
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 02 February 2023
Sec. Pathology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1066021
This article is part of the Research Topic Global Excellence in Pathology: Europe View all 5 articles
 Camilla Palumbo1
Camilla Palumbo1 Monica Benvenuto1,2
Monica Benvenuto1,2 Chiara Focaccetti1
Chiara Focaccetti1 Loredana Albonici1
Loredana Albonici1 Loredana Cifaldi1,3
Loredana Cifaldi1,3 Alessandra Rufini2,4
Alessandra Rufini2,4 Daniela Nardozi5
Daniela Nardozi5 Valentina Angiolini5
Valentina Angiolini5 Arianna Bei6
Arianna Bei6 Laura Masuelli5
Laura Masuelli5 Roberto Bei1*
Roberto Bei1*Head and neck squamous cell carcinoma (HNSCC) is the sixth most common cancer type, has often an aggressive course and is poorly responsive to current therapeutic approaches, so that 5-year survival rates for patients diagnosed with advanced disease is lower than 50%. The Epidermal Growth Factor Receptor (EGFR) has emerged as an established oncogene in HNSCC. Indeed, although HNSCCs are a heterogeneous group of cancers which differ for histological, molecular and clinical features, EGFR is overexpressed or mutated in a percentage of cases up to about 90%. Moreover, aberrant expression of the other members of the ErbB receptor family, ErbB2, ErbB3 and ErbB4, has also been reported in variable proportions of HNSCCs. Therefore, an increased expression/activity of one or multiple ErbB receptors is found in the vast majority of patients with HNSCC. While aberrant ErbB signaling has long been known to play a critical role in tumor growth, angiogenesis, invasion, metastatization and resistance to therapy, more recent evidence has revealed its impact on other features of cancer cells’ biology, such as the ability to evade antitumor immunity. In this paper we will review recent findings on how ErbB receptors expression and activity, including that associated with non-canonical signaling mechanisms, impacts on prognosis and therapy of HNSCC.
Head and neck (HN) cancers include a heterogeneous group of cancers which differ for histological, molecular and clinical features (1–3). More than 90% of HN cancers are squamous cell carcinomas (HNSCCs) arising from the mucosal epithelium of the oral cavity, pharynx and larynx, while much less common HN subtypes originate from the salivary glands, sinuses, muscles or nerves in the head and neck1 (2–4).
HNSCC is the sixth most common cancer type, with a worldwide incidence lately reported to range from about 500,000 to 900,000 cases per year, and with a further 30% increase expected in the next decade (1, 2).
These tumors often have an aggressive course and are poorly responsive to current therapeutic approaches. Indeed, more than half of HNSCC patients is diagnosed with advanced disease for which 5-year survival rates are lower than 50% (4). Moreover, the quality of life of HNSCC patients is often severely compromised as a consequence of both neoplastic growth and multimodality treatments causing pain, impairment of basic functions including eating and speaking, physical disfigurement and psychosocial distress (4).
Epidemiological studies and the definition of risk factors for HNSCC development, have led to the identification of two main subtypes, i.e., Human Papilloma Virus (HPV)-positive and HPV-negative tumors (4). That HNSCC are to be considered different biological entities on the basis of their being associated or not with HPV infection has been further validated by genome sequencing, RNA profiling and clinical data (1–4).
HPV-positive HNSCCs mainly arise in the oropharyngeal region following a latency of 10–30 years from oral infection with high-risk oncogenic HPV strains, primarily HPV-16, and have a more favorable prognosis (3, 4). As for their prevalence, HPV positivity is reported in a percentage of oropharyngeal cancers up to 70% in high income countries (2, 5). Conversely, HPV-negative HNSCCs mainly originate in the oral cavity, hypopharynx and larynx, in most cases in association with longterm tobacco use and alcohol consumption (3, 4).
Onset and progression of HPV-positive and HPV-negative HNSCCs are driven by (partly) different oncogenic pathways. In fact, loss of p53 and pRb tumor suppressor function occurs in both subtypes but as a result of different mechanisms: in HPV-positive tumors these tumor suppressor proteins are inactivated/targeted for degradation by the viral oncoproteins E6 and E7, whereas HPV-negative tumors show frequent mutations in TP53 and CDKN2A genes (~60–80% and ~ 20% of cases, respectively) (1–3). Notably, CDKN2A encodes for the pRb pathway regulator p16INK4A, whose inactivation promotes cell-cycle progression via the increased phosphorylation of pRb by cyclin-dependent protein kinases CDK4 and CDK6 (3).
Studies aimed at characterizing the molecular drivers of HNSCCs have identified further differences between HPV-positive and -negative tumors, as reviewed in (1–3), and have also revealed a substantial heterogeneity in the HPV-negative subgroup (3, 6, 7). In this complex landscape, the Epidermal Growth Factor Receptor (EGFR) has emerged as an established oncogene in HNSCC, being overexpressed or mutated in a percentage of cases up to about 90% (2, 7, 8).
The EGFR (HER1, ErbB1) is the prototypical member of the ErbB/HER family of receptor tyrosine kinases, which also includes ErbB2, ErbB3 and ErbB4 (a.k.a. HER2-4) (9). When bound by the cognate ligands, these receptors form homo- and hetero-dimers/oligomers in various combinations. This leads to receptor trans/autophosphorylation and the ensuing activation of interconnected and overlapping signaling cascades (reviewed in 9, 10), resulting in multiple biological responses, including proliferation, differentiation, cell motility, and cell death inhibition (Figure 1).
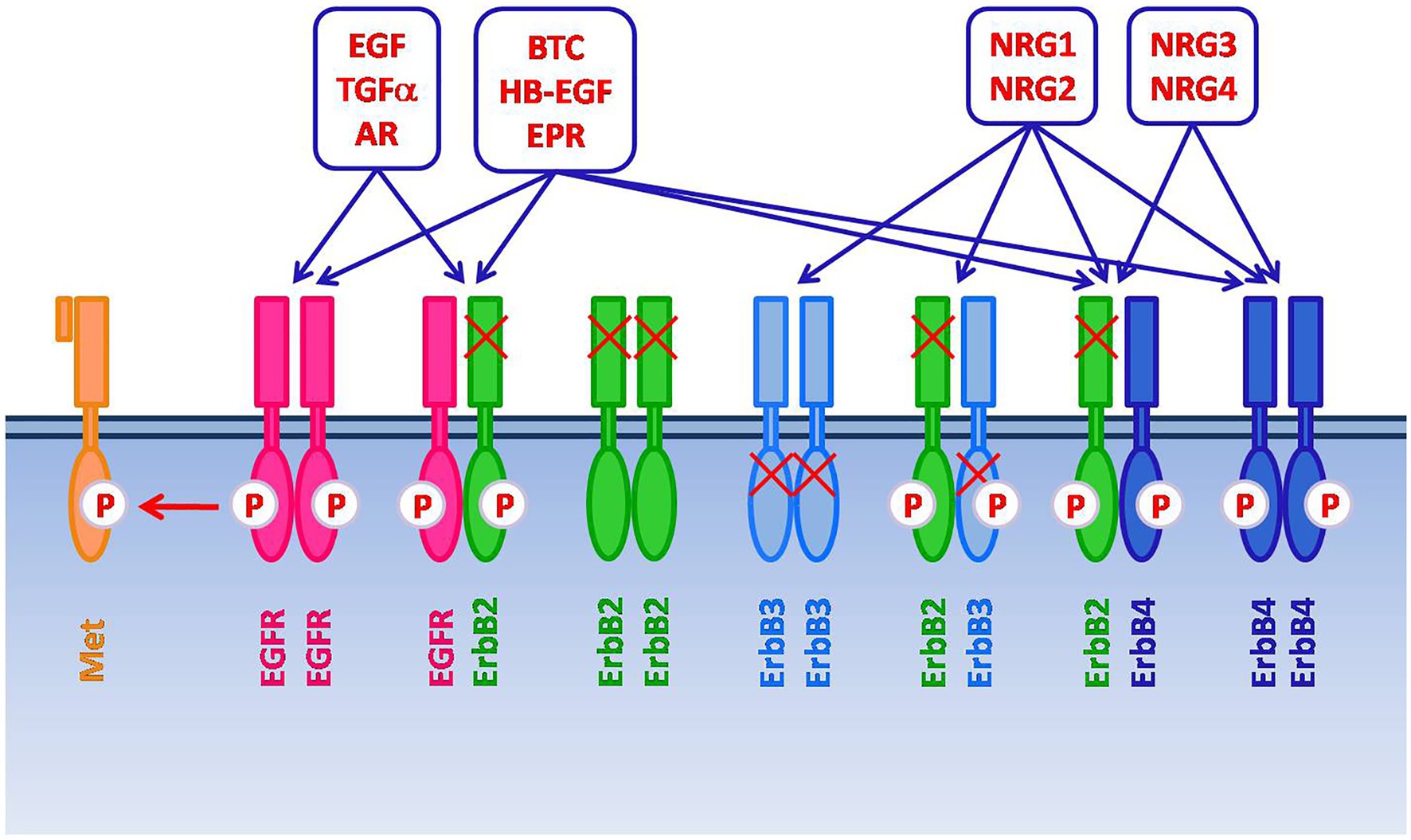
Figure 1. The ErbB/HER family of receptor tyrosine kinases includes four members: EGFR (HER1, ErbB1), ErbB2, ErbB3, and ErbB4 (HER2-4). After the binding with the cognate ligands, ErbB receptors form homo- and heterodimers/oligomers in various combinations. This leads to receptor trans/autophosphorylation and the activation of signaling cascades, resulting in proliferation, differentiation, cell motility, and cell death inhibition. Several ligands have been described, including Epidermal Growth Factor (EGF), Transforming Growth Factor-alpha (TGF-α), Amphiregulin (AR), Betacellulin (BTC), Heparin Binding Epidermal Growth Factor (HB-EGF), Epiregulin (EPR), and Neuregulins (NRGs). The specific ligands for EGFR are EGF, TGF-α and AR. NRG1-2 bind to ErbB3. BTC, HB-EGF, EPR, NRG1-4 bind to ErbB4. BTC, HB-EGF and EPR can also bind to EGFR. ErbB2, which does not have a direct ligand, and ErbB3, which has impaired kinase activity, signal via heterodimerization with other members of the family. Moreover, spontaneous dimerization of ErbB2 can occur due to the receptor overexpression. EGFR can also form heterodimers with receptor tyrosine kinases that do not belong to the ErbB family, such as the HGF receptor Met.
Beside the seven ligands known to bind the EGFR, which include the Epidermal Growth Factor (EGF) and Transforming Growth Factor α (TGF-α), additional growth factors can lead to EGFR activation via its hetero-oligomerization with different ErbB receptor partners (9, 11). In addition, EGFR has been reported to form heterodimers with receptor tyrosine kinases that do not belong to the ErbB family, such as the Hepatocyte Growth Factor (HGF) receptor Met, which also appears frequently overexpressed in HNSCC (1, 3, 11). Such promiscuity partly accounts for the complexity and diversification of the signaling outputs that can be elicited through the EGFR (9). Additional levels of complexity are related to non-canonical mechanisms of EGFR signaling, such as those associated with its nuclear translocation or with the recently reported release of EGFR-containing exosomes from cancer cells (12, 13).
In addition to EGFR, expression of the ErbB2 orphan receptor, which does not have a direct ligand but signals via heterodimerization with other members of the family, and of ErbB3 and ErbB4 has also been reported in variable proportions of HNSCCs (6, 14, 15).
While aberrant ErbB signaling has long been known to play a critical role in tumor growth, angiogenesis, invasion, metastatization and resistance to therapy (10), more recent evidence has revealed its impact on other aspects of cancer cells’ biology, such as the ability to evade antitumor immunity (16).
In this paper we will review recent findings on how ErbB receptors expression and activity, including that associated with non-canonical signaling mechanisms, impacts on prognosis and therapy of HNSCC.
Aberrant expression of ErbB tyrosine kinase receptors can result from a number of genetic alterations, such as gene amplification, mutation and translocation, and is often associated with aberrant activation of downstream signaling pathways involved in cancer onset and progression. Indeed, ErbB receptors quantitative and qualitative alterations are frequently found in different solid tumors, including HNSCC (2, 17).
