- 1The Graduate School, Tianjin Medical University, Tianjin, China
- 2The Graduate School, Qinghai University, Xining, China
- 3Clinical Laboratory, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 4National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, China
- 5Department of Ophthalmology, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 6Shunde Hospital of Southern Medical University, Foshan, China
Objective: To objectively evaluate the efficacy of the Zilongjin tablets in non-small cell lung cancer (NSCLC) and to explore its potential mechanism of action against NSCLC and COVID-19 based on network pharmacology.
Methods: The database was searched for randomized controlled trials (RCTs) of the Zilongjin tablets for NSCLC published up to 22 August 2022. The quality of included trials was assessed using Cochrane standard guidelines, and a meta-analysis was performed using Rev Man 5.3. Gene targets for intersections of NSCLC and COVID-19 (the NC) and drugs were obtained from the TCMSP database, HERB database, GeneCards database, and the NCBI database for network pharmacology research.
Results: Meta-analysis included 14 articles with 2,430 patients. The meta-analysis showed that the Zilongjin tablets combined with conventional chemotherapy were significantly more effective than chemotherapy alone in the treatment of NSCLC. A total of 29 drug-disease intersecting targets were identified in the network pharmacology. The “ingredient-target-pathway” diagram component-target-pathway network contained 119 nodes and 429 edges, with the majority of targets associated with inflammatory responses.
Conclusion: The efficacy and quality of life of the Zilongjin tablets combined conventional chemotherapy for NSCLC were significantly better than chemotherapy alone, alleviating various adverse effects. At the same time, the Zilongjin tablets may modulate the inflammatory response to alleviate NSCLC and COVID-19.
1. Introduction
Lung cancer has a high incidence and mortality rate, with approximately 1.8 million new cases and 1.6 million deaths per year, and the 5-year survival rate of lung cancer ranges from 4 to 17%. Moreover, it is the leading cause of cancer-related death among men; and the second leading cause of cancer-related death among women worldwide (1, 2). Non-small cell lung cancer (NSCLC) accounts for about 84% of all types of lung cancer and has a poor prognosis, especially in advanced stages (3, 4). Treatment of lung cancer includes surgery, chemotherapy, radiotherapy, molecular targeted therapy, and immunotherapy (5). Currently, chemotherapy is still the first choice for lung cancer treatment, however, the more severe the disease progression, the less tolerant patients are to chemotherapy, due to the painful disease symptoms and severe adverse effects caused by chemotherapy.
At the time of the COVID-19 pandemic, a large number of lung cancer patients face difficulty in admission to a hospital for treatment, and their risk of COVID-19 infection is 2.31 times higher than that of the general population, due to immunosuppression and respiratory infections (6–9). When lung cancer patients are infected, they have a higher risk of disease progression, so COVID-19 combined with NSCLC leads to a higher mortality rate and a worse prognosis than the general population. Currently, the National Institute for Health and Care Excellence (NICE) has published a clinical guideline for standard anticancer treatment for cancer patients with COVID-19 while the available studies do not appear specific values for the effect of COVID-19 treatment (10–12, 14). Additionally, patients may experience lung damage symptoms for a while after the COVID-19 fever goes away, including fatigue, shortness of breath, drowsiness, dizziness, loss of appetite, shortness of breath, sweating, coughing, and chest pain—the same side effects as those brought on by chemotherapy for NSCLC. The pathogenesis of both conditions is characterized by qi and blood insufficiency, unexhausted residual heat, and a connection between phlegm and blood stasis. Therefore, the similarity of symptoms and pathogenesis may suggest that they are mechanistically related.
A large number of clinical studies have shown that traditional Chinese medicine combined with chemotherapy can improve not only the anti-tumor effect but also enhance the immune function of the body (11). The Zilongjin tablet is a tonifying formula that has the advantageous effects of regulating qi, nourishing blood, clearing heat, detoxifying, and removing phlegm, having an anti-tumor function, improving the quality of life of tumor patients, reducing the adverse effects of chemotherapy, etc. Its ingredients mainly include the following eight herbs, Astragali Radix (AR, Huangqi in Chinese), Angelicae Sinensis Radix (ASR, Danggui in Chinese), Herba Solani Lyrati (HSL, Baimaoteng in Chinese), Solanum Nigrum Linn (SNL, Longkui in Chinese), Salviae liguliobae Radix (SLR, Danshen in Chinese), Scutellariae Barbatae Herba (SBH, Banzhilian in Chinese), Curcumae Radix (CR, Yujin in Chinese), and Duchesnea indica (DI, Shemei in Chineses) (12). In order to assess the efficacy of the Zilongjin tablets on NSCLC more objectively and provide evidence-based medical evidence, we comprehensively evaluated the efficacy of the Zilongjin tablets for NSCLC by meta-analysis. Besides, the study is a basis for follow-up studies to analyze the potential mechanism of the Zilongjin tablets on the intersection gene of NSCLC and COVID-19 (the NC) based on network pharmacology.
2. Method
2.1. Database and search strategy
The following electronic databases were searched to identify eligible trials: Chinese National Knowledge Infrastructure (CNKI), Wan-fang Database, China BioMedical Literature (CBM), MEDLINE (PubMed), EMBASE, Google Scholar, World Health Organization International Clinical Trials Registry (http://www.who.int/ictrp/en/), and US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov). Meanwhile, eligible trials were retrieved from the initial date until August 22, 2022. The following keywords and phrases, and their abbreviations or derivatives, were used separately or in combination: “the Zilongjin tablets” and “non-small cell lung cancer” or “NSCLC” and “RCT”. The search terms were adapted to different databases with a reliable search strategy developed by the Cochrane Collaboration (13). Finally, two reviewers managed and analyzed all studies by Zotero independently.
2.2. Study selection
2.2.1. Inclusion criteria: type of studies
All RCTs, published before August 22, 2022, were considered regardless of blinding.
2.2.2. Type of participants
Any patients with NSCLC of any ethnicity, gender, profession, and age were included.
2.2.3. Type of interventions
The experimental group was the Zilongjin tablets combined with a chemotherapy drug, and the chemotherapy drug combined with and without a placebo group was the control group.
2.3. Outcomes
The primary outcome includes: Objective response rate (ORR); disease control rate (DCR); Quality of life; and Adverse reactions.
2.4. Studies selection and data extraction
Two reviewers independently screened the titles and abstracts of searching results against pre-specified inclusion criteria to identify potential relevance. Disagreements were resolved by consensus. The third reviewer judged all articles included. Two reviewers systematically extracted data regarding study design, participant characteristics, interventions, and outcomes independently. Similarly, discrepancies were resolved by discussion between the two reviewers and consultation with the third reviewer.
2.4.1. Types of study designs to be included
We included randomized controlled trials (RCTs) irrespective of blinding or language. We excluded quasi-randomized trials.
2.4.2. Data extraction and management
Two reviewers independently extracted data, including details of the study population, intervention, and outcome, using a predesigned data extraction form. The following data were extracted: gross trial characteristics (title, authors, and year); baseline of the patient and disease data (gender, age, and sample size); interventions ways; and outcomes (outcome measures and the rate of adverse events). We resolved differences in data extraction by consensus or a third party. One reviewer entered data into Cochrane software Review Manager 5.3 (RevMan5.3) (14), and checked the data to reduce data entry errors.
2.5. Quality assessment
The risk of bias evaluation tool proposed by the Cochrane Collaboration was utilized to address the following seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other bias” (15). The risk of bias for each item was summarized into three categories: low, unclear, and high. The first reviewer performed the quality assessment with supervision from the other two reviewers.
2.6. Data synthesis and measures of treatment effect
The data were analyzed using Review Manager 5.3 software (14). Risk ratios (RRs) were calculated using the Mantel–Haenszel method for dichotomous outcomes, and weighted mean differences (MDs) were calculated using the inverse variance method for continuous variables. We used I2 statistics to assess the heterogeneity. A fixed-effect (FE) model was used if there was no significant heterogeneity in the data (I2 < 50%), and a random-effect (RE) model was used if significant heterogeneity was present (I2 > 50%) (16).
2.7. Screening of drug components
The names of all the ingredients of Zilongjin Tablets were retrieved through the TCMSP database (https://tcmspw.com/tcmsp.php), HERB database (http://herb.ac.cn/). Furthermore, all the drug components were collated and retrieved in the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) to obtain the CID numbers, Smiles numbers, and canonical molecular formulas of the active ingredients by Python. The detected drug compounds were imported into the STITCH database (http://stitch.embl.de/cgi/input.pl) to obtain the gene targets interacting with the active ingredient of the Zilongjin tablets, selecting “Homo sapiens”, and screening the targets with a combined score >0.95. We also obtained the gene targets from the HERB database (P < 0.05). Then, we collated all gene targets and screened out duplicate items.
2.8. Retrieval of disease gene targets
The disease targets about the NSCLC and COVID-19 were retrieved from the GeneCards database (https://www.genecards.org) and the NCBI database (https://www.ncbi.nlm.nih.gov) respectively. Additionally, we de-duplicated them with scores >10 in the GeneCards database. Bioinformatics & Evolutionary Genomics (http://bioinformatics.psb.ugent.be/webtools/Venn/) was used to intersect the genes of NSCLC and COVID-19, and the two disease intersections are referred to as the NC.
2.9. Screening of the gene targets of the drug-disease
Using the Bioinformatics & Evolutionary Genomics website, the gene targets of the Zilongjin tablets were intersected with the NC disease targets to obtain the targets of the Zilongjin tablets for both diseases. The 29 targets obtained by screening were imported into the STRING database (http://string-db.org) for the protein-protein interaction (PPI) network. We selected the species as “Homo sapiens”, and the protein interaction score as “high confidence > 0.9”, hiding the unlinked nodes, and kept the rest of the parameters unchanged to obtain the PPI network map. Furthermore, we obtained the interactions information file “string_interactions.tsv” from the String database, imported the data into Cytoscape 3.8.2 software to restring the topology of analysis results by “Network Analyzer” function, and made the PPI network map according to the degree value.
2.10. Enrichment analysis
The intersecting targets of the Zilongjin tablets drug components and the NC were imported into The Database for Annotation, Visualization, and Integrated Discovery (DAVID, https://david.ncifcrf.gov/home.jsp) for Gene Ontology (GO) analysis, including biological process (BP), cellular component (CC), and molecular function (MF), and Kyoto Encyclopedia of Genes and Genomes (KEGG). The results were visualized and analyzed using a microbiology letter (http://www.bioinformatics.com.cn/login/) (17, 18). Meanwhile, we performed a visual topological analysis of the KEGG pathway by applying Cytoscape 3.8.2.
