
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 06 March 2023
Sec. Ophthalmology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1085280
This article is part of the Research TopicAddressing the Unmet Needs of Cataract Patients: When Quality of Vision Can Make the Difference in Quality of LifeView all 10 articles
 Emilio Pedrotti†
Emilio Pedrotti† Erika Bonacci†
Erika Bonacci† Raphael Kilian
Raphael Kilian Camilla Pagnacco*
Camilla Pagnacco* Marco Anastasi
Marco Anastasi Mariacarmela Ventura
Mariacarmela Ventura Giorgio Marchini
Giorgio MarchiniPurpose: To analyze the objective and subjective visual performances of a new hybrid refractive/aspheric extended depth of focus (EDOF) intraocular lens (IOL).
Methods: In this monocentric prospective study patients with bilateral cataracts underwent cataract surgery and were implanted with a Lucidis IOL (SAV-IOL SA, Neuchâtel, Switzerland) in both eyes, 1 week apart from each other. At 3 months from implantation postoperative evaluations included monocular and binocular uncorrected and distance-corrected distant (4 m), intermediate (80 cm, 67 cm) and near (40 cm) visual acuities (UDVA/DCVA, UI80-67VA/DCI80-67VA, UNVA/DCNVA), binocular defocus curves, halometry, contrast sensitivity and objective quality-of-vision measurements. Also, patients were also asked to complete the national eye institute refractive error quality of life (NEI-RQL-42) questionnaire.
Results: Twenty-five patients (50 eyes) were included. The mean postoperative binocular UDVA, UI80VA, UI67VA and UNVA were-0.02 ± 0.13, 0.05 ± 0.09, 0.05 ± 0.08 and 0.03 ± 0.1 LogMar, and did not significantly differ from their corrected counterparts. On binocular defocus curves a VA ≥0.05LogMar was found between +0.50 and − 2.50 D of vergence, whereas the mean distance from the central stimulus on halometry was 1.23 ± 0.01. Mean ocular and corneal radical mean square at 4 mm were 0.31 ± 0.28 and 0.19 ± 0.07, respectively; whereas the mean Strehl ratio was 0.2 ± 0.09.
Conclusion: Lucidis IOLs demonstrated excellent visual performances, especially at close distances while maintain good quality of vision, contrast sensitivity, and overall patient-satisfaction.
In the last decades premium multifocal intraocular lenses (MFIOL) have been designed to meet the patients’ need for spectacle independence, however, these lenses frequently led to a bad quality of vision, especially at near and/or far distances. Other issues that have emerged through the years with these lenses consisted in the decrease of both contrast sensitivity and night vision, as well as in the frequent manifestation of visual phenomena such as halos, glare and starburst (especially with diffractive MFIOLs) (1–4). Recently, the need to overcome these concerns has led to the development of new technologies able to generate a single focal point with an extended depth of focus (EDOF). While improving far- and intermediate-distance spectacle independence, EDOF-IOLs are also said to be able to induce fewer visual phenomena (5, 6). However, these lenses are also known for the need of a small amount of positive spectacle correction at close distances (7).
The Lucidis IOL (Swiss Advanced Vision, SAV-IOL SA, Neuchâtel, Switzerland) is a new special hybrid refractive/aspheric EDOF IOL that has been created to overcome the limitation of near vision. However, until now only few studies have analyzed the outcomes of this lens and none of these has examined neither the defocus curve, nor the objective visual quality (8–10). The aim of this study was to examine the visual performances of the Lucidis IOL focusing on near vision, defocus curves, subjective and objective quality of vision and on the patient’s satisfaction 3 months after the surgery.
This prospective interventional monocentric study adhered to the tenets of the Declaration of Helsinki and was approved by the local Ethics Committee (protocol 54,139). A written informed consent was obtained from all participating subjects after thorough explanation of the benefits and the risks related to the implantation of the IOL in study.
Inclusion criteria were the presence of significant bilateral cataracts, defined by a preoperative corrected distance visual acuity (DCVA) of 0.20 logMAR (20/32 Snellen) or worse, availability to undergo both surgeries 1 week apart from each other, an axial length between 22 and 23 mm and a preoperative regular corneal astigmatism of less than 1.00 diopter (D). We excluded patients younger than 18, those with any other concomitant or previous ocular disease, irregular astigmatism and those who had undergone previous ocular surgeries. Patients that had experienced intraoperative complications were excluded from the final analysis.
