
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 09 March 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1041505
This article is part of the Research TopicRecent Advances and New Biomarkers in Ulcerative ColitisView all 10 articles
 Maojuan Li1,2†
Maojuan Li1,2† Yan Tao1,2†
Yan Tao1,2† Yang Sun1,2
Yang Sun1,2 Jing Wu1,2
Jing Wu1,2 Fengrui Zhang1,2
Fengrui Zhang1,2 Yunling Wen1,2
Yunling Wen1,2 Min Gong1,2
Min Gong1,2 Jingxian Yan1,2
Jingxian Yan1,2 Hao Liang1,2
Hao Liang1,2 Xinyu Bai1,2
Xinyu Bai1,2 Junkun Niu1,2*‡
Junkun Niu1,2*‡ Yinglei Miao1,2*‡
Yinglei Miao1,2*‡Aim: This study aimed to determine the factors affecting the quality of life of patients with inflammatory bowel disease (IBD) and to construct a disease recurrence prediction model based on these influencing factors.
Methods: A prospective, single-center study in China was conducted between October 2020 and March 2021. The quality of life of patients was assessed using the Inflammatory Bowel Disease Questionnaire (IBDQ). Multiple stepwise regression analysis was used to analyze the factors influencing the quality of life of patients with IBD. The chi-square test and the point-biserial correlation analysis were performed to identify factors associated with clinical recurrence. A binary logistic regression model was constructed to predict the recurrence. The receiver operating characteristic curve was used to evaluate the prediction model. Patients with IBD from April 2021 to June 2021 were randomly included for model verification to evaluate the disease recurrence prediction model.
Results: The average IBDQ score of patients with IBD was 172.2 ± 35.0 (decreased by 23.2%). The scores of all dimensions of the IBDQ were decreased, especially emotional function and systemic symptoms. Disease activity, age, extraintestinal manifestations (EIMs), and annual household income were important factors influencing the IBDQ scores of patients with ulcerative colitis, and these accounted for ~57.0% of the factors affecting the quality of life. Disease activity, EIMs, and occupational stress were important factors influencing the IBDQ scores of patients with Crohn's disease, and they accounted for approximately 75.1% of the factors affecting the quality of life. Annual household income, occupational stress, and IBDQ scores were independent risk factors for recurrence. The area under the curve of the recurrence prediction model was 81.1%. The sensitivity and specificity were 81.7 and 71.7%, respectively. The Youden index of the model was 0.534. The established recurrence prediction model has good discriminant validity in the validation cohort.
Conclusion: The quality of life of patients with IBD was generally poor. The use of factors affecting the quality of life to predict disease recurrence has high predictive value and can support the management of IBD by selecting patients at a higher risk for relapse.
Inflammatory bowel disease (IBD) is a chronic inflammatory disorder of the gastrointestinal tract that is believed to be caused by an inappropriate inflammatory response to intestinal microbes in a genetically susceptible host. The most common forms of IBD include ulcerative colitis (UC) and Crohn's disease (CD) (1). IBD is a global disease, and its incidence and prevalence are still increasing worldwide (2, 3). The incidence of IBD in China is generally lower than that in developed countries, such as Western Europe and North America, but epidemiological studies show a significant increase in the incidence of IBD in China (4). The most common clinical symptoms of IBD are abdominal pain, diarrhea, and bloody stools, often accompanied by extraintestinal manifestations (EIMs). Some patients also suffer from complications such as abscesses, fistulas, and stenosis (5).
Due to its chronic, relapsing nature and therapeutic complexity, IBD requires long-term medical management and imposes significant costs on individuals and society. Several studies have reported that patients with IBD have lower employment rates and a higher percentage of work disability than the general population (6). With long-term medical care and productivity loss, the economic burden of patients with IBD is increasing (7). In addition, IBD has been shown to have a psychological impact on daily life, with a significant increase in the incidence of psychological disorders (8). This psychological load adds to the physical burden of the disease and is associated with direct and indirect costs (9). In short, the physical, economic, and psychological burden, as well as the progression of the disease, all affect the quality of life of patients (10, 11). A previous study revealed that one-third of patients with UC relapse within the first year of diagnosis and 70–82% of the patients relapse within 5 years. Additionally, 85% of patients with CD relapse at least one time within 5 years from diagnosis (10). Since patients with severe relapse need intensive treatment, which may accrue high costs and increase the risk of adverse events, detection of relapse and early therapeutic interventions before the severe progression of activity are desirable (12). The current treatment strategies for patients with IBD not only alleviate symptoms and reduce complications but also improve the quality of life and reduce recurrence rates (13).
In the past few years, more attention has been paid to the role of age, C-reactive protein (CRP), fecal calprotectin (FC), erythrocyte sedimentation rate (ESR), endoscopy, EIMs, diet, and pathological scores in the prediction of recurrence (14–17). However, the predictive values of these different parameters in identifying patients at risk of recurrence have been disappointing. Some studies indicated that standard laboratory parameters (e.g., CRP) did not prove to be useful predictors of clinical relapse in IBD as a whole (18, 19). In those studies, the predictive value of FC in patients with UC with clinical remission for relapse was not so prominent (AUC = 0.60–0.70) (20). The predictability of recurrence in CD has also been reported, and the ability to predict recurrence at 1 year was slightly higher than that in UC (AUC = 0.75–0.79) (21, 22). However, due to its relatively high cost, FC is not so frequently measured in patients. In addition, endoscopy for this purpose is sometimes invasive and burdensome for patients (12).
Therefore, prediction tools for disease recurrence need to be developed. The construction of a prediction model of recurrence based on the factors affecting the quality of life of patients with IBD is a new exploration. Based on data from an IBD center in Southwest China, we reported factors influencing the quality of life of patients with IBD and constructed a model to predict disease recurrence, which is of great value for understanding the characteristics of patients with IBD in China and improving their quality of life and outcomes.