2.11. “Component-target-pathway” networks of the Zilongjin tablets and the NC
We obtained the drug components corresponding to the intersecting targets from the HERB database, and afterward, we docked it with the specific active ingredients of the Zilongjin tablets. Cytoscape 3.8.2 software was used to construct the active ingredient-the NC target network of the Zilongjin tablets, and “merge” and “group attributes layout” were used to cluster the active ingredients. The node size was described by “degree.”
3. Results
3.1. Literature search process
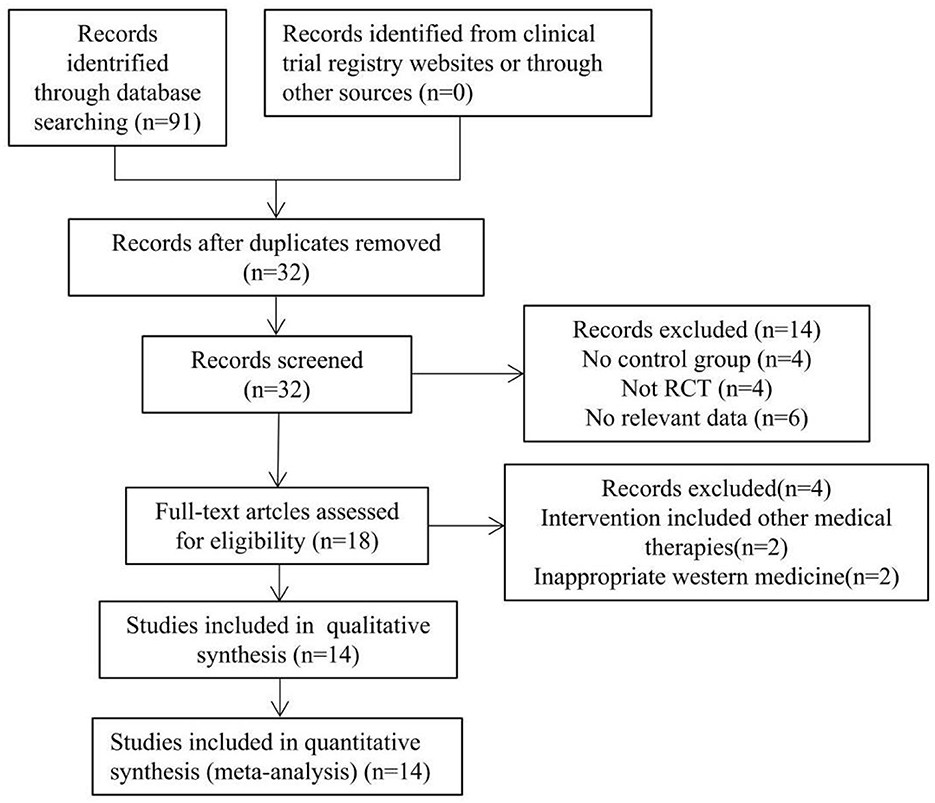
The flowchart of the study search results is shown in Figure 1. According to the inclusion and exclusion criteria, by reading the title, abstract, and full text of the literature, and excluding the repeated studies, cell and animal experiments, reviews and literature with inconsistent research contents, 14 RCTs that met the criteria was finally included (19–32), all of which were Chinese kinds of literature. The basic information of the included studies is shown in Table 1.
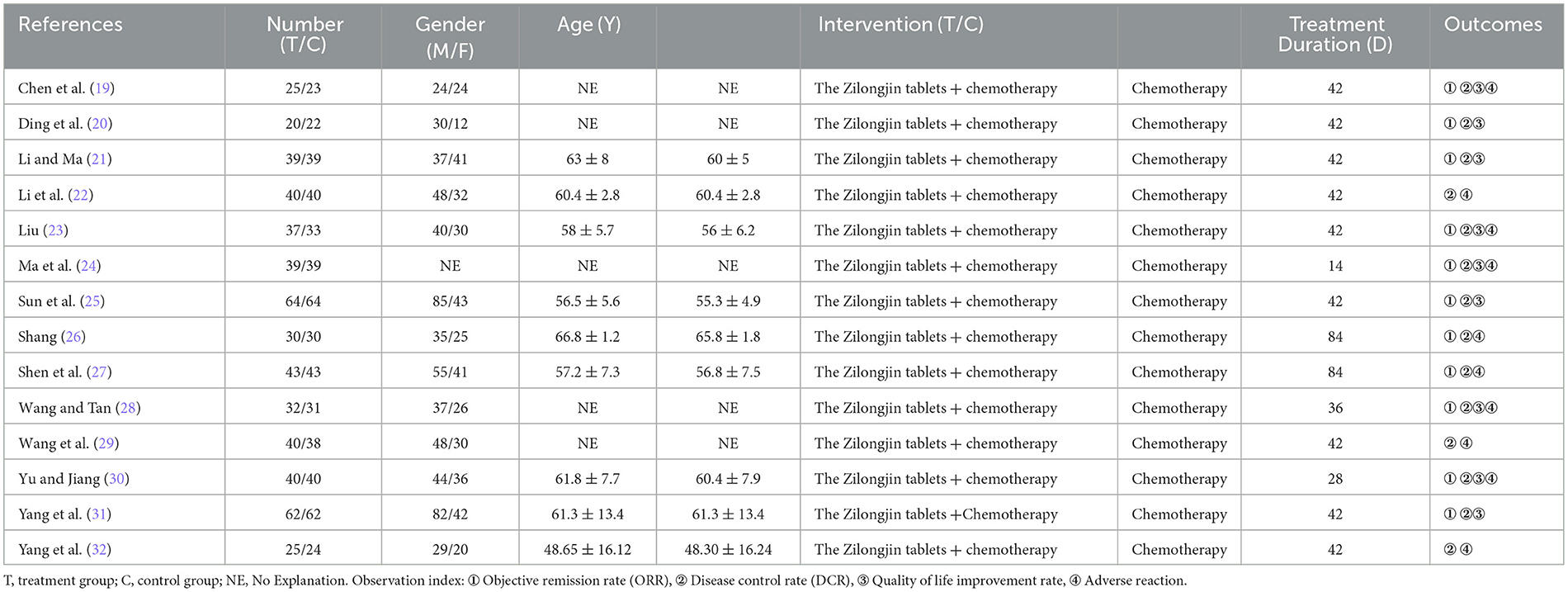
3.2. Characteristics of the included trials
The characteristics of the included 14 trials are summarized in Table 1. A total of 1,064 participants were involved, of which 536 and 528 were in the treatment and control groups.
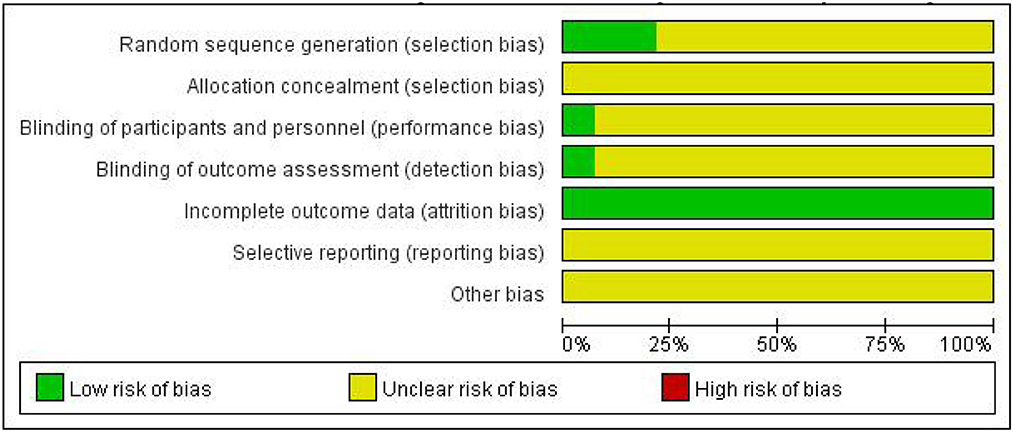
3.3. Quality assessment of included studies
As shown in Figures 2, 3, unclear risk of bias is in the majority as minimal information was available in many studies to permit a judgment of whether the risk of bias existed. All trials claimed that they were RCTs and described inclusion/exclusion criteria as well as grouping methods. Of the 14 RCTs, 3 three studies specified that they used a random number table to realize the randomization, while the others did not report the randomization procedures. Only 1 trial reported double-blind.
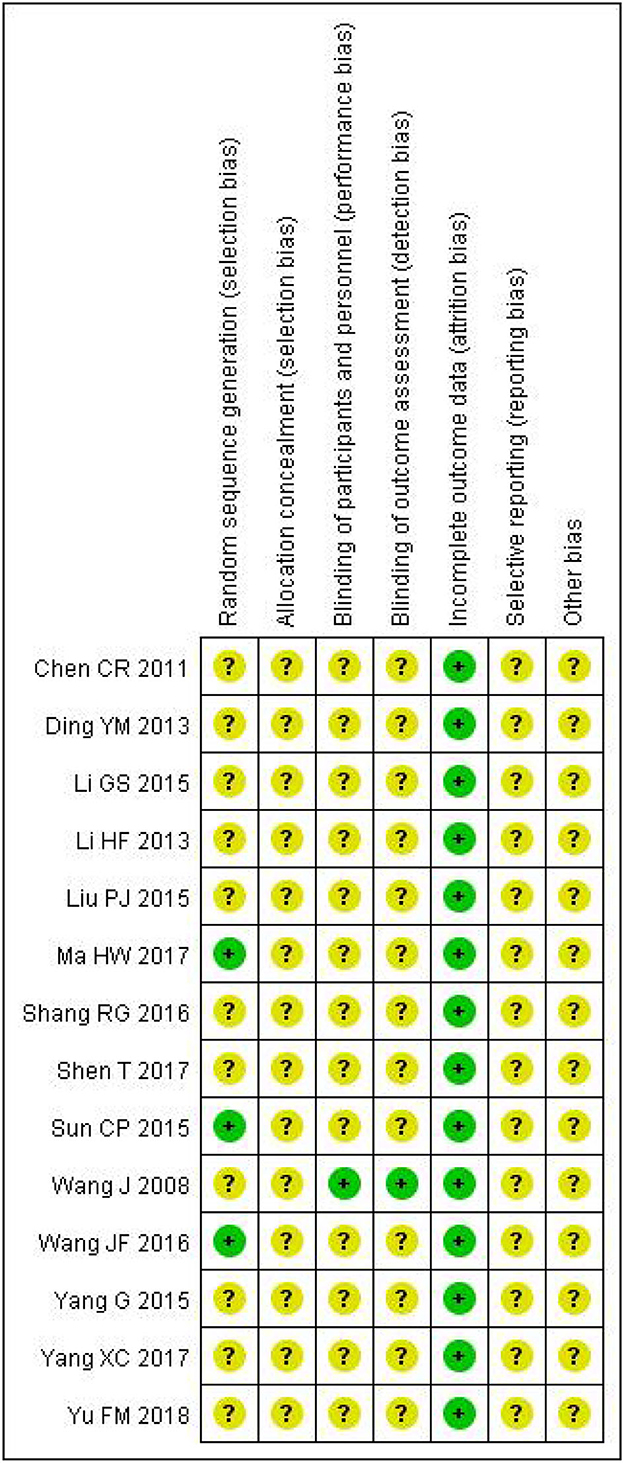
Figure 3. Risk of bias summary. The symbol “+”, low risk; the symbol “?”, unclear; the symbol “-”, high risk.