All patients underwent a thorough ophthalmological examination before surgery and 3 months after IOL implantation. The preoperative evaluation included measurement of monocular and binocular uncorrected and distance-corrected distant and near visual acuity (UDVA/DCVA at 4 m and UNVA/DCNVA at 40 cm, respectively) using the CSO Vision Charts V14.0 (CSO, Florence, Italy), measurement of the subjective refractive error, corneal tomography (MS-39, CSO, Firenze, Italy), optical biometry (Lenstar 900; Haag-Streit Diagnostics, Koeniz, Switzerland), Goldmann applanation tonometry, slit-lamp anterior segment examination, fundus examination under dilation and optical coherence tomography at the retinal plane (Spectralis OCT Heidelberg Engineering Inc., Heidelberg, Germany). Biometric values were used as inputs in the Kane formula to calculate the lens power, which in turn was selected targeting emmetropia (11).
Besides the binocular and monocular UDVA and DCVA at 4 m and the UNVA and DCNVA at 40 cm, the 3 months-postoperative visit, also included the uncorrected and best distance corrected intermediate visual acuity at 80 cm and 67 cm (UI80VA, DCI80VA, UI67VA and DCI67VA), binocular defocus curves, contrast sensitivity (CS) testing under photopic (80 cd/m2), mesopic (6 cd/m2), and scotopic (3 cd/m2) light conditions (CSV 1000 HGT; Vector Vision, Greenville, OH), ocular optical quality analysis by Pyramidal WaveFront-based sensor aberrometer (Osiris T Aberrometer, CSO, Firenze, Italy) and the halo test (Aston Halometer). After a slit lamp examination (to exclude the presence of posterior capsular opacity - PCO), patients were also asked to complete the National Eye Institute Refractive Error Quality of Life Instrument 42 (NEI-RQL-42) questionnaire.
Binocular defocus curves were obtained between +1.50 to −3.50 D using regular shifts of 0.50 D with respect to the 4 m DCVA and recording the best visual acuity for each step. To avoid memory effects, presenting letter sequences were randomized and patients’ eyes were occluded between each lens presentation (12). To analyze the ocular optical quality we used the Osiris T Aberrometer studying the ocular Root Means Square (RMS) and the Point-Spread-Function Strehl ratio (PSF Strehl ratio), which is defined as the ratio between the peak image intensity of the patient’s eye and that of an ideal eye (i.e., maximal intensity), limited only by diffraction (13). On the other hand, the purpose of the halo test is to measure in degrees how much a glaring source of light clouds a target. The halometer consisted of a light source (LED, Golden Dragon Pluc LCW W5AM.PC, 5000 K color temperature; Osram Licht AG, Munich, Germany) located in the center of an iPad4 tablet on which 0.3 logMAR (Snellen 20/40) letters were presented and moved toward the light source in 0.05-degree steps (14). To identify the halo area, patients stayed at 2 meters from the halometer in a dark room and were asked to recognize in succession the letters in six directions of orientation and separated by 60°. The cut-off value was collected for each direction. On slit lamp examination, if a grade 3 or higher PCO (According to Congdon’s study), (15) was detected, this was treated by YAG-laser capsulotomy and the 3 months evaluation was postponed 10 days thereafter. Finally, patients completed the NEI RQL-42 questionnaire to evaluate their quality of life in relation to their refractive error correction and visual acuity recovery (16). The questionnaire consists of 13 subscales with 42 items in 16 different question/response category formats.
All cataract surgeries were performed by the same surgeon (E.P.) under topical anesthesia. A 2.2 mm corneal tunnel was created on the steepest meridian and was followed by a standard phaco-chop technique-surgery using the Stellaris phaco-platform (Bausch & Lomb Inc., Rochester, NY). The 12.4 mm Lucidis IOL was then placed in the capsular bag. The second surgical procedure was performed within 7 days from the first one. Prophylaxis consisted of an antibiotic and a nonsteroidal anti-inflammatory eye drop whereas the postoperative therapy also included topical steroid drops.