When investigating the copy number of ErbB receptors genes in HNSCCs, several studies reported the presence of gene amplification or polysomy. On the other hand, protein expression levels are regarded as a more reliable marker of aberrant receptor activity as compared to quantitative genetic alterations. Indeed, in addition to genetic alterations, several mechanisms may contribute to ErbB protein overexpression, including transcriptional and post-translational mechanisms (17). Relevant studies in this regard, performed in different cohorts of HNSCC patients and published in the last two decades, are reported in Table 1. Overall, the results of these studies indicate that the EGFR protein is overexpressed in 18–90% of patients (median value: 58%), while the gene copy number is increased in 5–55% of patients (median value: 21%) (see Table 1). ErbB2 protein overexpression ranges from 1 to 35% of patients (median value: 7%), with gene copy number alterations found in 2–46% of patients (median value 7%) (see Table 1). Also, ErbB3 and ErbB4 protein are overexpressed in HNSCC patients, with percentages of 21–54% (median value: 43%) and 26% of patients, respectively (see Table 1). Collectively, the available studies clearly indicate that increased expression/activity of one or multiple ErbB receptors is found in the vast majority of patients with HNSCC.
Conversely, it has been reported that the expression of EGFR can be negatively regulated by the methylation of its promoter, which is more often associated with HPV infection (24). In this regard, it is of note that HPV-positive HNSCCs have a tendency to express lower levels of EGFR as compared to HPV-negative tumors (29, 52, 69, 70). On the other hand, as regards the impact of HPV status on the expression levels of ErbB receptors, HPV-positive HNSCC have been found to express higher levels of ErbB2, ErbB3 and ErbB2:ErbB3 heterodimers as compared to HPV-negative tumors (70). This finding is remarkable, since it suggests that patients with HPV-positive HNSCC may benefit from therapeutic regimens based on agents able to target simultaneously multiple ErbB receptors.
Clearly, the evaluation of the phosphorylated receptors levels would represent an even better biomarker of their activity as compared to the mere expression levels and, accordingly, different authors argue against the predictive value of ErbB protein amounts in tumor tissues in the absence of data on their tyrosine kinase activity (34). Furthermore, beside the reported alterations in expression levels, ErbB activity is known to be affected by polymorphisms and mutations in the encoding genes. In this regard, different authors have reported that, albeit at an overall low frequency, EGFR mutations affect a proportion of HNSCCs (24, 56, 57, 59). These mutations frequently occur in the intracellular kinase domain (ICD), in particular in exons 18–21, often leading to aberrant receptor signaling and conferring resistance to targeted therapies (8; Table 1). As for their frequency, a study reported the presence of EGFR ICD mutations in 57% of samples from a cohort of Saudi HNSCC patients (71). However, in most studies EGFR ICD mutations have been found in a percentage of cases lower than 10% (72; Table 1). Regarding the mutational status of the EGFR extracellular domain (ECD), this has been investigated in a limited number of studies in HNSCC, in spite of its potential therapeutic implications (73). In fact, the ECD mutations G33S, N56K and G465R have been found in HNSCC cells and reported to prevent Cetuximab binding to the receptor (73, 74). A larger number of studies has instead focused on the expression of EGFRvIII, a mutant EGFR with in-frame deletion of exons 2–7, which is incapable of binding ligands due to the lack of a portion of the ligand-binding domain, but is characterized by low levels of constitutive activity (54). The expression of EGFRvIII in HNSCC cells has been linked to enhanced growth and resistance to both cisplatin and agents targeting wild-type EGFR (75). However, while the expression of this mutant has been found in approximately 20% of HNSCC patients by different authors (54, 55), conflicting results obtained in other studies have raised doubts on its clinical relevance in HNSCC (6, 58).
Polymorphic variants of the EGFR are also known to differ for expression levels, function and sensitivity to targeted agents (17, 76). The EGFR-K521 (K-allele), resulting from a single nucleotide polymorphism which involves the EGFR ECD, has been found in 56% of HNSCC patients and shown to display a reduced affinity for Cetuximab (60). At the opposite, the EGFR Q787Q synonymous polymorphism appears to confer greater sensitivity to EGFR tyrosine kinase inhibitors (TKIs), due to a long noncoding RNA-mediated mechanisms, and has been reported in 17% of HPV-related and 32% of HPV-unrelated oropharyngeal HNSCC patients (24).
Studies aimed at correlating ErbB protein expression levels and prognosis of HNSCC have reported variable results. Multiple factors are responsible for this inherent variability, including differences in tumor sites, use of different antibodies, detection techniques, immunostaining scoring systems and also the different evaluation of the receptors subcellular distribution (17, 62). Nonetheless, there is a rather general consensus on the correlation between EGFR expression levels, poor prognosis and worse treatment outcomes in patients with HNSCC, while there is no definitive evidence that EGFR gene copy number and mutations may have prognostic value in this group of cancers (1, 2, 8, 17, 33). In particular, EGFR overexpression has been associated with radiotherapy resistance, loco-regional treatment failure, higher rates of metastatization, and with reduced disease-free, progression-free and overall survival in different cohorts of patients (8, 17, 77, 78). Worthy of note, several authors remarked that the significance of the observed correlations is critically dependent on the assessment of EGFR protein levels in cancer tissues by means of quantitative image analysis and scoring systems, taking into account both intensity and extent of the immunostaining (17, 78). Even though HPV-positive HNSCCs have a tendency to express lower levels of EGFR, according to some authors this receptor holds prognostic value also in this subgroup of tumors. In fact, it has been reported that the outcome of HPV-positive tumors with higher EGFR levels is worse as compared to that of HPV-positive tumors with low EGFR (29, 52, 69). However, others reported that the impact of EGFR on outcome may be limited to HPV-negative HNSCCs (32, 79).
It should also be also considered that an aberrant EGFR signaling may result from additional mechanisms beside its overexpression or mutation, such as transactivation by different receptors, including for instance the already mentioned Met receptor, or increased expression of the cognate ligands (3, 11, 17). Consistent with this consideration, in a study performed on HNSCC cell lines and tumor specimens it was observed that EGFR expression levels were not correlated with EGFR activity, evaluated via its phosphorylation status at multiple tyrosine residues (34). Accordingly, rather than EGFR protein levels, phosphorylated receptor levels should represent a better biomarker for EGFR pathway activation in tumor samples (80). However, the levels of activated EGFR in HNSCC have been investigated in a limited number of studies, with partly conflicting results regarding the impact of receptor activity on clinical outcome (44, 53).
While HER2 amplification/overexpression is a marker of poor prognosis in different cancers, including breast, ovary and lung carcinomas, the prognostic value of this receptor in HNSCC is still a matter of debate (61, 62, 81). By the way, even though ErbB2 is overexpressed in a fraction of HNSCCs, its expression levels appear on the whole lower in this type as compared to other types of cancer (62). A recently published study highlights how the association between ErbB2 overexpression and clinical outcome can be dependent on the use of different systems for scoring the receptor levels in HNSCC tissue samples (62). In this study the authors investigated ErbB2 expression by immunohistochemistry in 120 HNSCC tissue sections including laryngeal, oral cavity and oropharyngeal squamous cell carcinomas, and evaluated ErbB2 immunostaining using two systems: the conventional scoring system approved by the FDA, which takes into account the degree of membrane staining in >10% of cells, and an H-score-based system, in which an H-score value is obtained for each section by multiplying the intensity score by a proportion score based on the percentage of stained cells. According to both scoring systems, the majority of ErbB2-positive tumors were poorly differentiated, stage IV tumors with lymph nodal involvement. However, a different percentage of ErbB2-positive tumors was obtained using the H-score system as compared to the conventional system (19% vs. 11%, respectively), and ErbB2 overexpression was associated with decreased overall survival when evaluated by H-score only. In particular, median survival was 11 months for ErbB2-positive patients and 49 months for ErbB2-negative patients by H-score. In the same study it was evaluated the association between ErbB2 levels and clinical outcome based on data downloaded from The Cancer Proteome Atlas2 and it was found that ErbB2 protein expression had no effect on survival of patients with oral and oropharyngeal squamous cell carcinoma, while it was associated with improved survival in patients with laryngeal HNSCC. These results, coupled with the conflicting findings obtained in other studies, indicate that it is unlikely that ErbB2 could be a useful prognostic marker for HNSCC (61, 62, 81–83).
As compared with EGFR and ErbB2, a smaller number of studies have investigated the association between the expression of ErbB3 and ErbB4 and prognosis of HNSCC. As regards ErbB3, different authors agree on its value as a predictor of poor clinical outcome in HNSCC. In a study performed on a large cohort of HNSCC patients, membranous ErbB3 overexpression was associated with worse overall survival and was significantly increased in metastatic lesions as compared to primary tumors (84). In different cohorts of HNSCC patients, ErbB3 expression levels have also been found to correlate with nodal stage, poor relapse-free, disease-free, and overall survival (68, 85, 86).
Still, a more complex scenario emerges from a recent study, where the subcellular distribution of ErbB3 in laryngeal HNSCC cells was taken into account (87). Indeed, ErbB3, as well as ErbB4, appears to play different roles in HNSCC progression depending on its membranous/cytoplasmic vs. nuclear localization (87, 88). This aspect, which will be discussed in the next paragraph in the context of non-canonical mechanisms of ErbB signaling, can also be partly responsible for the discordant data regarding ErbB4 as prognostic marker for HNSCC. Actually, only sparse data are available on the impact of ErbB4 status on HNSCC, and the discordant results in this regard may also be ascribed to the different site of origin of the tumors investigated. In fact, a correlation has been found by different authors between ErbB4 membranous/cytoplasmic expression, lymph node metastasis and risk of recurrence in oral HNSCC (89, 90). Conversely, ErbB4 overexpression has been reported as a favorable prognostic factor in tongue and, in association with its nuclear localization, in laryngeal HNSCC (85, 88, 91).
On the other hand, many lines of evidence indicate that, beside the expression of individual ErbB receptors, the simultaneous expression of multiple ErbB family members can be a stronger predictor for outcome of HNSCC (92, 93). Indeed, co-expression of different ErbBs allows receptor heterodimerization, which in turn activates cooperative and diversified downstream signaling cascades (9, 10). In an early report, the expression of each ErbB family member was significantly associated with shortened survival in patients with oral HNSCC, but the co-expression of EGFR, ErbB2 and ErbB3 had an improved predicting power (93). In another study aimed at investigating the relationship between clinical parameters and single versus paired overexpression of ErbB family members in patients with oral HNSCC, overexpression of ErbB1 and ErbB4 was associated with a lower survival, but the simultaneous overexpression of both receptors predicted the worst overall and disease-free survivals (90). Consistent with these findings, in a previous study by our group performed on oral cavity epithelium samples, including invasive and in situ carcinomas, benign lesions and normal mucosa, the simultaneous expression of three or four ErbB receptors was correlated with tumor invasion (94). Finally, in a recent paper the prognostic stratification of patients with EGFR-positive advanced laryngeal squamous cell carcinoma was improved by taking into account the simultaneous expression of nuclear ErbB3 (as further detailed in the next paragraph) (87). On the whole, these findings indicate that cooperative signaling by ErbB receptors plays a significant role in the pathogenesis and outcome of HNSCC, which deserves further investigation.
In addition to receptor overexpression or mutation, more recent findings point to non-canonical mechanisms of ErbB signaling as important players in different cancers (12, 95). Non-canonical ErbB signaling involves for instance mechanisms mediated by different subcellular localizations of the receptors, and most studies in this respect have been focused on nuclear EGFR (12, 95).
Different stimuli, that include (but are not limited to) ligand-binding, can induce EGFR nuclear translocation (Figure 2). Once in the nucleus, one of the functions of EGFR is to promote cell proliferation by phosphorylating and stabilizing the proliferating cell nuclear antigen (PCNA). Accordingly, nuclear EGFR is typically found in rapidly dividing cells (95). In addition, nuclear EGFR can promote DNA damage repair and act as a co-transcription factor leading to the increased expression of oncogenes (e.g., Cyclin D1, c-Myc, B-Myb, Aurora Kinase A). Remarkably, these latter functions are mediated by protein–protein interactions and do not appear to require the receptor tyrosine kinase activity (95). This finding and the subcellular localization of nuclear EGFR suggest that its expression could predict clinical resistance to both EGFR-targeted therapeutic antibodies and TKIs (95).
