- 1Nuffield Department of Clinical Medicine (NDM), University of Oxford, Oxford, United Kingdom
- 2Oxford NIHR Biomedical Research Centre, University of Oxford, Oxford, United Kingdom
- 3Department of Physiology, Anatomy and Genetics, University of Oxford, Oxford, United Kingdom
- 4Wellcome Centre for Human Genetics, NDM, University of Oxford, Oxford, United Kingdom
- 5Oxford University Hospitals (OUH) NHS Foundation Trust, Oxford, United Kingdom
- 6Radcliffe Department of Medicine, University of Oxford, Oxford, United Kingdom
- 7Chinese Academy of Medical Sciences Oxford Institute, University of Oxford, Oxford, United Kingdom
Background and aim: In acute severe COVID-19, patients present with lung inflammation and vascular injury, accompanied by an exaggerated cytokine response. In this study, our aim was to describe the inflammatory and vascular mediator profiles in patients who were previously hospitalized with COVID-19 pneumonitis, months after their recovery, and compare them with those in patients recovering from severe sepsis and in healthy controls.
Methods: A total of 27 different cytokine, chemokine, vascular endothelial injury and angiogenic mediators were measured in the plasma of forty-nine patients 5.0 ± 1.9 (mean ± SD) months after they were hospitalized with COVID-19 pneumonia, eleven patients 5.4 ± 2.9 months after hospitalization with acute severe sepsis, and 18 healthy controls.
Results: Compared with healthy controls, IL-6, TNFα, SAA, CRP, Tie-2, Flt1, and PIGF were significantly increased in the post-COVID group, and IL-7 and bFGF were significantly reduced. While IL-6, PIGF, and CRP were also significantly elevated in post-Sepsis patients compared to controls, the observed differences in TNFα, Tie-2, Flt-1, IL-7 and bFGF were unique to the post-COVID group. TNFα levels significantly correlated with the severity of acute COVID-19 illness (spearman’s r = 0.30, p < 0.05). Furthermore, in post-COVID patients, IL-6 and CRP were each strongly negatively correlated with gas transfer factor %predicted (spearman’s r = –0.51 and r = –0.57, respectively, p < 0.002) and positively correlated with computed tomography (CT) abnormality scores at recovery (r = 0.28 and r = 0.46, p < 0.05, respectively).
Conclusion: A unique inflammatory and vascular endothelial damage mediator signature is found in plasma months following acute COVID-19 infection. Further research is required to determine its pathophysiological and clinical significance.
Introduction
Clinical outcomes in acute coronavirus disease 2019 (COVID-19) are highly dependent upon the cytokine response in the host (1). The entry of SARS-Cov-2 virions into pulmonary epithelial cells via the angiotensin converting enzyme (ACE2), triggers a wave of pro-inflammatory cytokines and chemokines (2). In the healthy immune response infected cells are cleared and this inflammatory cascade recedes. In patients with more severe disease, however, an exaggerated elevation of these mediators has been observed, termed “cytokine release syndrome” (1, 3–5), which may lead to immunopathogenesis by causing tissue damage. These inflammatory pathways are the target of several successful treatments in the acute setting, such as dexamethasone and the anti-IL6R monoclonal antibody tocilizumab (6, 7). In addition, the vascular endothelium is also dysregulated in acute COVID-19 and microvascular thrombosis and endothelial inflammation contribute significantly to the pathology (8–10).
Contrary to the acute effects, our understanding of the longer-term effects of COVID-19 on inflammatory mediators and vascular function remains opaque, and other follow-up studies often have lacked an appropriate control group (11, 12). In this study, we examine levels of cytokine, chemokine and markers of vascular injury and angiogenesis in the peripheral blood of patients recovering from COVID-19 pneumonia many months after their acute infection, and compare their profiles to those of patients recovering from severe sepsis and to those of healthy controls.
