- 1Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, University of Gondar, Gondar, Ethiopia
- 2Department of Medical Laboratory Science, College of Health Sciences, Mizan-Tepi University, Mizan, Ethiopia
- 3University of Gondar Comprehensive Specialized Hospital Laboratory, Gondar, Ethiopia
- 4College of Medicine and Public Health, Flinders University, Adelaide, SA, Australia
Background: Anemia is highly prevalent globally and disproportionately affects postnatal women. It is a significant cause of maternal mortality and morbidity globally.
Objective: The main aim of this study was to determine the extent of postpartum anemia and associated factors among postnatal women in two selected health facilities in Gondar, Northwest Ethiopia.
Methods: A facility-based, cross-sectional study was conducted among 282 postnatal women from March to May 2021. A systematic sampling technique was used to recruit study participants from each institute. Sociodemographic, obstetric, and clinical data were collected through a semi-structured questionnaire. A venous blood sample was collected to determine the red blood cell parameters. A thin blood smear preparation was performed to examine blood morphology. In addition, direct wet mount and formalin-ether sedimentation techniques were used for stool examination to identify intestinal parasites. Data were entered into EpiData and exported to Stata 14 for statistical analysis. Descriptive statistics were presented in text, tables, and figures. A binary logistic regression model was used to identify factors associated with postpartum anemia. A p-value <0.05 was considered statistically significant.
Results: The proportion of postpartum anemia was 47.16%; 95% CI; 41.30–53.03 with moderate, mild, and severe anemia accounting for 45.11, 42.86, and 12.03%, respectively. The majority of the anemia (94%) was of the normocytic normochromic type. It was associated with postpartum hemorrhage (AOR = 2.23; 95% CI: 1.24–4.01), cesarean section (AOR = 4.10; 95% CI: 2.11–7.78), lack of iron and folate supplementation during pregnancy (AOR = 2.12; 95% CI: 1.17–4.02), and low diet diversity level (AOR = 1.83; 95% CI: 1.05–3.18).
Conclusion: The prevalence of anemia was found to be a major public health concern. Iron and folate supplementation during pregnancy, improved management of PPH, an effective cesarean section with post-operative care, and taking a diversified diet will reduce the burden. Therefore, identified factors should be considered to prevent and control postpartum anemia.
Introduction
Anemia is defined as a decrease in the mass of red blood cells (RBCs) or a low level of hemoglobin (Hb) relative to the normal reference range. The World Health Organization (WHO) defines it as Hb < 12.0 gm/dl in adult, non-pregnant women. However, there is no well-defined agreement for postpartum anemia (PPA), nonetheless, which is still defined as Hb < 12 gm/dl (1, 2). The postpartum period is defined as the first 6 weeks following childbirth (3).
Anemia is also defined as tissue hypoxia due to inadequate oxygen delivery in the tissue. The pathophysiology of anemia is multi-factorial and complex, which might be associated with a predisposition in genes for Hb, enzymatic deficiencies, chronic and acute blood loss, nutrient deficiencies, infection, hemorrhage, chronic disease, bone marrow disorders, and other factors. This may be due to inadequate or defective erythropoiesis from food shortages, bone marrow infiltration, inflammation, or hereditary Hb disorders and severe erythrocyte loss related to hemolysis and acute blood bleeding (4, 5).
Indeed, iron deficiency is the most common micronutrient deficiency and the most common cause of anemia in the world. About half (50%) of the cases of anemia are attributed to iron deficiency. Iron deficiency anemia (IDA) is more prevalent in low-income countries (6). Anemia is a serious problem and challenge in the world that can affect about one-third of people around the world (women, young children, and individuals with long-term diseases) in both developed and developing countries (7, 8). It is a fact that anemia is more prevalent among children and women of reproductive age, including postnatal women, and can cause morbidity and mortality in women in addition to fetal consequences (9).
Anemia is a significant cause of maternal mortality and morbidity on a global scale. It affects 38% of pregnant women and 29% of non-pregnant women, with the highest proportion in central and west Africa (56%) (10). Around 500 million women of reproductive age are affected by anemia, which is a major public health challenge for low- and middle-income countries (11). One of the developing countries, Ethiopia, had the highest rates of maternal deaths in 2016, with 412 women dying for every 10,000 live births (12). This mortality is mainly associated with prolonged iron loss due to postpartum hemorrhage (PPH), which is a leading predictor of cardiac arrhythmias (13, 14). It is also associated with lower global household income, cognitive and psychological impairment, poor quality of life, emotional instability, and postpartum depression (1, 15).
In addition, PPA has the highest risk of endometritis and thrombotic complications secondary to iron deficiency and local ischemia (16, 17). Furthermore, the prevalence of anemia among lactating women has increased from 23.03 to 28.3% in Ethiopia over the past two and a half decades (2011–2016) (18). PPA is primarily linked to poor economic development, obstetric problems, and low nutritional status. Evidence suggests that obstetric factors are a significant source of PPA, leading to morbidity and mortality in women of reproductive age (19). During delivery, women experienced traumatic processes, leading to PPH. Different studies investigated the risk factors for PPA, including PPH, maternal residence, blood loss, and maternal level of education. This could be attributed to active bleeding during delivery, which causes a drop in Hb levels both before and after delivery (20).
It is also characterized by their socioeconomic context. Higher prevalence rates (57.2%) of anemia among rural women aged 20 years and older were observed in India (21). PPA is also a common problem in countries with stronger economies (22). Low maternal education levels, poor socioeconomic status, and living in rural areas all play a significant role in the development of anemia in women (23). Women with low dietary diversity had a higher risk of anemia (24). During pregnancy, improved and increased adherence to iron and folic acid was found to reduce the possible risk of anemia in postnatal women. Vitamin and mineral deficiencies may worsen during pregnancy due to increased energy and nutrient demands, causing adverse outcomes for both mother and child (25).
In addition to this, anemia in women of reproductive age is a significant issue, and the WHO has set a global goal of attaining a 50% reduction in anemia in women of reproductive age by 2025 (26). To achieve the WHO target, addressing anemia in postnatal women is of great importance. However, there are only a few rigorous studies conducted on PPA in Africa, particularly in Ethiopia. Although blood morphology is helpful in the diagnosis and categorization of the pathophysiology of anemia (27). In addition, parasitic infection is an independent predictor of anemia. However, the available studies did not reveal this. As a result, this study highlighted important findings that can help improve the maternal healthcare system.
Methods
Study area, design, and period
A facility-based cross-sectional study was conducted in the town of Gondar, in the Amhara regional state, from March to May 2021. Gondar has one hospital and eight public health centers. The estimated population projection for Gondar was 362,000 in 2020 (28). Gondar is situated about 727 kilometers (km) northwest of Addis Ababa and 185 km from the city of Bahir Dar. It is located at an altitude of 2,133 meters above sea level at 12° 36′ north and 37° 28′ east, latitude and longitude, respectively (29).
Population
Source and study population: All postnatal women in Gondar were considered as the source population. All postnatal women who gave birth and attended the postnatal care (PNC) clinic at the Gondar Health Center and the University of Gondar Comprehensive Specialized Hospital (UGCSH) and were available during the data collection period were considered as the study population.
Sample size determination
The sample size was determined by a single population proportion formula, considering a 5% margin of error at 95% CI, and by taking the 24.3% prevalence reported in the previous study (30):
where N = the desired sample size, z = 1.96, standard normal distribution value at 95% CI corresponding to a significant level of alpha, and d = acceptable margin of error.
The total sample size calculated for this study was 282 postnatal women.
