
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 03 May 2023
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1117474
This article is part of the Research Topic Case Reports in Infectious Diseases – Surveillance, Prevention and Treatment View all 26 articles
Introduction: A rare pathogen of Infective Endocarditis (IE), the Abiotrophia defectiva, has been known to trigger life-threatening complications. The case discussed here is of a teenager with brain infarction and subarachnoid hemorrhage caused by IE due to A. defectiva.
Case report: A 15-year-old girl with movement disorders involving the left limbs and intermittent fevers was admitted to the hospital. A head CT scan revealed cerebral infarction in the right basal ganglia and subarachnoid hemorrhage. Moreover, vegetation on the mitral valve were confirmed by echocardiography. The blood cultures were found to be positive for Gram-positive streptococcus and identified by Vitek mass spectrometry as A. defectiva. She was prescribed vancomycin antibacterial therapy and underwent a surgical mitral valve replacement.
Conclusion: This case is suggestive of the fact that A. defectiva is a rare but crucial pathogen of IE-associated stroke. Obtaining early blood cultures and using microbial mass spectrometry could help achieve an accurate diagnosis. Moreover, reasonable anti-infective medications and surgical interventions need to be combined to avoid and/or manage severe complications.
Abiotrophia defectiva (A. defectiva), was originally known to be a Nutritionally Variant Streptococci (NVS). This NVS was subsequently reclassified as Abiotrophia defectiva, Granulicatella adiacens, Granulicatella elegans, and Granulicatella balaenopterae through 16S rRNA gene sequencing. These organisms are normal colonization bacteria of the human body. A. defectiva needs L-cysteine, pyridoxal, and other such factors for its proper growth. In earlier case reports, the subacute IE were the most frequently reported cases of A. defectiva due to their indolent clinical course. Nevertheless, A. defectiva led to severe complications including valvular damage, congestive heart failure, and events of embolisation. Thus, early diagnosis and effective treatment strategy for A. defectiva become crucial in clinical practice.
A 15-year-old girl was admitted to Shenzhen People's Hospital Neurology Intensive Care Unit (NICU) due to the sudden onset of a left-sided movement disorder. She experienced a sudden episode of weakness in her left lower limb one and a half months prior to hospitalization, which resolved spontaneously 3 days later. A month ago, she developed weakness in her left upper extremity, which disappeared after 5 days. Intermittent fevers of 39°C started 1 week ago, along with mild shortness of breath but no chills, cough, diarrhea, or bladder irritation symptoms. She noticed the sudden onset of a persistent left-limb movement disorder 3 h before being admitted to the NICU. She claimed to have lost 7.5 kilograms (17 percent of her body weight) in 3 months. She underwent orthodontic treatment a year ago and subsequently wore braces. She denied having a history of hypertension, diabetes, cigarette smoking, alcohol consumption, intravenous drug use, immunosuppressant use, atrial fibrillation, and inherited diseases.
She was conscious on physical examination, and her verbal responses to questions were appropriate. Her face was pale, her left lower limb muscle strength was grade II, her left upper limb muscle strength was grade III, and both Babinski and Brudzinski's signs were positive. In addition, a grade 4–6 systolic murmur was heard in the mitral region, splenomegaly was confirmed by abdominal palpation, and clubbed fingers were observed upon careful inspection of the hands.
Laboratory analysis showed the following: a normal leukocyte count, but mild normocytic anemia (hemoglobin, 91 g/L); an elevated C-reactive protein level of 32.05 mg/L (normal range, 0–5 mg/L); and a slightly elevated procalcitonin level (0.07 ng/mL; normal range, <0.05 ng/mL). The NT-proBNP level was 1,123 pg/m (normal range, <125 pg/mL). The cerebrospinal fluid (CSF) was red, the pressure was 180 mm H2O, the glucose level decreased (1.79 mmol/L; normal range, 3.9–6.1 mmol/L), the protein concentration increased (0.55 g/L; normal range, 0.15-0.45 g/L), the nucleated cell number increased (280 /μL; normal range, <20 /μL), and the CSF culture was negative.
