- 1Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 3Students’ Scientific Research Center, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 4Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran
- 5Student Research Committee, Zahedan Medical Sciences Branch, Islamic Azad University, Zahedan, Iran
- 6Student Research Committee, School of Paramedical Sciences, Mashhad University of Medical Sciences, Mashhad, Iran
- 7School of Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- 8Student Research Committee, Fasa University of Medical Sciences, Fasa, Iran
- 9Bitab Knowledge Enterprise, Fasa University of Medical Sciences, Fasa, Iran
- 10Department of Clinical Biochemistry, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran
- 11Student Research Committee, Semnan University of Medical Sciences, Semnan, Iran
Laser-assisted in situ keratomileusis (LASIK) is a unique corneal stromal laser ablation method that uses an excimer laser to reach beneath corneal dome-shaped tissues. In contrast, surface ablation methods, such as photorefractive keratectomy, include removing epithelium and cutting off the Bowman’s layer and the stromal tissue of the anterior corneal surface. Dry eye disease (DED) is the most common complication after LASIK. DED is a typical multi-factor disorder of the tear function and ocular surface that occurs when the eyes fail to produce efficient or adequate volumes of tears to moisturize the eyes. DED influences quality of life and visual perception, as symptoms often interfere with daily activities such as reading, writing, or using video display monitors. Generally, DED brings about discomfort, symptoms of visual disturbance, focal or global tear film instability with possible harm to the ocular surface, the increased osmolarity of the tear film, and subacute inflammation of the ocular surface. Almost all patients develop a degree of dryness in the postoperative period. Detection of preoperative DED and committed examination and treatment in the preoperative period, and continuing treatments postoperatively lead to rapid healing, fewer complications, and improved visual outcomes. To improve patient comfort and surgical outcomes, early treatment is required. Therefore, in this study, we aim to comprehensively review studies on the management and current treatment options for post-LASIK DED.
Introduction
Refractive surgery is a non-invasive method for correcting or improving the refractive state of the eye (1). This surgery has recently become specialized for non-essential and non-sight-threatening conditions (2). Today, ophthalmic surgeons have multiple refractive surgical choices to ameliorate refractive errors (1). In the last two decades, refractive surgery has advanced highly beyond corrective laser therapy. Progress in surgical technologies has also been conducted to introduce intraocular phakic implants, intracorneal implants, and a new minimally invasive refractive surgery approved by the Food and Drug Administration (FDA) (3). The speed and accuracy of laser devices have progressed over the past decade, and the success of laser vision correction is highly related to the precision of these platforms (4).
Refractive surgeries widely divide into refractive corneal lenticular surgery (a kind of stromal ablation that does not need a flap), corneal stromal surgery (making of a corneal flap), and corneal surface ablation surgery (5). Laser-assisted in situ keratomileusis (LASIK) is a unique corneal stromal laser ablation method that uses an excimer laser to reach beneath corneal dome-shaped tissues, while surface ablation methods, such as photorefractive keratectomy, include removing epithelium and cutting of the Bowman’s layer and the stromal tissue of the anterior corneal surface (4, 6, 7). On the other hand, other refractive surgeries, such as small incision lenticule extraction (SMILE), suggest a paradigm change in laser vision correction through a less invasive method that creates a lenticule in the intact cornea (< 4 mm) and removes it through an incision (8). These methods’ visual and refractive outcomes have been demonstrated to be similar to LASIK; however, there is some evidence for the advantages of SMILE over LASIK, such as an intact anterior stroma, better biomechanics, and fewer dry eye disease (DED) symptoms and corneal reinnervation (9, 10).
The Bowman’s layer is the cornea’s most vital part, followed by the central tightly interwoven anterior stroma (100–120 μm) (11). Flap thickness in the LASIK group is between 90 and 110 μm, and cap thickness in the SMILE group is between 100 and 120 μm (12). Therefore, the SMILE method removes the deeper stroma, leaving the anterior-most stroma intact and maintaining more structural integrity (13). However, LASIK involves flap creation by cutting the peripheral collagen fibers, thus causing lower stability (12). The lesser inflammatory response in wound healing after SMILE may contribute to better biomechanics (14).
Recently, the morbidity of DED has increased and has demonstrated a younger trend (15). Recent research revealed that DED occurs in up to 87.5% of the global population (mostly in women). On the other hand, the prevalence of DED multiplies every five years after the age of 50 (16). Therefore, the management of LASIK-induced DED (Li-DED) complications attracted much attention in recent studies. Here, we aim to review published studies on Li-DED to understand what approaches can be taken to decrease laser-surgery disorders, especially DED.
LASIK vs. PRK vs. SMILE
LASIK, the most common method of vision correction treatment, like photorefractive keratectomy (PRK), is used for various types of myopia, hyperopia, and astigmatism cases. SMILE is the newest method of vision correction treatment that is performed for types of myopia and astigmatism.
In the LASIK method, the eye’s cornea is reshaped by creating a thin circular flap on the external tissue of the cornea, and as a result, the incoming light becomes well-focused. PRK is similar to LASIK, with the difference that in this method, instead of using a corneal flap, the upper layer of the cornea, called the epithelium, is removed to expose the inner layer. This method is usually used for patients for whom the LASIK method is unsuitable, such as patients with thin corneas. In SMILE, instead of using an excimer laser, a femtosecond laser creates a small lens-shaped piece of tissue, known as a lenticule, within the cornea. Then, this lens is removed by making an incision in the cornea’s surface. In terms of recovery, the LASIK and SMILE methods are similar, whereas the PRK method requires more recovery time due to the destruction of the corneal epithelium layer.
Few studies have examined PRK and LASIK methods in terms of dry eye parameters; Lee et al. (17) reported a shorter tear break-up time (TBUT) and lower Schirmer test in LASIK than in PRK at 3-month follow-up; however, Bower et al. (18) found more reduction in the Schirmer test in PRK than in LASIK at postoperative 1 and 3 months. On the other hand, several studies have examined SMILE and LASIK methods in terms of dry eye parameters (19–21). The results of a recently published meta-analysis study show that SMILE had better corneal sensitivity, longer TBUT, and lower ocular surface disease index (OSDI) at postoperative 1, 3, and 6 months. However, Schirmer test 3–5 and tear osmolarity 4,5 showed no difference within 6 months of follow-up (20). However, long-term studies have not observed any superiority between these methods (22).
Post-LASIK complications
Over the past decade, it has been reported that LASIK surgery is the most common vision correction surgery in the world, and this is due to not only high patient satisfaction but also to its rare postoperative complications. However, many patients have complained of dry eye symptoms, namely, irritation, dryness, red eye, and ocular fatigue, the so-called post-LASIK tear dysfunction that disappears over time. Only a few patients complain of these symptoms as long-term problems (23). Although the mechanism involved in post-LASIK eye dryness is not clearly understood, flap creation leads to temporal denervation of the cornea, which causes decreased corneal sensitivity, resulting in this condition. It is reported that corneal sensitivity decreases significantly for 3 months after LASIK surgery, but intracorneal nerves regenerate approximately 3 to 6 months post-operation. Decreased or absent corneal sensitivity is a neurodegenerative disease called neurotrophic keratitis (NK). NK is also known as neurotrophic keratopathy. It is a degenerative corneal disease caused by damage to the trigeminal nerve. Corneal nerves are essential for maintaining normal metabolism, tear production, and ocular surface function. Loss of sensitivity results in loss or reduction of corneal sensation (CS). It can impair corneal wound healing and lead to changes in the epithelium, including persistent epithelial defects, punctate epithelial keratopathy, and corneal ulceration. Due to the low sensitivity of the cornea, patients might have no symptoms such as pain or discomfort in their eyes. However, eye redness and dryness, blurred vision, and decreased clarity of vision might appear (24, 25). NK is classified with an estimated prevalence of fewer than 5/10,000 people (26). Although NK can be stimulated by any condition that affects the nerves serving the cornea, two fundamental causes are the herpes simplex virus and the herpes zoster virus. In addition, surgeries involving the cornea or that are performed around the eye, namely LASIK, cataract surgery, orbital surgery, and corneal transplants, have the potential to damage the cornea, thus leading to NK (25).
The diagnosis of NK requires a careful review of the ocular and systemic clinical history, a thorough eye examination, and an evaluation of corneal sensitivity. Complementary examinations such as in vivo confocal microscopy and all diagnostic methods are considered to achieve NK’s correct diagnosis and classification (26). The function of the corneal nerve can be examined and tested quantitatively and qualitatively. Quantitative testing requires the use of a corneal esthetic meter. With the Cochet-Bonnet esthetic meter, mechanical contact is made on the corneal surface with different lengths of nylon thread. The filament is applied to the eye’s surface, and gentle pressure bends the filament. In a qualitative test, dental floss, cotton swabs, or tissue tips stimulate the corneal surfaces bilaterally, and the response is compared in each eye. The response is measured by eliciting the guard blink reflex. The quality of this response can be described as absent from hypostatic to normal. The more significant function of the trigeminal nerve is evident when the patient perceives the cord to be longer. Belmonte’s non-contact gas esthetic meter, which emits carbon dioxide at different temperatures and flow rates, can measure different aspects of corneal sensitivity.
Neurotrophic keratitis (NK) severity correlates with corneal nerve dysfunction during testing. NK is unlikely to occur if the patient has normal corneal sensitivity. However, more work is indicated if the response to eye surface stimuli is reduced (27).
Neurotrophic keratitis (NK) treatment approach should be rapid and based on the severity and stage of the disease. Despite the availability of various medical and surgical treatments, the treatment of NK still needs to be improved, and a lack of positive response is usually observed in clinical practice (25). NK treatment should be based on the severity of the disease. The goal of treating stage 1 disease is to improve the quality and transparency of the epithelium and prevent epithelial breakdown. In the presence of pigment epithelial detachment (PED), treatment is performed to prevent the formation of corneal ulcers and stroma involvement and promote corneal healing. More severe cases with stromal melting and corneal ulceration require immediate attention to prevent perforation and stop stromal lysis (26). In stage 2, the treatment promotes epithelial defect healing and prevents corneal ulcer progression. The patient should be monitored continuously, as progression to asymptomatic perforation and stromal melting may occur. Topical antibiotics are recommended at this stage to prevent infection. Conversely, topical steroids should be used with caution because they may increase the risk of corneal melting and inhibit the healing process. Therapeutic contact lenses improve the cornea, keep the fluid layer in regular contact with the cornea, and protect against eyelid rubbing. However, patients should be careful due to the increased risk of infection (25). An essential feature of stage 3 is the presence of corneal ulceration. Stromal melting may progress to corneal thinning and eventual perforation. This issue may occur without multiple ocular symptoms due to corneal sensitivity disorder. However, patients may complain of blurred vision in edema, corneal ulceration, or scarring (25, 27). Cenegermin (oxervate) is an FDA-approved eye drop for treating NK (28).
Corneal dysesthesia happens when the regeneration of nerve endings results in the exaggeration of stimulus (29–31). However, dry eye signs and symptoms had an incomplete disparity in some observations (32). Although it had been hypothesized that the decreased sensation of the dry eye would ameliorate in some months after the operation due to the regeneration of nerve endings, the slow improvement of thermoreceptors (the nerves involved in the sensation of eye dryness) can explain the reason of this condition (33, 34). In addition, when the osmolarity of the tear increases in patients with preoperative dry eye, the cold thermoreceptors exaggerate the minimal changes after LASIK (33, 35).
After LASIK, some patients experience intense symptoms while they do not indicate any abnormal findings (36). On the other hand, some patients with obvious changes in the epithelium do not complain of any DED symptoms (37). This discrepancy may be due to nervous etiologies rather than tear production (38, 39). Generally, neuralgia is a pain in the nerve distribution with simultaneous nerve damage symptoms. Corneal neuralgia (CN) is a chronic condition in which patients complain of pain and foreign body sensation, often variable and affected by environmental conditions. Persistent DED symptoms following LASIK may be due to CN, which may be a variation of complex regional pain syndrome (CRPS) type 2 (40). Patients with CN indicate poor response to monotherapy with conventional treatments of DED (41). CN is considered to be significantly rare and as a result, its exact incidence is ill-defined (42). This disease is associated with certain comorbidities, such as fibromyalgia, anxiety, depression, headache, HIV, celiac disease, idiopathic small fiber neuropathies, and diabetes. Women have a higher incidence of CN as well as its related comorbidities (43).