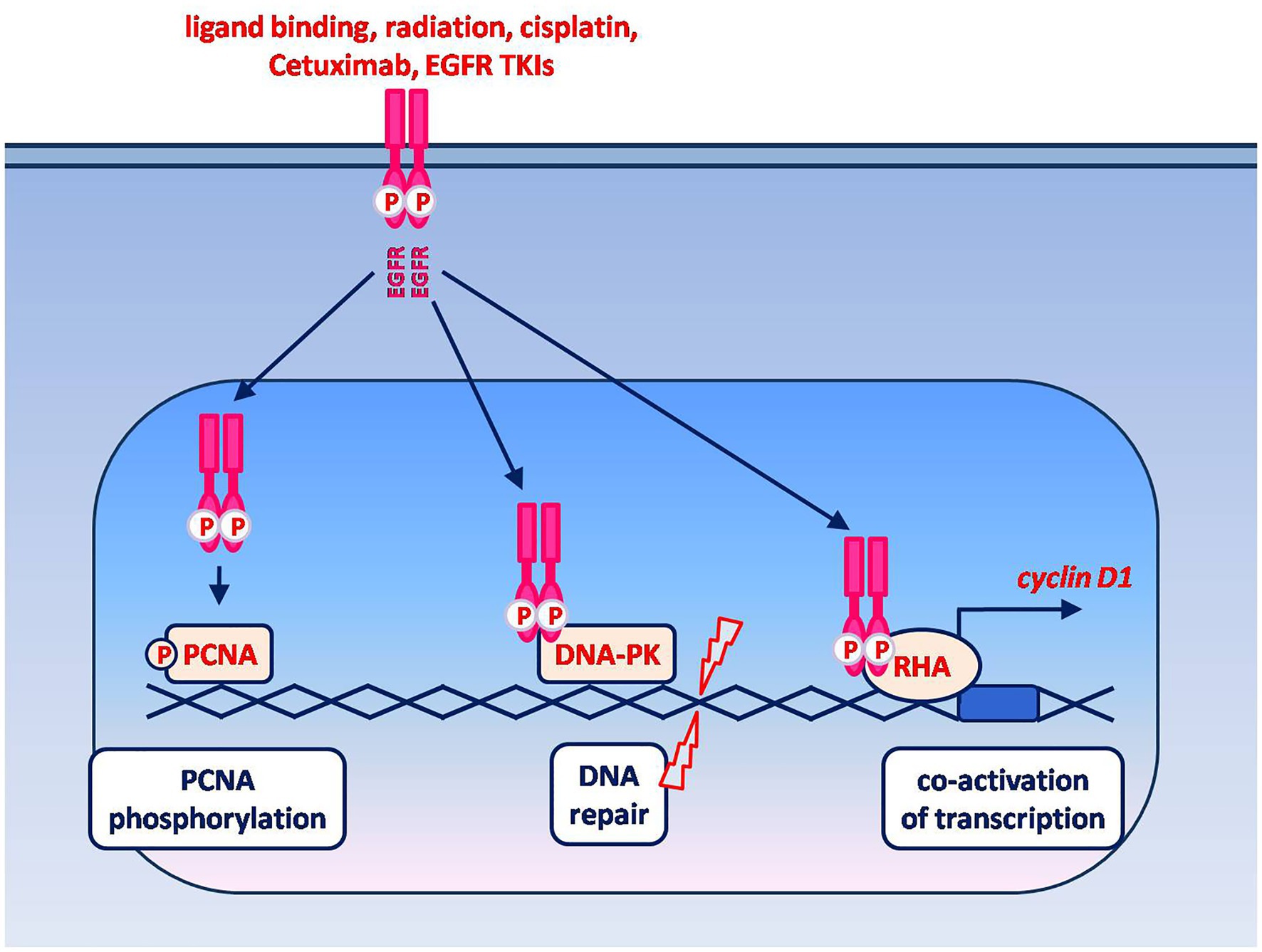
Figure 2. Non-canonical signaling by nuclear EGFR. EGFR nuclear translocation is known to be induced by ligand binding as well as by cell exposure to radiations, drugs and EGFR-targeting agents. The known oncogenic functions of nuclear EGFR include: phosphorylation and stabilization of chromatin-bound PCNA, which in turn plays an essential role in DNA replication and repair; promotion of DNA repair via protein–protein interaction with the DNA-dependent protein kinase (DNA-PK); co-activation of transcription via the interaction with different transcriptional regulators. The interaction between nuclear EGFR and RNA helicase A (RHA) leads to an enhanced expression of cyclin D1. Among the other genes whose expression is enhanced by nuclear EGFR, in association with various transcription factors, are those encoding for c-Myc, B-Myb, Aurora Kinase A and thymidylate synthase.
A correlation between nuclear EGFR expression, poor survival and chemo-radiation resistance has been in fact demonstrated in various types of cancer, including HNSCC (95). In particular, nuclear EGFR appears associated with induced chemo-radiation resistance, since its level was found to increase following in vitro irradiation, cisplatin exposure, or treatment of HNSCC cells with Cetuximab or with the EGFR TKIs Erlotinib and Lapatinib (12, 96–98). Moreover, an association was found between increased sensitivity of cultured HNSCC cells to the cytotoxic effects of irradiation, cisplatin and Cetuximab, and reduced levels of EGFR nuclear translocation (96, 98).
A correlation between nuclear EGFR expression and HNSCC clinical parameters has also been reported by several authors. In a study on laryngeal HNSCC, a higher frequency of strong nuclear EGFR was found in invasive tumors compared to laryngeal dysplasia and vocal cord polyps, and high nuclear EGFR expression levels correlated with worse overall cancer patients’ survival (39). In oropharyngeal HNSCC high nuclear EGFR expression levels were associated with reduced responses to radiation therapy, higher risk of local recurrence and lower overall survival (99, 100). Finally, in a study on the expression of total and nuclear EGFR in relation to the HPV infection surrogate marker p16, it was shown that both total and nuclear EGFR levels were higher in p16-negative tumors compared to p16-positive tumors (69). On the whole, the reported findings indicate that EGFR nuclear localization is a negative prognostic factor in HNSCC and suggest that it may be regarded as a biomarker for clinical resistance as well as a potential therapeutic target (95).
Worthy of note, also the other ErbB receptors are known to translocate to the nucleus and play a role in transcriptional regulation (95, 101). However, unlike nuclear EGFR, localization of ErbB3 and ErbB4 in the nucleus of HNSCC cells appears associated with a more favorable prognosis. Evidence in this respect comes from studies on laryngeal HNSCC in which ErbB3 was primarily observed in the nuclear compartment of tumor cells in association with variable cytoplasmic staining, and low expression levels of the receptor were associated with high proliferative indices, and with shorter relapse-free and overall survival (88, 91). Further, EGFR-positive laryngeal tumors co-expressing nuclear ErbB3 had a better prognosis as compared to those that expressed EGFR without ErbB3 or in association with cytoplasmic ErbB3 (87). Moreover, based on the observation that ErbB3 was never expressed alone, but always co-expressed with ErbB2, both in the presence and absence of EGFR, the authors of these studies speculated that ErbB3 nuclear localization may play a favorable role by preventing the formation of ErbB heterodimers at the cell membrane and the ensuing activation of pro-tumoral downstream signaling pathways (87).
As for ErbB4, this receptor is known to undergo ligand-induced intramembrane-regulated proteolysis (RIP) leading to the nuclear translocation of its cytoplasmic domain (101, 102). In a study by our group comparing the expression and distribution of ErbB receptors between HNSCCs and adjacent normal mucosa, ErbB4 was overexpressed in ~26% of the investigated carcinomas and, in addition to cytoplasmic staining, a strong nuclear ErbB4 immunostaining was observed in ~18% of carcinomas (26). Such nuclear localization of ErbB4 was found in tumors graded from 1 to 3, according to WHO classification, thus appearing unrelated to the degree of tumor differentiation (26).
More recently, both nuclear and cytoplasmic positivity for ErbB4 has also been reported in a proportion of laryngeal HNSCCs (~43%, 67 cases) and a more prominent localization of ErbB4 in the nuclear compartment was associated with longer relapse-free and overall survival (88, 91). This finding is consistent with the reported role of nuclear ErbB4 in mediating growth arrest or apoptosis in various tissues, even though its impact on the biological and clinical behavior of different cancer types remains controversial (102, 103).
Recently, the multifaceted function of tumor cell-derived exosomes has emerged as an important field in cancer research, and in this regard novel, non-canonical mechanisms of action have been reported for both the EGFR and ErbB2. Exosomes, i.e., nanovesicles released by cells in the interstitial space and found in various bodily fluids, such as serum, plasma, saliva, urine, etc., act as mediators of intercellular communication by delivering a complex cargo of sorted proteins, lipids, nucleic acids and metabolites from donor to recipient cells (104). A growing number of studies is demonstrating that the cargo of tumor-derived exosomes is specifically enriched in signaling molecules able to promote cancer progression and remodeling of the tumor microenvironment (104), and different cancer types have been reported to release EGFR- or ErbB2-containing exosomes (13, 95).
Indeed, exosomal transfer of tumor-derived EGFR to both local and distant recipient cells has been found to promote cancer cell growth, metastases and drug resistance as well as angiogenesis, metastatic niche formation, and suppression of anti-tumor immunity (13, 95, 105). Although reported in a more limited number of studies, similar findings have been published as regards the exosomal transfer of tumor-derived ErbB2 (95, 106, 107).
As in other cancer types, exosomes are deeply involved in HNSCC progression, and the correlation between exosome release and HNSCC aggressiveness is supported by multiple lines of evidence (108, 109). In this context, the release of EGFR-containing exosomes from HNSCCs has been demonstrated to occur both in vitro and in vivo. Indeed, HNSCC cultured cells have been reported to release both EGFR- and phospho-EGFR containing exosomes (110, 111). In addition, tumor-derived exosomes immunocaptured from HNSCC patients’ plasma have been found to contain high amounts of immunosuppressive and tumor growth-promoting mediators such as PD-L1, FasL, TGF-β and EGFR. Remarkably, the exosomal levels of these proteins, including the EGFR, correlated with clinicopathological parameters, being higher in patients with stage III/IV disease and lymph node metastases versus those with stage I/II disease and without lymph node metastases (112).
Fujiwara and colleagues showed that in Cetuximab-treated oral HNSCC cells the therapeutic antibody was secreted within EGFR-containing vesicles (including exosomes and microvesicles), thereby providing evidence of a mechanism by which the release of EGFR-containing vesicles may reduce the antibody therapeutic efficacy (110). Further, they demonstrated that the release of EGFR-containing vesicles by oral squamous cell carcinoma was increased by EGF stimulation and was able to drive carcinogenic epithelial-mesenchymal transition in recipient immortalized oral epithelial cells (113).
On the whole, these findings highlight that more research is warranted to fully define the involvement of ErbB receptors-containing exosomes in HNSCC, to validate their possible relevance as non-invasive diagnostic/prognostic markers and to explore their potential as therapeutic targets (13, 108, 109).
Early-stage HNSCCs (30–40%) are currently managed with single modality approaches (mainly surgical resection for oral cavity cancers and radiation for larynx and pharynx cancers), with long-term survival rates achieved in about 70–90% of patients (2, 4). However, the majority of patients are diagnosed with advanced disease, requiring multimodality interventions. These usually consist of surgery followed by radiation with or without (platinum-based) chemotherapy for cancers of the oral cavity, and primary chemoradiation for cancers arising in the pharynx or larynx (2, 4, 5). Beside their severe impact on quality of life, such interventions have limited effects, recurrent/metastatic disease affects more than 65% of patients, and 5-year survival rates following a diagnosis of advanced HNSCC are lower than 50% (1, 4).