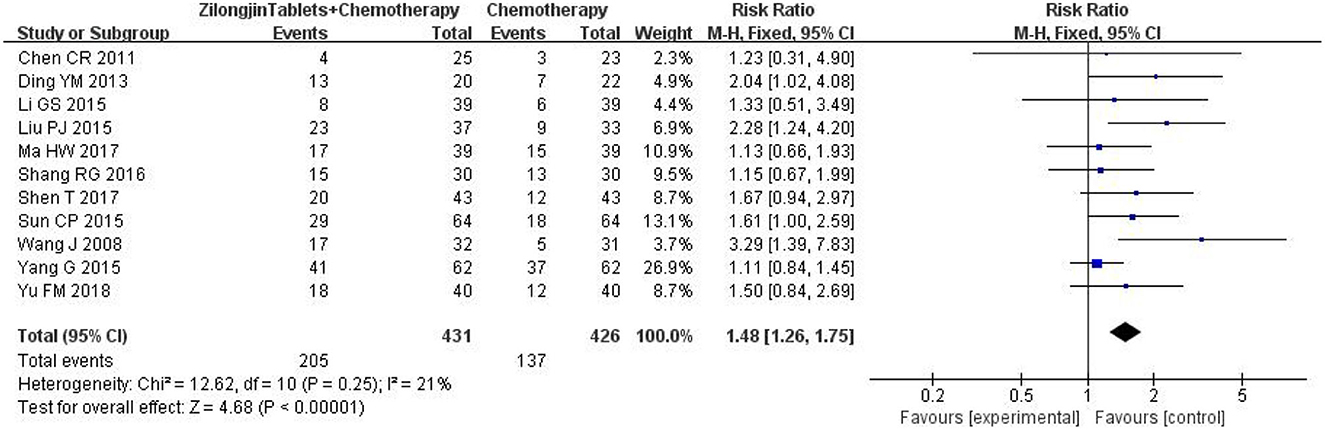
3.4. Objective response rate
A total of 11 studies with 857 subjects were included. There was no statistical heterogeneity among the 11 studies [Chi2 = 12.62, DF = 10 (P = 0.25); I2 = 21%] (Figure 4). Meta-analysis using the fixed-effect model showed that the improvement ORR of the Zilongjin tablets combined with chemotherapy in the treatment of NSCLC was significantly better than that of chemotherapy alone [OR = 1.48, 95%CI (1.26, 1.75), OR combined hypothesis test Z = 4.68, P < 0.0001].
3.5. Disease control rate
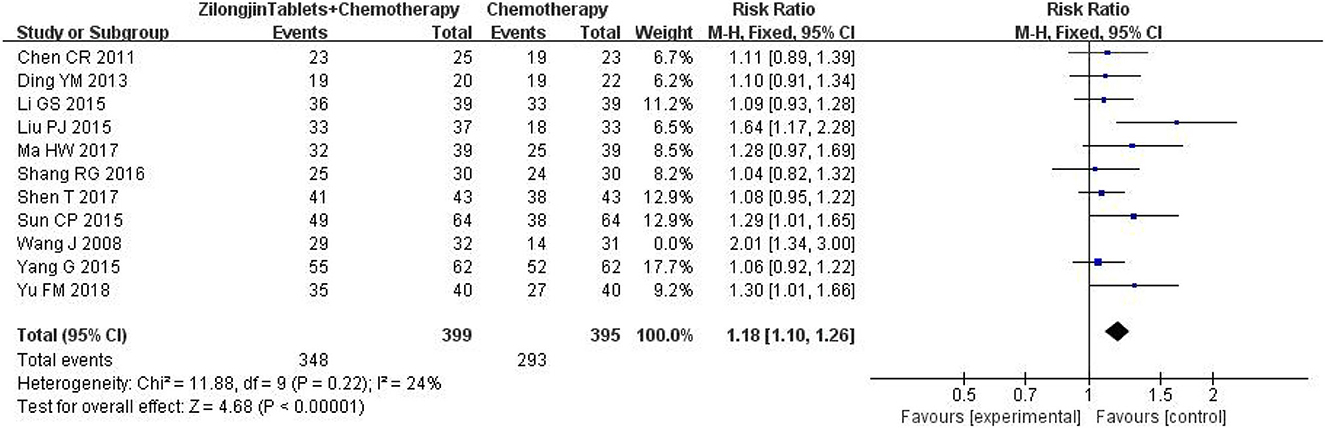
A total of 11 studies with 857 subjects were included by heterogeneity test, I2 = 54 > 50%, indicating heterogeneity among studies. The sensitivity analysis of 11 kinds of literature showed that the study of Wang J greatly impacted heterogeneity. After removing this research, the heterogeneity test was conducted again, and the results showed that there was no heterogeneity in the remaining literatures (I2 = 24%) (Figure 5). Meta-analysis using the fixed-effect model showed that the improvement DCR of the Zilongjin tablets combined with chemotherapy in the treatment of NSCLC was significantly better than that of chemotherapy alone [OR = 1.18, 95%CI (1.10, 1.26) OR combined hypothesis test Z = 4.68, P < 0.0001].
3.6. Quality of life improvement rate
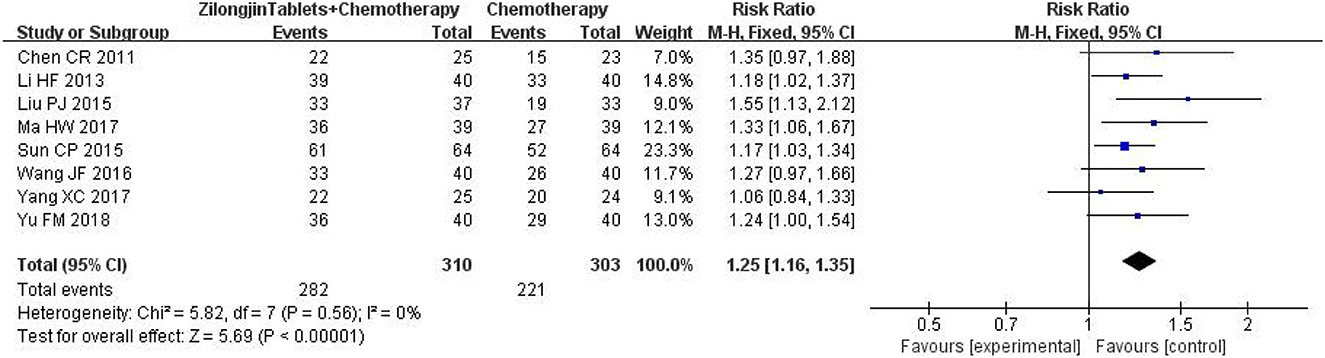
A total of 8 studies with 313 subjects were included. There was no statistical heterogeneity among the 8 studies [Chi2= 5.82, DF = 7 (P = 0.56); I2 = 0%] (Figure 6). Meta-analysis using the fixed-effect model showed that the improved quality of life of the Zilongjin tablets combined with chemotherapy in the treatment of NSCLC was significantly better than that of chemotherapy alone [OR = 1.25, 95%CI (1.16, 1.35), OR combined hypothesis test Z = 5.69, P < 0.0001].
3.7. The adverse event
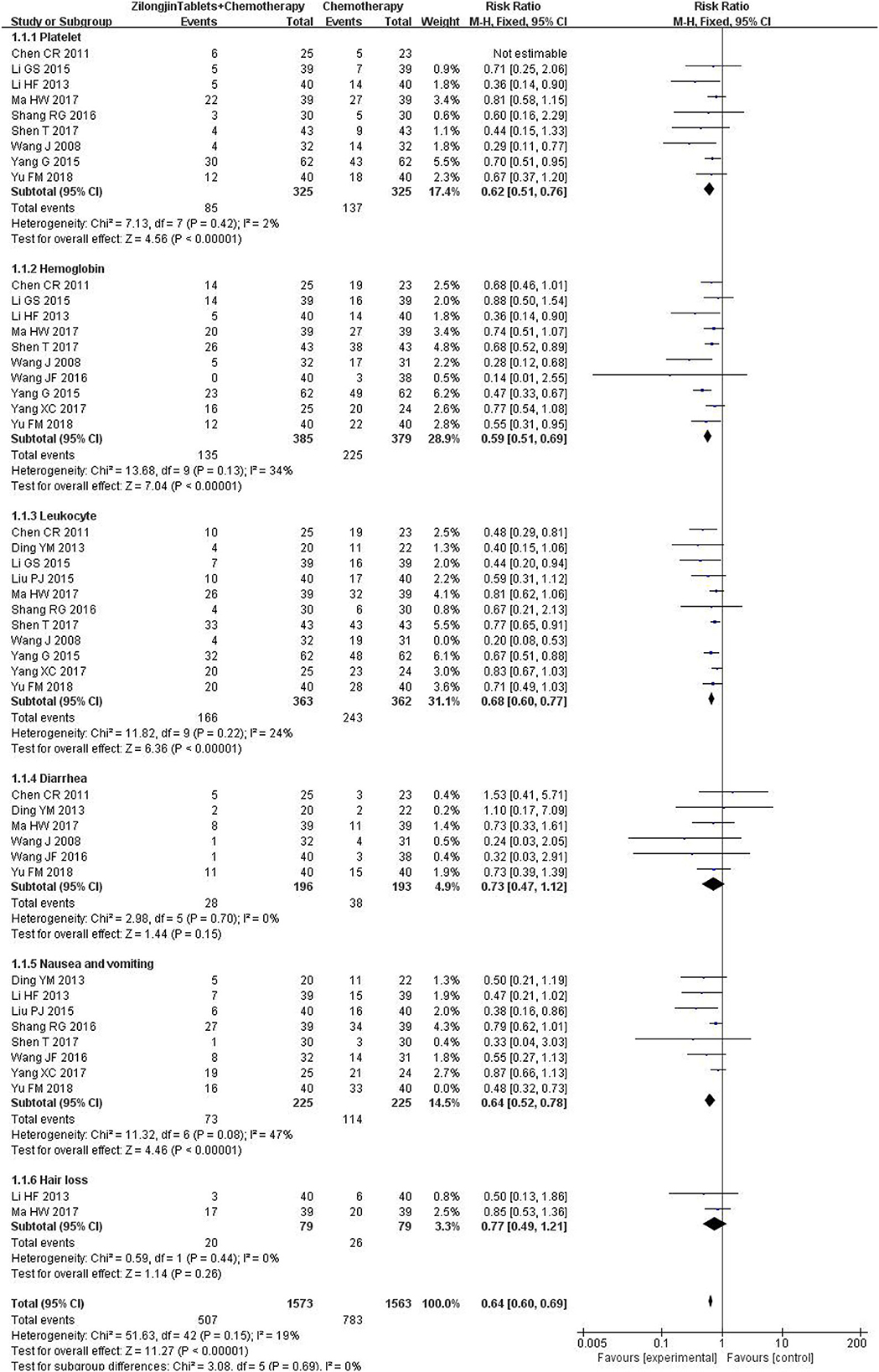
Platelet counts were reported in 9 studies (650 cases), and there was no statistical heterogeneity [Chi2 = 7.13, DF =7 (P = 0.42); I2 = 2%). The fixed effect model was used for meta-analysis (Figure 7). The results of the meta-analysis showed that the platelet count of the experimental group was significantly higher than that of the control group, and the difference was statistically significant [RR = 0.62, 95%CI (0.51, 0.76), Z = 4.56, P < 0.00001].
