- 1Centre for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 2Enteric Diseases and Vaccines Research Unit, Centre for Infectious Disease Research in Zambia (CIDRZ), Lusaka, Zambia
- 3Department of Nursing, College of Health Science and Medicine, Dilla University, Dilla, Ethiopia
- 4Levy Mwanawasa University Teaching Hospital, Department of Internal Medicine, Lusaka, Zambia
- 5School of Public Health, Saint Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia
Background: Cryptococcal meningitis (CM) is a leading cause of adult meningitis in countries with a high burden of HIV. It has remained a significant cause of morbidity and mortality in Africa despite the extensive rollout of HIV antiretroviral therapy (ART). This study aimed to systematically synthesize the evidence on the prevalence of CM among people living with HIV (PLWH) and its predictors of mortality among adults who are on induction antifungal therapy in Africa.
Methods: PubMed/MEDLINE, Embase, and Google Scholar were searched for randomized clinical trials or observational studies published in Africa from 1995 to April 2021. Pooled prevalence of CM among PLWH was calculated using R-studio Version 1.4.1717 software and the data extracted from eligible studies were pooled as percentage with a 95% confidence interval (CI). Predictors of mortality among adults on induction antifungal therapy were synthesized narratively.
Results: Out of 364 studies identified, 17 eligible articles were included in the analysis. The prevalence of CM among PLWH in Africa was 5.11% (95% CI 2.71–9.43%; participants = 10,813; studies = 9; I2 = 97%). In the subgroup analysis, the prevalence was 12.9% (95% CI 4.883–30.0; participants = 533; studies = 3; I2 = 63%) in the years 1995–2010 and 3.18% (95% CI 1.54–6.45; participants = 10,280; studies = 6; I2 = 98%) in the years 2011–2021, with the prevalence significantly decreased by 51% (p = 0.02). Predictors of mortality were fluconazole monotherapy, focal neurological signs, low Glasgow coma scale, and delayed diagnosis of CM at varied timepoint.
Conclusion: Prevalence of CM has significantly decreased from 1996–2010 to 2011–2021 among PLWH on induction therapy in Africa. Fluconazole monotherapy, focal neurological symptoms, diastolic blood pressure < 60 mmHg, and concurrent tuberculosis coinfection were significant predictors of mortality at 2- and 10-weeks timepoints. CM remains a major concern among PLWH despite increases in ART coverage. Improved access to effective antifungal therapies is needed in Africa for timely initiation of combination induction therapy and better treatment outcomes of PLWH.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=254113], identifier [CRD42021254113].
Introduction
Cryptococcal Meningitis (CM) is one of the top causes of meningitis in adults in Sub-Saharan Africa (SSA) and other regions with a high prevalence of Human Immuno-deficiency Virus (HIV), accounting for over 100,000 incident cases of meningitis per year in the region. Globally, about 15% of HIV-related deaths are attributable to CM and 75% of these occur in SSA (1–3). It was further estimated that 10–20% of HIV-related deaths in Africa are due to CM (4). Although the global incidence and deaths from HIV have continued to decline owing to increased access to antiretroviral therapy (ART), including pre- and post-exposure prophylaxis, the prevalence of the disease has not declined (5).
CM, an immune-compromise-associated fungal infection caused by Cryptococcus neoformans or Cryptococcus gattii, has a high mortality rate in both HIV-negative and HIV-positive individuals, with a cumulative mortality of about 65% at 1 year (4, 6, 7). It is commonly diagnosed in HIV-positive patients with a CD4 count lower than 100 cells/μl. Mortality from the disease has remained high despite increased ART access and this is true for 20–50% of hospitalized patients (6, 8, 9) and commonly in the immuno-compromised (7, 10) and those with a history of defaulting or other comorbid conditions (11).