A head CT scan urgently performed revealed a cerebral infarction in the right basal ganglia and a subarachnoid hemorrhage (Figure 1). A transthoracic echocardiogram (TTE) revealed vegetation on the mitral leaflets measuring 2.3 × 1.3 cm (Figure 2). Before administering ceftriaxone to the patient, three blood cultures (aerobic and anaerobic bottles from peripheral blood once every half an hour) were obtained. After 9.5 h, the blood culture was positive for facultative anaerobic Gram-positive streptococcus, which was immediately identified as A. defectiva (Figure 3) with the Vitek MS using library v3.2 (bioMérieux, Marcy-l'Étoile, France) and the percent of identity is 99.9%. According to antimicrobial susceptibility testing (Supplementary Figure 1), the bacteria were susceptible to vancomycin, clindamycin, and linezolid but resistant to penicillin, ceftriaxone, and cefepime. All three blood cultures tested positive for A. defectiva. The girl met two of the modified Duke diagnostic criteria for IE. Based on the susceptibility test results, the antibiotic therapy was immediately changed to vancomycin, 3 days later, the girl's temperature turned normal. The girl was transferred to a higher-level hospital for cardiovascular surgery on the tenth hospital day. Three weeks later, she underwent mitral valve replacement surgery. Upon discharge, two months after the onset of symptoms, her left lower limb muscle strength was grade V, while the left upper limb muscle showed a strength of grade IV.
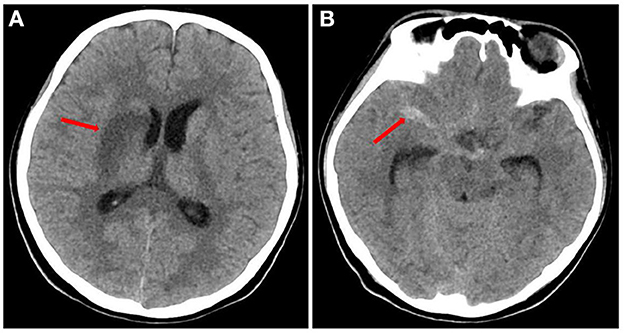
Figure 1. Head CT of the patient (A) the red arrow indicates infarction of the right basal ganglia. (B) The red arrow indicates subarachnoid hemorrhage.

Figure 2. Transthoracic echocardiography examination of the heart in the patient. (A) THE red arrow indicates the vegetation (2.3 × 1.3 cm) on the mitral valve. (B) The red arrow indicates another position of the vegetation (2.3 × 0.7 cm) on the mitral valve. LA: left atrium; LV: left ventricle.
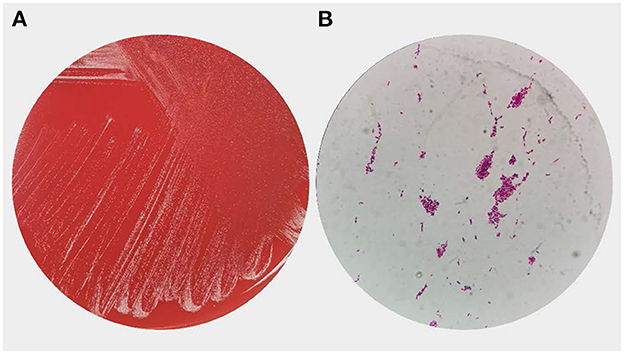
Figure 3. Overview of Abiotrophia defectiva of growth morphology (A) Colony growth of A. defectiva on blood agar. (B) Smear showing gram-positive cocci in chains.
A. defectiva is a facultative anaerobic Gram-positive coccus that was first reported in a case of IE in 1961 (1). A. defectiva normally afflicts the respiratory, urogenital, and gastrointestinal tracts (2). The IE caused by A. defectiva usually occurs in patients with congenital heart disease or a history of previous cardiac surgery (3). Moreover, oral hygiene, and a dental procedure could also be important causes of A. defectiva related IE (4, 5). In the pertinent case, the girl had a history of an orthodontics procedure, which could have contributed to IE. Compared to other pathogens leading to IE, A. defectiva is more likely to form valvular vegetations and lead to cardiogenic emboli (6, 7). Literature suggests that due to the production of a considerable amount exopolysaccharides, the organism has a higher affinity for the endocardium and the ability to bind with fibronectin in the extracellular matrix, further contributes to their virulence (8, 9). Colonization and infection of corneas, joints and heart valves may indicate a significant tendency of the bacterium to colonize vascular free collagen tissue (10). Besides, A. defectiva endocarditis has an indolent course and is often culture-negative, contributing to a delayed diagnosis, with high mortality, and complication rates (6). The NVS are estimated to cause approximately 5%−6% of all cases of IE (11), the mortality rate of NVS IE is 9.2% (12). In the patient in the pertinent case, the disease course continued for 6 weeks. Unfortunately, the IE was complicated by heart failure, a brain infarction, and subarachnoid hemorrhage when diagnosed.