Sampling technique
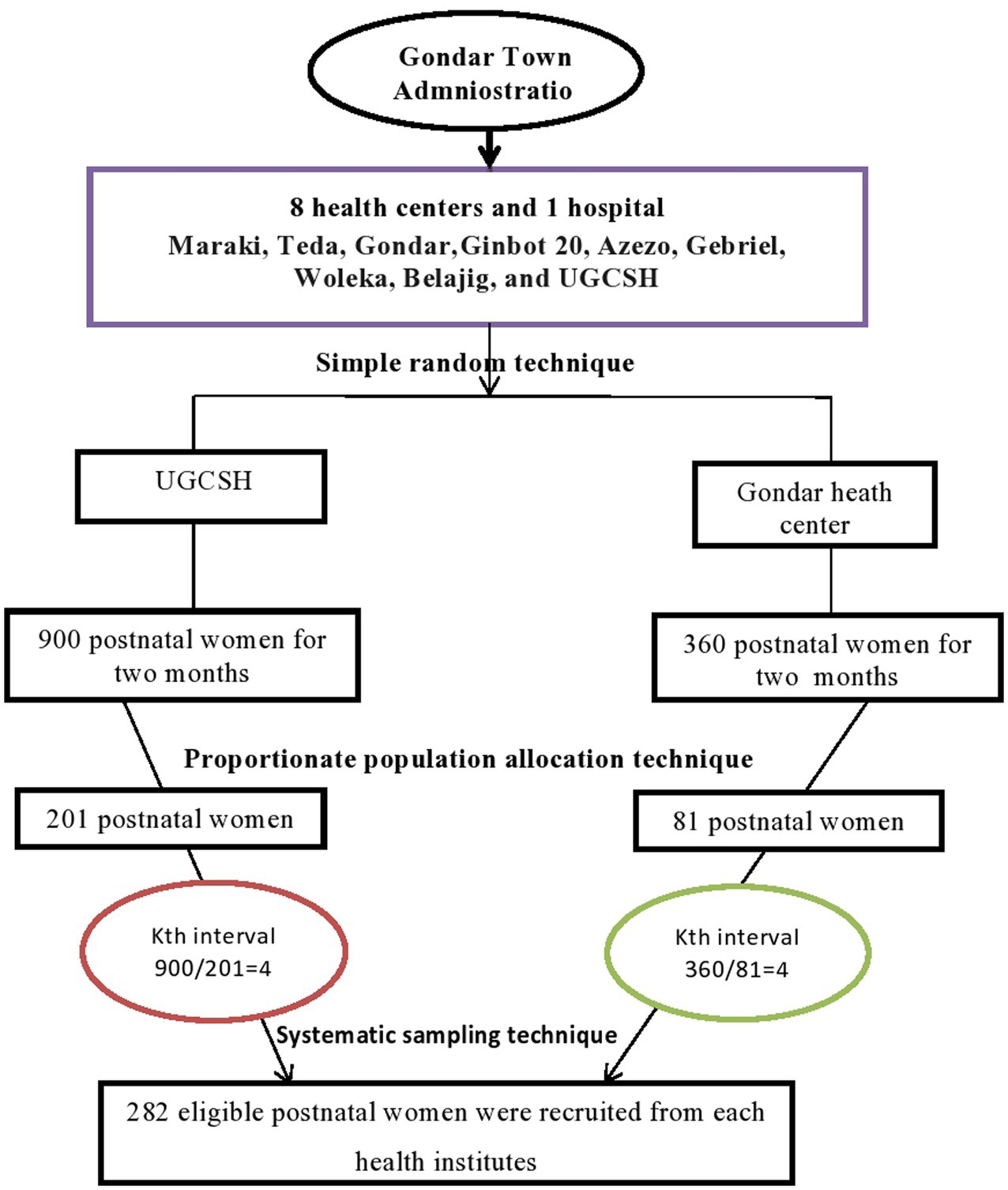
Of the nine public health facilities, two of them, the Gondar Health Center and the UGCSH, were selected using a simple random sampling technique. Using the monthly postnatal women numbers from both facilities (450 from UGCSH and 180 from the Gondar Health Center), the interval value was calculated by dividing the estimated total number of women within the calculated sample size (1890/282 = 6.7) by the proportionate population size in each health facility. For instance, the estimated total number of women in UGCSH during the study period (900) was divided by the interval (4.46) to yield a proportionate sample, which was approximately 201, and the same method was used for the Gondar Health Center, where 81 postnatal women were recruited. Then, a systematic sampling technique was employed, using an interval of four based on the corresponding sample size from each health facility. The lottery method was applied to select the first four participants, and every four women were interviewed until the allocated sample size was achieved (Figure 1).
Inclusion criteria and exclusion criteria: All postnatal women who delivered in the selected health institutions and volunteered to participate were included in the study. Women with a medical history of hypertension, kidney disease, human immunodeficiency virus (HIV), or hepatitis B, too ill to give informed consent, receiving any form of hematinic, or those who had been recently transfused were excluded from the study.
Variables
Dependent variables: Anemia was a dependent variable. Before determining whether a woman was anemic or not, altitude was considered. The adjustment for altitude was made to account for the decrease in blood oxygen saturation. The Hb level was adjusted using the following formula: (adjustment = −0.032 (altitude * 0.0032808) + 0.022 (altitude * 0.0032808)2 and adjHb = Hb – adjustment (when adjustment >0)) (31).
Independent variables: Socio-demographic variables (maternal age, educational level, residence, marital status, religion, family size, occupation), obstetric variables (parity, frequency of antenatal care (ANC) visits, history of abortion and malaria infection, place and mode of delivery, iron and folate supplementation during pregnancy, PPH, intestinal parasites (IP), family planning, birth within 5 years), and nutritional variables such as dietary diversity, weekly meat consumption, daily fruit and vegetable consumption, coffee and tea drinking habits were considered as independent variables.
Operational definitions
High dietary diversity: Consumption of at least 7 foods from 10 food groups (32, 33).
Low dietary diversity: Consumption of four or fewer foods from 10 food groups (32, 33).
Minimal dietary diversity: Consumption of at least five foods from 10 food groups (33, 34).
Postpartum anemia: Adjusted Hb levels of postnatal women below 11 gm/dl within 24 h and 12gm/dl up to 6 weeks after the birth period result in postpartum anemia, which is further classified as mild (adjHb = 11–11.9 gm/dl), moderate (adjHb = 8–10.9gm/dl) and severe (adjHb <8 gm/dl) (1, 2).
Postpartum hemorrhage: Bleeding during delivery is classified as mild (500-1,000 ml), moderate (1000–2000 ml), and heavy (>2000 ml) postpartum hemorrhage.
Data collection techniques
After obtaining written informed consent, socio-demographic variables such as age, educational attainment, marital status, occupation, and related characteristics of postnatal women were collected through face-to-face interviews using a pre-tested, semi-structured questionnaire at the obstetric and gynecological wards and PNC units of UGCSH and the Gondar Health Center. Obstetric and clinical data were collected using physical examinations, chart reviews, and laboratory tests. Detailed information on the history of abortion and malaria infection, and iron and folic acid supplements during pregnancy, were collected from the charts. According to the guidelines of the Food and Agriculture Organization (FAO), a Dietary Diversity Score (DDS) was assessed.
Blood sample collection and analysis: A 5 ml venous blood sample was collected by well-trained laboratory personnel using standard aseptic procedures and sent to the hematology laboratory. The blood sample was placed in a labeled tri-potassium ethylenediaminetetraacetic acid (K3-EDTA) tube. An automated, compact 5-part differential hematology analyzer (Beckman Coulter UniCel DxH 800) was used to determine hematological parameters. A drop of blood from a confirmed anemic woman was put on a microscopic slide, and a thin smear was prepared. The smear was stained with undiluted Wright’s staining solution based on standard operating procedures. It was then air-dried, labeled with an identification number, and examined under a microscope with a 100X oil immersion objective by well-trained hematologists. RBC shape, size, color, and cellular inclusions were carefully evaluated for morphologic examination.
Stool sample collection and examination: Pea-sized stool samples were collected from each study participant using a clean, wide-mouth, leak-proof stool cup. Wet mount preparation and the formol-water concentration technique were used to detect IP. Fresh stool samples were mounted on a microscope slide with a wooden applicator stick and emulsified with a drop of physiological saline (0.85% w/v NaCl). The concentration technique was used on the remaining samples for the concentration of IP.
The fecal sample was emulsified in 10 ml of formol-water (10% v/v) in a screw-cap bottle. The suspension was strained into a conical tube by using gauze to extract large fecal particles and centrifuged at 1500 rpm for 3 min. Then, it was re-suspended by using 4–3 ml of formol water and 3–4 ml of ether or ethyl acetate and centrifuged at 3,000 rpm for 1 min. Finally, the supernatant was removed, and the sediment was examined under the microscope. A stool examination was performed according to standard operating procedures. Any helminth eggs, larvae, or cyst stages were detected. All samples were collected through laboratory tests using the participants’ laboratory information checklist.
Data quality management: The questionnaire was prepared in English, translated into the local language (Amharic), and then translated back into English for consistency. Prior to data collection, the data collection tool was pretested on 14 volunteer postnatal women at UGSCH. There were inaccuracies in the measurement of body mass index (BMI), which was therefore omitted. Prior to data collection, orientation was provided to data collectors. During the data collection process, there was close monitoring to ensure data accuracy and consistency. Following blood collection, to avoid hemolysis, the blood was applied to the wall of the test tube. Data were collected by well-trained health professionals (five midwives and three laboratory staff).
Prior to running the participants’ sample, the accuracy of the hematology analyzer was checked with high-, normal-, and low-quality control (QC) materials. All reagents were checked for expiration dates and prepared according to the manufacturer’s instructions. A morphological examination was carried out on the known mean corpuscular volume (MCV) and mean corpuscular Hb concentration (MCHC) of healthy individuals to ensure the quality of the staining reagent. The stool wet mount and suspension were made within the appropriate concentration of sample and reagents. Fresh stool samples were observed within 30 min of collection. Finally, all results were documented and registered with the correct value and units.
Data processing, analysis, and interpretation
The data were checked for completeness and consistency. Then, they were coded and double-entered into EpiData, version 4.6.0.0, before being imported into Stata, version 14.0, for analysis. The data was cleaned, and preliminary analyses were carried out. Descriptive statistics were summarized using frequency, percentage, median, mean, interquartile range (IQR), and standard deviation for presentation in text, tables, and figures. A binary logistic regression model was used to assess factors associated with the outcome variable. Variables with a p-value of ≤0.25 were adjusted for multivariable analysis. In the model, a backward variable selection was used. The Hosmer and Lemeshow post-estimation statistical test was performed to check the goodness of fit. A p-value 0.05 was considered statistically significant.
