
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 24 March 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1126491
This article is part of the Research TopicEndocrinology and COVID-19: A Cross-Disciplinary Topic, volume IIView all 17 articles
The COVID-19 pandemic is ongoing and places a substantial burden on healthcare systems worldwide. As we further shed light on different disease characteristics, we identify more and more groups of people at higher risk of poor COVID-19 outcomes. Metabolic-associated fatty liver disease (MAFLD) (previously non-alcoholic fatty liver disease or NAFLD) is a common metabolic disorder characterized by fat accumulation and liver fibrosis. Given its close correlation with metabolic syndrome, an established risk factor for severe COVID-19, it is necessary to investigate its interplay with the novel coronavirus. In this study, we review the available data on COVID-19 prognosis, treatment and prevention options in patients with MAFLD, and the effect that the disease and the pandemic have on MAFLD care. Furthermore, we point out the gaps in the current literature to accentuate the work that needs to be done to improve MAFLD care during the pandemic and beyond.
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has posed a daunting challenge since late 2019, with approximately 600 million confirmed cases and 7 million deaths as of September 1st, 2022 (1). Early on, we learned that while respiratory symptoms may be predominant in COVID-19, the disease affects various organ systems, with gastrointestinal, cardiovascular, neurological, hematological, and renal involvement (2–10). With the growing knowledge of the disease, we learned that in addition to the acute phase, COVID-19 might induce multi-system long-term consequences (i.e., long COVID), such as fatigue, myalgia, psychological symptoms, and hepatitis (11–13).
Liver damage is one of the most important aspects of COVID-19, with elevated liver enzymes appearing in approximately 15–65% of patients in the acute phase (14, 15) and prolonged hepatobiliary complications in some cases (16, 17). In critically ill COVID-19 patients, pathological studies have revealed mild lobular and portal inflammation as well as moderate macrovesicular steatosis (18, 19). Direct viral cytotoxic effects, systemic inflammation, hypoxia, coagulopathy, and drug-induced liver injury are all potential causes of liver damage (4). Notably, viral RNA has been detected in liver samples, and SARS-CoV-2 isolated from liver tissue is infectious (20–22). Angiotensin-converting enzyme 2 (ACE2) is the primary viral receptor for SARS-COV-2. The host transmembrane serine protease 2 (TMPRSS2) is also crucial for viral infectiousness. ACE2 and TMPRSS2 were found to be highly expressed in the liver. Cholangiocytes had the highest levels of ACE2 expression, followed by hepatocytes. Transmembrane serine protease 2 was found to be mainly expressed in cholangiocytes, hepatocytes, periportal liver sinusoidal endothelial cells, and erythroid cells. (23, 24). Interestingly, hypoxia and inflammatory conditions were found to upregulate ACE-2 expression (25, 26).
The liver injury could be more severe in patients with pre-existing chronic liver diseases. This can be partly explained by the increased expression of ACE2 in these patients (26–28). Non-alcoholic fatty liver disease (NAFLD), recently known as metabolic-associated fatty liver disease (MAFLD), is a spectrum of diseases ranging from simple steatosis with or without mild inflammation to a necroinflammatory subtype with the presence of hepatocellular injury (non-alcoholic steatohepatitis (NASH)) and cirrhosis (29, 30). NAFLD is the most common cause of chronic liver disease and is estimated to have affected a quarter of the global population (31, 32). Of note, given the role of cardiometabolic risk factors in the development and progression of the disease, two new position papers (29, 30) proposed the terminology of MAFLD instead of NAFLD in 2020 to better capture the pathophysiology of the disease (33, 34).
Though controversial, early reports during the pandemic indicated that patients with NAFLD have a greater risk of developing a more severe disease course (35–37). Given the association of MAFLD with other cardiometabolic risk factors, which are also well-established predictors of poor prognosis in COVID-19, it remains unclear whether MAFLD is merely associated with poor outcomes or plays a causal role. Moreover, not only can MAFLD influence the course of COVID-19, but it is also important to recognize the effects of the COVID-19 pandemic on the care of patients with MAFLD and the epidemiology of the disease. In addition, given the global scale of COVID-19 vaccination, the focus of research should shift to the safety and efficacy of COVID-19 vaccines in patients with MAFLD.
In this review, we provide a concise yet comprehensive overview of the interplay between MAFLD and the COVID-19 pandemic, focusing on the COVID-19 outcomes in patients with MAFLD, the impact of the COVID-19 pandemic on the care of patients with MAFLD and the epidemiology of the disease, and COVID-19 vaccination in patients with MAFLD (Figure 1). We also pave the way for future research by highlighting the current gaps in the field’s knowledge.