Hemoglobin was reported in 11 studies (849 cases), and there was no statistical heterogeneity [Chi2 = 13.68, DF = 9 (P = 0.13); I2 = 34%]. The fixed effect model was used for meta-analysis. The results of the meta-analysis showed that the hemoglobin of the experimental group was significantly higher than that of the control group, and the difference was statistically significant [RR = 0.59, 95%CI (0.51, 0.69), Z = 7.04, P < 0.000 01].
Leukocytes were reported in 11 studies (725 cases), and there was no statistical heterogeneity [Chi2 = 11.82, DF = 9 (P = 0.22); I2 = 24%]. The fixed effect model was used for meta-analysis. The results of the meta-analysis showed that the leukocyte of the experimental group was significantly higher than that of the control group, and the difference was statistically significant [RR = 0.68, 95%CI (0.60, 0.77), Z = 6.36, P < 0.00001].
Diarrhea was reported in 6 studies (389 cases), and there was no statistical heterogeneity [Chi2 = 2.98, DF = 5 (P = 0.70); I2 = 0%]. The fixed effect model was used for meta-analysis. The results of the meta-analysis showed that there was no significant difference in diarrhea between the experimental group and the control group [RR = 0.73, 95%CI (0.47,1.12), Z = 1.44, P = 0.15 > 0.05].
Nausea and vomiting were reported in 8 studies (450 cases) [Chi2 = 11.32, DF = 6 (P = 0.08); I2 = 47%]. The fixed effect model was used for meta-analysis. The results of the meta-analysis showed that nausea and vomiting of the experimental group were significantly lower than that of the control group, and the difference was statistically significant [RR = 0.64, 95%CI (0.52,0.78), Z = 4.46, P < 0.00001].
Hair loss was reported in 2 studies (158 cases), and there was no statistical heterogeneity [Chi2 = 0.59, DF = 1 (P = 0.44); I2 = 0%]. The fixed effect model was used for meta-analysis. The results of the meta-analysis showed that there was no significant difference in hair loss between the experimental group and the control group [RR = 0.77, 95%CI (0.49,1.21), Z = 1.14, P < 0.26 > 0.05].
3.8. Drug' number in network pharmacology database
AR (Herb id, HERB002560; TCMSP id, 187). ASR (Herb id, HERB001210; TCMSP id, 95). HSL (Herb id, HERB000246; TCMSP id, 16). SNL (Herb id, HERB003482; TCMSP id, 252; SymMap id, SMHB00252). SLR (Herb id, HERB001193; TCMSP id, 93; SymMap id, SMHB00093). SBH (Herb id, HERB000338; TCMSP id, 36; SymMap id: SMHB00036). CR (Herb id; HERB006718; TCMSP id, 469; SymMap id: SMHB00469). DI (Herb id, HERB004977).
3.9. Results of drug component
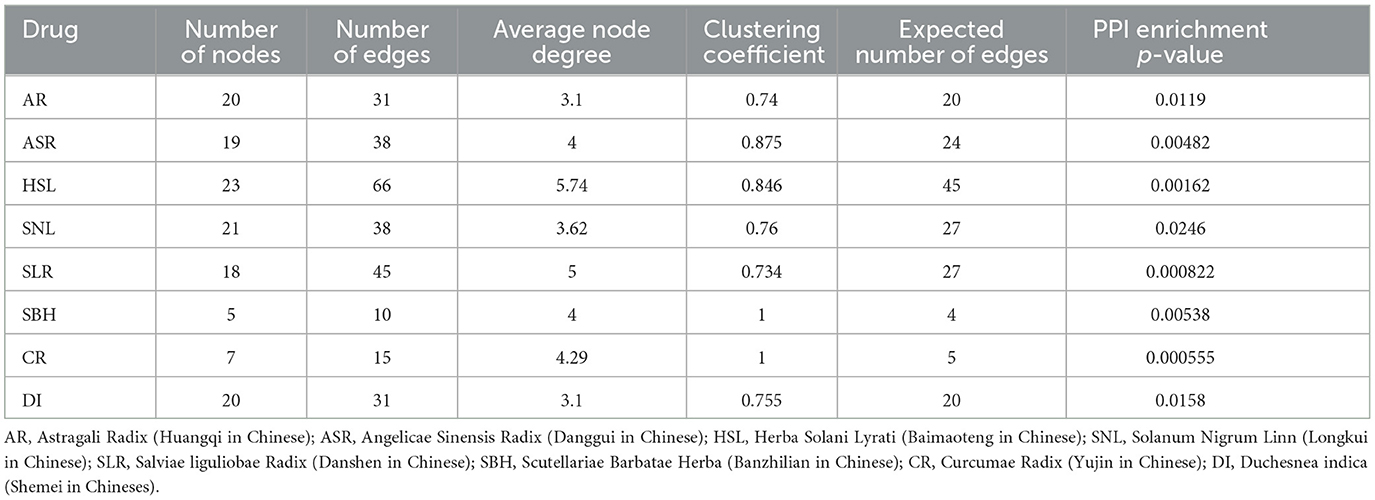
A total of 149 active ingredients of AR, 276 of ASR, 104 of HSL, 94 of SNL, 253 of SLR, 113 of SBH, 279 of CR, and 21 of DI. Additionally, a total of 787 after de-weighting. Then, we obtained the stitch-PPI networks of the drugs (Table 2).
3.10. Disease target search results
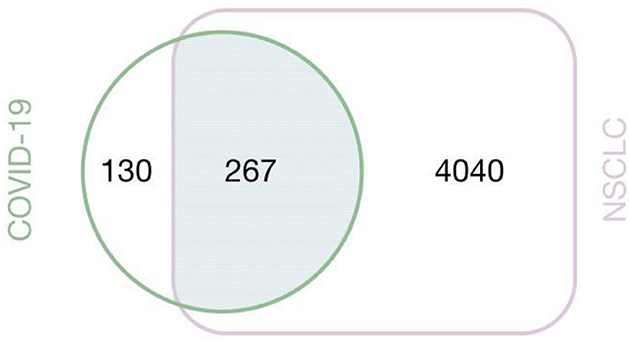
We obtained 397 COVID-19-related targets and 4,307 NSCLC-related targets in total, of which 267 were associated with COVID-19 and NSCLC (Figure 8).
3.11. TARGET-INGREDIENT-DRUG of the Zilongjin tablets on the NC
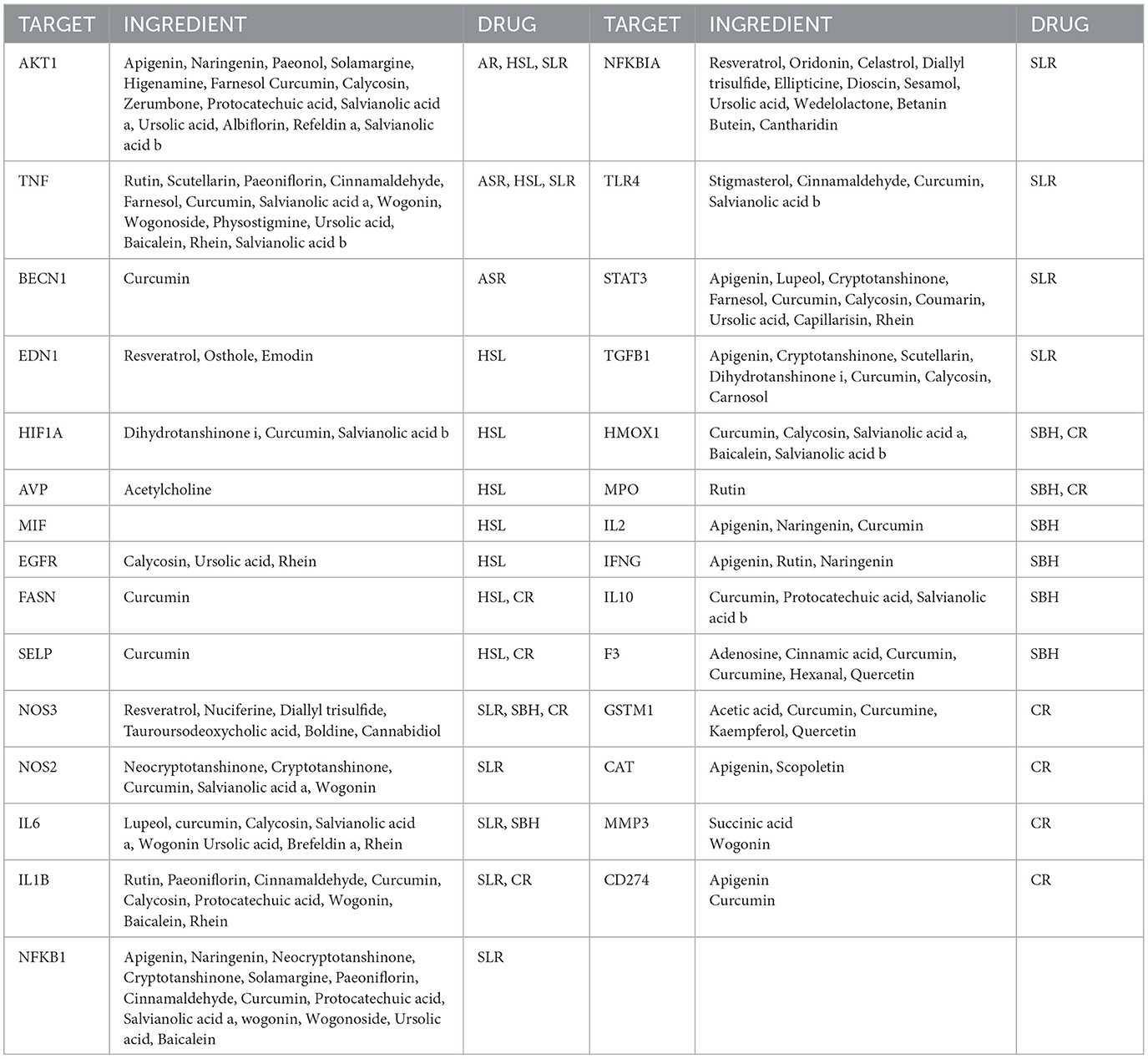
There are 29 targets where the gene targets of the Zilongjin tablets intersect with the NC (Table 3).