Results
Socio-demographic characteristics
A total of 282 postpartum women were included in this study. The median age of the participants was 28 years, with an IQR of 24–30. Approximately 62.8% of the participants were between the ages of 25 and 34. Three-fourths (75.9%) of the respondents were from urban areas. One-third (29.4%) of respondents were illiterate. Of the total participants, 242 (85.8%) were married (Table 1).
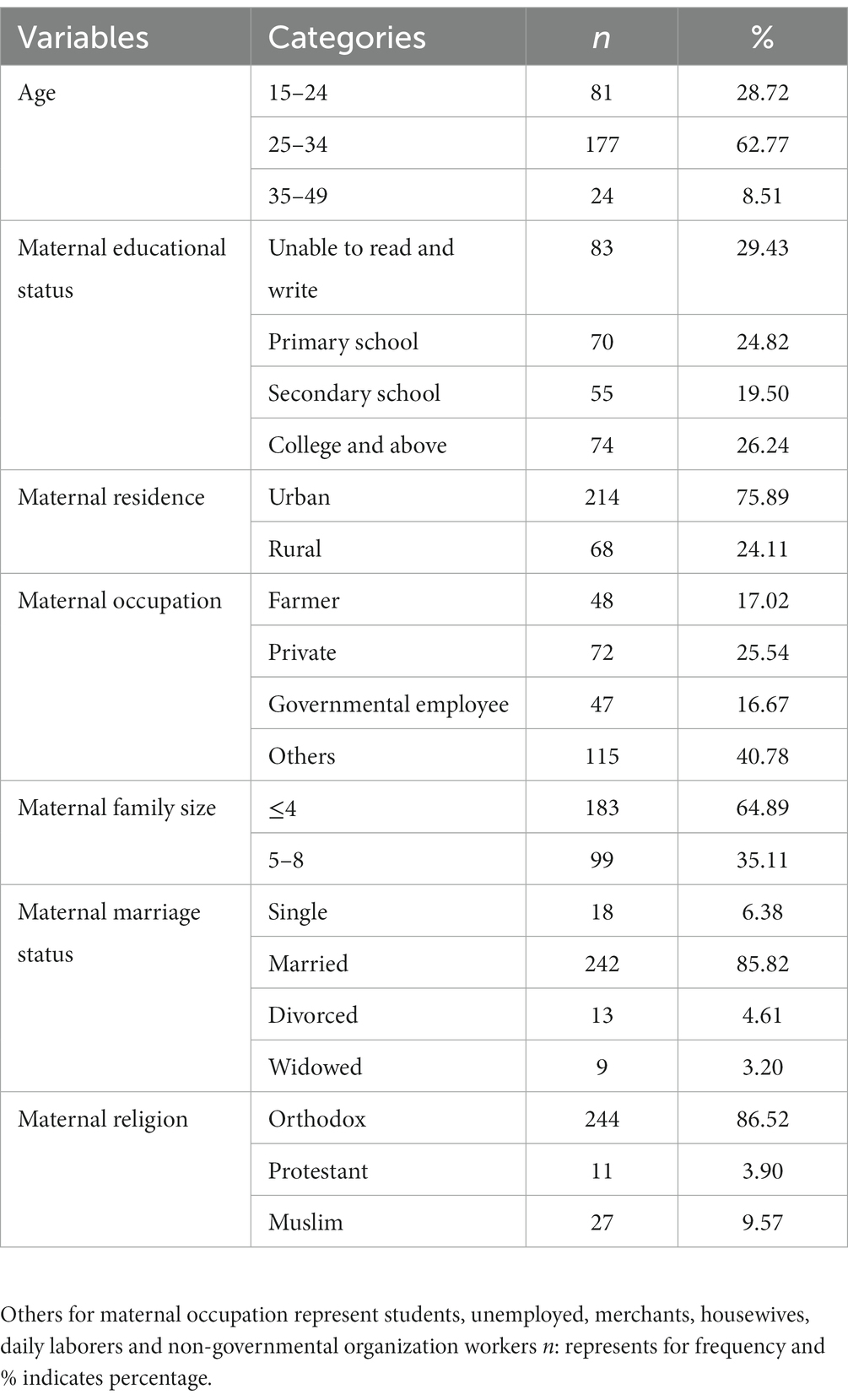
Table 1. Socio-demographic characteristics of post-natal women in Gondar town, Northwest Ethiopia, 2021 (n = 282).
Obstetric and clinically related characteristics
Of the total study population, 162 (57.5%) women were multiparous. A total of 159 (56.4%) of the participants had experienced PPH. The majority of women, 256 (90.8%) individuals, had at least one ANC visit during pregnancy, with 62.9% of them having at least 4. Most (98.2%) of the women had delivered at a health facility. A total of 209 (74.1%) had a vaginal delivery (Table 2).
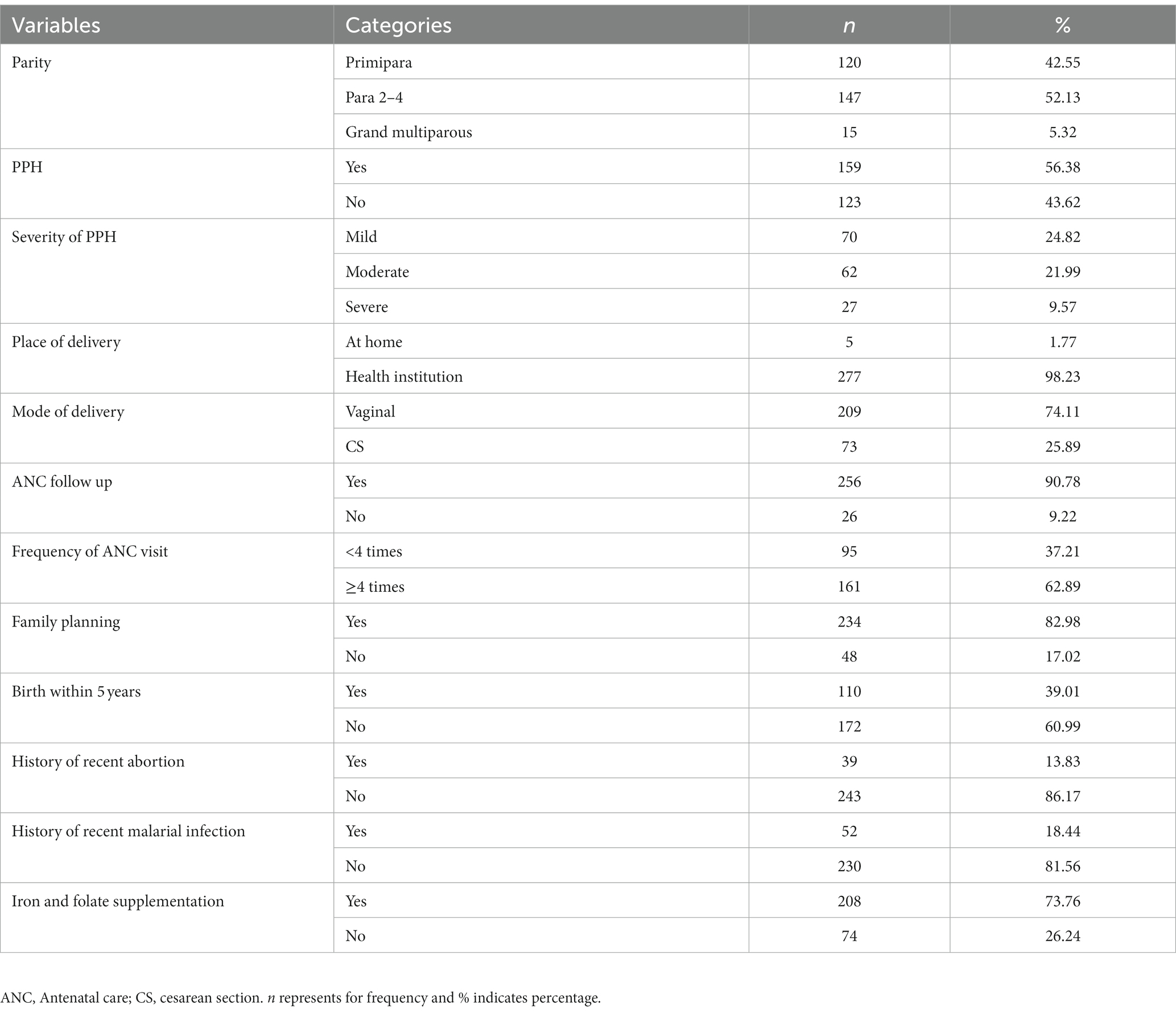
Table 2. Obstetric and clinical characteristics of postnatal women in Gondar town, Northwest Ethiopia, 2021 (n = 282).
