
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 14 March 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1084479
This article is part of the Research Topic Cross-Talk between Cellular and Molecular Compartments during Liver Injury, Volume II View all 4 articles
Ferroptosis is a type of regulated cell death caused by iron overload and lipid peroxidation, and its core is an imbalance of redox reactions. Recent studies showed that ferroptosis played a dual role in liver diseases, that was, as a therapeutic target and a pathogenic factor. Therefore, herein, we summarized the role of ferroptosis in liver diseases, reviewed the part of available targets, such as drugs, small molecules, and nanomaterials, that acted on ferroptosis in liver diseases, and discussed the current challenges and prospects.
Liver disease is a general term for inflammatory and non-inflammatory diseases of the liver, including hepatitis, cirrhosis, steatosis, liver cancer, and others. It is a common and extremely harmful disease that adversely affects human health. Currently, the treatment of end-stage liver disease mainly consists of liver transplantation and symptomatic treatment. Although the quality of life of patients has been greatly improved, the survival rate is still unsatisfactory. Therefore, it is necessary to find more effective treatment strategies for liver diseases.
Ferroptosis is a type of regulated cell death caused by iron overload and lipid peroxidation, which is distinct from pyroptosis, necrosis, and apoptosis. It is involved in various metabolic pathways, including iron, glutathione (GSH), and coenzyme Q metabolism (1). And studies have shown that ferroptosis is associated with the occurrence and development of liver diseases, including cancer, liver fibrosis, and ischemia-reperfusion injury (IRI) (1) (Figure 1). The nuclear factor erythroid 2-related factor 2 (Nrf2)- glutathione peroxidase 4 (GPx4) axis was involved in carbon tetrachloride (CCl4)-mediated acute liver injury in mice (2). Furthermore, the acyl-CoA synthetase long chain family member 4 (ACSL4)-mediated ferroptosis showed a tumor-promoting role in the progression of hepatocellular carcinoma (HCC) from liver injury and a tumor-suppressing role in HCC treatment (3). However, there are still few clinical applications of ferroptosis targets in liver diseases. Therefore, an in-depth understanding of the ferroptosis mechanism will promote the development of therapeutics for ferroptosis in liver diseases. The molecular mechanisms related to ferroptosis have been reviewed by other studies. Thus, herein, we review the role of ferroptosis in liver diseases and summarize the part of available therapeutic targets for ferroptosis in liver diseases.
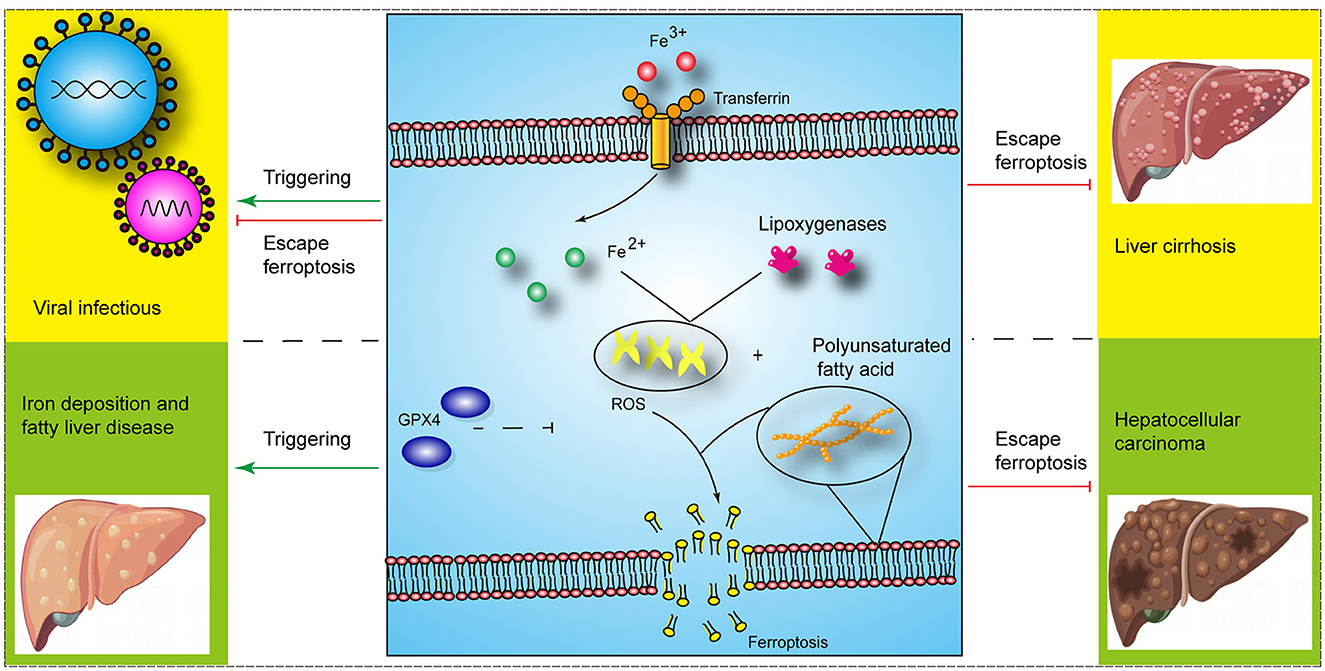
Figure 1. The role of ferroptosis in liver diseases (Some image elements came from Figdraw with permissions).