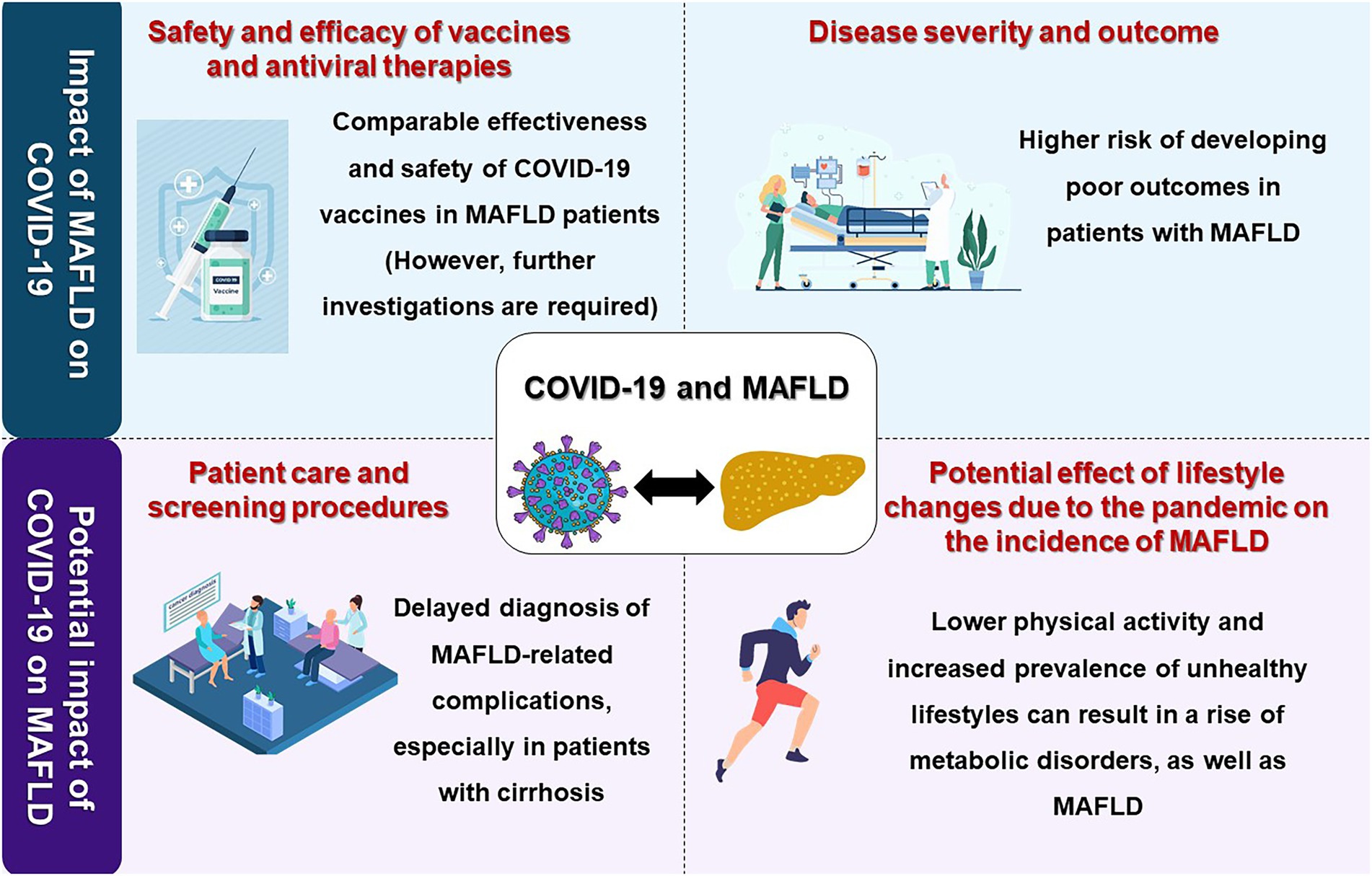
Figure 1. A visual summary of the interplay of the COVID-19 pandemic and metabolic-associated fatty liver disease (MAFLD). Image made using material provided by pch.vector on Freepik.
Metabolic factors such as obesity and diabetes are established risk factors for severe COVID-19 (38). Hence, it is only logical to assume that MAFLD is associated with a worse prognosis for COVID-19 (39). While almost all studies show that patients with MAFLD are at a higher risk of severe disease, it is not yet well understood whether MAFLD-related changes can act as an independent prognostic factor and, if they do, to what extent they can impact the clinical course of COVID-19. The following sections review the various aspects of the MAFLD-COVID-19 interaction.
According to an analysis of a Korean nationwide cohort, patients with NAFLD had a 35–41% increased disease risk of severe COVID-19 (40), and in another study, patients with MAFLD were four times more likely to acquire severe disease (adjusted odds ratio (OR) = 4.07, p = 0.02) (41). A study in Turkey (42) showed that patients with hepatosteatosis (HS) had significantly higher pneumonia severity scores compared with non-HS patients (p < 0.001). Among hospitalized SARS-CoV-2 infected patients with NAFLD, diabetes and advanced liver fibrosis were independent predictors of progression to severe disease (adjusted ORs = 8.26 and 11.06 (p = 0.03 for both), respectively) (43). Targher et al. (44) reported that patients with intermediate and high Fibrosis-4 (FIB-4) scores had more than four and five times higher risk of severe COVID-19, respectively, compared with patients without MAFLD. Even when adjusted for sex, obesity, and prior diabetes history, the odds of severe disease remained high (OR = 2.59, p = 0.03 for intermediate and OR = 4.04, p = 0.02 for high FIB-4 scores). Intermediate and high FIB-4 scores had a combined 3-fold increase in severe COVID-19 risk (adjusted OR = 2.95, p < 0.005). Similarly, in a study in Italy (45), the FIB-4 score < 1.45 was associated with lower disease severity (adjusted OR = 0.3, p = 0.01) and mortality (adjusted OR = 0.4, p = 0.04). Contrary to the previous results, do Amaral e Castro and colleagues (46) did not find an association between HS and worse COVID-19 outcomes, although HS was more common among patients with worse outcomes. Furthermore, one study (47) found increased risks of intensive care unit (ICU) admission and mortality with increasing liver fibrosis degree in univariable analysis, although they became insignificant when the risk was adjusted for other factors. A recent meta-analysis of 16 studies by Hayat et al. (48) showed a three-fold increase in severe COVID-19 risk in patients with MAFLD compared with controls. ICU admission was also more incident in patients with MAFLD; however, mortality was similar to the control group. Similar results were achieved in a 2021 analysis of adjusted risks (37), with an adjusted OR of 2.6 (p < 0.001) for severe disease, 1.66 (p < 0.001) for ICU admission, and 1.01 (p = 0.96) for mortality.