This study was performed in the First Affiliated Hospital of Kunming Medical University, which is a tertiary hospital and an IBD center in Southwest China. We recruited patients with IBD who were hospitalized between October 2020 and March 2021 as a training cohort. Patients with IBD diagnosed from April 2021 to June 2021 were selected to establish a validation cohort. The inclusion criteria were as follows: (1) the diagnosis was confirmed according to the ECCO guidelines (23, 24) and (2) patients gave informed consent. The exclusion criteria were as follows: (1) patients with severe cognitive and mental disorders and (2) patients with other chronic serious diseases, such as heart, kidney, or liver failure, stroke, and serious lung disease. We followed the participants for 1 year and identified recurrence through telephone appointments and outpatient visits. The process for patient inclusion and exclusion is presented in Figure 1.
Demographics, including age, gender, nature of occupation, occupational stress, education level, marital status, family size, annual family income, and type of medical insurance, were obtained by a general information questionnaire. Clinical information, including diagnosis, disease activity, disease localization (the Montreal classification of IBD) (25), disease duration, EIMs (e.g., peripheral and axial arthritis, pyoderma gangrenosum, erythema nodosum, Sweet syndrome, aphthous stomatitis, primary sclerosing cholangitis and episcleritis, anterior uveitis, and iritis), complications (e.g., intestinal obstruction or perforation, massive hemorrhage of the gastrointestinal tract, colon cancer, or toxic megacolon), intestinal surgery, and biologics (e.g., infliximab, vedolizumab, adalimumab, and ustekinumab) were collected from medical records. The disease activity of UC was assessed using the Mayo scores (a Mayo score of ≤ 2 with no single item score of >1 was classified as clinical remission, ranging between 3 and 5 as mild activity, ranging between 6 and 10 as moderate activity, and ranging between 11 and 12 as severe activity) (26). Clinical relapse was defined as the occurrence of symptoms accompanied by an increase in the partial Mayo score of 2 or more, which required a change in therapy, such as the escalation of ongoing therapy with the introduction of steroids and/or immunosuppressive biological drugs (27). The Crohn's disease activity index (CDAI) was used to assess disease activity in CD (CDAI < 150 was classified as remission, 150 to 220 as mild activity, 221 to 450 as moderate activity, and >450 as severe activity) (28). Recurrence was defined as a CDAI of more than 220, an increase of at least 70 from the baseline between 150 and 220, or the need for a surgical procedure (29).
The quality of life of the patients was assessed using the Chinese translation of an Italian version of the Inflammatory Bowel Disease Questionnaire (IBDQ) (30), which is a widely recognized disease-specific questionnaire used to measure the quality of life. The IBDQ consists of 32 questions and four subscales, namely, bowel symptoms (e.g., bloody stools, abdominal pain), systemic symptoms (e.g., fatigue, sleep problems), emotional function (e.g., anxiety, anger, and depression), and social function (e.g., limited social activities, school, or work attendance). The score of each question ranges from 1 point (worst condition) to 7 points (best condition), with a total score ranging from 32 to 224, with a higher score indicating a better quality of life. All patients were asked to complete the IBDQ.
Data were analyzed using the Statistical Package for the Social Sciences, version 26.0 for Mac (IBM SPSS Statistics 26). Continuous variables were presented as the mean ± standard deviation (SD), and the counting data were expressed as frequencies and percentages. The Mann–Whitney U-tests were used to compare the mean values between the two groups. The mean values of multiple groups were compared using a one-way analysis of variance. Factors affecting the quality of life were analyzed by multiple linear stepwise regression. The chi-square test and point-biserial correlation analysis were used to screen relapse-related factors, and binary logistic regression analysis was used to construct predictive models of relapse. To assess the performance of the resulting predictions, receiver operating characteristic (ROC) curves were plotted to calculate the area under the curve (AUC). A P-value of < 0.05 was considered statistically significant.
A total of 212 questionnaires were distributed in the training cohort. Questionnaires were considered invalid if any question was unanswered. Finally, a total of 191 valid questionnaires were recovered, yielding a response rate of 90.1% (191/212). UC (132, 69.1%) was the most common type, followed by CD (55, 28.8%) and IBD-U (4, 2.1%). We only analyzed the data of patients with UC and CD to explore factors affecting the quality of life and recurrence. The demographic and clinical characteristics of patients are shown in Table 1. Among the patients with UC, 80 of them (60.6%) were men. The average age was 43.7 ± 12.8 years. The median duration of the disease was 6 years. EIMs occurred in 58 (43.9%) patients and 17 (12.9%) patients had complications. Among the patients with CD, 38 of them (69.1%) were men. The average age was 36.0 ± 13.5 years. The median duration of the disease was 7 years. A total of 24 (43.6%) patients had EIMs. Complications occurred in 24 (43.6%) patients.
In the training cohort (Table 2), 41 (31.1%) patients with UC were engaged in manual labor and 19 (14.4%) patients with UC felt that their occupation was stressful. The annual household income of 25 patients (18.9%) was < RMB¥ 10,000. Among the patients with CD, the occupation of 19 (34.5%) patients was manual labor, and 11 (20.0%) patients felt that their occupation was stressful. The annual household income of 11 patients (20.0%) with CD was < RMB¥ 10,000.
We used the IBDQ to investigate the quality of life of patients with IBD (Table 3). The results showed that all dimensions of the IBDQ were decreased, with the total scores reduced by 23.2%. The score of systemic symptoms decreased the most. There were no significant differences in the score of intestinal symptoms, systemic symptoms, emotional functioning, and social functioning between UC and CD patients. It showed that the quality of life of patients with IBD was decreased but was not affected by the type of disease.
This study analyzed the factors influencing the quality of life of patients with UC and CD (Table 4). The related factors with a P-value of < 0.1 in univariate analysis were included in the multiple regression analyses. Disease activity, age, EIMs, and annual household income were the factors that affected the total score of the IBDQ in patients with UC. Several factors affect intestinal symptoms, systemic symptoms, emotional function, and social function in patients with UC to varying degrees. Disease activity, EIMs, and occupational stress were the factors affecting the total scores and scores of all four dimensions of the IBDQ in patients with CD.