The Lucidis lens (Swiss Advanced Vision, SAV-IOL SA, Neuchâtel, Switzerland)is a single-piece foldable hydrophilic acrylic lens with an optical diameter of 6.0 mm and a total diameter of 10.8 mm or 12.4 mm. The IOL has square edges with closed loop haptics and is designed to be implanted in the capsular bag. Its hybrid refractive/aspheric design, where a 1-mm aspheric central zone is surrounded by a 6-mm refractive ring (Figure 1), allows for a + 3.0 D addition power on top of the normal distance power, which ranges from +5.0 D to +30.0 D. In this study only the 12.4 mm-IOL was implanted in order to avoid IOL decentration.
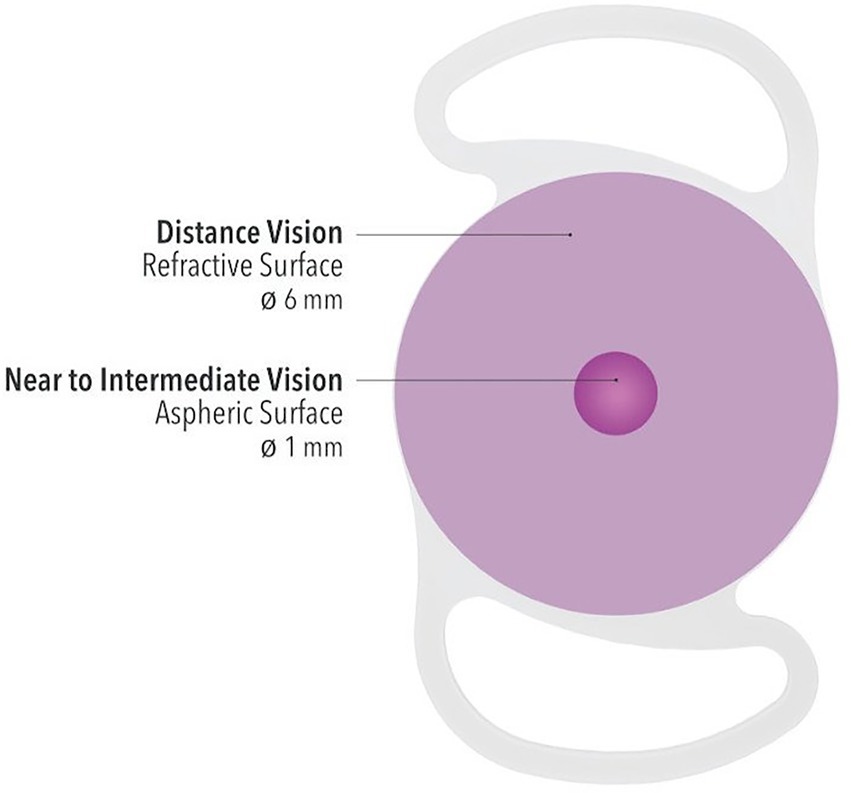
Figure 1. Schematic graph of the optic technology of Lucidis IOL with a central aspheric surface for near to intermediate vision and a larger refractive surface for distance vision.
Statistical analysis was performed using the IBM SPSS software version 24 for MacIntosh (IBM-SPSS). The Shapiro–Wilk test was used to determine data distribution. All quantitative results are reported as mean ± standard deviation for parametric distribution and as median ± interquartile range for non-parametric distribution. The t test for parametric distribution and the Mann–Whitney test for non-parametric distribution were used to compare the data. A p value lower than 0.05 was considered statistically significant.
The sample size was calculated based on monocular and binocular DCNVAs obtained from previous studies. With an estimated standard deviation of 0.13, a sample size of 50 patients produces a 95% confidence interval in DCNVA of 0.037. When the estimated standard deviation is 0.14, a sample size of 25 gives a 95% confidence interval of 0.06 (17). Postoperative data are presented at 3 months from implantation.
Twenty-five patients (50 eyes) with a mean age (±SD) of 68 ± 10 years were included. Thirty-six percent of patients were male and 64% were female. The average spherical dioptric power of the implanted IOLs was 19.01 ± 4.29 D (median: 19.0 D, range: 12.5 to 26.5 D).
There were no major postoperative or intraoperative complications.