Corapli et al. (42) observed that patients with HS were more likely to be admitted (65% vs. 48%, p = 0.003), with similar ward (p = 0.93) and ICU (p = 0.50) stay durations in HS and non-HS groups nonetheless. In a preprint (49), each additional year of having NAFLD/NASH was associated with an 86% increase in the risk of hospitalization (p < 0.01). An interesting result of this study was that when patients were adjusted for NAFLD/NASH, obesity decreased the chance of hospitalization by almost 60% (p < 0.01), pointing toward the important role of liver fibrosis in COVID-19 prognosis in obese patients. While using medications in the 3 months leading to the COVID-19 diagnosis did not result in less hospitalization, those who had undergone bariatric surgery were less likely to be admitted (OR = 0.22, p < 0.05). Furthermore, patients with NAFLD were much more likely to experience disease progression when hospitalized (OR = 6.4) (50). Additionally, these patients recover 36% slower (p < 0.001, based on time to or readiness for discharge) and are more likely to face pulmonary thromboembolism (OR = 2.15, p = 0.04) (51).
In addition to being an independent predictor, MAFLD appears to increase the effect of obesity on the prognosis of COVID-19. While obese patients are 1.5 times more likely to acquire severe disease (52), Zheng and colleagues (53) demonstrated that in patients with MAFLD, obesity was associated with six times higher risk for severe COVID-19. Moreover, prolonged viral shedding might also be present in this population (50).
Secondary sclerosing cholangitis (SSC) is a hepatic complication of COVID-19, with an incidence of 11.8% in patients with severe disease (54) and 2.0% among all hospitalized patients (55). Hartl et al. (55) found that among 10 hospitalized patients with COVID-19 who developed SSC, seven (70%) were because of NAFLD/NASH.
The reason behind these worse outcomes is a matter of debate, with several possible mechanisms involved. Some propose that MAFLD exacerbates COVID-19’s cytokine storm by increasing the release of pro-inflammatory cytokines from the liver (44, 56). In contrast, others hypothesize that innate immunity diminishes with the liver’s immune cell shift from pro-inflammatory M1 macrophages to regulatory M2 macrophages (57), leading to the deterioration of the patient’s condition (50). A recent study (58) has confirmed both of these findings and demonstrated that patients with MAFLD expressed higher levels of some inflammatory cytokines [such as interleukin-6 (IL-6), which has been shown to play an important role in severe disease and its treatment (59)] and lower levels of interferon-γ (IFN-γ), which is crucial to macrophage activity. Another involved mechanism might be the upregulation of SARS-CoV-2 entry proteins (i.e., ACE2 and TMPRSS2) in obese patients with NASH (28). Furthermore, since fatty liver diseases are closely intertwined with metabolic syndrome, similar detrimental pathophysiological pathways are likely involved (42).
Few studies are available on how MAFLD affects COVID-19 vaccination outcomes.
Wang et al. (60) found that 24.9% of patients with MALFD who received the Sinopharm (BBIBP-CorV) vaccine (inactivated virus) showed adverse reactions seven days post-inoculation, which is lower than the vaccine’s phase 3 results (more than 40%) (61). In another study (62), patients receiving either Comirnaty (BNT162b2) or CoronaVac were divided into HS (those with moderate/severe hepatosteatosis) and control groups. Patients in the HS group showed fewer adverse reactions after the first and second doses of CoronaVac. In contrast, Comirnaty resulted in a higher rate of systemic reactions in the HS group after the first dose (58% vs. 39%, p = 0.008), especially fatigue (40% vs. 27%, p = 0.07), and also a higher rate of joint pain after the second dose (13% vs. 1%, p < 0.001).
In the study by Wang and colleagues (60) (Sinopharm vaccine), seroconversion was observed in 95.5% of the patients, which is comparable to the nearly 100 percent achieved in the phase 3 trial. Moreover, Cheung et al. (62) observed that on day 56 after the first dose, all cases receiving Comirnaty had achieved seroconversion with similar titer levels (p = 0.68). However, the best-responding cases (top 25% of virus microneutralization titer levels) were more prevalent in the control group (p = 0.04). All HS patients and all controls, except one, remained seroconverted on day 180 after the first dose, with similar titers (p = 1.00 for both). CoronaVac also produced similar seroconversion rates in HS and control groups on day 56 (p = 0.13); however, the geometric mean titer was lower in the HS group (p = 0.02). Similar to Comirnaty, the best responders were mostly from the control group (p = 0.04).