Methods
Our post-COVID cohort consisted of 49 patients [aged 60 ± 9 years (mean ± SD), 13 females] from whom venous blood was collected 5.0 ± 1.9 months after hospitalization with acute COVID-19 pneumonia. These patients were recruited from a post-COVID-19 follow-up respiratory clinic, having previously been hospitalized with acute COVID-19 pneumonia in the period between March 2020 and Jan 2021. For comparison, blood was obtained from a group of 11 patients 5.4 ± 2.9 months after hospitalization with severe sepsis (age 66 ± 17 years, seven females) (13) and 18 healthy control participants (age 47 ± 16 years, two females). Plasma was obtained by centrifugation of blood collected in EDTA-lined tubes and stored at –80°C prior to measurement of 27 different cytokine, chemokine, angiogenic and vascular injury markers [Meso Scale Discovery (MSD) V-PLEX multiplex assays using a Meso-Scale Discovery SQ120 device]: Interferon gamma, Interleukin 1B (IL-1B), IL-4, IL-6, IL-10, Tumor Necrosis Factor alpha (TNF alpha), Granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-17A, Interleukin 12 (IL-12/23p40), IL-7, Macrophage Inflammatory protein 1A (MIP-1A), MIP-1B, Monocyte Chemoattractant Protein 1 (MCP-1), MCP-4, Interferon-inducible protein 10 (IP-10), Thymus and activation regulated chemokine (TARC), Vascular Endothelial Growth Factor A (VEGF-A), VEGF-C, VEGF-D, Placental Growth Factor (PIGF), Vascular Endothelial Growth Factor Receptor 1 (Flt-1), Angiopoietin 1 receptor (Tie-2), basic Fibroblast Growth Factor (bFGF), Serum Amyloid A protein (SAA), C-reactive protein (CRP), Vascular Cell Adhesion Molecule 1 (VCAM-1), Intercellular Adhesion Molecule 1 (ICAM-1) (see Table 1 for more details). All participants provided informed written consent. The studies were approved by the North-West Preston (20/NW/0235) and Oxford C (19/SC/0296) Research Ethics Committees.