Dietary characteristics
More than half of the women (150, or 53.2%) had low dietary diversity. A total of 114 (40.4%) women ate meat at least once per week. More than half of the women (158, or 56%) consumed fruit and vegetables at least once a day. A total of 229 women (81.2%) had the habit of drinking coffee; 183 of them (79.9%) preferred to do so 30 min after food consumption (Table 3).
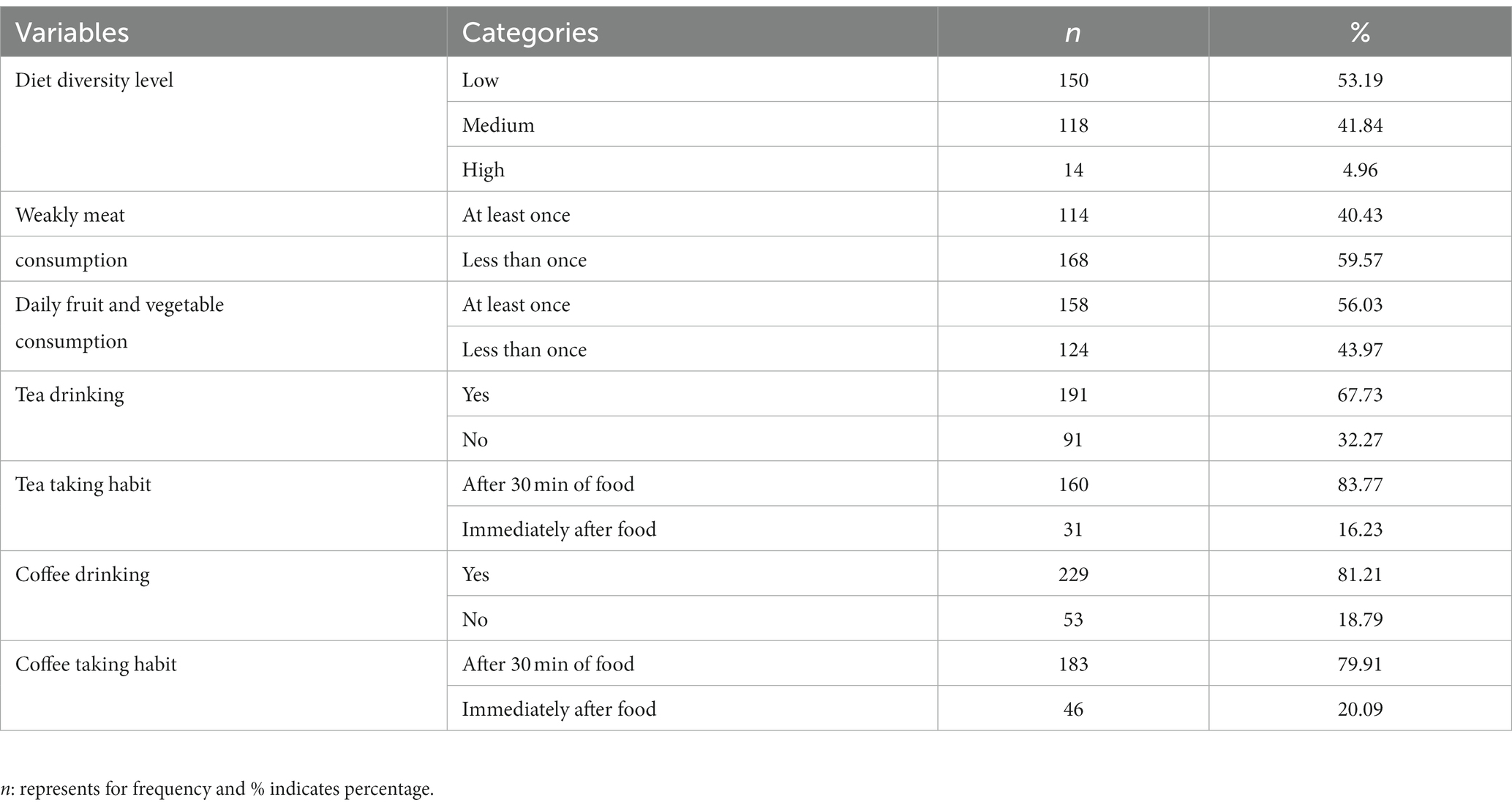
Table 3. Nutritional characteristics of postnatal women in Gondar town, Northwest Ethiopia, 2021 (n = 282).
Laboratory findings
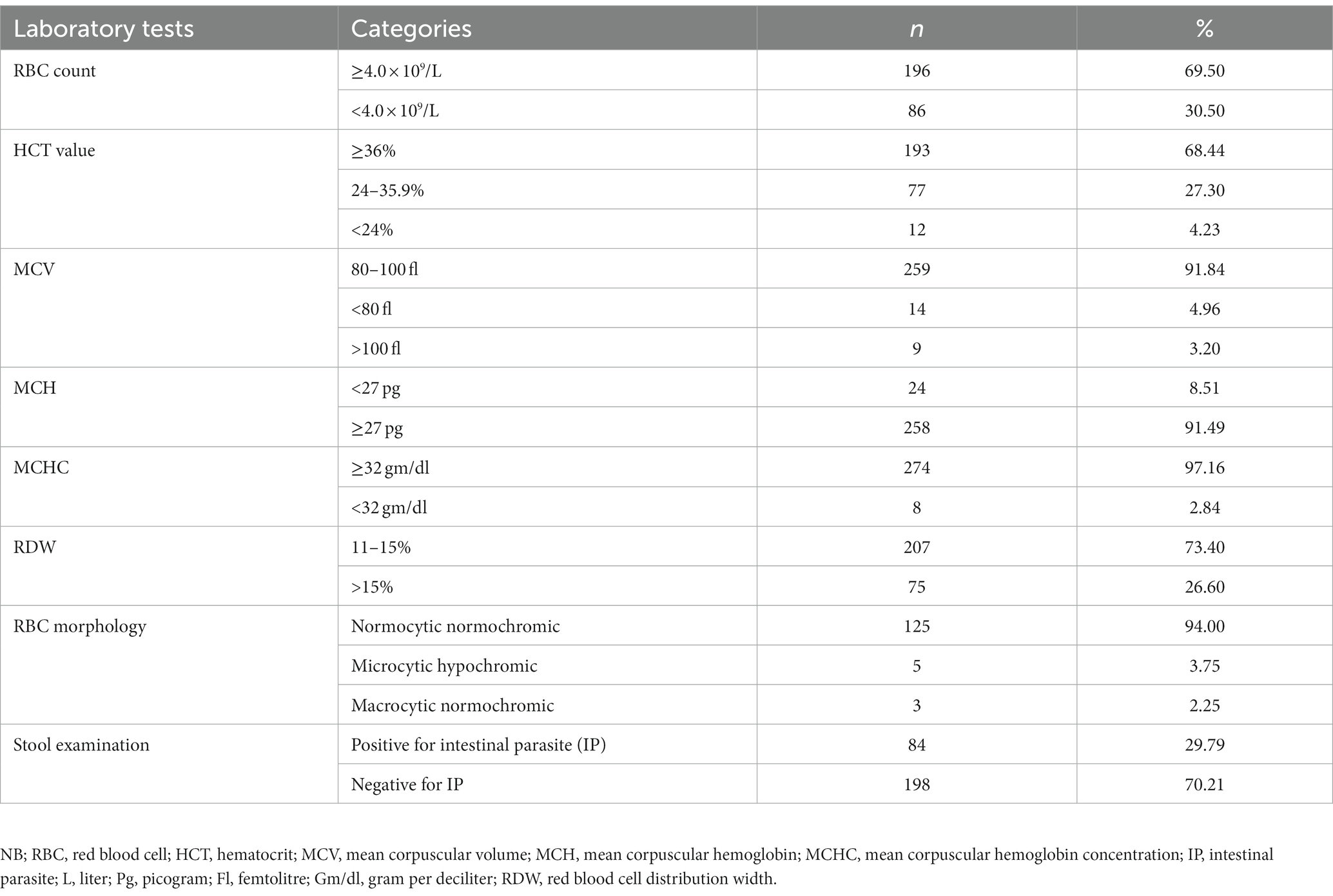
The median adjusted Hb value of the women was 12.1 gm/dl (IQR: 10.8–13.1 gm/dl). A total of 126 (69.5%) women had an RBC count ≥4.0 × 109/l with a mean value of 4.21 ± 0.78 × 109/l. The median HCT value was 38.3% (IQR = 34.8–41.5%) with 68.4% ≥ 36%. The result of the stool examination showed that 84 (29.8%) of them were positive for at least one IP. A. lumbercoid was the predominant one, with a proportion of 46.4% (Table 4 and Figure 2).