Acute liver injury without any underlying liver diseases includes drug-induced liver injury, IRI, and liver injury caused by acute viral infection. Liver injury caused by acetaminophen (APAP) is a common drug-induced liver injury, and IR-induced liver injury mainly includes insufficient perfusion injury of the liver caused by liver transplantation and cardiovascular diseases. A study showed that ferroptosis initiated APAP-induced liver injury, and ferroptosis inhibitors, such as ferrostatin-1 and iron chelator deferoxamine, alleviated APAP-induced liver injury (4). In addition, ferroptosis involved in IRI-induced liver injury, and HECT, UBA and WWE domain containing E3 ubiquitin protein ligase 1 (HUWE1) could antagonize abnormal iron accumulation and ferroptosis to alleviate IRI-induced liver injury (5). Further research showed that ferroptosis-related liver injury employed a variety of regulatory mechanisms, such as exosomes. Mesenchymal stem cells attenuated ferroptosis in acute liver injury by promoting the stability of SLC7A11 via exosomes (6). Moreover, exosome-delivered miR-124-3p and miR-29a-3p from heme oxygenase-1-modified bone marrow mesenchymal stem cells inhibited ferroptosis to alleviate IRI in steatotic grafts (7, 8). Although research on liver injury and ferroptosis has shown promising results, it is mainly at the level of animal models, and there is a long way to go before clinical translation. In addition, studies showed that certain necrosis inhibitors, such as necrostatin-1 (nec1) could also partially inhibit ferroptosis. Therefore, the interaction between ferroptosis and other types of cell death needs to be further explored to better guide ferroptosis treatment strategies. Moreover, inflammatory cells involved in acute liver injury, and ferroptosis have been shown to affect the function of inflammatory cells. For example, the kinase complex mTORC2 promoted longevity of virus-specific memory CD4+ T cells by inhibiting ferroptosis (9) and ferroptosis could affect the ability of macrophages to kill intracellular bacteria (10). Thus, whether and how hepatocytes and inflammatory cells ferroptosis affect each other during liver injury requires further research.
Chronic liver injury refers to long-term and chronic damage to liver function caused by multiple factors, and it is mainly seen in chronic viral hepatitis, long-term heavy drinking, and fatty liver. Hepatic stellate cells (HSCs) were rich in iron, which provided the basis for ferroptosis regulation in cirrhosis (11). The study also showed that ferroptosis was closely related to liver cirrhosis. Therefore, elastin and sorafenib treatment could alleviate liver fibrosis in mice by inducing ferroptosis. In addition, sorafenib, a well-known ferroptosis inducer, attenuated liver injury and extracellular matrix accumulation in CCl4-induced fibrotic livers by reducing SLC7A11 and GPX4 proteins (12). Ferroptosis affects multiple pathways in liver cirrhosis and is regulated in multiple ways. For instance, ferroptosis could regulate the autophagy signaling pathway in HSCs and dihydroartemisinin attenuated liver fibrosis via m6A methylation-involved ferroptosis in HSCs (13). The researchers also investigated ferroptosis in cirrhosis that develops from viral hepatitis. Chen et al. found that chronic hepatitis B virus and/or hepatitis C virus (HCV) infection altered iron metabolism and related genes in the liver (14), and ferroptosis could affect HCV replication and therapeutic efficacy (15). Moreover, hepatitis B virus X protein (HBx) could alleviate the cell death of HSCs by inhibiting ferroptosis, thereby promoting the progression of liver cirrhosis (16). In addition, steatosis and steatohepatitis have been confirmed to be related to ferroptosis, and ferroptosis inhibitors could alleviate liver injury (17), however, the underlying molecular mechanisms are not completely known. Therefore, further research is needed to reveal the role of ferroptosis in steatosis and steatohepatitis. In summary, although several potential ferroptosis targets, such as P53 and ELAVL1, have been identified, the associated studies used only cellular and animal models and the relevant molecular mechanisms are not completely clear. Therefore, further research efforts are needed.