Statistical comparisons between groups were performed using Kruskal-Wallis tests for non-normally distributed data and one-way ANOVA for normally distributed data. To allow for multiple testing, false discovery rate (FDR) correction was performed using the Benjamini Hochberg method (FDR-adjusted q-value of 0.05). Where appropriate, pair-wise comparisons were undertaken using Dunn’s (or Tukey’s) multiple comparison tests. Associations between variables are given as Spearman correlation coefficients. Analyses were undertaken using Prism (version 8) and RStudio (version 1.2.5033).
Results
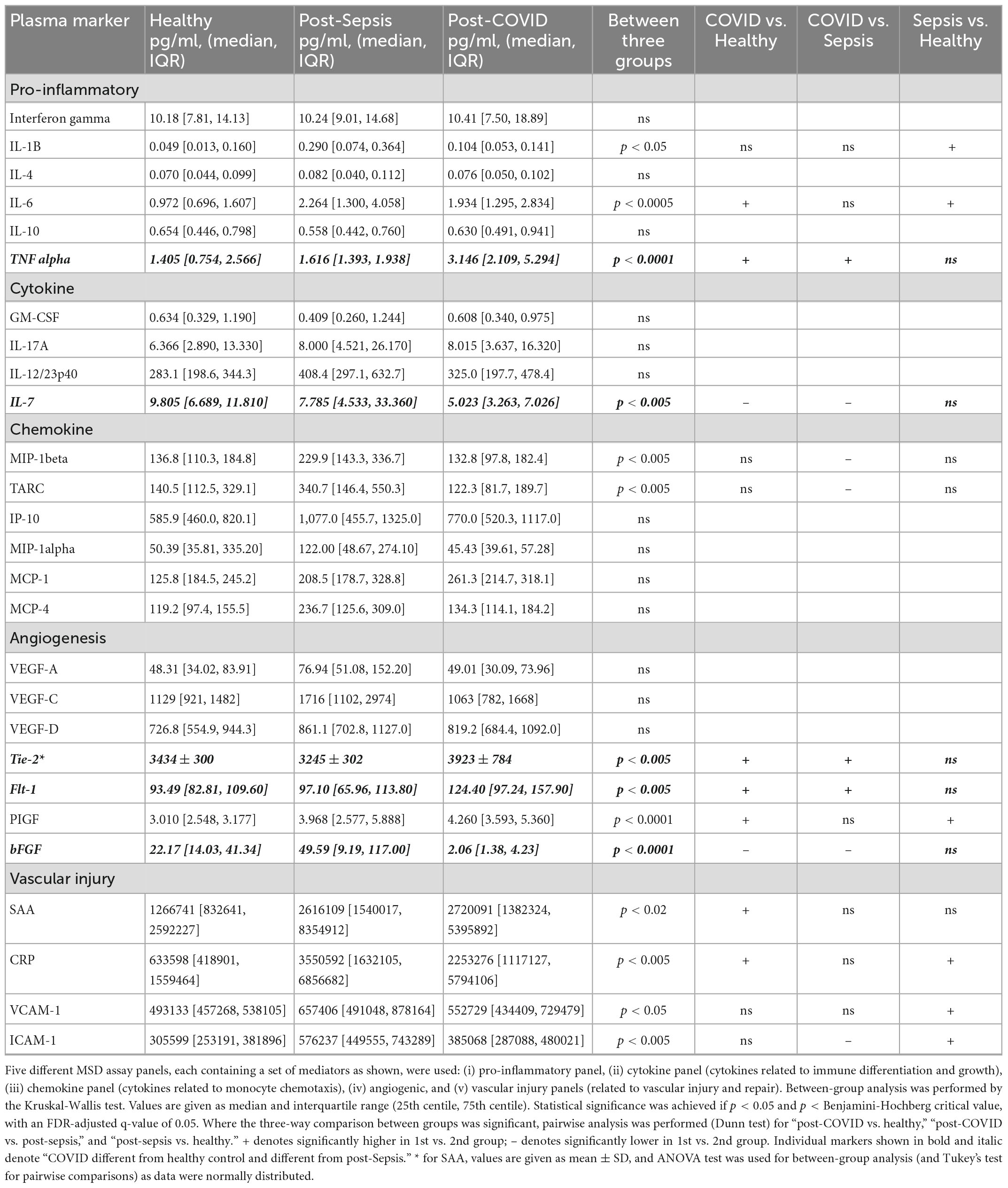
Table 1 summarizes the assay results for all the mediators tested in the three participant groups, showing a three-way comparison between groups.
In keeping with previous reports of abnormal cytokine profiles months after COVID-19 infection (11, 12, 14), we demonstrate persistent significant elevation of IL-6, TNFα, Tie-2, Flt-1, PIGF, SAA, and CRP in our post-COVID cohort, compared with healthy controls, and persistent significant suppression of IL-7 and bFGF, as shown in Figure 1.
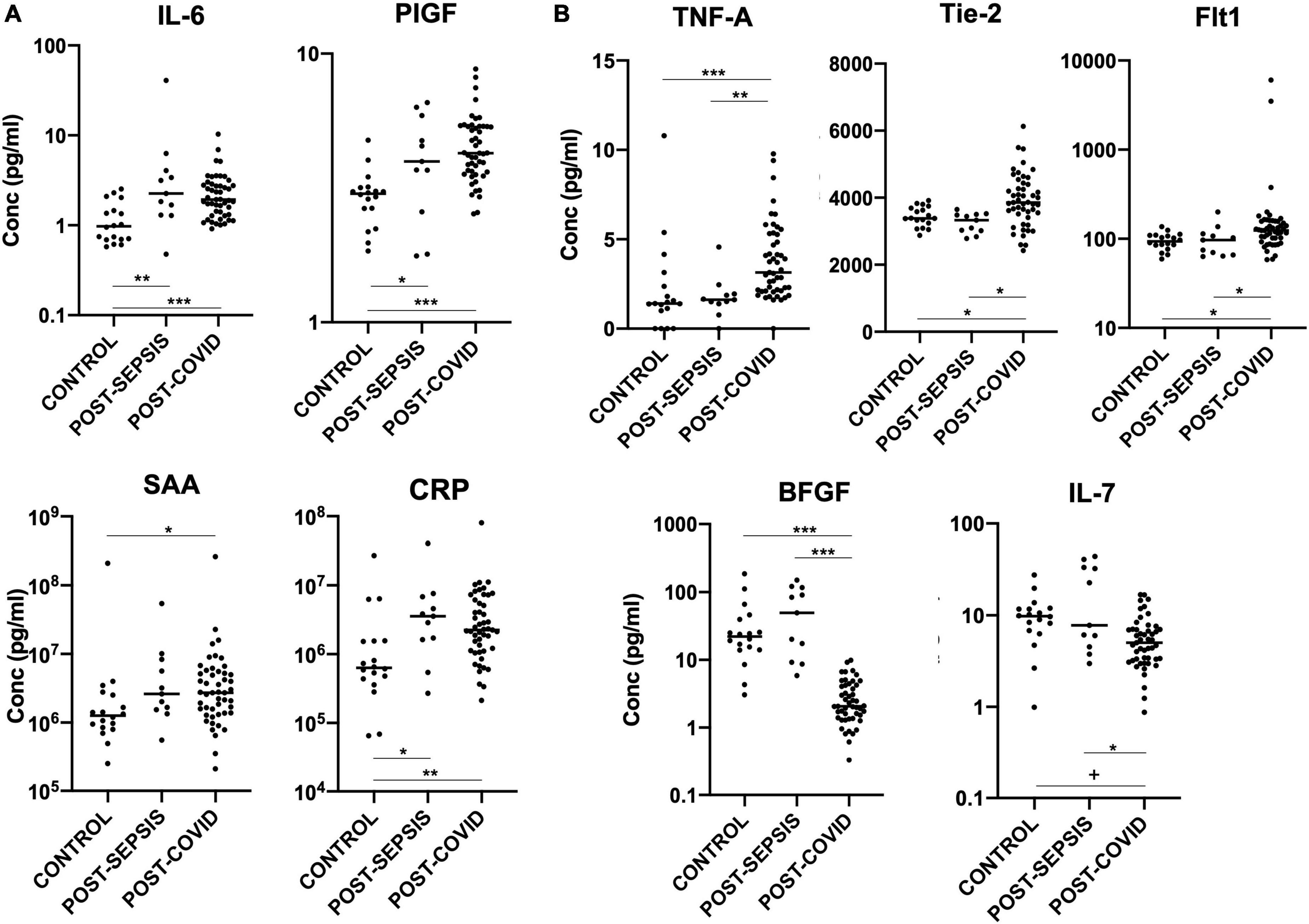
Figure 1. (A) Plasma markers elevated in both post-COVID and post-Sepsis groups, compared to healthy controls: IL-6, PIGF, SAA, and CRP. (B) Plasma markers uniquely different in post-COVID patients but not in post-Sepsis patients compared with healthy controls: TNFα, Tie-2, and Flt-1 are elevated while bFGF and IL-7 are depressed in post-COVID patients compared to healthy controls and compared to post-Sepsis patients. Horizontal bars indicate medians. +p < 0.01, *p < 0.05, **p < 0.005, ***p < 0.0005.
We are unable to determine whether these mediators are markers of previous disease in these patients or mediators of ongoing pathology. However, in a recent UK study of >2,000 patients, only 26% of patients felt fully recovered 5 months after COVID-19 infection, and IL-6 and CRP were among the cytokines persistently upregulated