
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Med., 22 March 2023
Sec. Intensive Care Medicine and Anesthesiology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1124743
This article is part of the Research TopicInsights in Intensive Care Medicine and Anesthesiology: 2022View all 9 articles
There are presently no consensuses on the optimal sedation strategy for obese patients during gastrointestinal endoscopy. This study aim to explore the effects of opioid-free propofol or remimazolam balanced anesthesia on hypoxemia incidence in patients with obesity. A total of 264 patients were randomized to remimazolam + esketamine group (group R) or propofol + esketamine group (group P). Anesthesia in group P was administrated by propofol, esketamine and in group R by remimazolam, esketamine. The primary outcome was incidence of hypoxemia. Secondary outcomes were the time to loss of consciousness (LoC) and to recovery and the incidence of intraoperative and postoperative adverse reactions. We found the incidence of mild hypoxemia in group R was similar to that in group P (14.2% vs. 11.5%, p = 0.396). The incidence of severe hypoxemia in group R was significantly lower than Group P (4.2% vs. 9.2%, p = 0.019). The time to LoC in group R was longer than group P [Median (interquartile range, IQR): 53 s (45 to 61) vs. 50 s (42 to 54), p = 0.001]. The time to recovery from anesthesia in group R was less than group P [Median (IQR): 48 min (41 to 58) vs. 55.5 min (46 to 67), p<0.001]. There was no significant difference in the incidence of adverse events (p > 0.05 for all). We concluded that compared with propofol combined with esketamine, remimazolam combined with esketamine can reduce the incidence of severe hypoxemia during gastrointestinal endoscopy in obese patients.
Clinical Trial Registration: www.chictr.org.cn, Identifier: ChiCTR2200065575.
To eliminate discomforts from mechanical stimulation, gastrointestinal endoscopy procedures are usually performed under deep sedation. Propofol combined with short-acting opioids is currently the preferred anesthetic strategy for endoscopy, due to its reliable sedative and analgesic effects. A large observational study showed that the incidence of hypoxemia with upper gastrointestinal endoscopy in the general population was approximately 15%, and that up to 23.5% of patients with hypoxemia require at least two or more airway interventions (1). Unsuprisely, the incidence of adverse respiratory events may be higher in obese patients (2). Notebly, Patients with obesity have a higher incidence of hypoxemia under deep sedation because they have a relatively low functional residual capacity and higher closed volume (3, 4). A high prevalence of hypoxemia is associated with interoperative adverse outcomes like brain injury (5, 6). Even though hypoxemia is common in endoscopy, this adverse event may cause tachycardia and coronary ischemia during sedation (7). Thus, administering sedation safely in patients with obesity remains a challenge (8).
Opioid-free balanced anesthesia minimizes opioid-related adverse effects (9). Esketamine is the S-enantiomer of ketamine and it has fewer mental side effects, higher clearance rate and receptor affinity. Current researches suggest that esketamine is a more attractive sedative adjunct to propofol compared with opioids (10–12). Esketamine not only has a synergistic effect on sedation, its use greatly reduces the incidences of respiratory and circulatory depression, mental side effects, and vomiting (13). Nevertheless, the effectiveness of this strategy in populations at high airway management risk remains inconclusive. In addition, propofol has obvious disadvantages so as to limit its use, including a narrow therapeutic window, strong injection pain, highly hepatic active enzyme-dependent metabolism, and potential protein allergens (14).
Researches have confirmed that remimazolam, a new type of benzodiazepine, has a stronger and faster sedative effect compared with midazolam, and causes less respiratory and circulatory depression (15, 16). However, its sedative safety and efficacy in those with obesity has not been fully evaluated. The purpose herein was to determine whether remimazolam combined with esketamine is safer and more effective than propofol in patients with obesity. This single-center randomized controlled trial assessed patients at risk of hypoxemia, to explore the effects of these two opioid-free balanced anesthesia strategies on the incidence of hypoxemia in patients with obesity during sedation for endoscopy.
This prospective, randomized, double-blind, controlled trial was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2022-KL291-02) and was registered at the Chinese Clinical Trial Registry (ChiCTR2200065575). This study strictly followed the guidelines of the Declaration of Helsinki. This study was conducted at the Gastrointestinal Endoscopy Center of the Affiliated Hospital of Xuzhou Medical University, which is the largest gastrointestinal endoscopy center in Xuzhou, Jiangsu Province, China. Nearly 1,000 gastrointestinal endoscopy procedures are performed monthly in this center’s operating room by experienced gastroenterologists and nursing teams. All patients signed informed consent before received allocated intervention.
Patients scheduled for gastrointestinal endoscopy were eligible to participate if they were aged 18 to 65 years, had a body mass index (BMI) between 30 and 40 kg/m2, had American Society of Anesthesiologists Physical Status (ASA PS) grade II or III, had no history of cardiac insufficiency (Including left ventricular ejection fraction >55%, the peak velocity of blood flow of diastolic mitral valve: E/A > 1 and the tircuspid annular plane systolic excursion >13 mm) and were able to communicate normally. Exclusion criteria were known allergy to planned medications, severe hypertension, history of myocardial infarction, severe hepatic or renal dysfunction, mental illness, pregnancy, epileptic disorder, elevated intracranial pressure, use of medication that affects the central nervous system, or modified Mallampati class IV. Secondary exclusion criteria were termination of the clinical protocol for any reason and patients request to withdrawal.