HCC is one of the important causes of tumor-related deaths, and ferroptosis has been recognized as a tumor suppressor. Therefore, researchers investigated the induction of ferroptosis in HCC cells. Sorafenib is currently used as the first-line drug for the treatment of advanced HCC, and studies have shown that it could induce ferroptosis of HCC cells and thereby inhibit their proliferation (18). And the efficacy of sorafenib was affected by intracellular genetic status; for example, Rb-negative hepatoma cells were more sensitive to sorafenib-induced ferroptosis (19). Additionally, ferroptosis induced by sorafenib could be reversed by ferroptosis inhibitors, such as ferrostatin-1 and activation of p62-Keap1-Nrf2 axis (20, 21). Moreover, sorafenib-induced increase in metallothionein-1G (MT-1G) promoted the resistance of HCC cells to sorafenib (22). This suggests that secondary resistance to sorafenib and gene expression of HCC cells might affect the therapeutic effect of sorafenib; therefore, mechanisms underlying the resistance to sorafenib need to be further elucidated. In addition, ferroptosis-inducing nanoparticles, extracellular lactate levels, and antipsychotic drug haloperidol also participated in inducing ferroptosis of HCC cells (23–25). Taken together, seeking more effective ferroptosis therapeutic targets and strategies is beneficial for HCC.
The present therapeutic strategies for ferroptosis mainly include genes, RNAs, proteins, small-molecule compounds and nanomaterials. Ferroptosis related nanomaterials consisted of both iron-based and non-iron-based nanomaterials, and they induced ferroptosis by scavenging GSH and inducing the degradation of GPx4 (26). Currently, targets for ferroptosis are mainly divided into ferroptosis inducers and inhibitors. There are four types of ferroptosis-inducing compounds, including those: inhibiting the cysteine (Cys)/glutamate (Glu) reverse exchange (Xc−) system, directly or indirectly inactivating GPX4, causing iron overload and activating heme oxygenase 1 (HMOX1) (27). At present, ferroptosis was often regarded as a detrimental factor in certain liver diseases, ferroptosis-inducing agents were mainly used to treat HCC and liver fibrosis (28). Table 1 lists the part of available ferroptosis inducers in liver diseases.
Ferroptosis inhibitors work by reducing iron overload and peroxidation levels and scavenging peroxidation products (81). Of these, the prominent ones are iron chelators that reduce iron overloads, such as deferoxamine and deferiprone. A small number of iron chelators are used in the clinic or clinical trials. And the library of novel deferoxamine compounds is created and updated regularly (40). Table 1 lists the part of available ferroptosis inhibitors in liver diseases. Furthermore, nec1 inhibited necrosis and ferroptosis in primary renal tubular and mouse heart transplantation models (82), but its use in liver disease is not reported. So far, the role of ferroptosis inhibitors in liver diseases has mainly been reported in animal models, and there are only a few reports about its clinical application. Therefore, additional research is needed to provide evidence for targeted ferroptosis in liver diseases. And although nanomaterials targeting ferroptosis have improved their targeting properties through special modifications such as biofilm and PUFA modification (83), their safety for in vivo application has not been effectively demonstrated.
Ferroptosis plays an important role in the occurrence, development, and treatment of liver diseases. Researchers have screened and identified several ferroptosis inducers and inhibitors using animal experiments and small molecule compound libraries. However, evidence regarding the distribution, metabolism, excretion and use of these targets is lacking. Secondly, the role of these targets in the progression of chronic liver disease to HCC may be variable, and the underlying mechanisms are unknown. Additionally, how can targets be adjusted at different stages of liver diseases is not clear. Thirdly, the targeting of these agents needs to be improved. Studies have shown that these targets promote or inhibit ferroptosis of inflammation cells and thus affect their immune function. At present, there is little relevant evidence and the underlying mechanism needs to be elucidated. Finally, current nanomedicine technologies enable highly specific targeting of these agents, and nanomaterial-related ferroptosis promoters and inhibitors have been studied in cancers. There is a long way to go before we can effectively target ferroptosis to treat liver diseases, but we believe that combining efforts from over the world will help us realize the therapeutic strategy.
XX and JG designed the study. DSu, XX, and DSh revised the manuscript. All authors contributed to the article and approved the submitted version.
