- Department of Plastic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Prostaglandin analogs have been found to have more versatile uses: treatment of open-angle glaucoma, high intraocular pressure, vitiligo, and other treatments. And prostaglandin analogs have been found to have an important role in the hair growth cycle. However, prostaglandin analogs have not been sufficiently studied for hair (including hair, eyelashes, and eyebrows) regeneration. In this study, a systematic review and meta-analysis of topical prostaglandin analogs on hair loss was performed.
Objective: The purpose of this meta-analysis is to determine the efficacy and safety of topical prostaglandin analogs for treating hair loss.
Methods: We searched PubMed, Embase, and Cochrane Library databases comprehensively. Data were pooled using Review Manager 5.4.1, and subgroup analyses were performed if necessary.
Results: There were six randomized controlled trials included in this meta-analysis. All studies compared prostaglandin analogs with placebo, and one trial consisted of two sets of data. The results showed that prostaglandin analogs could significantly improve the hair length and density (p < 0.001). As far as adverse events are concerned, there was no significant difference between the experimental group and the control group.
Conclusion: In patients with hair loss, the topical prostaglandin analogs have better therapeutic efficacy and safety than placebo. However, the best dose and frequency of experimental treatment require further studies.
Introduction
Hair loss is one of the most anxiety-provoking and emotionally distressing dermatologic conditions. Psychological distress can be caused by hair loss. In addition to hair loss, eyelashes and eyebrows can also be lost. There is a range of severity when it comes to hair loss, from a single patch to multiple patches or more than 50% of the scalp. Hair loss not only affects esthetics but also profoundly affects a person’s mental state, and people with hair loss often lack self-confidence. It is important to differentiate between non-cicatricial and cicatricial alopecia. Non-cicatricial alopecia occurs more frequently. It is common for non-cicatricial alopecia to result from androgenetic alopecia, trichotillomania, and telogen effluvium. There are several types of cicatricial hair loss, including frontal fibrosing alopecia, folliculitis decalvans, and discoid lupus erythematosus (1). Hair loss also includes loss of eyelashes and loss of eyebrows. Hypotrichosis of the eyelashes refers to an insufficient amount of eyelashes (2). Eyelash hypotrichosis can be caused by many factors, including hereditary, age-related, chemotherapy, and unknown factors (2). There are other factors that can cause thin or absent lashes, including physical trauma to the face, eye surgery, trichotillomania and alopecia areata (3–5). In addition endocrinopathies, primary dermatoses, autoimmune conditions, infections, neoplasms, genetic conditions, and exogenous agents can cause eyebrow hair loss (6).
To date, there are several non-surgical ways to regrow hair, but the results are variable. These include topical or oral finasteride, minoxidil, dexpanthenol treatment, PRP, microneedle therapy, laser therapy, etc. (7–9). Although the above treatments are in some ways better (10, 11) for hair loss, they still have many shortcomings. It has been shown that finasteride increases the risk of sexual dysfunction in multiple randomized trials involving placebo controls (12, 13). In the beginning of minoxidil treatment, paradoxical hair shedding may occur (14). Contact irritant dermatitis and facial hirsutism (15, 16) are also seen after topical application. PRP efficacy data cannot be compared between studies, which is a major limitation in interpreting them. The effectiveness of micro-needling as a monotherapy is uncertain, though it may have some benefit when combined with other hair growth stimulants (17, 18).
Due to the lack of proven therapies, we thought of prostaglandin analogs. Treatment with topical prostaglandin analogs has recently shown better efficacy than conventional therapies (19–27).
Prostaglandins produced by almost all tissues and organs in the body are local hormones, except red blood cells. They are active at the location of their secretion or near the cells that secrete them. It is thought that prostaglandins play an important biological role in both physiological and pathological processes (28, 29). Prostaglandin analogs have a similar chemical structure to prostaglandins and have similar biological activity. Prostaglandin analogs are possible treatments for eyelash alopecia. These drugs can also affect fibrosis alopecia and alopecia areata, such as eyebrow hypotrichosis. Hypertrichosis induced by prostaglandins appears to occur by inducing follicles into anagen phase. It is essential for hair growth to be regulated by prostaglandins (PGs) (30). The scalp of people with AGA has decreased levels of PGE2 and increased levels of PGD2. Hair growth is inhibited by PGD2, while it is stimulated by PGE2 and PGF2a (31). Clinically known prostaglandin analogs include Bimatoprost, Latanoprost, and Cetirizine (32, 33). Synthetic analogs of PGE2 are known as Bimatoprost. The topical application of bimatoprost 0.03% lotion daily for 12 and 16 weeks has been found to significantly increase the diameter of vellus hair and the number of vellus hairs (34).
Synthetic analogs of PGF2a are known as Latanoprost. It was found that latanoprost 0.1% applied daily during 24 weeks resulted in significant improvements in hair density compared with baseline in 16 male patients suffering from AGA (35).
Studies have shown that cetirizine decreases PGD2 production. The effectiveness of topical 1% cetirizine applied every day for 6 months was evaluated in pilot studies on 85 patients with hair loss (36). According to the results, cetirizine could significantly increase both terminal hair density and total hair density.
However, the number of patients in all these studies was small. Therefore, there is some evidence that these drugs are effective, but further research is needed. In order to answer this question, randomized controlled trials were conducted to evaluate the efficiency and security of topical prostaglandin analogs. The purpose of this study was to use a systematic review and meta-analysis to identify a more novel approach for the treatment of hair loss and to analyze the efficacy and safety of this method.
Methods
Meta-analysis was carried out in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. And Our meta-analysis had been registered on the official PROSPERO website with the registration number CRD42022365811.