The cornea is one of the tissues with the most nervous density. It is among the tissues with the strongest pain sensation. It has virtually 7,000 nerve terminals in each square millimeter. As a result, corneal sensitivity is 300–600 times stronger than skin sensitivity (44). Tactile, temperature, and pain sensations are carried by the nerves of the cornea. Mechanical, chemical, and thermal triggers are identified by these nerves. These triggers are perceived as a form of dysesthesia or pain. Dysesthesia causes unpleasant sensations, including dryness, grittiness, photograph allodynia, irritation, and burning. Pain is a protective mechanism from injury. Nociceptors detect pain stimuli and transmit this signal through action potentials to higher centers for perception. Iatrogenic complications, trauma, and inflammatory states of the ocular surface may impair these nerves, resulting in their hypersensitivity. This sensitization reinforces the signals of pain. Chronic provocation sensitizes the central nervous system and thereby photograph allodynia and higher pain perception occur (43). Peripheral nerve damage might take place during LASIK. CN or corneal neuropathic pain would occur due to a set of adversely regenerated nerve endings after LASIK. The nociceptors become upregulated, bringing about increased responsivity (43, 45).
Patients with CN might report a variety of symptoms, such as aching, boring, burning, foreign body sensation, and photophobia. These manifestations can be debilitating. Blepharospasm may also be present with CN (43). Confirmation of a diagnosis can be achieved through a confocal microscopic view of the cornea, which reveals the abnormal conditions of the nerves. Esthesiometry is another approach to evaluating the response and function of nerve fibers (44). However, these methods are not accessible to all physicians, making CN a diagnosis by exclusion. The identification of CN is very challenging because of a lack of clinical exam signs. Hence, CN can frequently be mistaken for DED (43).
These patients mention increased pain reaction to drops, touch, and air. Cataract or refractive surgery should be taken into account in patient history. Practitioners often disregard the patients’ condition because of scarce clinical findings (38). Normal DED evaluations are initially performed for the patient. The eye surface indicates healthy in CN while patients with DED have impaired tear osmolarity and surface staining (46). Subjective reports of pain without objective DED evidence may be indicative of CN. Of note, DED can be accompanied by CN, which further complicates accurate diagnosis (43).
Severe photophobia and ocular pain can significantly hinder everyday function. Suicidal thoughts can also be present in intense cases (44). A proparacaine challenge test can be used to differentiate between central and peripheral nervous impairment, directing toward the correct treatment option. In this test, 0.5% proparacaine hydrochloride is applied topically. A complete response shows peripheral nerve damage. Additionally, cases with both types show partial response. If there is no alleviation of symptoms, central nerves have been sensitized, making pain management very difficult. There are many proposed therapeutic options for CN (43). Autologous serum can precipitate the regeneration of nerves. Prosthetic replacement of the ocular surface ecosystem (PROSE) may decrease signals of the peripheral nervous system. Topical anti-inflammatory treatments may lower ocular inflammation. Systemic drugs might also hamper or alter pain signals. Neurotrophic factors [especially nerve growth factors (NGFs)] have been reported to reduce pain. Regeneration of impaired nerves of the cornea may alleviate symptoms. In this regard, neurotrophic factors have been evidenced to heal the injured peripheral nerves with improvement in their function. Autologous serum therapy might have the same effect (40).
The prognosis differs for each individual. Chronic CN symptoms usually demand combinational therapy with different methods (38, 44). Early treatment brings about a more encouraging prognosis (43). Regarding CN side effects, anxiety arises in cases with chronic pain and causes even more pain. Furthermore, persistent pain causes physical exhaustion. Depression, fatigue, and joint pain are also found to be associated with CN (43).
All in all, even though post-LASIK dry eye is usually a temporary condition, some severe symptoms are not entirely resolved, which affects patients’ satisfaction and decreases vision quality; therefore, an accurate diagnosis and preventive methods might be effective.
Dry eye disease (DED)
According to the Tear Film and Ocular Surface Society dry eye workshop II (TFOS DEWS II) definition, dry eye is a disease of the ocular surface caused by different etiological factors. In DED, the tear film homeostasis is compromised with concurrent ocular manifestations. The tear film’s unstable and hyperosmolar state, inflammation, impairment of the ocular surface, and neurosensory dysfunctions contribute to these ocular symptoms (47).
Dry eye disease (DED) is a typical multi-factor disorder of the tear function and ocular surface that occurs when the eyes fail to produce efficient or adequate volumes of tears to moisturize the eyes (47, 48). DED influences quality of life and visual perception, as symptoms often interfere with daily activities, such as reading, writing, or using video display monitors (49). The ocular surface and lacrimal gland are composed of a functional unit where the afferent sensory nerves of the ocular surface and the efferent autonomic nerves to the lacrimal gland help to modulate tear production and secretion. When this interaction is damaged, tear film osmolarity rises and induces corneal epithelium apoptosis and inflammation (50).
Generally, DED brings about discomfort, symptoms of visual disturbance, focal or global tear film instability with possible harm to the ocular surface, increased osmolarity of the tear film, and subacute inflammation of the ocular surface (47, 48). Moreover, DED can result in other symptoms, such as positive vital staining of the ocular surface, receded TBUT and Schirmer test values, decreased corneal sensitivity, ocular dysesthesias, and fluctuating or blurry vision (41, 51).
Although we have several available methods to diagnose and measure DED severity, such as Schirmer’s test, fluorescein break-up time (FBUT), and validated questionnaires, for instance, the OSDI (52), and also reliable therapies to decrease DED complications, such as anti-inflammatory medication, topical ophthalmic drops, autologous serum treatment, and punctual plugs, patients face significant DED symptoms after laser-based operations (53–58). Studies on laser-induced DED provided unalike incidences of this disorder ranging from 20% to more than 50% in patients who underwent LASIK surgery. Patients who underwent SMILE also contracted postoperative DED but the risk of contraction was lower (59).
Risk factors
Generally, the severity and incidence of Li-DED mostly rely on patients’ characteristics and history. The most significant risk factor for Li-DED is the preexisting experience of DED (60–62). Studies on laser-induced complications showed that ocular surface recovery after LASIK is affected by preoperative tear volume (63). Edward et al. (64) reported that patients with less than 10 mm in the Schirmer test before the operation were more susceptible to DED induced by LASIK. Moreover, refractive surgery, women (especially in premenopausal ages), postmenopausal estrogen therapy, and older age increase the risk of incidence and severity of Li-DED (65, 66). Those of Asian origin are also more at risk of developing DED. Increased rates of attempted refractive corrections, dynamics of blinking, tear film parameters, and anatomy of orbit and eyelid in Asian eyes promote DED incidence in Asian populations (67). A genetic effect with polymorphism in the thrombospondin-1 gene can also cause chronic inflammation of the ocular surface (68).
Several studies in the last decades showed the importance of comorbidities in DED complications. Collagen vascular diseases, such as seronegative spondyloarthropathies, systemic lupus erythematosus, Sjogren syndrome, and rheumatoid arthritis, are the other risk factors that usually result in Li-DED (69). Other factors that may facilitate the development of Li-DED are diabetes mellitus, previous blepharoplasty, and lagophthalmos (62, 70).
The parameters used in the LASIK surgery (including the ablation zone and suction time) can induce DED (71, 72). The ablation depth and thickness of the flap are the other surgical factors that cause DED through corneal nerve transection (73, 74). Besides, patients that require more refractive correction are more prone to developing DED (75–77). Patients who undergo LASIK for high myopia, from –9.10 to –14.00 D, can experience DED a few years after the operation (76). On the other hand, a thinner flap may preserve blink rate and CS and diminish corneal nerve invasion, reducing Li-DED duration and severity (78).
At an early post-LASIK stage, ablation depth is associated with densities of the corneal nerve or meniscus of the tear; however, at later stages, goblet cell destiny can affect Li-DED (79). Moreover, wearing contacts for long periods results in delayed recovery of tear secretion, pro-inflammatory cytokines elevation, and reduced corneal sensitivity (80–83). Ocular allergy may also contribute to Li-DED (84).
Surgical changes to the ocular surface often induce Li-DED. Several studies demonstrated that inflammation induced by altered levels of cytokines, damage to the corneal afferent nerve with resultant hypoesthesia, reduced production of tears, distribution of abnormal tears from changes in corneal contour, reduced rates of blinks, and loss of conjunctival goblet cells by the suction ring of LASIK remarkably increase DED severity (28, 85, 86). Sensory denervation instigated by excimer photoablation and transection of nerve throughout the flap creation can lead to Li-DED (87). In addition, some studies indicated a correlation between corneal nerve impairment and Li-DED (87, 88).
Hinge position is another risk factor for Li-DED because most corneal nerves go into the stroma in the horizontal meridians (60). Feng et al. (89) showed that the horizontal hinge flap improved TBUT, corneal sensitivity, and corneal fluorescein staining 3 months after LASIK compared to horizontally hinged flaps. However, the same after 6 months.
Flap/hinge characteristics
The corneal sensory nerves have a crucial role in tear production, initiating the blink reflex and keeping the corneal epithelium alive (74). Since corneal nerves are meant to be injured during modern refractive surgeries, the corneal innervation subject has been brought to attention (90). Although LASIK disrupts corneal innervation, it has become one of the most reliable refractive surgeries in the last decades (90, 91). It seems that decreased CS due to this disruption may be determinative in the Li-DED condition (90, 91).
There have been two models of corneal innervation proposed. Some studies indicate a dominancy of corneal nerves entering the eye from the medial and lateral rather than superior or inferior sides (92). Using in vivo confocal microscopy, other models suggest an inferior-superior orientation of sub-basal nerve fibers with other leashes in different directions (93). As there is variability in the patterns through which corneal nerves run into the cornea, changing flap characteristics such as flap thickness, hinge position, hinge width, and hinge angle during LASIK surgery may have different outcomes in postoperative CS and DED (94).
To date, many studies have been trying to figure out the association between flap/hinge characteristics and Li-DED (74, 90, 91, 94–100), and while studies suggest a relationship between flap/hinge characteristics and Li-DED, others mention no such relationship.
The pros for this relationship would be divided into hinge position, hinge width, and flap thickness. DED is more prominent in eyes with a superior hinge flap than those with a nasal hinge flap (95, 96). Besides, other studies showed that eyes with nasal hinge flaps usually demonstrated better CS recovery (96, 97). In contrast, De Paiva et al. (99) reported that nasal hinge flaps show more reduction in CS than the superior hinge flaps one week after surgery, although CS returned to comparable preoperative levels at 6 months.
Studies on hinge width showed that in wider hinge flap eyes, patients had a better CS, especially 1 and 3 months after surgery, and less DED (74). Moreover, DED is correlated with the degree of preoperative myopia, the laser ablation depth, and flap thickness (99). A thicker flap might increase the volume of nerve regeneration and thereby slow neurosensory corneal recovery (94). Several studies have reported thinner flaps to cause a significantly lower rate of CS loss in microkeratome and femtosecond laser LASIK (97). Another study suggested faster DED healing in thinner flaps with higher Schirmer test scores and longer TBUT when investigating 130 μm and 100 μm flaps. Nonetheless, the other DED parameters were the same, and both groups experienced mild symptoms, which might suggest the existence of a threshold (greater than 130 μm) for flap thickness regarding significant differences in DED symptoms (94).
Tai and Sun (101) conducted a comparative study, investigating the effects of flap diameter in 113 eyes. Interestingly, the results indicated that flap size alone could not be regarded as a single determinant of DED. There was no critical difference regarding all DED parameters between different flap size groups. However, the strong association of the ratio of the diameter of the flap/cornea with DED was noted. The group with a larger flap/cornea diameter ratio experienced decreased Schirmer test and longer TBUT than the other group. This suggests the importance of individualized flap diameter measurement for surgeons (101).
In addition, the angle of flap side cuts is another factor that may affect post-LASIK DED. Evidence suggests that flaps with inverted side-cuts improved the process of wound healing and CS compared to conventional side-cut flaps (102, 103). One prospective study with 120 eyes reported better corneal neurosensory recovery in the eyes with inverted side cuts (130°) than with standard traditional side cuts (70°) at 1, 3, 6, and 12 months after surgery. Nevertheless, the subjective DED symptoms were not notably different in the eyes with different side-cut angles at any follow-up time points (104).
Several studies reported that the hinge position did not affect CS or DED symptoms (91, 98, 100). Vroman et al. (90) suggested no difference between these two groups in the occurrence of DED (although the nasal hinge group significantly had a better nasal CS at 1 month). In another study, Mian et al. (94) showed that hinge position, hinge angle, and flap thickness did not affect CS or DED.
Therefore, flap/hinge characteristics may affect CS and Li-DED, but in most cases, the long-term results are not significantly different between the two groups (74, 90, 91, 94, 97–100). Further studies on corneal innervation maps and regrowth patterns would play an essential role in determining the connection between these two procedures and developing reliable methods to minimize Li-DED. Besides, more expanded studies on some other known risk factors, such as preoperative neurotrophic corneas or DED, and also pre/post-surgery actions taken into consideration are required to achieve the best results in the future (Table 1).