3.12. PPI data analysis
The intersection targets of drug components and the NC of the Zilongjin tablets were imported into the STRING database, and 74 proteins were obtained (PPI enrichment p-value: < 1.0e-16) (Figure 9A). In Cytoscape 3.8.2 software, 74 nodes and 289 edges were obtained using “Analyze network”, sorted by degree value, and the color and size evolved from pink to green with increasing degree value (Figure 9B). TNF (Degree = 79), IL6 (Degree = 76), IL1B (Degree = 70), STAT3 (Degree = 68), TP53 (Degree = 68), EGFR (Degree = 67), EGF (Degree = 62), TLR4 (Degree = 59), IL10 (Degree = 58), HSP90AA1 (Degree = 55), HIF1A (Degree = 55), IFNG (Degree = 54), NFKBIA (Degree = 52), IL2 (Degree = 51), and MTOR (Degree = 50) were the predicted important target proteins.
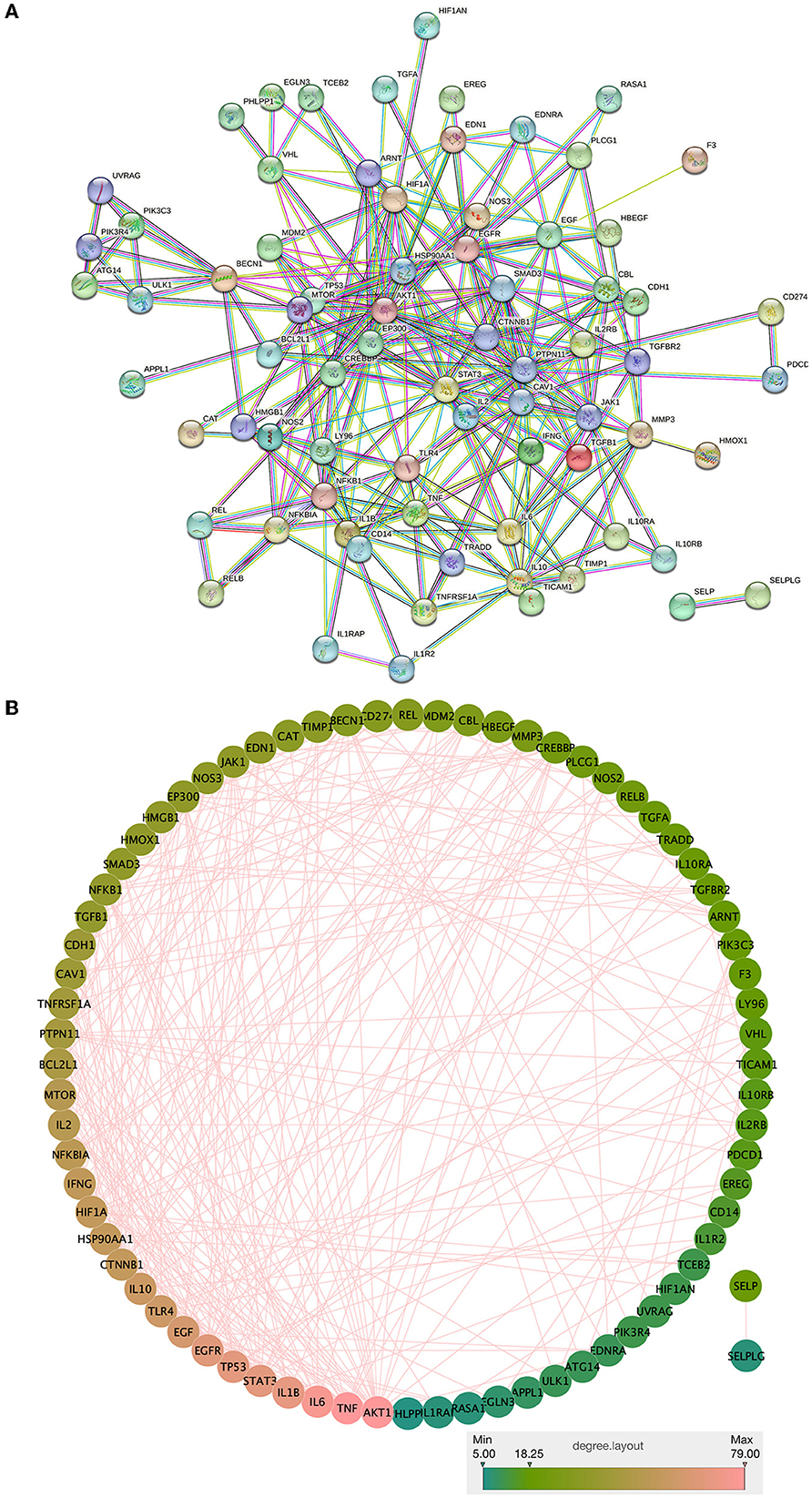
Figure 9. PPI network analysis and visualization of the intersection targets of Zilongjin tablets and the NC.
3.13. GO and KEGG enrichment analysis
Twenty-nine intersecting genes of the Zilongjin tablets acting on the NC were imported into the David data platform for GO analysis (BP, CC, and MF). A total of 285 entries in total for BP, mainly involving lipopolysaccharide-mediated signaling pathway (P < 0.01, count = 7, fold enrichment = 119.50), positive regulation of MAP kinase activity (P < 0.01, count = 6, fold enrichment = 58.26) and positive regulation of chemokine production (P < 0.01, count = 7, fold enrichment = 88.77), positive regulation of interleukin-8 production (P < 0.01, count = 7, fold enrichment = 71.70), et al. (Figure 10A). There were 21 entries for CC, with extracellular space (P < 0.01, count = 17, fold enrichment = 6.22), extracellular region (P < 0.01, count = 14, fold enrichment = 4.66), and secretory granule (P < 0.01, count = 4, fold enrichment = 28.65), et al. related (Figure 10A). MF had a total of 26 entries, including cytokine activity (P < 0.01, count = 9, fold enrichment = 30.50), enzyme binding (P < 0.01, count = 9, fold enrichment = 15.02), identical protein binding (P < 0.01, count = 13, fold enrichment = 4.93), heme binding (P < 0.01, count = 5, fold enrichment = 21.54) et al. (Figure 10A). KEGG pathways were enriched with a total of 102 entries, mainly involving Malaria (p < 0.01, count = 8, fold enrichment = 45.04), Inflammatory bowel disease (P < 0.01, count = 10, fold enrichment = 43.31), African trypanosomiasis (P < 0.01, count = 5, fold enrichment = 38.04), Antifolate resistance (P < 0.01, count = 4, fold enrichment = 37.54), et al. (Figure 10B).
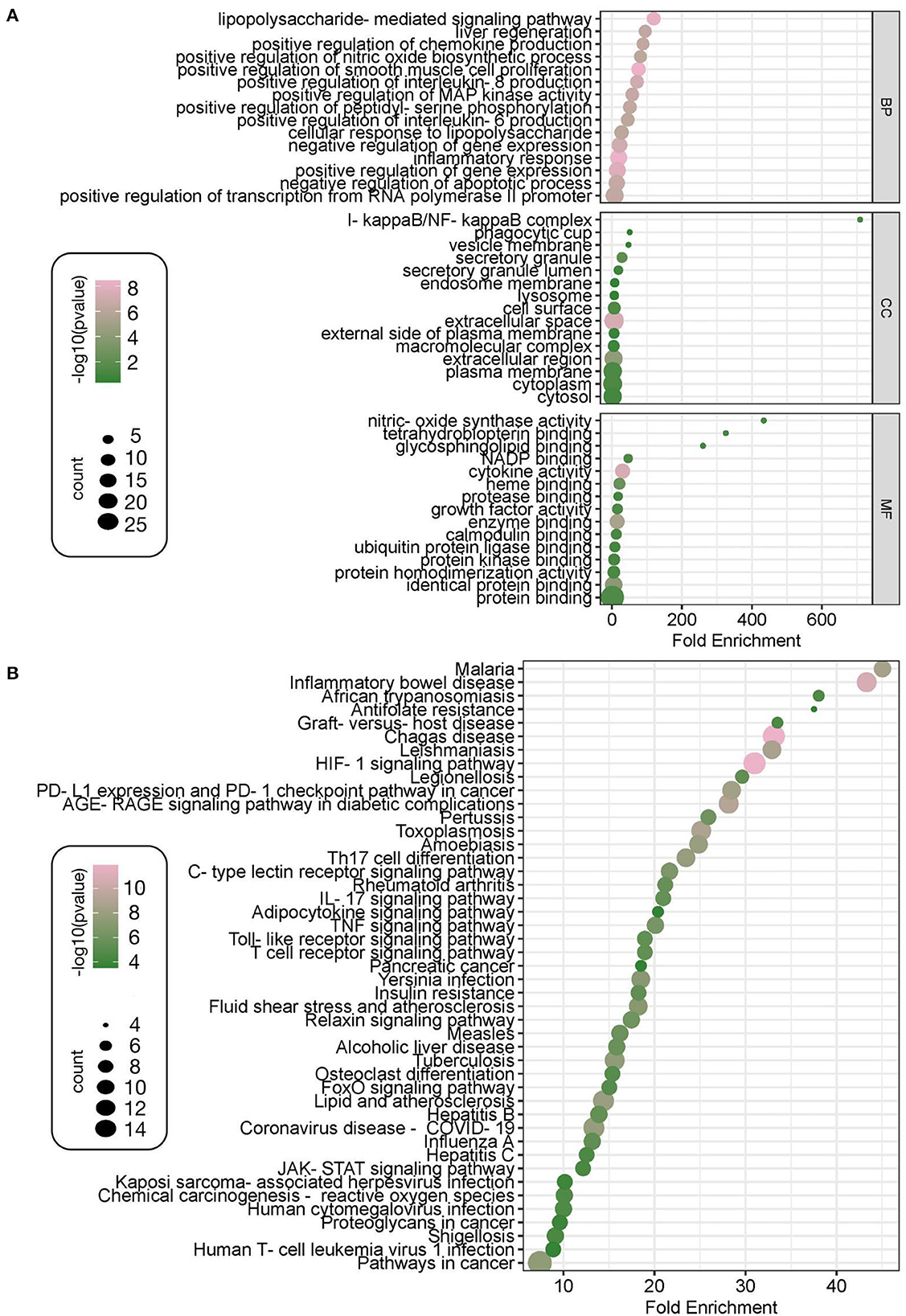
Figure 10. GO and KEGG enrichment analysis. The GO enriched terms of Zilongjin tablets in treating the NC include three parts, the enrichment content of BP (biological process), CC (cellular component), and MF (molecular function), and the 15 results are shown according to the high to low P values, respectively. The X-coordinate indicates the fold enrichment, and the color, corresponding to the value of -log10, of the dot represents the P value of the terms, the color is pinker to indicate the more significant difference [-log10 (p-value)>0.41 means p < 0.05 and -log10 (p-value) >0.88 means p < 0.01]. Besides, the “count” represents the number of genes enriched to this entry, and a bigger dot indicates that more genes are enriched (A). The 45 results of KEGG are shown according to the high to low enrichment of the KEGG pathway (B).