NASH is one of the most common causes of cirrhosis and the second leading indication for a liver transplant. Patients with cirrhosis require prompt diagnosis and treatment of the relevant complications. Considering the annual cumulative hepatocellular carcinoma (HCC) incidence rate of 2.6% for NASH-related cirrhosis, these patients need routine screening for HCC (31). Notably, a multicenter investigation found a significantly decreased number of HCC diagnoses and an increased rate of HCC treatment delay compared to the same period in the previous year during a high prevalence of COVID-19 (63). Moreover, regular screening for esophageal varices, given the high risk of mortality, is also required in patients with NASH-related cirrhosis (64); however, during the COVID-19 pandemic, most screening procedures were delayed, which presumably has led to undiagnosed cases as we are recovering from the pandemic (65). One such example is screening endoscopy, which was recommended to be performed only in urgent circumstances by the pandemic guidelines. This has most likely resulted in missed esophageal varices due to delayed screening (66, 67), especially in earlier periods of the pandemic, though to the best of our knowledge, no studies have reported the corresponding data. The COVID-19 pandemic has also adversely affected transplantation activity and, in turn, affected the care of patients with NASH-related cirrhosis (68).
From another perspective, the COVID-19 pandemic caused considerable behavioral changes due to the restrictions, including lockdown, home confinement, and closure of sports facilities. Several studies have shown decreased physical activity and increased prevalence of unhealthy lifestyles, including increased dietary intake, decreased sleep, and increased smoking during COVID-19 lockdowns (69–71). A longitudinal investigation of NAFLD patients showed that the more active patients had lower physical activity than before during the lockdown, while inactive people had higher physical activity (72). Importantly, cohorts of patients with NAFLD showed that COVID-19 lockdown caused a significant increase in body weight, body mass index (BMI), insulin resistance, cholesterol levels, low-density lipoprotein (LDL) levels, and glucose levels. It also led to a reduction in high-density lipoprotein (HDL) levels alongside with progression of fatty liver (73–75). In addition, population-based analyses using the United States (US) national mortality records revealed that the steady increase in NAFLD mortality prior to the COVID-19 pandemic sped up during the pandemic (76). A recently published cohort study comparing patients before and after a COVID-19 lockdown grouped patients with NAFLD according to the level of physical activity. They found that the fatty liver index (FLI) increased in all groups after the lockdown. However, the elevation in the FLI was higher in the medium physical activity than in the low physical activity group (72). In addition to worsening metabolic risk factors and liver involvement in patients with NAFLD, such lifestyle changes may increase the likelihood of an increased incidence of NAFLD in the coming years as we recover from the pandemic, given the strong association of metabolic risk factors with NAFLD development and progression (32).
The COVID-19 pandemic, although catastrophic, provided and continues to provide many valuable lessons to experts and policymakers of all fields, especially medicine. First and foremost, healthcare providers must always anticipate a sudden crisis that halts delivering health services. Although the COVID-19 pandemic pressured organizations into identifying actions and developing protocols to counter the effect of this global crisis, the effort must be continuous and the results have to be updated regularly based on most recent evidence. Telemedicine is an example of a tool that has been extensively studied during the ongoing pandemic and shown to be effective (77, 78) and satisfactory (79); hence, we suggest healthcare facilities commence and test out different telemedicine approaches to identify the most suitable one for their use. Rapid re-initiation of medical practices during crises is of utmost importance; however, this must not result in the normalization of the ongoing calamity. While continuing care for some patients is necessary, some medical practices could be postponed with no or minimal adverse outcomes (80). Comprehensive crisis-management guidelines and protocols are required to stratify medical services based their delay capacity. Undoubtedly, in the unfortunate event of a similar disaster in the future, the world’s response would be much more appropriate given the experience gained from COVID-19, just as it was for COVID-19 because of the previous outbreaks such as Middle East Respiratory Syndrome (MERS) and SARS (81).
Current studies, as presented in this manuscript, provide strong evidence that patients with MAFLD tend to experience worse COVID-19 outcomes. However, literature is short of approaches to mitigate this risk. Consequently, to the best of our knowledge, no specific COVID-19 guidelines have been developed for patients with MAFLD. We believe the focus of future related studies should be on evaluating different care strategies in these patients.
Vaccination and specific antiviral treatments are the current trend in COVID-19 research. To the best of our knowledge, very few studies have evaluated COVID-19 vaccine effectiveness in patients with MAFLD and no studies have evaluated antiviral treatments (such as remdesivir and Paxlovid) in this population. Investigating these subjects in patients with MAFLD is of utmost importance, given the crucial role of the liver in drug metabolization.
Delay in health care and shift of resources toward managing COVID-19 is the pandemic’s predominant impact on other diseases. No comprehensive analyses have yet been performed to check whether MAFLD prevalence and incidence have increased in this period. Furthermore, there are no studies investigating whether MAFLD complications such as esophageal varices and HCC have significantly increased, given the cardinal role of screening in their detection and prevention.
Almost 3 years after the emergence of COVID-19, we very well know that it can affect the liver, and patients with certain comorbidities, such as metabolic dysfunction, are at higher risk for severe disease or mortality. Herein, we concisely reviewed the substantial evidence supporting the mutual association between MAFLD and COVID-19. Patients with MAFLD are at a higher risk of poor outcomes during COVID-19, even after controlling for the confounding effect of the other metabolic abnormalities. COVID-19 caused drastic changes in human lifestyle, screening programs, and transplantation programs, which could adversely affect the care of patients with MAFLD, especially those with NAFLD cirrhosis, or even potentially increase the incidence rate of MAFLD in years to come. Assessment of the efficacy and safety of COVID-19 vaccines in this group of patients has garnered attention recently, with the wide global vaccination showing comparable results with the healthy population. Further investigations are required for development of guidelines for management of MAFLD during the COVID-19 pandemic, and assessment of efficacy of vaccination and antiviral therapies in this group of patients.