Search strategy
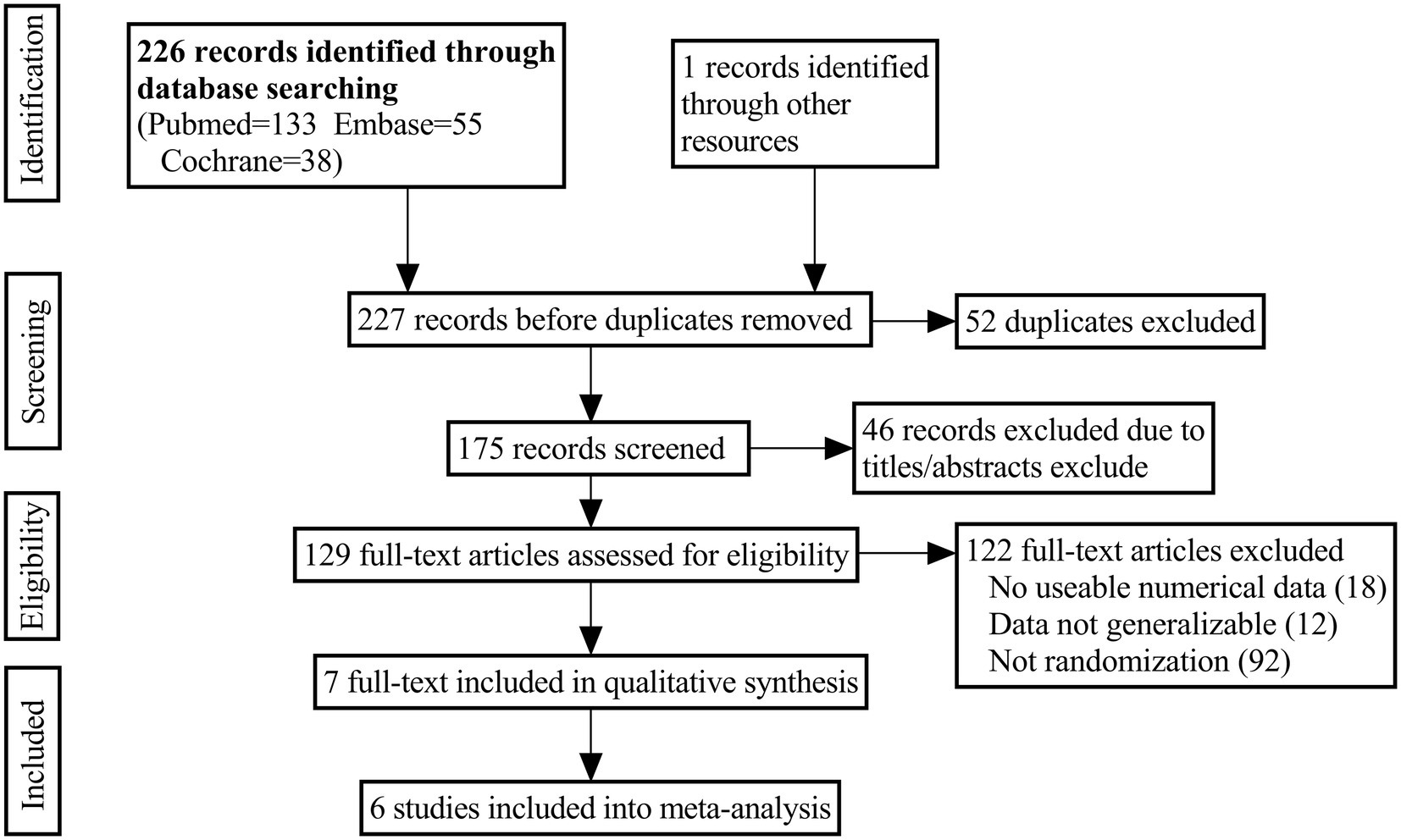
A number of electronic databases were searched to find potentially relevant studies, including PubMed, Embase, and the Cochrane Library, from inception to September 2022. Combining these keywords with the Boolean operators “AND” or “OR” yielded the following results: “alopecia OR hair loss,” “prostaglandins,” “bimatoprost,” “cetirizine,” “latanoprost.” Language restrictions were not imposed. Additional publications were identified by cross-referencing other related publications and trials retrieved in the bibliographies. The search process was shown in Figure 1.
Inclusion criteria
Those trials meeting the PICOS criteria (patients, intervention, comparator, outcome, study design) were included in this meta-analysis. (1) Patients: Men or women with hair thinning. (2) Intervention: using topical prostaglandin analog (Bimatoprost, Latanoprost, Cetirizine). (3) Comparator: Topical treatment with placebo alone. (4) outcomes: change in hair length or density (5) Study design: randomized controlled trial(RCT). A retrospective study, a letter, a guideline, a surgical registry, a review paper, or a study with insufficient outcome data were excluded from the analysis.
Data extraction
Two reviewers independently extracted related information from the articles with a standard data extraction table. Data extracted included information about authors, study designs, sample sizes, publication dates, ages, and outcomes. The primary outcomes included the change in hair length or density, which was a comprehensive photographic assessment performed by the investigators at the end of treatment. In cases where the eligibility criteria were unclear, we contacted authors for additional information. Whenever there was disagreement between the two authors, a consensus was reached through discussion.
Assessment of methodological quality
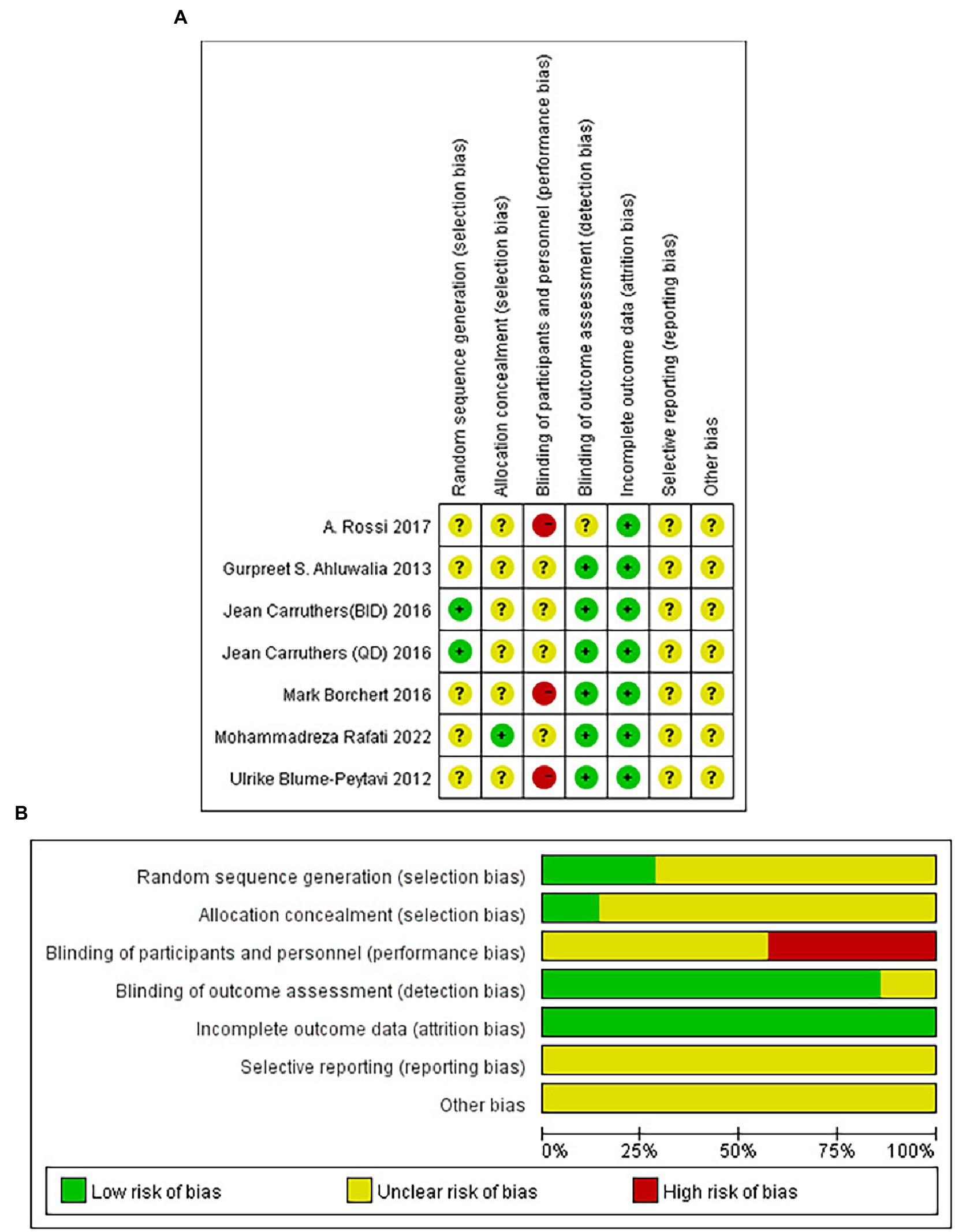
Based on the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions, independent assessment of the quality of the included studies was conducted by the authors. The following contents were included in our “risk-of-bias” table: selection bias(random sequence generation and allocation concealment), blinding, reporting bias(selective reporting), attrition bias(incomplete outcome data), and other bias. There were three categories of bias risk evaluated in each study: “low risk of bias,” “unclear risk of bias,” or “high risk of bias.”