in those with more significant impairment (12). In our study, IL-6 and CRP levels in the post-COVID patients correlated positively with the thoracic computed tomography (CT) abnormality score (r = 0.28, p < 0.05 for IL-6, r = 0.46, p < 0.002 for CRP) and negatively with gas transfer factor (DLCO %predicted; r = –0.51, p < 0.002 for IL-6, r = –0.57, p < 0.0005 for CRP), which were performed at around the same time that blood was obtained for this study. Details of thoracic CT scores and lung function test results (DLCO and spirometry) are shown in Table 2. However, there was no correlation between breathlessness measured by the MRC dyspnea score (defined in Table 2) and levels of these or other measured mediators. Within the post-COVID group, we found a significant correlation between the severity of the acute illness (as defined in Table 2) and levels of TNFα (r = 0.30, p < 0.05).
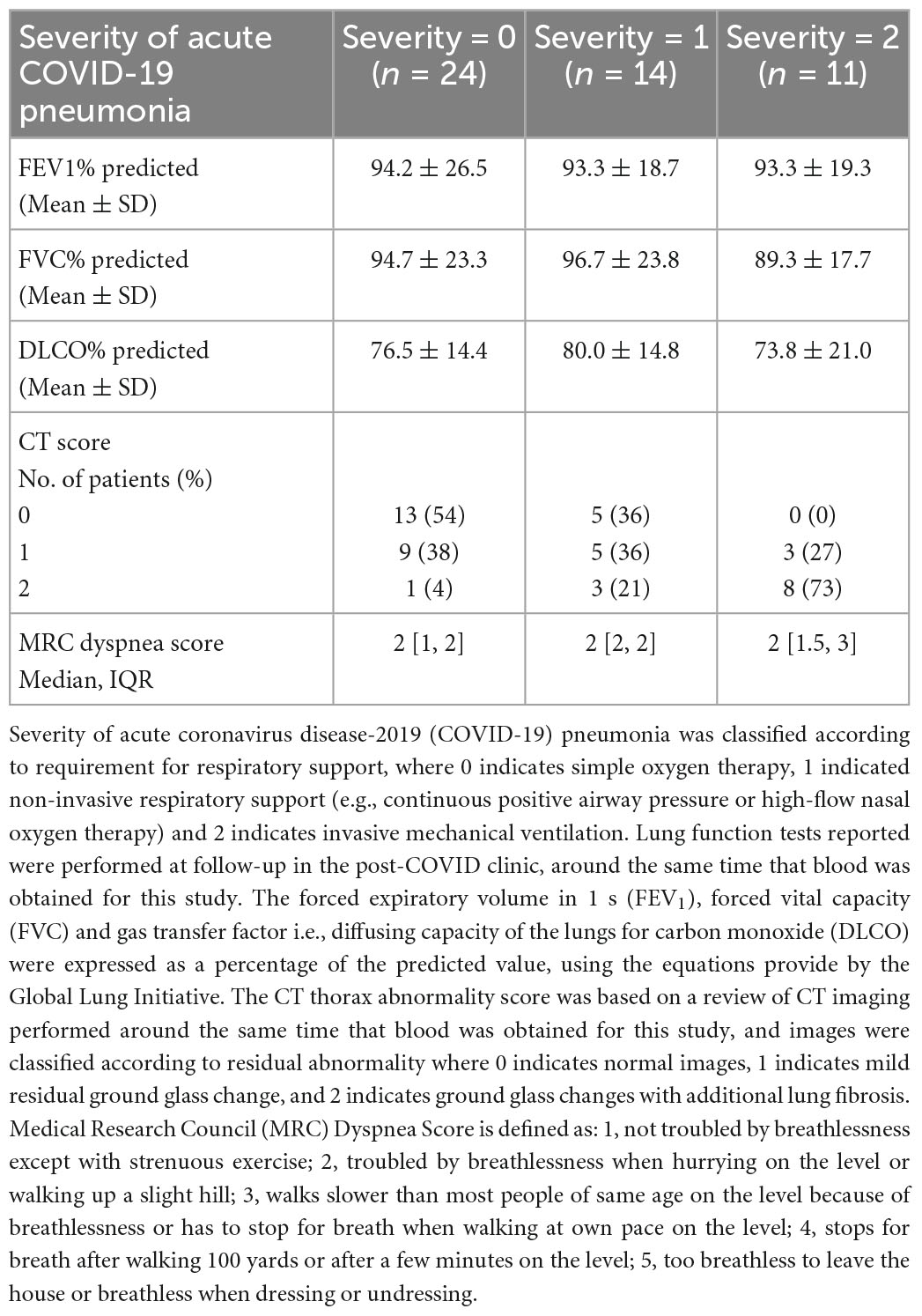
Table 2. Severity scores of acute coronavirus disease-2019 (COVID-19) pneumonia and details of follow-up thoracic computed tomography (CT) and lung function tests in the post-COVID patients.
Importantly, our study also significantly adds to previous findings by identifying mediators for which expression is persistently abnormal in patients recovering from COVID-19, but not in those recovering from another pathology characterized by acute inflammation, namely severe sepsis. This group of mediators, which therefore constitutes a specific post-infection inflammatory/vascular injury signature of COVID-19, include the angiogenic factors Tie-2, Flt-1, and bFGF, and the inflammatory markers IL-7 and TNFα, as shown in Figure 1 and Table 1.
Discussion
In this study we found persistent elevation of multiple mediators – IL-6, TNFα, SAA, Tie-2, Flt1, PIGF, and CRP – and persistent depression of IL-7 and bFGF in patients recovering from COVID-19 several months following their acute infection. Importantly, we also showed for the first time that persistent changes in Tie-2, Flt-1, bFGF, IL-7, and TNFα were uniquely seen in patients following recovery following COVID-19, but not in patients recovering from severe sepsis.