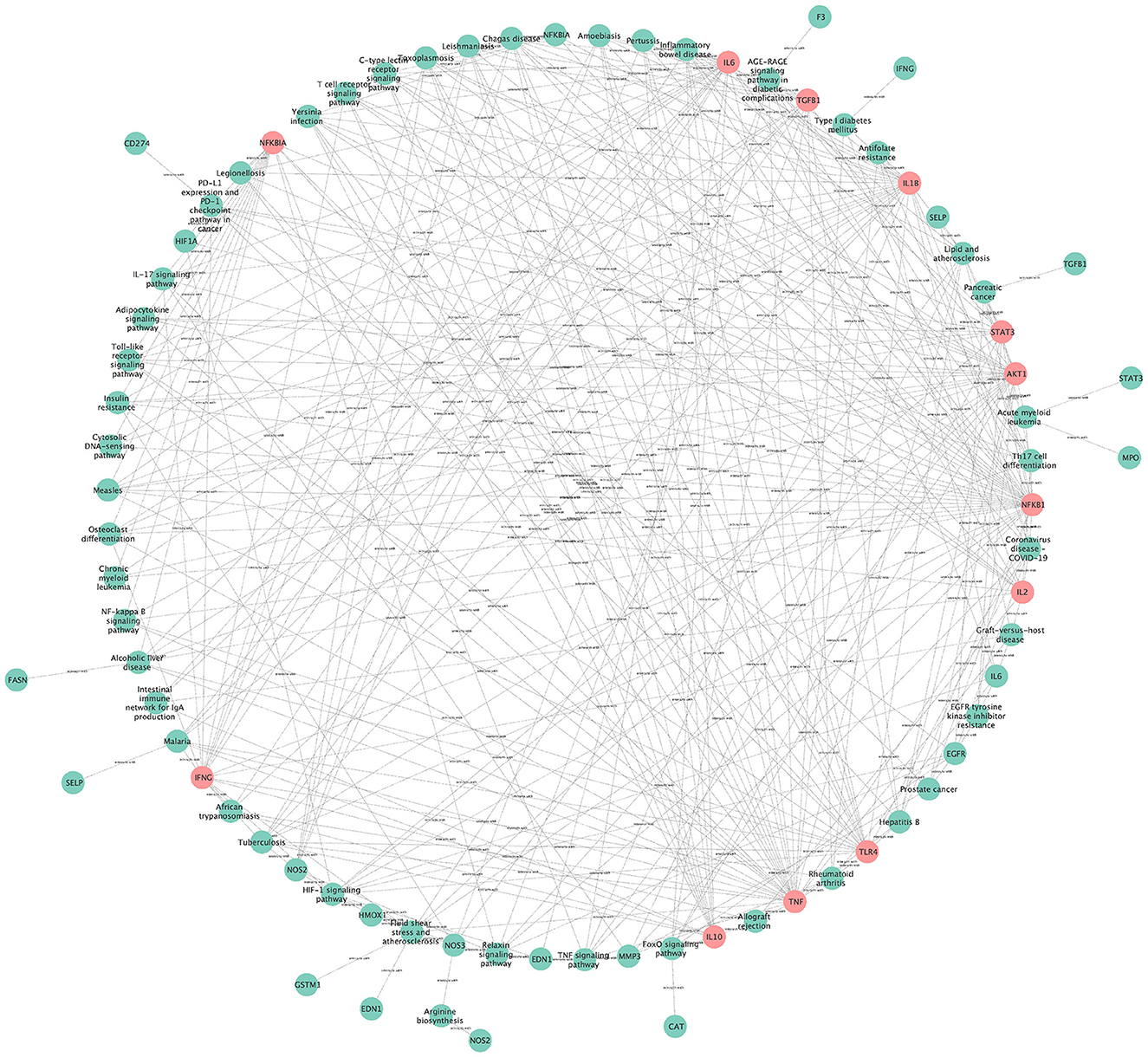
3.14. The network for visual topology analysis of KEGG pathways
The network for visual topology analysis of KEGG pathways contains 79 nodes with 322 edges (Figure 11), the green nodes are pathway genes (Degree < 10), and the pink nodes indicate pathway genes (Degree ≥ 10). The pathways with high degree values include Chagas disease (Degree = 12), Hypoxia-inducible factor 1 (HIF-1) signaling pathway (Degree = 12), Lipid and atherosclerosis (Degree = 11), Coronavirus disease COVID-19 (Degree = 11), Inflammatory bowel disease (Degree = 10), AGE-RAGE signaling pathway in diabetic complications (Degree = 10), Toxoplasmosis (Degree = 10), Tuberculosis (Degree = 10). In addition, targets with Degree values ≥10 include NFKB1 (Degree = 35), TNF (Degree = 31), IL1B (Degree = 28), AKT1 (Degree = 26), IL6 (Degree = 23), TLR4 (Degree = 20), IFNG (Degree = 18), NFKBIA (Degree = 17), TGFB1 (Degree = 16), IL10 (Degree = 15), STAT3 (Degree = 15), and IL2 (Degree = 12).
3.15. “Component-target-pathway” network for Zilongjin tables and the NC
The component-target-pathway network consists of 119 nodes and 429 edges. Among them, the 42 green nodes represent the active ingredients of the Zilongjin tablets, with high degree values such as lupeol, curcumin, and apigenin; the 25 pink nodes represent the targets of the Zilongjin tablets on the NC; the 52 purple nodes represent the pathways of the Zilongjin tablets on the NC (Figure 12). Lupeol (degree = 30), curcumin (degree = 19), apigenin (degree = 8), and calycosin (degree = 7) are the main components of the Zilongjin tablets acting on the NC. While Chagas disease (degree = 13), Tuberculosis (degree = 12), HIF-1 signaling pathway (degree = 11), Amoebiasis (degree = 11), and Inflammatory bowel disease [NFKB1 (degree = 48), TNF (degree = 45), AKT1 (degree = 41), IL1B (degree = 37), IL6 (degree = 37), and STAT3 (degree = 36)] are the main pathways through which the active ingredients of the Zilongjin tablets act on the NC. are the main targets of the Zilongjin tablets-the NC.
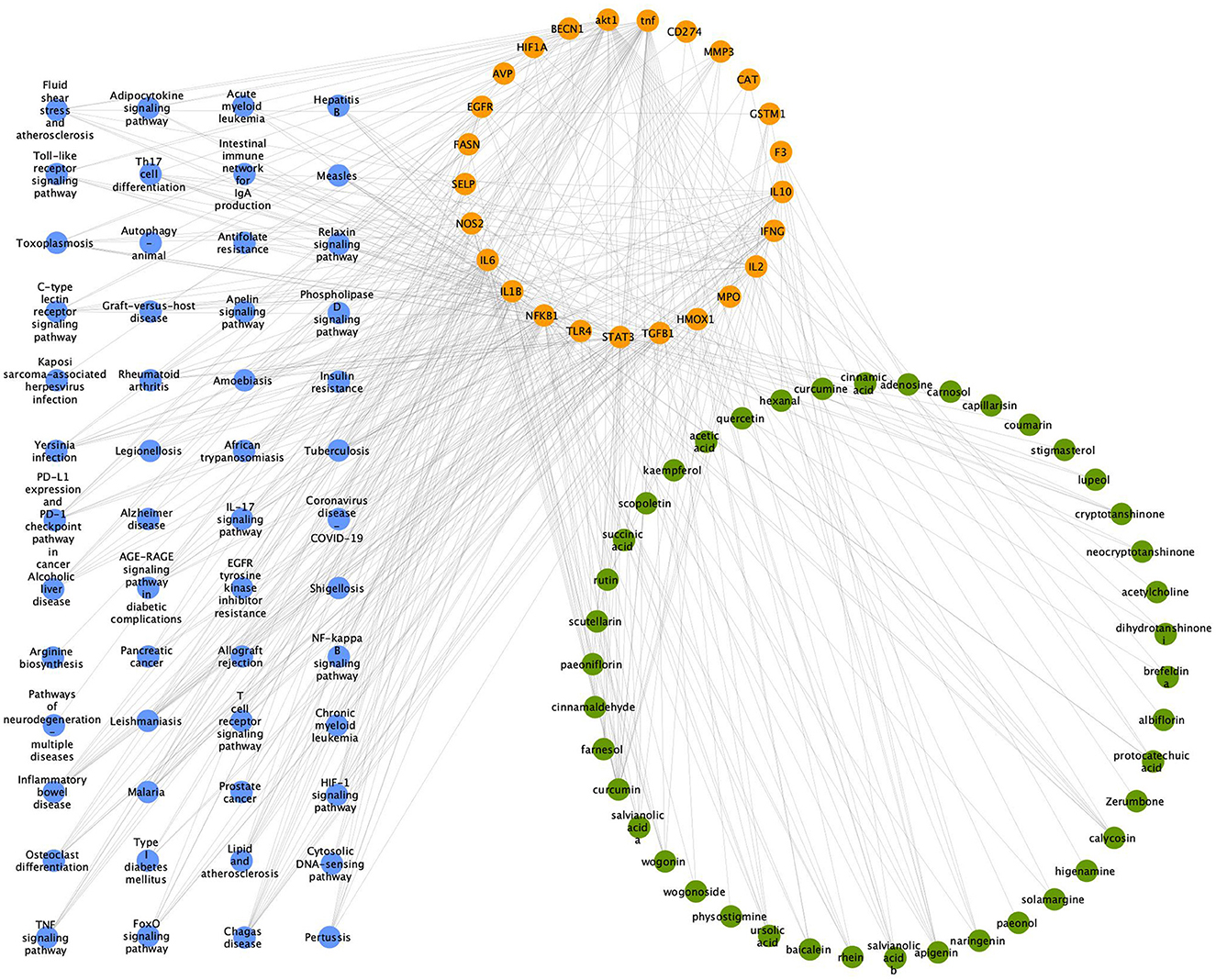
Figure 12. “Component-target-pathway” network for the Zilongjin tablets and the NC. The green nodes represent the drug component, the orange nodes represent the target, and the purple nodes represent the pathway.