All enrolled patients were randomly allocated in a 1: 1 ratio to either propofol + esketamine group (group P) or remimazolam + esketamine group (group R) using the randomization tool at www.randomization.com, with a random block size of six. Randomization was completed by an independent investigator, and the results sealed in an opaque envelope and delivered to the anesthesiologist responsible for sedation. Normally, two researchers who did not know the specific grouping were present during each procedure, to ensure protocol adherence and record intraprocedural data. The researchers were only responsible for instructing anesthesiologist about the protocol before induction of anesthesia. After the completion of the sedation, the anesthesiologist was responsible for informing the researchers about the intraoperative information after the operation, and the researchers recorded it. Anesthesiologist and investigators were not in the same work area. Except for the occurrence of unforeseen serious adverse events during the procedure, investigators were blind to randomization results.
Anesthesiologist assessed enrolled patients before the procedure and recorded basic demographic characteristics. They also carefully assessed patient airway safety and screened patients for OSA using the STOP-BANG (SB) questionnaire (17). All patients underwent the same preprocedural preparation, including strict fasting for 6 h. Patients entered the preparation room and a peripheral venous channel was established in the right upper extremity. Next, 500 ml of balanced salt solution was infused at a rate of 250 ml/h. Electrocardiogram and heart rate (HR) monitoring were performed and pulse oxygen saturation (SpO2) and mean arterial pressure (MAP) were measured. The pulse oximeter was not placed on the same upper extremity as the blood pressure cuff, to prevent false drops in saturation during cuff inflation. All patients received oxygen via nasal cannula at a flow rate of 4 to 6 l/min, and an airflow detector at the tip of the nasal cannula detected respiratory rate. At the beginning of the gastrointestinal endoscopy, patients were positioned in the left lateral decubitus.
According to their adjusted body weight (ideal body weight + 0.4 × [total body weight − ideal body weight]), the two patient groups were intravenously administered a bolus of esketamine solution (5 mg/ml, 0.5 mg/kg, completed within 10 s). After injection, group P received 1 mg/kg of propofol intravenously (10 mg/ml) and group R recieved 0.1 mg/kg of remimazolam intravenously (1 mg/ml). Both groups were injected slowly, at a rate of 0.5 ml/s until the eyelash reflex disappeared. An anesthetist was responsible for appropriate sedative rescue. When patients had swallowing reflex or Modified Observer’s Assessment Alert/Sedation (MOAA/S) score > 2, groups P and R were administered a single bolus of 1 mg/kg propofol and 0.05 mg/kg remimazolam, respectively and repeated the process if necessary. The MOAA/S scale is a modified OAA/S scale which describes sedation level in greater detail, with a score range from 5 to 0. Whether to perform sedation rescue was determined by the anesthesiologist and endoscopist according to the MOAA/S score. Sedation failure was defined as insufficient sedation after the initial dose and up to four additional doses of remimazolam/propofol within 15 min.
During the procedure, HR, SpO2, and MAP were continuously monitored. The management of hypoxemia was shown in Figure 1. If an adverse cardiovascular reaction lasted >60 s, the anesthesiologist intervened with appropriate vasoactive drugs. Post-procedure, patients were sent to the post-anesthesia care unit (PACU) for anesthesia recovery monitoring. The modified Aldrete scale was used to assess the quality of patient recovery on five dimensions: respiration, blood pressure, SpO2, activity, and consciousness (18). Patients were allowed to leave the PACU when their Aldrete score was ≥9 or equal to their pre-procedure level. The quality of patient recovery and any adverse reactions within 24 h post-procedure (e.g., dizziness, nausea, vomiting, chills, headache, drowsiness) were registered by the postoperative investigators.
For ethical and safety reasons, we chose hypoxemia incidence and type in both groups as the primary endpoints (i.e., the primary outcome was SpO2 < 90%, regardless of duration). Hypoxemia was further divided into mild (SpO2 75 to 90% for <60 s) and severe (SpO2 < 75% at any time, or SpO2 75 to 90% for >60 s). We instructed the team to perform rapid airway intervention (including chin lift, mask ventilation) when SpO2 < 90% for ≥10 s to avoid patient harm from prolonged hypoxemia (19). Secondary outcomes were: incidence of adverse cardiovascular event (including bradycardia, tachycardia, hypotension, or hypertension [>20% change from baseline]); incidence of accidental intubations; incidence of sedation failure; and requirement of sedative rescues (Refers to the proportion of patients who received the maximum number of remedial sedation allowed within 15 min of induction). All adverse respiratory and cardiovascular events were defined according to the World SIVA International Sedation Task Force (20). Other outcomes included: time to loss of consciousness (LoC, defined as the time from induction to disappearance of the eyelash reflex); time to leave the PACU; and incidence of any adverse effects during follow-up.
We enrolled 20 patients with obesity received sedation strategy of group P in a small preliminary trial, in which we found a total hypoxemia incidence of 28%. In this prospective clinical trial, we assumed that patients in group R would have a 14% incidence of hypoxemia compared with group P. With a one-sided alpha of 2.5% and power of 80%, 129 patients were needed in each group. We increased the sample size to 282 in both groups to accommodate dropouts and study terminations for any reason. Sample size estimates were calculated using PASS version 15.0 (NCSS, Kaysville, UT, United States).