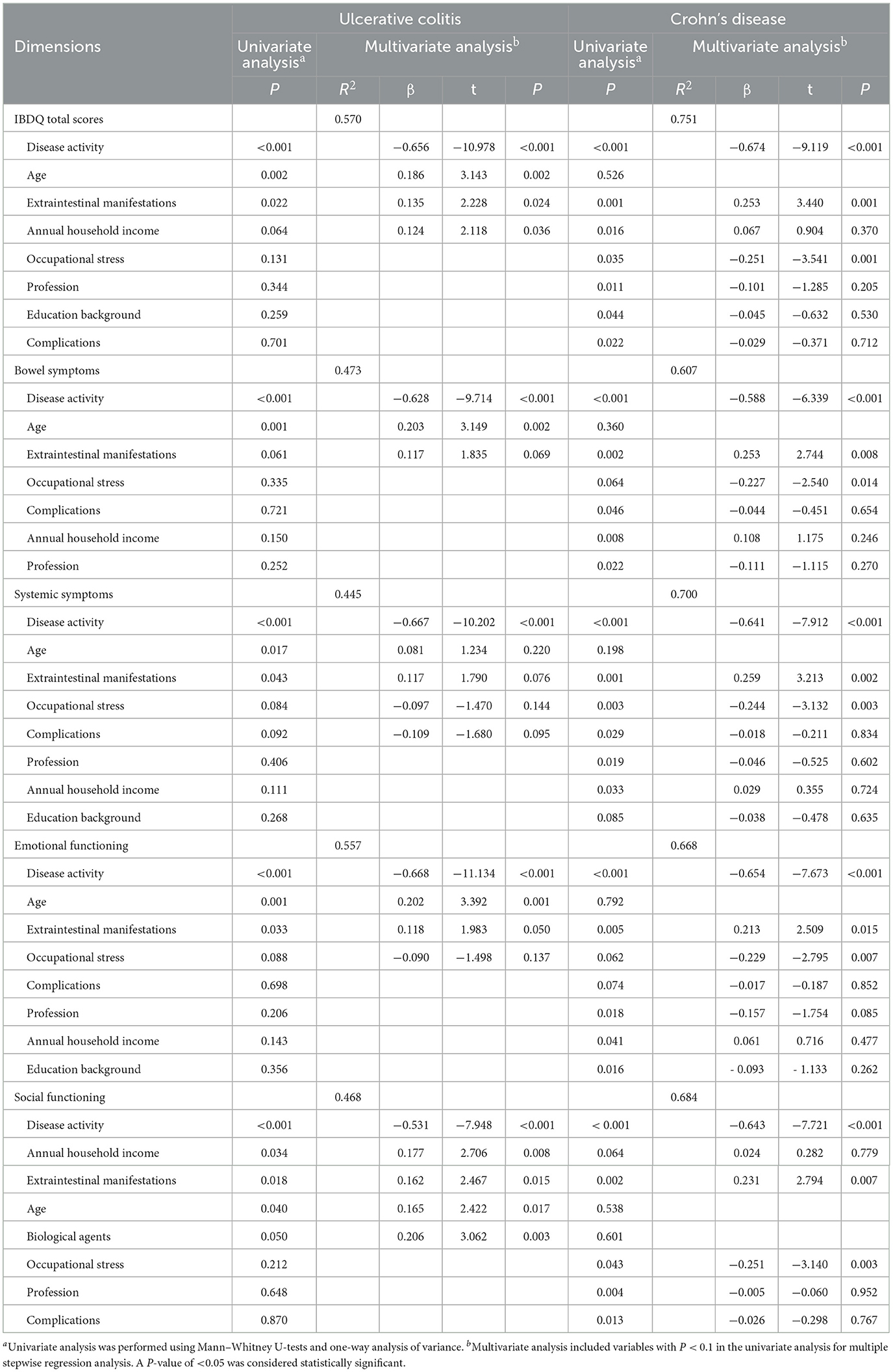
Table 4. Univariate analysis and multivariate stepwise regression analysis of factors affecting the quality of life of patients with ulcerative colitis and Crohn's disease.
To analyze the factors associated with the recurrence of IBD in patients and construct a prediction model for disease recurrence, 191 patients with IBD in the training cohort were followed up for 1 year. In this period, these participants included 82 patients with recurrence, 99 patients without recurrence, and 10 (5.2%) patients lost to follow-up. The chi-square test and point-biserial correlation analysis were used to screen relapse-related factors. We included age, EIMs, profession, biological agents, complications, disease activity, annual household income, occupational stress, and IBDQ scores for analysis. Finally, several potential predictors for relapse were identified in our cohort. Disease activity, annual household income, occupational stress, and IBDQ scores were found to be associated with short-term recurrence in patients with IBD (Table 5).
The relapse-related factors were included in the binary logistic regression analysis. The Hosmer–Lemeshow test was used to test the model fit and goodness, and the results showed that the model fit and goodness were good (P = 0.529). The linear predictor was 5.036 + (-0.026) × IBDQ + 1.976 × annual household income (RMB¥ 10,000 or less) + 0.898 × annual household income (RMB¥ 10,000 50,000) + (-2.067) × occupational stress (relaxed) + (-1.609) × occupational stress (standard) (Table 6). Predicted probabilities were calculated using the linear predictor in the formula: 1/(1+e−linear predictor). When the P-value was >0.5, the patient was predicted to relapse. In this study, the model correctly predicted 72.9% of patient outcomes. The results revealed that 79.8% of the patients without recurrence predicted by the model did not have a recurrence and that 64.6% of the patients with recurrence predicted by the model had a recurrence.