Statistical analysis
To conduct the meta-analysis, we used Review Manager 5.4.1 software. Continuous outcomes were assessed with mean differences (MD) and 95% confidence intervals, and outcomes were assessed with odds ratios (OR). Meta-analysis of variables was performed when the outcome was reported by two or more studies. The heterogeneity of studies included in this study was assessed using p and I2, with a fixed-effects model when I2 < 50% and p > 0.1; otherwise, a random-effects model was used. Statistical significance was determined when the p value was less than 0.05.
Results
Search results
Figure 1 illustrated the PRISMA flow chart used for the study search. A total of 227 studies from the database were searched from the search strategy. A total of 98 articles were excluded after removing duplicates and screening by titles and abstracts. Eighteen studies were excluded because they lacked usable numerical data after a detailed reading of their full texts. Ninety two studies were excluded due to non-RCT. And 12 studies were excluded because the experimental data were not generalizable. Finally, six studies (35–40) met the inclusion criteria, including 683 patients.
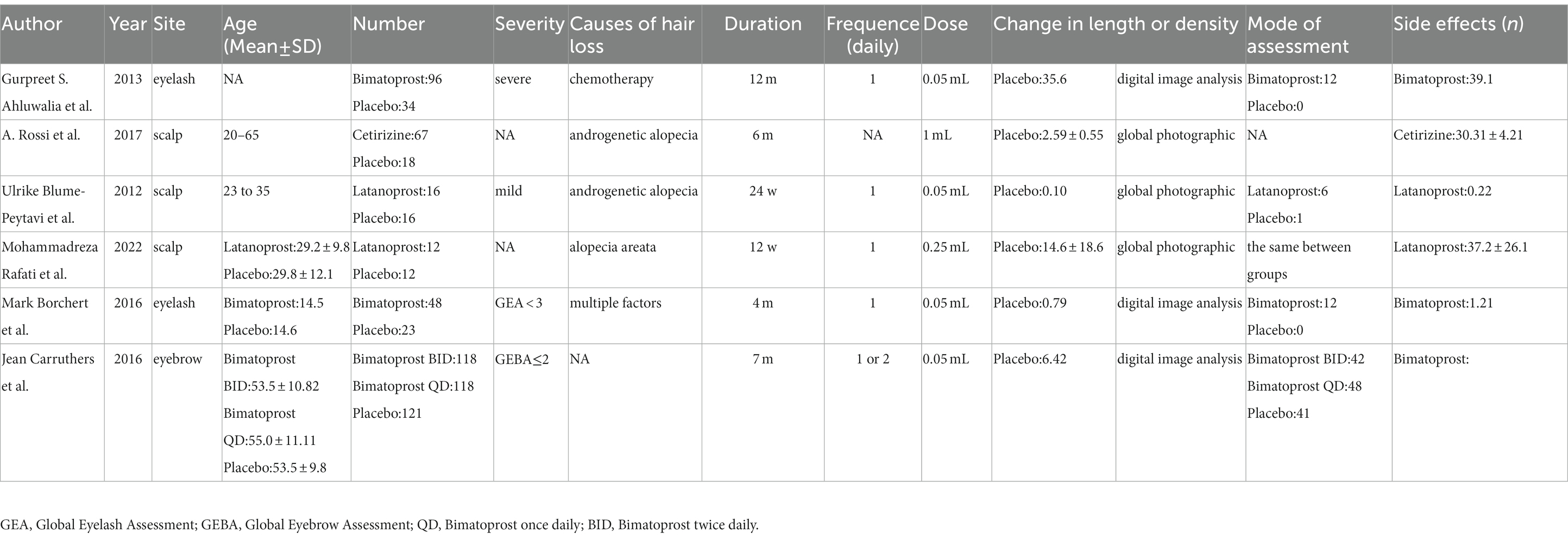
As shown in Table 1, an overview of the basic characteristics and interventions was provided. Three RCTs (37–39) compared the efficacy of Bimatoprost with that of the control group, two RCTs (35, 40) compared the efficacy of Latanoprost with that of the control group, and one RCT (36) compared the efficacy of Cetirizine with that of the control group. In all relevant RCTs, topical Bimatoprost, Latanoprost, and Cetirizine were administered 1 or 2 times/day at doses of 0.05 mL, 0.25 mL, and 1 mL. Changes in hair length or density were calculated in 6 RCTs. Six RCTs had varying end-of-follow-up times, ranging from as short as 12 weeks to as long as 12 months.
Risk-of-bias assessment
Figure 2 illustrated the risk of bias risk assessment. Among the six RCTs, only one (39) used the method of randomizing based on a random number table, and the other five (35–38, 40) did not describe how randomization was generated. Allocation concealment was not mentioned in any of these studies except for Mohammadreza Rafati’s experiment (40). Five studies (35, 37–40) described that the study results were assessed by two observers who were unaware of the trial method, and one study was not mentioned (36). A total of six studies submitted complete data, and none of the included studies showed selective reporting. A meta-analysis that included only 6 RCTs is difficult to assess in terms of publication bias since there were only 6 included.