Distribution of continuous variables was examined using the Kolmogorov–Smirnov method. Normally distributed continuous variables are expressed as mean ± standard deviation (SD) and were compared using the Student’s t-test. If not normally distributed, the Mann–Whitney U method was used and expressed as median and interquartile range (IQR). The Hodges–Lehmann method was used to estimate the median difference (MD) and 95% confidence interval (CI) was used to estimate heterogeneity. Categorical variables are expressed as numbers (%) and were compared using the χ2 test or Fisher’s exact probability test, as appropriate. The number to treat (NNT, 1/[event rate in control group − event rate in experimental group]) and 95% CI were used to assess treatment effects on randomized groups. Kaplan–Meier survival analysis and log-rank test were used to evaluate the effects of sedation strategy on induction and recovery times, and to examine possible heterogeneity of different baseline variables by stratification.
We assessed treatment effects with post hoc analyses by stratifying binary variables that may influence hypoxemia. Multiple mixed-effects logistic regression was used to explore independent factors for hypoxemia. Stratified variables like age, gender, BMI, ASA PS, anamnesis, and endoscopy type were included in univariate logistic regression models. If the regression model likelihood ratio test (model fit) p < 0.100, it was included in the mixed-effects multivariate logistic regression. All included variables were tested for collinearity, and variance inflation factor > 5 indicated statistically significant multicollinearity among variables. Interactions among stratified variables and randomized groups were tested with logistic regression. Confidence interval estimates in the bootstrap method were restricted to the 2.5th and 97.5th percentiles. Unless otherwise indicated, the odds ratio (OR) and its 95% CI values were obtained by replicating a mixed-effects logistic model on a bootstrapped sample of 2,000 iterations. A two-tailed p < 0.05 was considered statistically significant. p values were not adjusted for multiple testing. Data analysis followed the intention-to-treat principle. SPSS v23.0 (IBM, Armonk, NY, United States) was used for all analyses.
A total of 261 patients were included in the final analyses: 131 in group R and 130 in group P (Figure 2). Despite the limited sample size, randomization appeared to lead to well-balanced groups. There were no significant between-group differences in age, BMI, comorbidities, or endoscopy type. Respiratory system-related parameters including proportion of patients with neck circumference (NC) > 40 cm, different Mallampati grades, SB score > 5, and lung diseases with different pathophysiological characteristics were also similar between the groups (Table 1).
The incidence of mild hypoxemia was similar in groups R and P (14.2% vs. 11.5%, p = 0.396). However, the incidence of severe hypoxemia in group R was significantly lower than in group P (4.2% vs. 9.2%, p = 0.019, NNT = 10 [95% CI, 5 to 55)]) (Table 2). The time to LoC in group R was longer than in group P (median [IQR]: 53 s [45 to 61] vs. 50 s [42 to 54], p = 0.001, MD = 4 s [95% CI, 1 to 6]) (Table 2; Figure 3). There were no accidental endotracheal intubations in either group. Group R required more sedation rescue (19.1% vs. 7.7%, p = 0.007, NNT = −9 [95% CI, −31 to −5]) and less airway support than did group P (mask ventilation, 5.3% vs. 13.1%, p = 0.031, NNT = 12 [95% CI, 7 to 127]; chin lift, 19.1% vs. 30.8%, p = 0.029, NNT = 9 [95% CI, 5 to 78]) (Table 2). There was not a significant difference in the incidence of adverse hemodynamic events during sedation between the groups, including tachycardia, bradycardia, hypertension, and hypotension (p > 0.05). There were no significant differences in the incidence of adverse mental events, including headache, hallucinations, and dizziness during recovery (p > 0.05). However, the time to recovery from anesthesia in group R was shorter than in group P (median [IQR]: 48 min [41 to 58] vs. 55.5 min [46 to 67], P<0.001, MD = −8 min [95% CI, −11 to −4]) (Table 2; Figure 3). Neither time to LoC nor recovery showed significant heterogeneity across subgroups (Supplemental Digital Content 1, Supplementary Table S1). There were no significant interactions between endoscopy type and intervention (Supplemental Digital Content 2, Supplementary Table S2). All adverse events resolved after intervention according to the protocol.
