P + Cis was more well-tolerated as illustrated in the trial by Hattori et al. (33). Our findings were similar to those that have been reported.
Patients significantly benefit in terms of ORR after nab-P + C/Cis chemotherapy (12, 17). The mechanism of action of albumin combined with carboplatin/cisplatin for intervention against NSCLC may be to utilize albumin features for increasing the antitumor role of the drug. Tumors may be fed by albumin as a nutritive substance in the tumor microenvironment and it possibly promotes tumor growth (34). Nab-P, an albumin-bound drug, leverages these mechanisms to enhance the delivery specifically to tumors that an affinity for albumin. The gp60 receptor-specific endothelial cells activate transmembrane transport, and albumin accumulates in the tumor environment by the EPR effect to reach tumors (8, 10, 11). The studies show that the coadministration of albumin-bound paclitaxel with gemcitabine enhances the gemcitabine levels in tumors of a mouse model of pancreatic cancer (35, 36). This supports the possibility that albumin-bound paclitaxel could increase the level of combined drugs reaching the tumor, but more research data are required to support this.
This meta-analysis included clinical studies of nab-P in combination with cisplatin, and it had a higher total sample size. Second, contour-enhanced funnel plots and Begg's and Egger's tests demonstrated that no evidence of publication bias was observed in this study. Besides, outcome quality was graded according to grade guidelines, and correlations for ORR, PFS, and OS with intervention dose and intervention duration were estimated using a regression analysis. However, there are several limitations to this analysis. First, the study shows considerable heterogeneity with respect to ORR, PFS, OS, neutropenia, anemia, and thrombocytopenia. Although using the subgroup analysis and a random-effects model, there is no way to decrease heterogeneity. In the second place, with two of the accepted articles, no HR was provided and calculated using Kaplan–Meier survival curves, which may cause a potential risk of bias (22, 26). Finally, most of the studies of nab-P + Cis in terms of primary data are unavailable or unable to calculate HR for PFS and OS, and AEs are not graded.
5. Conclusion
In general, this meta-analysis demonstrates that nab-P combined with carboplatin/cisplatin in patients with NSCLC could significantly increase ORR and prolong PFS and OS. However, PFS and OS do not show any particularly visible benefit; therefore, a greater number of studies will be demanded to explore further. Based on the efficacy and tolerability of nab-P combined with carboplatin/cisplatin, it may provide an economically disadvantaged patient with an affordable treatment option.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
TT and SL were responsible for drafting the initial text and making equivalent revisions. WH and TY conducted data analysis for the revised manuscript, while QZ and XZ extracted and analyzed data from the original manuscript. XC, XZ, and TX played significant roles in the conceptualization and design of the study. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Barta JA, Powell CA, Wisnivesky JP. Global epidemiology of lung cancer. Ann Glob Health. (2019) 85:2419. doi: 10.5334/aogh.2419
3. Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Prim. (2021) 7:1–20. doi: 10.1038/s41572-020-00235-0
4. Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. (2013) 31:992–1001. doi: 10.1200/JCO.2012.46.9270
5. Ardizzoni A, Boni L, Tiseo M, Fossella FV, Schiller JH, Paesmans M, et al. Cisplatin- versus carboplatin-based chemotherapy in first-line treatment of advanced non-small-cell lung cancer: an individual patient data meta-analysis. J Natl Cancer Inst. (2007) 99:847–57. doi: 10.1093/jnci/djk196
6. Ettinger DS, Akerley W, Borghaei H, Chang SC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. (2012) 10:1236–71. doi: 10.6004/jnccn.2012.0130