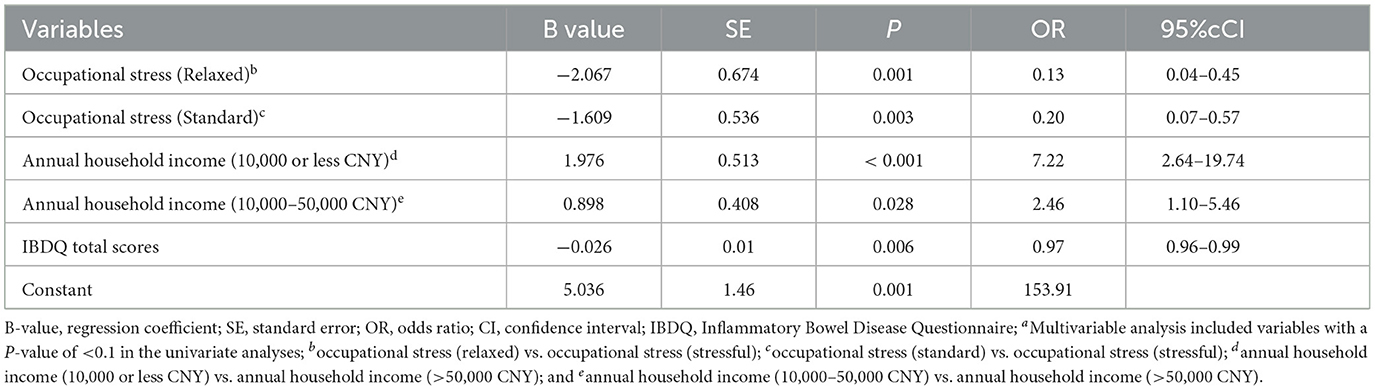
Table 6. Associations between influencing factors and relapse of inflammatory bowel disease using binary logistic regressiona.
The discriminant ability was evaluated according to the AUC (Figure 2; Table 7). The results indicated that the predictive effect of the model was better than that of the independent predictors, and the predictive effect of the IBDQ scores on recurrence was better than that of annual household income and occupational stress. The AUC of the prediction model of this study was 81.1%, and the 95% confidence interval (CI) was 74.8–87.5%. The maximum value of the Youden index (a measure of the authenticity of a screening test, also known as the correctness index) was the best critical value of the prediction model. The Youden index of this study was 0.534. The sensitivity and specificity were 81.7 and 71.7%, respectively. The model could be considered to have a good discriminant effect.
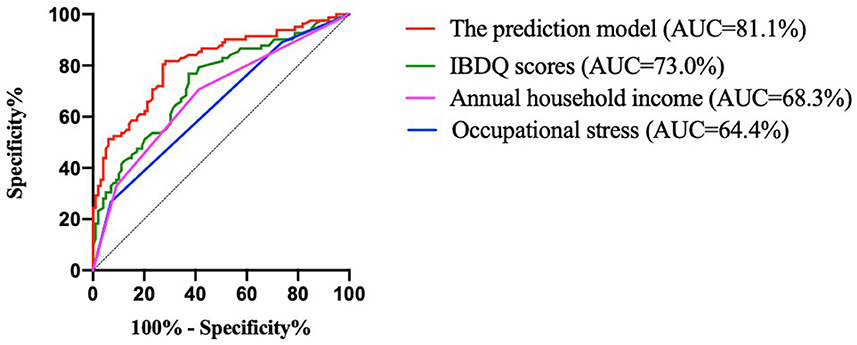
Figure 2. ROC curves for the developed model for predicting recurrence and variables. IBDQ, Inflammatory Bowel Disease Questionnaire; and AUC, area under the curve.
Another 100 patients with IBD diagnosed in the hospital from April 2021 to June 2021 were selected to establish a validation group. The demographic, clinical, and general characteristics of the patients in the validation cohort are shown in Tables 1, 2. Among these, 38 patients had recurrence within 1 year of follow-up and 62 patients had no recurrence. The successful modeling formula from the first stage was used to predict recurrence, with a sensitivity of 81.6%, a specificity of 83.9%, and an accuracy of 85.0%. The positive predictive value was 81.6%. The negative predictive value was 87.1%. The false-negative rate was 13.7%, and the false-positive rate was 20.5%. The ROC curve was drawn by the same method. The results revealed that the AUC was 87.4%, and the 95% CI was 79.9–94.8%. The Youden index was 0.655 (Figure 3). This indicated that the established recurrence prediction model also had good discriminant validity in the validation cohort.
Inflammatory bowel disease (IBD) is comprehensively affected by environmental factors, individual genotypes, intestinal microecology, autoimmunity, and other factors. Prognosis and recurrence prediction are of great significance for disease control. In recent years, the quality of life and social psychology of patients with IBD has drawn extensive concern. Due to the differences in clinical manifestations, treatment options, economic situation, cultural backgrounds, and lifestyles of patients with IBD, studies from other countries cannot reflect the quality of life and short-term recurrence of patients with IBD in China. In this study, patients with IBD in a tertiary hospital in Southwest China were selected as the research subjects. The results showed that the quality of life of patients with IBD was generally poor. Moreover, a prediction model of disease recurrence based on factors affecting the quality of life had a high predictive value. This study is of great significance for understanding the characteristics and quality of life of patients with IBD in China. The model established in this study can effectively guide treatment monitoring and follow-up.
The chronic progressive course, the side effects associated with medications, and the increasing financial burden reduce the quality of life of patients with IBD. A recent systematic review found that the quality of life of patients with IBD is worse than that of healthy people (31). We also found that patients with IBD had decreased quality of life in all dimensions, especially emotional functioning and systemic symptoms. This suggests that the therapeutic goals of IBD are not only mucosal healing but also an improved quality of life. Traditionally, patients with CD were believed to have a poorer quality of life than patients with UC due to worse disease behavior (32–34). Our study did not show a statistical difference between the two conditions. This may be because frequent diarrhea and bloody stool in patients with UC lead to lower intestinal symptom scores, and these symptoms are more likely to affect the physical health and social activities of the patients. In addition, patients with CD had a higher proportion of biologics than patients with UC in our study. Although our data revealed no significant association between biologics and the quality of life, Zhang et al. (35) found that the use of biologics had a positive effect on the quality of life of patients with CD. The differences may be due to the heterogeneity of patients and studies. Most patients treated with biologics have severe or complex conditions, and their emotional experiences may be completely different in the early stages of treatment and the period after disease control. This suggests that both UC and CD seriously affect the quality of life. Multivariate analysis showed that the independent factors influencing the quality of life of patients with UC were disease activity, annual household income, EIMs, and age, which explained ~57.0% of the quality of life score. However, disease activity, occupational stress, and EIMs were independent factors influencing the quality of life of patients with CD, accounting for ~75.1%.