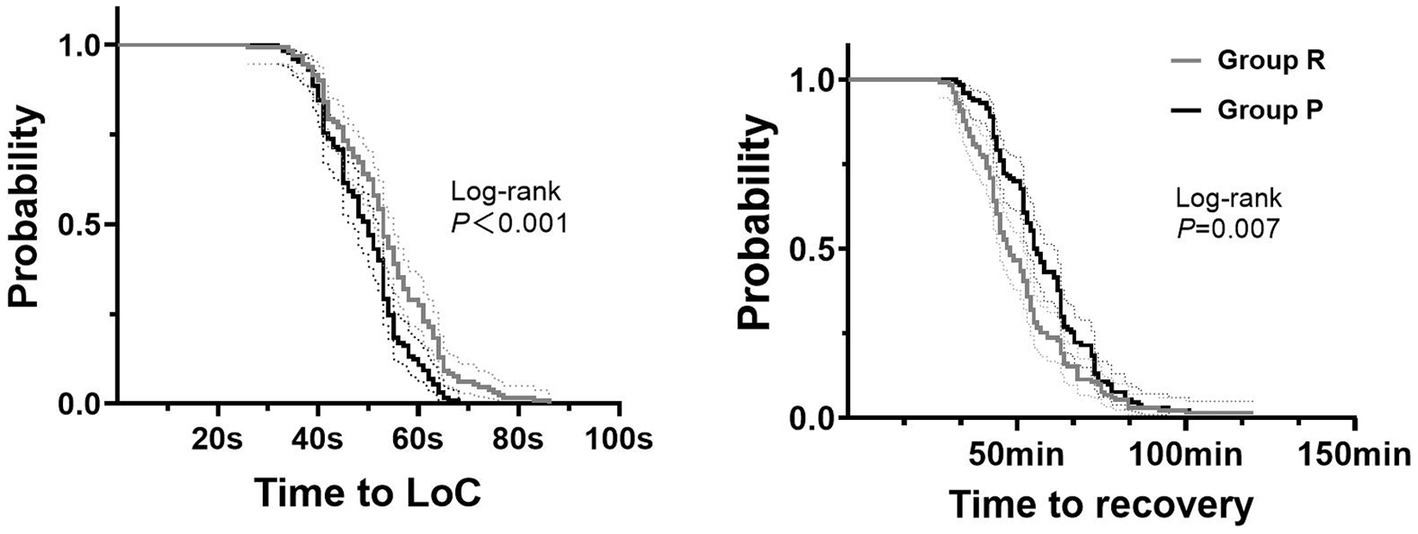
Figure 3. Kaplan–Meier curves for time to induction and recovery. 1. Group R = propofol + esketamine group; Group P = remimazolam + esketamine group; 2. Time to Loc: time to loss of consciousness; 3. The dashed lines represent the 95% confidence interval.
The effect of the intervention on incidence of mild hypoxemia was consistent across all subgroups. However, there was heterogeneity in the treatment effect for severe hypoxemia based on gender, age, NC, ischemic heart disease, existence of respiratory comorbidities, and endoscopy type (Figure 4). Univariate logistic regression analysis showed that gender, age, BMI, SB score, NC, and cardiac comorbidities may be independent risk factors for development of hypoxemia (p < 0.100). There was no significant interaction between the intervention and specific binary variables (Supplemental Digital Content 2, Supplementary Table S2). Multivariate mixed-effects logistic regression analysis showed that, with patients without obesity or hypoxemia as a reference, in obese patients with mild hypoxemia, NC ≤ 40 cm was a significant independent protective factor (adjusted OR [aOR]: 0.140, [95% CI: 0.071 to 0.275]; p < 0.001). Likewise, SB score ≤ 5 (aOR: 0.279, [95% CI: 0.102 to 0.761]; p = 0.008), NC ≤ 40 cm (aOR: 0.050, [95% CI: 0.014 to 0.180]; p = 0.001), and no history of ischemic heart disease (aOR: 0.342, [95% CI: 0.138 to 0.851]; p = 0.029) were also independent protective factors against developing severe hypoxemia (Supplemental Digital Content 3, Supplementary Table S3).
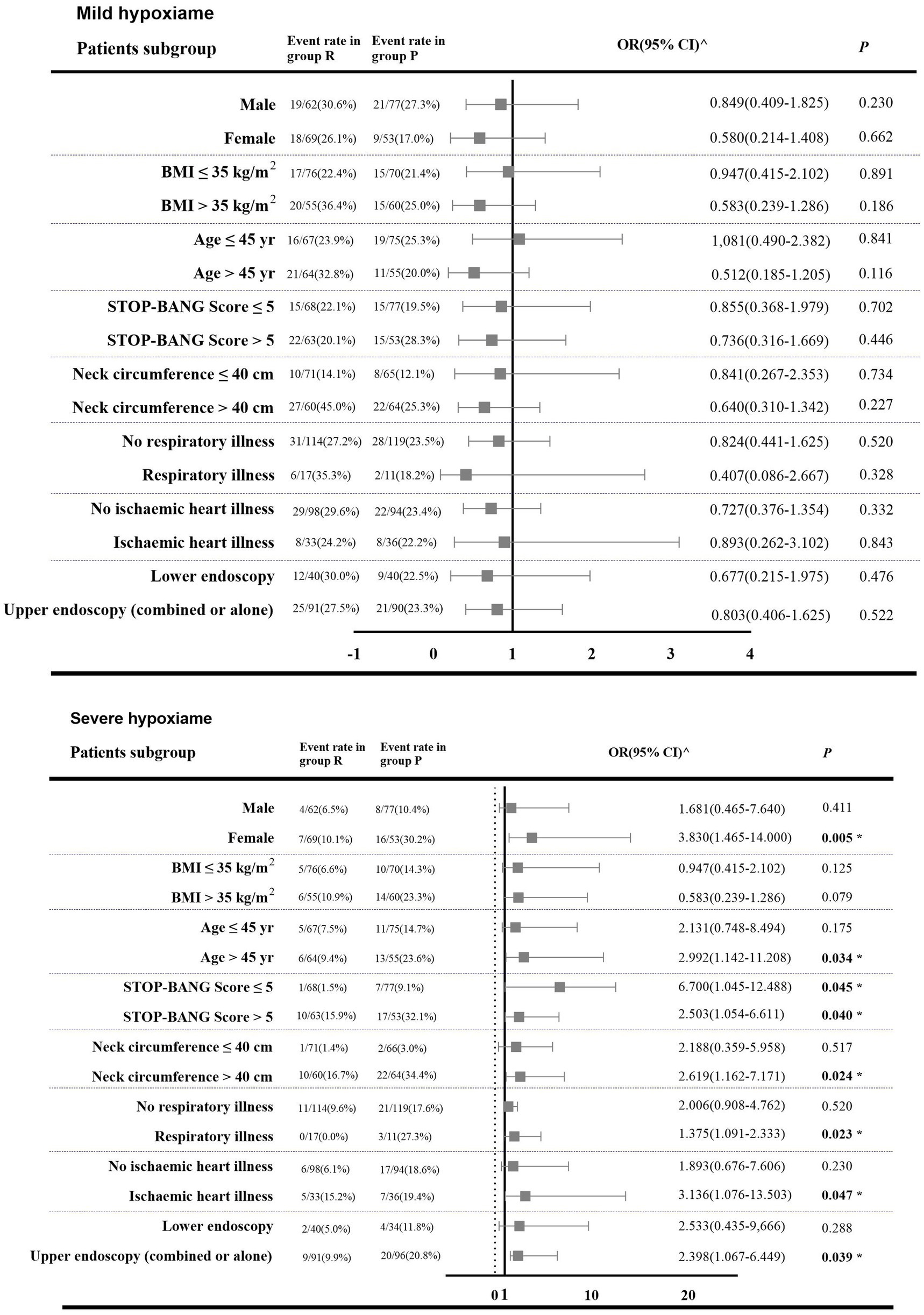
Figure 4. Forest plot showing odds ratiao in the occurrence of hypoxemia in subgroups of patients in both intervention groups. 1. Group R = propofol + esketamine group; Group P = remimazolam + esketamine group; 2. BMI (body mass index) is the weight in kilograms divided by the square of the height in meters; 95% CI (95% confidence interval). 3. ^: The number of outcome events in these patient categories is too small so that bootstrapping and logistic regression are not feasible; OR (95% CI) were obtained from Mantel–Haenszel (MH) estimates (21). 4. The p-values of some cases were obtained by the Fisher’s exact test. 5. *: A two-tailed test p < 0.05 was considered statistically significant.
Given increasing demands for safe and comfortable endoscopy, more work needs to be done by anesthesiologists to explore optimal sedation strategies for specific high-risk populations. Increased airway fragility and reduced chest wall compliance make patients with obesity particularly prone to desaturation under traditional opioid-based balanced anesthesia (3). Although high-flow nasal cannula (HFNC) decreases the incidence of desaturation in sedated patients with obesity (22), the high cost of these systems and the training needed for professional practitioners to operate them has limited widespread adoption (22, 23). Thus, lower cost and easier use may facilitate implementation of improved clinical protocols, while maintaining safety. By comparing the effectiveness of two opioid-free balanced anesthesia approaches to painless gastrointestinal endoscopy in patients with obesity, we found that compared with propofol + esketamine, remimazolam + esketamine reduced the incidence of severe hypoxemia. NNT results suggest that using remimazolam + esketamine may prevent severe hypoxemia in 1 in 10 patients with obesity.