AN: conceptualization, investigation, writing—original draft, and writing—review & editing. SM: conceptualization, investigation, writing—original draft, writing—review & editing, and visualization. NR: conceptualization, writing—review & editing, and supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACE2, angiotensin-converting enzyme 2, BMI, body mass index, COVID-19, coronavirus disease 2019, FIB-4, fibrosis-4, FLI, fatty liver index, HCC, hepatocellular carcinoma, HS, hepatosteatosis, ICU, intensive care unit, IFN-γ, interferon-γ, IL-6, interleukin-6, MAFLD, metabolic associated fatty liver disease, NAFLD, Non-alcoholic fatty liver disease, NASH, non-alcoholic steatohepatitis, OR, odds ratio, SARS-COV-2, severe acute respiratory syndrome coronavirus 2, TMPRSS2, transmembrane serine protease 2,
1. World Health Organization. (2022). Coronavirus disease (COVID-19) pandemic. Available at: https://covid19.who.int/
2. Momtazmanesh, S, Shobeiri, P, Hanaei, S, Mahmoud-Elsayed, H, Dalvi, B, and Malakan, RE. Cardiovascular disease in COVID-19: a systematic review and meta-analysis of 10,898 patients and proposal of a triage risk stratification tool. Egypt Heart J. (2020) 72:41. doi: 10.1186/s43044-020-00075-z
3. Harapan, BN, and Yoo, HJ. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19). J Neurol. (2021) 268:3059–71. doi: 10.1007/s00415-021-10406-y
4. Nardo, AD, Schneeweiss-Gleixner, M, Bakail, M, Dixon, ED, Lax, SF, and Trauner, M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. (2021) 41:20–32. doi: 10.1111/liv.14730
5. Yang, X, Tian, S, and Guo, H. Acute kidney injury and renal replacement therapy in COVID-19 patients: a systematic review and meta-analysis. Int Immunopharmacol. (2021) 90:107159. doi: 10.1016/j.intimp.2020.107159
6. Bertolini, A, van de Peppel, IP, Bodewes, FAJA, Moshage, H, Fantin, A, Farinati, F, et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. (2020) 72:1864–72. doi: 10.1002/hep.31480
7. Hundt, MA, Deng, Y, Ciarleglio, MM, Nathanson, MH, and Lim, JK. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1,827 patients in a major U.S. hospital network. Hepatology. (2020) 72:1169–76. doi: 10.1002/hep.31487
8. Alqahtani, JS, Oyelade, T, Aldhahir, AM, Alghamdi, SM, Almehmadi, M, Alqahtani, AS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. (2020) 15:e0233147. doi: 10.1371/journal.pone.0233147
9. Oyelade, T, Alqahtani, J, and Canciani, G. Prognosis of COVID-19 in patients with liver and kidney diseases: an early systematic review and meta-analysis. Trop Med Infect Dis. (2020) 5:80. doi: 10.3390/tropicalmed5020080
10. Kullar, R, Patel, AP, and Saab, S. Hepatic injury in patients with COVID-19. J Clin Gastroenterol. (2020) 54:841–9. doi: 10.1097/MCG.0000000000001432
11. Higgins, V, Sohaei, D, Diamandis, EP, and Prassas, I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. (2021) 58:297–310. doi: 10.1080/10408363.2020.1860895
12. Sykes, DL, Holdsworth, L, Jawad, N, Gunasekera, P, Morice, AH, and Crooks, MG. Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung. (2021) 199:113–9. doi: 10.1007/s00408-021-00423-z
13. Kolesova, O, Vanaga, I, Laivacuma, S, Derovs, A, Kolesovs, A, Radzina, M, et al. Intriguing findings of liver fibrosis following COVID-19. BMC Gastroenterol. (2021) 21:370. doi: 10.1186/s12876-021-01939-7
14. Marjot, T, Webb, GJ, Barritt, AS, Moon, AM, Stamataki, Z, Wong, VW, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. (2021) 18:348–64. doi: 10.1038/s41575-021-00426-4
15. Zhang, C, Shi, L, and Wang, F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. (2020) 5:428–30. doi: 10.1016/S2468-1253(20)30057-1
16. Roth, NC, Kim, A, Vitkovski, T, Xia, J, Ramirez, G, Bernstein, D, et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. (2021) 116:1077–82. doi: 10.14309/ajg.0000000000001154
17. Cooper, S, Tobar, A, Konen, O, Orenstein, N, Kropach, N, Landau, Y, et al. Long COVID-19 liver manifestation in children. J Pediatr Gastroenterol Nutr. (2022) 75:244–51. doi: 10.1097/MPG.0000000000003521
18. Xu, Z, Shi, L, Wang, Y, Zhang, J, Huang, L, Zhang, C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
19. Lagana, SM, Kudose, S, Iuga, AC, Lee, MJ, Fazlollahi, L, Remotti, HE, et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. (2020) 33:2147–55. doi: 10.1038/s41379-020-00649-x
20. Wanner, N, Andrieux, G, Badia-i-Mompel, P, Edler, C, Pfefferle, S, Lindenmeyer, MT, et al. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. (2022) 4:310–9. doi: 10.1038/s42255-022-00552-6
21. Puelles, VG, Lütgehetmann, M, Lindenmeyer, MT, Sperhake, JP, Wong, MN, Allweiss, L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. (2020) 383:590–2. doi: 10.1056/NEJMc2011400