Thanks to the He'nan Key Laboratory of Digestive Organ Transplantation and Medicial 3D printing center of He'nan province supporting this work. In addition, we thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Chen J, Li X, Ge C, Min J, Wang F. The multifaceted role of ferroptosis in liver disease. Cell Death Differ. (2022) 29:467–80. doi: 10.1038/s41418-022-00941-0
2. Zhao T, Yu Z, Zhou L, Wang X, Hui Y, Mao L, et al. Regulating Nrf2-GPx4 axis by bicyclol can prevent ferroptosis in carbon tetrachloride-induced acute liver injury in mice. Cell Death Discov. (2022) 8:380. doi: 10.1038/s41420-022-01173-4
3. Grube J, Woitok MM, Mohs A, Erschfeld S, Lynen C, Trautwein C, et al. ACSL4-dependent ferroptosis does not represent a tumor-suppressive mechanism but ACSL4 rather promotes liver cancer progression. Cell Death Dis. (2022) 13:704. doi: 10.1038/s41419-022-05137-5
4. Niu B, Lei X, Xu Q, Ju Y, Xu D, Mao L, et al. Protecting mitochondria via inhibiting VDAC1 oligomerization alleviates ferroptosis in acetaminophen-induced acute liver injury. Cell Biol Toxicol. (2022) 38:505–30. doi: 10.1007/s10565-021-09624-x
5. Wu Y, Jiao H, Yue Y, He K, Jin Y, Zhang J, et al. Ubiquitin ligase E3 HUWE1/MULE targets transferrin receptor for degradation and suppresses ferroptosis in acute liver injury. Cell Death Differ. (2022) 29:1705–18. doi: 10.1038/s41418-022-00957-6
6. Lin F, Chen W, Zhou J, Zhu J, Yao Q, Feng B, et al. Mesenchymal stem cells protect against ferroptosis via exosome-mediated stabilization of SLC7A11 in acute liver injury. Cell Death Dis. (2022) 13:271. doi: 10.1038/s41419-022-04708-w
7. Li X, Wu L, Tian X, Zheng W, Yuan M, Tian X, et al. miR-29a-3p in Exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells alleviates steatotic liver ischemia-reperfusion injury in rats by suppressing ferroptosis via iron responsive element binding protein 2. Oxid Med Cell Longev. (2022) 2022:6520789. doi: 10.1155/2022/6520789
8. Wu L, Tian X, Zuo H, Zheng W, Li X, Yuan M, et al. miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia-reperfusion injury in steatotic grafts. J Nanobiotechnology. (2022) 20:196. doi: 10.1186/s12951-022-01407-8
9. Wang Y, Tian Q, Hao Y, Yao W, Lu J, Chen C, et al. The kinase complex mTORC2 promotes the longevity of virus-specific memory CD4(+) T cells by preventing ferroptosis. Nat Immunol. (2022) 23:303–17. doi: 10.1038/s41590-021-01090-1
10. Ma R, Fang L, Chen L, Wang X, Jiang J, Gao L. Ferroptotic stress promotes macrophages against intracellular bacteria. Theranostics. (2022) 12:2266–89. doi: 10.7150/thno.66663
11. Zhang Z, Yao Z, Wang L, Ding H, Shao J, Chen A, et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. (2018) 14:2083–103. doi: 10.1080/15548627.2018.1503146
12. Yuan S, Wei C, Liu G, Zhang L, Li J, Li L, et al. Sorafenib attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis via HIF-1alpha/SLC7A11 pathway. Cell Prolif. (2022) 55:e13158. doi: 10.1111/cpr.13158
13. Shen M, Guo M, Li Y, Wang Y, Qiu Y, Shao J, et al. m(6)A methylation is required for dihydroartemisinin to alleviate liver fibrosis by inducing ferroptosis in hepatic stellate cells. Free Radic Biol Med. (2022) 182:246–59. doi: 10.1016/j.freeradbiomed.2022.02.028
14. Chen X, Kang R, Kroemer G, Tang D. Ferroptosis in infection, inflammation, and immunity. J Exp Med. (2021) 218:e20210518. doi: 10.1084/jem.20210518
15. Yamane D, Hayashi Y, Matsumoto M, Nakanishi H, Imagawa H, Kohara M, et al. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem Biol. (2022) 29:799–810.e794. doi: 10.1016/j.chembiol.2021.07.022
16. Kuo CY, Chiu V, Hsieh PC, Huang CY, Huang SJ, Tzeng IS, et al. Chrysophanol attenuates hepatitis B virus X protein-induced hepatic stellate cell fibrosis by regulating endoplasmic reticulum stress and ferroptosis. J Pharmacol Sci. (2020) 144:172–82. doi: 10.1016/j.jphs.2020.07.014