Hypoxemia severity depends on both oxygen reserve capacity and degree of respiratory center depression during sedation. High respiratory vulnerability in patients with obesity is mainly attributed to fatty deposits in the soft tissues of the pharyngeal wall and large tongue bodies that are prone to sinking (24). Decreased pharyngeal wall muscle tone and collapse of the upper airway during sedation further exacerbate difficult airway management (24). Guo et al. (25) explored the efficacy of an initial remimazolam bolus of 0.15 mg/kg in gastrointestinal endoscopy sedation in older adults; compared with propofol, this significantly reduced the incidence of hypoxemia. Unlike those with obesity, older adults have low respiratory reserve capacity, high airway sensitivity, increased respiratory center sensitivity, and weakened respiratory protective reflexes. Thus, the protection provided by remimazolam for respiratory center sensitivity may be especially meaningful, partly explaining why the incidence herein of severe hypoxemia in group R was lower than that in group P. Interestingly, propofol has the property of dilating the bronchi in ex vivo animal models, under both normal and hypoxic conditions, related to its inhibition of the release of active substances like cabergoline, histamine, and prostaglandins (26). However, the advantages of these properties for patients with obesity and airway diseases remain to be confirmed by extensive mechanistic and clinical studies.
The anesthesia recovery time in group R was shorter than that in group P. While we found no sedation failure in either group, group R required more frequent sedation rescue, similar to findings by Chen et al. (27). Remimazolam can be rapidly hydrolyzed into inactive metabolites by non-organ-dependent metabolism, while pharmacokinetic models indicate that total remimazolam clearance is independent of body weight (28). This may explain the difference in pharmacokinetics and apparent distribution volumes between remimazolam and propofol in patients with obesity. Although obesity increases both fat and muscle, the proportion of adipose tissue is higher, and provides a marked reservoir for high-fat-soluble anesthetics. Although propofol clearance and peripheral compartment volume are significantly higher in patients with morbid obesity (MO), the half-life concentration in these patients is significantly lower than that of patients with standard weights (by ~37.9 to 38.6%), suggesting that the population with MO may be more sensitive to propofol (29). Consequently, increasing the initial dose of remimazolam, for more rapid induction and less rescue sedation, may be feasible in patients with obesity.