Due to the growing body of evidence on the involvement of EGFR in HNSCC, many preclinical and clinical study have investigated the therapeutic potential of EGFR-targeting agents in this group of tumors. These studies have led to the approval of Cetuximab in combination with radiation therapy (with or without chemotherapy) for patients with locally advanced HNSCC (2, 114). Cetuximab, a chimeric monoclonal antibody that competes with natural ligands for binding to the extracellular region of EGFR, was approved for HNSCC by the European Medicines Agency (EMA) in 2004 and by the Food and Drug Administration (FDA) in 2006, remaining the only approved targeted agent for HNSCC until the recent introduction of immune checkpoint inhibitors (ICIs) (1, 2, 114). Indeed, HNSCCs, and in particular HPV-negative tumors, are highly radioresistant, and Cetuximab was proven to act as a radiosensitizer able to improve loco-regional control as well as to increase overall survival from 29.3 to 49.0 months, when combined with radiation alone (1, 2, 114, 115). Over time, however, resistance to both radiotherapy and Cetuximab develops in the majority of HNSCC patients, hence allowing for relapse and disease progression (1, 114, 115). The mechanisms responsible for Cetuximab resistance are multifactorial and appear to involve, among the others, the increased expression/activity of different receptor tyrosine kinases (including other ErbB family members and Met) or of the same EGFR, increased production of EGFR ligands, increased EGFR subcellular localization in the nuclear compartment (12, 97, 114, 116). Due to the involvement of Met receptor in Cetuximab resistance, many studies have investigated the dual targeting of Met and EGFR as a therapeutic strategy for HNSCC. However, although preclinical data support the co-targeting of EGFR and Met in these tumors (117), according to the recently published results of a phase II trial performed in patients with recurrent/metastatic HNSCC, the combination between Cetuximab and the Met inhibitor Tivantinib did not improve response rates or patients survival compared with Cetuximab alone, while it was associated with increased toxicities (118–120).
In addition to Cetuximab, therapeutic regimens including different ErbB-targeting agents have been and are being investigated in HNSCC patients (115). However, the results of the clinical studies published so far show that many among these agents have an overall modest clinical efficacy, that does not appear on the whole superior to that of Cetuximab (1, 115). In this regard, a modest efficacy in terms of clinical outcomes has been reported for the fully human anti-EGFR antibodies Panitumumab and Zalutumumab, and for Duligotuzumab, a dual-action antibody directed against EGFR and ErbB3 (115). Among the anti-EGFR therapeutic antibodies, some encouraging results have instead been obtained with Nimotuzumab, whose binding requires that EGFR is expressed at high density on the surface of the target cells, thus resulting in selective activity on EGFR-overexpressing tumor cells as compared to normal cells (115, 121). As for ErbB receptors TKIs, Gefitinib and Erlotinib, which act as reversible inhibitors of EGFR, and Lapatinib, a reversible inhibitor of both EGFR and ErbB2, have also shown a modest activity in HNSCC patients (1, 115). Conversely, Afatinib, a multi-targeted and irreversible TKI targeting EGFR, ErbB2 and ErbB4, has shown clinical benefits in different cohorts of HNSCC patients, comparable or superior to that of Cetuximab (1, 115). A list of selected, recently completed, recruiting and active trials using ErbB-targeted agents, alone or in combination with different treatments, is reported in Table 2.
In 2016 Immune Checkpoint Inhibitors (ICIs) entered the clinical practice for HNSCC with the approval of the anti-Programmed cell Death protein 1 (PD-1) antibodies Nivolumab and Pembrolizumab for patients with recurrent or metastatic tumors (5). Indeed, promising results were obtained in clinical trials based on these agents, both in terms of improved survival and limited treatment-related toxicities (1, 2, 5).
The PD-1/PD-L1 checkpoint is a central mediator of immunosuppression in the tumor immune microenvironment. The PD-1 receptor is expressed, among the others, on the surface of T cells, and its binding to PD-L1 ligands on the surface of tumor cells (and other cells in the tumor microenvironment) negatively regulates T cells functions and allows tumor evasion of immune surveillance (138). By blocking the PD-1/PD-L1 interaction, ICIs can release T cell inhibition, thus restoring antitumor immunity (138). Preclinical data also suggest that the combined blockade of PD-1 and Cytotoxic T Lymphocyte Antigen-4 (CTLA-4) immune checkpoints could be beneficial in HN cancers (139). In this context, the evidence that ErbB receptors have important immune-modulatory effects has provided the rational basis for combining ICIs and ErbB-targeting agents in HNSCC patients.
Indeed, abnormal signals by ErbB family members, in addition to playing an essential role in tumorigenesis, are accountable for the evasion of antitumor immunity in many cancers, including HNSCC (16). Although the mechanisms are still incompletely understood, inhibitory effects on the adaptive antitumor immune response are associated with the creation of a tumor microenvironment conditioned by many factors affected by aberrant ErbB receptor signaling. In HNSCC the main cellular components of the tumor microenvironment are T lymphocytes, tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), natural killer cells (NKs), and cancer-associated fibroblasts (CAF) (140). Several cell types in the microenvironment of HNSCC tumors, including regulatory T cells (Tregs), CAFs, and TAMs have been shown to mediate immunosuppression and dysfunction of antitumor immunity (141). In fact, HNSCCs, in particular those of the inflamed/mesenchymal subtype (6, 142), often have a high infiltrate of CD8+ T cells, which, however, is not always linked to a favorable prognosis (143). Indeed, although higher numbers of CD8+ T lymphocytes have been correlated with improved outcomes of HNSCC by some authors (144–146), often T cells present in HNSCC microenvironment are dysfunctional or “exhausted.” In this regard, high numbers of immunosuppressive Tregs are found among tumor infiltrating lymphocytes in HNSCC, and their presence has been associated with unfavorable prognosis and resistance to radiotherapy (147).
Actually, several findings support a role for ErbB receptors in mediating tumor immune escape. Oncogenic signals via EGFR and ErbB2 can induce the overexpression of PD-L1 and the production of immunosuppressive cytokines including transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF) or IL-10 (148, 149). Furthermore, EGFR signaling can participate to the suppression of immune responses via recruitment and activation of Tregs as well as through the reduction of T cell chemo-attractants (149). Moreover, both EGFR and ErbB2 have been reported to downregulate HLA class-I mediated peptide presentation (148). Further, aberrant ErbB signaling has been associated with the decrease of Th1 response, the induction of the exhaustive phenotype of CD8+ T lymphocytes and the immune cells switch from a pro-inflammatory to a pro-tumor phenotype (143).
The generation of abnormal proteins, which have not been previously recognized by the immune system (neoantigens), derived by HNSCC cells with inherent genetic instability could trigger CD8+ T cell responses and contribute to the elimination of cancer cells (150). In this regard, the lack or reduced presentation of neoantigens via MHCI or MHCII can adversely affect the adaptive antitumor immune response. Conversely, the presentation of neoantigens via MHC molecules can have a significant impact on clinical responses to immunotherapies, such as those targeting immune checkpoints mediated by CTLA-4 signaling and the PD-1/PD-L1 axis (151). In this context, aberrant ErbB signaling can also alter the tumor cells’ transcriptome, including the expression of tumor-associated antigens (TAA) and tumor-specific antigens (TSA), therefore influencing both the pool of processing peptides available for antigen presentation and the MHC repertoire present at the surface of tumor cells (152).
Interesting results have also been reported on the link between ErbB receptors expression and spontaneous immune responses in HNSCC. Different studies have demonstrated that aberrant expression of ErbB receptors can spontaneously induce immune response by breaking tolerance for these self-antigens in patients with several cancers, including breast, lung, colon, prostate, pancreas, stomach, bladder, liver, testis, and lymphoma (153). However, despite the high level of ErbB receptors expression in HNSCC, it was reported that natural tumor-specific humoral immune responses in HNSCC patients are poor (26). On the other hand, the presence of EGFR-specific CD8+ T cells was observed in the circulation of HNSCC patients with high EGFR scores, suggesting that EGFR overexpression on tumor cells can elicit specific T cell responses (154). In this regard, the observation that elevated circulating EGFR-specific T cells are found in HNSCC patients treated with the anti-EGFR antibodies Cetuximab and Nimotuzumab (155, 156), suggests that the presence of specific immune responses against ErbB receptors could be boosted by EGFR-targeted treatments. Actually, the efficacy of Cetuximab appears to involve its ability to trigger antibody-dependent cell-mediated cytotoxicity (ADCC) on NK cells and to promote crosstalk between NK and antigen presenting cells, thereby leading to the generation of EGFR-specific T cells (157). Furthermore, recent evidence obtained in preclinical models of different cancers, including HNSCC, demonstrate that Cetuximab can induce immunogenic cell death, i.e., a type of cell death involving the release of damage-associated molecular patterns (DAMPs) capable to trigger the generation of CD8+ T lymphocytes and lead to tumor-specific immunological memory (158, 159).
The therapeutic potential of regimens based on the combination of ICIs and ErbB-targeting agents has been investigated in several studies and many clinical trials are also underway in this regard (160; Table 2). The results of a recent study performed on a small number of PD-L1-positive oral HNSCC patients indicate that the combination of PD-1 inhibitors (Nivolumab or Sintilimab), anti-EGFR targeted therapy (Nimotuzumab) and chemotherapy (Paclitaxel) could improve the response rate and survival outcome (161). In a case report of a patient with recurrent/metastatic oral squamous cell carcinoma, the efficacy of the PD-1 inhibitor Nivolumab in combination with the anti-EGFR antibody Nimotuzumab and radiotherapy has also been described in terms of decreased metabolic activity in cancer cells, reduction of lung lesions dimensions, progression-free survival and tolerable safety profile (162). To mention a few of the ongoing studies, the triple combination of ICIs, Cetuximab and radiotherapy for patients with advanced HNSCC is being investigated in clinical trials employing the anti-PD-L1 antibodies Avelumab and Durvalumab and the anti-CTLA-4 antibody Ipilimumab (Table 2; 157). As regards the Cetuximab/Ipilimumab/radiotherapy combination, preliminary results indicate that the efficacy of this regimen is comparable to that of the standard cisplatin/radiotherapy combination in terms of progression-free and overall survival but offers the advantage of avoiding the administration of the heavily cytotoxic cisplatin (125). The combination of Cetuximab and ICIs such as Pembrolizumab or Avelumab is also investigated in patients with recurrent/metastatic HNSCC (Table 2), and promising clinical activity has been reported for the Cetuximab/Pembrolizumab combination (126). Beside Cetuximab, other ErbB-targeting agents are being evaluated in combination with ICIs for HNSCC, including the anti-ErbB2 antibodies Margetuximab, BDC-1001 and SBT6050, and the EGFR/ErbB2/ErbB4 inhibitor Afatinib (Table 2).
In the search for increasingly effective immunotherapeutic strategies for HNSCC, other approaches are also being investigated, such as those based on chimeric antigen receptor (CAR)-immune cell therapy, oncolytic virus therapy, and vaccines (160). In this regard, CAR-immune cell therapies are under evaluation in HNSCC patients, in trials based on the transfer of different types of immune cells armed with chimeric receptors able to target one or multiple ErbBs. One of such trials is underway to test the safety of the intratumoral administration of autologous T-cells engineered to express a second generation CAR able to engage multiple ErbB dimers that are commonly upregulated in HNSCC (163; Table 2). Trials based on the adoptive transfer of immune cells engineered to target ErbB2, such as NK cells, macrophages or T cells, are also underway for patients with ErbB2-expressing tumors, including HNSCC (Table 2).
As a final remark, the clinical evidence accumulated so far indicates that the response to immunotherapy in HNSCC patients appears on the whole variable (160). This evidence highlights the urge to identify biomarkers able to predict clinical responses in order to select the best therapeutic regimen for personalized, tailored treatments.
While the incidence of HNSCC is raising worldwide, overall outcomes remain poor due to the limited tumor responsivity to radiation and drug regimens, leading to treatment failure, relapses and disease progression, thus highlighting the urge for novel, more effective therapeutic strategies. Although the EGFR has long been recognized as an established oncogene and a therapeutic target, the full potential of targeting this receptor and the related ErbBs in HNSCC is yet to be fully explored. For instance, although most studies have been focused on the involvement of the EGFR in HNSCCs, the available data indicate that a good proportion of these tumors may simultaneously express multiple ErbB receptors. In this regard, the impact of ErbB receptors cooperative signaling on HNSCC pathogenesis, outcomes and therapeutic responses is an issue which will deserve further investigation. Another open field of research is based on the evidence that non-canonical ErbB signaling mechanisms are involved in HNSCC progression and resistance to therapy, which may lead to the development of therapeutic agents aimed at targeting EGFR nuclear translocation or EGFR/ErbB2 exosomal transfer. Future studies in these fields may also provide new important findings on the involvement of canonical and non-canonical ErbB signaling in the modulation of the tumor microenvironment, useful for the optimization of therapeutic strategies based on the combination of ErbB-targeting agents and immunotherapy approaches.
CP, MB, CF, LA, LC, AR, DN, VA, and AB were responsible for conceptualization, methodology, formal analysis, writing, figure, and original draft preparation. LM and RB were responsible for conceptualization, review and editing and for funding acquisition. All authors contributed to the article and approved the submitted version.