In our study, disease activity affecting all dimensions of the IBDQ was an independent predictor of poor IBDQ scores, and the amount of explanation reached 50.7% (UC) and 62.3% (CD), respectively. This finding was consistent with other studies (36, 37). Zhao et al. (38) found that high disease activity scores were associated with the early recurrence of IBD after fecal bacteria transplantation. Our data also confirmed that disease activity was associated with the recurrence of IBD. Patients in remission or relapse experience different levels of anxiety, depression, sleep problems, and stress (39). Effective treatment and psychological intervention can reduce disability and improve the quality of life of patients with IBD (40). The reported prevalence of EIMs ranges from 6 to 47% (41). These EIMs regularly result in significant morbidity in patients with IBD, even more so than the intestinal disease itself (42). We found that EIMs simultaneously affected the four dimensions of the quality of life of patients with CD and affected the total IBDQ scores by reducing the social functioning scores in patients with UC. Ott et al. (43) also found that EIMs significantly influence the quality of life. Multidisciplinary management of IBD and EIMs can simultaneously improve outcomes and the quality of life (44). Therefore, disease activity and EIMs should be assessed in patients with IBD on a regular basis as prevention and/or specific treatment can have a major benefit on patients' quality of life and outcomes. Although it was recognized that elderly patients have poorer physiological conditions and higher treatment risks, univariate analysis reported that age could affect several dimensions of the IBDQ in patients with UC, and it was confirmed that age could affect the total IBDQ score in multivariate analysis. Advanced age did positively impact the quality of life of patients with UC, but it was not evident in CD. Perera et al. also confirmed that advanced age did not have a negative impact on the health-related quality of life of patients with IBD (45). The elderly population may receive more social and family support to cope with the condition. We found that low annual household income reduced total IBDQ scores in patients with UC. Two studies, by Liu et al. (46) and Yoon et al. (47), found that annual income was an independent predictor of reduced quality of life of patients with IBD. In addition, low annual household income was found to be a risk factor for disease recurrence in our predictive model. This may be because patients with low income experienced more stress and had fewer treatment options available. Patients with IBD are often impaired in their ability to be employed due to their morbidity. A study from Germany showed that more than half of the patients with IBD had a negative subjective prognosis for employment and experienced daily work-related problems, including reduced work ability, fear of not being fully productive, and work stress (48). Occupational stress was associated with the total IBDQ scores reflecting a decrease in all dimensions in patients with CD based on our results. Occupational stress was an independent risk factor for disease recurrence. Compared to patients with high occupational stress, patients with the standard [OR 0.20 (0.07–0.57)] and relaxed [OR 0.13 (0.04–0.45)] occupational stress had a lower risk of recurrence. For this complex condition, rehabilitation programs and support services that meet patients' needs should be implemented for work-related problems.
At least ~10–50% of patients with CD undergo one or more surgical procedures during their lifetime and ~5–10% of patients with UC require surgery within 5 years (10, 49). Studies suggest that surgery and the use of biologics were associated with the quality of life of patients with IBD (47, 50). Another study found that lower education levels and socioeconomic levels were related factors for the poor quality of life of patients with IBD (51). Our multivariate analysis did not show that intestinal surgery or biologics were independent predictors of the quality of life. This can be explained by the low rate of surgery and the use of biologics. In univariate analysis, we found that complications, profession, and educational background could affect several dimensions of scores of the IBDQ, but there was no statistical significance in multivariate analysis. Several studies have reported a significant correlation between the quality of life and the duration of disease, indicating better quality of life with a greater duration of IBD (50, 52, 53). Knowles et al. argued that, although symptoms persisted, patients no longer reacted negatively to them, viewing this condition as the new normal (54). This change reflects a process of self-adaptation to the chronic condition (55). Our results did not indicate a relationship between the duration of diseases and the quality of life. This may be because the time of our study was concentrated, and long-term longitudinal changes in patients were not visible.
Previous studies rarely considered the impact of disease burden on the recurrence of IBD. In this study, binary logistic regression analysis indicated that the quality of life, occupational stress, and annual household income were independent risk factors for recurrence, among which the quality of life was the most significant factor affecting recurrence. The higher the IBDQ scores, the lower the recurrence probability. Previous studies suggested that relapse-prone patients had a lower quality of life than patients in long-term remission (56, 57). This study found that patients with poor quality of life were more likely to relapse in the short term. These psychological factors will not trigger IBD, but they may have a negative effect on the progression and recurrence of the disease (58, 59). Patients with IBD with lower quality of life and higher occupational stress may be more likely to experience more anxiety, depression, sleep problems, and stress, and thus be more likely to relapse. Studies revealed that the medication compliance of patients with IBD is generally poor, ranging from 25.0 to 40.9%. Poor treatment compliance was closely related to recurrence (60, 61). Low annual household income may be a key factor affecting the treatment compliance of patients, resulting in a relatively high recurrence rate in the short term. The prediction model established by the risk factors affecting the quality of life had a good discriminative effect and could effectively screen for a population at high risk of recurrence. Our study analyzed the four dimensions of the IBDQ and found that they were affected by different factors. Compared with CRP, FC, ESR, endoscopy, or pathology scores, the model established based on the factors influencing the quality of life in this study is more practical, non-invasive, and cost-effective. Clinicians should pay more attention to the quality of life, occupational stress, and low income of patients with IBD. Early detection of patients at high risk of recurrence, as well as enhanced education and follow-up, will have a positive effect on disease control, show an improvement in the quality of life, and lead to a reduction of recurrence in patients with IBD.