Our findings suggest that processes of endothelial injury and repair persist months after acute COVID-19 infection. The soluble angiopoietin 1 receptor (Tie-2), which has been previously reported increased following COVID-19 infection (11), is nearly exclusive to endothelial cells and has a critical role in antithrombotic signaling (15). Higher circulating levels of this receptor may reflect a homeostatic response to increased levels of its prothrombotic antagonist, angiopoietin-2 (15). Alternatively, it may reflect loss or cleavage of Tie-2 from the endothelial surface during ongoing inflammatory states. It could be a consequence of ongoing downregulation and shedding of angiotensin converting enzyme 2 (ACE2), the receptor for SARS-CoV-2, and a resulting accumulation of angiotensin 2 which has inflammatory and thrombotic effects when bound to the endothelial receptor AT1R (8, 16, 17). This final suggested mechanism may also explain our finding of increased levels of soluble vascular endothelial growth factor receptor (Flt1). Flt-1, an inhibitor of the vascular endothelial growth factor pathway which promotes endothelial dysfunction, has been shown to be increased acutely in COVID-19 (18) but its persistent elevation in the post-COVID setting is a novel finding of our study. The interaction between angiotensin 2 and the receptor AT1R has also been found to promote local Flt-1 upregulation in hypoxia (19). Therefore, dysregulation of the renin/angiotensin system is a potential unifying mechanism for our findings of increased Tie-2 and Flt-1 in post-COVID-19 patients. Unfortunately, we do not have data on tissue expression of ACE2, angiopoietin-2 or angiotensin 2 to explore this further.