The patient, in this case, the young girl, was admitted to the NICU with symptoms associated with brain infarction, hence, urgent brain imaging was essential to confirm the stroke diagnosis and guide the therapy strategies. Moreover, the etiology of brain infarction determines the subsequent prevention strategies. Stroke, cardioembolic causes, and arterio-pathic causes are the most frequent etiologies in childhood (defined as 29 days to 18 years of age). The reasons for arterial ischemic stroke include congenital or acquired heart disease, non-atherosclerotic arteriopathies (arterial dissection, focal cerebral arteriopathy, and Moyamoya), and sickle cell diseases (13).
For the diagnosis of IE, an echocardiogram and laboratory testing (especially blood cultures) would be needed. In the current case, timely blood cultures and bacterial mass spectrometry facilitated the rapid identification of a rare pathogen, like A. defectiva. The 16S rRNA sequencing also played an important role in verification of A. defective (14). Moreover, Metagenomic Next-Generation Sequencing (mNGS) has been reported in the clinical diagnosis of IE (15). Compared to conventional cultures, the mNGS has been found to be more sensitive and particularly suitable for rare or culture-negative pathogens (16). Recently, Du reported a case of IE caused by A. defectiva, with the diagnosis being assisted by mNGS (17).
The administration of proper antibiotics is the foundation of the IE treatment. According to the 2015 European Society of Cardiology (ESC) guidelines, penicillin G, ceftriaxone, or vancomycin need to be used for 6 weeks, combined with an aminoglycoside for at least 2 weeks (18). The American Heart Association guidelines recommend the administration of a combination regimen that includes ampicillin (12 g/d in divided doses) or penicillin (18–30 million U/D in divided doses or by continuous infusion) plus gentamicin (3 mg/kg/day in 2–3 divided doses) with infectious diseases consultation to determine the duration of the therapy (19). Almost all susceptibility studies have reported that A. defectiva was susceptible to vancomycin. Although A. defectiva susceptibility to ceftriaxone was 92%–100% (20), in this case study, the bacteria were resistant to ceftriaxone. In IE caused by A. defectiva, approximately 27% of patients need prosthetic valve replacement (21). Due to the large mitral valve vegetations (>1 cm) and severe embolic events, the pertinent patient had to undergo surgical mitral valve replacement.
This case study indicated that A. defectiva is an important cause of IE, which could lead to severe complications. Thus, timely application of blood cultures and rapid identification of A. defectiva would enable the proper prescription of antibiotics. Moreover, effective anti-infective medications and surgical interventions need to be combined to avoid and/or manage serious complications.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
MY: diagnosis and treatment of the patient and conception and creation of the manuscript. YL: conceptualization and original draft preparation. XP: writing. JW: microbial identification. BH: clinical consultation. YH: reviewing. JL: supervision and reviewing. All authors contributed to the article and approved the submitted version.
This study was supported by Shenzhen Basic Research Key projects, Shenzhen Science and Technology Innovation Commission (JCYJ20200109144220704), and Natural Science Foundation of Shenzhen University General Hospital (SUGH2018QD047).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1117474/full#supplementary-material
Supplementary Figure 1. Antimicrobial susceptibility test results of Abiotrophia defectiva according to CLSI M45 criterion. MIC, Minimum Inhibitory Concentration; KB, Kirby-Bauer Test; I, Intermediate; R, Resistant; S, Susceptible.
1. Frenkel A, Hirsch W. Spontaneous development of L forms of streptococci requiring secretions of other bacteria or sulphydryl compounds for normal growth. Nature. (1961) 191:728–30. doi: 10.1038/191728a0
2. Al-Jasser AM, Enani MA, Al-Fagih MR. Endocarditis caused by Abiotrophia defectiva. Libyan J Med. (2007) 2:43–5. doi: 10.3402/ljm.v2i1.4691
3. Baltimore RS, Gewitz M, Baddour LM, Beerman LB, Jackson MA, Lockhart PB, et al. Infective endocarditis in childhood: 2015 update: a scientific statement from the american heart association. Circulation. (2015) 132:1487–515. doi: 10.1161/CIR.0000000000000298
4. Blochowiak KJ. Dental treatment and recommended management in patients at risk of infective endocarditis. Kardiochir Torakochirurgia Pol. (2019) 16:37–41. doi: 10.5114/kitp.2019.83944
5. Birlutiu V. and Birlutiu RM, Endocarditis due to Abiotrophia defectiva, a biofilm-related infection associated with the presence of fixed braces: a case report. Medicine (Baltimore). (2017) 96:e8756. doi: 10.1097/MD.0000000000008756
6. Kalogeropoulos AS, Siva A, Anderson L. Abiotrophia defectivus infectious endocarditis: think beyond the heart. Lancet. (2015) 385:1044. doi: 10.1016/S0140-6736(15)60090-3
7. Tellez A, Ambrosioni J, Llopis J, Pericas JM, Falcas C, Almela M, et al. Epidemiology, clinical features, and outcome of infective endocarditis due to abiotrophia species and granulicatella species: report of 76 Cases, 2000-2015. Clin Infect Dis. (2018) 66:104–11.