Presurgical evaluation
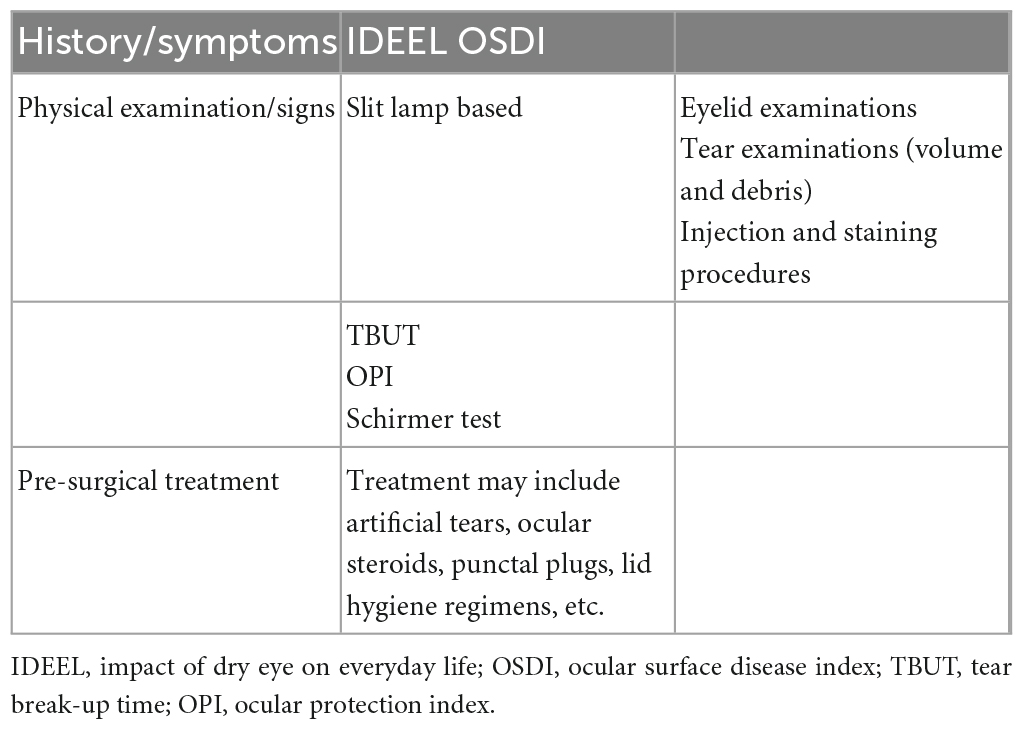
Before LASIK, patients with refractive disorders pass a complete evaluation of DED and the ocular surface. The most crucial preoperative evaluation is the Schirmer testing, tear quality, presence of lid disease, TBUT, tear meniscus, and any ocular surface staining (105, 106). Moreover, feelings of dryness, grittiness, fluctuations of vision (particularly in conjunction with blinking), and tired eyes are the other vital symptoms in the clinical process of DED (107).
The complaints of blurry vision by patients with cataracts should be differentiated from DED-associated fluctuating blurriness. This information can be collected by utilizing questionnaires, including the impact of dry eye on everyday life (IDEEL) and the OSDI (108, 109). Other examinations for DED are lactoferrin measurement, tear osmolarity, goblet cell count, tear lysozyme measurement, and tear mucin measurement (110). Corneal fluorescein staining assesses the corneal epithelial defects. Anterior segment spectral-domain optical coherence tomography is used to assess the tear meniscus. Corneal confocal microscopy, a less invasive method (111), measures the density of the sub-nasal nerve and evaluates corneal wound healing. Patients diagnosed with persistent corneal staining or reduced vision because of external ocular surface disease and severe DED should not undergo LASIK surgery (112).
Before surgery, external ocular surface abnormalities should be treated (107). Patients with a high risk of DED should begin a regimen and control their therapeutic efficacy. In the pre-surgery term, all patients should be aware of enough lubrication of the external ocular surface. Punctal plugs decrease the drainage rate of tears preoperatively. Moreover, corticosteroids or cyclosporine eyedrops treat dry eye inflammatory components before surgery (113). Ultimately, Lid margin disease should be treated before operation (107).
In terms of medical history and demographic data, it has been reported that women, older age, long-term contact lenses use, having a lower preoperative best-corrected visual acuity (BCVA), and lower preoperative refractive error are associated with higher risks of developing DED within 6 months after surgery.
As a person ages, the sensitivity of the cornea decreases, increasing the risk of developing DED. However, there have been controversies regarding the effect of age on developing DED after surgery. Additionally, some discrepancies have been reported on the association between refractive correction, ablation depth, and postoperative DED, which can be due to differences in the criteria defining DED (50). As for contact lenses, wearing them for a long time increases proinflammatory cytokines and tear film instability and decreases corneal sensitivity.
Some diseases have been suggested to be associated with developing DED postoperatively. Collagen vascular diseases, including systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome, and seronegative spondyloarthropathies, have established ocular complications such as DED. However, some studies have shown good postoperative results and minimum ophthalmic complications in well-controlled patients (84).
Considering neural disorders, reduced corneal sensitivity is associated with post-LASIK DED. Also, a loss of neural stimulation decreases aqueous tear production, thus, increasing ocular surface inflammation and tear osmolarity. Incomplete blinking and reduced blink frequency increase tear evaporation, exacerbating aqueous production deficiency (114).
In essence, the clinician should be fully prepared to perform surgery, assessing the patient with three tools: history taking, clinical examination, and a short period of pre-surgical treatment. Among these, the most practical, available, and effective examinations and tools are mentioned in Table 2.
Differences regarding surgery methods
Popular refractive surgery includes microkeratome (MK), femtosecond laser (FS), and SMILE. Li-DED incidence is more significant in MK than in FS (71). In addition, FS causes a more prominent reduction in the population of goblet cells than MK due to the difference in the mean period of suction ring activity on the conjunctiva (71, 90). FS has thinner flaps, leading to fewer Li-DED and less afferent sensory damage in the anterior stroma (71, 90). In a novel study, Xia et al. (115) showed that MK and FS for LASIK flap cutting correct myopia, with no remarkable difference throughout 6 months of follow-up. They indicated that MK and FS are safe for LASIK flap cutting, and refractive outcomes stayed stable after a month post-operation. They also showed that FS has more advantages in longer TBUT, predictability of flap thickness, and better cerebero spinal fluid (pressure) (CSF) (115).
In another study, Salomão et al. (71) reported that FS flaps have fewer Li-DED than MK ones, and also required less treatment compared with MK. The reason behind this condition is that FS generates thinner flaps. Thus, afferent sensory nerves have less damage in the anterior stroma. Since FS causes more mean spherical equivalent correction than MK, more Li-DED and more ablation of the stromal nerve are expected in FS. However, MK has a Li-DED incidence. Sun et al. (116) stated that MK and FS increase OSDI scores and corneal staining but decrease TBUT and CS. However, post-operation TBUT is remarkably higher in FS.
On the other hand, Golas and Manche (66) indicated no remarkable difference in self-reported DED symptoms between FS and MK. Several studies showed that SMILE is correlated with a higher score in the DED questionnaire, reduced corneal epitheliopathy risk, elevated Schirmer test value, faster nerve growth factor, lower interleukin-6, less CS and sub-basal nerve density reduction, and longer TBUT in comparison to FS and MK (117–123). SMILE corrects myopia and myopic astigmatism. Cai et al. (124) determined that, mainly within 3 months, recovery of dry eye is better in SMILE than FS and that, in the first 3 months post-surgery, the cornea’s sensitivity recovers better in SMILE than in FS.
Treatment approaches
Cyclosporine A
Cyclosporine A is a calcineurin-inhibiting anti-inflammatory peptide that exerts its immunosuppressing effects by preventing T-lymphocyte activation, leading to the restriction of inflammatory cytokine production. This agent is produced as a metabolite of the fungi Beauveria nevus and Tolypocladium inflatum (114–116). Topical use of cyclosporine A enhances goblet cell density and tear generation (125). Cyclosporine A also blocks the permeability transition pores of the mitochondria and subsequently hinders apoptosis (126). In addition, this agent is regarded to increase corneal sensitivity by seemingly inducing the reproduction of corneal axons (125).
Cyclosporine A is implemented as an FDA-approved agent for many inflammation-associated diseases, including persistent nummular keratitis, ocular inflammation, and ocular-inflammation-induced DED (54, 127). Several clinical studies have confirmed the efficacy of topical cyclosporine A (0.05% emulsion) in treating Li-DED (54, 128–130). Moreover, cyclosporine A is more beneficial in treating DED patients than artificial tears and diquafosol (128, 131). However, temporary pain, irritation, lacrimation, and sensation of a foreign body are seen to be the local complications caused by the topical use of this drug on the eye (131). Supplementary Table 1 summarizes the potential treatments for DED.
Diquafosol
Another topical drop employed in Li-DED has been diquafosol tetrasodium solution (132, 133). Diquafosol activates P2Y2 receptors and elevates intracellular Ca2+ to promote fluid conveyance by epithelium cells, conjunctiva mucin production by goblet cells, and the provocation of lipid generation (134, 135). Diquafosol (3% solution) has indicated promising outcomes in the DED treatment of different entities, including cataract-surgery-associated, LASIK-associated, contact-lens-associated, aqueous deficiency, meibomian gland dysfunction (MGD), TBUT, and computer-display-use-associated DED (136).
Both monotherapies with diquafosol and their combination with sodium hyaluronate have demonstrated encouraging therapeutic benefits in treating Li-DED (132, 133). Nevertheless, a combination regimen of diquafosol with sodium hyaluronate appears more efficacious in improving DED symptoms and tear production (132). Major adverse complications of this drug in DED include irritation, discharge, conjunctiva injection, pain, pruritus, foreign body sensation, and eye discomfort. Most of these adverse reactions emerged in modest intensity, were reversible, and were eventually eliminated. Hence, diquafosol is considered to have no significant drawbacks (137, 138) (Supplementary Table 1).
Artificial tear therapy
Artificial teardrops are a traditional treatment for DED that help reduce symptoms (139). Ingredients of artificial tears are composed of two main parts: emollients and demulcents. Emollients are natural fats or oils, such as flaxseed oil or castor oil, which are used to increase the thickness of the fat layer and thus maintain the stability of the tear film and reduce evaporation. Demulcents are water-soluble polymers that help maintain and lubricate the eye’s mucous membrane. The most common of these is carboxymethylcellulose (CMC), which increases viscosity and more extended corneal coverage. In the composition of many artificial tears, in addition to the above (emollient-demulcents), preservatives, such as benzalkonium chloride, polixetonium, polyquaternium (Polyquad), and OcuPure, reduce the growth of bacteria.
Several studies have shown that artificial tears significantly improve DED symptoms compared with placebo (140–143). However, the variety of artificial tears and the lack of randomized clinical trials to compare different types of artificial tears make it difficult to determine which combination is superior to the other (144). Investigations have also caused side effects for this treatment, the most common of which are blurred vision, foreign body sensation, eye discomfort, and irritation (140, 142, 145–147) (Supplementary Table 1).
Autologous serum
Tears are antimicrobial, mechanical, nourishing, and optical components. They include many active components, such as fibronectin, growth factors, and various vitamins, to provide growth, differentiation, and migration of both conjunctival and corneal epithelium. The absence of these epitheliotropic components in some diseases, such as Li-DED, leads to more severe side effects on the ocular surface (148). Autologous serums are blood derivatives in the shape of eye drops that are naturally non-allergic and biomedically similar to natural tears (148, 149).
IV cell culture and many clinical cohort studies showed that the function and morphology of epithelial cells in the cornea are noticeably preserved (148). In addition, many studies proved the powerful therapeutic effect of the serum on accelerating the curative response and reducing symptoms in moderate to severe DED. This enhancing impact is induced by bioactive proteins and growth factors produced, synthesized, and available in the blood, mainly by activated alpha-granules of platelets (55, 149, 150). New studies aim to evaluate the effectiveness of autologous E-PRP (platelet-rich plasma eye drop) monotherapy of post-LASIK chronic ocular surface syndrome (OSS) in large numbers of cases. In the short term, autologous serum combined with artificial eye drops showed more promising outcomes (151). However, autologous serum therapy had no significantly different outcomes compared with artificial eye drops, but autologous serum results showed fewer adverse effects (152). In a prospective controlled randomized study, E-PRP as a postoperative LASIK treatment was beneficial for improving epithelial healing. However, it appeared to have no “extra” positive effect on corneal nerve regeneration or sensitivity. In addition, recent trials introduced autologous platelet-rich plasma as a novel therapy for patients with severe DED, which performed better than autologous serums (153).
Although a positive serology induced some potential complications, such as scleral vasculitis in some patients with rheumatoid arthritis and some immune-induced inflammatory response correlated with the circulating of antibodies in the serum and antibodies of the cornea, this method has no other serious complications (148, 154–156). However, the abovementioned complications could have happened in the natural course of rheumatoid arthritis (148) (Supplementary Table 1).