The safety of remimazolam for procedural sedation in colonoscopy with the ASA III/IV population was confirmed by Rex et al. in the only open-label clinical study to date (30). Dai et al. (31) also confirmed that when the initial bolus of remimazolam is within 0.2 to 0.4 mg/kg, the time to LoC remains similar to a propofol bolus of 2 mg/kg. Even among populations with hepatic or renal impairment, remimazolam retains predictable pharmacokinetic properties and dosage adjustments are unnecessary (32). Unfortunately, few studies have examined the effects of obesity on the pharmacokinetics of remimazolam in detail. Superior sedation strategies benefit both the anesthesiologist and the patients. Of course, considering the patient’s own interests, we must consider the possible increased cost of group R sedation strategy. However, in busy units, shorter PACU discharge times and higher respiratory protection are clearly valuable. Thus compared to HFNC, which is costly, there is reason to believe that the group R sedation strategy has a huge potential advantage in anesthesia outside the operating room.
Exploratory analyses showed that patients with obesity and ischemic heart disease are more likely to develop severe hypoxemia. Pre-existing cardiac comorbidities increase susceptibility to hypoxemia during gastrointestinal endoscopy because ischemic myocardial tissue is more prone to pump dysfunction, which in turn reduces the oxygen exchange rate between the pulmonary capillaries and alveolus (3, 33). This context may deteriorate when ischemic myocardial tissue is subjected to hypoxia. Thus, there is support for the notion that our group R sedation protocol can be preferentially recommended for patients with these risk factors.
We also found that SB score > 5 and NC > 40 cm are independent risk factors for hypoxemia during sedation in patients with obesity. Logically, patients with BMI > 30 kg/m2 should also be among the high-priority patients (24, 33). However, our analyses did not show significant heterogeneity of treatment between patient groups with a BMI of 30 to 40 kg/m2, thus these data do not support BMI > 35 kg/m2 as independent risk factor for hypoxemia. Low sensitivity of BMI may be attributed to large individual differences in body fat percentage, age, gender, muscle mass, and ethnicity (34). NC measurements has been confirmed to be practical, simple, inexpensive, timesaving, and less invasive than BMI. Kroll et al. (35) have suggested that NC is an accurate tool for assessing/screening obesity across age, gender, and weight categories. However, in females larger NC is more likely to be associated with disproportionately accumulated fat, in contrast to lean tissue, which is a significant contributor to NC in male (36). The proportion of lean tissue in the necks of females may be lower than that of males with otherwise relatively consistent NC; thus, supporting tension for the pharynx of females with obesity would be relatively weaker, increasing their likelihood of upper airway collapse. This may partly explain between-group treatment heterogeneity among females. Further, NC is closely related to the occurrence and development of obstructive sleep apnea (37). Thus for patients with obesity, NC and SB score may be more effective than BMI for assessing the risk of hypoxemia, and are important factors in deciding whether to implement protective sedation strategies.
Several important limitations must be considered when interpreting these results. First, although all patients received low-flow nasal cannula oxygen (4 to 6 l/min) during sedation, FiO2 changes were unpredictably influenced by patient-related factors like open mouth (38). Second, we did not include patients with BMI > 40 kg/m2 or Mallampati class IV, for ethical and safety reasons, limiting both sample size and generalizability. Third, the specificity of the sample and avoidance of unpredictable risk events meant that dose restrictions in both groups were relatively stringent. Finally, some subgroups lacked sufficient statistical power; further evidence from larger samples and multi-center studies is thus needed, and we did not describe trends in pulse oxygen saturation during sedation in both groups. Besides, the guiding significance of using the bispectral index (BIS) to study remimazolam was not clear; therefore, we had no confidence in using BIS and chose the MOAA/S score instead. Meanwhile, We must admitted that due to the subjective nature of the scale, we did not choose to record the MOSS/A scores in real time. Therefore, we could not completely rule out the clinical outcomes we observed were due to differences in the sedative efficacy of remimazolam and propofol in obese patients.
In summary, we concluded that the sedation strategy of remimazolam combined with esketamine shows a higher safety profile for gastrointestinal endoscopy in patients with obesity compared with propofol combined with esketamine. However, the advantages of this strategy in this and other high-risk populations remains to be confirmed by larger clinical trials.
The data analyzed in this study is subject to the following licenses/restrictions: The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to Z2FvZmFuZ3h6QDEyNi5jb20=.
This prospective, randomized, double-blind, controlled trial was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (XYFY2022-KL291-02) and strictly followed the guidelines of the Declaration of Helsinki.
KZ: proposing initial conception and methods and writing original manuscript. YB: statistical analysis and editing the manuscript. XH: intraoperative trial protocol supervision and security management. WZ: intraoperative trial protocol supervision. YY: postoperative follow-up and investigation. ML: responsible for patients recruitment and grouping. FG: concept optimization and reviewing manuscript. All authors contributed to the article and approved the submitted version.