7. Besse B, Adjei A, Baas P, Meldgaard P, Nicolson M, Paz-Ares L, et al. 2nd ESMO Consensus Conference on Lung Cancer: non-small-cell lung cancer first-line/second and further lines of treatment in advanced disease. Ann Oncol. (2014) 25:1475–84. doi: 10.1093/annonc/mdu123
8. Yardley DA. Nab-P mechanisms of action and delivery. J Control Rel. (2013) 170:365–72. doi: 10.1016/j.jconrel.2013.05.041
9. Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Rel. (2008) 132:171–83. doi: 10.1016/j.jconrel.2008.05.010
10. Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. (2006) 12:1317–24. doi: 10.1158/1078-0432.CCR-05-1634
11. John TA, Vogel SM, Tiruppathi C, Malik AB, Minshall RD. Quantitative analysis of albumin uptake and transport in the rat microvessel endothelial monolayer. Am J Physiol Lung Cell Mol Physiol. (2003) 284:L187–96. doi: 10.1152/ajplung.00152.2002
12. Fang Y, Wang L, Xia G-H, Shi M-Q. Clinical investigation of efficacy of albumin bound paclitaxel plus platinum compounds as first-line chemotherapy for stage III/IV squamous non-small cell lung cancer. Asian Pacific J Cancer Prev. (2014) 15:7453–7. doi: 10.7314/APJCP.2014.15.17.7453
13. Langer C, Hirsh V, Okamoto I, Lin FJ, Wan Y, Whiting S, et al. Survival, quality-adjusted survival, and other clinical end points in older advanced non-small-cell lung cancer patients treated with albumin-bound paclitaxel. Br J Cancer. (2015) 113:20–9. doi: 10.1038/bjc.2015.181
14. Langer CJ, Hirsh V, Ko A, Renschler MF, Socinski MA. Weekly nab-P in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: analysis of safety and efficacy in patients with renal impairment. Clin Lung Cancer. (2015) 16:112–20. doi: 10.1016/j.cllc.2014.09.003
15. Socinski MA, Okamoto I, Hon JK, Hirsh V, Dakhil SR, Page RD, et al. Safety and efficacy analysis by histology of weekly nab-P in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer. Ann Oncol. (2013) 24:2390–6. doi: 10.1093/annonc/mdt235
16. Socinski MA, Langer CJ, Okamoto I, Hon JK, Hirsh V, Dakhil SR, et al. Safety and efficacy of weekly nab®-paclitaxel in combination with carboplatin as first-line therapy in elderly patients with advanced non-small-cell lung cancer. Ann Oncol. (2013) 24:314–21. doi: 10.1093/annonc/mds461
17. Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okomoto I, et al. Weekly nab-P in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. (2012) 30:2055–62. doi: 10.1200/JCO.2011.39.5848
18. Satouchi M, Okamoto I, Sakai H, Yamamoto N, Ichinose Y, Ohmatsu H, et al. Efficacy and safety of weekly nab-P plus carboplatin in patients with advanced non-small cell lung cancer. Lung Cancer. (2013) 81:20. doi: 10.1016/j.lungcan.2013.02.020
19. Hirsh V, Ko A, Pilot R, Renschler MF, Socinski MA. Weekly nab-P in combination with carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: analysis of safety and efficacy in patients with diabetes. Clin Lung Cancer. (2016) 17:367–74. doi: 10.1016/j.cllc.2016.04.002
20. Wang Z, Huang C, Yang J-J, Song Y, Cheng Y, Chen GY, et al. A randomised phase II clinical trial of nab-P and carboplatin compared with gemcitabine and carboplatin as first-line therapy in advanced squamous cell lung carcinoma (C-TONG1002). Eur J Cancer. (2019) 109:183–91. doi: 10.1016/j.ejca.2019.01.007
21. Xie Y, Wang B-Q. Clinical efficacy of albumin-bound paclitaxel combined with cisplatin in the treatment of advanced non-small cell lung cancer (in Chinese). Clin Res. (2021) 29:81–2. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMwNDI2Eg55eXliajIwMjEwMTAzORoIOW16cjZsZW4%3D
22. Gao Y, Zhu Z-T. Paclitaxel liposome combined with cisplatin versus albumin-bound paclitaxel combined with cisplatin as first-line treatment for advanced non-small cell lung cancer (in Chinese). J Nanchang Univ. (2014) 54:32–46. Available online at: https://kns.cnki.net/kcms2/article/abstract?v=3uoqIhG8C44YLTlOAiTRKgchrJ08w1e7M8Tu7YZds88zQog2Wexm_4dtFsnviolRMBAaIiuZuTdW3_RhdOyQHvrMwkyY8zb0&uniplatform=NZKPT&src=copy
23. Zhu J, Liu Y, Zhang R, Xin Y, Cheng Y. Albumin paclitaxel in combination with platinum versus gemcitabine in combination with platinum in first-line treatment Efficacy and safety analysis of advanced squamous lung cancer (in Chinese). World Lat Med Inform. (2018) 18:1. doi: 10.19613/j.cnki.1671-3141.2018.100.097