22. Sonzogni, A, Previtali, G, Seghezzi, M, Grazia Alessio, M, Gianatti, A, Licini, L, et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. (2020) 40:2110–6. doi: 10.1111/liv.14601
23. Pirola, CJ, and Sookoian, S. SARS-CoV-2 virus and liver expression of host receptors: putative mechanisms of liver involvement in COVID-19. Liver Int. (2020) 40:2038–40. doi: 10.1111/liv.14500
24. Qi, F, Qian, S, Zhang, S, and Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. (2020) 526:135–40. doi: 10.1016/j.bbrc.2020.03.044
25. Suarez-Farinas, M, Tokuyama, M, Wei, G, Huang, R, Livanos, A, Jha, D, et al. Intestinal inflammation modulates the expression of ACE2 and TMPRSS2 and potentially overlaps with the pathogenesis of SARS-CoV-2-related disease. Gastroenterology. (2021) 160:287–301.e20. doi: 10.1053/j.gastro.2020.09.029
26. Paizis, G, Tikellis, C, Cooper, ME, Schembri, JM, Lew, RA, Smith, AI, et al. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. (2005) 54:1790–6. doi: 10.1136/gut.2004.062398
27. Soldo, J, Heni, M, Königsrainer, A, Häring, H-U, Birkenfeld, AL, and Peter, A. Increased hepatic ACE2 expression in NAFL and diabetes—a risk for COVID-19 patients? Diabetes Care. (2020) 43:e134–6. doi: 10.2337/dc20-1458
28. Fondevila, MF, Mercado-Gómez, M, Rodríguez, A, Gonzalez-Rellan, MJ, Iruzubieta, P, Valentí, V, et al. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J Hepatol. (2021) 74:469–71. doi: 10.1016/j.jhep.2020.09.027
29. Eslam, M, Newsome, PN, Sarin, SK, Anstee, QM, Targher, G, Romero-Gomez, M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9. doi: 10.1016/j.jhep.2020.03.039
30. Eslam, M, Sanyal, AJ, and George, J, International Consensus P. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. (2020) 158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312
31. Younossi, ZM, Koenig, AB, Abdelatif, D, Fazel, Y, Henry, L, and Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
32. Powell, EE, Wong, VW-S, and Rinella, M. Non-alcoholic fatty liver disease. Lancet. (2021) 397:2212–24. doi: 10.1016/S0140-6736(20)32511-3
33. Fouad, Y, Dufour, JF, Zheng, MH, Bollipo, S, Desalegn, H, Gronbaek, H, et al. The NAFLD-MAFLD debate: is there a consensus-on-consensus methodology? Liver Int. (2022) 42:742–8. doi: 10.1111/liv.15197
34. Farahat, TM, Ungan, M, Vilaseca, J, Ponzo, J, Gupta, PP, Schreiner, AD, et al. The paradigm shift from NAFLD to MAFLD: a global primary care viewpoint. Liver Int. (2022) 42:1259–67. doi: 10.1111/liv.15188
35. Younossi, ZM, Stepanova, M, Lam, B, Cable, R, Felix, S, Jeffers, T, et al. Independent predictors of mortality among patients with NAFLD hospitalized with COVID-19 infection. Hepatol Commun. (2021) 6:3062–72. doi: 10.1002/hep4.1802
36. Forlano, R, Mullish, BH, Mukherjee, SK, Nathwani, R, Harlow, C, Crook, P, et al. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. (2020) 15:e0240400. doi: 10.1371/journal.pone.0240400
37. Singh, A, Hussain, S, and Antony, B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: a comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. (2021) 15:813–22. doi: 10.1016/j.dsx.2021.03.019
38. National Institutes of Health. (2023). Clinical Spectrum of SARS-CoV-2 infection. Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/
39. Gonzales Yovera, JG, Concepcion-Zavaleta, MJ, Coronado Arroyo, J, and Moreno, MD. Confluence of obesity and MAFLD during Covid-19 pandemic in a developing country. Endocrinol Diabetes Metab. (2021) 4:e00189. doi: 10.1002/edm2.189
40. O'Gorman, P, and Norris, S. Exercising in the COVID-19 era: implications in non-alcoholic fatty liver disease (NAFLD). BMJ Open Gastroenterol. (2021) 8:e000568. doi: 10.1136/bmjgast-2020-000568
41. Zhou, YJ, Zheng, KI, Wang, XB, Sun, QF, Pan, KH, Wang, TY, et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. (2020) 40:2160–3. doi: 10.1111/liv.14575
42. Corapli, M, Cil, E, Oktay, C, Kacmaz, H, Corapli, G, and Bulut, HT. Role of hepatosteatosis in the prognosis of COVID 19 disease. Clin Imaging. (2021) 80:1–5. doi: 10.1016/j.clinimag.2021.06.034
43. Yao, R, Zhu, L, Wang, J, Liu, J, Xue, R, Xue, L, et al. Risk of severe illness of COVID-19 patients with NAFLD and increased NAFLD fibrosis scores. J Clin Lab Anal. (2021) 35:e23880. doi: 10.1002/jcla.23880
44. Targher, G, Mantovani, A, Byrne, CD, Wang, XB, Yan, HD, Sun, QF, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. (2020) 69:1545–7. doi: 10.1136/gutjnl-2020-321611
