
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 16 February 2023
Sec. Nephrology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1017459
This article is part of the Research Topic Endocrine Abnormalities and Renal Complications View all 17 articles
 Juyeon Lee1,2,3
Juyeon Lee1,2,3 Eun Hui Bae4
Eun Hui Bae4 Soo Wan Kim4
Soo Wan Kim4 Wookyung Chung5
Wookyung Chung5 Yeong Hoon Kim6
Yeong Hoon Kim6 Yun Kyu Oh7
Yun Kyu Oh7 Yong-Soo Kim8
Yong-Soo Kim8 Kook-Hwan Oh9*
Kook-Hwan Oh9* Sue K. Park1,3,10*
Sue K. Park1,3,10*Backgrounds: Some observational studies have suggested a possible association between vitamin D deficiency and CKD. However, in most studies, the causality between low levels of vitamin D and risk of renal events could not be explained. We investigated the relationship between vitamin D deficiency and risk of severe CKD stage and renal event in a large-scale prospective cohort study.
Methods: We used data from a prospective cohort of 2,144 patients with available information on serum 25-hydroxyvitamin D (25(OH)D) levels at baseline from KNOW-CKD, 2011-2015 were included. Vitamin D deficiency was defined as serum 25(OH)D levels < 15 ng/mL. We performed a cross-sectional analysis to elucidate the relationship between 25(OH)D and CKD stage using baseline CKD patient data. We further examined a cohort analysis to clarify the association between 25(OH)D and risk of renal event. Renal event was a composite of the first occurrence of a 50% decline in eGFR from the baseline value or the onset of CKD stage 5 (initiation of dialysis or kidney transplantation) across the follow-up period. We also investigated the associations of vitamin D deficiency with risk of renal event according to diabetes and overweight status.
Results: Vitamin D deficiency were significantly associated with an increased risk of severe CKD stage – 1.30-fold (95% CI: 1.10-1.69) for 25(OH)D. Deficiency of 25(OH)D with 1.64-fold (95% CI: 1.32-2.65) was related to renal event compared with the reference. Furthermore, vitamin D deficiency patients with presence of DM and overweight status also displayed higher risk than non-deficient patients for risk of renal event.
Conclusion: Vitamin D deficiency is associated with significantly increased risk of severe CKD stage and renal event.
Chronic kidney disease (CKD) is defined as the existence of structural or functional abnormalities of the kidney, with or without decreased estimated glomerular filtration rate (eGFR < 60 mL/min/1.73m2), lasting over three months (1); it is considered a significant worldwide health problem (2). The estimated prevalence of CKD in Korean adults is 8.2% according to the Korean National Health and Nutritional Examination Surveys (KNHANES) (3). In addition, Vitamin D deficiency is high among Korean adults (4), both physicians and patients are questioning whether supplement of vitamin D are needed.
Vitamin D, a steroid hormone known for its importance in bone metabolism, can be supplemented with diet, supplement, or produced in the body by dermal synthesis with UV from the precursor (5). Vitamin D deficiency has been related to numerous conditions such as fracture risk, diabetes, fractures, renal disease, cardiovascular disease, auto-immune disease, depression, and cancer (6, 7).
The causal relationship between vitamin D deficiency and decreased kidney function remains debates. Decreased kidney function is related to lower enzyme 1a-hydroxylase activity in the proximal tubule, which leads to decreased activation of 25(OH)D to 1,25(OH)2D (8). However, increasing evidence from scientific approaches has shown an association between low vitamin D levels and decreased estimated glomerular filtration (eGFR) (9, 10). Vitamin D is known to play an important role in maintaining homeostasis, which is associated with the renin-angiotensin system (RAS) (11). Several studies have shown that activation of the RAS system due to low vitamin D level leads to kidney disease, hypertension, cardiovascular disease, insulin resistance, and increased mortality (12, 13). In fact, 2017 Kidney Disease Improving Global Outcomes (KDIGO) experts carefully suggested (level of evidence 2C) checking and supplementing low vitamin D levels in CKD and dialysis patients (14).
Some observational studies and guidelines have suggested a possible association between low vitamin D level and CKD (15–17). However, in most studies and guidelines, the causality between low vitamin D levels and risk of renal event could not be explained because this relationship was analyzed through a cross-sectional study or focused on dialysis patients, or did not distinguish the stage of CKD (early versus advanced stage) (15–17).
The purpose of this study was twofold. First, to evaluate the relationship between patients with 25(OH)D deficiency and CKD stages at baseline. Second, to analyze longitudinal follow-up data to elucidate the association between patients with 25(OH)D deficiency and risk of renal event, which is defined as a 50% decline in eGFR from the baseline value or the onset of CKD stage 5 across the follow-up. Further, controlling the effects of confounding on vitamin D levels using propensity score matching analysis (PSM), we evaluated the relationship between patients with 25(OH)D deficiency and severe CKD stage and renal event. We also investigated the associations of patients with 25(OH)D deficiency and risk of renal event according to diabetes and overweight status.
We collected baseline data for 2,238 non-dialysis dependent patients with CKD from stage G1 to 5, who were enrolled in a prospective cohort study [KoreaN Cohort Study for Outcome in Patients With Chronic Kidney Disease] from 2011 to 2015 in collaboration with a multicenter, patient-based, prospective-cohort study. The detailed design and methods were previously published (18). Among 2,238 (KNOW-CKD) cohort study patients, a total of 2,144 patients with serum 25(OH)D was included in this study (Supplementary Figure 1). This study was conducted according to guidelines established by the Declaration of Helsinki. All patients gave written informed consent for inclusion before they participated in the study. The study protocol was approved by the institutional review board of each participating clinical center: Seoul National University Hospital (1104-089-359), Seoul National University Bundang Hospital (B-1106/129–008), Kangbuk Samsung Medical Center (2011–01-076), Yonsei University Severance Hospital (4-2011-0163), Seoul St. Mary’s Hospital (KC11OIMI0441), Eulji General Hospital (201105-01), Gil Hospital (GIRBA2553), Pusan Paik Hospital (11-091), Chonnam National University Hospital (CNUH-2011-092) in 2011.
Data regarding personal and family history, anthropometric measurements, cardiac evaluation, radiological imaging, and baseline laboratory results were extracted from the electronic data management system (PhactaX: http://www.phactaX.org) with assistance from the Division of data management at Seoul National University Medical Research Collaborating Center. Serum samples were harvested and sent immediately to the central laboratory of Lab Genomics, Seongnam, Republic of Korea. Urine samples were also immediately sent to the central laboratory for KNOW-CKD. The reliability of biomarker analysis was previously reported (18).
Serum 25(OH)D level was measured by electrochemiluminescence immunoassay (ECLIA), using an ADVIA Centaur Vitamin D Total assay reagents (Siemens, NY, USA). 25(OH)D deficiency was defined as level < 15 ng/mL, as considered in the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (19). Non-deficient levels of 25(OH)D was ≥ 15 ng/mL. Limit of detection for 25(OH)D level was 3.20 ng/mL (18).