Detrimental inflammatory and cytotoxic effects of angiotensin 2 binding to AT1R might also play a role in our finding of reduced levels of the basic fibroblastic growth factor (bFGF) in post-COVID-19 patients. BFGF is present in basement membranes, activated during wound healing, and has mitogenic effects on endothelial cells (20). Reduced levels of bFGF after COVID-19 could represent reduced production or increased consumption during healing from COVID-19 pneumonitis, or other endothelial injuries. This result, reported also by (11), shows an interesting contrast with the finding of elevated levels of bFGF in a large cohort of young adults with acute COVID who did not require hospitalization (21). It is possible that reduced levels of bFGF in our post-COVID-19 are a finding specific to more severe disease requiring hospitalization and post COVID-19 pneumonitis. It is noted, however, that no correlation is found here between reduced bFGF with raised inflammatory mediators IL-6 or CRP (which are associated with reduced gas transfer factor) or with TNF alpha (which correlates with severity of acute illness within our hospitalized cohort). Further exploration of the role and utility of bFGF in patients with post-COVID syndrome would be of interest.
We also found a persistent elevation of PIGF in post-COVID patients, which in the acute setting has been shown to correlate with in-hospital mortality (22), but did not see any differences (between COVID-19 patients and healthy controls) in levels of the vascular intercellular adhesion molecules ICAM-1 and VCAM-1 (important in inflammatory cell recruitment to the lung), nor in the endothelial growth factors VEGF-A, VEGF-C, or VEGF-D. The acute phase inflammatory proteins serum amyloid A and CRP are elevated in our post-COVID-19 cohort, in common with post-sepsis patients; but there are no differences in the levels of the leukocytic pyrogen and component of the inflammasome complex, IL-1β, from healthy control patients, in contrast with the findings of another study of patients reporting post-acute sequelae of COVID-19 (23).
The observed persistent suppression of IL-7 in our post-COVID cohort is also in keeping with previous reports (11), but contrasts the elevation of IL-7 in the acute setting (24, 25). IL-7 is critical for the development, maturation and survival of lymphocytes, and prevents memory cell apoptosis (26). In a recent study, recombinant IL-7 increased CD8+ and CD4+ T cell proliferation (ex vivo) in critically ill COVID-19 patients (27), and IL-7 has been suggested as immunotherapy and/or a vaccine adjuvant for COVID-19 (27–29). Reduced IL-7 after COVID-19 could reflect persistent lymphocyte exhaustion, contributing to inefficient viral clearance and chronic immune stimulation.
Tumor necrosis factor alpha, a pro-inflammatory cytokine, is elevated both in acute COVID-19 and acute sepsis and is linked to more severe disease and worse prognosis (4, 30, 31). In addition to its persistent elevation in our post-COVID patients, we identified a positive correlation between TNFα plasma levels after discharge and acute COVID-19 disease severity score (p < 0.05). No such correlation was identified for any other mediator in the current study. Others have found elevated TNFα in those with persistent symptoms many months after mild COVID-19 (14), and suggested it has a role in sustaining macrophage activation and cellular inflammation (14). These findings, as well the distinct TNFα elevation in post-COVID but not in post-Sepsis patients in our study, merit further study and consideration given the availability of established anti-TNF therapies.
Finally, of particular interest is the observation that elevation of IL-6 seen in our post-COVID cohort, and reported by others (11, 12, 14), is not specific to post-COVID but is also seen in a post-sepsis cohort. This has implications when considering anti-IL6 therapies in the post-COVID setting.
As noted above, a strength of our study is the control group consisting of patients recovering from sepsis, as well as a second group of healthy controls. Limitations include the relatively small numbers in the post-sepsis group, and the fact that patients were recruited early in the pandemic, prior to the emergence of later variants of the SARS-CoV-2 virus, such as the omicron variant. Notwithstanding these limitations, our key finding is that COVID-19 appears to be associated with a post-inflammatory signature that persists for at least 5 months, and that is distinct from the profile seen in patients recovering from sepsis. Understanding this signature may be important both for understanding the pathophysiology of the long-term effects of COVID-19, and for development or targeting of effective therapy. Further, more targeted study is required, for example in those who may suffer with prolonged symptoms (post-COVID syndrome), to understand the pathophysiological significance and potential clinical utility of theses uniquely persistent mediators.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the North West Preston (20/NW/0235) and Oxford C (19/SC/0296) Research Ethics Committees. The patients/participants provided their written informed consent to participate in this study.