This study was funded by grants from Ministero dell’Università e della Ricerca, PRIN 2020 (BeiR20Prin, CUP: E85F22000060006 to RB), and by a grant from the University of Rome “Sapienza,” Ateneo 2019 (#RM11916B74788236 to LM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1.Alsahafi, E, Begg, K, Amelio, I, Raulf, N, Lucarelli, P, Sauter, T, et al. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. (2019) 10:540. doi: 10.1038/s41419-019-1769-9
2.Johnson, DE, Burtness, B, Leemans, CR, Lui, VWY, Bauman, JE, and Grandis, JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. (2020) 6:92. doi: 10.1038/s41572-020-00224-3
3.Leemans, CR, Braakhuis, BJM, and Brakenhoff, RH. The molecular biology of head and neck cancer. Nat Rev Cancer. (2011) 11:9–22. doi: 10.1038/nrc2982
5.Adelstein, D, Gillison, ML, Pfister, DG, Spencer, S, Adkins, D, Brizel, DM, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Cancer Netw. (2017) 15:761–70, 770. doi: 10.6004/jnccn.2017.0101
6.Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinoma. Nature. (2015) 517:576–82. doi: 10.1038/nature14129
7.Chung, CH, Parker, JS, Karaca, G, Wu, J, Funkhouser, WK, Moore, D, et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. (2004) 5:489–500. doi: 10.1016/s1535-6108(04)00112-6
8.Nair, S, Bonner, JA, and Bredel, M. EGFR mutations in head and neck squamous cell carcinoma. Int J Mol Sci. (2022) 23:3818. doi: 10.3390/ijms23073818
9.Roskoski, R. The ErbB/HER family of protein tyrosine kinases and cancer. Pharmacol Res. (2014) 79:34–74. doi: 10.1016/j.phrs.2013.11.002
10.Wang, Z. ErbB receptors and cancer. Methods Mol Biol. (2017) 1652:3–35. doi: 10.1007/978-1-4939-7219-7_1
11.Kennedy, SP, Hastings, JF, Han, JZR, and Croucher, DR. The under-appreciated promiscuity of the epidermal growth factor receptor family. Front Cell Dev Biol. (2016) 4:88. doi: 10.3389/fcell.2016.00088
12.Brand, TM, Iida, M, Luthar, N, Starr, MM, Huppert, EJ, and Wheeler, DL. Nuclear EGFR as a molecular target in cancer. Radiother Oncol. (2013) 108:370–7. doi: 10.1016/j.radonc.2013.06.010
13.Frawley, T, and Piskareva, O. Extracellular vesicle dissemination of epidermal growth factor receptor and ligands and its role in cancer progression. Cancers. (2020) 12:3200. doi: 10.3390/cancers12113200
14.Lee, JH, Heo, SG, Ahn, BC, Hong, MH, Cho, BC, Lim, M, et al. A Phase II study of poziotinib in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Cancer Med. (2021) 10:7012–20. doi: 10.1002/cam4.4231
15.Steuer, CE, Griffith, CC, Nannapaneni, S, Patel, MR, Liu, Y, Magliocca, KR, et al. A correlative analysis of PD-L1, PD-1, PD-L2, EGFR, HER2, and HER3 expression in oropharyngeal squamous cell carcinoma. Mol Cancer Ther. (2018) 17:710–6. doi: 10.1158/1535-7163.MCT-17-0504
16.Kumagai, S, Koyama, S, and Nishikawa, H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer. (2021) 21:181–97. doi: 10.1038/s41568-020-00322-0
17.Bossi, P, Resteghini, C, Paielli, N, Licitra, L, Pilotti, S, and Perrone, F. Prognostic and predictive value of EGFR in head and neck squamous cell carcnoma. Oncotarget. (2016) 7:74362–79. doi: 10.18632/oncotarget.11413
18.Hashmi, AA, Hussain, ZF, Aijaz, S, Irfan, M, Khan, EY, Nax, S, et al. Immunohistochemical expression of epidermal growth factor receptor (EGFR) in South Asian head and neck squamous cell carcinoma: association with various risk factors and clinico-pathologic and prognostic parameters. World J Surg Oncol. (2018) 16:118. doi: 10.1186/s12957-018-1425-3
19.Jia, J, Cui, Y, Lu, M, Wang, X, Li, J, Li, J, et al. The relation of EGFR expression by immunohistochemical staining and clinical response of combination treatment of nimotuzumab and chemotherapy in esophageal squamous cell carcinoma. Clin Transl Oncol. (2016) 18:592–8. doi: 10.1007/s12094-015-1406-8
20.Janecka-Widla, A, Majchrzyk, K, Mucha-Malecka, A, and Biesaga, B. EGFR/PI3K/Akt/mTOR pathway in head and neck squamous cell carcinoma patients with different HPV status. Pol J Pathol. (2021) 72:296–314. doi: 10.5114/pjp.2021.113073
21.Ang, KK, Zhang, Q, Rosenthal, DI, Nguyen-Tan, PF, Sherman, EJ, Weber, RS, et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. (2014) 32:2940–50. doi: 10.1200/JCO.2013.53.5633
22.Dionysopoulos, D, Pvalakis, K, Kotoula, V, Fountzilas, E, Markou, K, Karasmanis, I, et al. Cyclin D1, EGFR, and Akt/mTOR pathway. Potential prognostic markers in localized laryngeal squamous cell carcinoma. Strahlenther Onkol. (2013) 189:202–14. doi: 10.1007/s00066-012-0275-0
23.Rodríguez, MO, Rivero, TC, del Castillo-Bahi, R, Muchuli, CR, Bilbao, MA, Vinageras, AN, et al. Nimotuzumab plus radiotherapy for unresectable squamous-cell carcinoma of the head and neck. Cancer Biol Ther. (2010) 9:343–9. doi: 10.4161/cbt.9.5.10981
24.Suzuki, Y, Fukumura, Y, Asahina, M, Fujimaki, M, Ohba, S, Matsumoto, F, et al. EGFR protein expression relates with tumor histology, methylation status of EGFR and HPV16 E6 viral load in oropharyngeal carcinoma. Head Neck Pathol. (2021) 15:743–56. doi: 10.1007/s12105-020-01261-w
25.de Andrade, AL, Ferreira, SJ, Ferreira, SM, Ribeiro, CM, Freitas Rde, A, and Galvão, HC. Immunoexpression of EGFR and EMMPRIN in a series of cases of head and neck squamous cell carcinoma. Pathol Res Pract. (2015) 211:776–81. doi: 10.1016/j.prp.2015.07.005
26.Bei, R, Budillon, A, Masuelli, L, Cereda, V, Vitolo, D, Di Gennaro, E, et al. Frequent overexpression of multiple ErbB receptors by head and neck squamous cell carcinoma contrasts with rare antibody immunity in patients. J Pathol. (2004) 204:317–25. doi: 10.1002/path.1642
27.Huang, SF, Cheng, SD, Chien, HT, Liao, CT, Chen, IH, Wang, HM, et al. Relationship between epidermal growth factor receptor gene copy number and protein expression in oral cavity squamous cell carcinoma. Oral Oncol. (2012) 48:67–72. doi: 10.1016/j.oraloncology.2011.06.511
28.Hitt, R, Irigoyen, A, Cortes-Funes, H, Grau, JJ, García-Sáenz, JA, Cruz-Hernandez, JJ, et al. Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol. (2012) 23:1016–22. doi: 10.1093/annonc/mdr367
29.Kontić, M, Čolović, Z, Paladin, I, Gabelica, M, Barić, A, and Pešutić-Pisac, V. Association between EGFR expression and clinical outcome of laryngeal HPV squamous cell carcinoma. Acta Otolaryngol. (2019) 139:913–7. doi: 10.1080/00016489.2019.1651938
30.Owusu-Afriyie, O, Owiredu, WKBA, Owusu-Danquah, K, Kormarck, C, Foltin, SK, Larsen-Reindorf, R, et al. Expression of immunohistochemical markers in non-oropharyngeal head and neck squamous cell carcinoma in Ghana. PLoS One. (2018) 13:e0202790. doi: 10.1371/journal.pone.0202790
31.Pectasides, E, Rampias, T, Kountourakis, P, Sasaki, C, Kowalski, D, Fountzilas, G, et al. Comparative prognostic value of epidermal growth factor quantitative protein expression compared with FISH for head and neck squamous cell carcinoma. Clin Cancer Res. (2011) 17:2947–54. doi: 10.1158/1078-0432.CCR-10-2040
32.Vainshtein, JM, Spector, ME, McHugh, JB, Wong, KK, Walline, HM, Byrd, SA, et al. Refining risk stratification for locoregional failure after chemoradiotherapy in human papillomavirus-associated oropharyngeal cancer. Oral Oncol. (2014) 50:513–9. doi: 10.1016/j.oraloncology.2014.02.001
33.Alterio, D, Marvaso, G, Maffini, F, Gandini, S, Chiocca, S, Ferrari, A, et al. Role of EGFR as prognostic factor in head and neck cancer patients treated with surgery and postoperative radiotherapy: proposal of a new approach behind the EGFR overexpression. Med Oncol. (2017) 34:107. doi: 10.1007/s12032-017-0965-7
34.Kriegs, M, Claudits, TS, Hoffer, K, Bartels, J, Buhs, S, Gerull, H, et al. Analysing expression and phosphorylation of the EGF receptor in HNSCC. Sci Rep. (2019) 9:13564. doi: 10.1038/s41598-019-49885-5
35.Watson, RF, Chernock, RD, Zhang, KH, Michel, LS, Adkins, DR, El-Moftly, SK, et al. Epidermal growth factor receptor expression in spindle cell carcinomas of the head and neck. Head Neck Pathol. (2015) 9:360–8. doi: 10.1007/s12105-014-0604-y
36.Costa, V, Kowalski, LP, Coutinho-Camillo, CM, Begnami, MD, Calsavara, VF, Neves, JI, et al. EGFR amplification and expression in oral squamous cell carcinoma in young adults. Int J Oral Maxillofac Surg. (2018) 47:817–23. doi: 10.1016/j.ijom.2018.01.002
37.Gröbe, A, Eichhorn, W, Fraederich, M, Kluwe, L, Vashist, Y, Wikner, J, et al. Immunohistochemical and FISH analysis of EGFR and its prognostic value in patients with oral squamous cell carcinoma. J Oral Pathol Med. (2014) 43:205–10. doi: 10.1111/jop.12111
38.Lundberg, M, Leivo, I, Saarilahti, K, Mäkitie, AA, and Mattila, PS. Transforming growth factor beta 1 genotype and p16 as prognostic factors in head and neck squamous cell carcinoma. Acta Otolaryngol. (2012) 132:1006–12. doi: 10.3109/00016489.2012.678944
39.Marijić, B, Braut, T, Babarović, E, Krstulja, M, Maržić, D, Avirović, M, et al. EGFR expression is associated with poor survival in laryngeal carcinoma. Appl Immunohistochem Mol Morphol. (2021) 29:576–84. doi: 10.1097/PAI.0000000000000932
40.Cohen, ER, Reis, IM, Gomez, C, Pereira, L, Freiser, ME, Hoosien, G, et al. Immunohistochemistry analysis of CD44, EGFR, and p16 in oral cavity and oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. (2017) 157:239–51. doi: 10.1177/0194599817700371
41.Licitra, L, Störkel, S, Kerr, KM, Van Cutsem, E, Pirker, R, Hirsh, FR, et al. Predictive value of epidermal growth factor receptor expression for first-line chemotherapy plus cetuximab in patients with head and neck and colorectal cancer: analysis of data from the EXTREME and CRYSTAL studies. Eur J Cancer. (2013) 49:1161–8. doi: 10.1016/j.ejca.2012.11.018
42.Mehta, A, Chowdhary, M, and Sinha, R. Immunoscoring of epidermal growth factor receptor expression in recurrent cases of oral squamous cell carcinoma. J Oral Pathol Med. (2015) 44:818–22. doi: 10.1111/jop.12303
43.Tinhofer, I, Klighammer, K, Weichert, W, Knödler, M, Stenzinger, A, Gauler, T, et al. Expression of amphiregulin and EGFRvIII affect outcome of patients with squamous cell carcinoma of the head and neck receiving cetuximab-docetaxel treatment. Clin Cancer Res. (2011) 17:5197–204. doi: 10.1158/1078-0432.CCR-10-3338
44.Romanitan, M, Näsman, A, Munck-Wikland, E, Dalianis, T, and Ramqvist, T. EGFR and phosphorylated EGFR in relation to HPV and clinical outcome in tonsillar cancer. Anticancer Res. (2013) 33:1575–83.