Serum c-terminal FGF23 level was measured using enzyme-linked immunosorbent assay (ELISA; Immunotopics, San Clemente, CA, USA). Serum levels of klotho were measured using an ELISA kit (Immuno-Biological Laboratories Co., Gunma, Japan). (18).
Serum creatinine was measured by an IDMS-traceable method at a central laboratory and eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula (CKD-EPI) (20). Mild CKD stage (Stage 1/2) was defined as eGFR ≥ 60 mL/min/1.73 m2. Moderate CKD stage (Stage 3A/3B) was defined as 30 ≤ eGFR < 60 mL/min/1.73 m2 and severe CKD stage (Stage 4/5) was defined as eGFR of less than 30 mL/min/1.73 m2.
Renal event was a composite of the first occurrence of a 50% decline in eGFR from the baseline value or the onset of CKD stage 5 (initiation of dialysis or kidney transplantation) across the follow-up period.
Characteristics of patients are described by binary of 25(OH)D, before and after propensity score matching analysis. Continuous variables are expressed as mean and standard deviation for t-tests, and categorical variables are reported as number of patients and percentage for the chi-squared test. To examine the association of 25(OH)D with CKD stages and risk of renal event, polytomous logistic regression and Cox proportional hazard models were constructed to calculate odds ratio (ORs) and hazard ratio (HRs), using non-deficiency vitamin D levels as the reference. Patients who were lost to follow-up were censored at the date of the last hospital examination. This study was adjusted for clinically important confounding factors: age, sex, baseline eGFR, serum phosphorus levels, serum intact parathyroid hormone (iPTH), serum fibroblast growth factor 23 (FGF23), klotho, random urine albumin-to-creatinine ratio (UACR), 24-h urine protein, diabetes mellitus (DM), cause of CKD, use of vitamin D supplements, and angiotensin converting enzyme inhibitors(ACEi)/angiotensin receptor blocker (ARBs) medications. Further, controlling the effects of confounding on vitamin D levels using propensity score matching analysis (PSM), we evaluated the relationship between vitamin D deficiency and severe CKD stage and risk of renal event. Confounding variables affecting age, sex, PTH, serum phosphorus, serum klotho, FGF23, and cause of CKD were matched in the two study groups using 1:1 propensity score matching analysis (PSM) and a caliper width of 0.01. We also examined multi-collinearity between independent variables with Pearson correlation coefficients and variance inflation factor. We also performed stratified analysis by DM (No vs. Yes), and overweight (BMI < 23 kg/m2 vs. BMI ≥ 23 kg/m2) status using Cox regression models. P-interaction term was calculated to determine interaction between DM, overweight and each vitamin D level. The spline model was adjusted for age, sex, and confounding factors. The survival probability was calculated with the Kaplan-Meier (KM) method. To analyze the association with vitamin D on the risk of renal events, a Cox regression model was constructed by controlling the following 8 risk factors, which were statistically significant in each univariable analysis: age, sex, baseline eGFR, cause of CKD, 24-h proteinuria, intact PTH and serum klotho. Each model was constructed by backward Cox regression model. In order to observe the effect of vitamin D on the risk of renal events under adjustment for 25(OH)D levels and other risk factors, we constructed a vitamin D-epidemiologic-clinical model which was a full multivariable model. In addition, we evaluated the discriminatory accuracy of the only vitamin D model, vitamin D-epidemiological model, and a vitamin D-epidemiologic-clinical model in predicting risk of renal events using Harrell’s C index.
P-value less than 0.01 were considered statistically significant. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and spline plots were drawn by R version 4.2.1 (http://www.r-project.org). P-values < 0.05 were considered statistically significant.
Baseline characteristics of the study population are described Supplementary Table 1. Compared with non-deficient vitamin D patients, patients with deficiency were more likely to be male; have higher SBP, DBP, UACR, UPCR, 24-h urine protein, serum creatinine, phosphorus, potassium, total cholesterol, i-PTH, FGF23, klotho, hepcidin, angiotensin levels; have lower eGFR, hemoglobin, uric acid, albumin, calcium and sodium levels. The PSM study showed that all variables were not statistically different according to vitamin D levels.
Table 1 presents the associations of patients with 25(OH)D deficiency with severe CKD stage. We found increased odds for severe CKD stage in patients with vitamin D deficiency levels compared to patients with non-deficient levels (OR = 1.30, 95% CI = 1.01-1.69). In addition, Table 1 shows the likelihood of having severe CKD stage relative to mild CKD stage by serum levels of vitamin D biomarkers using PSM. Patients with 25(OH)D deficiency levels were not statistically significant associated with increased odds of severe CKD stage compared to patients with non-deficient levels (OR = 1.44, 95% CI = 0.98-2.13).
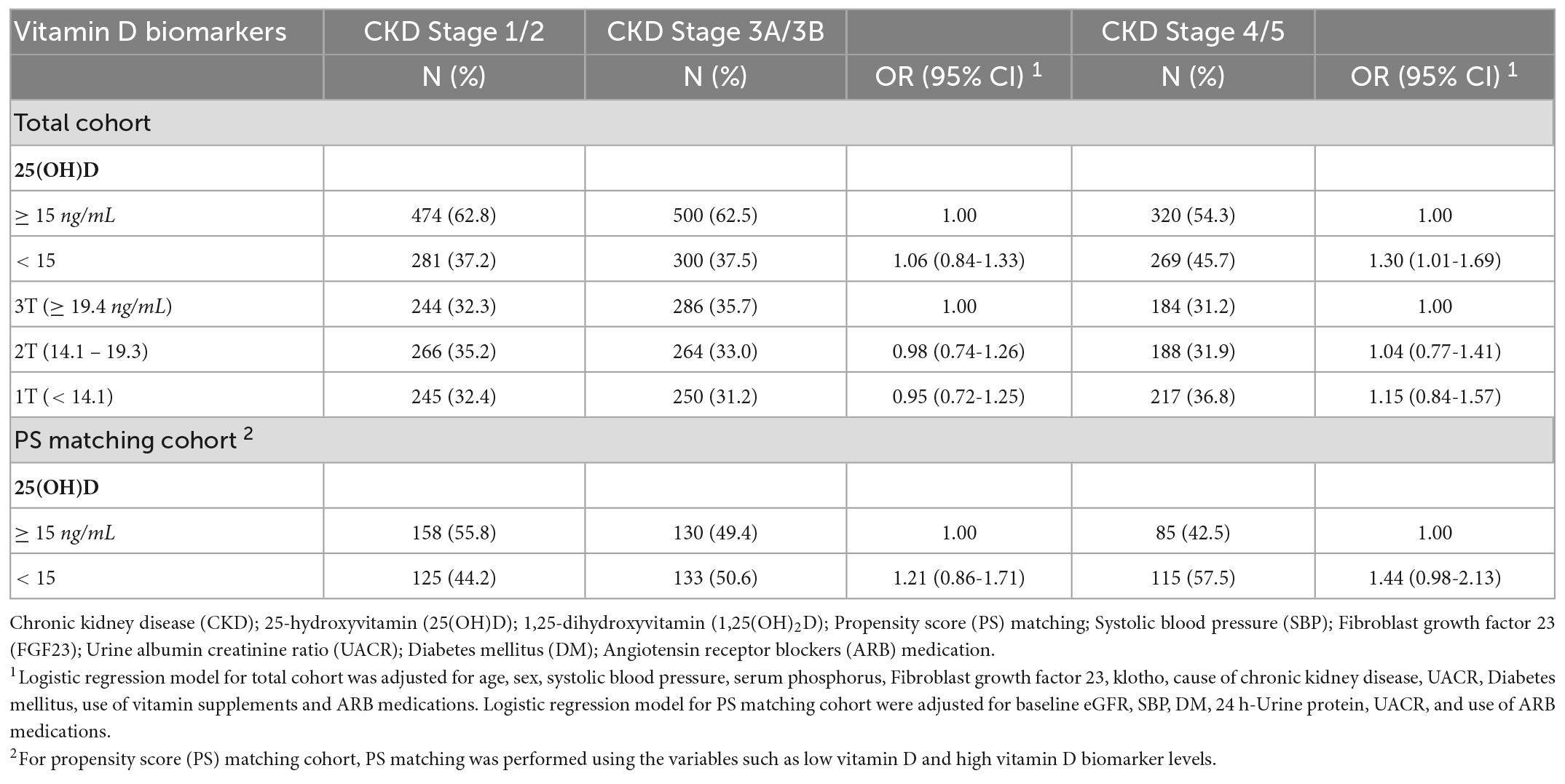
Table 1. Association between serum vitamin D levels and moderate (Stage 3A/3B) and severe CKD (Stage 4/5) stage in the Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) study, 2011-2015.