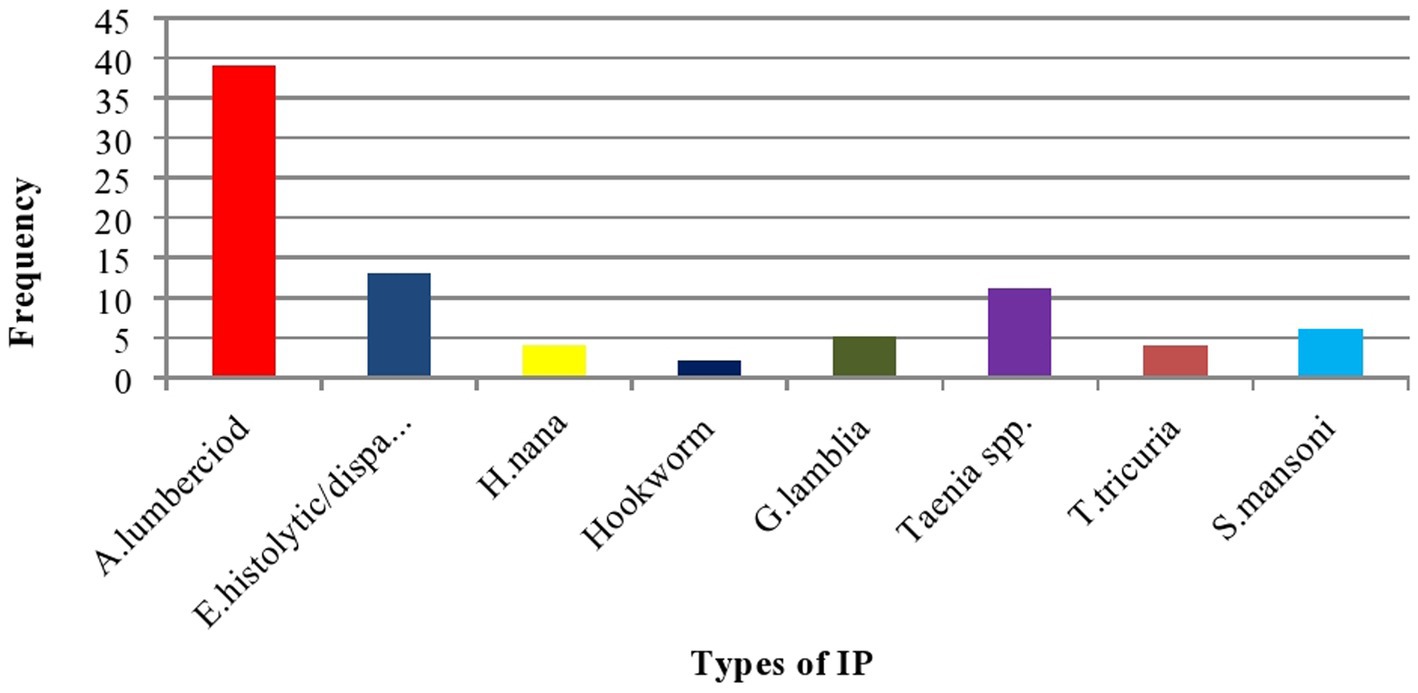
Figure 2. Bar graph showing the type of identified IP among postnatal women in Gondar town, Northwest Ethiopia, 2021 (n = 282).
Prevalence, severity, and morphologic characteristics of PPA
Prevalence and severity: The overall prevalence of PPA anemia was 133 (47.16%; 95% CI: 41.30–53.03). Individuals with anemia were further classified into three categories: mild anemia (57, or 42.86%), moderate anemia (60 or 45.11%), and severe anemia (16, or 12.03%) (Figure 3).
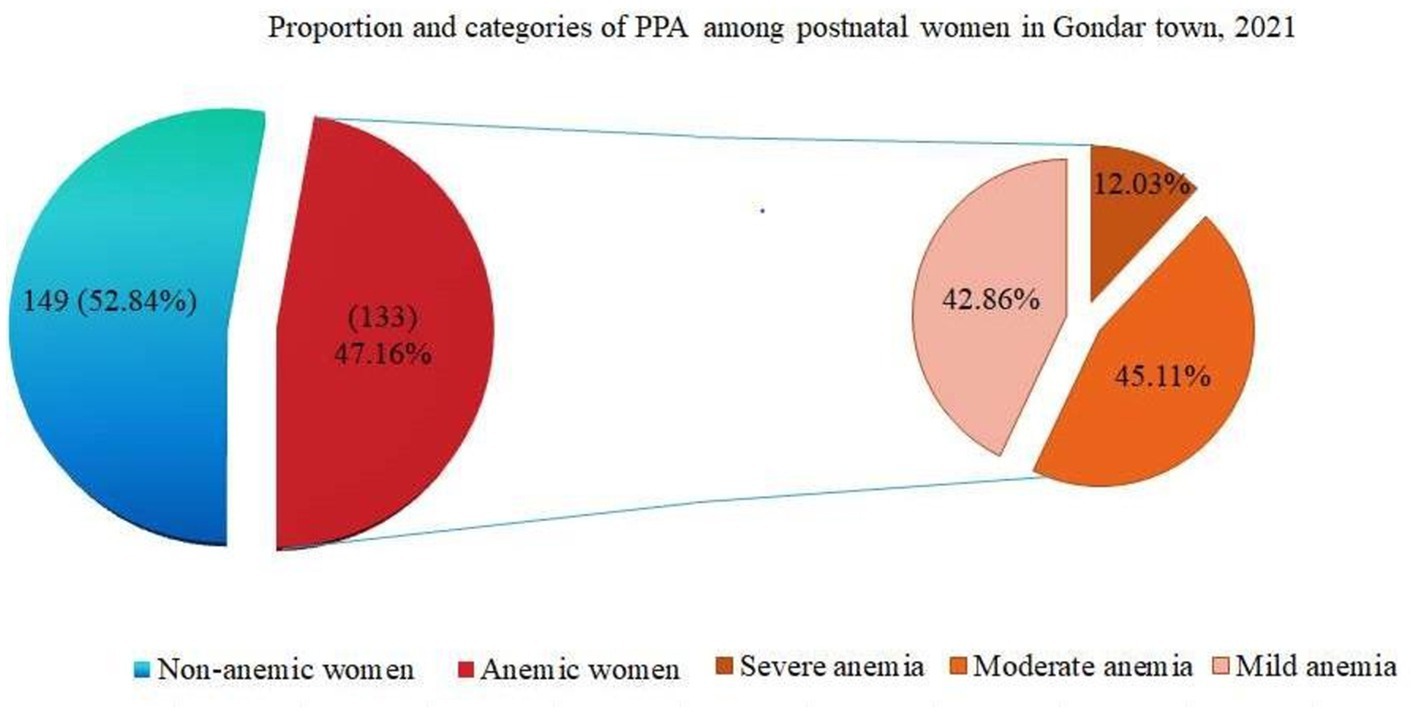
Figure 3. Pie of pie chart showing proportion and severity of PPA among postnatal women in Gondar town, 2021 (n = 282).
Morphological characteristics of PPA
Based on the blood film reports, out of the total of 133 anemic women, the majority (125, or 94%) had a normocytic, normochromic blood picture. A total of 5 (3.76%) had a microcytic hypochromic blood picture, while 3 (2.24%) had a macrocytic blood picture (Table 4).
Determinants of PPA in postpartum women
In univariate analysis, all variables were insignificant except PPH, mode of delivery, iron and folate supplementation during pregnancy, and level of dietary diversity. Women who experienced PPH (AOR = 2.23; 95% CI: 1.24–4.01), delivered via CS (AOR = 4.10; 95% CI: 2.11–7.78), did not take iron and folate supplementation during pregnancy (AOR = 2.12; 95% CI: 1.17–4.02) and had a low dietary diversity level (AOR = 1.83; 95% CI: 1.05–3.18) were at higher risk of PPA (Table 5).