17. Qi J, Kim JW, Zhou Z, Lim CW, Kim B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation-mediated cell death in mice. Am J Pathol. (2020) 190:68–81. doi: 10.1016/j.ajpath.2019.09.011
18. Fouquet G, Marie C, Collet L, Vilpoux C, Ouled-Haddou H, Nguyen-Khac E, et al. Rescuing SLAMF3 expression restores sorafenib response in hepatocellular carcinoma cells through the induction of mesenchymal-to-epithelial transition. Cancers. (2022) 14:910. doi: 10.3390/cancers14040910
19. Louandre C, Marcq I, Bouhlal H, Lachaier E, Godin C, Saidak Z, et al. The retinoblastoma (Rb) protein regulates ferroptosis induced by sorafenib in human hepatocellular carcinoma cells. Cancer Lett. (2015) 356:971–7. doi: 10.1016/j.canlet.2014.11.014
20. Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. (2016) 63:173–84. doi: 10.1002/hep.28251
21. Lu Y, Chan YT, Tan HY, Zhang C, Guo W, Xu Y, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res. (2022) 41:3. doi: 10.1186/s13046-021-02208-x
22. Sun X, Niu X, Chen R, He W, Chen D, Kang R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. (2016) 64:488–500. doi: 10.1002/hep.28574
23. Nie J, Lin B, Zhou M, Wu L, Zheng T. Role of ferroptosis in hepatocellular carcinoma. J Cancer Res Clin Oncol. (2018) 144:2329–37. doi: 10.1007/s00432-018-2740-3
24. Bebber CM, Muller F, Prieto Clemente L, Weber J, Von Karstedt S. Ferroptosis in cancer cell biology. Cancers. (2020) 12:164. doi: 10.3390/cancers12010164
25. Zhao Y, Li M, Yao X, Fei Y, Lin Z, Li Z, et al. HCAR1/MCT1 Regulates tumor ferroptosis through the lactate-mediated AMPK-SCD1 activity and its therapeutic implications. Cell Rep. (2020) 33:108487. doi: 10.1016/j.celrep.2020.108487
26. Fei W, Zhang Y, Ye Y, Li C, Yao Y, Zhang M, et al. Bioactive metal-containing nanomaterials for ferroptotic cancer therapy. J Mater Chem B. (2020) 8:10461–73. doi: 10.1039/D0TB02138E
27. Feng H, Stockwell BR. Unsolved mysteries: how does lipid peroxidation cause ferroptosis? PLoS Biol. (2018) 16:e2006203. doi: 10.1371/journal.pbio.2006203
28. Bekric D, Ocker M, Mayr C, Stintzing S, Ritter M, Kiesslich T, et al. Ferroptosis in hepatocellular carcinoma: mechanisms, drug targets and approaches to clinical translation. Cancers. (2022) 14:164. doi: 10.3390/cancers14071826
29. Xu Y, Li Y, Li J, Chen W. Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol. (2022) 53:102349. doi: 10.1016/j.redox.2022.102349
30. Luo P, Liu D, Zhang Q, Yang F, Wong YK, Xia F, et al. Celastrol induces ferroptosis in activated HSCs to ameliorate hepatic fibrosis via targeting peroxiredoxins and HO-1. Acta Pharm Sin B. (2022) 12:2300–14. doi: 10.1016/j.apsb.2021.12.007
31. Liu G, Wei C, Yuan S, Zhang Z, Li J, Zhang L, et al. Wogonoside attenuates liver fibrosis by triggering hepatic stellate cell ferroptosis through SOCS1/P53/SLC7A11 pathway. Phytother Res. (2022) 36:4230–43. doi: 10.1002/ptr.7558
32. Li L, Wang K, Jia R, Xie J, Ma L, Hao Z, et al. Ferroportin-dependent ferroptosis induced by ellagic acid retards liver fibrosis by impairing the SNARE complexes formation. Redox Biol. (2022) 56:102435. doi: 10.1016/j.redox.2022.102435
33. Yuan Y, Yucai L, Lu L, Hui L, Yong P, Haiyang Y. Acrylamide induces ferroptosis in HSC-T6 cells by causing antioxidant imbalance of the XCT-GSH-GPX4 signaling and mitochondrial dysfunction. Toxicol Lett. (2022) 368:24–32. doi: 10.1016/j.toxlet.2022.08.007
34. Tan Y, Huang Y, Mei R, Mao F, Yang D, Liu J, et al. HucMSC-derived exosomes delivered BECN1 induces ferroptosis of hepatic stellate cells via regulating the xCT/GPX4 axis. Cell Death Dis. (2022) 13:319. doi: 10.1038/s41419-022-04764-2
35. Liu GZ, Xu XW, Tao SH, Gao MJ, Hou ZH. HBx facilitates ferroptosis in acute liver failure via EZH2 mediated SLC7A11 suppression. J Biomed Sci. (2021) 28:67. doi: 10.1186/s12929-021-00762-2