Author contributions
BR, AM, NT, and NP conceived and designed the study. JM, AA, AM, AF, EF, AK, JK, and NP collected data. JM and NP performed experiments and analyzed data. JM, NT, and NP drafted the manuscript. All authors contributed to the interpretation of data, revision of the manuscript and approved the final version submitted for publication.
Funding
This study was funded by the University of Oxford’s COVID-19 Research Response Fund and the Oxford NIHR Biomedical Research Centre through a BRC pump-priming award. NP was funded by an OUH NHS Foundation Trust NIHR Research Capability Fund. AM was a NIHR Academic Clinical Lecturer. BR was funded by the British Heart Foundation Oxford Centre of Research Excellence (RE/18/3/34214). JK was funded by a Wellcome Trust Investigator Award (204969/Z/16/Z), Oxford NIHR Biomedical Research Centre, and CAMS IFMS (2018-I2M-2-002).
Acknowledgments
We thank the participants for taking part in the study. We thank the clinicians and nurses from the Emergency Medicine Research Oxford (EMROx) department, the Oxford Respiratory Trials Unit and the NIHR Clinical Research Network Thames Valley and South Midlands, who contributed to patient recruitment, sampling and clinical data collection, and to members of the COVID-19 Multi-omics Blood Atlas (COMBAT) Consortium who contributed to sample processing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol. (2021) 21:245–56. doi: 10.1038/s41577-021-00522-1
2. Tay M, Poh C, Renia L, MacAry P, Ng L. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
3. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. (2020) 130:2620–9. doi: 10.1172/JCI137244
4. Darif D, Hammi I, Kihel A, El Idrissi Saik I, Guessous F, Akarid K. The pro-inflammatory cytokines in COVID-19 pathogenesis: what goes wrong? Microb Pathog. (2021) 153:104799. doi: 10.1016/j.micpath.2021.104799
5. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
6. Group R. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. (2021) 397:1637–45.
7. Group R, Horby P, Lim W, Emberson J, Mafham M, Bell J, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. (2021) 384:693–704. doi: 10.1056/NEJMoa2021436
8. Bernard I, Limonta D, Mahal L, Hobman T. Endothelium infection and dysregulation by SARS-CoV-2: evidence and caveats in COVID-19. Viruses (2020) 13:29. doi: 10.3390/v13010029
9. Leisman D, Deutschman C, Legrand M. Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. (2020) 46:1105–8. doi: 10.1007/s00134-020-06059-6
10. Ackermann M, Verleden S, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. (2020) 383:120–8. doi: 10.1056/NEJMoa2015432
11. Zhou M, Yin Z, Xu J, Wang S, Liao T, Wang K, et al. Inflammatory profiles and clinical features of Coronavirus 2019 survivors 3 months after discharge in Wuhan, China. J Infect Dis. (2021) 224:1473–88. doi: 10.1093/infdis/jiab181
12. The Phosp-Covid Collaborative Group. Clinical characteristics with inflammation profiling of long COVID and association with 1-year recovery following hospitalisation in the UK: a prospective observational study. Lancet Respir Med. (2022) 10:761–75.
13. COvid-19 Multi-Omics Blood ATlas (COMBAT) Consortium. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. (2022) 185:916–938.e58.
14. Schultheiss C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes S, et al. The IL-1beta, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. (2022) 3:100663.