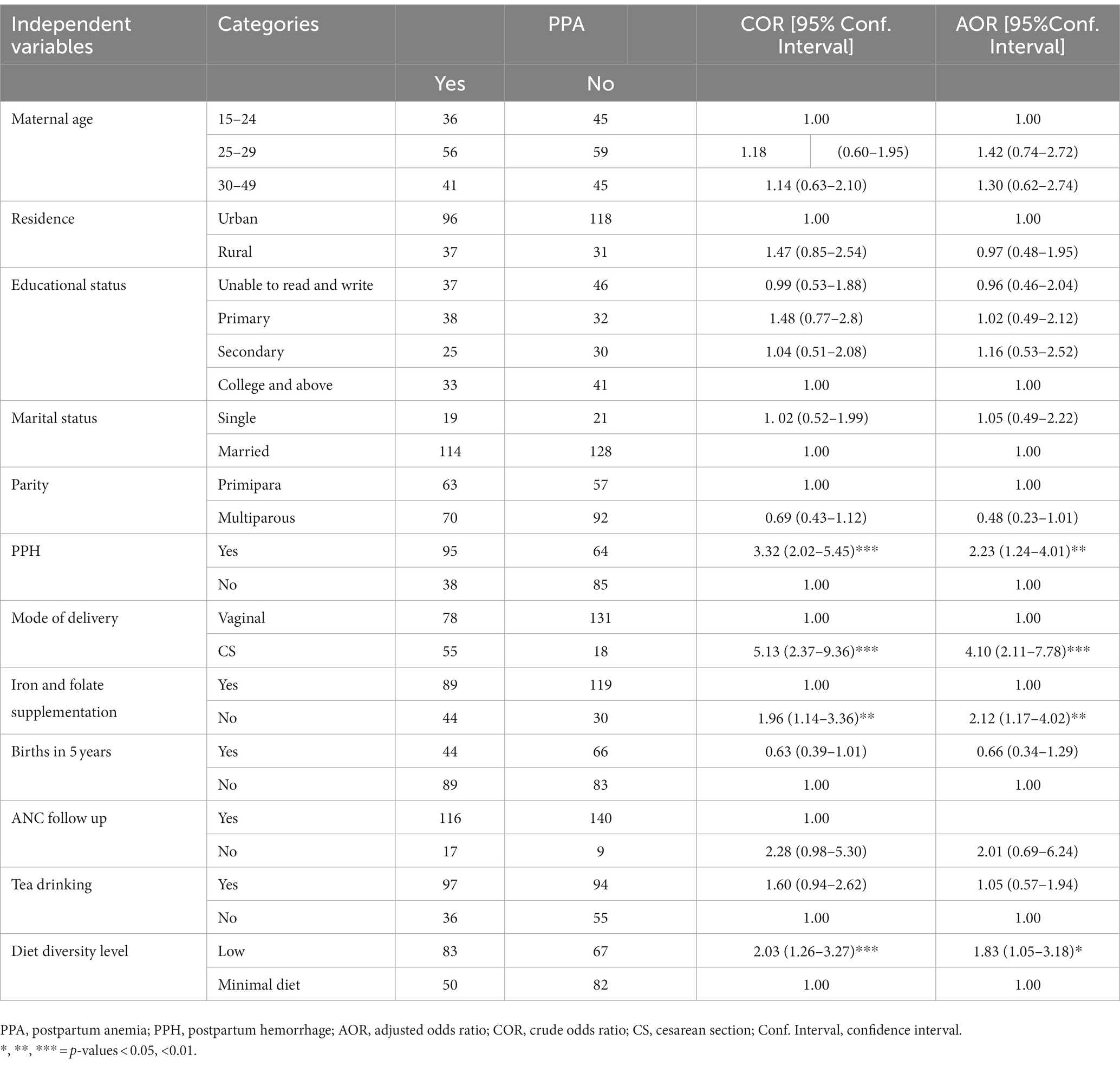
Table 5. Logistic regression showing factors associated with PPA in Gondar town, Northwest Ethiopia, 2021 (n = 282).
Discussion
A number of factors have been associated with PPA. The change in Hb during the postpartum period would play an important role in providing baseline information. Thus, the current study aimed to assess the magnitude and factors associated with PPA among postnatal women in two selected health facilities in Gondar.
In this study, the prevalence of anemia among postnatal women was 47.16% (95% CI: 41.30–53.03). The finding was consistent with the results of studies conducted in southern India (47.3%) (35) and southeastern Kokang, Myanmar (56.3%) (36). In terms of severity, moderate anemia (45.11%) was common in this study. This finding was in line with a similar study conducted in India, which found 49.8% of moderate anemia types and 26% of mild anemia types (37).
On the other hand, the prevalence was lower than in studies conducted in Asia [eastern rural Myanmar (60.3%) (38) and India (76.2%; 95% CI; 70.4–81.4%) (38)]. This difference may be due to teff injera, a staple food consumed by most Ethiopian mothers (39), which contains more iron. In addition, it could be the coexistence of numerous contributing factors in rural Myanmar women like low family income, lack of primary education, hunger, drinking spring or river water, or drinking unboiled water, which could lead to anemia (38). It could be due to the fact that women in rural areas have poor socioeconomic status and, therefore, do not have access to iron-rich foods (40). Instead, given that the postpartum period is characterized by accelerated erythropoiesis and RBC mass expansion, it is possible that the hemodilution effect subsides and Hb levels return to normal in the participants in this study (35). Another reason for the discrepancy could be the difference in sample sizes, which were 227 and 733 in the India and Myanmar studies, respectively (38, 41).
The prevalence reported from the current study was higher than studies conducted in Japan (10.5%) (42), Spain, Mancha-Centro Hospital (16.4%) (22), northern Kenya (25%) (43), Ghana (16%) (44), Bhaktar, Nepal (20%) (45), Ethiopia – in Tigray (16.5%) (46), Sidama (19%) (47), Addis Ababa – at the Tikur Anbessa Specialized and Gandhi Memorial Hospitals (30%) (48), and in Debre Markos (24.3%) (30). The disparity may be due to differences in the geographical, cultural, clinical, and nutritional factors of women. In addition, different cutoff points were used to define PPA. Since there is no universally accepted definition of PPA, different researchers use different cutoff points, such as Hgb < 11 mg/dl at 24 h in Spain [Mancha-Centro Hospital (22) and in Debre Markos (30)]. In Madrid, Hgb < 10 mg/dl is used to indicate PPA for up to 6 weeks (49). In addition, there may be a difference in postpartum screening time. If the postpartum period is extended, mothers will have more time to recover from anemia or have their Hb levels increase (30). Iron is expected to improve in lactating women due to decreased iron requirements and reduced blood loss associated with amenorrhea (50).
According to RBC morphological findings, the majority of anemic women had a normocytic normochromic blood picture (94%), followed by a microcytic hypochromic (3.24%) and a macrocytic blood picture (2.24%). Possible causes include acute bleeding, or early iron deficiency (51).
Postpartum hemorrhage, iron and folate supplementation during pregnancy, CS, and low dietary diversity were found to be significantly associated with PPA. In this study, the prevalence of anemia was found to increase in women who experienced PPH. The odds of PPA were 2.2 times higher among women who had experienced PPH (AOR = 2.23; 95% CI: 1.24–4.01) than their counterparts. Probably, uterine atony, uterine inversion, coagulopathy, vaginal tears, uterine laceration, and retained tissue or placenta may expose women to cycles of bleeding, which can lead to Hb declines. The majority of studies showed Hb and HCT declines in the context of overt PPH. Excessive blood loss during and after delivery, in addition to insufficient erythropoiesis, may also cause a drop in Hb throughout the postpartum period (35).
Compared to mothers who gave birth via the vaginal route, mothers who gave birth via CS had four times higher odds of anemia (AOR = 4.10; 95% CI: 2.11–7.78). The findings were in agreement with a similar study conducted in Tigray, which discovered that vaginal birth was associated with a lower risk of anemia (AOR = 0.13; 95% CI: 0.038–0.454) (52). This potentially traumatic surgical procedure could cause women to suffer a major hemorrhage, leading to PPH. Surgery is a key determinant of blood loss. Postoperative anemia, defined as a blood loss of more than 500 ml, is a common complication that affects 80–90% of people who have had major surgery (53).
The results of this study also suggested that women who were not supplemented with iron and folate during their pregnancy had twice as many chances of developing PPA as those who were (AOR = 2.12; 95% CI: 1.17–4.02). This conclusion was supported by studies from India (AOR = 3.53; 95% CI: 1.18–11.37) and Ethiopia (35, 54). The possible explanation would be that iron is the most important nutrient for hematopoiesis and that, when taken throughout pregnancy, it has the capacity to reduce anemia, even during delivery. The depletion of stored iron during pregnancy may also be a factor because of the high demand for iron during childbirth (30).
Women with a low level of dietary diversity were 83% more likely to suffer from anemia than those with a minimum level of dietary diversity (AOR = 1.83; 95% CI: 1.05–3.18). This finding was consistent with a study conducted among lactating women in Jimma District (AOR = 2.32; 95% CI: 1.65–5.72) (24). This could be due to inadequate dietary intake, leading to an iron, vitamin B12, folate, and vitamin A deficiency. Another reason could be a lack of protein and iron-containing foods such as eggs and meat, which could lead to an iron deficiency (36).
Many studies from Ethiopia and abroad discovered that inadequate and missed ANC follow-ups, multiparity, and low education levels were independent predictors of PPA (24, 54–58). However, these factors were not associated with PPA in this study.
Strengths and limitations
The women’s hemoglobin concentration was adjusted for altitude. To make the sampling more representative, the probability sampling technique was used. Furthermore, the study estimates were done with the appropriate statistical analysis. As a result, we are confident that this study provides more precise and generalizable results that policymakers and program managers may use to develop action strategies for this issue.
Furthermore, anemia has been associated with antepartum hemorrhage (30), but this association has not been investigated. Due to budgetary constraints, we were unable to measure anemia indicators for further categorization. As a result, we were unable to identify the type of anemia.