The investigators are grateful to all participants for their cooperation in the study. The authors also appreciate for the support from the Endoscopic physicians and nursing teams of the Affiliated Hospital of Xuzhou Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1124743/full#supplementary-material
1. Coté, AG, Hovis, RM, Ansstas, MA, Waldbaum, L, Azar, RR, Early, DS, et al. Incidence of sedation-related complications with propofol use during advanced endoscopic procedures. Clin Gastroenterol Hepatol. (2010) 8:137–2. doi: 10.1016/j.cgh.2009.07.008
2. Laffin, AE, Kendale, SM, and Huncke, TK. Severity and duration of hypoxemia during outpatient endoscopy in obese patients: a retrospective cohort study. Can J Anaesth. (2020) 67:1182–9. doi: 10.1007/s12630-020-01737-x
3. Qadeer, MA, Rocio Lopez, A, Dumot, JA, and Vargo, JJ. Risk factors for hypoxemia during ambulatory gastrointestinal endoscopy in ASA I-II patients. Dig Dis Sci. (2009) 54:1035–40. doi: 10.1007/s10620-008-0452-2
4. Sugerman, HJ, Fairman, RP, Baron, PL, and Kwentus, JA. Gastric surgery for respiratory insufficiency of obesity. Chest. (1986) 90:81–6. doi: 10.1378/chest.90.1.81
5. Abdelmalak, BB, Cata, JP, Bonilla, A, You, J, Kopyeva, T, Vogel, JD, et al. Intraoperative tissue oxygenation and postoperative outcomes after major non-cardiac surgery: an observational study. Br J Anaesth. (2013) 110:241–9. doi: 10.1093/bja/aes378
6. Casati, A, Fanelli, G, Pietropaoli, P, Proietti, R, Tufano, R, Danelli, G, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. (2005) 101:740. doi: 10.1213/01.ane.0000166974.96219.cd
7. Qadeer, MA, Lopez, AR, Dumot, JA, and Vargo, JJ. Hypoxemia during moderate sedation for gastrointestinal endoscopy: causes and associations. Digestion. (2011) 84:37–45. doi: 10.1159/000321621
8. Jirapinyo, P, and Thompson, CC. Sedation challenges: obesity and sleep apnea. Gastrointest Endosc Clin N Am. (2016) 26:527–7. doi: 10.1016/j.giec.2016.03.001
9. Lavand’homme, P, and Steyaert, A. Opioid-free anesthesia opioid side effects: tolerance and hyperalgesia. Best Pract Res Clin Anaesthesiol. (2017) 31:487–8. doi: 10.1016/j.bpa.2017.05.003
10. Eberl, S, Koers, L, van Hooft, J, Jong, ED, Hermanides, J, Hollmann, MW, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. (2020) 37:394–1. doi: 10.1097/EJA.0000000000001134
11. Xin, N, Yan, W, and Jin, S. Efficacy of analgesic propofol/esketamine and propofol/fentanyl for 352 painless induced abortion: a randomized clinical trial. Biomed Res Int. (2022) 2022:5095282. doi: 10.1155/2022/5095282
12. Eberl, S, Koers, L, van Hooft, JE, Jong, E, Schneider, T, Hollmann, MW, et al. Sedation with propofol during ERCP: is the combination with esketamine more effective and safer than with alfentanil? Study protocol for a randomized controlled trial. Trials. (2017) 18:472. doi: 10.1186/s13063-017-2197-8
13. Nakao, S, Nagata, A, Miyamoto, E, Masuzawa, M, Murayama, T, and Shingu, K. Inhibitory effect of propofol on ketamine-induced c-Fos expression in the rat posterior cingulate and retrosplenial cortices is mediated by GABAA receptor activation. Acta Ichthyol Piscat. (2003) 47:284–10. doi: 10.1034/j.1399-6576.2003.00040.x
14. Sahinovic, MM, Struys, M, and Absalom, AR. Clinical pharmacokinetics and pharmacodynamics of propofol. Clin Pharmacokinet. (2018) 57:1539–58. doi: 10.1007/s40262-018-0672-3
15. Rex, DK, Bhandari, R, Desta, T, DeMicco, MP, Schaeffer, C, Etzkorn, K, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. (2018) 88:427. doi: 10.1016/j.gie.2018.04.2351
16. Doi, M, Morita, K, Takeda, J, Sakamoto, A, Yamakage, M, and Suzuki, T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. (2020) 34:543–3. doi: 10.1007/s00540-020-02788-6
17. Chung, F, Yegneswaran, B, Liao, P, Chung, SA, Vairavanathan, S, Lslam, S, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. (2008) 108:812–1. doi: 10.1097/ALN.0b013e31816d83e4
18. Aldrete, JA. The post-anesthesia recovery score revisited. J Clin Anesth. (1995) 7:89–91. doi: 10.1016/0952-8180(94)00001-k
19. Kulkas, A, Duce, B, Leppänen, T, Hukins, C, and Töyräs, J. Severity of desaturation events differs between hypopnea and obstructive apnea events and is modulated by their duration in obstructive sleep apnea. Sleep Breath. (2017) 21:829–5. doi: 10.1007/s11325-017-1513-6
20. Mason, KP, Green, SM, and Piacevoli, Q. Adverse event reporting tool to standardize the reporting and tracking of adverse events during procedural sedation: a consensus document 380 from the world SIVA international sedation task force. Br J Anaesth. (2012) 108:13–20. doi: 10.1093/bja/aer407