Results of the meta-analysis
Change in hair length or density
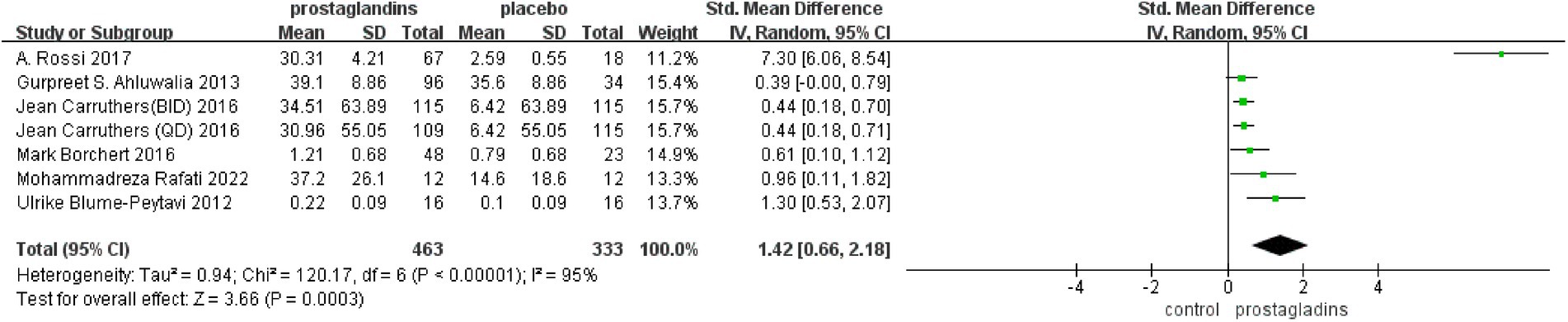
There were six studies that reported changes in hair length or density from baseline to the end of treatment (35–40), including 683 patients who compared placebo treatment. The experimental data from one paper were divided into two groups because of the difference in the frequency of dosing (39), so that we had a total of seven data sets for analysis. Based on a random-effects model, the meta-analysis found significant differences between the two groups[SMD =1.42, 95%CI (0.66, 2.18), p = 0.0003; Figure 3]. All test groups had more hair length or density growth than the control group. For example, Gurpreet S. Ahluwalia’s trial (37) showed a 39.1% increase, higher than the 35.6% increase in the control group. The subjects in A. Rossi’s test group (36) had a hair growth of 30.31 ± 4.21 mm compared to 2.59 ± 4.21 mm in the control group.
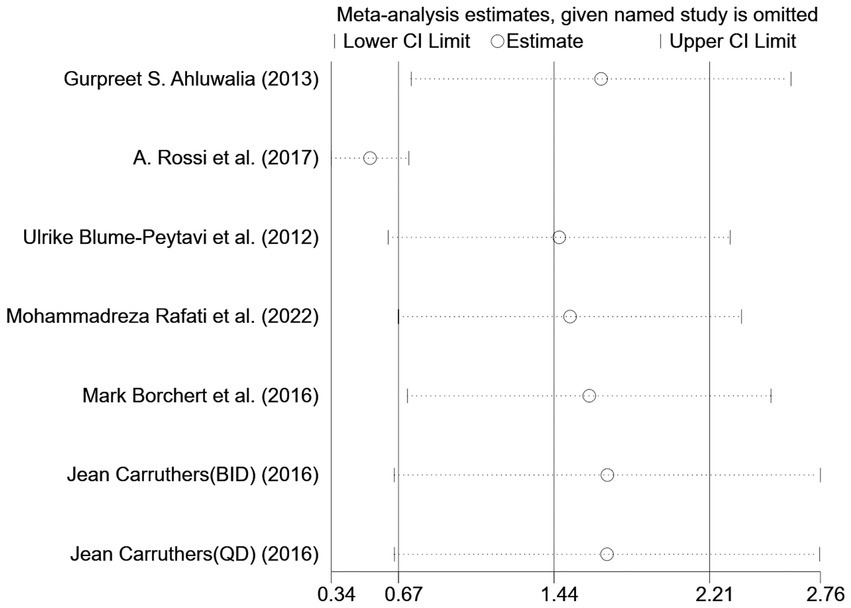
However, the I2 statistic indicated excessive heterogeneity was existing among these six studies. We then performed a sensitivity analysis (Figure 4). We could find a significant deviation between the data of the article published by A Rossi et al. in 2017 and other data. After excluding this literature, the I2 value was 18%, which was less than 50%. After a closer look at A Rossi’s experiment, we speculated that it is the significant difference between the dose of their experiment and the other groups; they used a 1 mL experimental reagent, which is much larger than the other groups, which may have led to the bias of the data.
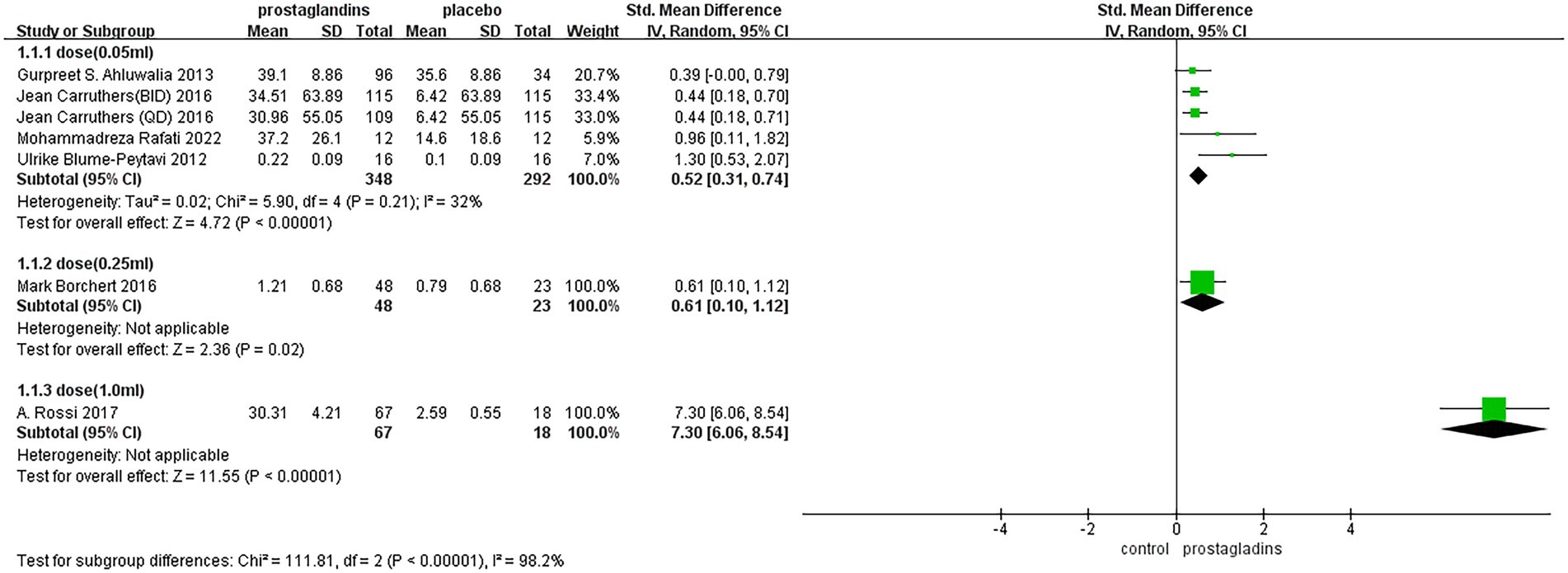
The subgroup analysis of the dose was performed in each group (Figure 5), with 0.05 mL of experimental group reagent in all four experimental groups (35, 37, 39, 40), 0.25 mL of reagent in one experimental group (38), and 1.0 mL of reagent in one experimental group (36). The results showed that the dose of the 1.0 mL group had the largest effect size [SMD = 7.30, 95% CI (6.06, 8.54), p < 0.00001; Figure 5]. In contrast, in the dose of the 0.25 mL group, the effect size was not significantly different from that of the dose of the 0.05 mL group.