Growth factors
The NGF is a binding polypeptide that adjusts the growth and survival of mature neurons in the nervous system (157). It has become known as an active mediator in many vital functions in mammals’ central nervous system (158). It also performs as a differentiating and survival factor for sympathetic neurons and neural crest sensory (1). NGF induces neurite outgrowth by neuronal cells and reinstates the function of damaged neurons (157, 159).
In a previous study, Bonini et al. (160) indicated that topical NGF eyedrops develop corneal sensitivity and the healing process of corneal epithelial in patients with severe and moderate NK. The assessment of the ocular surface following LASIK condition in patients after prescribing plasma rich in growth factor (PGRF) or artificial tears notably improved all clinical variables (except for best-corrected visual acuity or BCVA). These findings propose the possible effectiveness of PGRF eyedrops in preventing Li-DED compared with conventional eyedrops. Additionally, these results have shown that improving dry eye syndrome PGRF is a lot more effective than conventional treatment. Hence PGRF is suggested for patients suffering from postoperative DED (56) (Supplementary Table 1).
Punctal plugs
Punctal plugs provide a safe procedure to ameliorate moderate and severe DED (161, 162). They are divided into several types based on their duration of placement (short-term, long-term, or permanent placement) or material (silicone, collagen, hydrogel, polydioxanone, and acrylic) (57, 161). Short-term plugs are usually made of collagen (collected from animals) and can be used for 4–14 days. On the other hand, long-term plugs are made of silicone, hydrogel, polydioxanone, and acrylic and last up to 6 months. Therefore, long-term plugs provide a better choice for postoperative terms (161). Since laser-related DED is temporary after laser procedures, such as LASIK, punctual plugs are urged (162).
The surgeons diminish tear drainage by inserting punctal plugs into the inferior eye punctual opening (161–163). The insertion can be performed at the beginning of the operation and after it (57, 163). This occlusion boosts and maintains natural tears and eye lubricants (57). Although numerous articles have supported the efficacy of punctual plugs in treating DED, some complications, such as allergic reaction, canaliculitis, migration, chronic irritation, conjunctivitis extrusion, and epiphora, can develop from this procedure (57, 161–167) (Supplementary Table 1).
Omega-3 fatty acids
Omega-3 fatty acids (v3FA), including eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid, are essential fatty acids for body metabolisms. Since these essential fatty acids cannot be synthesized in our bodies, we have to obtain them through our diet. Upregulation of NGF secretion is induced by injury to the cornea (168). In animal models, regeneration of post-injury nerve to the cornea in the eye is improved by DHA plus pigment epithelial-derived factor (PEDF) combinations and DHA plus NGF combinations (169, 170). Therefore, supplementation with v3FA would play an essential role in LASIK.
Several studies demonstrated that high v3FA consumption also causes a significant proportion of these accumulations in cell membrane phospholipids. The v3FA substrates-generated eicosanoids include thromboxane (TX) A3 and leukotriene (LT) B5. They have an anti-inflammatory role against arachidonic acid-generated eicosanoids, such as LTB4, PG E2, and TX A2 (arachidonic acid-generated eicosanoids have a pro-inflammatory role) (171–173). Moreover, v3FA administration improves goblet cell density in patients with DED (174, 175). Therefore, v3FA positively affects the goblet cells, regenerates nerves, and has an anti-inflammatory role in Li-DED pathophysiology (Supplementary Table 1).
Eyelid-warming devices
The efficacy of many types of warming devices on tear function and the ocular surface have been considered and these devices have been divided into “non-wet warming” and “wet warming” according to the remaining water on the lid surface after or during warming (176). Eye warmer, a disposable eyelid-warming device based on iron oxidation, significantly increases eyelid temperature and melts the meibomian gland lipid. Thus, MGD can be treated (177). Blephasteam, an eyelid-warming device, provides humidity and warmth for eyelids to liquefy meibum and decreases conjunctival hyperemia and ocular symptoms, including sensitivity to light and burning sensation (178). Di Pascuale et al. (179) used eye-feel, an eye-warming device that focuses on the role of lipids in tear insufficiency-1480408721-1480408721, for the treatment of persistent Li-DED (Supplementary Table 1).
Thermal pulsation
After prevalent therapies such as omega-3 supplementation, eyelid hygiene, and hot compresses, thermal pulsation is now the primary treatment for evaporative DED and MGD (180). Vectored thermal pulsation (VTP) can transfer heat by overcoming obstacles and discharging the gland’s contents. It is done by a device, called Lipiflow, that heats (42.5°C) the interior of the upper and lower eyelids to insulate both eyes and concurrently apply pressure by distending and compacting an inflatable air bladder to the outer of the eyelids. This device should be used by physicians only in adult patients (180, 181) (Supplementary Table 1).
Some studies confirm the efficiency and safety of thermal pulsation for treating dry eye disease by improving the TBUT after laser vision correction and refractive surgery (181–183). Adverse events of the device were temporary, predictable, and not serious (180).
Intense pulsated light
The Intense Regulated Pulsed Light is a polychromatic pulsed light generator that can produce homogeneous and calibrated light impulses (184). The sculpted pulses are released in the form of pulse trains whose distance, energy, and spectrum are precisely determined to stimulate the meibomian glands and make them return to normal function (184). Wavelengths of light ranging from 400 to 1,200 nm generated by xenon flashlamps of intense pulsated light (IPL) and are limited to visible light by a filter (185). Fuentes Páez et al. (186) suggested that IPL can reduce symptoms and signs of chronic evaporative DED in patients who sustain LASIK surgery; however, more studies are required with more patients and longer follow-up times. Swelling, light sensitivity, floaters, blistering, and facial redness have been reported, but they are not earnest adverse events by the definition of the FDA (185) (Supplementary Table 1).
Acupuncture
Acupuncture is an old Chinese treatment that has been effective for some diseases, such as ophthalmologic diseases (187, 188). Previous studies showed that acupuncture diminishes Li-DED symptoms (189) and that it is almost better than artificial tears for DED treatment (188). Acupuncture increases the secretion of the lacrimal gland and ameliorates DED through stimulation of the immune system and autonomic nerves (187). There are limited studies on the effects of acupuncture on DED after refractive surgery, and it is necessary to research more about the physiology of this condition (187). Few studies reported on the side effects of this treatment method. For example, Kim et al. (190) reported three cases of hematoma with different intensities. Jang et al. (189) described local, general, and psychological adverse events that may be related to acupuncture (Supplementary Table 1).
Other studied treatments
Prosthetic replacement of the ocular surface ecosystem (PROSE)
Prosthetic replacement of the ocular surface ecosystem is a type of FDA-validated contact lens fabricated from a fluorosilicone-acrylate polymer that is permeable to gas. These scleral lenses are custom-made by computer software for each patient’s eyes. This device has indicated efficacy in ocular surface diseases and intense ectasia (191, 192). Scleral lenses save the patient’s eyesight when every other therapy method has failed (193). PROSE devices have been proven advantageous for patients with Li-DED (194) (Supplementary Table 1).
Pregabalin and gabapentin
Several studies approved that anti-nociceptive analgesics decrease post-operation chronic pain. Oral pregabalin and gabapentin are α2δ ligands used to reduce the occurrence and extent of post-surgical lingering pain following several surgeries (195). In a prospective randomized study, Galor et al. (196) demonstrated that pregabalin perioperatively administered to patients with Li-DED did not decrease the severity or frequency of their symptoms at a six-month follow-up after LASIK. Supporting previous results, Paik et al. (197) proved that pregabalin makes no reliable changes in nerve intensity or cornea sensitivity. On the other hand, we have limited information about the efficacy of pregabalin and gabapentin in treating Li-DED. Nevertheless, the existing data does not introduce them as significantly promising monotherapies (196, 197) (Supplementary Table 1). Figure 1 summarizes current treatment options for post-LASIK DED.
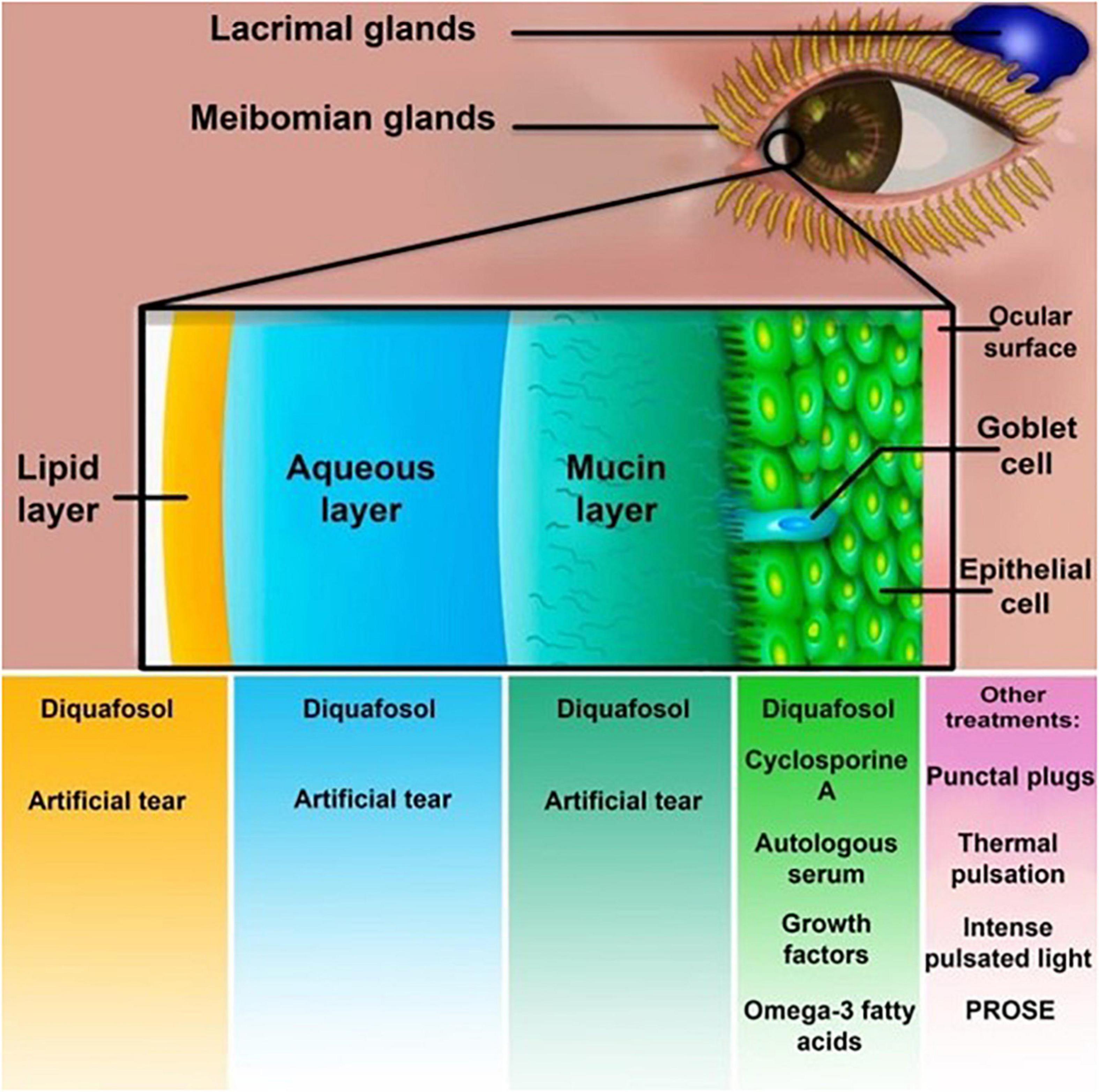
Figure 1. Treatment methods for post-laser-assisted in situ keratomileusis (LASIK) dry eye syndrome. Cyclosporine A, diquafosol, artificial tears, autologous serum, growth factors, and omega-3 fatty acids are dry eye disease (DED) treatments affecting different layers of the tear film. Punctual plugs, thermal pulsation, and prosthetic replacement of the ocular surface ecosystem (PROSE) are other treatment options.
Conclusion
Dry eye disease is among the most common complications in many refractive surgeries, particularly after LASIK. This disorder affects the vast majority of patients up to one year after the surgery. Moreover, DED can also turn into a chronic disease in some patients. Therefore, providing suitable methods would enhance visual results and ameliorate dryness and discomfort.