15. Schmaier A, Hurtado G, Manickas-Hill Z, Sack K, Chen S, Bhambhani V, et al. Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19. medRxiv [Preprint]. (2021). doi: 10.1101/2021.05.13.21257070
16. Osman I, Melenotte C, Brouqui P, Million M, Lagier J, Parola P, et al. Expression of ACE2, soluble ACE2, angiotensin I, angiotensin II and angiotensin-(1-7) is modulated in COVID-19 patients. Front Immunol. (2021) 12:625732. doi: 10.3389/fimmu.2021.625732
17. Zoufaly A, Poglitsch M, Aberle J, Hoepler W, Seitz T, Traugott M, et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir Med. (2020) 8:1154–8. doi: 10.1016/S2213-2600(20)30418-5
18. Dupont V, Kanagaratnam L, Goury A, Poitevin G, Bard M, Julien G, et al. Excess soluble fms-like tyrosine kinase 1 correlates with endothelial dysfunction and organ failure in critically Ill coronavirus disease 2019 patients. Clin Infect Dis. (2021) 72:1834–7. doi: 10.1093/cid/ciaa1007
19. Zhou C, Ahmad S, Mi T, Xia L, Abbasi S, Hewett P, et al. Angiotensin II induces soluble fms-Like tyrosine kinase-1 release via calcineurin signaling pathway in pregnancy. Circ Res. (2007) 100:88–95. doi: 10.1161/01.RES.0000254703.11154.18
20. Burgess W, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. (1989) 58:575–606. doi: 10.1146/annurev.bi.58.070189.003043
21. Lund Berven L, Selvakumar J, Havdal L, Stiansen-Sonerud T, Einvik G, Leegaard T, et al. Inflammatory markers, pulmonary function, and clinical symptoms in acute COVID-19 among non-hospitalized adolescents and young adults. Front Immunol. (2022) 13:837288. doi: 10.3389/fimmu.2022.837288
22. Smadja D, Philippe A, Bory O, Gendron N, Beauvais A, Gruest M, et al. Placental growth factor level in plasma predicts COVID-19 severity and in-hospital mortality. J Thromb Haemost. (2021) 19:1823–30. doi: 10.1111/jth.15339
23. Schultheiß C, Willscher E, Paschold L, Gottschick C, Klee B, Henkes S, et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep Med. (2022) 3:100663.
24. Chi Y, Ge Y, Wu B, Zhang W, Wu T, Wen T, et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J Infect Dis. (2020) 222:746–54. doi: 10.1093/infdis/jiaa363
25. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
26. Mackall C, Fry T, Gress R. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol. (2011) 11:330–42. doi: 10.1038/nri2970
27. Bidar F, Hamada S, Gossez M, Coudereau R, Lopez J, Cazalis M, et al. Recombinant human interleukin-7 reverses T cell exhaustion ex vivo in critically ill COVID-19 patients. Ann Intensive Care. (2022) 12:21. doi: 10.1186/s13613-022-00982-1
28. Bekele Y, Sui Y, Berzofsky J. IL-7 in SARS-CoV-2 infection and as a potential vaccine adjuvant. Front Immunol. (2021) 12:737406. doi: 10.3389/fimmu.2021.737406
29. Laterre P, Francois B, Collienne C, Hantson P, Jeannet R, Remy K, et al. Association of Interleukin 7 immunotherapy with lymphocyte counts among patients with severe Coronavirus Disease 2019 (COVID-19). JAMA Netw Open. (2020) 3:e2016485. doi: 10.1001/jamanetworkopen.2020.16485
30. Gogos C, Drosou E, Bassaris H, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. (2000) 181:176–80. doi: 10.1086/315214
Keywords: COVID-19, inflammation, cytokines, vascular injury, post-COVID
Citation: Melhorn J, Alamoudi A, Mentzer AJ, Fraser E, Fries A, Cassar MP, Kwok A, Knight JC, Raman B, Talbot NP and Petousi N (2023) Persistence of inflammatory and vascular mediators 5 months after hospitalization with COVID-19 infection. Front. Med. 10:1056506. doi: 10.3389/fmed.2023.1056506
Received: 08 November 2022; Accepted: 09 January 2023;
Published: 10 February 2023.
Edited by:
Luis Garcia De Guadiana-Romualdo, Santa Lucía University General Hospital, SpainReviewed by:
Guenter Weiss, Innsbruck Medical University, AustriaChristian Albert Devaux, Centre National de la Recherche Scientifique (CNRS), France
Copyright © 2023 Melhorn, Alamoudi, Mentzer, Fraser, Fries, Cassar, Kwok, Knight, Raman, Talbot and Petousi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nayia Petousi,  bmF5aWEucGV0b3VzaUBuZG0ub3guYWMudWs=
bmF5aWEucGV0b3VzaUBuZG0ub3guYWMudWs=
†These authors share senior authorship
 James Melhorn1,2
James Melhorn1,2 Asma Alamoudi
Asma Alamoudi Alexander J. Mentzer
Alexander J. Mentzer Mark Philip Cassar
Mark Philip Cassar Julian Charles Knight
Julian Charles Knight Nick P Talbot
Nick P Talbot Nayia Petousi
Nayia Petousi