Table 2 shows that 342 patients reached the composite outcome of renal event. Patients with 25(OH)D deficiency levels had a significantly increased risk of renal events compared to patients with non-deficient levels (HR = 1.64, 95% CI = 1.32-2.05). Table 2 also shows the risk of renal event according to serum levels of vitamin D biomarkers using PSM. After PSM, patients with 25(OH)D deficiency levels had no significant association with renal event (HR = 1.52, 95% CI = 0.88-2.65). The relationship between serum vitamin D levels (continuous scale) and severe CKD stage and risk of renal event were non-linear after multivariate adjustment (Figure 1). The Kaplan-Meier survival curves show statistically significant difference in renal event probability among the binary and tertiles of serum 25(OH)D levels (p < 0.001) (Figure 2). Thus, this model has a certain practicability and reliability in evaluating prognosis.

Table 2. Association between serum vitamin D levels and renal event in the Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) study, 2011-2015.
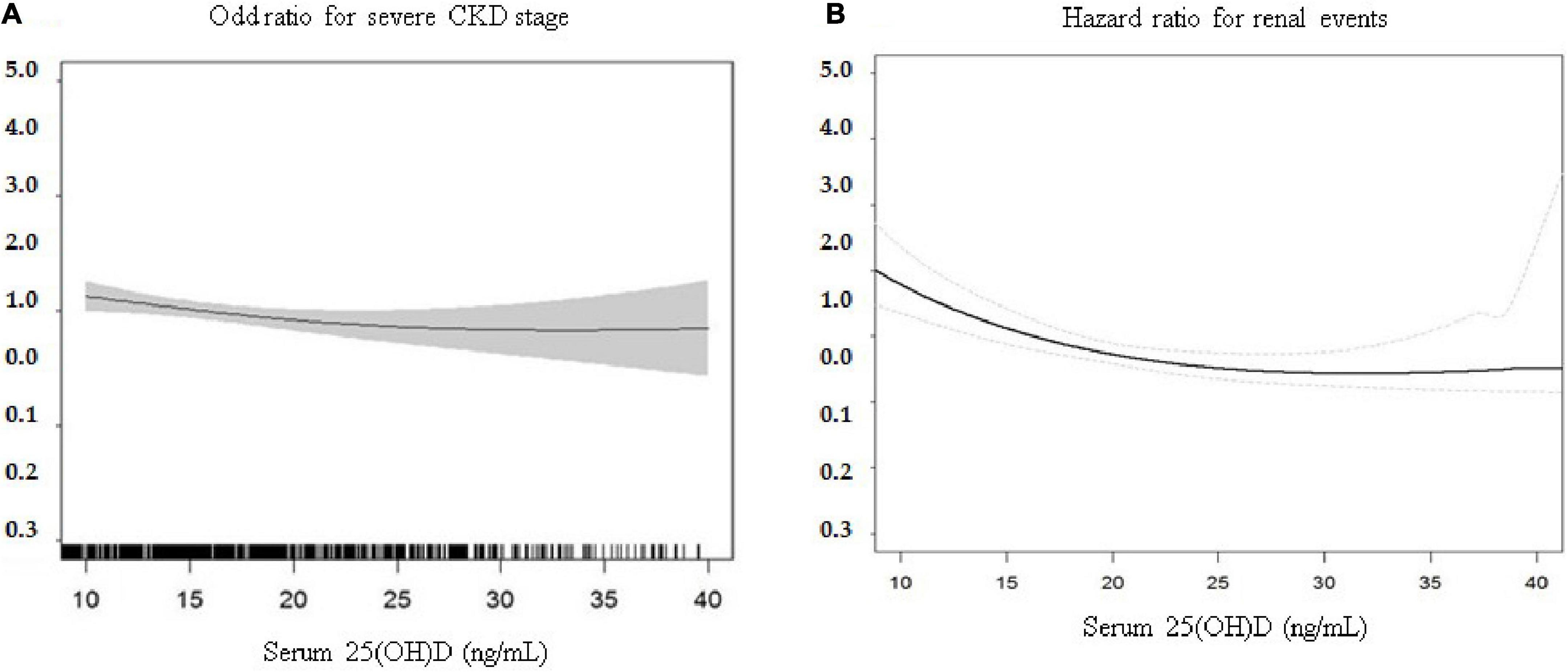
Figure 1. Multivariate association of continuously measured 25(OH)D levels (ng/mL) and (A) severe CKD stages and (B) risk of renal events. The relationship between serum vitamin D levels (continuous scale) and risk of renal event were non-linear after multivariate adjustment. CKD, Chronic kidney disease; 25(OH)D, 25-hydroxyvitamin D.