Conclusion and recommendations
The prevalence of PPA in this study was a major public health concern. One in two postnatal women was found to be anemic with an adjusted Hb concentration below 12 g/dl. Iron and folate supplementation and the administration of uterotonics such as oxytocin during the third stage of labor will prevent PPH and PPA. To reduce the burden of anemia among postnatal women, health education and promotion of iron and folate supplements during pregnancy, in addition to dietary diversity practices, need to be combined with women’s long-term income-generating activities. Anemia in women who have had cesarean deliveries may also be avoided by efficient CS delivery, a positive long-term health outlook following CS, and postoperative monitoring. Thus, due attention must be given to reduce the magnitude of PPA through effective antepartum, intrapartum, and postpartum maternal care. In addition, further research is required to address the limitations of this study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethical approval and consent to participate. The research was conducted after ethical clearance was secured with reference number of SBMLS/2747 from Ethical Review Committee of School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. In addition, permission was obtained from each selected health institution. Moreover, informed consent was secured from each study participants and the obtained data were strictly confidential. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
GB conceived the idea, wrote the proposal, performed the data analysis and interpretation, wrote the initial draft, and revised subsequent drafts. MM and ES provided advice on study design, data analysis, and interpretation, and reviewed and commented on subsequent drafts. SK and CS participated in data collection and laboratory processing, and assisted in drafting the manuscript. MM and ES revised and edited the manuscript. All authors approved the submitted version of the manuscript.
Funding
Funding was obtained from the University of Gondar, College of Medicine and Health Sciences.
Acknowledgments
We thank the University of Gondar for its financial support for the completion of this study. We would like to thank the study participants for being part of the study. We acknowledge the data collectors for providing accurate data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANC, Antenatal Care; AOR, Adjusted Odds Ratio; BMI, Body Mass Index; CI, Confidence Interval; COR, Crude Odds Ratio; CS, Cesarean Section; DDS, Dietary Diversity Score; FAO, Food and Agricultural Organization; G/dl, gram per deciliter; Hb, hemoglobin; HCT, hematocrit; HIV, human immunodeficiency virus; IDA, iron deficiency anemia; IP, intestinal parasite; IQR, interquartile range; K3-EDTA, tri-potassium ethylene diamine tetra-acetic acid; KM, kilometer; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; MCV, mean corpuscular volume; Ml, milliliter; PNC, postnatal care; PPA, postpartum anemia; PPH, postpartum hemorrhage; QC, quality control; RBC, red blood cell; RDW, red cell distribution width; RPM, revolutions’ per minute; UGCSH, University of Gondar Comprehensive Specialized Hospital; WHO, World Health Organization.
References
1. Milman, N. Postpartum anemia: definition, prevalence, causes, and consequences. Ann Hematol. (2011) 90:1247–53. doi: 10.1007/s00277-011-1279-z
2. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. (2011). Available at: https://www.who.int/vmnis/indicators/haemoglobin.pdf
3. Dutta, D. Perinatology and contraception. Text book of obstetrics. 7th, Jaypee Brothers Medical Publishers (P) Ltd. (2013). 148–151.
4. Elaine Keohane, CO, and Walenga, J. Clinical principles and applications In: Rodak’s Hematology. 6th ed: Elsevier, My Evolve (2019). 252–61.
5. Chaparro, CM, and Suchdev, PS. Anemia epidemiology, pathophysiology, and etiology in low-and middle-income countries. Ann N Y Acad Sci. (2019) 1450:15–31. doi: 10.1111/nyas.14092
6. World Health Organization. Nutritional anaemias: Tools for effective prevention and control. (2017) 1–6.
7. Antwi-Bafour, S, Hammond, S, Adjei, JK, Kyeremeh, R, Martin-Odoom, A, and Ekem, I. A case–control study of prevalence of anemia among patients with type 2 diabetes. J Med Case Rep. (2016) 10:110–8. doi: 10.1186/s13256-016-0889-4
8. Klinkenberg, EF, Huis In’t Veld, EM, De Wit, PD, De Kort, WL, and Fransen, MP. Barriers and motivators of Ghanaian and African-Surinamese migrants to donate blood. Health Soc Care Community. (2019) 27:748–56. doi: 10.1111/hsc.12692
9. Baig, M, Habib, H, Haji, AH, Alsharief, FT, Noor, AM, and Makki, RG. Knowledge, misconceptions and motivations towards blood donation among university students in KSA. Pak J Med Sci. (2013) 29:1295–9. doi: 10.12669/pjms.296.4137
10. Stevens, GA, Finucane, MM, de-Regil, LM, Paciorek, CJ, Flaxman, SR, Branca, F, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. (2013) 1:e16–25. doi: 10.1016/S2214-109X(13)70001-9
11. Woldu, B, Enawgaw, B, Asrie, F, Shiferaw, E, Getaneh, Z, and Melku, M. Prevalence and associated factors of anemia among reproductive-aged women in Sayint Adjibar town, Northeast Ethiopia: community-based cross-sectional study. Anemia. (2020) 2020:e8683946:1–8. doi: 10.1155/2020/8683946
12. Yadeta, TA, Mengistu, B, Gobena, T, and Regassa, LD. Spatial pattern of perinatal mortality and its determinants in Ethiopia: data from Ethiopian demographic and health survey 2016. PLoS One. (2020) 15:e02424499. doi: 10.1371/journal.pone.0242499
13. Kassebaum, NJ, Jasrasaria, RN, Naghavi, M, Wulf, SK, Johns, N, Lozano, R, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. (2014) 123:615–24. doi: 10.1182/blood-2013-06-508325
14. Kim, M, Hong, M, Kim, JY, Kim, IS, Yu, HT, Kim, TH, et al. Clinical relationship between anemia and atrial fibrillation recurrence after catheter ablation without genetic background. IJC Heart Vasc. (2020) 27:100507. doi: 10.1016/j.ijcha.2020.100507
15. Förster, J, Peltonen, S, Kaaja, R, Lampinen, K, and Pettilä, V. Plasma exchange in severe postpartum HELLP syndrome. Acta Anaesthesiol Scand. (2002) 46:955–8. doi: 10.1034/j.1399-6576.2002.460805.x
16. Galambosi, PJGM, Kaaja, RJ, and Ulander, VM. Incidence and risk factors of venous thromboembolism during postpartum period: a population-based cohort-study. Acta Obstet Gynecol Scand. (2017) 96:852–61. doi: 10.1111/aogs.13137
17. Axelsson, D, Brynhildsen, J, and Blomberg, M. Postpartum infection in relation to maternal characteristics, obstetric interventions and complications. J Perinat Med. (2018) 46:271–8. doi: 10.1515/jpm-2016-0389
18. Liyew, AMKS, Kebede, SA, Agegnehu, CD, Teshale, AB, Alem, AZ, Yeshaw, Y, et al. Spatiotemporal patterns of anemia among lactating mothers in Ethiopia using data from Ethiopian demographic and health surveys (2005, 2011 and 2016). PLoS One. (2020) 15:e0237147. doi: 10.1371/journal.pone.0237147
19. Gonzalo-Carballes,, Ríos-Vives, MÁ, Fierro, EC, Azogue, XG, Herrero, SG, Rodríguez, AE, et al. A pictorial review of postpartum complications. Radiographics. (2020) 40:2117–41. doi: 10.1148/rg.2020200031
20. Atukunda, E, Mugyenyi, OC, Obua, C, Atuhumuza, EB, Musinguzi, N, Tornes, YF, et al. Measuring post-partum haemorrhage in low-resource settings: the diagnostic validity of weighed blood loss versus quantitative changes in hemoglobin. PLoS One. (2016) 11:e0152408. doi: 10.1371/journal.pone.0152408
21. Chauhan, G, and Tadi, P. Physiology, postpartum changes. Treasure Island, FL: StatPearls Publishing (2021).
22. Urquizu, X, Rodriguez, M, Fernández, AG, and Picañol, EP. Anaemia in pregnancy and in the immediate postpartum period, prevalence and risk factors in pregnancy. Childbirth Med Clín. (2016) 146:429–35. doi: 10.1016/j.medcli.2016.01.029
23. Kibret, Kelemu Tilahun, Chojenta, Catherine, and D’Arcy, Ellie, Deborah Loxton. Population attributable fraction estimates for factors associated with different types of anaemia among women in Ethiopia: Multilevel multinomial analysis. Cambridge University Press. (2020) (6):1–11.
24. Alemayehu, MAA, and Mariam, AG. Factors associated with malnutrition among lactating women in subsistence farming households from Dedo and Seqa-Chekorsa districts, Jimma zone. Dev Ctry Stud. (2015) 5:117–8.
25. Say, L, Chou, D, Gemmill, A, Tunçalp, Ö, Moller, A-B, Daniels, J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. (2014) 2:e323–33. doi: 10.1016/S2214-109X(14)70227-X
26. WHO. Global Nutrition Targets 2025. AnaemiaPolicy brief. Geneva: World Health Organization. 2014 p.
27. Gaspar, BL, Sharma, P, and Das, R. Anemia in malignancies: pathogenetic and diagnostic considerations. Hematology (Amsterdam, Netherlands). (2015) 20:18–25. doi: 10.1179/1607845414Y.0000000161
28. Gondar, Ethiopia Metro Area Population 1950-2021 (2020). Available at: https://www.macrotrends.net/cities/20926/gondar/population.