This study has several limitations. The long-term quality of life and recurrence rate were not available during the observation period of this study. Due to the differences in socioeconomic factors, the cutoff values of factors affecting the quality of life, such as income, need to be adjusted. In addition, questionnaire participants in this study were mainly young and middle-aged, with the elderly and children being underrepresented. To reduce the selection bias, the study randomly investigated hospitalized patients with IBD at an IBD center and a tertiary hospital in Southwest China. Therefore, the results of the study are still reliable.
In conclusion, quality of life and recurrence rate are prognostic indicators of patients with IBD. The prediction model of recurrence based on factors affecting the quality of life can support the management of IBD by selecting patients at a higher risk of relapse. Interventions targeting the four dimensions of quality of life can be beneficial in reducing relapse and improving the quality of life of patients with IBD. Subsequently, longitudinal studies on the quality of life throughout treatment and after interventions can provide effective social support approaches and optimal management strategies for patients with IBD.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The study involving human participants was approved by the Ethics Committee of Kunming Medical University, Kunming, Yunnan province, China (the approval number: KMMU20192032). Informed consent was obtained from all the included patients.
JN, YM, ML, and YT contributed to the study design. ML, YT, YS, JW, FZ, YW, MG, JY, HL, and XB wrote and revised the manuscript. JN and YM reviewed the manuscript. All the authors have read and approved the final version of the manuscript.
This study was supported by the Natural Science Foundation of China (U1802282, 81960108, 82170550, and 82160107); Yunnan Health Training Project of High Level Talents (H-2019050); Yunnan Ten Thousand Talents Plan Young and Elite Talents Project; Applied Basic Research Projects of Yunnan Province (202201AY070001-086).
We acknowledge the staff who have contributed to this study and thank all funding.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Park J, Park S, Lee SA, Park SJ, Cheon JH. Improving the care of inflammatory bowel disease (IBD) patients: perspectives and strategies for IBD center management. Korean J Intern Med. (2021) 36:1040–8. doi: 10.3904/kjim.2021.114
2. Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology. (2019) 156:1345–53.e4. doi: 10.1053/j.gastro.2019.01.002
3. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. (2017) 390:2769–78. doi: 10.1016/S0140-6736(17)32448-0
4. Cui G, Yuan A. A systematic review of epidemiology and risk factors associated with Chinese inflammatory bowel disease. Front Med. (2018) 5:183. doi: 10.3389/fmed.2018.00183
5. Guo BJ, Bian ZX, Qiu HC, Wang YT, Wang Y. Biological and clinical implications of herbal medicine and natural products for the treatment of inflammatory bowel disease. Ann Ny Acad Sci. (2017) 1401:37–48. doi: 10.1111/nyas.13414
6. Netjes JE, Rijken M. Labor participation among patients with inflammatory bowel disease. Inflamm Bowel Dis. (2013) 19:81–91. doi: 10.1002/ibd.22921
7. Kawalec P. Indirect costs of inflammatory bowel diseases: crohn's disease and ulcerative colitis. a systematic review. Arch Med Sci. (2016) 12:295–302. doi: 10.5114/aoms.2016.59254
8. Lo B, Julsgaard M, Vester-Andersen MK, Vind I, Burisch J. Disease activity, steroid use and extraintestinal manifestation are associated with increased disability in patients with inflammatory bowel disease using the inflammatory bowel disease disability index: a cross-sectional multicentre cohort study. Eur J Gastroen Hepat. (2018) 30:1130–6. doi: 10.1097/MEG.0000000000001199
9. Marrie RA, Graff LA, Fisk JD, Patten SB, Bernstein CN. The relationship between symptoms of depression and anxiety and disease activity in IBD over time. Inflamm Bowel Dis. (2021) 27:1285–93. doi: 10.1093/ibd/izaa349
10. Zhao M, Gönczi L, Lakatos PL, Burisch J. The burden of inflammatory bowel disease in Europe in 2020. J Crohns Colitis. (2021) 15:1573–87. doi: 10.1093/ecco-jcc/jjab029
11. Aladraj H, Abdulla M, Guraya SY, Guraya SS. Health-related quality of life of patients treated with biological agents and new small-molecule drugs for moderate to severe crohn's disease: a systematic review. J Clin Med. (2022) 11:3743. doi: 10.3390/jcm11133743
12. Kato J, Yoshida T, Hiraoka S. Prediction of treatment outcome and relapse in inflammatory bowel disease. Expert Rev Clin Immu. (2019) 15:667–77. doi: 10.1080/1744666X.2019.1593140
13. Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. (2021) 160:1570–83. doi: 10.1053/j.gastro.2020.12.031
14. Fukuda T, Naganuma M, Sugimoto S, Nanki K, Mizuno S, Mutaguchi M, et al. The risk factor of clinical relapse in ulcerative colitis patients with low dose 5-aminosalicylic acid as maintenance therapy: a report from the IBD registry. PLoS ONE. (2017) 12:e0187737. doi: 10.1371/journal.pone.0187737