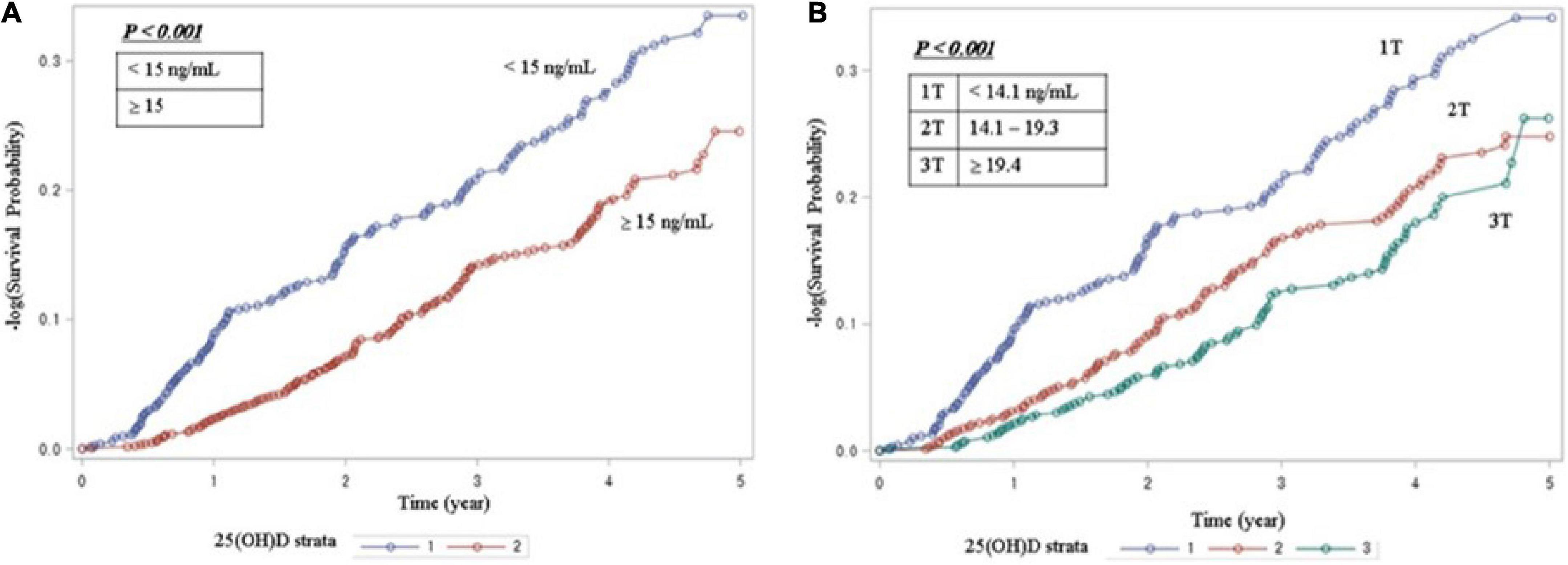
Figure 2. Kaplan-Meier survival analysis for serum vitamin D levels (A) Survival curve for renal event by binary of 25(OH)D. (B) Survival curve for renal event by tertiles of 25(OH)D. The Kaplan-Meier curves show that the patient with vitamin D deficiency group had a significantly higher cumulative incidence of renal events (P < 0.001). 25(OH)D, 25-hydroxyvitamin D.
Compared to the non-DM, the diabetic CKD showed stronger associations between CKD stage 4 and 5 and patients with 25(OH) deficiency (OR = 1.59, 95% CI = 1.13-2.25, p-interaction = 0.02).
In this study, compared to the non-overweight (BMI < 23), the patients with overweight (BMI ≥ 23) status demonstrated stronger associations between CKD stage 4 and 5 and patients with 25(OH) deficiency (OR = 1.41, 95% CI = 1.03-1.92, p-interaction = 0.30). On the other hand, none of the patients with 25(OH)D deficiency showed a significant association with the risk of CKD stage 3A and 3B according to DM and overweight status (Table 3).
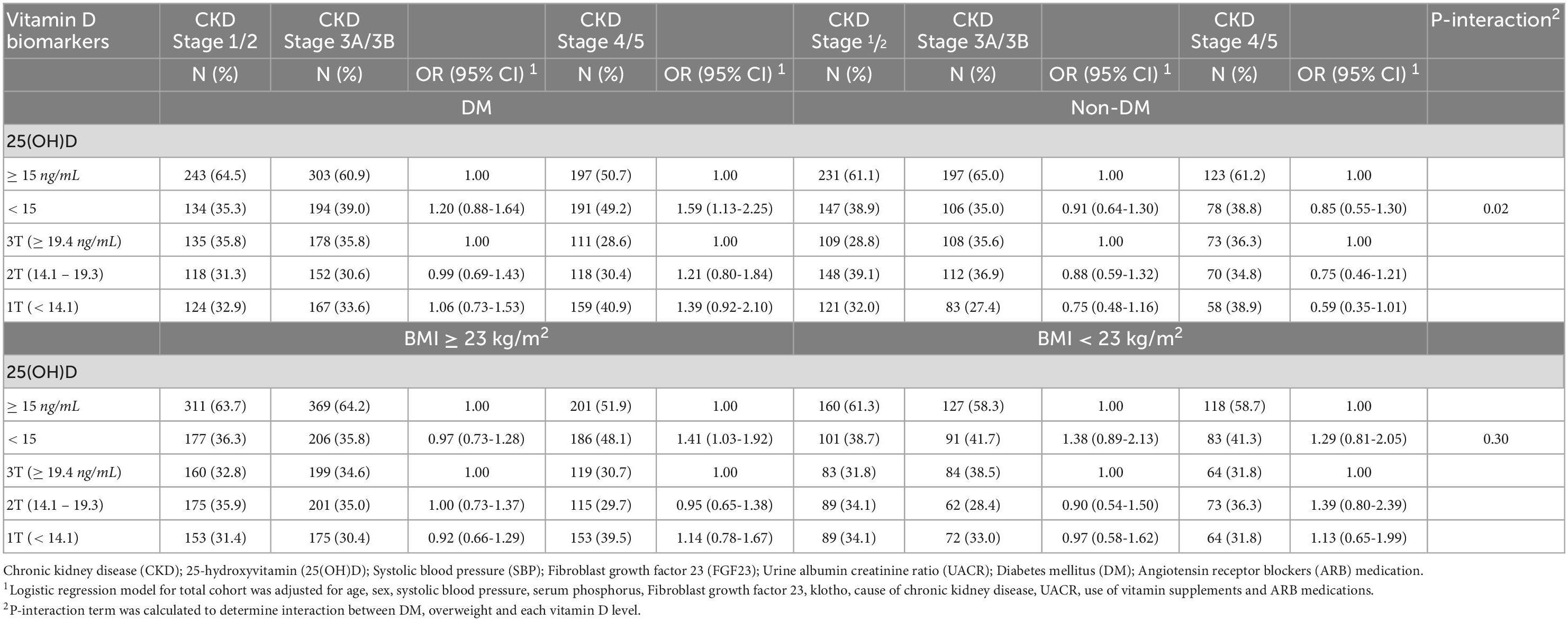
Table 3. Association between serum vitamin D levels and moderate (Stage 3A/3B) and severe CKD (Stage 4/5) stage according to diabetes and overweight status in the Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) study, 2011-2015.