4. Discussion
Lung cancer continues to be the most lethal disease in the world (33). The 5-year survival rate for lung cancer has increased over the past 10 years as a result of improvements in the treatment paradigm (34–39). Given that chemotherapy has considerable toxic side effects, including hematological and digestive side effects, it is imperative to consider combining it with other treatment alternatives to reduce the side effects and improve therapeutic outcomes. Adverse effects like vomiting, constipation, diarrhea, weakness, and poor appetite during treatment leave patients with profound memories of harm and affect their subsequent tolerance and adherence to treatment or even abandonment of treatment. It is true even though potent anti-emetic drugs, leukocyte- and platelet-raising drugs after myelosuppression ensure that chemotherapy is administered on time and in adequate doses. There is a dearth of evidence-based evidence, even though the Zilongjin tablets have been demonstrated to be beneficial in treating lung cancer. Comparing chemotherapy alone to chemotherapy plus the Zilongjin tablets, we found that combination therapy had a better impact on NSCLC outcomes and patients' quality of life and that treatment-induced hematological side effects, digestive system side effects, and hair loss were less severe.
Phlegm buildup and blood stagnation, which prevent the Qi of the lung from spreading and purifying, are the fundamental pathogenic factors for lung cancer. Cancer patients were experiencing immunity declining as the disease and its treatments and were often in touch with medical facilities during the COVID-19 outbreak because they needed therapy. Owing to hypo immunity and the lung damage caused by their lung cancer, patients are more susceptible to the SARS-CoV-2 of the COVID-19 virus, which can further exacerbate their condition. According to some disease reports, COVID-19 can cause severe infections and even death (40, 41). With the mortality rate ranging from 3 to 6%, multi-organ failure, acute respiratory distress syndrome, and septic shock occur in almost 20% of patients with COVID-19 (42). Although several therapies have been tried, none of them succeeded. Clinical care focuses more on symptom management, including organ transplantation in the ICU for patients who are severely sick.
According to the theory of traditional Chinese medcine (TCM), “Yili's qi” is the main cause of COVID-19. Moreover, COVID-19's occurrence and development are affected by several pathogenic variables, including moisture, toxicity, cold, heat, and stasis, and the interpretation of the cause is intricate. Many researchers have dissected the interpretation of the cause of COVID-19 from the perspectives of the “warm and turbid,” “heat-toxin,” “cold-dampness,” “cold-damp, the volatile-dry combo,” and “plague-toxicity”, but the conclusion has not been unified. Nowadays, the majority of studies categorize COVID-19 into four phases: (1) Early-stage symptoms might include cold-dampness invading the lungs, heat-dampness invading the lungs, etc. (2) Middle-late-stage symptoms gradually deepen the degree, “appearing to become heat due to depression” (the pathogenic factor transforms into the heat-toxin due to long-term stagnation), “the stagnation of pathogenic dampness and heat” (the combination of dampness and heat damages the body), “phlegm obstructs the lung” (due to difficulty to remove, the dampness gradually develops into a new pathogenic factor “phlegm-dampness”, which obstructs the function of the lung and causes lung damage), and “syndrome of lung collaterals injured by heat” (because the pathogenic factor “warmth-hotness” is more powerful, it depletes the lung fluid and burns the blood vessels in the lungs, forming blood stasis.). (3) In the recovery period, there is “intermingled deficiency and excess”. Although the healthy qi expelling pathogen, the qi is insufficient and the qi, blood, yin, and yang are deficient. Meanwhile, there is a possibility of recurrence of the disease due to the small amount of pathogen vested in the body. (4) In the critical stage, there are several symptoms, such as “syndrome of epidemic toxin blocking lung” (the pathogenic factor “epidemic toxin” blocking the lung qi, resulting in more serious lung ventilation and systemic symptoms).
Yet, there is no variation in the viral strains' capacity to infect various groups when people are infected with the same type of COVID-19 virus (SARS-CoV-2). The varying immunological capacities of various people are an influencing factor in disease severity. Because of high-level immunity, the general population with COVID-19 often has milder symptoms. In contrast, patients with COVID-19 combined NSCLC generally have more severe symptoms due to low-level immunity, and the disease symptoms are mainly intermingled deficiency and excess. The following explanations apply: (1) NSCLC patients have lung damage, so lung qi is weaker to resist pathogenic factors (The lung controls the Qi of the entire body, and the lung Qi has the function to resist pathogenic factors according to TCM theory). Additionally, the DUXIE (pathogenic factor) invading their bodies may even be guided by the patient's inherent pathogenic factors, aggravating the inherent condition. Then the pathogenic factors directly cause serious damage to the lungs, resulting in symptoms such as “phlegm obstructs the lung”, “syndrome of lung collaterals injured by heat”, and “syndrome of epidemic toxin blocking lung”. (2) NSCLC patients who have undergone chemotherapy have a deficient constitution, an imbalance of qi, blood, yin and yang, and dysfunction of the viscera. They are less able to fend off the virus than healthy individuals, which causes their disease state to change more quickly and unstablely than healthy individuals and increases their risk of the disease.
The primary goal of treatment in the early, middle and late phases of the disease in healthy individuals with COVID-19 is to eradicate the DUXIE (pathogenic factor), avoiding the use of tonic formulations or medications that may induce DUXIE (pathogenic factor) stacking. Patients with COVID-19 and NSCLC, on the other hand, require a mix of corrective (the application of tonics) and detoxifying (the removal of pathogenic elements) treatment.
The Zilongjin tablets, as a TCM treatment for lung cancer, have the ability to benefit Qi and nourish the blood, clear heat and detoxify the blood, and regulate Qi and resolve stasis. In this study, the mechanism of action of the Zilongjin tablet on COVID-19 combined with NSCLC was discussed through network pharmacology. The top three major drug components of the Zilongjin tablets are lupeol, curcumin, and apigenin, as shown in the “Component-Target-Pathway” diagram. Lupeol is a natural product found in the AR and other organisms with data available. It acts through numerous signaling pathways such as Inflammatory bowel disease and HIF-1 signaling pathway, and target IL6, STAT3. Curcumin is a natural dyestuff found in the ASR and CR, and it has a role as an anti-inflammatory agent, an antineoplastic agent, a hepatoprotective agent, and an anti-inflammatory agent. Additionally, antifolate resistance, the HIF-1 signaling pathway, and legionellosis are a few of the many signaling pathways through which it functions, and it targets NOS2, IL6, IL1B, NFKB1, TLR4, STAT3, BECN1, HIF1A, FASN, SELP, and other molecules. A natural substance called apigenin is present in SLR, SBH, and other organisms for which data are available, and functions as a metabolite modulator and antineoplastic agent, targeting NFKB1, STAT3, TGFB1, IL2, IFNG, CAT, CD274, and IFNG. These genes are involved in the inflammatory response, and inflammation-triggered immunoreaction plays a significant role in both NSCLC and COVID-19. Moreover, COVID-19-induced endothelial dysfunction will further exacerbate pathological transformation and cause more pronounced inflammation. A cohort study reported that the Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio were discovered to be separate predictors of mortality in patients with NSCLC neo-coronary pneumonia (43). The Zilongjin tablets will relieve disease progression and reduce patient death brought on by overly aggressive inflammatory responses when they act on the NC. Additionally, several studies have hypothesized that the increased risk for NC patients may relate to lung damage and the elevated immunosuppression (42). The Zilongjin tablets act on the NC via a bidirectional immunoregulation in which BP includes both positive and negative regulation of the inflammatory response. It can inhibit the excessive proliferation of inflammatory cells such as T cells or inflammatory factors, thus suppressing the excessive inflammatory response and positively regulating the inflammatory response to avoid immunosuppression. The further effect of the Zilongjin tablets on the NC needs to be verified by subsequent experiments.
There are some limitations to this study. Fourteen RCTs were national studies and may be subject to some bias. Three included studies explicitly stated that they used a randomization table to achieve randomization, while other studies did not report randomization procedures, and only 1 article reported a double-blind approach, factors that may contribute to implementation bias, and measurement bias. Although objective response rate analyses reported that the Zilongjin tablets in combination with chemotherapeutic agents were significantly more effective than chemotherapy alone in the treatment of NSCLC. According to objective response rate analyses, due to the small sample sizes of the included studies, there was some clinical heterogeneity that affected the analysis's outcome. The dosage of the Zilongjin tablets was consistent across the RCTs (2.6 g/dose, orally, 3 times/d), but the chemotherapy regimens used varied, with two chemotherapy regimens set up in Wang and Yan study (28) (i) norethindrone (VNB) 25 mg/m2 + cisplatin 25 mg/m2; (ii) mitomycin (MMC) 6 mg/m2 + vincristine (VDS) 3 mg/m2 + cisplatin (DDP). The other 10 studies designed only one chemotherapy regimen, such as irinotecan 175 mg/m2 + carboplatin (AUC = 5), docetaxel 75 mg/m2 + cisplatin 20 mg/m2, gemcitabine hydrochloride 1,000 mg/m2 + cisplatin 60 mg/m2, etc. The chemotherapy regimens were not consistent across studies and may have an impact on treatment outcomes, as well as on the results of this study.
5. Conclusion
In this study, we comprehensively evaluated the efficacy of the Zilongjin tablets in the treatment of NSCLC. The efficacy and the degree of improvement in the quality of life of the Zilongjin tablets combined with chemotherapy in the treatment of NSCLC were significantly better than those of chemotherapy alone, and they could attenuate various adverse effects brought about by the disease or chemotherapy. In the network pharmacology, it reported that the efficacy of the Zilongjin tablets against NSCLC combined with COVID-19 might be achieved through the bidirectional regulation of inflammation, while further laboratory analysis was required for subsequent efficacy verification.