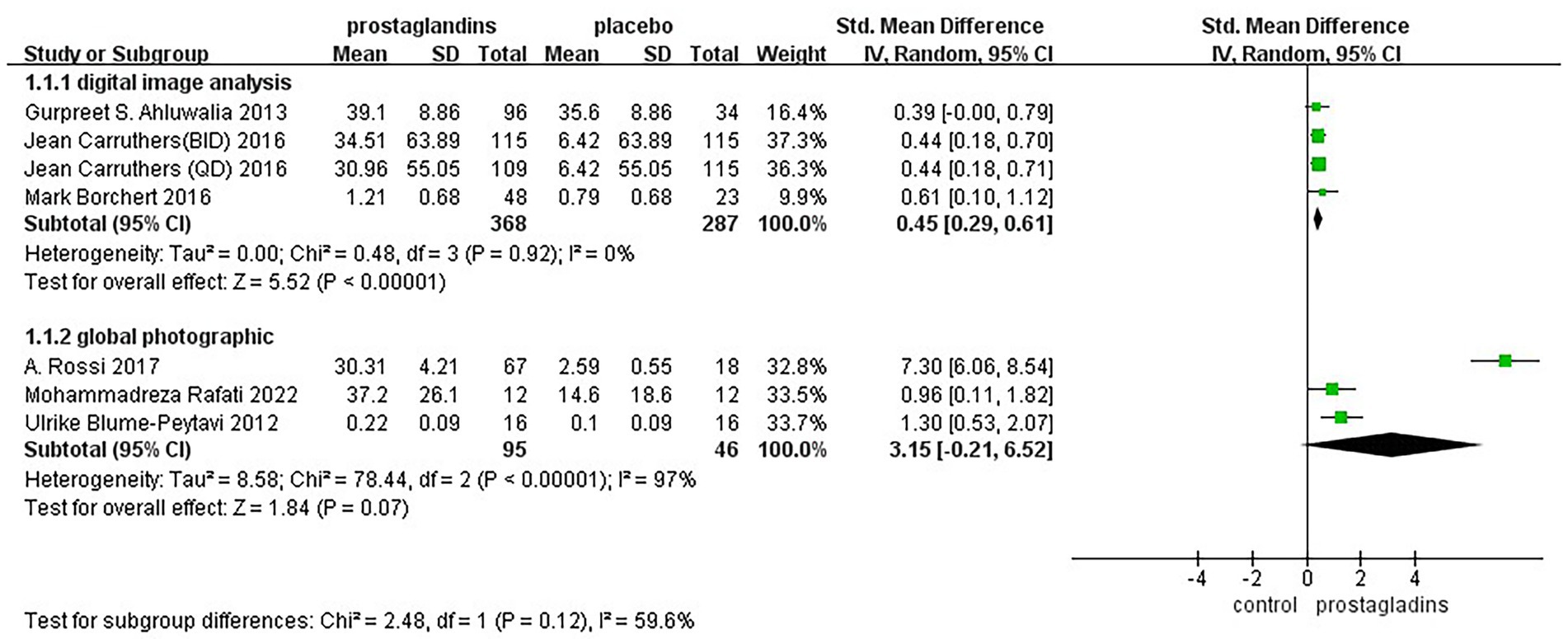
Considering that the way in which the efficacy was assessed may affect the results of the experiment, we performed a subgroup analysis of mode of assessment as well (Figure 6). It was divided into two groups, digital image analysis group and global photographic group. The results of the analysis showed low heterogeneity of results in digital image analysis group [SMD = 0.45, 95% CI (0.29, 0.61), p < 0.00001; Figure 6].
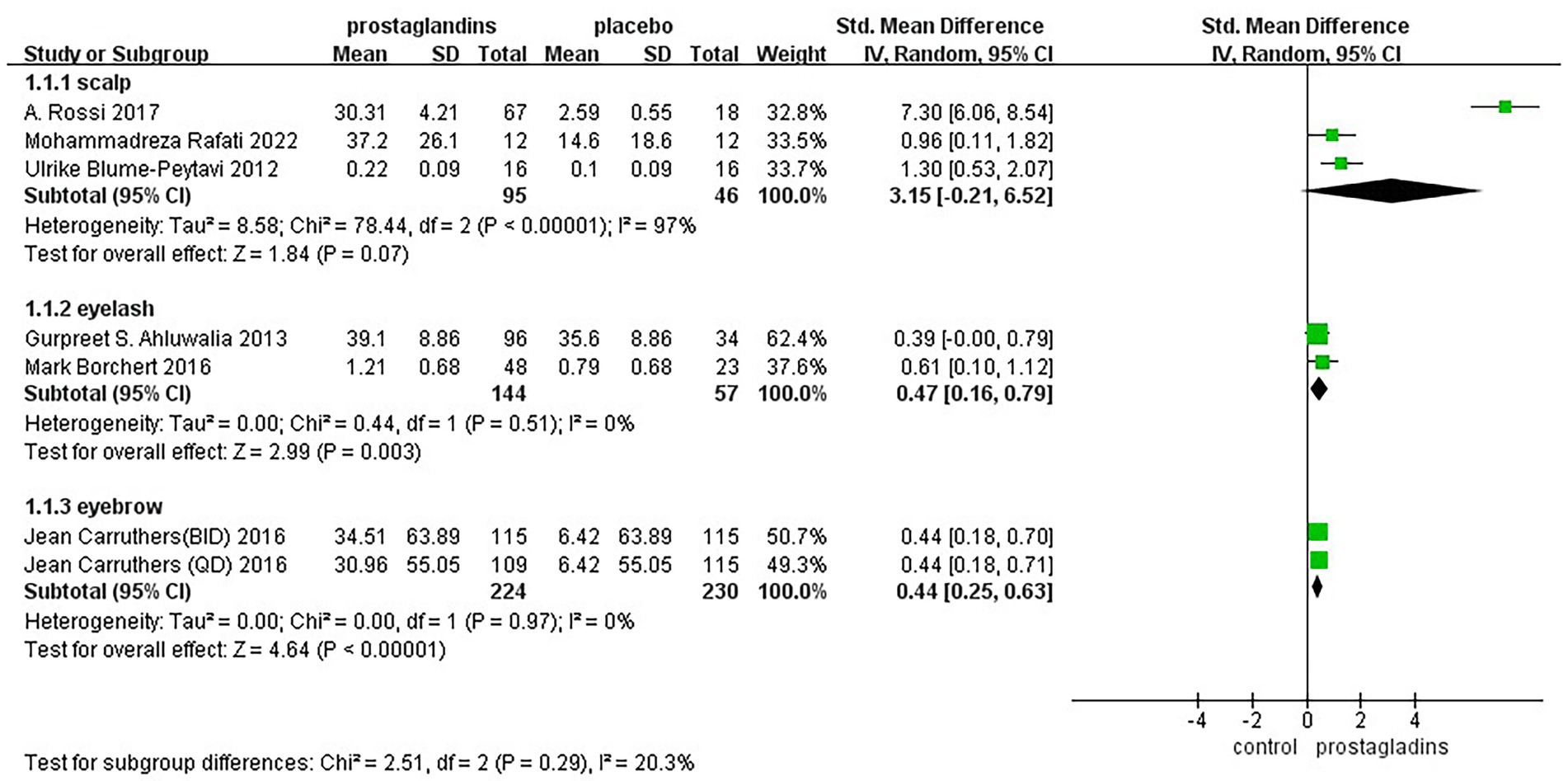
To assess whether hair loss at different sites could be combined for analysis, we performed a subgroup analysis between groups for eyebrows, eyelashes, and scalp (Figure 7). The results showed fair heterogeneity between groups(I2 = 20.3%).