45.Rössle, M, Weber, CS, Züllig, L, Graf, N, Jochum, W, Stöckli, SJ, et al. EGFR expression and copy number changes in low T-stage oral squamous cell carcinomas. Histopathology. (2013) 63:271–8. doi: 10.1111/his.12175
46.Hongo, T, Yamamoto, H, Jiromaru, R, Yasumatsu, R, Kuga, R, Nozaki, Y, et al. PD-L1 expression, tumor-infiltrating lymphocytes, mismatch repair deficiency, EGFR alteration and HPV infection in sinonasal squamous cell carcinoma. Mod Pathol. (2021) 34:1966–78. doi: 10.1038/s41379-021-00868-w
47.Lassen, P, Overgaard, J, and Eriksen, JG. Expression of EGFR and HPV-associated p16 in oropharyngeal carcinoma: correlation and influence on prognosis after radiotherapy in the randomized DAHANCA 5 and 7 trials. Radiother Oncol. (2013) 108:489–94. doi: 10.1016/j.radonc.2013.08.036
48.Nakata, Y, Uzawa, N, Takahashi, KI, Sumino, J, Michikawa, C, Sato, H, et al. EGFR gene copy number alteration is a better prognostic indicator than protein overexpression in oral tongue squamous cell carcinomas. Eur J Cancer. (2011) 47:2364–72. doi: 10.1016/j.ejca.2011.07.006
49.Feldman, R, Gatalica, Z, Knezetic, J, Reddy, S, Nathan, CA, Javadi, N, et al. Molecular profiling of head and neck squamous cell carcinoma. Head Neck. (2016) 38:E1625–38. doi: 10.1002/hed.24290
50.Licitra, L, Mesia, R, Rivera, F, Remenár, E, Hitt, R, Erfám, J, et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Ann Oncol. (2011) 22:1078–87. doi: 10.1093/annonc/mdq588
51.Braut, T, Krstulia, M, Rukavina, KM, Joniić, N, Kjundžić, M, Manestar, ID, et al. Cytoplasmic EGFR staining and gene amplification in glottic cancer: a better indicator of EGFR-driven signaling? Appl Immunohistochem Mol Morphol. (2014) 22:674–80. doi: 10.1097/PAI.0000000000000014
52.Young, R, Rischin, D, Fisher, R, McArthur, GA, Fox, SB, Peters, LJ, et al. Relationship between epidermal growth factor receptor status, p16(INK4A), and outcome in head and neck squamous cell carcinoma. Cancer Epidemiol Biomark Prev. (2011) 20:1230–7. doi: 10.1158/1055-9965.EPI-10-1262
53.Wheeler, S, Siwak, DR, Chai, R, LaValle, C, Seethala, RR, Wang, L, et al. Tumor epidermal growth factor receptor (EGFR) and EGFR PY1068 are independent prognostic indicators for head and neck squamous cell carcinoma. Clin Cancer Res. (2012) 18:2278–89. doi: 10.1158/1078-0432.CCR-11-1593
54.Wheeler, SE, Egloff, AM, Wang, L, James, CD, Hammerman, PS, and Grandis, JR. Challenges in EGFRvIII detection in head and neck squamous cell carcinoma. PLoS One. (2015) 10:e0117781. doi: 10.1371/journal.pone.0117781, eCollection 2015
55.Szabò, B, Nelhubel, GA, Kárpáti, A, Kenessey, I, Jóri, B, Székely, C, et al. Clinical significance of genetic alterations and expression of epidermal growth factor receptor (EGFR) in head and neck squamous cell carcinomas. Oral Oncol. (2011) 47:487–96. doi: 10.1016/j.oraloncology.2011.03.020
56.Cabal, VN, Menendez, M, Vivanco, B, Potes-Ares, S, Riobello, C, Suarez-Fernandez, L, et al. EGFR mutation and HPV infection in sinonasal inverted papilloma and squamous cell carcinoma. Rhinology. (2020) 58:368–76. doi: 10.4193/Rhin19.371
57.Kaur, G, Phogat, D, and Manu, V. Study of EGFR mutations in head and neck squamous cell carcinomas. Autops Case Rep. (2021) 11:e2021251. doi: 10.4322/acr.2021.251
58.Koch, DT, Pickhard, A, Gebel, L, Buchberger, AMS, Bas, M, Mogler, C, et al. Epidermal growth factor receptor variant III in head and neck squamous cell carcinoma is not relevant for targeted therapy and irradiation. Oncotarget. (2017) 8:32668–82. doi: 10.18632/oncotarget.15949
59.Smilek, P, Neuwirthova, J, Jarkovsky, J, Dusek, L, Rottenberg, J, Kostrica, R, et al. Epidermal growth factor receptor (EGFR) expression and mutations in the EGFR signaling pathway in correlation with anti-EGFR therapy in head and neck squamous cell carcinomas. Neoplasma. (2012) 59:508–15. doi: 10.4149/neo_2012_065
60.Braig, F, Kriegs, M, Voigtlaender, M, Habel, B, Grob, T, Biskup, K, et al. Cetuximab resistance in head and neck cancer is mediated by EGFR-K521 polymorphism. Cancer Res. (2017) 77:1188–99. doi: 10.1158/0008-5472.CAN-16-0754
61.Hanken, H, Gaudin, R, Gröbe, A, Fraederich, M, Eichhorn, W, Smeets, R, et al. Her2 expression and gene amplification is rarely detectable in patients with oral squamous cell carcinomas. J Oral Pathol Med. (2014) 43:304–8. doi: 10.1111/jop.12173
62.Warren, EAK, Anil, J, Castro, PD, Kemnade, J, Suzuki, M, Hedge, M, et al. Human epidermal growth factor receptor 2 expression in head and neck squamous cell carcinoma: variation within and across primary tumor sites, and implications for antigen-specific immunotherapy. Head Neck. (2021) 43:1983–94. doi: 10.1002/hed.26662
63.Cierpikowski, P, Lis-Nawara, A, Gaidzis, P, and Bar, J. PDGFRα/HER2 and PDGFRα/p53 co-expression in oral squamous cell carcinoma. Anticancer Res. (2018) 38:795–802. doi: 10.21873/anticanres.12286
64.Birkeland, AC, Yanik, M, Tillman, BN, Scott, MV, Foltin, SK, Mann, JE, et al. Identification of targetable ERBB2 aberrations in head and neck squamous cell carcinoma. JAMA Otolaryngol Head Neck Surg. (2016) 142:559–67. doi: 10.1001/jamaoto.2016.0335
65.Cortelazzi, B, Verderio, P, Ciniselli, CM, Pizzamiglio, S, Bossi, P, Gloghini, A, et al. Receptor tyrosine kinase profiles and human papillomavirus status in oropharyngeal squamous cell carcinoma. J Oral Pathol Med. (2015) 44:734–45. doi: 10.1111/jop.12301
66.Wei, Q, Sheng, L, Shui, Y, Hu, Q, Nordgren, H, and Carlsson, J. EGFR, HER2, and HER3 expression in laryngeal primary tumors and corresponding metastases. Ann Surg Oncol. (2008) 15:1193–201. doi: 10.1245/s10434-007-9771-3
67.Rautava, J, Jee, KJ, Miettinen, PJ, Nagy, B, Myllykangas, S, Odell, EW, et al. ERBB receptors in developing, dysplastic and malignant oral epithelia. Oral Oncol. (2008) 44:227–35. doi: 10.1016/j.oraloncology.2007.02.012
68.Kim, H, Choi, JY, Rah, YC, Ahn, JC, Kim, H, Jeong, WJ, et al. ErbB3, a possible prognostic factor of head and neck squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. (2020) 129:377–87. doi: 10.1016/j.oooo.2019.12.006
69.Husain, H, Psyrry, A, Markovic, A, Rampias, T, Pectasides, E, Wang, H, et al. Nuclear epidermal growth factor receptor and p16 expression in head and neck squamous cell carcinoma. Laryngoscope. (2012) 122:2762–8. doi: 10.1002/lary.23647
70.Pollock, NI, Wang, L, Wallweber, G, Gooding, WE, Huang, W, Chenna, A, et al. Increased expression of HER2, HER3, and HER2:HER3 heterodimers in HPV-positive HNSCC using a novel proximity-based assay: implications for targeted therapies. Clin Cancer Res. (2015) 21:4597–606. doi: 10.1158/1078-0432.CCR-14-3338
71.Vatte, C, Al Amri, AM, Cyrus, C, Chathoth, S, Acharya, S, Hashim, TM, et al. Tyrosine kinase domain mutations of EGFR gene in head and neck squamous cell carcinoma. Onco Targets Ther. (2017) 2017:1527–33. doi: 10.2147/OTT.S132187.eCollection
72.Perisanidis, C. Prevalence of EGFR tyrosine kinase domain mutations in head and neck squamous cell carcinoma: cohort study and systematic review. In Vivo. (2017) 31:23–34. doi: 10.21873/invivo.11020
73.Nair, S, Trummell, HQ, Rajbhandari, R, Thudi, NK, Nozell, SE, and Warram, JM. Novel EGFR ectodomain mutations associated with ligand-independent activation and cetuximab resistance in head and neck cancer. PLoS One. (2020) 15:e0229077. doi: 10.1371/journal.pone.0229077
74.Khattri, A, Sheikh, N, Acharya, R, Tan, YHC, Kochanny, S, Lingen, MW, et al. Mechanism of acquired resistance to cetuximab in head and neck cancer. J Clin Oncol. (2018) 36:e18061–1. doi: 10.1200/JCO.2018.36.15_suppl.e18061
75.Sok, JC, Coppelli, FM, Thomas, SM, Lango, MN, Xi, S, Hunt, JL, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. (2006) 12:5064–73. doi: 10.1158/1078-0432.CCR-06-0913
76.Alaoui-Jamali, MA, Morand, GB, and da Silva, SD. ErbB polymorphisms: insights and implications for response to targeted cancer therapeutics. Front Genet (2015) 6:17. doi: 10.3389/fgene.2015.00017.eCollection 2015.