At 3-months from implantation, a grade 3 PCO was found in 1 eye (2.1%) and a YAG-laser capsulotomy was performed.
The mean postoperative subjective refractive spherical equivalent was −0.36 ± 0.39 D and laid within ±0.51 D in 58% of eyes and within ±1.00 D in 100% of cases.
Table 1 summarizes both the monocular and binocular uncorrected and distance corrected VA-results.
The differences between the mean binocular and monocular UDVA and UI80VA, UI67VA and UNVA were not statistically significant (p = 0.26, p = 0.24 and p = 0.24 and p = 0.31, p = 0.83 and p = 0.84, respectively).
Figure 2 shows the mean binocular defocus curve at 3 months after surgery. Visual acuity was found to be higher than or equal to 0.05 logMar between +0.50 and − 2.50 D of vergence, showing the deepest point at −1.50 D. However, neither the difference in VA between 0.00 and − 1.5 D, nor that between −1.5 and − 2 D, were statistically significant (p = 0.08 and p = 0.11, respectively).
Figure 3 presents the binocular CS function measured under scotopic, mesopic, and photopic light conditions. There were no statistically significant differences among the three conditions at any of the studied spatial frequencies (e.g., in the scotopic vs. photopic condition at 12 cpg the p value was 0.26). Mean CS values of a population ranging from 50 to 75 years of age were also taken into account and the performance of this IOL was statistically significant better at 3 cpd in photopic, mesopic and scotopic condition p = 0.01, p = 0.004 and p = 0.03, respectively (18).
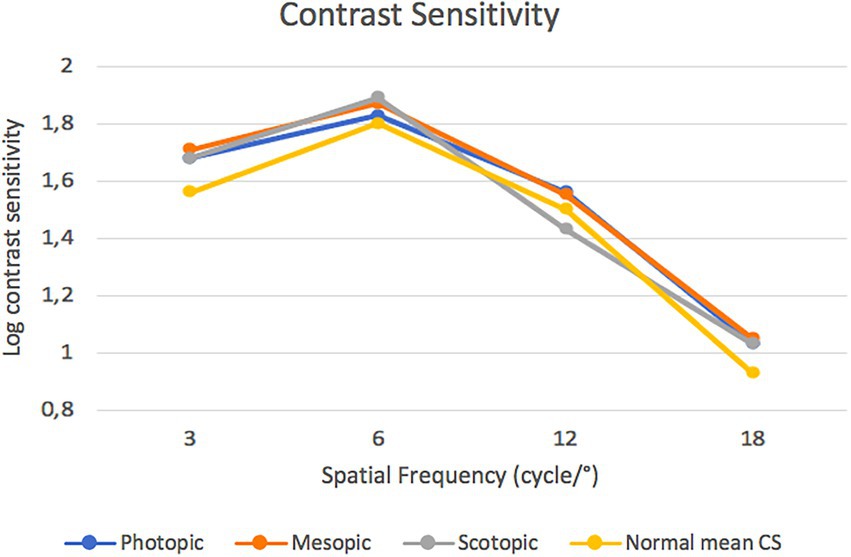
Figure 3. Postoperative contrast sensitivity function measured under scotopic, mesopic and photopic conditions.
The mean distance from the central stimulus was 1.23 ± 0.01. Figure 4 presents the mean halometric cut-off values for each of the six axes.
At 3 months from the operation the mean ocular and corneal RMS at 4 mm were 0.31 ± 0.28 (range: 0.09–1.5) and 0.19 ± 0.07 (range: 0.07–0.5), respectively; whereas the mean PSF Strehl ratio was 0.2 ± 0.09 (range: 0.03–0.41).
The NEI RQL-42 evidenced high subjective satisfaction results for all the items, especially for suboptimal correction, activity limitations, glare, appearance, far vision, dependence on correction and satisfaction with correction (Table 2).
Extended depth of focus technology is among the most effective proposed methods to enhance spectacle independence after cataract surgery. Nevertheless, when it comes to near vision, these lenses are usually outperformed by MFIOLs, which, however, are often burdened by annoying light phenomena (1, 2).
