- 1Liver Research Unit, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2Department of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
Concurrent hepatitis B virus (HBV) and hepatitis C virus (HCV) infection is not uncommon as the two viruses shared the similar transmission routes. HCV is usually the dominant virus to suppress HBV, and HBV reactivation may occur during or after the course of anti-HCV treatment. By contrast, HCV reactivation after anti-HBV therapy in the concurrent HBV- and HCV-infected patients was rarely noted. Here, we reported the unusual viral evolutions of a patient with concurrent HBV and HCV infection, in whom HCV reactivation occurred during the entecavir therapy to rescue the severe HBV flare, while the following anti-HCV combination therapy with pegylated interferon and ribavirin elicited the second HBV flare despite sustained virological response to HCV infection, and further entecavir therapy healed the flare.
Introduction
Concurrent infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) infection is not uncommon in highly endemic areas as both HBV and HCV infections share the common transmission routes (1). The patients dually infected with HCV and HBV carry a higher risk of developing liver cirrhosis and hepatocellular carcinoma than those with either infection alone (2). The primary goal of the treatment of concurrent HCV and HBV infection is to eliminate or permanently suppress both viruses, and the dominant virus should be identified and treated first (3). HCV is usually the dominant virus (4) as it inhibits HBV gene expression and replication by its core protein (5), but reciprocal suppression is also possible (4). HBV reactivation following direct-acting antiviral (DAA) anti-HCV therapy thus has been reported in many patients with concurrent HBV and HCV infection (1). By contrast, HCV reactivation following anti-HBV therapy is never reported in the patients concurrently infected with HBV and HCV, although we ever noticed an episode of HCV reactivation in a dually-infected patient who achieved HBV viral response following lymphoblastoid interferon therapy (6).
Here, we reported an unusual case with concurrent HBV and HCV infection, in whom HCV reactivation occurred during entecavir therapy for the severe HBV flare, while the subsequent pegylated interferon (pegIFN)-based therapy for HCV reactivation elicited the second HBV flare despite sustained virological response (SVR) to HCV infection, and the HBV flare was successfully controlled by another entecavir therapeutic course.
Case presentation
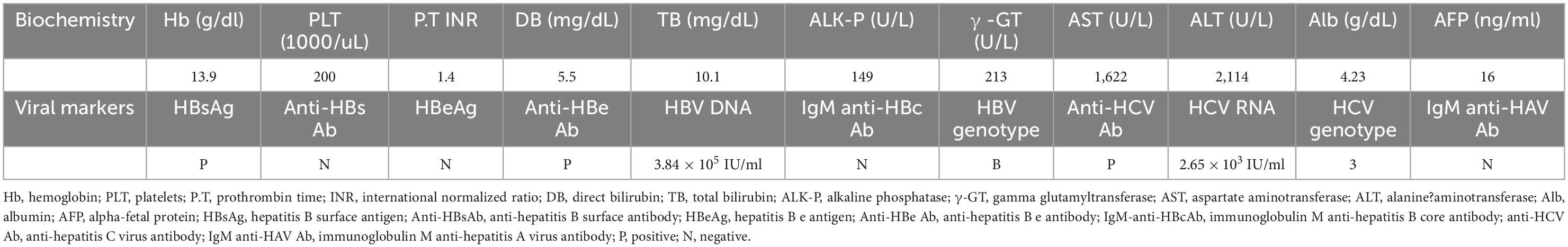
A 37-year-old male treatment-naive patient with chronic HBV (genotype B) and HCV (genotype 3) infection was treated with entecavir for severe HBV flare, presented with alanine aminotransferase (ALT) of 2,114 U/L, total bilirubin of 10.1 mg/dL, HBV DNA of 3.84 × 105 IU/ml and HCV RNA of 2.65 × 103 IU/ml, as shown in Table 1. He denied any prior alcohol consumption or drug abuse. Sonography disclosed a non-cirrhotic liver. The clinical course is shown in Figure 1, ALT and bilirubin levels progressively declined after entecavir therapy. The 3-year therapy was terminated in March 2016, when bilirubin level was normal and HBV DNA level was undetectable for over 1 year. However, the ALT levels were rarely under the upper limit of normal (ULN) (36 U/L) during the therapeutic course and was 49 U/L at the end of therapy (EOT), when HCV RNA level (7.02 × 105 IU/ml) was > 2 log higher than its baseline level. Two months later, a combination therapy with 24-week PegIFN α-2a and ribavirin was initiated since May, 2016 and achieved rapid, early and sustained HCV virological responses. ALT levels decreased progressively in the first 12 weeks to a nadir (ALT: 28 U/L) but increased progressively in the last 12 weeks during the therapy, with HBV DNA and ALT levels of 8.08 × 105 IU/ml and 82 U/L, respectively, at EOT, and increased to 7.23 × 106 IU/ml and 249 U/L, respectively, at 3 months after EOT of anti-HCV therapy. He received second course of 3-year entecavir therapy to treat the second HBV flare. HBV DNA levels decreased progressively and became undetectable within 1 year and HCV RNA levels were never detectable again. The ALT levels were mostly under ULN during the entecavir therapy.