36. Zhu Y, Zhang C, Huang M, Lin J, Fan X, Ni T. TRIM26 induces ferroptosis to inhibit hepatic stellate cell activation and mitigate liver fibrosis through mediating SLC7A11 Ubiquitination. Front Cell Dev Biol. (2021) 9:644901. doi: 10.3389/fcell.2021.644901
37. Peng Y, Li N, Tang F, Qian C, Jia T, Liu J, et al. Corosolic acid sensitizes ferroptosis by upregulating HERPUD1 in liver cancer cells. Cell Death Discov. (2022) 8:376. doi: 10.1038/s41420-022-01169-0
38. Liu Y, Ouyang L, Mao C, Chen Y, Li T, Liu N, et al. PCDHB14 promotes ferroptosis and is a novel tumor suppressor in hepatocellular carcinoma. Oncogene. (2022) 41:3570–83. doi: 10.1038/s41388-022-02370-2
39. Iseda N, Itoh S, Toshida K, Tomiyama T, Morinaga A, Shimokawa M, et al. Ferroptosis is induced by lenvatinib through fibroblast growth factor receptor-4 inhibition in hepatocellular carcinoma. Cancer Sci. (2022) 113:2272–87. doi: 10.1111/cas.15378
40. Yang X, Xiao J, Jiang L, Ran L, Fan Y, Zhang M, et al. A multifunctional vanadium-iron-oxide nanoparticle eradicates hepatocellular carcinoma via targeting tumor and endothelial cells. ACS Appl Mater Interfaces. (2022) 14:28514–26. doi: 10.1021/acsami.2c03474
41. Yang M, Wu X, Hu J, Wang Y, Wang Y, Zhang L, et al. COMMD10 inhibits HIF1alpha/CP loop to enhance ferroptosis and radiosensitivity by disrupting Cu-Fe balance in hepatocellular carcinoma. J Hepatol. (2022) 76:1138–50. doi: 10.1016/j.jhep.2022.01.009
42. He GN, Bao NR, Wang S, Xi M, Zhang TH, Chen FS. Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther. (2021) 15:3965–78. doi: 10.2147/DDDT.S332847
43. Wang Z, Li M, Liu Y, Qiao Z, Bai T, Yang L, et al. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein responseinduced upregulation of CHAC1 expression. Oncol Rep. (2021) 46:240. doi: 10.3892/or.2021.8191
44. Su Y, Zhao D, Jin C, Li Z, Sun S, Xia S, et al. Dihydroartemisinin induces ferroptosis in HCC by promoting the formation of PEBP1/15-LO. Oxid Med Cell Longev. (2021) 2021:3456725. doi: 10.1155/2021/3456725
45. Zhu G, Murshed A, Li H, Ma J, Zhen N, Ding M, et al. O-GlcNAcylation enhances sensitivity to RSL3-induced ferroptosis via the YAP/TFRC pathway in liver cancer. Cell Death Discov. (2021) 7:83. doi: 10.1038/s41420-021-00468-2
46. Sun J, Zhou C, Zhao Y, Zhang X, Chen W, Zhou Q, et al. Quiescin sulfhydryl oxidase 1 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by driving EGFR endosomal trafficking and inhibiting NRF2 activation. Redox Biol. (2021) 41:101942. doi: 10.1016/j.redox.2021.101942
47. Li K, Xu K, He Y, Lu L, Mao Y, Gao P, et al. Functionalized tumor-targeting nanosheets exhibiting Fe(II) overloading and GSH consumption for ferroptosis activation in liver tumor. Small. (2021) 17:e2102046. doi: 10.1002/smll.202102046
48. Liu X, Zhu X, Qi X, Meng X, Xu K. Co-administration of iRGD with sorafenib-loaded iron-based metal-organic framework as a targeted ferroptosis agent for liver cancer therapy. Int J Nanomedicine. (2021) 16:1037–50. doi: 10.2147/IJN.S292528
49. Tian H, Zhao S, Nice EC, Huang C, He W, Zou B, et al. A cascaded copper-based nanocatalyst by modulating glutathione and cyclooxygenase-2 for hepatocellular carcinoma therapy. J Colloid Interface Sci. (2022) 607:1516–26. doi: 10.1016/j.jcis.2021.09.049
50. Zhang Y, Luo M, Cui X, O'connell D, Yang Y. Long noncoding RNA NEAT1 promotes ferroptosis by modulating the miR-362-3p/MIOX axis as a ceRNA. Cell Death Differ. (2022) 29:1850–63. doi: 10.1038/s41418-022-00970-9
51. Asperti M, Bellini S, Grillo E, Gryzik M, Cantamessa L, Ronca R, et al. H-ferritin suppression and pronounced mitochondrial respiration make Hepatocellular Carcinoma cells sensitive to RSL3-induced ferroptosis. Free Radic Biol Med. (2021) 169:294–303. doi: 10.1016/j.freeradbiomed.2021.04.024