Adverse events
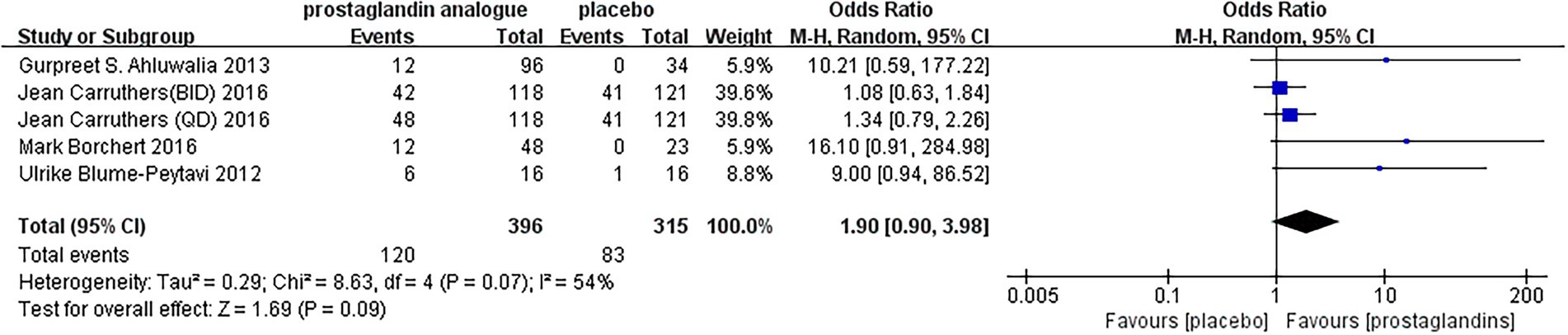
During the follow-up period, adverse events were reported in five RCTs. As seen by the forest plot (Figure 8), MD = 1.90, 95% CI (0.90, 3.98), p = 0.07, I2 = 54%. It could be seen that there was still a slight increase in the incidence of adverse reactions after using prostaglandin analogs compared to using a placebo. But the gap and the severity of the adverse reactions were still within the acceptable range. Upper respiratory tract infections were reported in Two studies (37, 39), including 332 patients. There was 1 study with unknown adverse reactions (36). The remaining studies (35, 38, 40) reported local adverse reactions to drug administration, including conjunctival hyperemia, punctate keratitis, erythema, folliculitis, the sensation of bilateral burning, and application site pruritus. There were also rare side effects reported by enrolled studies, such as urinary tract infections, other body hair increase, influenza and actinic keratosis.
Discussion
Psychological distress can be caused by hair loss, which is a common condition. Recently, the previous literature suggested that prostaglandin analogs, including Bimatoprost (34), Latanoprost (41), and Cetirizine (42), could promote hair growth in clinical trials. As far as we know, We have conducted the first systematic review and meta-analysis of prostaglandin analog therapy for hair loss on this topic. The aim of this meta-analysis was to assess the efficacy and safety of topical prostaglandin analog treatments in patients with a lack of hair(scalp, eyelash and eyebrow).
When searching the literature, we excluded case–control studies retrieved because historical information is often not verifiable and it is sometimes impossible to guarantee the authenticity of the information. Similarly, we excluded cohort studies because they are prone to missing visit bias, and changes in known variables or the introduction of unknown variables can also produce bias, thus reducing the credibility of the original data. Our meta-analysis involved 6 RCTs (35–40)(7 data sets). The data showed more significant improvement in hair regrowth in the topical prostaglandin analog treatment group, with more patients having a substantial increase in hair and a similar incidence of adverse events, suggesting that topical prostaglandin analog treatment is a better option for patients with a lack of hair (p < 0.001).
As shown by whole-body photographic evaluation, changes in hair length or density were a common way of evaluating efficacy. Prostaglandin analogs were found to promote superior hair growth in all studies compared to controls. Thus, our meta-analysis showed a significant advantage of topical prostaglandin analog treatment in hair regrowth (p < 0.001). It is possible that including more randomized controlled trials would not change this result. Using fixed-effects and random-effects models, we found utterly different results for hair density changes. This may be due to the fact that the number of randomized controlled trials is limited, and therefore, there was still a need for more studies to confirm these findings. In conclusion, our pooled data suggest that the topical prostaglandin analog treatment group has excellent results in hair regeneration and offers a new possible therapeutic prospect for patients with hair loss.
We likewise found that the effects of different doses of prostaglandin analogs on hair differed considerably. We performed a subgroup analysis of dose and could see that dose and effect size were positively correlated within the statistical range. However, we have less data to find the most appropriate dose.
The hair follicles are complex micro-organisms with constant cycling behavior associated with growth, quiescence, and involution (31). The growth and cycle of hair is influenced by numerous gene products and growth factors. The prostaglandins are unsaturated carboxylic acids composed of 20 carbon skeletons that are synthesized by biochemical means from arachidonic acid. According to Colombe (2007), human hair follicles are capable of metabolizing PGE2 and PGF2a (43). It was found in hair follicles a year later that all four major PG receptors are present and according to a 2012 study, PGD2 and its enzyme synthase were elevated in skin biopsies taken from male bald head scalps (44, 45). Then, the results of Eleni Chovarda’s study (46) indicated that a potential treatment for hair loss might target prostaglandin receptors, which possibly contributing to hair loss pathogenesis. We then analyzed the data from existing clinical studies and concluded that topical application of prostaglandin analogs may have a positive improvement in hair loss.
Generally, hair growth evaluation and follow-up are limited to individual physician and patient assessments. The investigators’ hair growth assessments were confirmed by global photographic assessment and Automatic digitalized photographic systems. In global photographic assessment of hair growth, experts are blinded to treatment and time and evaluate global photographs. With the help of a digital image analysis system, it is possible to quantify hair density, hair thickness, anagen hair ratio in the survey area (14). As with the results of the subgroup analysis described above, it was evident that the use of digital image technology allowed for a more accurate assessment of efficacy.