The main therapeutic options encompass punctual plugs, autologous serum, cyclosporin, artificial tear therapy, sclera lenses, and oral medication. Nonetheless, the currently existing data on the usage of pregabalin and gabapentin post-refractive surgeries needs more investigation. Overall, the most essential post-surgical approach is to provide the eye with a stable environment by increasing the production of tears and maintaining the tear surface. As a result, different types of ophthalmic drops, depicted in Figure 1, play a pivotal role in the post-surgical management of the condition. Assuming that most patients would experience Li-DED, we recommend using at least preservative-free artificial tears for a specific period, depending on the conditions of each patient. Besides, the severe or chronic forms of Li-DED may benefit from more invasive approaches such as punctual plugs. Other treatments may be beneficial, but at the surgeon’s discretion, and each treatment may be applied based on the patient’s condition. The most significant factor that predisposes patients to Li-DED is preexistent DED, which makes a preoperative assessment of each patient essential. DED management before surgery enhances wound healing and decreases the chance of DED and flap side effects. Prediction and prevention of DED, along with the provision of the right choice of treatment to each patient, is crucial to achieving better outcomes.
Author contributions
ND: concept, design, analysis, or interpretation. AT, SE, MI, and PP: data collection or processing and literature search. AT, SE, MI, PP, AF, MP, FS, ZK, and HP: writing. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank kind contribution and suggestions of Dr. Ashkan Rahmati, Dr. Masoud Noroozi, Dr. Mohammadreza Rahmani, Dr. Amir Abdi, Dr. Zahra Sheikh, Dr. Pooya Behfarnia, Dr. Saba Mehrtabar, and Dr. Amirhossein Yousefi that made impactful touches to our work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1057685/full#supplementary-material
References
1. Kohnen T, Strenger A, Klaproth OK. Basic knowledge of refractive surgery: correction of refractive errors using modern surgical procedures. Dtsch Ärztebl Int. (2008) 105:163. doi: 10.3238/arztebl.2008.0163
3. del Barrio JLA, Wilkins M, Cochener B, Ang M. Refractive surgery. Lancet. (2019) 393:2085–98. doi: 10.1016/S0140-6736(18)33209-4
4. Barsam A, Allan BD. Excimer laser refractive surgery versus phakic intraocular lenses for the correction of moderate to high myopia. Cochrane Database Syst Rev. (2014) 6:CD007679. doi: 10.1002/14651858.CD007679.pub4
5. McAlinden C, Moore J. Laser-assisted subepithelial keratectomy retreatment surgery. J Cataract Refract Surg. (2011) 37:358–63. doi: 10.1016/j.jcrs.2010.11.009
6. McAlinden C. Corneal refractive surgery: past to present. Clin Exp Optom. (2012) 95:386–98. doi: 10.1111/j.1444-0938.2012.00761.x
7. Amm M, Wetzel W, Winter M, Uthoff D, Duncker GI. Histopathological comparison of photorefractive keratectomy and laser in situ keratomileusis in rabbits. J Refract Surg. (1996) 12:758–66. doi: 10.3928/1081-597X-19961101-07
8. Chansue E, Tanehsakdi M, Swasdibutra S, McAlinden C. Efficacy, predictability and safety of small incision lenticule extraction (SMILE). Eye Vis. (2015) 2:14. doi: 10.1186/s40662-015-0024-4
9. Reinstein DZ, Archer TJ, Gobbe M. Small incision lenticule extraction (SMILE) history, fundamentals of a new refractive surgery technique and clinical outcomes. Eye Vis. (2014) 1:3. doi: 10.1186/s40662-014-0003-1
10. Shah R. History and results; indications and contraindications of SMILE compared with LASIK. Asia Pac J Ophthalmol. (2019) 8:371. doi: 10.1097/01.APO.0000580132.98159.fa
11. Chang J-Y, Lin P-Y, Hsu C-C, Liu CJ-L. Comparison of clinical outcomes of LASIK, Trans-PRK, and SMILE for correction of myopia. J Chin Med Assoc. (2022) 85:145–51. doi: 10.1097/JCMA.0000000000000674
12. Guo H, Hosseini-Moghaddam SM, Hodge W. Corneal biomechanical properties after SMILE versus FLEX, LASIK, LASEK, or PRK: a systematic review and meta-analysis. BMC Ophthalmol. (2019) 19:167. doi: 10.1186/s12886-019-1165-3
13. Yan H, Gong L-Y, Huang W, Peng Y-L. Clinical outcomes of small incision lenticule extraction versus femtosecond laser-assisted LASIK for myopia: a meta-analysis. Int J Ophthalmol. (2017) 10:1436.
14. Luft N, Schumann RG, Dirisamer M, Kook D, Siedlecki J, Wertheimer C, et al. Wound healing, inflammation, and corneal ultrastructure after SMILE and femtosecond laser–assisted LASIK: a human ex vivo study. J Refract Surg. (2018) 34:393–9. doi: 10.3928/1081597X-20180425-02
15. Lou L, Fu H, Liu H. The clinical efficiency of lycium-rehmannia pills in treating dry eye symptom: a meta-analysis. Medicine. (2020) 99:e20887. doi: 10.1097/MD.0000000000020887
16. Zhang X, Wang L, Zheng Y, Deng L, Huang X. Prevalence of dry eye disease in the elderly: a protocol of systematic review and meta-analysis. Medicine. (2020) 99:e22234. doi: 10.1097/MD.0000000000022234
17. Lee JB, Ryu CH, Kim J-H, Kim EK, Kim HB. Comparison of tear secretion and tear film instability after photorefractive keratectomy and laser in situ keratomileusis. J Cataract Refract Surg. (2000) 26:1326–31. doi: 10.1016/S0886-3350(00)00566-6
18. Bower KS, Sia RK, Ryan DS, Mines MJ, Dartt DA. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: manifestations, incidence, and predictive factors. J Cataract Refract Surg. (2015) 41:2624–34. doi: 10.1016/j.jcrs.2015.06.037
19. Liu M, Chen Y, Wang D, Zhou Y, Zhang X, He J, et al. Clinical outcomes after SMILE and femtosecond laser-assisted LASIK for myopia and myopic astigmatism: a prospective randomized comparative study. Cornea. (2016) 35:210–6. doi: 10.1097/ICO.0000000000000707
20. Shen Z, Shi K, Yu Y, Yu X, Lin Y, Yao K. Small incision lenticule extraction (SMILE) versus femtosecond laser-assisted in situ keratomileusis (FS-LASIK) for myopia: a systematic review and meta-analysis. PLoS One. (2016) 11:e0158176. doi: 10.1371/journal.pone.0158176
21. Wang B, Naidu RK, Chu R, Dai J, Qu X, Zhou H. Dry eye disease following refractive surgery: a 12-month follow-up of SMILE versus FS-LASIK in high myopia. J Ophthalmol. (2015) 2015:132417. doi: 10.1155/2015/132417
22. Lim EWL, Lim L. Review of laser vision correction (LASIK, PRK and SMILE) with simultaneous accelerated corneal crosslinking–long-term results. Curr Eye Res. (2019) 44:1171–80. doi: 10.1080/02713683.2019.1656749
23. Toda I. LASIK and the ocular surface. Cornea. (2008) 27(Suppl. 1):S70–6. doi: 10.1097/ICO.0b013e31817f42c0
24. Das S, Garg P, Mullick R, Annavajjhala S. Keratitis following laser refractive surgery: clinical spectrum, prevention and management. Indian J.Ophthalmol. (2020) 68:2813. doi: 10.4103/ijo.IJO_2479_20
25. Versura P, Giannaccare G, Pellegrini M, Sebastiani S, Campos EC. Neurotrophic keratitis: current challenges and future prospects. Eye Brain. (2018) 10:37. doi: 10.2147/EB.S117261
26. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. (2014) 8:571. doi: 10.2147/OPTH.S45921
27. Duncan NW, Houser KH, Khandelwal SS. Neurotrophic keratitis: a review. Rev Ophthalmol. (2022) 16:67–71. doi: 10.17925/USOR.2022.16.2.67
28. Nettune GR, Pflugfelder SC. Post-LASIK tear dysfunction and dysesthesia. Ocul Surf. (2010) 8:135–45. doi: 10.1016/S1542-0124(12)70224-0
29. Rozsa AJ, Guss RB, Beuerman RW. Neural remodeling following experimental surgery of the rabbit cornea. Invest Ophthalmol Vis Sci. (1983) 24:1033–51.
30. Chan KY, Järveläinen M, Chang JH, Edenfield M. A cryodamage model for studying corneal nerve regeneration. Invest Ophthalmol Vis Sci. (1990) 31:2008–21.
31. Bridges D, Thompson SW, Rice AS. Mechanisms of neuropathic pain. Br J Anaesth. (2001) 87:12–26. doi: 10.1093/bja/87.1.12
32. Belmonte C. Eye dryness sensations after refractive surgery: impaired tear secretion or” phantom” cornea? J Refract Surg. (2007) 23:598–602. doi: 10.3928/1081-597X-20070601-11
33. Alio JL, Teus MA, Alio del Barrio JL, Katsanos A. Post-LASIK Corneal Dysesthesia. Management of Complications in Refractive Surgery. Berlin: Springer (2018). p. 113–6. doi: 10.1007/978-3-319-60561-6_14
34. Belmonte C, Gallar J. Cold thermoreceptors, unexpected players in tear production and ocular dryness sensations. Invest Ophthalmol Vis Sci. (2011) 52:3888–92. doi: 10.1167/iovs.09-5119
35. Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain. (2014) 155:1481–91. doi: 10.1016/j.pain.2014.04.025
36. Wilson SE, Ambrósio R Jr. Laser in situ keratomileusis-induced neurotrophic epitheliopathy. Am J Ophthalmol. (2001) 132:405–6. doi: 10.1016/S0002-9394(01)00995-3
37. Hovanesian JA, Shah SS, Maloney RK. Symptoms of dry eye and recurrent erosion syndrome after refractive surgery. J Cataract Refract Surg. (2001) 27:577–84. doi: 10.1016/S0886-3350(00)00835-X
38. Rosenthal P, Baran I, Jacobs DS. Corneal pain without stain: is it real? Ocul Surf. (2009) 7:28–40. doi: 10.1016/S1542-0124(12)70290-2
39. Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle. Ocul Surf. (2012) 10:2–14. doi: 10.1016/j.jtos.2012.01.002
40. Theophanous C, Jacobs DS, Hamrah P. Corneal neuralgia after LASIK. Optom Vis Sci. (2015) 92:e233–40. doi: 10.1097/OPX.0000000000000652
41. Galor A, Moein H-R, Lee C, Rodriguez A, Felix ER, Sarantopoulos KD, et al. Neuropathic pain and dry eye. Ocul Surf. (2018) 16:31–44. doi: 10.1016/j.jtos.2017.10.001
42. Moshirfar M, Bhavsar UM, Durnford KM, McCabe SE, Ronquillo YC, Lewis AL, et al. Neuropathic corneal pain following LASIK surgery: a retrospective case series. Ophthalmol Ther. (2021) 10:677–89. doi: 10.1007/s40123-021-00358-x
43. Moshirfar M, Benstead EE, Sorrentino PM, Tripathy K. Ocular Neuropathic Pain: StatPearls. Treasure Island, FL: StatPearls Publishing (2019).
44. Goyal S, Hamrah P, editors. Understanding neuropathic corneal pain–gaps and current therapeutic approaches. Semin Ophthalmol. (2016) 31:59–70. doi: 10.3109/08820538.2015.1114853
45. Cohen M, Quintner J, van Rysewyk S. Reconsidering the international association for the study of pain definition of pain. Pain Rep. (2018) 3:e634. doi: 10.1097/PR9.0000000000000634
46. Dieckmann G, Goyal S, Hamrah P. Neuropathic corneal pain: approaches for management. Ophthalmology. (2017) 124:S34–47. doi: 10.1016/j.ophtha.2017.08.004
47. Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo C-K, et al. TFOS DEWS II definition and classification report. Ocul Surf. (2017) 15:276–83. doi: 10.1016/j.jtos.2017.05.008
48. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Ärztebl Int. (2015) 112:71. doi: 10.3238/arztebl.2015.0071
49. Rouen PA, White ML. Dry eye disease: prevalence, assessment, and management. Home Healthc Now. (2018) 36:74–83. doi: 10.1097/NHH.0000000000000652
50. Shehadeh-Mashor R, Mimouni M, Shapira Y, Sela T, Munzer G, Kaiserman I. Risk factors for dry eye after refractive surgery. Cornea. (2019) 38:1495–9. doi: 10.1097/ICO.0000000000002152
51. Toda I. Dry eye after LASIK. Invest Ophthalmol Vis Sci. (2018) 59:DES109–15. doi: 10.1167/iovs.17-23538
52. Ræder S, Klyve P, Utheim TP. Dry eye disease–diagnosis and treatment. Tidsskrift Norske Laegeforening. (2019) 139:11.