15. Bots SJ, Kuin S, Ponsioen CY, Gecse KB, Duijvestein M, D'Haens GR, et al. Relapse rates and predictors for relapse in a real-life cohort of IBD patients after discontinuation of anti-TNF therapy. Scand J Gastroentero. (2019) 54:281–8. doi: 10.1080/00365521.2019.1582693
16. Connelly TM, Messaris E. Predictors of recurrence of Crohn's disease after ileocolectomy: a review. World J Gastroentero. (2014) 20:14393–406. doi: 10.3748/wjg.v20.i39.14393
17. Liverani E, Scaioli E, Digby RJ, Bellanova M, Belluzzi A. How to predict clinical relapse in inflammatory bowel disease patients. World J Gastroentero. (2016) 22:1017–33. doi: 10.3748/wjg.v22.i3.1017
18. Langhorst J, Boone J, Lauche R, Rueffer A, Dobos G. Faecal lactoferrin, calprotectin, PMN-elastase, CRP, and white blood cell count as indicators for mucosal healing and clinical course of disease in patients with mild to moderate ulcerative colitis: post hoc analysis of a prospective clinical trial. J Crohns Colitis. (2016) 10:786–94. doi: 10.1093/ecco-jcc/jjw044
19. Däbritz J, Langhorst J, Lügering A, Heidemann J, Mohr M, Wittkowski H, et al. Improving relapse prediction in inflammatory bowel disease by neutrophil-derived S100A12. Inflamm Bowel Dis. (2013) 19:1130–8. doi: 10.1097/MIB.0b013e318280b1cd
20. De Vos M, Louis EJ, Jahnsen J, Vandervoort JG, Noman M, Dewit O, et al. Consecutive fecal calprotectin measurements to predict relapse in patients with ulcerative colitis receiving infliximab maintenance therapy. Inflamm Bowel Dis. (2013) 19:2111–7. doi: 10.1097/MIB.0b013e31829b2a37
21. Naismith GD, Smith LA, Barry SJ, Munro JI, Laird S, Rankin K, et al. A prospective evaluation of the predictive value of faecal calprotectin in quiescent Crohn's disease. J Crohns Colitis. (2014) 8:1022–9. doi: 10.1016/j.crohns.2014.01.029
22. García-Sánchez V, Iglesias-Flores E, González R, Gisbert JP, Gallardo-Valverde JM, González-Galilea A, et al. Does fecal calprotectin predict relapse in patients with Crohn's disease and ulcerative colitis? J Crohns Colitis. (2010) 4:144–52. doi: 10.1016/j.crohns.2009.09.008
23. Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. (2017) 11:649–70. doi: 10.1093/ecco-jcc/jjx008
24. Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European evidence-based consensus on the diagnosis and management of crohn's disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. (2017) 11:3–25. doi: 10.1093/ecco-jcc/jjw168
25. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. (2006) 55:749–53. doi: 10.1136/gut.2005.082909
26. D'Haens G, Sandborn WJ, Feagan BG, Geboes K, Hanauer SB, Irvine EJ, et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology. (2007) 132:763–86. doi: 10.1053/j.gastro.2006.12.038
27. Scaioli E, Sartini A, Bellanova M, Campieri M, Festi D, Bazzoli F, et al. Eicosapentaenoic acid reduces fecal levels of calprotectin and prevents relapse in patients with ulcerative colitis. Clin Gastroenterol Heptol. (2018) 16:1268–75.e2. doi: 10.1016/j.cgh.2018.01.036
28. Best WR, Becktel JM, Singleton JW, Kern F. Development of a Crohn's disease activity index. national cooperative crohn's disease study. Gastroenterology. (1976) 70:439–44. doi: 10.1016/S0016-5085(76)80163-1
29. Bourreille A, Cadiot G, Le Dreau G, Laharie D, Beaugerie L, Dupas JL, et al. Saccharomyces boulardii does not prevent relapse of Crohn's disease. Clin Gastroenterol H. (2013) 11:982–7. doi: 10.1016/j.cgh.2013.02.021
30. Ciccocioppo R, Klersy C, Russo ML, Valli M, Boccaccio V, Imbesi V, et al. Validation of the Italian translation of the inflammatory bowel disease questionnaire. Dig Liver Dis. (2011) 43:535–41. doi: 10.1016/j.dld.2010.12.014
31. Knowles SR, Graff LA, Wilding H, Hewitt C, Keefer L, Mikocka-Walus A. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part I. Inflamm Bowel Dis. (2018) 24:742–51. doi: 10.1093/ibd/izx100
32. Marinelli C, Savarino E, Inferrera M, Lorenzon G, Rigo A, Ghisa M, et al. Factors influencing disability and quality of life during treatment: a cross-sectional study on IBD patients. Gastroent Res Pract. (2019) 2019:5354320. doi: 10.1155/2019/5354320
33. Casellas F, López-Vivancos J, Casado A, Malagelada JR. Factors affecting health related quality of life of patients with inflammatory bowel disease. Qual Life Res. (2002) 11:775–81. doi: 10.1023/A:1020841601110
34. Casellas F, López Vivancos J, Malagela JR. Previous experience and quiality of life in patients with inflammatory bowel disease during relapse. Rev Esp Enferm Dig. (2003) 95:476–9.
35. Zhang CK, Hewett J, Hemming J, Grant T, Zhao H, Abraham C, et al. The influence of depression on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. (2013) 19:1732–9. doi: 10.1097/MIB.0b013e318281f395
36. Gower-Rousseau C, Sarter H, Savoye G, Tavernier N, Fumery M, Sandborn WJ, et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut. (2017) 66:588–96. doi: 10.1136/gutjnl-2015-310151
37. Soares JB, Pereira R, Costa JM, Arroja B, Gonçalves R. The inflammatory bowel disease-disability index: validation of the portuguese version according to the cosmin checklist. Eur J Gastroen Hepat. (2016) 28:1151–60. doi: 10.1097/MEG.0000000000000701
38. Zhao D, Ye C, Zhang S, Lv X, Yang B. Analysis of risk factors for early clinical recurrence of inflammatory bowel disease after fecal microbiota transplantation. Am J Transl Res. (2021) 13:12875–86.