The cause of hair loss may influence treatment, and the causes of hair loss we included in our study were chemotherapy (37), alopecia areata (40), androgenic alopecia (35, 36), and a mixture of factors (38). The cause of hair loss in one of the included studies was unknown (39). For the included studies, prostaglandin analogs can treat the above causes of hair loss, but more data were needed to support this.
A few limitations are present in our review. First, we did not include all medical databases, such as Web of Science, in our search, and we included only English-language publications. As a result, this could lead to a selection bias. Second, the number of limited trials for each result prevented us from performing a funnel plot analysis. Third, the small number of our study and the small sample of two types of hairs, eyelashes and eyebrows, may affect the credibility of the results.
Conclusion
Based on the six RCTs clinical trials with small sample sizes, the prostaglandin analogs showed better efficacy than placebo in hair loss treatment. However, the optimal dose of prostaglandin analogs treatment requires additional studies. In addition, the safety of topical application of prostaglandin analogs cannot be better guaranteed. Considering its efficacy and safety, hair loss disorder may be treated with topical prostaglandin analogs, particularly in patients who have not responded well to other forms of treatment. Further studies are also needed to better understand factors influencing prostaglandin analogs treatment outcomes in patients with hair loss. Among the concerns that might be taken into account are: sufficient sample size, optimal concentrations and regimens, and duration of topical prostaglandin analogs, adequate follow-up time, and standardized and appropriate outcomes, including patient satisfaction evaluations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
SJ: conceptualization, methodology, software, and writing–original draft. ZH: validation, data curation, and supervision. WQ: validation and resources. ZW: formal analysis and resources. MZ: writing–review and editing. NG: visualization and writing–review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Natural Science Foundation of Hubei Province, China (No. 2019CFB561) and Youth Fund of National Natural Science Foundation of China (02.07.21020088).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alessandrini, A, Bruni, F, Piraccini, BM, and Starace, M. Common causes of hair loss - clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. (2021) 35:629–09. doi: 10.1111/jdv.17079
2. Law, SK. Bimatoprost in the treatment of eyelash hypotrichosis. Clin Ophthalmol. (2010) 4:349–8. doi: 10.2147/opth.s6480
3. Pucci, N, Novembre, E, Lombardi, E, Massai, C, Bernardini, R, Caputo, R, et al. Long eyelashes in a case series of 93 children with vernal keratoconjunctivitis. Pediatrics. (2005) 115:e86–91. doi: 10.1542/peds.2004-1555
4. Woodson, SA. Latisse: empirical discovery yields treatment for sparse eyelashes. Nurs Womens Health. (2009) 13:243–8. doi: 10.1111/j.1751-486X.2009.01426.x
5. van der Velden, EM, Drost, BH, Ijsselmuiden, OE, Baruchin, AM, and Hulsebosch, HJ. Dermatography as a new treatment for alopecia areata of the eyebrows. Int J Dermatol. (1998) 37:617–1. doi: 10.1046/j.1365-4362.1998.00540.x
6. Velez, N, Khera, P, and English, JC. Eyebrow loss: clinical review. Am J Clin Dermatol. (2007) 8:337–6. doi: 10.2165/00128071-200708060-00003
7. York, K, Meah, N, Bhoyrul, B, and Sinclair, R. A review of the treatment of male pattern hair loss. Expert Opin Pharmacother. (2020) 21:603–2. doi: 10.1080/14656566.2020.1721463
8. Shin, JY, Kim, J, Choi, Y-H, Kang, N-G, and Lee, S. Dexpanthenol promotes cell growth by preventing cell senescence and apoptosis in cultured human hair follicle cells. Curr Issues Mol Biol. (2021) 43:1361–73. doi: 10.3390/cimb43030097
9. Kutlu, Ö, and Metin, A. Systemic dexpanthenol as a novel treatment for female pattern hair loss. J Cosmet Dermatol. (2021) 20:1325–30. doi: 10.1111/jocd.13729
10. Lee, SW, Juhasz, M, Mobasher, P, Ekelem, C, and Mesinkovska, NA. A systematic review of topical Finasteride in the treatment of Androgenetic alopecia in men and women. J Drugs Dermatol. (2018) 17:457–3.
11. Goren, A, Shapiro, J, Roberts, J, McCoy, J, Desai, N, Zarrab, Z, et al. Clinical utility and validity of minoxidil response testing in androgenetic alopecia. Dermatol Ther. (2015) 28:13–6. doi: 10.1111/dth.12164
12. Irwig, MS, and Kolukula, S. Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. (2011) 8:1747–53. doi: 10.1111/j.1743-6109.2011.02255.x
13. Irwig, MS. Persistent sexual side effects of finasteride: could they be permanent? J Sex Med. (2012) 9:2927–32. doi: 10.1111/j.1743-6109.2012.02846.x
14. Kanti, V, Messenger, A, Dobos, G, Reygagne, P, Finner, A, Blumeyer, A, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men - short version. J Euro Acad Dermatol Venereol. (2018) 32:11–22. doi: 10.1111/jdv.14624
15. Ebner, H, and Müller, E. Allergic contact dermatitis from minoxidil. Contact Dermatitis. (1995) 32:316–7. doi: 10.1111/j.1600-0536.1995.tb00798.x
16. Olsen, EA, Whiting, D, Bergfeld, W, Miller, J, Hordinsky, M, Wanser, R, et al. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. (2007) 57:767–4. doi: 10.1016/j.jaad.2007.04.012
17. Fertig, RM, Gamret, AC, Cervantes, J, and Tosti, A. Microneedling for the treatment of hair loss? J Euro Acad Dermatol Venereol. (2018) 32:564–9. doi: 10.1111/jdv.14722
18. Dhurat, R, Sukesh, M, Avhad, G, Dandale, A, Pal, A, and Pund, P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: a pilot study. Int J Trichol. (2013) 5:6–11. doi: 10.4103/0974-7753.114700
19. Barton, VR, Toussi, A, Awasthi, S, and Kiuru, M. Treatment of pediatric alopecia areata: a systematic review. J Am Acad Dermatol. (2022) 86:1318–34. doi: 10.1016/j.jaad.2021.04.077
20. Yadav, S, Dogra, S, and Kaur, I. An unusual anatomical colocalization of alopecia areata and vitiligo in a child, and improvement during treatment with topical prostaglandin E2. Clin Exp Dermatol. (2009) 34:e1010–1. doi: 10.1111/j.1365-2230.2009.03677.x
21. Mehta, JS, Raman, J, Gupta, N, and Thoung, D. Cutaneous latanoprost in the treatment of alopecia areata. Eye (Lond). (2003) 17:444–6. doi: 10.1038/sj.eye.6700354
22. Coronel-Pérez, IM, Rodríguez-Rey, EM, and Camacho-Martínez, FM. Latanoprost in the treatment of eyelash alopecia in alopecia areata universalis. J Euro Acad Dermatol Venereol. (2010) 24:481–5. doi: 10.1111/j.1468-3083.2009.03543.x
23. Li, AW, and Antaya, RJ. Successful treatment of pediatric alopecia Areata of the scalp using topical Bimatoprost. Pediatr Dermatol. (2016) 33:e282–3. doi: 10.1111/pde.12920
24. Zaheri, S, and Hughes, B. Successful use of bimatoprost in the treatment of alopecia of the eyelashes. Clin Exp Dermatol. (2010) 35:e161–2. doi: 10.1111/j.1365-2230.2009.03755.x
25. Hossein Mostafa, D, Samadi, A, Niknam, S, Nasrollahi, SA, Guishard, A, and Firooz, A. Efficacy of cetirizine 1% versus Minoxidil 5% topical solution in the treatment of male alopecia: a randomized, single-blind controlled study. J Pharm Pharm Sci. (2021) 24:191–9. doi: 10.18433/jpps31456
26. Chen, X, Xiang, H, and Yang, M. Topical cetirizine for treating androgenetic alopecia: a systematic review. J Cosmet Dermatol. (2022) 21:5519–26. doi: 10.1111/jocd.15309
27. Saceda-Corralo, D, Domínguez-Santas, M, Vañó-Galván, S, and Grimalt, R. What’s new in therapy for male Androgenetic alopecia? Am J Clin Dermatol. (2022) 24:15–24. doi: 10.1007/s40257-022-00730-y
28. Yazdanian, N, Mozafarpoor, S, and Goodarzi, A. Phosphodiesterase inhibitors and prostaglandin analogues in dermatology: a comprehensive review. Dermatol Ther. (2021) 34:e14669. doi: 10.1111/dth.14669