As shown in Table 4, patients with DM, and overweight (BMI ≥ 23) status appeared to have a greater risk for renal event in the context of vitamin D deficiency. The associations of 25(OH)D deficiency with risk of renal event were stronger in patients with DM (HR = 1.81, 95% CI = 1.38-2.38, p < 0.01 for interaction between 25(OH)D and DM for renal event; and overweight status (HR = 1.89, 95% CI = 1.44-2.48, p < 0.01 for interaction between 25(OH)D and overweight for renal event.
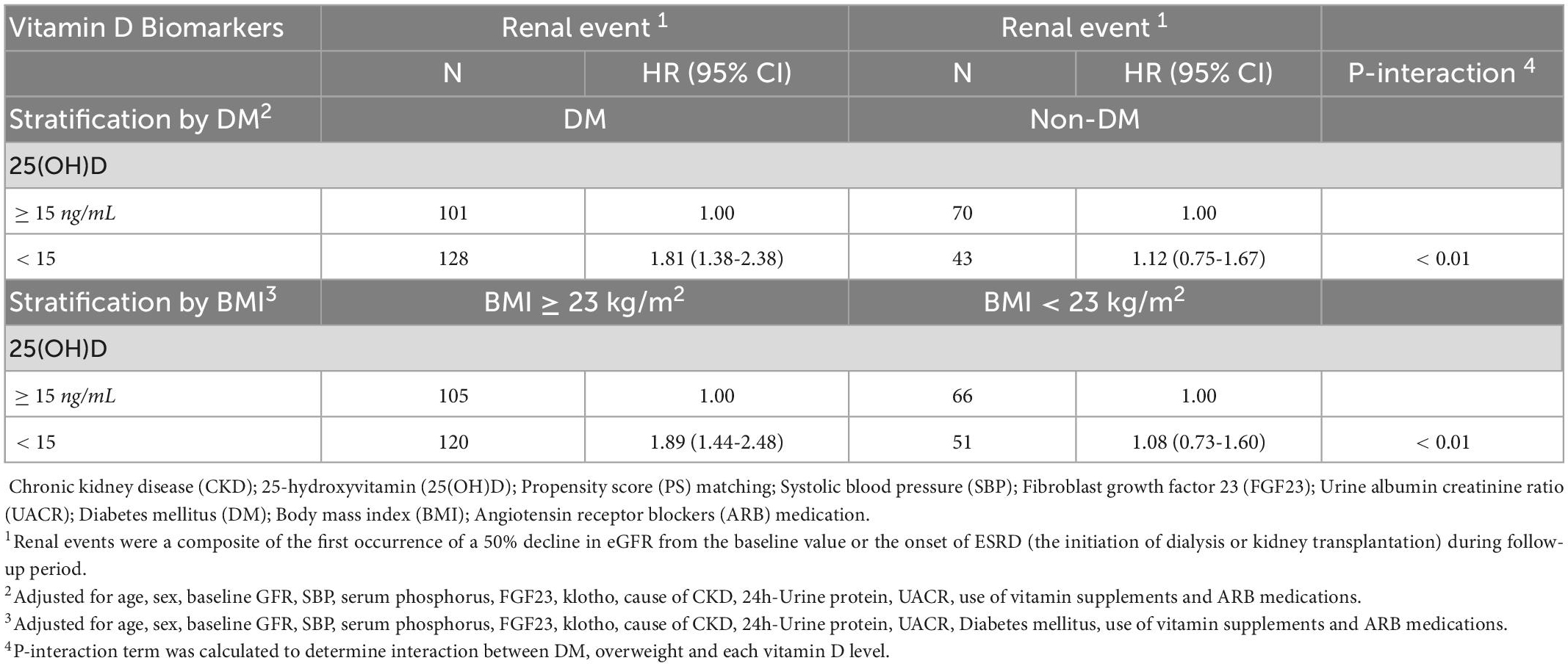
Table 4. Association between serum vitamin D levels and renal event according to diabetes and overweight status in the Korean Cohort Study for Outcome in Patients With Chronic Kidney Disease (KNOW-CKD) study, 2011-2015.
With the ability to discriminate renal events for the epidemiological model, a multivariate model controlled by 25(OH)D levels and other risk factors, the Harrell’s C-index at long term follow up was as follow: Model 1 (vitamin D only) was constructed with 1 variable and the Harrell’s C-index was 0.5822. Model 2 (vitamin D-epidemiological model) was constructed with 4 variables and the Harrell’s C-index was 0.8386. Model 3 (vitamin D-epidemiologic-clinical model) was constructed with 8 variables and the Harrell’s C-index was 0.8916. A full multivariable model shows the highest differential accuracy among the three models predicting the risk of renal events. The C-index of the model was significantly different from that of the vitamin D only model (p-value < 0.01). (Figure 3).
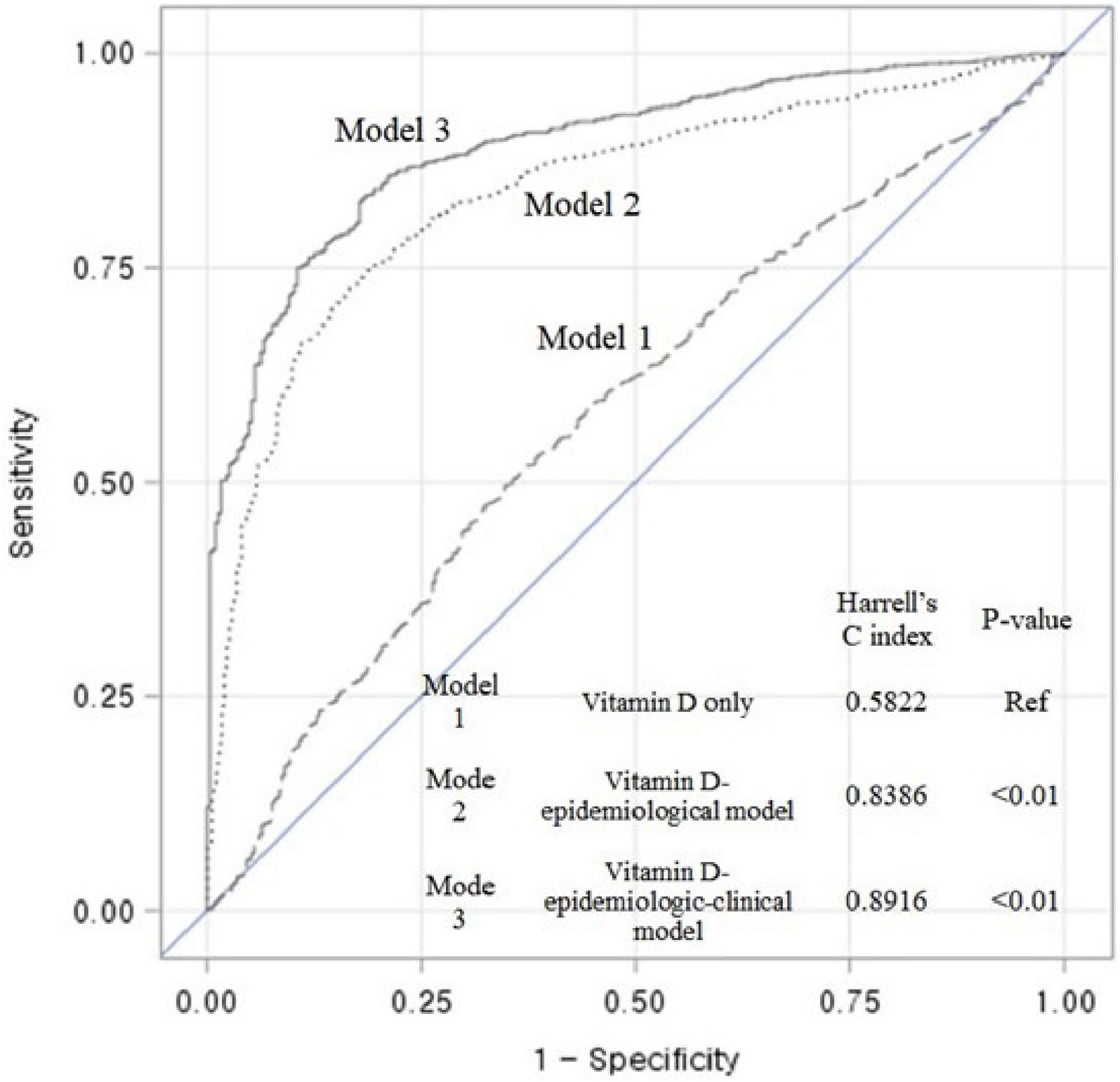
Figure 3. Receiver operating characteristic (ROC) curves and Harrell’s C-index showing the discriminant accuracy of each model for the ability to distinguish renal events in entire cohort; Model 1 (vitamin D model): Function(Y) = β1[25(OH)D], Function(Y) = Log (( Hazard
Hazard  _Exposed)/
_Exposed)/ Hazard
Hazard  _(Non-Exposed)); Model 2 (Vitamin D-epidemiological model): Function(Y) = β1[25(OH)D] + β2[Age] + β3[Sex] + β4[Baseline eGFR]; Model 3 (Vitamin D-epidemiologic -clinical model): Function(Y) = β1[25(OH)D] + β2[Age] + β3[Sex] + β4[Baseline eGFR] + β5[Cause of CKD] + β6[24-h proteinuria] + β7[intact PTH] + β8 [Klotho]; A full multivariable model shows the highest differential accuracy among the three models predicting the risk of renal events. The C-index of the model was significantly different from that of the vitamin D only model (p-value < 0.01). 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration.
_(Non-Exposed)); Model 2 (Vitamin D-epidemiological model): Function(Y) = β1[25(OH)D] + β2[Age] + β3[Sex] + β4[Baseline eGFR]; Model 3 (Vitamin D-epidemiologic -clinical model): Function(Y) = β1[25(OH)D] + β2[Age] + β3[Sex] + β4[Baseline eGFR] + β5[Cause of CKD] + β6[24-h proteinuria] + β7[intact PTH] + β8 [Klotho]; A full multivariable model shows the highest differential accuracy among the three models predicting the risk of renal events. The C-index of the model was significantly different from that of the vitamin D only model (p-value < 0.01). 25(OH)D, 25-hydroxyvitamin D; eGFR, estimated glomerular filtration.