In this study, the Lucidis IOL has shown to be able to strengthen the near-distance VA at the expense of a slight decrease in the intermediate vision. Indeed, 74, 57 and 42% of patients reached a binocular VA higher than 20/25 at 40, 67 and 80 cm, respectively. This result was confirmed by the trend of the defocus curve and seems to be in accordance with the current literature (4–6). Authors would like to underline that these results appear to be in agreement with the available literature on Lucidis IOLs, as to our knowledge currently no study has ever reported the DCIVA, but only the UIVA (without specifying how many cm it was run) and none performed defocus curves.
Although a direct comparison was not performed, when considering the results of other EDOF IOLs, it is striking how these are usually characterized by a regular downslope in the myopic portion of the defocus curve, reaching the lowest performances around −2.50 D; indeed, patients often need a spherical addition of 1 D in order to achieve the optimal near-distance VA (7, 19, 20). Meanwhile, in our study, at −2.50D of vergence, the defocus curve showed a mean VA of little less than 0.05 LogMar. With regards to intermediate VAs, on the other hand, our results do not significantly differ with those of other EDOF IOLs.
When considering an extended range of vision (ERV) IOL (21) such as the TECNIS Symphony, it seems like Lucidis IOLs perform better at far and near distances, whereas the former performs better at intermediate distances (22, 23).
Surprisingly, the Lucidis IOL showed comparable performances to the tri-quadrifocal Enlighten Panoptix IOL at the 40 cm distance (0.14 ± 0.04 and 0.00 ± 0.08 for Enlighten and hybrid IOLs, respectively) and performed even better than this IOL at intermediate distances (0.10 ± 0.03 and 0.04 ± 0.09 for Enlighten and Lucidis IOL, respectively) (22).
This outstanding performance is probably related to the special hybrid design of these lenses. The main optical propriety is due to the central aspheric portion of this IOL which is able to create a peak of light via constructive light wave interference, whereas the periphery maintains a refractive surface. The lens therefore acts as an axicon (Bessel like ray of light). The system altogether results in the formation of a pseudo non diffracting beam which starts to diverge after some distance from the lens itself, therefore covering the whole range of vision (i.e., from near-intermediate to far distances). An axicon lens is an optical element first introduced in 1954 by McLeod, (24) able to transform a laser beam into a ring-shaped distribution, resulting in a beam of focal fields that allow a continuous vision from intermediate to short distances.
Interestingly, these visual performances are achieved while preserving a good quality of vision. Indeed, the ocular RMS was 0.31 ± 0.28, with corneal component of 0.19 ± 0.07 and a mean internal RMS of 0.12 (i.e., ocular RMS – corneal RMS). The RMS is closely related to Zernike polynomials and its minimum value is 0, which represents the ideal wavefront condition. Even though the measurement was taken using different instruments, the internal RMS of the Lucidis IOL results to be lower than both the ZXR00s-TECNIS Symphony’s (0.15 ± 0.06) and the tri-quadrifocal Enlighten Panoptix’s (0.18 ± 0.06), measured in a previous study of ours (22). The RMS results of the current study differ from those found by Rabinovich et al. (10) on Lucidis IOLs. However, the latter study has several limitations, such as its retrospective design and the absence of a precise description of what RMS evaluation had been carried out and what instrumentation was used, so a reliable comparison between our results is not feasible. Nonetheless, the total RMS found in this study (i.e., 0.18 ± 0.1) seems to be better than that obtained with aberrometric EDOFs, multifocal diffractive and refractive IOLs (13).
Lucidis IOLs however, showed worse performances than the aberrometric EDOF Mini Well IOL (SIFI S.p.A., Catania, Italy) and the Enlighten IOL in terms of mean PSF Strehl ratio and CS at the lower spatial frequencies (3, 22).
In addition, halometry results show that the Lucidis IOL performs very similarly to monofocal IOLs for all mean cut-off values (17). Even though no direct comparison has been performed, the NEI RQL-42 questionnaire-results seem to show higher subjective satisfaction with the Lucidis IOL than with aberrometric EDOF, ERV and Enlighten IOLs with regards to “glare” evaluation. Despite patients reporting good levels of satisfaction after bilateral implantation of this hybrid lens, all other items in the questionnaire seem to show better results with aberrometric EDOF IOLs (17, 22).
Among the limitations of this study it is worth mentioning its limited number of patients and the absence of a direct comparison with the other type of IOLs.