29. Pustisek, N, and Lipozencić, J. Prostaglandins in dermatology. Acta Dermatovenerol Croat. (2001) 9:291–8.
30. Xu, XG, and Chen, HD. Prostanoids and hair follicles: implications for therapy of hair disorders. Acta Derm Venereol. (2018) 98:318–3. doi: 10.2340/00015555-2843
31. Johnstone, MA, and Albert, DM. Prostaglandin-induced hair growth. Surv Ophthalmol. (2002) 47:S185–202. doi: 10.1016/s0039-6257(02)00307-7
32. Roch-Arveiller, M, Tissot, M, Idohou, N, Sarfati, G, Giroud, JP, and Raichvarg, D. In vitro effect of cetirizine on PGE2 release by rat peritoneal macrophages and human monocytes. Agents Actions. (1994) 43:13–6. doi: 10.1007/BF02005756
33. Charlesworth, EN, Kagey-Sobotka, A, Norman, PS, and Lichtenstein, LM. Effect of cetirizine on mast cell-mediator release and cellular traffic during the cutaneous late-phase reaction. J Allergy Clin Immunol. (1989) 83:905–2. doi: 10.1016/0091-6749(89)90104-8
34. Barrón-Hernández, YL, and Tosti, A. Bimatoprost for the treatment of eyelash, eyebrow and scalp alopecia. Expert Opin Investig Drugs. (2017) 26:515–2. doi: 10.1080/13543784.2017.1303480
35. Blume-Peytavi, U, Lönnfors, S, Hillmann, K, and Garcia, BN. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J Am Acad Dermatol. (2012) 66:794–09. doi: 10.1016/j.jaad.2011.05.026
36. Rossi, A, Campo, D, Fortuna, MC, Garelli, V, Pranteda, G, De Vita, G, et al. A preliminary study on topical cetirizine in the therapeutic management of androgenetic alopecia. J Dermatolog Treat. (2018) 29:149–1. doi: 10.1080/09546634.2017.1341610
37. Ahluwalia, GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. (2013) 16:S73–s76. doi: 10.1038/jidsymp.2013.30
38. Borchert, M, Bruce, S, Wirta, D, Yoelin, SG, Lee, S, Mao, C, et al. An evaluation of the safety and efficacy of bimatoprost for eyelash growth in pediatric subjects. Clin Ophthalmol. (2016) 10:419–9. doi: 10.2147/opth.S89561
39. Carruthers, J, Beer, K, Carruthers, A, Coleman, WP, Draelos, ZD, Jones, D, et al. Bimatoprost 0.03% for the treatment of eyebrow Hypotrichosis. Dermatol Surg. (2016) 42:608–7. doi: 10.1097/DSS.0000000000000755
40. Rafati, M, Mahmoudian, R, Golpour, M, Kazeminejad, A, Saeedi, M, and Nekoukar, Z. The effect of latanoprost 0.005% solution in the management of scalp alopecia areata, a randomized double-blind placebo-controlled trial. Dermatol Ther. (2022) 35:e15450. doi: 10.1111/dth.15450
41. Ocampo-Garza, J, Griggs, J, and Tosti, A. New drugs under investigation for the treatment of alopecias. Expert Opin Investig Drugs. (2019) 28:275–4. doi: 10.1080/13543784.2019.1568989
42. Caro, G, Fortuna, MC, Magri, F, Federico, A, Carlesimo, M, and Rossi, A. A new treatment of alopecia induced by palbociclib: topical cetirizine. J Oncol Pharm Pract. (2021) 27:460–3. doi: 10.1177/1078155220930334
43. Colombe, L, Vindrios, A, Michelet, JF, and Bernard, BA. Prostaglandin metabolism in human hair follicle. Exp Dermatol. (2007) 16:762–9. doi: 10.1111/j.1600-0625.2007.00586.x
44. Colombe, L, Michelet, JF, and Bernard, BA. Prostanoid receptors in anagen human hair follicles. Exp Dermatol. (2008) 0:747001. doi: 10.1111/j.1600-0625.2007.00639.x
45. Garza, LA, Liu, Y, Yang, Z, Alagesan, B, Lawson, JA, Norberg, SM, et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Trans Med. (2012) 4:126ra34. doi: 10.1126/scitranslmed.3003122
Keywords: hair loss, prostaglandin analog, Bimatoprost, Latanoprost, cetirizine, meta-analysis
Citation: Jiang S, Hao Z, Qi W, Wang Z, Zhou M and Guo N (2023) The efficacy of topical prostaglandin analogs for hair loss: A systematic review and meta-analysis. Front. Med. 10:1130623. doi: 10.3389/fmed.2023.1130623
Edited by:
Devinder Mohan Thappa, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaReviewed by:
Gulhima Arora, Mehektagul Dermaclinic, IndiaÖmer Kutlu, Tokat Gaziosmanpaşa University, Türkiye
Maryam Nasimi, Tehran University of Medical Sciences, Iran
Copyright © 2023 Jiang, Hao, Qi, Wang, Zhou and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muran Zhou, bXVyYW5femhvdUAxNjMuY29t; Nengqiang Guo, Z3VvbnEyMDEyQGhvdG1haWwuY29t
†These authors share first authorship
 Shangxuan Jiang
Shangxuan Jiang Zhuolun Hao
Zhuolun Hao Wenli Qi
Wenli Qi