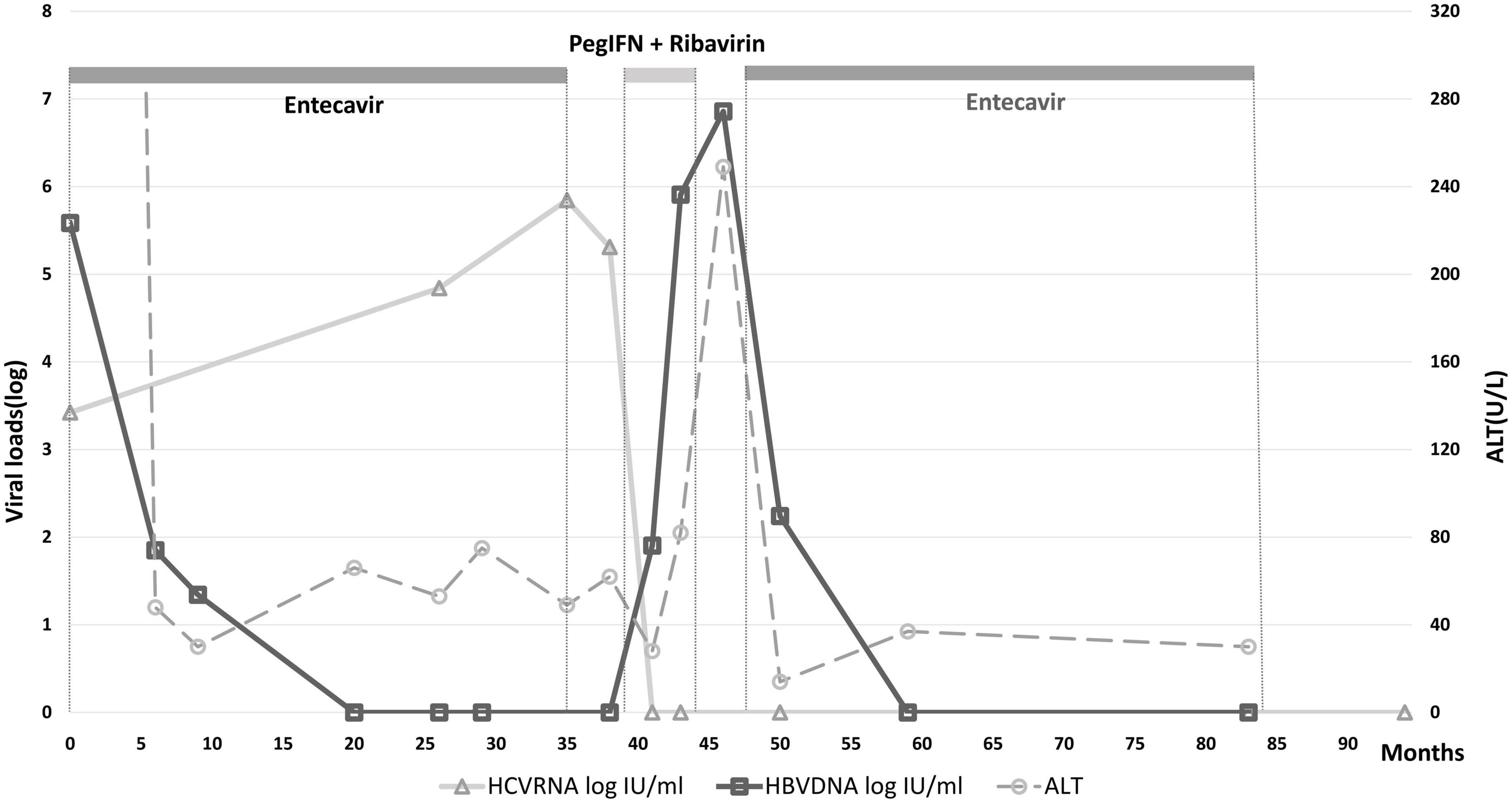
Figure 1. Longitudinal changes of HBV DNA, HCV RNA, and ALT levels of the HBV and HCV-coinfected patient. PegINT, pegylated interferon.
Discussion
The clinical course of the current case demonstrates HCV reactivation during anti-HBV therapy followed by HBV reactivation during anti-HCV therapy. These reciprocal changes reflect the reciprocal suppressive effects between HBV and HCV (4). The case is unique in that initially he suffered from the HBV-dominant severe hepatitis, which was rescued by entecavir. Anti-HCV therapy was not prescribed simultaneously because DAA therapy was not available until 2017 in Taiwan and peg-IFN-based therapy is contraindicated for severe hepatitis (3). Interestingly, the suppressive effect of HBV on HCV replication, although less effective (4), was evidenced by the increase of HCV level during the first entecavir therapeutic course. In contrast to HBV, HCV infection rarely induces severe acute exacerbation (7), that HBV is the most likely etiology of severe hepatitis flare in this patient. However, in the dually infected patient with efficient HBV suppression, the possibility of HCV-related hepatitis cannot be excluded even the ALT levels were only mildly elevated. During 24-week pegIFN-based therapy to treat HCV infection, the mildly elevated ALT levels returned to normal in the mid-term of therapy but elevated progressively afterward coinciding with the emergence of HBV flare. Indeed, although the pegIFN-based therapy might be effective for treating chronic concurrent HBV and HCV infection (2), HBV reactivation did