53. Wallerstein A, Jackson WB, Chambers J, Moezzi AM, Lin H, Simmons PA. Management of post-LASIK dry eye: a multicenter randomized comparison of a new multi-ingredient artificial tear to carboxymethylcellulose. Clin Ophthalmol. (2018) 12:839. doi: 10.2147/OPTH.S163744
54. Iqbal MS, Latif K. Topical cyclosporine in treatment of post-LASIK symptomatic dry eye. Pak J Ophthalmol. (2020) 36:381–5. doi: 10.36351/pjo.v36i4.1078
55. Wang L, Cao K, Wei Z, Baudouin C, Labbé A, Liang Q. Autologous serum eye drops versus artificial tear drops for dry eye disease: a systematic review and meta-analysis of randomized controlled trials. Ophthalmic Res. (2020) 63:443–51. doi: 10.1159/000505630
56. Sanchez-Avila RM, Merayo-Lloves J, Fernandez ML, Rodriguez-Gutierrez LA, Jurado N, Muruzabal F, et al. Plasma rich in growth factors for the treatment of dry eye after LASIK surgery. Ophthalmic Res. (2018) 60:80–6. doi: 10.1159/000487951
57. Jehangir N, Bever G, Mahmood S, Moshirfar M. Comprehensive review of the literature on existing punctal plugs for the management of dry eye disease. J Ophthalmol. (2016) 2016:9312340. doi: 10.1155/2016/9312340
58. Epitropoulos AT, Donnenfeld ED, Shah ZA, Holland EJ, Gross M, Faulkner WJ, et al. Effect of oral re-esterified omega-3 nutritional supplementation on dry eyes. Cornea. (2016) 35:1185. doi: 10.1097/ICO.0000000000000940
59. Kobashi H, Kamiya K, Shimizu K. Dry eye after small incision lenticule extraction and femtosecond laser–assisted LASIK: meta-analysis. Cornea. (2016) 36:85–91. doi: 10.1097/ICO.0000000000000999
60. Ambrósio R Jr., Tervo T, Wilson SE. LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg. (2008) 24:396. doi: 10.3928/1081597X-20080401-14
61. Quinto GG, Camacho W, Behrens A. Postrefractive surgery dry eye. Curr Opin Ophthalmol. (2008) 19:335–41. doi: 10.1097/ICU.0b013e3283009ef8
62. Huh D, Kay K, Kim WJ. Superficial punctate keratopathy after laser in situ keratomileusis. J Refract Surg. (2004) 20:835–6. doi: 10.3928/1081-597X-20041101-13
63. Konomi K, Chen L-L, Tarko RS, Scally A, Schaumberg DA, Azar D, et al. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci. (2008) 49:168–74. doi: 10.1167/iovs.07-0337
64. Edward Y, Leung A, Rao S, Lam DS. Effect of laser in situ keratomileusis on tear stability. Ophthalmology. (2000) 107:2131–5. doi: 10.1016/S0161-6420(00)00388-2
65. Paulsen AJ, Cruickshanks KJ, Fischer ME, Huang G-H, Klein BE, Klein R, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. (2014) 157:799–806. doi: 10.1016/j.ajo.2013.12.023
66. Golas L, Manche EE. Dry eye after laser in situ keratomileusis with femtosecond laser and mechanical keratome. J Cataract Refract Surg. (2011) 37:1476–80. doi: 10.1016/j.jcrs.2011.03.035
67. Albietz JM, Lenton LM, McLennan SG. Dry eye after LASIK: comparison of outcomes for Asian and Caucasian eyes. Clin Exp Optom. (2005) 88:89–96. doi: 10.1111/j.1444-0938.2005.tb06673.x
68. Contreras-Ruiz L, Ryan DS, Sia RK, Bower KS, Dartt DA, Masli S. Polymorphism in THBS1 gene is associated with post-refractive surgery chronic ocular surface inflammation. Ophthalmology. (2014) 121:1389–97. doi: 10.1016/j.ophtha.2014.01.033
69. Simpson RG, Moshirfar M, Edmonds JN, Christiansen SM, Behunin N. Laser in situ keratomileusis in patients with collagen vascular disease: a review of the literature. Clin Ophthalmol. (2012) 6:1827. doi: 10.2147/OPTH.S36690
70. Fraunfelder FW, Rich LF. Laser-assisted in situ keratomileusis complications in diabetes mellitus. Cornea. (2002) 21:246–8. doi: 10.1097/00003226-200204000-00002
71. Salomão MQ, Ambrósio R Jr., Wilson SE. Dry eye associated with laser in situ keratomileusis: mechanical microkeratome versus femtosecond laser. J Cataract Refract Surg. (2009) 35:1756–60. doi: 10.1016/j.jcrs.2009.05.032
72. Bragheeth M, Dua H. Corneal sensation after myopic and hyperopic LASIK: clinical and confocal microscopic study. Br J Ophthalmol. (2005) 89:580–5. doi: 10.1136/bjo.2004.046888
73. Toda I, Asano-Kato N, Komai-Hori Y, Tsubota K. Dry eye after laser in situ keratomileusis. Am J Ophthalmol. (2001) 132:1–7. doi: 10.1016/S0002-9394(01)00959-X
74. Donnenfeld ED, Ehrenhaus M, Solomon R, Mazurek J, Rozell JC, Perry HD. Effect of hinge width on corneal sensation and dry eye after laser in situ keratomileusis. J Cataract Refract Surg. (2004) 30:790–7. doi: 10.1016/j.jcrs.2003.09.043
75. Shoja M, Besharati M. Dry eye after LASIK for myopia: incidence and risk factors. Eur J Ophthalmol. (2007) 17:1–6. doi: 10.1177/112067210701700101
76. Tuisku IS, Lindbohm N, Wilson SE, Tervo TM. Dry eye and corneal sensitivity after high myopic LASIK. J Refract Surg. (2007) 23:338–42. doi: 10.3928/1081-597X-20070401-05
77. Kim W-S, Kim J-S. Change in corneal sensitivityfollowing laser in situ keratomileusis. J Cataract Refract Surg. (1999) 25:368–73. doi: 10.1016/S0886-3350(99)80085-6
78. Hatch BB, Moshirfar M, Ollerton AJ, Sikder S, Mifflin MD. A prospective, contralateral comparison of photorefractive keratectomy (PRK) versus thin-flap LASIK: assessment of visual function. Clin Ophthalmol. (2011) 5:451. doi: 10.2147/OPTH.S18967
79. Hu L, Xie W, Liu J, Zhou Y, Zhou Q, Yu Y, et al. Tear menisci and corneal subbasal nerve density in patients after laser in situ keratomileusis. Eye Contact Lens. (2015) 41:51–7. doi: 10.1097/ICL.0000000000000062
80. Albietz JM, Lenton LM, McLennan SG. Chronic dry eye and regression after laser in situ keratomileusis for myopia. J Cataract Refract Surg. (2004) 30:675–84. doi: 10.1016/j.jcrs.2003.07.003
81. Benitez-del-Castillo JM, del Rio T, Iradier T, Hernández JL, Castillo A, Garcia-Sanchez J. Decrease in tear secretion and corneal sensitivity after laser in situ keratomileusis. Cornea. (2001) 20:30–2. doi: 10.1097/00003226-200101000-00005
82. Yüksel Elgin C, Ískeleli G, Talaz S, Akyol S. Comparative analysis of tear film levels of inflammatory mediators in contact lens users. Curr Eye Res. (2016) 41:441–7. doi: 10.3109/02713683.2015.1037001
83. Patel SV, McLaren JW, Hodge DO, Bourne WM. Confocal microscopy in vivo in corneas of long-term contact lens wearers. Invest Ophthalmol Vis Sci. (2002) 43:995–1003.
84. Dohlman TH, Lai EC, Ciralsky JB. Dry eye disease after refractive surgery. Int Ophthalmol Clin. (2016) 56:101–10. doi: 10.1097/IIO.0000000000000104
85. Garcia-Zalisnak D, Nash D, Yeu E. Ocular surface diseases and corneal refractive surgery. Curr Opin Ophthalmol. (2014) 25:264–9. doi: 10.1097/ICU.0000000000000077
86. Raoof D, Pineda R. Dry eye after laser in-situ keratomileusis. Semin Ophthalmol. (2014) 29:358–62. doi: 10.3109/08820538.2014.962663
87. Chao C, Golebiowski B, Stapleton F. The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul Surf. (2014) 12:32–45. doi: 10.1016/j.jtos.2013.09.001
88. Labbé A, Liang Q, Wang Z, Zhang Y, Xu L, Baudouin C, et al. Corneal nerve structure and function in patients with non-Sjögren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. (2013) 54:5144–50. doi: 10.1167/iovs.13-12370
89. Feng Y-F, Yu J-G, Wang D-D, Li J-H, Huang J-H, Shi J-L, et al. The effect of hinge location on corneal sensation and dry eye after LASIK: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. (2013) 251:357–66. doi: 10.1007/s00417-012-2078-5
90. Vroman DT, Sandoval HP, de Castro LEF, Kasper TJ, Holzer MP, Solomon KD. Effect of hinge location on corneal sensation and dry eye after laser in situ keratomileusis for myopia. J Cataract Refract Surg. (2005) 31:1881–7. doi: 10.1016/j.jcrs.2005.03.074
91. Mian SI, Shtein RM, Nelson A, Musch DC. Effect of hinge position on corneal sensation and dry eye after laser in situ keratomileusis using a femtosecond laser. J Cataract Refract Surg. (2007) 33:1190–4. doi: 10.1016/j.jcrs.2007.03.031
92. Müller L, Pels L, Vrensen G. Ultrastructural organization of human corneal nerves. Invest Ophthalmol Vis Sci. (1996) 37:476–88.
93. Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. (2003) 76:521–42. doi: 10.1016/S0014-4835(03)00050-2
94. Mian SI, Li AY, Dutta S, Musch DC, Shtein RM. Dry eyes and corneal sensation after laser in situ keratomileusis with femtosecond laser flap creation: effect of hinge position, hinge angle, and flap thickness. J Cataract Refract Surg. (2009) 35:2092–8. doi: 10.1016/j.jcrs.2009.07.009
95. Lee K-W, Joo C-K. Clinical results of laser in situ keratomileusis with superior and nasal hinges. J Cataract Refract Surg. (2003) 29:457–61. doi: 10.1016/S0886-3350(02)01738-8
96. Donnenfeld ED, Solomon K, Perry HD, Doshi SJ, Ehrenhaus M, Solomon R, et al. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology. (2003) 110:1023–9. doi: 10.1016/S0161-6420(03)00100-3
97. Nassaralla BA, McLeod SD, Boteon JE, Nassaralla JJ Jr. The effect of hinge position and depth plate on the rate of recovery of corneal sensation following LASIK. Am J Ophthalmol. (2005) 139:118–24. doi: 10.1016/j.ajo.2004.08.057
98. Ghoreishi M, Aidenloo NS, Peyman A, Peyman M, Haghdoustoskoey M. Does hinge position affect dry eye after laser in situ keratomileusis? Ophthalmologica. (2005) 219:276–80. doi: 10.1159/000086111
99. De Paiva CS, Chen Z, Koch DD, Hamill MB, Manuel FK, Hassan SS, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. (2006) 141:438–45. doi: 10.1016/j.ajo.2005.10.006
100. Huang JC-C, Sun C-C, Chang C-K, Ma DH-K, Lin Y-F. Effect of hinge position on corneal sensation and dry eye parameters after femtosecond laser–assisted LASIK. J Refract Surg. (2012) 28:625–31. doi: 10.3928/1081597X-20120815-07
101. Tai YC, Sun CC. Effects of flap diameter on dry eye parameters and corneal sensation after femtosecond laser-assisted LASIK. Taiwan J Ophthalmol. (2019) 9:166–72. doi: 10.4103/tjo.tjo_59_19
102. Donnenfeld E. Preservation of corneal innervation with femtosecond laser inverted sidecut flaps. Invest Ophthalmol Vis Sci. (2010) 51:2855.