To conclude, Lucidis IOLs demonstrated a good safety profile and excellent visual performances at all distances, but especially at near distances, while also allowing a good quality of vision.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Comitato etico per la Sperimentazione Clinica (CESC) delle Province di Verona e Rovigo. The patients/participants provided their written informed consent to participate in this study.
All authors made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data. They all took part in drafting the article or revising it critically for important intellectual content and agreed to submit it to the current journal. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. de Vries, NE, Webers, CA, Touwslager, WR, Bauer, NJC, de Brabander, J, Berendschot, TT, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. (2011) 37:859–5. doi: 10.1016/j.jcrs.2010.11.032
2. de Silva, SR, Evans, JR, Kirthi, V, Ziaei, M, and Leyland, M. Multifocal versus monofocal intraocular lenses after cataract extraction. Cochrane Database Syst Rev. (2016) 2016:CD003169. doi: 10.1002/14651858.CD003169.pub4
3. Puell, MC, Pérez-Carrasco, MJ, Hurtado-Ceña, FJ, and Álvarez-Rementería, L. Disk halo size measured in individuals with monofocal versus diffractive multifocal intraocular lenses. J Cataract Refract Surg. (2015) 41:2417–23. doi: 10.1016/j.jcrs.2015.04.030.26703491
4. Ukai, Y, Okemoto, H, Seki, Y, Nakatsugawa, Y, Kawasaki, A, Shibata, T, et al. Quantitative assessment of photic phenomena in the presbyopia-correcting intraocular lens. PLoS One. (2021) 16:e0260406. doi: 10.1371/journal.pone.0260406
5. Kanclerz, P, Toto, F, Grzybowski, A, and Alio, JL. Extended depth-of-field intraocular lenses: an update. Asia Pac J Ophthalmol (Phila). (2020) 9:194–2. doi: 10.1097/APO.0000000000000296.32511121; PMCID: PMC7299221
6. Kohnen, T, and Suryakumar, R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. (2020) 46:298–4. doi: 10.1097/j.jcrs.0000000000000109.32126045
7. Pedrotti, E, Neri, E, Bonacci, E, Barosco, G, Galzignato, A, Montresor, A, et al. Extended depth of focus versus Monofocal IOLs in patients with high myopia: objective and subjective visual outcomes. J Refract Surg. (2022) 38:158–6. doi: 10.3928/1081597X-20211220-01
8. Gillmann, K, and Mermoud, A. Visual performance, subjective satisfaction and quality of life effect of a new refractive intraocular lens with central extended depth of focus. Klin Monatsbl Augenheilkd. (2019 Apr) 236:384–06. English. doi: 10.1055/a-0799-9700. Epub 2019 Feb 14
9. Ozulken, K, Kiziltoprak, H, Yuksel, E, and Mumcuoğlu, T. A comparative evaluation of diffractive trifocal and new refractive/extended depth of focus intraocular lenses for refractive lens exchange. Curr Eye Res. (2021) 46:811–7. doi: 10.1080/02713683.2020.1833347
10. Rabinovich, M, Ceresara, G, Aramburu Del Boz, A, Al Khatib, D, Crespe, M, and Bovet, J. Visual outcomes after implantation of Lucidis EDOF IOL. J Ophthalmol. (2022) 2022:1–5. doi: 10.1155/2022/5100861
11. Chung, J, Bu, JJ, and Afshari, NA. Advancements in intraocular lens power calculation formulas. Curr Opin Ophthalmol. (2022) 33:35–40. doi: 10.1097/ICU.0000000000000822.34854826
12. Gupta, N, Wolffsohn, JS, and Naroo, SA. Optimizing measurement of subjective amplitude of accommodation with defocus curves. J Cataract Refract Surg. (2008) 34:1329–38. doi: 10.1016/j.jcrs.2008.04.031.18655984
13. Alio, JL, D'Oria, F, Toto, F, Balgos, J, Palazon, A, Versaci, F, et al. Retinal image quality with multifocal, EDoF, and accommodative intraocular lenses as studied by pyramidal aberrometry. Eye Vis (Lond). (2021) 8:37. doi: 10.1186/s40662-021-00258-y