8. Park S, Ann H, Ahn J, Ku N, Han S, Hong G, et al. A case of infective endocarditis caused by Abiotrophia defectiva in Korea. Infect Chemother. (2016) 48:229–33. doi: 10.3947/ic.2016.48.3.229
9. Pinkney JA, Nagassar RP, Green KJ, Ferguson T. Abiotrophia defectiva endocarditis. BMJ Case Rep. (2014) 201:bcr20142073614. doi: 10.1136/bcr-2014-207361
10. Abry F, Sauer A, Riegel P, Saleh M, Gaucher D, Schatz C, et al. Infectious crystalline keratopathy caused by Streptococcus Abiotrophia defective. Cornea. (2010) 29:934–6. doi: 10.1097/ICO.0b013e3181ca2e8f
11. Ruoff KL. Nutritionally variant streptococci. Clin Microbiol Rev. (1991) 4:184–90. doi: 10.1128/CMR.4.2.184
12. Garcia-Granja PE, Lopez J, Vilacosta I, Sarria C, Ladroon R, Olmos C, et al. Nutritionally variant streptococci infective endocarditis: a different view. Clin Infect Dis. (2018) 67:1800–1. doi: 10.1093/cid/ciy444
13. Sporns PB, Fullerton H, Lee S, Kim H, Lo W, Mackay M, et al. Childhood stroke. Nat Rev Dis Primers. (2022) 8:12. doi: 10.1038/s41572-022-00337-x
14. Rozemeijer W, Jiya TU, Rijnsburger M, Heddema E, Savelkoul P, Ang W. Abiotrophia defectiva infection of a total hip arthroplasty diagnosed by 16S rRNA gene sequencing. Diagn Microbiol Infect Dis. (2011) 70:142–4. doi: 10.1016/j.diagmicrobio.2010.11.016
15. Tattevin P, Watt G, Revest M, Arvieux C, Fournier P-E. Update on blood culture-negative endocarditis. Med Mal Infect. (2015) 45:1–8. doi: 10.1016/j.medmal.2014.11.003
16. Fukui Y, Aoki K, Okuma S, Sato T, Ishii Y, Tateda K. Metagenomic analysis for detecting pathogens in culture-negative infective endocarditis. J Infect Chemother. (2015) 21:882–4. doi: 10.1016/j.jiac.2015.08.007
17. Du Y, Zhang Z, Chen C, Xia H, Zhang H, Guo Z, et al. Case report: report of infective endocarditis caused by Abiotrophia defectiva and literature review. Front Pediatr. (2022) 10:894049. doi: 10.3389/fped.2022.894049
18. Habib G, Lancellotti P, Antunes MJ, Bongiomi MG, Casalta J, Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. (2015) 36:3075–128. doi: 10.1093/eurheartj/ehv319
19. Baddour LM, Wilson W, Bayer A, Fowler V, Tleyjeh I, Rybak M, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the american heart association. Circulation. (2015) 132:1435–86.
20. Dumm RE, Wing A, Richterman A, Jacob J, Glaser L, Rodino K. Closing the brief case: a variant on a classic-abiotrophia Defectiva endocarditis with discitis. J Clin Microbiol. (2021) 59:e0309420. doi: 10.1128/JCM.03094-20
Keywords: Abiotrophia defectiva, infective endocarditis, brain infarction, subarachnoid hemorrhage, microbial mass spectrometry
Citation: Yang M, Lin Y, Peng X, Wu J, Hu B, He Y and Lu J (2023) Abiotrophia defectiva causing infective endocarditis with brain infarction and subarachnoid hemorrhage: a case report. Front. Med. 10:1117474. doi: 10.3389/fmed.2023.1117474
Received: 06 December 2022; Accepted: 04 April 2023;
Published: 03 May 2023.
Edited by:
Francesco Paolo Bianchi, University of Bari Aldo Moro, ItalyReviewed by:
Lei Huang, First Hospital, Peking University, ChinaCopyright © 2023 Yang, Lin, Peng, Wu, Hu, He and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Lu, c3psdWppYW5Ac3p1LmVkdS5jbg==; Yitao He, aGV5aXRhb3Z2QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.