Data availability statement
All data used in this article are from public databases. The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WY and XX contributed to the conception and design of the study. WY, YZ, and YY organized the literature review database and performed the statistical analysis. JG, PH, and XX organized the pharmacological database and performed the statistical analysis. WY, YZ, and XX wrote the first draft of the manuscript. JG wrote sections of the manuscript. JG and XX contributed to the manuscript revision. All authors read and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol. (2016) 893:1–19. doi: 10.1007/978-3-319-24223-1_1
2. Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran Jr WJ, Wu Y-L, et al. Lung cancer: current therapies and new targeted treatments. Lancet. (2017) 389:299–311. doi: 10.1016/S0140-6736(16)30958-8
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
4. Cetin K, Ettinger DS, Hei YJ, O'Malley CD. Survival by histologic subtype in stage IV nonsmall cell lung cancer based on data from the Surveillance, Epidemiology and End Results Program. Clin Epidemiol. (2011) 3:139–48. doi: 10.2147/CLEP.S17191
5. Skričková J, Kadlec B, Venclíček O, Merta Z. Lung cancer. Karcinom plic Cas Lek Cesk. (2018) 157:226–36.
6. Passaro A, Bestvina C, Velez Velez M, Garassino MC, Garon E, Peters S. Severity of COVID-19 in patients with lung cancer: evidence and challenges. J Immunother Cancer. (2021) 9:e002266. doi: 10.1136/jitc-2020-002266
7. Rogado J, Pangua C, Serrano-Montero G, Obispo B, Marino AM, Pérez-Pérez M, et al. Covid-19 and lung cancer: A greater fatality rate? Lung Cancer. (2020) 146:19–22. doi: 10.1016/j.lungcan.2020.05.034
8. Sha Z, Chang K, Mi J, Liang Z, Hu L, Long F, et al. The impact of the COVID-19 pandemic on lung cancer patients. Ann Palliat Med. (2020) 9:3373–8. doi: 10.21037/apm-20-1662
9. Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. (2020) 395:1907–18. doi: 10.1016/S0140-6736(20)31187-9
10. National Institute for Health Care Excellence. COVID-19 rapid guideline: delivery of systemic anticancer treatments. (2020). Available online at: https://www.guidelines.co.uk/infection/covid-19-rapid-guideline-delivery-of-systemic-anticancer_x0002_treatments/455238.article (accessed March 26, 2020).
11. Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. (2020) 121:109570. doi: 10.1016/j.biopha.2019.109570
12. CAI QH, Zhang CC, Qiu YP. Zilongjin tablet combined with platinum chemotherapy in non-small cell lung cancer: A meta-analysis. World Chineses Med. (2019) 16:618–25. doi: 10.3969/j.issn.1673-7202.2021.04.017
13. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration (2011). Available online at: http://handbook-5-1.cochrane.org
14. Cochrane T. Review Manager (RevMan) 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre (2008).
15. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Datab System Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
16. Zhang ZN. Diagnosis and Efficacy Criteria for Hematological Diseases. Beijing: Science Press (1998). p. 171–3.
17. Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. (2012) 16:284–7. doi: 10.1089/omi.2011.0118
18. Luo W, Brouwer C. Pathview: an R/Biocondutor package for pathway-based data integration and visualization. Bioinformatics. (2013) 29:1830–1. doi: 10.1093/bioinformatics/btt285
19. Chen C, Zhang C, Wang J, Li C, Yu G. Clinical observation of Zirong gold tablets combined with docetaxel and cisplatin in the treatment of advanced non-small cell lung cancer. Chin Pract Med. (2011) 6:139–40. doi: 10.3969/j.issn.1673-7555.2011.30.118
20. Ding YM, Yu ZY, Xie H. Clinical study on the first-line treatment of extensive stage small-cell lung cancer with irinotecan and carboplatin plus zilongjin. J Zhejiang Chinese Med Univ. (2013) 6:745–7. doi: 10.3969/j.issn.1005-5509.2013.06.033
21. Li G, Ma S. Effect of zirong gold tablet combined with docetaxel and nedaplatin on locally advanced nonsmall cell lung cancer. Modern Drugs Clinic. (2015) 30:1506–10. doi: 10.7501/j.issn.1674-5515.2015.12.018
22. Li H, Wang H, Dai Y, Yu Q. Clinical experience of Zilong gold tablets in the treatment of advanced non-small cell lung cancer. Chinese Foreign Women's Health Res. (2015) 8:30. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMwNDI2EhF6d254amt5ajIwMTUwODAyNRoIZHdldG4ydjY%3D
23. Liu PJ. Efficacy of Zilongjin tablet combined with chemotherapy in treatment of advanced non-small cell lung cancer. Chinese J Pract Med. (2015) 11:114–5. doi: 10.3760/cma.J.SSN1674-4756.2015.11.062
24. Ma H, Fang J, Wang S, Wang D, Wang T, Wang E. Clinical efficacy of zilong gold tablets combined with chemotherapy for advanced lung cancer. Lab Med Clinic. (2017) 14:3318–20. doi: 10.3969/j.issn.1672-9455.2017.22.015
25. Sun CP, Wang JF, Chen XL. Study of zilongjin tablets combined with chemotherapy on advanced non small cell lung cancer. Chinese J Trad Chinese Med. (2015) 33:1145–7. doi: 10.13193/j.issn.1673-7717.2015.06.037
26. Shang RG. Clinical effect of Zilongjin Tablet combined with docetaxel plus cisplatin in treatment of advanced non-small cell lung cancer. Dr Trad Chinese Med Clin Res. (2016) 8:45–6. doi: 10.12677/WJCR.2019.91006
27. Shen T, Wei YS, Zhang HZ. Clinical effect of Zilongjin tablet combined with TP regimen in the treatment of locally advanced non-small cell lung cancer and its effect on T lymphocyte subsets. Hebei Chinese Med. (2017) 39:1539–42. (in Chinese). doi: 10.3969/j.issn.1002-2619.2017.10.025
28. Wang J, Yan WR. Study of Zilongjin tablets combined with chemotherapy on non small cell lung cancer in middle or advanced stage. J Mod Integrat Chinese Western Med. (2008) 17:3–4. doi: 10.3969/j.issn.1008-8849.2008.01.002
29. Wang JF, Chen XL, Sun CP. Clinical observation on treating 40 cases of local advanced non-small-cell lung cancer with the Zilongjin tablet. Clin Res Chinese Med. (2016) 8:15–17. doi: 10.3969/j.issn.1674-7860.2016.01.005
30. Yu FM, Jiang DL. Clinical observation on treating 40 cases of local advanced non-small-cell lung cancer with the Zilongjin tablet. Modern Med Clinic. (2018) 33:1184–8. doi: 10.7501/j.issn.1674-5515.2018.05.040
31. Yang G, Liu HN, Yang ZX. Effect of Zilongjin tablets on myelinosuppression by chemotherapy in small cell lung cancer. Tianjin Pharm. (2015) 27:54–6. doi: 10.3969/j.issn.1006-5687.2015.03.022
32. Yang XC, Tian F, Yu JC. Clinical study on treatment of primary non-small cell lung cancer with zilongjin tablets combined with PC chemotherapy. Chinese J Trad Chinese Med. (2017) 32:715–7. doi: 10.16368/j.issn.1674-8999.2017.05.187
33. Siegel R L, Miller K D, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. (2020) 70:7–30. doi: 10.3322/caac.21590
34. Fukuoka M, Wu Y-L, Thongprasert S, Sunpaweravong P, Leong S-S, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. (2011) 29:2866–74. doi: 10.1200/JCO.2010.33.4235
35. Yang JC-H, Sequist LV, Geater SL, Tsai C-M, Mok TSK, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. (2015) 16:830–8. doi: 10.1016/S1470-2045(15)00026-1
36. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
37. Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. (2018) 378:113–25. doi: 10.1056/NEJMoa1713137
38. Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, et al. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. (2020) 38:1580–90. doi: 10.1200/JCO.19.02446
39. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomized, open_x0002_label, phase 3 trial. Lancet Oncol. (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
40. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 out- break in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. (2020) 323:1545–6. doi: 10.1001/jama.2020.4031
41. Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
42. Baud D, Qi X, Nielsen-Saines K, Musso D, Pomar L, Favre G. Real estimates of mortality following COVID-19 infection. Lancet Infect Dis. (2020) 20:773. doi: 10.1016/S1473-3099(20)30195-X
43. Sutandyo N, Jayusman AM, Widjaja L, Dwijayanti F, Imelda P, Hanafi AR. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as mortality predictor of advanced stage non-small cell lung cancer (NSCLC) with COVID-19 in Indonesia. Eur Rev Med Pharmacol Sci. (2021) 25:3868–78. doi: 10.26355/eurrev_202105_25954
Keywords: Zilongjin tablets, non-small cell lung cancer (NSCLC), COVID-19, traditional Chinese medicine, meta-analysis, network pharmacology
Citation: Yang W, Zhang Y, Gao J, Hu P, Yang Y and Xu X (2023) A meta-analysis of the Zilongjin tablets for non-small cell lung cancer and its network pharmacology of action against NSCLC and COVID-19. Front. Med. 10:1080121. doi: 10.3389/fmed.2023.1080121
Received: 25 October 2022; Accepted: 05 July 2023;
Published: 01 August 2023.
Edited by:
Dawei Yang, Fudan University, ChinaReviewed by:
Vasiliki Epameinondas Georgakopoulou, Laiko General Hospital of Athens, GreecePrem P. Kushwaha, Case Western Reserve University, United States
Copyright © 2023 Yang, Zhang, Gao, Hu, Yang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoqing Xu, eHV4aWFvcWluZ0B0bXUuZWR1LmNu
†These authors have contributed equally to this work
‡ORCID: Wuxia Yang orcid.org/0000-0002-7967-9399
Yichao Zhang orcid.org/0009-0005-7433-6791
Jing Gao orcid.org/0009-0000-6858-2420
Pengcheng Hu orcid.org/0000-0002-3720-2488
Yanjie Yang orcid.org/0009-0002-1617-4374
Xiaoqing Xu orcid.org/0000-0001-7577-6362
 Wuxia Yang
Wuxia Yang Yichao Zhang2†‡
Yichao Zhang2†‡ Pengcheng Hu
Pengcheng Hu Yanjie Yang
Yanjie Yang Xiaoqing Xu
Xiaoqing Xu