29. Population EOot, Commission HC. Summary and statistical report of the 2007 population and housing census: population size by age and sex Federal Democratic Republic of Ethiopia Population Census Commission (2008). Open Africa https://africaopendata.org/dataset/ethiopia-summary-and-statistical-report-of-the-2007-population-and-housing-census
30. Abebaw, AG, Worku, T, and Bayew, K. Proportion of immediate postpartum anaemia and associated factors among postnatal mothers in Northwest Ethiopia: a cross-sectional study. Anemia. (2020) 2020:1–10. doi: 10.1155/2020/8979740
31. Sharma, AJ, Addo, OY, Mei, Z, and Suchdev, PS. Reexamination of hemoglobin adjustments to define anemia: altitude and smoking. Ann N Y Acad Sci. (2019) 1450:190–203. doi: 10.1111/nyas.14167
32. The Food and Agriculture Organization of the United Nations and USAID’s Food and Nutrition Technical Assistance III Project (FANTA) mbF. Minimum dietary diversity for women a guide to measurement. Rome: University of California (2016).
33. Gonete, KA, Tariku, A, Wami, SD, and Akalu, TY. Dietary diversity practice and associated factors among adolescent girls in Dembia district, Northwest Ethiopia, 2017. Public Health Rev. (2020) 41:23–3. doi: 10.1186/s40985-020-00137-2
34. Gómez, G, Nogueira Previdelli, Á, Fisberg, RM, Kovalskys, I, Fisberg, M, Herrera-Cuenca, M, et al. Dietary diversity and micronutrients adequacy in women of childbearing age: results from ELANS study. Nutrients. (2020) 12:1994. doi: 10.3390/nu12071994
35. Rakesh, PGV, Jamkhandi, D, Manjunath, K, George, K, and Prasad, J. Determinants of postpartum anemia among women from a rural population in southern India. Int J Women’s Health. (2014) 6:395–400. doi: 10.2147/IJWH.S58355
36. Zhang, Y. Anemia among lactating mothers in Kokang, Myanmar. Southeast Asian J Trop Med Public Health. (2016) 47:1298–305.
37. Selvaraj, R, Ramakrishnan, J, Sahu, S, Kar, SS, Laksham, KB, Premarajan, KC, et al. High prevalence of anemia among postnatal mothers in urban Puducherry: a community-based study. J Fam Med Prima Care. (2019) 8:2703–7. doi: 10.4103/jfmpc.jfmpc_386_19
38. Gao, H, Zhang, Y, Wang, P, Xue, Y, Zhao, A, Li, B, et al. Prevalence of anemia and its risk factors among lactating mothers in Myanmar. Am J Trop Med Hyg. (2014) 90:963–7. doi: 10.4269/ajtmh.13-0660
39. Lo, E, Yewhalaw, D, Zhong, D, Zemene, E, Degefa, T, Tushune, K, et al. Molecular epidemiology of plasmodium vivax and plasmodium falciparum malaria among Duffy-positive and Duffy-negative populations in Ethiopia. Malar J. (2015) 14:84. doi: 10.1186/s12936-015-0596-4
40. Addis Alene, K, and Mohamed, DA. Prevalence of anemia and associated factors among pregnant women in an urban area of eastern Ethiopia. Anmeia. (2014) 2014:1–8. doi: 10.1155/2014/561567
41. Das, MK, Singh, SS, Adak, T, Vasantha, K, and Mohanty, D. The Duffy blood groups of Jarawas - the primitive and vanishing tribe of Andaman and Nicobar Islands of India. Transfus Med (Oxford, England). (2005) 15:237–40. doi: 10.1111/j.1365-3148.2005.00583.x
42. Amano, I, and Murakami, A. Prevalence of infant and maternal anemia during the lactation period in Japan. Pediatr Int. (2019) 61:495–503. doi: 10.1111/ped.13833
43. Fujita, M, and Wander, K. A test of the optimal iron hypothesis among breastfeeding Ariaal mothers in northern Kenya. Am J Phys Anthropol. (2017) 164:586–97. doi: 10.1002/ajpa.23299
44. Kofie, PTE, Manu, E, Amu, H, Ayanore, MA, Aku, FY, Komesuor, J, et al. Prevalence and associated risk factors of anaemia among women attending antenatal and post-natal clinics at a public health facility in Ghana. BMC Nutr. (2019) 5:40. doi: 10.1186/s40795-019-0303-x
45. Chandyo, RK, Henjum, S, Ulak, M, Thorne- Lyman, AL, Ulvik, RJ, Shrestha, PS, et al. The prevalence of anemia and iron deficiency is more common in breastfed infants than their mothers in Bhaktapur Nepal. Eur J Clin Nutr. (2016) 70:456–62. doi: 10.1038/ejcn.2015.199
46. Wonde, TEMA. Maternofetal outcomes of obstructed labor among women who gave birth at general hospital in Ethiopia. BMC Res Notes. (2019) 12:1–5. doi: 10.1186/s13104-019-4165-8
48. Adere, A, Mulu, A, and Temesgen, F. Neonatal and maternal complications of placenta Praevia and its risk factors in Tikur Anbessa specialized and Gandhi memorial hospitals: unmatched case-control study. J Pregnancy. (2020) 2020:1–9. doi: 10.1155/2020/5630296
49. Medina Garrido, CLJ, and Romaní, VA. Maternal anaemia after delivery: prevalence and risk factors. J Obstet Gynaecol. (2018) 38:55–9. doi: 10.1080/01443615.2017.1328669
50. Akesson, A, Bjellerup, P, Berglund, M, Bremme, K, and Vahter, M. Soluble transferrin receptor: longitudinal assessment from pregnancy to postlactation. Obstet Gynecol. (2002) 99:260–6. doi: 10.1097/00006250-200202000-00017
51. Yan, G, Yewhalaw, D, Lo, E, Singh, SK, Hora, R, Belrhali, H, et al. Structural basis for Duffy recognition by the malaria parasite Duffy-binding-like domain. PLoS Negl Trop Dis. (2006) 439:741–4.
52. Gai, PP, van Loon, W, Siegert, K, Wedam, J, Kulkarni, SS, Rasalkar, R, et al. Duffy antigen receptor for chemokines gene polymorphisms and malaria in Mangaluru, India. Malar J. (2019) 18:328. doi: 10.1186/s12936-019-2966-9
53. Mosieri, C, Hyatali, F, Kallurkar, A, and Cornett, EM. Managing preoperative anemia: evolving concepts and strategies for improving patient outcomes. Best Pract Res Clin Anaesthesiol. (2020) 34:183–97. doi: 10.1016/j.bpa.2020.04.005
54. Gonzalez, L, Vega, J, Ramirez, JL, Bedoya, G, Carmona-Fonseca, J, and Maestre, A. Relationship between genotypes of the Duffy blood groups and malarial infection in different ethnic groups of Choco, Colombia. Colombia Medica (Cali, Colombia). (2012) 43:189–95. doi: 10.25100/cm.v43i3.933
55. Nankinga, OAD. Determinants of anemia among women in Uganda: further analysis of the Uganda demographic and health surveys. BMC Public Health. (2019) 19:1–9. doi: 10.1186/s12889-019-8114-1
56. Feleke, BEFT. Pregnant mothers are more anemic than lactating mothers, a comparative cross-sectional study, Bahir Dar, Ethiopia. BMC Hematol. (2018) 18:2. doi: 10.1186/s12878-018-0096-1
57. Sellami, MH, Kaabi, H, Midouni, B, Dridi, A, Mojaat, N, Boukef, MK, et al. Duffy blood group system genotyping in an urban Tunisian population. Ann Hum Biol. (2008) 35:406–15. doi: 10.1080/03014460802082127
Keywords: determinant factors, postpartum anemia, postnatal women, Ethiopia, postpartum hemarrhage
Citation: Bambo GM, Kebede SS, Sitotaw C, Shiferaw E and Melku M (2023) Postpartum anemia and its determinant factors among postnatal women in two selected health institutes in Gondar, Northwest Ethiopia: A facility-based, cross-sectional study. Front. Med. 10:1105307. doi: 10.3389/fmed.2023.1105307
Edited by:
Sanghamitra Pati, Regional Medical Research Center (ICMR), IndiaReviewed by:
Nihar Ranjan Mishra, All India Institute of Medical Sciences, Kalyani (AIIMS Kalyani), IndiaAfra Alkan, Ankara Yıldırım Beyazıt University, Türkiye
Copyright © 2023 Bambo, Kebede, Sitotaw, Shiferaw and Melku. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Getachew Mesfin Bambo,Z21lc2ZpbjAwN0BnbWFpbC5jb20=; Z2V0YWNoZXdtZXNmaW5AbXR1LmVkdS5ldA==
 Getachew Mesfin Bambo
Getachew Mesfin Bambo Samuel Sahile Kebede1,2
Samuel Sahile Kebede1,2 Elias Shiferaw
Elias Shiferaw Mulugeta Melku
Mulugeta Melku