24. Chen T, Sun Q. Clinical effects of albumin-bound paclitaxel combined with carboplatin in the treatment of advanced non-small cell lung cancer (in Chinese). Anti-tumor Pharm. (2022) 12:775–8. doi: 10.3969/j.issn.2095-1264.2022.06.14
25. Qin S, Yu H, Wu X, Luo Z, Wang H, Sun S, et al. Weekly albumin-bound paclitaxel/cisplatin versus gemcitabine/cisplatin as first-line therapy for patients with advanced non-small-cell lung cancer: a phase II open-label clinical study. Chin J Cancer Res Apr. (2019) 31:339–48. doi: 10.21147/j.issn.1000-9604.2019.02.08
26. Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z, et al. Tislelizumab plus chemotherapy vs. chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. J Am Med Assoc Oncol. (2021) 7:709–17. doi: 10.1001/jamaoncol.2021.0366
27. Kogure Y, Iwasawa S, Saka H, Hamamoto Y, Kada A, Hashimoto H, et al. Efficacy and safety of carboplatin with nab-P versus docetaxel in older patients with squamous non-small-cell lung cancer (CAPITAL): a randomised, multicentre, open-label, phase 3 trial. Lancet Healthy Longev Dec. (2021) 2:e791–800. doi: 10.1016/S2666-7568(21)00255-5
28. Su J-X. Clinical effect of albumin-bound paclitaxel combined with cisplatin in the treatment of advanced non-small cell lung cancer (in chinese). Clin Med Res Practice. (2022) 7:28–30. doi: 10.19347/j.cnki.2096-1413.202202008
29. Wang Y-J, Wang G-F, Ni X-Q. Clinical efficacy of albumin-bound paclitaxel combined with cisplatin in the treatment of advanced non-small cell lung cancer (in chinese). Chang Shou. (2021) 2021:83. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMwNDI2EhpRS0JKQkQyMDIxMjAyMTExMDUwMDAwMjQxOBoIOW16cjZsZW4%3D
30. Cao X, Fang J-H. Effectiveness and safety of cisplatin combined with gemcitabine versus combined albumin-bound paclitaxel in the treatment of advanced non-small cell lung cancer (in chinese). Contemp Med Symp. (2020) 18:58–60. doi: 10.3969/j.issn.2095-7629.2020.16.044
31. Tan H, Hu J, Liu S. Efficacy and safety of nanoparticle albumin-bound paclitaxel in non-small cell lung cancer: a systematic review and meta-analysis. Artif Cells Nanomed Biotechnol. (2019) 47:268–77. doi: 10.1080/21691401.2018.1552595
32. Chen Y, Li J, Chen S, Zhang Y, Hu Y, Zhang G, et al. Nab-P in combination with cisplatin versus docetaxel plus cisplatin as first-line therapy in non-small cell lung cancer. Sci Rep. (2017) 7:10760. doi: 10.1038/s41598-017-11404-9
33. Hattori Y, Kono Y, Itoh S, Inoue T, Urata Y, Kawa Y, et al. A phase I/II study of weekly nab-P plus cisplatin in chemotherapy-naïve patients with advanced non-small-cell lung cancer. BMC Cancer. (2020) 20:115. doi: 10.1186/s12885-020-6588-y
34. Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RT, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. (2002) 8:1038–44. Available online at: https://pubmed.ncbi.nlm.nih.gov/12006516/
35. Von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, et al. Gemcitabine plus nab-P is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. (2011) 29:4548–54. doi: 10.1200/JCO.2011.36.5742
Keywords: non-small cell lung cancer, nab-paclitaxel, chemotherapy, efficacy, safety, meta-analysis
Citation: Tan T, Li S, Hu W, Yue T, Zeng Q, Zeng X, Chen X, Zhao X and Xiao T (2023) Efficacy and safety of nab-paclitaxel plus platinum in non-small cell lung cancer: a meta-analysis. Front. Med. 10:1139248. doi: 10.3389/fmed.2023.1139248
Received: 06 January 2023; Accepted: 19 June 2023;
Published: 24 July 2023.
Edited by:
Dawei Yang, Fudan University, ChinaReviewed by:
Apurva Patel, Gujarat Cancer & Research Institute, IndiaYuanyong Wang, People's Liberation Army General Hospital, China
Copyright © 2023 Tan, Li, Hu, Yue, Zeng, Zeng, Chen, Zhao and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochao Chen, MjgxMzI0NTIzQHFxLmNvbQ==; Xiangdong Zhao, emhhb3hkbG92ZXl3QDEyNi5jb20=; Tianbao Xiao, cHJvZl94aWFvdGlhbmJhb0AxNjMuY29t
 Tianying Tan
Tianying Tan Shuangshuang Li
Shuangshuang Li Wenting Hu
Wenting Hu Tinghui Yue
Tinghui Yue Qi Zeng2
Qi Zeng2