14. Buckhurst, PJ, Naroo, SA, Davies, LN, Shah, S, Buckhurst, H, Kingsnorth, A, et al. Tablet app halometer for the assessment of dysphotopsia. J Cataract Refract Surg. (2015) 41:2424–9. doi: 10.1016/j.jcrs.2015.05.041.26703492
15. Congdon, N, Fan, H, Choi, K, Huang, W, Zhang, L, Zhang, S, et al. Impact of posterior subcapsular opacification on vision and visual function among subjects undergoing cataract surgery in rural China: study of cataract outcomes and up-take of services (SCOUTS) in the caring is hip project, report 5. Br J Ophthalmol. (2008) 92:598–3. doi: 10.1136/bjo.2007.126714.18441169
16. Nichols, JJ, Mitchell, GL, Saracino, M, and Zadnik, K. Reliability and validity of refractive error-specific quality-of-life instruments. Arch Ophthalmol. (2003) 121:1289–96. doi: 10.1001/archopht.121.9.1289.12963612
17. Pedrotti, E, Chierego, C, Talli, PM, Selvi, F, Galzignato, A, Neri, E, et al. Extended depth of focus versus Monofocal IOLs: objective and subjective visual outcomes. J Refract Surg. (2020) 36:214–2. doi: 10.3928/1081597X-20200212-01.32267951
18. Pomerance, GN, and Evans, DW. Test-retest reliability of the CSV-1000 contrast test and its relationship to glaucoma therapy. Invest Ophthalmol Vis Sci. (1994) 35:3357–61.8056510.
19. Kohnen, T, Petermann, K, Böhm, M, Hemkeppler, E, Ahmad, W, Hinzelmann, L, et al. Nondiffractive wavefront-shaping extended depth-of-focus intraocular lens: visual performance and patient-reported outcomes. J Cataract Refract Surg. (2022) 48:144–06. doi: 10.1097/j.jcrs.0000000000000826.34653094
20. Arrigo, A, Gambaro, G, Fasce, F, Aragona, E, Figini, I, and Bandello, F. Extended depth-of-focus (EDOF) AcrySof® IQ Vivity® intraocular lens implant: a real-life experience. Graefes Arch Clin Exp Ophthalmol. (2021) 259:2717–22. doi: 10.1007/s00417-021-05245-6
21. Chang, DH, Janakiraman, DP, Smith, PJ, Buteyn, A, Domingo, J, Jones, JJ, et al. Visual outcomes and safety of an extended depth-of-focus intraocular lens: results of a pivotal clinical trial. J Cataract Refract Surg. (2022) 48:288–7. doi: 10.1097/j.jcrs.0000000000000747
22. Pedrotti, E, Carones, F, Talli, P, Bonacci, E, Selvi, F, Galzignato, A, et al. Comparative analysis of objective and subjective outcomes of two different intraocular lenses: trifocal and extended range of vision. BMJ Open Ophthalmol. (2020) 5:e000497:e000497. doi: 10.1136/bmjophth-2020-000497
23. Pedrotti, E, Bruni, E, Bonacci, E, Badalamenti, R, Mastropasqua, R, and Marchini, G. Comparative analysis of the clinical outcomes with a Monofocal and an extended range of vision intraocular lens. J Refract Surg. (2016) 32:436–2. doi: 10.3928/1081597X-20160428-06.27400074
Keywords: cataract, spectacle independence, near vision, premium IOLs, extended depth of focus IOL
Citation: Pedrotti E, Bonacci E, Kilian R, Pagnacco C, Anastasi M, Ventura M and Marchini G (2023) Quality of vision and outcomes after bilateral implantation of pseudo-non diffracting beam IOL. Front. Med. 10:1085280. doi: 10.3389/fmed.2023.1085280
Received: 31 October 2022; Accepted: 15 February 2023;
Published: 06 March 2023.
Edited by:
Rita Mencucci, University of Florence, ItalyReviewed by:
Roberto Vignapiano, Area del Farmaco e Salute del Bambino, ItalyCopyright © 2023 Pedrotti, Bonacci, Kilian, Pagnacco, Anastasi, Ventura and Marchini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Camilla Pagnacco, Y2FtaWxsYS5wYWduYWNjb0BnbWFpbC5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.