21. Viwatwongkasem, C, Srisawad, S, Soontornpipit, P, Sillabutra, J, Satitvipawee, P, Kitidamrongsuk, P, et al. Minimum MSE weighted estimator to make inferences for a common risk ratio across sparse meta-analysis data. Open J Statistics. (2022) 12:49–69. doi: 10.4236/ojs.2022.121004
22. Nay, MA, Fromont, L, Eugene, A, Marcueyz, JL, Mfam, WS, Baert, O, et al. High-flow nasal oxygenation or standard oxygenation for gastrointestinal endoscopy with sedation in patients at risk of hypoxaemia: a multicentre randomised controlled trial (ODEPHI trial). Br J Anaesth. (2021) 127:133–2. doi: 10.1016/j.bja.2021.03.020
23. Riccio, CA, Sarmiento, S, Minhajuddin, A, Nasir, D, and Fox, AA. High-flow versus standard nasal cannula in morbidly obese patients during colonoscopy: a prospective, randomized clinical trial. J Clin Anesth. (2019) 54:19–24. doi: 10.1016/j.jclinane.2018.10.026
24. Murphy, C, and Wong, DT. Airway management and oxygenation in obese patients. Can J Anaesth. (2013) 60:929–5. doi: 10.1007/s12630-013-9991-x
25. Guo, J, Qian, Y, Zhang, X, Han, S, Shi, Q, and Xu, J. Remimazolam tosilate compared with propofol for gastrointestinal endoscopy in elderly patients: a prospective, randomized and controlled study. BMC Anesthesiol. (2022) 22:180. doi: 10.1186/s12871-022-01713-6
26. Pedersen, CM, Thirstrup, S, and Nielsen-Kudsk, JE. Smooth muscle relaxant effects of propofol and ketamine in isolated Guinea-pig trachea. Eur J Pharmacol. (1993) 238:75–80. doi: 10.1016/0014-2999(93)90507-e
27. Chen, SH, Yuan, TM, Zhang, J, Bai, H, Tian, M, Pan, CX, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, phase III trial. J Gastroenterol Hepatol. (2021) 36:474–1. doi: 10.1111/jgh.15188
28. Kim, SH, and Fechner, J. Remimazolam - current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J Anesthesiol. (2022) 75:307–5. doi: 10.4097/kja.22297
29. Dong, D, Peng, X, Liu, J, Qian, H, Li, J, and Wu, B. Morbid obesity alters both pharmacokinetics and pharmacodynamics of propofol: dosing recommendation for anesthesia induction. Drug Metab Dispos. (2016) 44:1579–83. doi: 10.1124/dmd.116.071605
30. Rex, DK, Bhandari, R, Lorch, DG, Meyers, M, Schippers, F, and Bernstein, D. Safety and efficacy of remimazolam in high-risk colonoscopy: a randomized trial. Dig Liver Dis. (2021) 53:94–1. doi: 10.1016/j.dld.2020.10.039
31. Dai, G, Pei, L, Duan, F, Liao, M, Zhang, Y, Zhu, M, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. (2021) 87:1073–9. doi: 10.23736/S0375-9393.21.15517-8
32. Stöhr, T, Colin, PJ, Ossig, J, Pesic, M, Borkett, K, Winkle, P, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. (2021) 127:415–3. doi: 10.1016/j.bja.2021.05.027
33. Long, Y, Liu, HH, Yu, C, Tian, X, Yang, TR, Wang, C, et al. Pre-existing diseases of patients increase susceptibility to hypoxemia during gastrointestinal endoscopy. PLoS One. (2012) 7:e37614. doi: 10.1371/journal.pone.0037614
34. Chooi, YC, Ding, C, and Magkos, F. The epidemiology of obesity. Metab Clin Exp. (2019) 92:6–10. doi: 10.1016/j.metabol.2018.09.005
35. Kroll, C, Mastroeni, S, Czarnobay, SA, Ekwaru, JP, Veugelers, PJ, and Mastroeni, MF. The accuracy of NC for assessing overweight and obesity: a systematic review and meta-analysis. Ann Hum Biol. (2017) 44:667–7. doi: 10.1080/03014460.2017.1390153
36. Simpson, L, Mukherjee, S, Cooper, MN, Ward, KL, Lee, JD, Fedson, AC, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. (2010) 33:467–4. doi: 10.1093/sleep/33.4.467
37. Onat, A, Hergenç, G, Yüksel, H, Can, G, Ayhan, E, Kaya, Z, et al. NC as a measure of central obesity: associations with metabolic syndrome and obstructive sleep apnea syndrome beyond waist circumference. Clin Nutr. (2009) 28:46–51. doi: 10.1016/j.clnu.2008.10.006
Keywords: propofol, obesity, ketamine, deep sedation, remimazolam, hypoxemia
Citation: Zhang K, Bao Y, Han X, Zhai W, Yang Y, Luo M and Gao F (2023) Effects of opioid-free propofol or remimazolam balanced anesthesia on hypoxemia incidence in patients with obesity during gastrointestinal endoscopy: A prospective, randomized clinical trial. Front. Med. 10:1124743. doi: 10.3389/fmed.2023.1124743
Received: 15 December 2022; Accepted: 03 March 2023;
Published: 22 March 2023.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Aranya Bagchi, Harvard Medical School, United StatesCopyright © 2023 Zhang, Bao, Han, Zhai, Yang, Luo and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Gao, Z2FvZmFuZ3h6QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
‡ORCID: Fang Gao, https://orcid.org/0000-0001-9861-506X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.