45. Lombardi, R, Mura, V, Cespiati, A, Iuculano, F, Sigon, G, Pallini, G, et al. Usefulness of fibrosis-4 (FIB-4) score and metabolic alterations in the prediction of SARS-CoV-2 severity. Intern Emerg Med. (2022) 17:1739–49. doi: 10.1007/s11739-022-03000-1
46. ECA, DA, Yokoo, P, Fonseca, E, Otoni, JC, Haiek, SL, Shoji, H, et al. Prognostic factors of worse outcome for hospitalized COVID-19 patients, with emphasis on chest computed tomography data: a retrospective study. Einstein (Sao Paulo). (2022) 20:eAO6953. doi: 10.31744/einstein_journal/2022AO6953
47. Lopez-Mendez, I, Aquino-Matus, J, Gall, SM, Prieto-Nava, JD, Juarez-Hernandez, E, Uribe, M, et al. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol. (2021) 20:100271. doi: 10.1016/j.aohep.2020.09.015
48. Hayat, U, Ashfaq, MZ, Johnson, L, Ford, R, Wuthnow, C, Kadado, K, et al. The Association of Metabolic-Associated Fatty Liver Disease with clinical outcomes of COVID-19: a systematic review and meta-analysis. Kans J Med. (2022) 15:241–6. doi: 10.17161/kjm.vol15.16522
49. Bramante, C, Tignanelli, CJ, Dutta, N, Jones, E, Tamariz, L, Clark, JM, et al. Non-alcoholic fatty liver disease (NAFLD) and risk of hospitalization for Covid-19. medRxiv. (2020):20185850. doi: 10.1101/2020.09.01.20185850
50. Ji, D, Qin, E, Xu, J, Zhang, D, Cheng, G, Wang, Y, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. (2020) 73:451–3. doi: 10.1016/j.jhep.2020.03.044
51. Vrsaljko, N, Samadan, L, Viskovic, K, Mehmedovic, A, Budimir, J, Vince, A, et al. Association of Nonalcoholic Fatty Liver Disease with COVID-19 severity and pulmonary thrombosis: CovidFAT, a prospective, observational cohort study. Open forum. Infect Dis. (2022) 9:ofac073. doi: 10.1093/ofid/ofac073
52. Singh, R, Rathore, SS, Khan, H, Karale, S, Chawla, Y, Iqbal, K, et al. Association of Obesity with COVID-19 severity and mortality: an updated systemic review, meta-analysis, and meta-regression. Front Endocrinol (Lausanne). (2022) 13:780872. doi: 10.3389/fendo.2022.780872
53. Zheng, KI, Gao, F, Wang, XB, Sun, QF, Pan, KH, Wang, TY, et al. Letter to the editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. (2020) 108:154244. doi: 10.1016/j.metabol.2020.154244
54. Butikofer, S, Lenggenhager, D, Wendel Garcia, PD, Maggio, EM, Haberecker, M, Reiner, CS, et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. (2021) 41:2404–17. doi: 10.1111/liv.14971
55. Hartl, L, Haslinger, K, Angerer, M, Semmler, G, Schneeweiss-Gleixner, M, Jachs, M, et al. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology. (2022) 76:1563–75. doi: 10.1002/hep.32582
56. Sharma, P, and Kumar, A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. (2020) 14:825–7. doi: 10.1016/j.dsx.2020.06.013
57. Lefere, S, and Tacke, F. Macrophages in obesity and non-alcoholic fatty liver disease: crosstalk with metabolism. JHEP Rep. (2019) 1:30–43. doi: 10.1016/j.jhepr.2019.02.004
58. Papic, N, Samadan, L, Vrsaljko, N, Radmanic, L, Jelicic, K, Simicic, P, et al. Distinct cytokine profiles in severe COVID-19 and non-alcoholic fatty liver disease. Life (Basel). (2022) 12:795. doi: 10.3390/life12060795
59. Tasoudis, PT, Arvaniti, CK, Adamou, AT, Belios, I, Stone, JH, Horick, N, et al. Interleukin-6 inhibitors reduce mortality in coronavirus disease-2019: an individual patient data meta-analysis from randomized controlled trials. Eur J Intern Med. (2022) 101:41–8. doi: 10.1016/j.ejim.2022.04.004
60. Wang, J, Hou, Z, Liu, J, Gu, Y, Wu, Y, Chen, Z, et al. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): a multicenter study. J Hepatol. (2021) 75:439–41. doi: 10.1016/j.jhep.2021.04.026
61. Al Kaabi, N, Zhang, Y, Xia, S, Yang, Y, Al Qahtani, MM, Abdulrazzaq, N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. (2021) 326:35–45. doi: 10.1001/jama.2021.8565
62. Cheung, KS, Lam, LK, Hui, RWH, Mao, X, Zhang, RR, Chan, KH, et al. Effect of moderate-to-severe hepatic steatosis on neutralising antibody response among BNT162b2 and CoronaVac recipients. Clin Mol Hepatol. (2022) 28:553–64. doi: 10.3350/cmh.2022.0082
63. Amaddeo, G, Brustia, R, Allaire, M, Lequoy, M, Hollande, C, Regnault, H, et al. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. (2021) 3:100199. doi: 10.1016/j.jhepr.2020.100199
64. Romano, J, Abdelfattah, T, Manka, PP, Fuchs, M, and Syn, WK. Non-invasive risk stratification in NAFLD/NASH patients for screening EGD. Clin Exp Gastroenterol. (2022) 15:1–3. doi: 10.2147/CEG.S339850
65. Akbulut, S, Garzali, IU, Hargura, AS, Aloun, A, and Yilmaz, S. Screening, surveillance, and Management of Hepatocellular Carcinoma during the COVID-19 pandemic: a narrative review. J Gastrointest Cancer. (2022):1–12. doi: 10.1007/s12029-022-00830-2 [Epub ahead of print].