77.Bossi, P, and Platini, F. Radiotherapy plus EGFR inhibitors: synergistic modalities. Cancers Head Neck. (2017) 2:2. doi: 10.1186/s41199-016-0020-y
78.Chung, CH, Zhang, Q, Hammond, EM, Trotti, AM III, Wang, H, Spencer, S, et al. Integrating EGFR assay with clinical parameters improves risk classification for relapse and survival in head and neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. (2011) 81:331–8. doi: 10.1016/j.ijrobp.2010.05.024
79.Hong, A, Dobbins, T, Lee, CS, Jones, D, Jackson, E, Clark, J, et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur J Cancer. (2010) 46:2088–96. doi: 10.1016/j.ejca.2010.04.016
80.Masuelli, L, Marzocchella, L, Quaranta, A, Palumbo, C, Pompa, G, Izzi, V, et al. Apigenin induces apoptosis and impairs head and neck carcinomas EGFR/ErbB2 signaling. Front Biosci. (2011) 16:1060–8. doi: 10.2741/3735
81.Pollock, NI, and Grandis, JR. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res. (2015) 21:526–33. doi: 10.1158/1078-0432.CCR-14-1432
82.Ali, MA, Gunduz, M, Gunduz, E, Tamamura, R, Beder, LB, Katase, N, et al. Expression and mutation analysis of her2 in head and neck squamous cell carcinoma. Cancer Investig. (2010) 28:495–500. doi: 10.3109/07357900903476778
83.Tse, GM, Yu, KH, Chan, AWH, King, AD, Chen, GG, Wong, KT, et al. HER2 expression predicts improved survival in patients with cervical node-positive head and neck squamous cell carcinoma. Otolaryngol Head Neck Surg. (2009) 141:467–73. doi: 10.1016/j.otohns.2009.06.747
84.Takikita, M, Xie, R, Chiung, JY, Cho, H, Ylaya, K, Hong, SM, et al. Membranous expression of Her3 is associated with a decreased survival in head and neck squamous cell carcinoma. J Transl Med. (2011) 9:126. doi: 10.1186/1479-5876-9-126
85.Del Sordo, R, Angiero, F, Bellezza, G, Cavaliere, A, Mameli, MG, Stefani, M, et al. HER family receptors expression in squamous cell carcinoma of the tongue: study of the possible prognostic and biological significance. J Oral Pathol Med. (2010) 39:79–86. doi: 10.1111/j.1600-0714.2009.00815.x
86.Qian, G, Jiang, N, Wang, D, Newman, S, Kim, S, Chen, Z, et al. Heregulin and HER3 are prognostic biomarkers in oropharyngeal squamous cell carcinoma. Cancer. (2015) 121:3600–11. doi: 10.1002/cncr.29549
87.Almadori, G, Coli, A, De Corso, E, Mele, DA, Settimi, S, Di Cintio, G, et al. Nuclear HER3 expression improves the prognostic stratification of patients with HER1 positive advanced laryngeal squamous cell carcinoma. J Transl Med. (2021) 19:408. doi: 10.1186/s12967-021-03081-0
88.Bussu, F, Ranelletti, O, Gessi, M, Graziani, C, Lanza, P, Lauriola, L, et al. Immunohistochemical expression patterns of the HER4 receptors in normal mucosa and in laryngeal squamous cell carcinomas: antioncogenic significance of the HER4 protein in laryngeal squamous cell carcinoma. Laryngoscope. (2012) 122:1724–33. doi: 10.1002/lary.23311
89.Ohashi, Y, Kumagai, K, Miyata, Y, Matsubara, R, Kitaura, K, Suzuki, S, et al. Overexpression of ErbB4 is an independent marker for lymph node metastasis in Japanese patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 122:313–21. doi: 10.1016/j.oooo.2016.04.017
90.Silva, SD, Alaoui-Jamali, MA, Hier, M, Soares, FA, Graner, E, and Kowalski, P. Cooverexpression of ERBB1 and ERBB4 receptors predicts poor clinical outcome in pN+ oral squamous cell carcinoma with extranodal spread. Clin Exp Metastasis. (2014) 31:307–16. doi: 10.1007/s10585-013-9629-y
91.Almadori, G, Bussu, F, Gessi, M, Ferrandina, G, Scambia, G, Lauriola, L, et al. Prognostic significance and clinical relevance of the expression of the HER family of type I receptor tyrosine kinases in human laryngeal squamous cell carcinoma. Eur J Cancer. (2010) 46:1144–52. doi: 10.1016/j.ejca.2010.01.018
92.Masuelli, L, Budillon, A, Marzocchella, L, Mrozek, MA, Vitolo, D, Di Gennaro, E, et al. Caveolin-1 overexpression is associated with simultaneous abnormal expression of the E-cadherin/α-β catenins complex and multiple ErbB receptors and with lymph nodes metastasis in head and neck squamous cell carcinomas. J Cell Physiol. (2012) 227:3344–53. doi: 10.1002/jcp.24034
93.Xia, W, Lau, YK, Zhang, HZ, Xiao, FY, Johnston, DA, Liu, AR, et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. (1999) 5:4164–74.
94.Bei, R, Pompa, G, Vitolo, S, Moriconi, L, Ciocci, M, Quaranta, L, et al. Co-localization of multiple ErbB receptors in stratified epithelium of oral squamous cell carcinoma. J Pathol. (2001) 195:343–8. doi: 10.1002/path.965
95.Lee, HH, Wang, YN, and Hung, MC. Non-canonical signaling mode of the epidermal growth factor receptor family. Am J Cancer Res. (2015) 5:2944–58.
96.Iannelli, F, Zotti, AI, Roca, MS, Grumetti, L, Lombardi, R, Moccia, T, et al. Valproic acid synergizes with cisplatin and cetuximab in vitro and in vivo in head and neck cancer by targeting the mechanisms of resistance. Front Cell Dev Biol. (2020) 8:732. doi: 10.3389/fcell.2020.00732
97.Li, C, Iida, M, Dunn, EF, and Wheeler, DL. Dasatinib blocks cetuximab- and radiation-induced nuclear translocation of the epidermal growth factor receptor in head and neck squamous cell carcinoma. Radiother Oncol. (2010) 97:330–7. doi: 10.1016/j.radonc.2010.06.010
98.Nowsheen, S, Bonner, JA, and Yang, ES. The poly(ADP-Ribose) polymerase inhibitor ABT-888 reduces radiation-induced nuclear EGFR and augments head and neck tumor response to radiotherapy. Radiother Oncol. (2011) 99:331–8. doi: 10.1016/j.radonc.2011.05.084
99.Psyrri, A, Yu, Z, Weinberger, PM, Sasaki, C, Haffty, B, Camp, R, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin Cancer Res. (2005) 11:5856–62. doi: 10.1158/1078-0432.CCR-05-0420
100.Psyrry, A, Egleston, B, Weiberger, P, Yu, Z, Kowalski, D, Sasaki, K, et al. Correlates and determinants of nuclear epidermal growth factor receptor content in an oropharyngeal cancer tissue microarray. Cancer Epidemiol Biomark Prev. (2008) 17:1486–92. doi: 10.1158/1055-9965.EPI-07-2684
101.Wang, SC, and Hung, MC. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin Cancer Res. (2009) 15:6484–9. doi: 10.1158/1078-0432.CCR-08-2813
102.Lucas, LM, Swivedi, V, Senfeld, JI, Cullum, RL, Mill, CP, Piassa, JT, et al. The yin and yang of ERBB4: tumor suppressor and oncoproteins. Pharmacol Rev. (2022) 74:18–47. doi: 10.1124/pharmrev.121.000381
103.Mohd Nafi, SN, Generali, D, Kramer-Marek, G, Gijsen, M, Strina, C, Cappelletti, M, et al. Nuclear HER4 mediates acquired resistance to trastuzumab and is associated with poor outcome in HER2 positive breast cancer. Oncotarget. (2014) 5:5934–49. doi: 10.18632/oncotarget.1904
104.Paskeh, MDA, Entezari, M, Mirzaei, S, Zabolian, A, Saleki, H, Naghdi, MJ, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. (2022) 15:83. doi: 10.1186/s13045-022-01305-4
105.Wu, S, Luo, M, To, KKW, Zhang, J, Su, C, Zhang, H, et al. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol Cancer. (2021) 10:17. doi: 10.1186/s12943-021-01307-9
106.Song, X, Ding, Y, Liu, G, Yang, X, Zhao, R, Zhang, Y, et al. Cancer cell-derived exosomes induce mitogen-activated protein kinase-dependent monocyte survival by transport of functional receptor tyrosine kinases. J Biol Chem. (2016) 291:8453–64. doi: 10.1074/jbc.M116.716316
107.Yoshida, K, Tsuda, M, Matsumoto, R, Semba, S, Wang, L, Sugino, H, et al. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. (2019) 110:2119–32. doi: 10.1111/cas.14080
108.Li, Y, Gao, S, Hu, Q, and Wu, F. Functional properties of cancer epithelium and stroma-derived exosomes in head and neck squamous cell carcinoma. Life. (2022) 12:757. doi: 10.3390/life12050757
109.Teng, Y, Gao, L, Loveless, R, Rodrigo, JP, Strojan, P, Willems, SM, et al. The hidden link of exosomes to head and neck cancer. Cancers. (2021) 13:5802. doi: 10.3390/cancers13225802
110.Fujiwara, T, Eguchi, T, Sogawa, C, Ono, K, Murakami, J, Ibaragi, S, et al. Anti-EGFR antibody cetuximab is secreted by oral squamous cell carcinoma and alters EGF-driven mesenchymal transition. Biochem Biophys Res Commun. (2018) 503:1267–72. doi: 10.1016/j.bbrc.2018.07.035
111.Raulf, N, Lucarelli, P, Thavaraj, S, Brown, S, Vicencio, JM, Sauter, T, et al. Annexin A1 regulates EGFR activity and alters EGFR-containing tumor-derived exosomes in head and neck cancers. Eur J Cancer. (2018) 102:52–68. doi: 10.1016/j.ejca.2018.07.123
112.Theodoraki, MN, Matsumoto, A, Beccard, I, Hoffmann, TK, and Whiteside, TL. CD44v3 protein-carrying tumor-derived exosomes in HNSCC patients’ plasma as potential noninvasive biomarkers of disease activity. Onco Targets Ther. (2020) 9:1747732. doi: 10.1080/2162402X.2020.1747732
113.Fujiwara, T, Eguchi, T, Sogawa, C, Ono, K, Murakami, J, Ibaragi, S, et al. Carcinogenic epithelial-mesenchynal transition initiated by oral cancer exosomes is inhibited by anti-EGFR antibody cetuximab. Oral Oncol. (2018) 86:251–7. doi: 10.1016/j.oraloncology.2018.09.030
114.Muraro, E, Fanetti, G, Lupato, V, Giacomarra, V, Steffan, A, Gobitti, C, et al. Cetuximab in locally advanced head and neck squamous cell carcinoma: biological mechanisms involved in efficacy, toxicity and resistance. Crit Rev Oncol Hematol. (2021) 164:103424. doi: 10.1016/j.critrevonc.2021.103424
115.Xu, MJ, Johnson, DE, and Grandis, JR. EGFR-targeted therapies in the post-genomic era. Cancer Metastatis Rev. (2017) 36:463–73. doi: 10.1007/s10555-017-9687-8
116.Patel, N, and Saba, NF. Current aspects and future considerations of EGFR inhibition in locally advanced and recurrent metastatic squamous cell carcinoma of the head and neck. Cancers. (2021) 13:3545. doi: 10.3390/cancers13143545
117.Centuori, SM, and Bauman, JE. c-Met signaling as a therapeutic target in head and neck cancer. Cancer J. (2022) 28:346–53. doi: 10.1097/PPO.0000000000000619
118.Kochanny, SE, Worden, FP, Adkins, DR, Lim, DW, Bauman, JE, Wagner, SA, et al. A randomized phase 2 network trial of tivantinib plus cetuximab versus cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Cancer. (2020) 126:2146–52. doi: 10.1002/cncr.32762
119.Szturz, P, Raymond, E, Abitbol, C, Albert, S, de Gramont, A, and Faivre, S. Understanding c-MET signalling in squamous cell carcinoma of the head & neck. Crit Rev Oncol Hematol. (2017) 111:39–51. doi: 10.1016/j.critrevonc.2017.01.004
120.Wang, D, Lu, Y, Nannapaneni, S, Griffith, CC, Steuer, C, Qian, G, et al. Combinatiorial approaches targeting the EGFR family and c-Met in SCCHN. Oral Oncol. (2021) 112:105074. doi: 10.1016/j.oraloncology.2020.105074
121.Ang, MK, Montoya, JE, Tharavichitkul, E, Lim, C, Tan, T, Wang, LY, et al. Phase II study of nimotuzumab (TheraCim-hR3) concurrent with cisplatin/radiotherapy in patients with locally advanced head and neck squamous cell carcinoma. Head Neck. (2021) 43:1641–51. doi: 10.1002/hed.26635
122.Temam, S, Spicer, J, Farzaneh, F, Soria, JC, Oppenheim, D, McGurk, M, et al. An exploratory, open-label, randomized, multicenter study to investigate the pharmacodynamics of a glycoengineered antibody (imgatuzumab) and cetuximab in patients with operable head and neck squamous cell carcinoma. Ann Oncol. (2017) 28:2827–35. doi: 10.1093/annonc/mdx489