52. Wang YF, Feng JY, Zhao LN, Zhao M, Wei XF, Geng Y, et al. Aspirin triggers ferroptosis in hepatocellular carcinoma cells through restricting NF-kappaB p65-activated SLC7A11 transcription. Acta Pharmacol Sin. (2023). doi: 10.1038/s41401-023-01062-1
53. Lee D, Xu IM, Chiu DK, Leibold J, Tse AP, Bao MH, et al. Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology. (2019) 69:1768–86. doi: 10.1002/hep.30467
54. Li D, Pan J, Zhang Y, Li Y, Jin S, Zhong C, et al. C8orf76 modulates ferroptosis in liver cancer via transcriptionally up-regulating SLC7A11. Cancers. (2022) 14:3410. doi: 10.3390/cancers14143410
55. Zhang Q, Qu Y, Zhang Q, Li F, Li B, Li Z, et al. Exosomes derived from hepatitis B virus-infected hepatocytes promote liver fibrosis via miR-222/TFRC axis. Cell Biol Toxicol. (2022). doi: 10.1007/s10565-021-09684-z
56. Yang W, Wang Y, Zhang C, Huang Y, Yu J, Shi L, et al. Maresin1 protect against ferroptosis-induced liver injury through ROS inhibition and Nrf2/HO-1/GPX4 activation. Front Pharmacol. (2022) 13:865689. doi: 10.3389/fphar.2022.865689
57. Xue M, Tian Y, Sui Y, Zhao H, Gao H, Liang H, et al. Protective effect of fucoidan against iron overload and ferroptosis-induced liver injury in rats exposed to alcohol. Biomed Pharmacother. (2022) 153:113402. doi: 10.1016/j.biopha.2022.113402
58. Ye J, Peng J, Liu K, Zhang T, Huang W. MCTR1 inhibits ferroptosis by promoting NRF2 expression to attenuate hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. (2022) 323:G283–93. doi: 10.1152/ajpgi.00354.2021
59. Cai X, Hua S, Deng J, Du Z, Zhang D, Liu Z, et al. Astaxanthin activated the Nrf2/HO-1 pathway to enhance autophagy and inhibit ferroptosis, ameliorating acetaminophen-induced liver injury. ACS Appl Mater Interfaces. (2022) 14:42887–903. doi: 10.1021/acsami.2c10506
60. Liu T, Yang L, Gao H, Zhuo Y, Tu Z, Wang Y, et al. 3,4-dihydroxyphenylethyl alcohol glycoside reduces acetaminophen-induced acute liver failure in mice by inhibiting hepatocyte ferroptosis and pyroptosis. PeerJ. (2022) 10:e13082. doi: 10.7717/peerj.13082
61. Xing G, Meng L, Cao S, Liu S, Wu J, Li Q, et al. PPARalpha alleviates iron overload-induced ferroptosis in mouse liver. EMBO Rep. (2022) 23:e52280. doi: 10.15252/embr.202052280
62. Wu A, Feng B, Yu J, Yan L, Che L, Zhuo Y, et al. Fibroblast growth factor 21 attenuates iron overload-induced liver injury and fibrosis by inhibiting ferroptosis. Redox Biol. (2021) 46:102131. doi: 10.1016/j.redox.2021.102131
63. Gao G, Xie Z, Li EW, Yuan Y, Fu Y, Wang P, et al. Dehydroabietic acid improves nonalcoholic fatty liver disease through activating the Keap1/Nrf2-ARE signaling pathway to reduce ferroptosis. J Nat Med. (2021) 75:540–52. doi: 10.1007/s11418-021-01491-4
64. Ji Y, Si W, Zeng J, Huang L, Huang Z, Zhao L, et al. Niujiaodihuang detoxify decoction inhibits ferroptosis by enhancing glutathione synthesis in acute liver failure models. J Ethnopharmacol. (2021) 279:114305. doi: 10.1016/j.jep.2021.114305
65. Cao F, Luo A, Yang C. G6PD inhibits ferroptosis in hepatocellular carcinoma by targeting cytochrome P450 oxidoreductase. Cell Signal. (2021) 87:110098. doi: 10.1016/j.cellsig.2021.110098
66. Zhang T, Sun L, Hao Y, Suo C, Shen S, Wei H, et al. ENO1 suppresses cancer cell ferroptosis by degrading the mRNA of iron regulatory protein 1. Nat Cancer. (2022) 3:75–89. doi: 10.1038/s43018-021-00299-1
67. Feng H, Liu Y, Gan Y, Li M, Liu R, Liang Z, et al. AdipoR1 regulates ionizing radiation-induced ferroptosis in HCC cells through Nrf2/xCT pathway. Oxid Med Cell Longev. (2022) 2022:8091464. doi: 10.1155/2022/8091464
68. Zhang X, Zheng Q, Yue X, Yuan Z, Ling J, Yuan Y, et al. ZNF498 promotes hepatocellular carcinogenesis by suppressing p53-mediated apoptosis and ferroptosis via the attenuation of p53 Ser46 phosphorylation. J Exp Clin Cancer Res. (2022) 41:79. doi: 10.1186/s13046-022-02288-3