66. Fix, OK, Hameed, B, Fontana, RJ, Kwok, RM, McGuire, BM, Mulligan, DC, et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID-19 pandemic: AASLD expert panel consensus statement. Hepatology. (2020) 72:287–304. doi: 10.1002/hep.31281
67. Repici, A, Maselli, R, Colombo, M, Gabbiadini, R, Spadaccini, M, Anderloni, A, et al. Coronavirus (COVID-19) outbreak: what the department of endoscopy should know. Gastrointest Endosc. (2020) 92:192–7. doi: 10.1016/j.gie.2020.03.019
68. Aubert, O, Yoo, D, Zielinski, D, Cozzi, E, Cardillo, M, Durr, M, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health. (2021) 6:e709–19. doi: 10.1016/S2468-2667(21)00200-0
69. Radwan, H, Al Kitbi, M, Hasan, H, Al Hilali, M, Abbas, N, Hamadeh, R, et al. Indirect health effects of COVID-19: unhealthy lifestyle Behaviors during the lockdown in the United Arab Emirates. Int J Environ Res Public Health. (2021) 18:1964. doi: 10.3390/ijerph18041964
70. Baceviciene, M, and Jankauskiene, R. Changes in sociocultural attitudes towards appearance, body image, eating attitudes and behaviours, physical activity, and quality of life in students before and during COVID-19 lockdown. Appetite. (2021) 166:105452. doi: 10.1016/j.appet.2021.105452
71. Caroppo, E, Mazza, M, Sannella, A, Marano, G, Avallone, C, Claro, AE, et al. Will nothing be the same again?: changes in lifestyle during COVID-19 pandemic and consequences on mental health. Int J Environ Res Public Health. (2021) 18:8433. doi: 10.3390/ijerph18168433
72. Mascaro, CM, Bouzas, C, Montemayor, S, Garcia, S, Mateos, D, Casares, M, et al. Impact of physical activity differences due to COVID-19 pandemic lockdown on non-alcoholic fatty liver parameters in adults with metabolic syndrome. Nutrients. (2022) 14:2370. doi: 10.3390/nu14122370
73. Cinque, F, Cespiati, A, Lombardi, R, Costantino, A, Maffi, G, Alletto, F, et al. Interaction between lifestyle changes and PNPLA3 genotype in NAFLD patients during the COVID-19 lockdown. Nutrients. (2022) 14:556. doi: 10.3390/nu14030556
74. Lopez-Gonzalez, AA, Altisench Jane, B, Masmiquel Comas, L, Arroyo Bote, S, Gonzalez San Miguel, HM, and Ramirez Manent, JI. Impact of COVID-19 lockdown on non-alcoholic fatty liver disease and insulin resistance in adults: a before and after pandemic lockdown longitudinal study. Nutrients. (2022) 14:2795. doi: 10.3390/nu14142795
75. Shanmugam, H, Di Ciaula, A, Di Palo, DM, Molina-Molina, E, Garruti, G, Faienza, MF, et al. Multiplying effects of COVID-19 lockdown on metabolic risk and fatty liver. Eur J Clin Investig. (2021) 51:e13597. doi: 10.1111/eci.13597
76. Kim, D, Alshuwaykh, O, Dennis, BB, Cholankeril, G, and Ahmed, A. Trends in Etiology-based mortality from chronic liver disease before and during COVID-19 pandemic in the United States. Clin Gastroenterol Hepatol. (2022) 20:2307–2316.e3. doi: 10.1016/j.cgh.2022.05.045
77. Saokaew, S, Kanchanasurakit, S, Kositamongkol, C, Chaiyo, K, Jirapisut, T, Aomsin, N, et al. Effects of telemedicine on obese patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Med (Lausanne). (2021) 8:723790. doi: 10.3389/fmed.2021.723790
78. Ma, Y, Zhao, C, Zhao, Y, Lu, J, Jiang, H, Cao, Y, et al. Telemedicine application in patients with chronic disease: a systematic review and meta-analysis. BMC Med Inform Decis Mak. (2022) 22:105. doi: 10.1186/s12911-022-01845-2
79. Pogorzelska, K, and Chlabicz, S. Patient satisfaction with telemedicine during the COVID-19 pandemic-a systematic review. Int J Environ Res Public Health. (2022) 19:6113. doi: 10.3390/ijerph19106113
80. Wong, L, Hollaway, M, Sanford, J, Sexton, K, Yu, F, and Jensen, H. Elective operations delay and emergency department visits and inpatient admissions during COVID-19. Surg Pract Sci. (2022) 10:100111. doi: 10.1016/j.sipas.2022.100111
Keywords: COVID-19, MAFLD (metabolic associated fatty liver disease), NAFLD (non alcoholic fatty liver disease), NASH (non-alcoholic steatohepatitis), metabolic syndome, vaccine, SARS-CoV-2
Citation: Nowroozi A, Momtazmanesh S and Rezaei N (2023) COVID-19 and MAFLD/NAFLD: An updated review. Front. Med. 10:1126491. doi: 10.3389/fmed.2023.1126491
Received: 08 February 2023; Accepted: 10 March 2023;
Published: 24 March 2023.
Edited by:
Jeff M. P. Holly, University of Bristol, United KingdomReviewed by:
Tope Oyelade, University College London, United KingdomCopyright © 2023 Nowroozi, Momtazmanesh and Rezaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Momtazmanesh, c21vbXRhem1hbmVzaEBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.