123.Oppelt, P, Ley, J, Daly, M, Rich, J, Paniello, R, Jackson, RS, et al. nab-Paclitaxel and cisplatin followed by cisplatin and radiation (Arm 1) and nab-paclitaxel followed by cetuximab and radiation (Arm 2) for locally advanced head and neck squamous-cell carcinoma: a multicenter, non-randomized phase 2 trial. Med Oncol. (2021) 38:35. doi: 10.1007/s12032-021-01479-w
124.Bonomo, P, Desideri, I, Mangoni, M, Saieva, C, Loi, M, Becherini, C, et al. Durvalumab with cetuximab and radiotherapy for locally advanced squamous cell carcinoma of the head and neck: a phase 1/2 trial. Radiother Oncol. (2022) 169:64–70. doi: 10.1016/j.radonc.2022.02.008
125.Ferris, RL, Moskovitz, J, Kunning, S, Ruffin, AT, Reedr, C, Ohr, J, et al. Phase I trial of cetuximab, radiotherapy, and Ipilimumab in locally advanced head and neck cancer. Clin Cancer Res. (2022) 28:1335–44. doi: 10.1158/1078-0432.CCR-21-0426
126.Sacco, AG, Chen, R, Worden, FP, Wong, DJL, Adkins, D, Swiecicki, P, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. Lancet Oncol. (2021) 22:883–92. doi: 10.1016/S1470-2045(21)00136-4
127.Tao, Y, Aupérin, A, Sun, X, Sire, C, Martin, L, Coutte, A, et al. Avelumab-cetuximab-radiotherapy versus standards of care in locally advanced squamous-cell carcinoma of the head and neck: the safety phase of a randomised phase III trial GORTEC 2017-01 (REACH). Eur J Cancer. (2020) 141:21. doi: 10.1016/j.ejca.2020.09.008
128.Giralt, J, Trigo, J, Nuyts, S, Ozsahin, M, Skladowski, K, Hatoum, G, et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): a randomised, controlled, open-label phase 2 trial. Lancet Oncol. (2015) 16:221–32. doi: 10.1016/S1470-2045(14)71200-8
129.Vermorken, JB, Stöhlmacher-Williams, J, Davidenko, I, Licitra, L, Winquist, E, Villanueva, C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. (2013) 14:697–710. doi: 10.1016/S1470-2045(13)70181-5
130.Machiels, JP, Specenier, P, Kraub, J, Dietz, A, Kaminsky, MC, Lalami, Y, et al. A proof of concept trial of the anti-EGFR antibody mixture Sym004 in patients with squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol. (2015) 76:13–20. doi: 10.1007/s00280-015-2761-4
131.Reynolds, KL, Bedard, PL, Lee, SH, Lin, CC, Tabernero, J, Alsina, M, et al. A phase I open-label dose-escalation study of the anti-HER3 monoclonal antibody LJM716 in patients with advanced squamous cell carcinoma of the esophagus or head and neck and HER2-overexpressing breast or gastric cancer. BMC Cancer. (2017) 17:646. doi: 10.1186/s12885-017-3641-6
132.Harrington, K, Berrier, A, Robinson, M, Remenar, E, Housset, M, de Mendoza, FH, et al. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. Eur J Cancer. (2013) 49:1609–18. doi: 10.1016/j.ejca.2012.11.023
133.Harrington, K, Temam, S, Mehanna, H, D’Cruz, A, Jain, M, D’Onofrio, I, et al. Lapatinib and concurrent chemoradiotherapy followed by maintenance lapatinib monotherapy in high-risk patients with resected squamous cell carcinoma of the head and neck: a phase III, randomized, double-blind. Placebo Controlled Study J Clin Oncol. (2015) 33:4202–9. doi: 10.1200/JCO.2015.61.4370
134.Kao, HF, Liao, BC, Huang, YL, Huang, HC, Chen, CN, Chen, TS, et al. Afatinib and pembrolizumab for recurrent or metastatic head and neck squamous cell carcinoma (ALPHA study): a phase II study with biomarker analysis. Clin Cancer Res. (2022) 28:1560–71. doi: 10.1158/1078-0432.CCR-21-3025
135.Chiu, JW, Chan, K, Chen, EX, Siu, LL, and Abdul Razak, AR. Pharmacokinetic assessment of dacomitinib (pan-HER tyrosine kinase inhibitor) in patients with locally advanced head and neck squamous cell carcinoma (LA SCCHN) following administration through a gastrostomy feeding tube (GT). Investig New Drugs. (2015) 33:895–900. doi: 10.1007/s10637-015-0245-3
136.Van Schalkwyk, MC, Papa, SE, Jeannon, JP, Guerrero Urbano, T, Spicer, JF, and Maher, J. Design of a phase I clinical trial to evaluate intratumoral delivery of the ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum Gene Ther Clin Dev. (2013) 14:134–42. doi: 10.1089/humc.2013.144
137.McGrath, K, and Dotti, G. Combining oncolytic viruses with chimeric antigen receptor T cell therapy. Hum Gene Ther. (2021) 32:150–7. doi: 10.1089/hum.2020.278
138.Park, R, Winnicki, M, Liu, E, and Chu, WM. Immune checkpoints and cancer in the immunogenomics era. Brief Funct Genomics. (2019) 18:133–9. doi: 10.1093/bfgp/ely027
139.Swanson, MS, and Sinha, UK. Rationale for combined blockade of PD-1 and CTLA-4 in advanced head and neck squamous cell cancer—review of current data. Oral Oncol. (2015) 51:12–5. doi: 10.1016/j.oraloncology.2014.10.010
140.Peltanova, B, Raudenska, M, and Masarik, M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Mol Cancer. (2019) 18:63. doi: 10.1186/s12943-019-0983-5
141.Wang, H, Chan, L, and Cho, S. Targeting the immune microenvironment in the treatment of head and neck squamous cell carcinoma. Front Oncol. (2019) 9:1084. doi: 10.3389/fonc.2019.01084
142.Keck, MK, Zuo, Z, Khattri, A, Stricker, TP, Brown, CD, Imanguli, M, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. (2015) 21:870–81. doi: 10.1158/1078-0432.CCR-14-2481
143.Farlow, JL, Brenner, JC, Lei, YL, and Chinn, SB. Immune deserts in head and neck squamous cell carcinoma: a review of challenges and opportunities for modulating the tumor immune microenvironment. Oral Oncol. (2021) 120:105420. doi: 10.1016/j.oraloncology.2021.105420
144.Benvenuto, M, Ciuffa, S, Focaccetti, C, Sbardella, D, Fazi, S, Scimeca, M, et al. Proteasome inhibition by bortezomib parallels a reduction in head and neck cancer cells growth, and an increase in tumor-infiltrating immune cells. Sci Rep. (2021) 11:19051. doi: 10.1038/s41598-021-98450-6
145.Focaccetti, C, Benvenuto, M, Ciuffa, S, Fazi, S, Scimeca, M, Nardi, A, et al. Curcumin enhances the antitumoral effect induced by the recombinant vaccinia neu vaccine (rV-neuT) in mice with transplanted salivary gland carcinoma cells. Nutrients. (2020) 12:1417. doi: 10.3390/nu12051417
146.Hoesli, R, Birkeland, AC, Rosko, AJ, Issa, M, Chow, KL, Michmerhuizen, NL, et al. Proportion of CD4 and CD8 tumor infiltrating lymphocytes predicts survival in persistent/recurrent laryngeal squamous cell carcinoma. Oral Oncol. (2018) 77:83–9. doi: 10.1016/j.oraloncology.2017.12.003
147.Oweida, AJ, Darragh, L, Phan, A, Binder, D, Bhatia, S, Mueller, A, et al. STAT3 modulation of regulatory T cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. (2019) 111:1339–49. doi: 10.1093/jnci/djz036
148.Concha-Benavente, F, and Ferris, RL. Oncogenic growth factor signaling mediating tumor escape from cellular immunity. Curr Opin Immunol. (2017) 45:52–9. doi: 10.1016/j.coi.2017.01.004
149.Saba, NF, Chen, ZG, Haigentz, M, Bossi, P, Rinaldo, A, Rodrigo, JP, et al. Targeting the EGFR and immune pathways in squamous cell carcinoma of the head and neck (SCCHN): forging a new alliance. Mol Cancer Ther. (2019) 18:1909–15. doi: 10.1158/1535-7163.MCT-19-0214
150.Benvenuto, M, Focaccetti, C, Izzi, V, Masuelli, L, Modesti, A, and Bei, R. Tumor antigens heterogeneity and immune response-targeting neoantigens in breast cancer. Semin Cancer Biol. (2021) 72:65–75. doi: 10.1016/j.semcancer.2019.10.023
151.Ward, JP, Gubin, MM, and Schreiber, RD. The role of neoantigens in naturally occurring and therapeutically induced immune responses to cancer. Adv Immunol. (2016) 130:25–74. doi: 10.1016/bs.ai.2016.01.001
152.Kersh, AE, Sasaki, M, Cooper, LA, Kissick, HT, and Pollack, BP. Understanding the impact of ErbB activating events and signal transduction on antigen processing and presentation: MHC expression as a model. Front Pharmacol. (2016) 7:327. doi: 10.3389/fphar.2016.00327
153.Bei, R, Masuelli, L, Moriconi, E, Visco, V, Moretti, A, Kraus, MH, et al. Immune responses to all ErbB family receptors detectable in serum of cancer patients. Oncogene. (1999) 18:1267–75. doi: 10.1038/sj.onc.1202442
154.Schuler, PJ, Boeckers, P, Engers, R, Boelke, E, Bas, M, Greve, J, et al. EGFR-specific T cell frequencies correlate with EGFR expression in head and neck squamous cell carcinoma. J Transl Med. (2011) 9:168. doi: 10.1186/1479-5876-9-168
155.Mazorra, Z, Lavastida, A, Concha-Benavente, F, Valdés, A, Srivastava, RM, García-Bates, TM, et al. Nimotuzumab induces NK cell activation, cytotoxicity, dendritic cell maturation and expansion of EGFR-specific T cells in head and neck cancer patients. Front Pharmacol. (2017) 8:382. doi: 10.3389/fphar.2017.00382
156.Srivastava, RM, Lee, SC, Andrade Filho, PA, Lord, CA, Jie, HB, Davidson, HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. (2013) 19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426
157.Taberna, M, Oliva, M, and Mesía, R. Cetuximab-containing combinations in locally advanced and recurrent or metastatic head and neck squamous cell carcinoma. Front Oncol. (2019) 9:383. doi: 10.3389/fonc.2019.00383. eCollection 2019
158.De Azevedo, J, Mourtada, J, Bour, C, Devignot, V, Schultz, P, Borel, C, et al. The EXTREME regimen associating cetuximab and cisplatin favors head and neck cancer cell death and immunogenicity with the induction of an anti-cancer immune response. Cells. (2022) 11:2866. doi: 10.3390/cells11182866
159.Pozzi, C, Cuomo, A, Spadoni, I, Magni, E, Silvola, A, Conte, A, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med. (2016) 22:624–31. doi: 10.1038/nm.4078
160.Parmar, K, Mohamed, A, Vaish, E, Thawani, R, Cetnar, J, and Thein, KZ. Immunotherapy in head and neck squamous cell carcinoma: an updated review. Cancer Treat Res Commun. (2022) 33:100649. doi: 10.1016/j.ctarc.2022.100649
161.Tian, Y, Zhang, L, Jin, N, Wan, Z, Zhang, H, Zhang, H, et al. Clinical response to neoadjuvant immunotherapy combined with targeted therapy and chemotherapy in oral squamous cell carcinoma: experience in three patients. Onco Targets Ther. (2022) 15:353. doi: 10.2147/OTT.S355349
162.Tang, X, Chen, S, Sui, Q, Li, X, Liu, Z, Zhu, F, et al. Response to nivolumab combining radiotherapy and nimotuzumab in metastatic oral squamous cell carcinoma patient with strong PD-L1 expression: a case report. Ann Transl Med. (2020) 8:402. doi: 10.21037/atm.2020.02.96
163.Van Schalkwyk, MC, Papa, SE, Jeannon, JP, Guerrero Urbano, T, Spicer, JF, and Maher, J. Design of a phase I clinical trial to evaluate intratumoral delivery of the ErbB-targeted chimeric antigen receptor T-cells in locally advanced or recurrent head and neck cancer. Hum Gene Ther Clin Dev. (2013) 24:134–42. doi: 10.1089/humc.2013.144
Keywords: head and neck squamous cell carcinoma, EGFR, ErbB receptors, Cetuximab, immune checkpoint inhibitors
Citation: Palumbo C, Benvenuto M, Focaccetti C, Albonici L, Cifaldi L, Rufini A, Nardozi D, Angiolini V, Bei A, Masuelli L and Bei R (2023) Recent findings on the impact of ErbB receptors status on prognosis and therapy of head and neck squamous cell carcinoma. Front. Med. 10:1066021. doi: 10.3389/fmed.2023.1066021
Received: 10 October 2022; Accepted: 13 January 2023;
Published: 02 February 2023.
Edited by:
Weiren Luo, The Second Affiliated Hospital of Southern University of Science and Technology, ChinaReviewed by:
Lorenzo Lo Muzio, University of Foggia, ItalyCopyright © 2023 Palumbo, Benvenuto, Focaccetti, Albonici, Cifaldi, Rufini, Nardozi, Angiolini, Bei, Masuelli and Bei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberto Bei, ✉ YmVpQG1lZC51bmlyb21hMi5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.