69. Huang W, Chen K, Lu Y, Zhang D, Cheng Y, Li L, et al. ABCC5 facilitates the acquired resistance of sorafenib through the inhibition of SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia. (2021) 23:1227–39. doi: 10.1016/j.neo.2021.11.002
70. Tiefenbach J, Magomedova L, Liu J, Reunov AA, Tsai R, Eappen NS, et al. Idebenone and coenzyme Q10 are novel PPARalpha/gamma ligands, with potential for treatment of fatty liver diseases. Dis Model Mech. (2018) 11:dmm034801. doi: 10.1242/dmm.034801
71. Tang C, Li S, Zhang K, Li J, Han Y, Zhan T, et al. Selenium deficiency-induced redox imbalance leads to metabolic reprogramming and inflammation in the liver. Redox Biol. (2020) 36:101519. doi: 10.1016/j.redox.2020.101519
72. Chen MT, Huang JS, Gao DD, Li YX, Wang HY. Combined treatment with FABP4 inhibitor ameliorates rosiglitazone-induced liver steatosis in obese diabetic db/db mice. Basic Clin Pharmacol Toxicol. (2021) 129:173–82. doi: 10.1111/bcpt.13621
73. Demirel-Yalciner T, Sozen E, Ozaltin E, Sahin A, Ozer NK. alpha-Tocopherol supplementation reduces inflammation and apoptosis in high cholesterol mediated nonalcoholic steatohepatitis. Biofactors. (2021) 47:403–13. doi: 10.1002/biof.1700
74. Adelusi OB, Ramachandran A, Lemasters JJ, Jaeschke H. The role of Iron in lipid peroxidation and protein nitration during acetaminophen-induced liver injury in mice. Toxicol Appl Pharmacol. (2022) 445:116043. doi: 10.1016/j.taap.2022.116043
75. Simao M, Cancela ML. Musculoskeletal complications associated with pathological iron toxicity and its molecular mechanisms. Biochem Soc Trans. (2021) 49:747–59. doi: 10.1042/BST20200672
76. Jiang H, Zhang X, Yang W, Li M, Wang G, Luo Q. Ferrostatin-1 ameliorates liver dysfunction via reducing iron in thioacetamide-induced acute liver injury in mice. Front Pharmacol. (2022) 13:869794. doi: 10.3389/fphar.2022.869794
77. Jiang JJ, Zhang GF, Zheng JY, Sun JH, Ding SB. Targeting mitochondrial ROS-mediated ferroptosis by quercetin alleviates high-fat diet-induced hepatic lipotoxicity. Front Pharmacol. (2022) 13:876550. doi: 10.3389/fphar.2022.876550
78. Salama SA, Abdel-Bakky MS, Mohamed AA. Upregulation of Nrf2 signaling and suppression of ferroptosis and NF-kappaB pathway by leonurine attenuate iron overload-induced hepatotoxicity. Chem Biol Interact. (2022) 356:109875. doi: 10.1016/j.cbi.2022.109875
79. Liu B, Yi W, Mao X, Yang L, Rao C. Enoyl coenzyme A hydratase 1 alleviates nonalcoholic steatohepatitis in mice by suppressing hepatic ferroptosis. Am J Physiol Endocrinol Metab. (2021) 320:E925–37. doi: 10.1152/ajpendo.00614.2020
80. Zhu Z, Zhang Y, Huang X, Can L, Zhao X, Wang Y, et al. Thymosin beta 4 alleviates non-alcoholic fatty liver by inhibiting ferroptosis via up-regulation of GPX4. Eur J Pharmacol. (2021) 908:174351. doi: 10.1016/j.ejphar.2021.174351
81. Angeli JPF, Shah R, Pratt DA, Conrad M. Ferroptosis inhibition: mechanisms and opportunities. Trends Pharmacol Sci. (2017) 38:489–98. doi: 10.1016/j.tips.2017.02.005
82. Tonnus W, Meyer C, Steinebach C, Belavgeni A, Von Massenhausen A, Gonzalez NZ, et al. Dysfunction of the key ferroptosis-surveilling systems hypersensitizes mice to tubular necrosis during acute kidney injury. Nat Commun. (2021) 12:4402. doi: 10.1038/s41467-021-24712-6
Keywords: ferroptosis, targets, application, advancement, liver diseases
Citation: Xiang X, Gao J, Su D and Shi D (2023) The advancements in targets for ferroptosis in liver diseases. Front. Med. 10:1084479. doi: 10.3389/fmed.2023.1084479
Received: 30 October 2022; Accepted: 27 February 2023;
Published: 14 March 2023.
Edited by:
Han Wu, Eastern Hepatobiliary Surgery Hospital, ChinaReviewed by:
Feng Zhang, Nanjing University of Chinese Medicine, ChinaCopyright © 2023 Xiang, Gao, Su and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaohong Xiang, MTYxNTcwNjIxNEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.