In this study, we found that patients with 25(OH)D deficiency levels was significantly associated with severe CKD stage and increased risk of renal event. Furthermore, vitamin D deficiency patients with presence of DM and overweight status (≥ 23 kg/m2) also displayed higher risk than non-deficient patients for severe CKD stage and risk of renal event. None of the patients with 25(OH)D deficiency showed a significant association with the risk of CKD stage 3A and 3B.
Our findings are consistent with a number of previous studies. A cross-sectional studies conducted in France and Thailand showed that 25(OH)D deficiency was related to decreased GFR and developing ESRD (15, 16). A cohort study in the United States found that low 25(OH)D level was associated with development of ESRD (21). Similarly, another cohort study in Italy reported that low 25(OH)D level is an independent predictor of CKD progression (22).
On the other hand, previous studies found no association between low 25(OH)D level and CKD progression. A longitudinal patient cohort study reported that African Americans with low 25(OH)D levels were not associated with ESRD or doubling of serum creatinine (23). The Framingham Offspring cohort study showed similar results (24). The biggest difference between the former studies (15, 16, 21, 22) including our study and the latter studies (23, 24), is that the former studies (15, 16, 21, 22) reported that 25(OH)D deficiency is associated with risk of renal event while the latter studies (23, 24) showed that 25(OH)D deficiency is not associated with the risk of ESRD in the general population, where incidence of ESRD is less common. These inconsistencies in results can be attributed to various reasons, including differences in the general characteristics of the study participants, outcome variables, and confounding variables.
In addition, we found interactions between vitamin D deficiency and DM or between vitamin D deficiency and overweight for the risk of renal event. DM is one of the most common causes of CKD. Low vitamin D levels are related to insulin resistance and impaired pancreatic islet B-cell function (25). The effect is on upregulation of the insulin receptor gene and calcium and phosphorus metabolism (26). Also, an adequate vitamin D levels suppresses RAS system by reducing the expression AT1 receptors and by inhibiting renin synthesis in the kidney. It could lead to nephron-protective effects by reducing proteinuria and decreasing CKD progression (27). Previous studies reported that 25(OH)D deficiency is associated with type 2 DM (28). Since DM can induce increased risk of renal event, patients with DM may be more susceptible to vitamin D deficiency with regards to CKD than those without DM. Also, high BMI is one of the strongest risk factors for CKD (29). Previous study has shown that adipose tissue may increase the secretion of inflammatory cytokines or chemokine (MCP-1, IL-6, IL-1beta, TNF-a) and its plausible influence on the course of CKD (30). Overweight or obesity status is directly related to hypertension and diabetes status, the metabolic disorders responsible for the ESRD (31). One animal study presents that association between vitamin D deficiency and obesity impairs the renal function, hemodynamics, and metabolic parameters in the High-fat vitamin D deficient (H + VDD) rats. Obesity associated to vitamin D deficiency aggravated the renal inflammation associated to renal progression (32).