39. Larsson K, Lööf L, Rönnblom A, Nordin K. Quality of life for patients with exacerbation in inflammatory bowel disease and how they cope with disease activity. J Psychosom Res. (2008) 64:139–48. doi: 10.1016/j.jpsychores.2007.10.007
40. van Gennep S, Evers SW, Rietdijk ST, Gielen ME, de Boer NKH, Gecse KB, et al. High disease burden drives indirect costs in employed inflammatory bowel disease patients: the WORK-IBD study. Inflamm Bowel Dis. (2021) 27:352–63. doi: 10.1093/ibd/izaa082
41. Greuter T, Rieder F, Kucharzik T, Peyrin-Biroulet L, Schoepfer AM, Rubin DT, et al. Emerging treatment options for extraintestinal manifestations in IBD. Gut. (2021) 70:796–802. doi: 10.1136/gutjnl-2020-322129
42. Rogler G, Singh A, Kavanaugh A, Rubin DT. Extraintestinal manifestations of inflammatory bowel disease: current concepts, treatment, and implications for disease management. Gastroenterology. (2021) 161:1118–32. doi: 10.1053/j.gastro.2021.07.042
43. Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastro Hepat. (2013) 10:585–95. doi: 10.1038/nrgastro.2013.117
44. Luchetti MM, Benfaremo D, Bendia E, Bolognini L, Fava G, Marini F, et al. Clinical and patient reported outcomes of the multidisciplinary management in patients with inflammatory bowel disease-associated spondyloarthritis. Eur J Intern Med. (2019) 64:76–84. doi: 10.1016/j.ejim.2019.04.015
45. Perera LP, Bhandari S, Liu R, Guilday C, Zadvornova Y, Saeian K, et al. Advanced age does not negatively impact health-related quality of life in inflammatory bowel disease. Digest Dis Sci. (2018) 63:1787–93. doi: 10.1007/s10620-018-5076-6
46. Liu R, Tang A, Wang X, Shen S. Assessment of quality of life in Chinese patients with inflammatory bowel disease and their caregivers. Inflamm Bowel Dis. (2018) 24:2039–47. doi: 10.1093/ibd/izy099
47. Yoon JY, Shin JE, Park SH, Park DI, Cha JM. Disability due to inflammatory bowel disease is correlated with drug compliance, disease activity, and quality of life. Gut Liver. (2017) 11:370–6. doi: 10.5009/gnl16422
48. Langbrandtner J, Steimann G, Reichel C, Bokemeyer B, Hüppe A. Inflammatory bowel disease - challenges in the workplace and support for coping with disease. Rehabilitation. (2022) 61:97–106. doi: 10.1055/a-1581-6497
49. Adamina M, Bonovas S, Raine T, Spinelli A, Warusavitarne J, Armuzzi A, et al. ECCO guidelines on therapeutics in crohn's disease: surgical treatment. J Crohns Colitis. (2020) 14:155–68. doi: 10.1093/ecco-jcc/jjz187
50. Burisch J, Weimers P, Pedersen N, Cukovic-Cavka S, Vucelic B, Kaimakliotis I, et al. Health-related quality of life improves during one year of medical and surgical treatment in a European population-based inception cohort of patients with inflammatory bowel disease–an ECCO-EpiCom study. J Crohns Colitis. (2014) 8:1030–42. doi: 10.1016/j.crohns.2014.01.028
51. Habibi F, Habibi ME, Gharavinia A, Mahdavi SB, Akbarpour MJ, Baghaei A, et al. Quality of life in inflammatory bowel disease patients: a cross-sectional study. J Res Med Sci. (2017) 22:104. doi: 10.4103/jrms.JRMS_975_16
52. Blondel-Kucharski F, Chircop C, Marquis P, Cortot A, Baron F, Gendre JP, et al. Health-related quality of life in Crohn's disease: a prospective longitudinal study in 231 patients. AmJ Gastroenterol. (2001) 96:2915–20. doi: 10.1111/j.1572-0241.2001.04681.x
53. McCombie AM, Mulder RT, Gearry RB. Coping strategies and psychological outcomes of patients with inflammatory bowel disease in the first 6 months after diagnosis. Inflamm Bowel Dis. (2015) 21:2272–80. doi: 10.1097/MIB.0000000000000476
54. Knowles SR, Keefer L, Wilding H, Hewitt C, Graff LA, Mikocka-Walus A. Quality of life in inflammatory bowel disease: a systematic review and meta-analyses-part II. Inflamm Bowel Dis. (2018) 24:966–76. doi: 10.1093/ibd/izy015
55. Stanton AL, Revenson TA, Tennen H. Health psychology: psychological adjustment to chronic disease. Annu Rev Psychol. (2007) 58:565–92. doi: 10.1146/annurev.psych.58.110405.085615
56. Luo XP, Mao R, Chen BL, Qiu Y, Zhang SH, He Y, et al. Over-reaching beyond disease activity: the influence of anxiety and medical economic burden on health-related quality of life in patients with inflammatory bowel disease. Patient Prefer Adherence. (2017) 11:23–31. doi: 10.2147/PPA.S118589
57. Moradkhani A, Beckman LJ, Tabibian JH. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis. (2013) 7:467–73. doi: 10.1016/j.crohns.2012.07.012
58. Neuendorf R, Harding A, Stello N, Hanes D, Wahbeh H. Depression and anxiety in patients with inflammatory bowel disease: a systematic review. J Psychosom Res. (2016) 87:70–80. doi: 10.1016/j.jpsychores.2016.06.001
59. Stone ML, Feng M, Forster EM. COVID-19 Pandemic increased anxiety among patients with inflammatory bowel disease: a patient survey in a tertiary referral center. Digest Dis Sci. (2022) 67:2876–81. doi: 10.1007/s10620-021-07095-y
60. Mitra D, Hodgkins P, Yen L, Davis KL, Cohen RD. Association between oral 5-ASA adherence and health care utilization and costs among patients with active ulcerative colitis. BMC Gastroenterol. (2012) 12:132. doi: 10.1186/1471-230X-12-132
Keywords: inflammatory bowel disease, quality of life, influencing factors, recurrence, prediction model
Citation: Li M, Tao Y, Sun Y, Wu J, Zhang F, Wen Y, Gong M, Yan J, Liang H, Bai X, Niu J and Miao Y (2023) Constructing a prediction model of inflammatory bowel disease recurrence based on factors affecting the quality of life. Front. Med. 10:1041505. doi: 10.3389/fmed.2023.1041505
Received: 11 September 2022; Accepted: 15 February 2023;
Published: 09 March 2023.
Edited by:
Xiang Xue, University of New Mexico, United StatesReviewed by:
Katalin Eszter Müller, University of Pécs, HungaryCopyright © 2023 Li, Tao, Sun, Wu, Zhang, Wen, Gong, Yan, Liang, Bai, Niu and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinglei Miao, bWlhb3lpbmdsZWlAa21tdS5lZHUuY24=; Junkun Niu, bml1anVua3VuQGttbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.