103. Knorz MC, Vossmerbaeumer U. Comparison of flap adhesion strength using the Amadeus microkeratome and the IntraLase iFS femtosecond laser in rabbits. J Refract Surg. (2008) 24:875. doi: 10.3928/1081597X-20081101-04
104. Kung JS, Sáles CS, Manche EE. Corneal sensation and dry eye symptoms after conventional versus inverted side-cut femtosecond LASIK: a prospective randomized study. Ophthalmology. (2014) 121:2311–6. doi: 10.1016/j.ophtha.2014.07.015
105. Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Vis Sci. (2010) 51:6125–30. doi: 10.1167/iovs.10-5390
106. Suzuki M, Massingale ML, Ye F, Godbold J, Elfassy T, Vallabhajosyula M, et al. Tear osmolarity as a biomarker for dry eye disease severity. Invest Ophthalmol Vis Sci. (2010) 51:4557–61. doi: 10.1167/iovs.09-4596
108. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. (2000) 118:615–21. doi: 10.1001/archopht.118.5.615
109. Abetz L, Rajagopalan K, Mertzanis P, Begley C, Barnes R, Chalmers R. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual Life Outcomes. (2011) 9:1–16. doi: 10.1186/1477-7525-9-111
110. Cohen E, Spierer O. Dry eye post-laser-assisted in situ keratomileusis: major review and latest updates. J Ophthalmol. (2018) 2018:4903831. doi: 10.1155/2018/4903831
111. Xie W. Recent advances in laser in situ keratomileusis-associated dry eye. Clin Exp Optom. (2016) 99:107–12. doi: 10.1111/cxo.12361
112. Rosenfeld SI. Evaluation and management of post-LASIK dry eye syndrome. Int Ophthalmol Clin. (2010) 50:191–9. doi: 10.1097/IIO.0b013e3181e2469b
113. Pflugfelder SC, Maskin SL, Anderson B, Chodosh J, Holland EJ, De Paiva CS, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. (2004) 138:444–57. doi: 10.1016/j.ajo.2004.04.052
114. McMonnies CW. How blink anomalies can contribute to post-LASIK neurotrophic epitheliopathy. Optom Vis Sci. (2015) 92:e241–7. doi: 10.1097/OPX.0000000000000567
115. Xia L-K, Yu J, Chai G-R, Wang D, Li Y. Comparison of the femtosecond laser and mechanical microkeratome for flap cutting in LASIK. Int J Ophthalmol. (2015) 8:784.
116. Sun C-C, Chang C-K, Ma DH-K, Lin Y-F, Chen K-J, Sun M-H, et al. Dry eye after LASIK with a femtosecond laser or a mechanical microkeratome. Optom Vis Sci. (2013) 90:1048–56. doi: 10.1097/OPX.0b013e31829d9905
117. Li M, Zhou Z, Shen Y, Knorz MC, Gong L, Zhou X. Comparison of corneal sensation between small incision lenticule extraction (SMILE) and femtosecond laser-assisted LASIK for myopia. J Refract Surg. (2014) 30:94–100. doi: 10.3928/1081597X-20140120-04
118. Wei S, Wang Y. Comparison of corneal sensitivity between FS-LASIK and femtosecond lenticule extraction (ReLEx flex) or small-incision lenticule extraction (ReLEx smile) for myopic eyes. Graefes Arch Clin Exp Ophthalmol. (2013) 251:1645–54. doi: 10.1007/s00417-013-2272-0
119. Li M, Niu L, Qin B, Zhou Z, Ni K, Le Q, et al. Confocal comparison of corneal reinnervation after small incision lenticule extraction (SMILE) and femtosecond laser in situ keratomileusis (FS-LASIK). PLoS One. (2013) 8:e81435. doi: 10.1371/journal.pone.0081435
120. Li M, Zhao J, Shen Y, Li T, He L, Xu H, et al. Comparison of dry eye and corneal sensitivity between small incision lenticule extraction and femtosecond LASIK for myopia. PLoS One. (2013) 8:e77797. doi: 10.1371/journal.pone.0077797
121. Xu Y, Yang Y. Dry eye after small incision lenticule extraction and LASIK for myopia. J Refract Surg. (2014) 30:186–90. doi: 10.3928/1081597X-20140219-02
122. Gao S, Li S, Liu L, Wang Y, Ding H, Li L, et al. Early changes in ocular surface and tear inflammatory mediators after small-incision lenticule extraction and femtosecond laser-assisted laser in situ keratomileusis. PLoS One. (2014) 9:e107370. doi: 10.1371/journal.pone.0107370
123. Denoyer A, Landman E, Trinh L, Faure J-F, Auclin F, Baudouin C. Dry eye disease after refractive surgery: comparative outcomes of small incision lenticule extraction versus LASIK. Ophthalmology. (2015) 122:669–76. doi: 10.1016/j.ophtha.2014.10.004
124. Cai W-T, Liu Q-Y, Ren C-D, Wei Q-Q, Liu J-L, Wang Q-Y, et al. Dry eye and corneal sensitivity after small incision lenticule extraction and femtosecond laser-assisted in situ keratomileusis: a meta-analysis. Int J Ophthalmol. (2017) 10:632. doi: 10.18240/ijo.2017.04.21
125. Kymionis GD, Bouzoukis DI, Diakonis VF, Siganos C. Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol. (2008) 2:829. doi: 10.2147/OPTH.S1409
126. Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. (2000) 47:119–25. doi: 10.1016/S0162-3109(00)00192-2
127. Schultz C. Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye Dis. (2014) 6:S16067. doi: 10.4137/OED.S16067
128. Salib GM, McDonald MB, Smolek M. Safety and efficacy of cyclosporine 0.05% drops versus unpreserved artificial tears in dry-eye patients having laser in situ keratomileusis. J Cataract Refract Surg. (2006) 32:772–8. doi: 10.1016/j.jcrs.2005.10.034
129. Kanellopoulos AJ. Incidence and management of symptomatic dry eye related to LASIK for myopia, with topical cyclosporine A. Clin Ophthalmol. (2019) 13:545. doi: 10.2147/OPTH.S188521
130. Torricelli AA, Santhiago MR, Wilson SE. Topical cyclosporine a treatment in corneal refractive surgery and patients with dry eye. J Refract Surg. (2014) 30:558–64. doi: 10.3928/1081597X-20140711-09
131. Park CH, Lee HK, Kim MK, Kim EC, Kim JY, Kim HK, et al. Comparison of 0.05% cyclosporine and 3% diquafosol solution for dry eye patients: a randomized, blinded, multicenter clinical trial. BMC Ophthalmol. (2019) 19:131. doi: 10.1186/s12886-019-1136-8
132. Toda I, Ide T, Fukumoto T, Ichihashi Y, Tsubota K. Combination therapy with diquafosol tetrasodium and sodium hyaluronate in patients with dry eye after laser in situ keratomileusis. Am J Ophthalmol. (2014) 157:616–22.e1. doi: 10.1016/j.ajo.2013.11.017
133. Mori Y, Nejima R, Masuda A, Maruyama Y, Minami K, Miyata K, et al. Effect of diquafosol tetrasodium eye drop for persistent dry eye after laser in situ keratomileusis. Cornea. (2014) 33:659–62. doi: 10.1097/ICO.0000000000000136
134. Byun Y-S, Yoo Y-S, Kwon J-Y, Joo J-S, Lim S-A, Whang W-J, et al. Diquafosol promotes corneal epithelial healing via intracellular calcium-mediated ERK activation. Exp Eye Res. (2016) 143:89–97. doi: 10.1016/j.exer.2015.10.013
135. Burnstock G, Williams M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J Pharmacol Exp Ther. (2000) 295:862–9.
136. Keating GM. Diquafosol ophthalmic solution 3%: a review of its use in dry eye. Drugs. (2015) 75:911–22. doi: 10.1007/s40265-015-0409-7
137. Nakamura M, Imanaka T, Sakamoto A. Diquafosol ophthalmic solution for dry eye treatment. Adv Ther. (2012) 29:579–89. doi: 10.1007/s12325-012-0033-9
138. Koh S. Clinical utility of 3% diquafosol ophthalmic solution in the treatment of dry eyes. Clin Ophthalmol. (2015) 9:865. doi: 10.2147/OPTH.S69486
139. Jones L, Downie LE, Korb D, Benitez-del-Castillo JM, Dana R, Deng SX, et al. TFOS DEWS II management and therapy report. Ocul Surf. (2017) 15:575–628. doi: 10.1016/j.jtos.2017.05.006
140. Sullivan LJ, McCurrach F, Lee S, Taylor HR, Rolando M, Marechal-Courtois C, et al. Efficacy and safety of 0.3% carbomer gel compared to placebo in patients with moderate-to-severe dry eye syndrome. Ophthalmology. (1997) 104:1402–8. doi: 10.1016/S0161-6420(97)30124-9
141. Bruix A, Adan A, Casaroli-Marano RP. [Efficacy of sodium carboxymethylcellulose in the treatment of dry eye syndrome]. Arch Soc Esp Oftalmol. (2006) 81:85–92. doi: 10.4321/S0365-66912006000200008
142. Baeyens V, Bron A, Baudouin C, Group VHS. Efficacy of 0.18% hypotonic sodium hyaluronate ophthalmic solution in the treatment of signs and symptoms of dry eye disease. J Fr Ophtalmol. (2012) 35:412–9. doi: 10.1016/j.jfo.2011.07.017
143. Aguilar AJ, Marquez MI, Albera PA, Tredicce JL, Berra A. Effects of Systane® Balance on noninvasive tear film break-up time in patients with lipid-deficient dry eye. Clin Ophthalmol. (2014) 8:2365. doi: 10.2147/OPTH.S70623
144. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. (2016) 2:CD009729. doi: 10.1002/14651858.CD009729.pub2
145. Dumbleton K, Woods C, Fonn D. An investigation of the efficacy of a novel ocular lubricant. Eye Contact Lens. (2009) 35:149–55. doi: 10.1097/ICL.0b013e3181a2c986
146. Simmons PA, Vehige JG. Clinical performance of a mid-viscosity artificial tear for dry eye treatment. Cornea. (2007) 26:294–302. doi: 10.1097/ICO.0b013e31802e1e04
147. Simmons PA, Liu H, Carlisle-Wilcox C, Vehige JG. Efficacy and safety of two new formulations of artificial tears in subjects with dry eye disease: a 3-month, multicenter, active-controlled, randomized trial. Clin Ophthalmol. (2015) 9:665. doi: 10.2147/OPTH.S78184
148. Geerling G, Hartwig D. Autologous Serum Eyedrops for Ocular Surface Disorders: Cornea and External eye Disease. Berlin: Springer (2006). p. 1–20. doi: 10.1007/3-540-31226-9_1
149. Alio JL, Rodriguez AE, Abdelghany AA, Oliveira RF. Autologous platelet-rich plasma eye drops for the treatment of post-lasik chronic ocular surface syndrome. J Ophthalmol. (2017) 2017:2457620. doi: 10.1155/2017/2457620
150. Alio JL, Pastor S, Ruiz-Colecha J, Rodriguez A, Artola A. Treatment of ocular surface syndrome after LASIK with autologous platelet-rich plasma. J Refract Surg. (2007) 23:617–9. doi: 10.3928/1081-597X-20070601-13
151. Berhuni M, Istek Ş, Tıskaoğlu NS. 20% autologous serum vs. 0.05% cyclosporine and preservative-free artificial tears in the treatment of Sjögren related dry eye. Arq Brasil Oftalmol. (2022) 4:S0004–27492022005011217. doi: 10.5935/0004-2749.2022-0192
152. Yarur FM, Ordenes G, Cruzat A. Autologous serum compared to artificial tear drops for dry eye disease. Suero Autól Comp. (2021) 21:e8213. doi: 10.5867/medwave.2021.05.8213
153. Mohammad EA, Lamie NM, Ismail AN, Mansour MN. Autologous platelet rich plasma versus autologous serum eye drops in treatment of severe dry eye disease. Int J Med Arts. (2022) 4:2542–9. doi: 10.21608/ijma.2022.152958.1490
154. Poon AC, Geerling G, Dart JK, Fraenkel GE, Daniels JT. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. Br J Ophthalmol. (2001) 85:1188–97. doi: 10.1136/bjo.85.10.1188
155. Rocha E, Pelegrino F, De Paiva C, Vigorito A, De Souza C. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplant. (2000) 25:1101–3. doi: 10.1038/sj.bmt.1702334
156. Takamura E, Shinozaki K, Hata H, Yukariand J, Hori S. Efficacy of autologous serum treatment in patients with severe dry eye. Adv Exp Med Biol. (2002) 506(Pt B):1247–50. doi: 10.1007/978-1-4615-0717-8_179
157. Levi-Montalcini R. The nerve growth factor 35 years later. Science. (1987) 237:1154–62. doi: 10.1126/science.3306916
158. Cacialli P, Gatta C, d’Angelo L, Leggieri A, Palladino A, de Girolamo P, et al. Nerve growth factor is expressed and stored in central neurons of adult zebrafish. J Anat. (2019) 235:167–79. doi: 10.1111/joa.12986
159. Riaz S, Tomlinson D. Neurotrophic factors in peripheral neuropathies: pharmacological strategies. Prog Neurobiol. (1996) 49:125–43. doi: 10.1016/0301-0082(96)00010-X
160. Bonini S, Lambiase A, Rama P, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for neurotrophic keratitis. Ophthalmology. (2000) 107:1347–51. doi: 10.1016/S0161-6420(00)00163-9
161. Fouda SM, Mattout HK. Comparison between botulinum toxin A injection and lacrimal punctal plugs for the control of post-LASIK dry eye manifestations: a prospective study. Ophthalmol Ther. (2017) 6:167–74. doi: 10.1007/s40123-017-0079-5
162. Alfawaz AM, Algehedan S, Jastaneiah SS, Al-Mansouri S, Mousa A, Al-Assiri A. Efficacy of punctal occlusion in management of dry eyes after laser in situ keratomileusis for myopia. Curr Eye Res. (2014) 39:257–62. doi: 10.3109/02713683.2013.841258
163. Nemet A, Mimouni M, Hecht I, Assad N, Kaiserman I. Post laser-assisted in-situ keratomileusis dry eye disease and temporary punctal plugs. Indian J Ophthalmol. (2020) 68:2960. doi: 10.4103/ijo.IJO_1664_20
164. Huang B, Mirza MA, Qazi MA, Pepose JS. The effect of punctal occlusion on wavefront aberrations in dry eye patients after laser in situ keratomileusis. Am J Ophthalmol. (2004) 137:52–61. doi: 10.1016/S0002-9394(03)00903-6
165. Song JS, Woo IH, Eom Y, Kim HM. Five misconceptions related to punctal plugs in dry eye management. Cornea. (2018) 37:S58–61. doi: 10.1097/ICO.0000000000001734
166. Comez AT, Karakilic AV, Yildiz A. Silicone perforated punctal plugs for the treatment of punctal stenosis. Arq Brasil Oftalmol. (2019) 82:394–9. doi: 10.5935/0004-2749.20190077
167. Scheepers M, Pearson A, Michaelides M. Bilateral canaliculitis following SmartPLUG insertion for dry eye syndrome post LASIK surgery. Graefes Arch Clin Exp Ophthalmol. (2007) 245:895–7. doi: 10.1007/s00417-006-0462-8
168. Lambiase A, Manni L, Bonini S, Rama P, Micera A, Aloe L. Nerve growth factor promotes corneal healing: structural, biochemical, and molecular analyses of rat and human corneas. Invest Ophthalmol Vis Sci. (2000) 41:1063–9.