occur while the patient achieved an SVR to HCV infection. It reflected the suppressed effect of HCV on HBV replication. In the era of DAA, which has no effect like Peg-IFN to suppress HBV replication, the risk of HBV reaction in concurrent HBV and HCV-infected patients with cured HCV infection is even higher and warrants further caution (8). We want to stress that entecavir withdrawal flare can occur after ceasing entecavir for few months (9), and pegylated interferon can only suppress HBV a few log IU/mL (10) and elicit immune modulation (9) may all account for the second HBV flare. Further anti-HBV therapy such as restarting entecavir should be prescribed as soon as indicated to prevent foreseeable complications including hepatic decompensation and even mortality (11). In conclusion, dually HBV and HCV-infected case treated with severe HBV flare may develop HCV reactivation which demands special caution, given that anti-HCV therapy potentially elicit further HBV flare.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data that support the findings of this study are available on request from the corresponding author. Requests to access these datasets should be directed to M-LC, bWxjaGFuZzgyMTBAZ21haWwuY29t.
Ethics statement
The studies involving human participants were reviewed and approved by Chang Gung Memorial Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Author contributions
Y-TS drafted the manuscript and analyzed the data. M-LC and Y-FL drafted the manuscript and critically revised it for intellectual content. All authors read and approved the final version of the manuscript.