While the exact mechanism for vitamin D deficiency and risk of renal event is unclear, several biological mechanisms have been proposed. Vitamin D is converted enzymatically in the liver to 25(OH)D, the major circulating form, and then in the kidney to 1,25(OH)2D, the active form of vitamin D (33). Low levels of 1,25(OH)2D levels in the body can lead to deterioration of the RAS system by inducing abnormal metabolic profiles such as increasing renin, proteinuria, blood pressure, insulin resistance, and renal injury (34–36). For example, vitamin D supplementation decreases renin receptor and renin expression in rat models of CKD (37). Another possible mechanism is that low levels of 25(OH)D may modulate inflammation and oxidative stress and reduce fibroblast activation (13). In addition, CKD progression has been related to lower 25(OH)D reuptake and reduction of intracrine 1,25(OH)2D levels in the renal proximal tubules (38). Also, 1,25(OH)2D levels are known to reduce expression of the nuclear factor kB and promote a shift in T-helper cell response from T-helper 1 cell to T-helper 2 cell (39). Therefore, this reduces T-helper 1 cell-mediated tissue damage and increases production of T-helper 2 cell immunomodulatory cytokines (40). A genetic predisposition that affects higher vitamin D binding protein (DBP) was suggested as a plausible mechanism in the association between vitamin D level and risk of renal events (41). DBP is facilitated by receptor-mediated endocytosis. In the renal proximal tubule, cublin and megalin induce uptake of extracellular ligands. Deficiency of these proteins results in increased vitamin D excretion in the urine (42). More studies are needed to understand these effects in humans.
Several limitations should be considered when interpreting our results. First, serum vitamin D levels are generally affected by seasonal variation and the extent of UV exposure should be considered (43). However, our study could not be adjusted for seasonal variation. Second, we did not adjust our data for patient nutritional status. Nutritional status may also contribute to vitamin D status in CKD patients. Malnutrition is a typical finding in patients with CKD. Uremia may be related to impaired gastrointestinal absorption of vitamin D (44). Third, we conducted a single measurement of vitamin D levels at baseline. Our data in the present study did not have enough repeated measures to perform the analysis. Fifth, this study is an observational study. Therefore, it is possible that potential confounding factors were not correctly adjusted. To reduce this possibility, we conducted PSM analysis and found consistent results. Finally, the KNOW-CKD cohort consists only of Korean CKD patients and our findings may not be generalizable to other ethnic groups.
Despite these limitations, the present study has several strengths. First, our study used a well-designed patient-based prospective cohort (the KNOW-CKD) representative of Korean CKD patients. In addition, serum vitamin D levels can be easily obtained in the clinical setting and treatment of vitamin D deficiency/insufficiency is simple and inexpensive. This study should be taken into consideration by researchers and clinicians in order to improve CKD patient outcomes.
The present study suggests that vitamin D deficiency is significantly associated with risk of severe CKD stage and renal event.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Seoul National University Hospital (1104-089-359), Seoul National University Bundang Hospital (B-1106/129–008), Kangbuk Samsung Medical Center (2011–01-076), Yonsei University Severance Hospital (4-2011-0163), Seoul St. Mary’s Hospital (KC11OIMI0441), Eulji General Hospital (201105-01), Gil Hospital (GIRBA2553), Pusan Paik Hospital (11-091), Chonnam National University Hospital (CNUH-2011-092). The patients/participants provided their written informed consent to participate in this study.
JL, EB, SK, K-HO, and SP designed the study. JL, K-HO, and SP conducted the data analysis and drafted the manuscript and wrote the manuscript. K-HO and SP provided the supervision and mentorship. All authors supported the interpretation of results, provided important intellectual content and revised the final version of the manuscript and also provided final approval of the version to be published.
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101, 2019E320102, and 2022-11-007). Funding sources had no role in the design and conduct of study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant no. HI16C1127). This study was supported by a grant from Seoul National University Hospital (2022).
We thank all the participants and researchers who participated in the KNOW-CKD study. We are grateful to all of those with whom we have had the pleasure to work during this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1017459/full#supplementary-material
1. Levey A, Eckardt K, Tsukamoto Y, Levin A, Coresh J, Rossert J, et al. Definition and classification of chronic kidney disease: a position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int. (2005) 67:2089–100. doi: 10.1111/j.1523-1755.2005.00365.x
2. Levey A, Atkins R, Coresh J, Cohen E, Collins A, Eckardt K, et al. Chronic kidney disease as a global public health problem: approaches and initiatives–a position statement from kidney disease improving global outcomes. Kidney Int. (2007) 72:247–59. doi: 10.1038/sj.ki.5002343
3. Park J, Baek H, Jung H. Prevalence of chronic kidney disease in Korea: the Korean national health and nutritional examination survey 2011–2013. J Korean Med Sci. (2016) 31:915–23. doi: 10.3346/jkms.2016.31.6.915
4. Park J, Hong I, Chung J, Choi H. Vitamin D status in South Korean population: seven-year trend from the KNHANES. Medicine. (2018) 97:e11032. doi: 10.1097/MD.0000000000011032
5. Heaney R. Vitamin D in health and disease. Clin J Am Soc Nephrol. (2008) 3:1535–41. doi: 10.2215/CJN.01160308
7. Plum L, DeLuca H. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. (2010) 9:941–55. doi: 10.1038/nrd3318
8. Bikle D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. (2014) 21:319–29. doi: 10.1016/j.chembiol.2013.12.016
9. Shroff R, Wan M, Nagler E, Bakkaloğlu S, Fischer D, Bishop N, et al. Clinical practice recommendations for native vitamin D therapy in children with chronic kidney disease Stages 2–5 and on dialysis. Nephrol Dial Transplant. (2017) 32:1098–113. doi: 10.1093/ndt/gfx065
10. de Boer I, Katz R, Chonchol M, Ix J, Sarnak M, Shlipak M, et al. Serum 25-hydroxyvitamin D and change in estimated glomerular filtration rate. Clin J Am Soc Nephrol. (2011) 6:2141–9. doi: 10.2215/CJN.02640311
11. Li Y. Vitamin D regulation of the renin-angiotensin system. J Cell Biochem. (2003) 88:327–31. doi: 10.1002/jcb.10343
12. Fanari Z, Hammami S, Hammami M, Hammami S, Abdellatif A. Vitamin D deficiency plays an important role in cardiac disease and affects patient outcome: still a myth or a fact that needs exploration? J Saudi Heart Assoc. (2015) 27:264–71. doi: 10.1016/j.jsha.2015.02.003
13. Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong J, et al. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. (2014) 348:g1903. doi: 10.1136/bmj.g1903
14. Wheeler D, Winkelmayer W. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) foreword. Kidney Int Suppl. (2017) 7:1–59. doi: 10.1016/j.kisu.2017.04.001
15. Urena-Torres P, Metzger M, Haymann J, Karras A, Boffa J, Flamant M, et al. Association of kidney function, vitamin D deficiency, and circulating markers of mineral and bone disorders in CKD. Am J Kidney Dis. (2011) 58:544–53. doi: 10.1053/j.ajkd.2011.04.029
16. Satirapoj B, Limwannata P, Chaiprasert A, Supasyndh O, Choovichian P. Vitamin D insufficiency and deficiency with stages of chronic kidney disease in an Asian population. BMC Nephrol. (2013) 14:206. doi: 10.1186/1471-2369-14-206
17. Koh J, Kwak I, Song S, Lee S, Rhee H, Seong E, et al. 25-hydroxyvitamin D status based on estimated glomerular filtration rate in patients with chronic kidney disease. Korean J Med. (2012) 83:740–51. doi: 10.3904/kjm.2012.83.6.740
18. Oh K, Park S, Park H, Chin H, Chae D, Choi K, et al. KNOW-CKD (KoreaN cohort study for outcome in patients with chronic kidney disease): design and methods. BMC Nephrol. (2014) 15:80. doi: 10.1186/1471-2369-15-80
19. Levey A, Coresh J, Bolton K, Culleton B, Harvey K, Ikizler T, et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. (2002) 39:S1–266.