169. Esquenazi S, Bazan HE, Bui V, He J, Kim DB, Bazan NG. Topical combination of NGF and DHA increases rabbit corneal nerve regeneration after photorefractive keratectomy. Invest Ophthalmol Vis Sci. (2005) 46:3121–7. doi: 10.1167/iovs.05-0241
170. Cortina MS, He J, Li N, Bazan NG, Bazan HE. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. (2010) 51:804–10. doi: 10.1167/iovs.09-3641
171. Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, Van Der Meer JW, et al. The effect of dietary supplementation with n–3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. (1989) 320:265–71. doi: 10.1056/NEJM198902023200501
172. Gibney M, Hunter B. The effects of short-and long-term supplementation with fish oil on the incorporation of n-3 polyunsaturated fatty acids into cells of the immune system in healthy volunteers. Eur J Clin Nutr. (1993) 47:255–9.
173. Goldman D, Pickett W, Goetzl E. Human neutrophil chemotactic and degranulating activities of leukotriene B5 (LTB5) derived from eicosapentaenoic acid. Biochem Biophys Res Commun. (1983) 117:282–8. doi: 10.1016/0006-291X(83)91572-3
174. Bhargava R, Kumar P, Phogat H, Kaur A, Kumar M. Oral omega-3 fatty acids treatment in computer vision syndrome related dry eye. Contact Lens Anterior Eye. (2015) 38:206–10. doi: 10.1016/j.clae.2015.01.007
175. Bhargava R, Kumar P. Oral omega-3 fatty acid treatment for dry eye in contact lens wearers. Cornea. (2015) 34:413–20. doi: 10.1097/ICO.0000000000000386
176. Arita R, Morishige N, Shirakawa R, Sato Y, Amano S. Effects of eyelid warming devices on tear film parameters in normal subjects and patients with meibomian gland dysfunction. Ocul Surf. (2015) 13:321–30. doi: 10.1016/j.jtos.2015.04.005
177. Mori A, Shimazaki J, Shimmura S, Fujishima H, Oguchi Y, Tsubota K. Disposable eyelid-warming device for the treatment of meibomian gland dysfunction. Jpn J Ophthalmol. (2003) 47:578–86. doi: 10.1016/S0021-5155(03)00142-4
178. Doan S, Chiambaretta F, Baudouin C. Evaluation of an eyelid warming device (Blephasteam§) for the management of ocular surface diseases in France: the ESPOIR study. J Fr Ophtalmol. (2014) 37:763–72. doi: 10.1016/j.jfo.2014.06.004
179. Di Pascuale MA, Liu TS, Trattler W, Tseng SC. Lipid tear deficiency in persistent dry eye after laser in situ keratomileusis and treatment results of new eye-warming device. J Cataract Refract Surg. (2005) 31:1741–9. doi: 10.1016/j.jcrs.2005.02.041
180. Blackie CA, Coleman CA, Holland EJ. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. (2016) 10:1385–96. doi: 10.2147/OPTH.S109663
181. Lane SS, DuBiner HB, Epstein RJ, Ernest PH, Greiner JV, Hardten DR, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. (2012) 31:396–404. doi: 10.1097/ICO.0b013e318239aaea
182. Schallhorn C, Schallhorn SC, Schallhorn JM. Effectiveness of an eyelid thermal pulsation procedure to treat recalcitrant dry eye symptoms after refractive surgery. Invest Ophthalmol Vis Sci. (2014) 55:3694.
183. Schallhorn CS, Schallhorn JM, Hannan S, Schallhorn SC. Effectiveness of an eyelid thermal pulsation procedure to treat recalcitrant dry eye symptoms after laser vision correction. J Refract Surg. (2017) 33:30–6. doi: 10.3928/1081597X-20161006-05
184. Guilloto Caballero S, García Madrona JL, Colmenero Reina E. Effect of pulsed laser light in patients with dry eye syndrome. Arch Soc Esp Oftalmol. (2017) 92:509–15. doi: 10.1016/j.oftale.2017.03.012
185. Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg. (2015) 33:41–6. doi: 10.1089/pho.2014.3819
186. Fuentes Páez G, Soler Tomas JR, Burillo S. Intense pulsed light: results in chronic dry eye syndrome after LASIK. Arch Soc Esp Oftalmol. (2020) 95:226–30. doi: 10.1016/j.oftale.2020.02.010
187. Lee J-H, Han K, Kim T-H, Kim A-R, Kwon O, Kim J-H, et al. Acupuncture for dry eye syndrome after refractive surgery: a randomized controlled pilot trial. Integr Med Res. (2021) 10:100456. doi: 10.1016/j.imr.2020.100456
188. Yang L, Yang Z, Yu H, Song H. Acupuncture therapy is more effective than artificial tears for dry eye syndrome: evidence based on a meta-analysis. Evid Based Complement Alternat Med. (2015) 2015:143858. doi: 10.1155/2015/1 43858
189. Jang H, Lee S, Kim TH, Kim AR, Lee M, Lee JH. Acupuncture for dry eye syndrome after refractive surgery: study protocol for a randomized controlled trial. Trials. (2013) 14:351. doi: 10.1186/1745-6215-14-351
190. Kim TH, Kang JW, Kim KH, Kang KW, Shin MS, Jung SY, et al. Acupuncture for, controlled ttodeamr, trial with active comparison intervention (artificial teardrops). PLoS One. (2012) 5:e36638. doi: 10.1371/journal.pone.0036638
191. Baran I, Bradley JA, Alipour F, Rosenthal P, Le H-G, Jacobs DS. PROSE treatment of corneal ectasia. Contact Lens Anterior Eye. (2012) 35:222–7. doi: 10.1016/j.clae.2012.04.003
192. Maharana PK, Dubey A, Jhanji V, Sharma N, Das S, Vajpayee RB. Management of advanced corneal ectasias. Br J Ophthalmol. (2016) 100:34–40. doi: 10.1136/bjophthalmol-2015-307059
193. Segal O, Barkana Y, Hourovitz D, Behrman S, Kamun Y, Avni I, et al. Scleral contact lenses may help where other modalities fail. Cornea. (2003) 22:308–10. doi: 10.1097/00003226-200305000-00006
194. Mian SZ, Agranat JS, Jacobs DS. Prosthetic replacement of the ocular surface ecosystem (PROSE) treatment for complications after LASIK. Eye Contact Lens. (2016) 42:371–3. doi: 10.1097/ICL.0000000000000303
195. Schmidt PC, Ruchelli G, Mackey SC, Carroll IR. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. (2013) 119:1215–21. doi: 10.1097/ALN.0b013e3182a9a896
196. Galor A, Patel S, Small LR, Rodriguez A, Venincasa MJ, Valido SE, et al. Pregabalin failed to prevent dry eye symptoms after laser-assisted in situ keratomileusis (LASIK) in a randomized pilot study. J Clin Med. (2019) 8:1355. doi: 10.3390/jcm8091355
197. Paik DW, Lim DH, Chung T-Y. Effects of taking pregabalin (Lyrica) on the severity of dry eye, corneal sensitivity and pain after laser epithelial keratomileusis surgery. Br J Ophthalmol. (2020) 106:474–9. doi: 10.1136/bjophthalmol-2020-317570
198. Eskina E, Maychuk N, Parshina V, Kukleva O. Medicamental correction of pathomorphological changes of the ocular surface in patients with steroid therapy intolerance after photorefractive keratectomy. Vestnik Oftalmol. (2019) 135:67–77. doi: 10.17116/oftalma201913503167
199. Astakhov YS, Astakhov SY, Lisochkina AB. Assessment of dry eye signs and symptoms and ocular tolerance of a preservative-free lacrimal substitute (Hylabak®) versus a preserved lacrimal substitute (Systane®) used for 3 months in patients after LASIK. Clin Ophthalmol. (2013) 7:2289. doi: 10.2147/OPTH.S50446
200. Panova I, Titov A, Golovatenko S, Mirsaitova D, Leshchik O, Pogosyan M. Artificial tear drops Thealoz in the correction of dry eye syndrome after keratorefractive surgery. Vestnik Oftalmol. (2019) 135:113–21. doi: 10.17116/oftalma2019135021113
201. Zheng X, Zhao S. Effect of 0.1% and 0.3% sodium hyaluronic ophthalmic solution on tear film stability and visual quality in FS-LASIK dry eye. Chin J Exp Ophthalmol. (2018) 36:373–9.
202. Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. J Refract Surg. (2006) 22:61–6. doi: 10.3928/1081-597X-20060101-13
203. Pan S, Li L, Xu Z, Zhao J. Effect of leukemia inhibitory factor on corneal nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci Lett. (2011) 499:99–103. doi: 10.1016/j.neulet.2011.05.042
204. Joo M-J, Yuhan KR, Hyon JY, Lai H, Hose S, Sinha D, et al. The effect of nerve growth factor on corneal sensitivity after laserin situ keratomileusis. Arch Ophthalmol. (2004) 122:1338–41. doi: 10.1001/archopht.122.9.1338
205. Wang C, Peng Y, Pan S, Li L. Effect of insulin-like growth factor-1 on corneal surface ultrastructure and nerve regeneration of rabbit eyes after laser in situ keratomileusis. Neurosci Lett. (2014) 558:169–74. doi: 10.1016/j.neulet.2013.10.063
206. Yung YH, Toda I, Sakai C, Yoshida A, Tsubota K. Punctal plugs for treatment of post-LASIK dry eye. Jpn J Ophthalmol. (2012) 56:208–13. doi: 10.1007/s10384-012-0125-8
Keywords: LASIK, laser-assisted in situ keratomileusis, dry eye disease, eye drops, eye disease
Citation: Tamimi A, Sheikhzadeh F, Ezabadi SG, Islampanah M, Parhiz P, Fathabadi A, Poudineh M, Khanjani Z, Pourmontaseri H, Orandi S, Mehrabani R, Rahmanian M and Deravi N (2023) Post-LASIK dry eye disease: A comprehensive review of management and current treatment options. Front. Med. 10:1057685. doi: 10.3389/fmed.2023.1057685
Received: 29 September 2022; Accepted: 14 March 2023;
Published: 11 April 2023.
Edited by:
Pedram Hamrah, Tufts Medical Center, United StatesReviewed by:
Mo Ziaei, The University of Auckland, New ZealandMatteo Posarelli, University of Liverpool, United Kingdom
Jorge L. Alio, Miguel Hernández University, Spain
Copyright © 2023 Tamimi, Sheikhzadeh, Ezabadi, Islampanah, Parhiz, Fathabadi, Poudineh, Khanjani, Pourmontaseri, Orandi, Mehrabani, Rahmanian and Deravi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niloofar Deravi, bmlsb29mYXJkZXJhdmlAc2JtdS5hYy5pcg==
†These authors have contributed equally to this work
 Atena Tamimi
Atena Tamimi Farzad Sheikhzadeh2†
Farzad Sheikhzadeh2† Amirhossein Fathabadi
Amirhossein Fathabadi Mohadeseh Poudineh
Mohadeseh Poudineh Hossein Pourmontaseri
Hossein Pourmontaseri Shirin Orandi
Shirin Orandi Mohammad Rahmanian
Mohammad Rahmanian Niloofar Deravi
Niloofar Deravi