Funding
This study was supported by grants from the Chang Gung Medical Research Program (CMRPG3I0413, CMRPG3L1191, CMRPG3M0211, and CMRPG1K0111-3) and the National Science Council, Taiwan (MOST 110-2629-B-182-001-, 110-2314-B-182-044-, 111-2314-B-182A-156-, and 111-2629-B-182-001-). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HBV, hepatitis B virus; HCV, hepatitis C virus; DAA, direct-acting antiviral agent; NUC, nucleos(t)ide analogue; THI, Taiwan health insurance; RVR, rapid virological response; SVR, sustained virological response.
References
2. Liaw Y, Chen Y, Sheen I, Chien R, Yeh C, Chu C. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. (2004) 126:1024–9.
3. Chien R, Kao J, Peng C, Chen C, Liu C, Huang Y, et al. Taiwan consensus statement on the management of chronic hepatitis B. J Formos Med Assoc. (2019) 118(1 Pt 1):7–38.
4. Liaw Y. Role of hepatitis C virus in dual and triple hepatitis virus infection. Hepatology. (1995) 22(4 Pt 1):1101–8. doi: 10.1016/0270-9139(95)90615-0
5. Chen S, Kao C, Chen C, Shih C, Hsu M, Chao C, et al. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem. (2003) 278:591–607.
6. Liaw Y, Chien R, Lin S, Yeh C, Tsai S, Sheen I, et al. Response of patients with dual hepatitis B virus and C virus infection to interferon therapy. J Interferon Cytokine Res. (1997) 17:449–52. doi: 10.1089/jir.1997.17.449
7. Sheen I, Liaw Y, Lin D, Chu C. Acute exacerbations in chronic hepatitis C: a clinicopathological and prognostic study. J Hepatol. (1996) 24:525–31. doi: 10.1016/s0168-8278(96)80136-x
8. Yeh M, Huang C, Huang C, Holmes J, Hsieh M, Tsai Y, et al. Hepatitis B-related outcomes following direct-acting antiviral therapy in Taiwanese patients with chronic HBV/HCV co-infection. J Hepatol. (2020) 73:62–71. doi: 10.1016/j.jhep.2020.01.027
9. Chang M, Liaw Y. Hepatitis B flare in hepatitis B e antigen-negative patients: a complicated cascade of innate and adaptive immune responses. Int J Mol Sci. (2022) 23:1552. doi: 10.3390/ijms23031552
10. Li M, Zhang L, Xie S, Sun F, Zeng Z, Deng W, et al. Dynamic changes of cytokine profiles and virological markers associated with HBsAg loss during peginterferon alpha-2a treatment in HBeAg-positive chronic hepatitis B patients. Front Immunol. (2022) 13:892031. doi: 10.3389/fimmu.2022.892031
Keywords: chronic hepatitis B, chronic hepatitis C, co-infection, reactivation, entecavir
Citation: Su Y-T, Chang M-L and Liaw Y-F (2023) Case report: Unusual viral evolutions following antiviral therapies in a patient with concurrent hepatitis B virus and hepatitis C virus infection. Front. Med. 10:1136111. doi: 10.3389/fmed.2023.1136111
Received: 02 January 2023; Accepted: 30 January 2023;
Published: 15 February 2023.
Edited by:
Wan-Long Chuang, Kaohsiung Medical University Hospital, TaiwanReviewed by:
Tawesak Tanwandee, Mahidol University, ThailandPonsiano Ocama, Makerere University, Uganda
Copyright © 2023 Su, Chang and Liaw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming-Ling Chang,  bWxjaGFuZzgyMTBAZ21haWwuY29t; Yun-Fan Liaw,
bWxjaGFuZzgyMTBAZ21haWwuY29t; Yun-Fan Liaw,  bGl2ZXJ5ZmxAZ21haWwuY29t
bGl2ZXJ5ZmxAZ21haWwuY29t
 Yi-Tse Su1,2
Yi-Tse Su1,2 Ming-Ling Chang
Ming-Ling Chang