20. Levey A, Stevens L, Schmid C, Zhang Y, Castro A, Feldman H, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
21. Melamed M, Astor B, Michos ED, Hostetter T, Powe N, Muntner P. 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol. (2009) 20:2631–9. doi: 10.1681/ASN.2009030283
22. Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. (2009) 75:88–95. doi: 10.1038/ki.2008.501
23. Scialla J, Astor B, Isakova T, Xie H, Appel L, Wolf M. Mineral metabolites and CKD progression in African Americans. J Am Soc Nephrol. (2013) 24:125–35. doi: 10.1681/ASN.2012070713
24. O’Seaghdha C, Hwang S, Holden R, Booth S, Fox C. Phylloquinone and vitamin D status: associations with incident chronic kidney disease in the Framingham offspring cohort. Am J Nephrol. (2012) 36:68–77. doi: 10.1159/000339005
25. Martin T, Campbell R. Vitamin D and diabetes. Diabetes Spectr. (2011) 24:113–8. doi: 10.2337/diaspect.24.2.113
26. Nada A, Shaheen D. Cholecalciferol improves glycemic control in type 2 diabetic patients: a 6-month prospective interventional study. Ther Clin Risk Manag. (2017) 13:813–20. doi: 10.2147/TCRM.S132344
27. Lang C, Wang M, Chiang C, Lu K. Vitamin D and the immune system from the nephrologist’s viewpoint. ISRN Endocrinol. (2014) 2014:105456. doi: 10.1155/2014/105456
28. Mattila C, Knekt P, Männistö S, Rissanen H, Laaksonen M, Montonen J, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. (2007) 30:2569–70. doi: 10.2337/dc07-0292
29. Kovesdy C, Furth S, Zoccali C. Obesity and kidney disease: hidden consequences of the epidemic. Braz J Nephrol. (2017) 39:1–10. doi: 10.5935/0101-2800.20170001
30. Mori J, Patel V, Ramprasath T, Alrob O, DesAulniers J, Scholey J, et al. Angiotensin 1–7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am J Physiol Renal Physiol. (2014) 306:F812–21. doi: 10.1152/ajprenal.00655.2013
31. Hall M, do Carmo J, da Silva A, Juncos L, Wang Z, Hall J. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis. (2014) 7:75–88. doi: 10.2147/IJNRD.S39739
32. Bernardo D, Carolina de Braganca A, Heloisa Massola Shimizu M, Carlos Seguro A, Moura Nascimento M, Canale D, et al. MO417: obesity associated with vitamin D deficiency impairs renal function, haemodynamics, metabolic parameters and aggravates the renal morphological damage in rats submitted to ischaemia-reperfusion injury. Nephrol Dial Transplant. (2022) 37(Suppl. 3):gfac070.31. doi: 10.1093/ndt/gfac070.031
33. Bouillon R, Norman A, Lips P. Vitamin D deficiency. N Engl J Med. (2007) 357:1980–1. doi: 10.1056/NEJMc072359
34. Santoro D, Caccamo D, Lucisano S, Buemi M, Sebekova K, Teta D, et al. Interplay of vitamin D, erythropoiesis, and the renin-angiotensin system. Biomed Res Int. (2015) 2015:145828. doi: 10.1155/2015/145828
35. Kim C, Kim S. Vitamin D and chronic kidney disease. Korean J Intern Med. (2014) 29:416–27. doi: 10.3904/kjim.2014.29.4.416
36. Vaidya A, Williams J. Vitamin D in the pathophysiology of hypertension, kidney disease, and diabetes: examining the relationship between vitamin D and the renin-angiotensin system in human diseases. Metabolism. (2012) 61:450–8. doi: 10.1016/j.metabol.2011.09.007
37. Freundlich M, Quiroz Y, Zhang Z, Zhang Y, Bravo Y, Weisinger J, et al. Suppression of renin-angiotensin gene expression in the kidney by paricalcitol. Kidney Int. (2008) 74:1394–402. doi: 10.1038/ki.2008.408
38. Takemoto F, Shinki T, Yokoyama K, Inokami T, Hara S, Yamada A, et al. Gene expression of vitamin D hydroxylase and megalin in the remnant kidney of nephrectomized rats. Kidney Int. (2003) 64:414–20. doi: 10.1046/j.1523-1755.2003.00114.x
39. Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Rheum Dis Clin North Am. (2012) 38:125–39. doi: 10.1016/j.rdc.2012.03.012
40. Chen S, Sims G, Chen X, Gu Y, Chen S, Lipsky P. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. (2007) 179:1634–47. doi: 10.4049/jimmunol.179.3.1634
41. Brown A, Coyne D. Bioavailable vitamin D in chronic kidney disease. Kidney Int. (2012) 82:5–7. doi: 10.1038/ki.2012.135
42. Goldsmith D. Pro: should we correct vitamin D deficiency/insufficiency in chronic kidney disease patients with inactive forms of vitamin D or just treat them with active vitamin D forms? Nephrol Dial Transplant. (2016) 31:698–705. doi: 10.1093/ndt/gfw082
43. Klingberg E, Olerod G, Konar J, Petzold M, Hammarsten O. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine. (2015) 49:800–8. doi: 10.1007/s12020-015-0548-3
Keywords: vitamin D deficiency (VDD), renal event, CKD stage, propensity score match, cohort study [or longitudinal study]
Citation: Lee J, Bae EH, Kim SW, Chung W, Kim YH, Oh YK, Kim Y-S, Oh K-H and Park SK (2023) The association between vitamin D deficiency and risk of renal event: Results from the Korean cohort study for outcomes in patients with chronic kidney disease (KNOW-CKD). Front. Med. 10:1017459. doi: 10.3389/fmed.2023.1017459
Received: 12 August 2022; Accepted: 31 January 2023;
Published: 16 February 2023.
Edited by:
Anil Kumar Pasupulati, University of Hyderabad, IndiaReviewed by:
Fabio Rosario Salerno, ASST Lecco, ItalyCopyright © 2023 Lee, Bae, Kim, Chung, Kim, Oh, Kim, Oh and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kook-Hwan Oh,  a2hvaEBzbnUuYWMua3I=; Sue K. Park,
a2hvaEBzbnUuYWMua3I=; Sue K. Park,  c3VlcGFya0BzbnUuYWMua3I=
c3VlcGFya0BzbnUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.