Adequate antifungal therapy and physical removal of excess cerebrospinal fluid (CSF) to relieve acute symptoms are the mainstays of CM treatment (12, 13). The World Health Organization 2018 guidelines targeting low- and middle-income countries recommend the use of amphotericin B (AmB) dosed at 1 mg/kg/day for a week with flucytosine (5-FC) in four divided doses of 100 mg/kg/day. To complete the induction therapy phase, fluconazole (FLU) at 1200 mg/day is also administered in the following week, and 800 mg/day of FLU for 8 weeks and 200 mg/day are given in the consolidation and maintenance phases, respectively (14). Though preferable, AmB has not been widely available across the SSA due to high cost, thereby making FLU monotherapy the mainstay of therapy (15, 16). Depending on availability, drugs recommended for the induction phase included a 2 weeks FLU and 5-FC or a 2 weeks AmB and FLU (14). Though AmB is one of the most effective, it has been associated with nephrotoxicity and anemia which have limited its use in patients with preexisting renal dysfunction or baseline hemoglobin level of < 8 g/dl (12, 17, 18).
Several studies have documented a significant association between early mortality and altered level of consciousness or sensorium. Patients who presented with either a low Glasgow coma scale, altered behavior, or simply altered mental status died or had a longer hospital admission (19–22). Furthermore, a CD4 count between 100 and 200 cells/mm3 predisposes one with CM to worse symptoms and clinical outcomes (23). Anemia is a hematological finding that has been reported as a significant contributor to 2 and 10-week mortality if present in the moderate to severe forms at baseline (24).
Survival of HIV-related CM patients has improved in the advent of ART compared to previous periods, while mortality in HIV-related CM has remained high (20, 25, 26). Individual studies on the subject of mortality predictors in HIV-related CM either used low sample sizes, compared only baseline variables at study entry points, considered patients not naïve to therapy, or focused on a single antifungal regimen (27, 28). Further, some studies have stated that CM is a metric of HIV treatment failure and so it becomes important to consider all the factors contributing to CM mortality to better assess HIV programming and make informed policy recommendations (3). Thus, it is important to understand the prevalence of CM among people living with HIV (PLWH) and predictors of mortality in patients on induction therapy in high HIV-endemic settings.
To the best of our knowledge, few reviews have been done documenting the pooled continental prevalence of the disease in Africa or short to medium-term mortality determinants of HIV-related CM among adults for the region (29–31). This review aimed to synthesize pooled prevalence of HIV-related CM and identify predictors of mortality among patients on induction antifungal therapy for the disease.
Methods
The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) database, ID: CRD42021254113. The methods and findings of the review have been reported according to preferred reporting for systematic reviews and meta-analyses (PRISMA 2020) (32).
Eligibility
Eligible studies were identified using the population, intervention, comparison, outcome and study design (PICOS) model.
• Participants
– Adult male and non-pregnant female with confirmed HIV and CM.
• Intervention
– Anti-CM therapy, which could be AmB based regimens, 5-FC, or FLU, or a combination of either of these drugs.
• Outcomes
– Primary outcome:
○ The prevalence of HIV-related CM in Africa. Prevalence data on CM was obtained by calculating the number of PLWH for whom CM was confirmed amongst the total number of PLWH included during the data collection period.
– Secondary outcomes:
○ Pooled prevalence of cryptococcal antigenemia;
○ All-cause mortality reported by 2 weeks (for short-term) and by 10-weeks (for medium-term) of therapy, and their association with identifiable predictors of mortality;
○ Predictors of mortality, including a history of or persisting headache, nausea and vomiting, visual disturbance, history of tuberculosis, and history of ART use.
○ Physical examination predictors include fever, Glasgow coma scale of less than 15/15, neck stiffness, altered mental status, and high opening pressure (> 25 mmH2O).
○ Other predictors including aneamia (heamo globin < 7.5 g/dl), low CD4 (200 copies/ml), high CSF white cell count (> 5 cells/mm3), low plasma glucose (< 0.6 mmol/l), high CSF protein (> 0.5 mg/dl), high fungal burden and evidence of renal dysfunction.
• Studies
– Randomized controlled trials and observational studies published from 1995 to April 2021 and written or translated into English.
Electronic searches
A systematic literature search was done to identify relevant articles from online databases PubMed/MEDLINE, Embase, and Google Scholar. Studies were retrieved using medical subject headings (MeSH) and a combination of subject words and free words. The search term for PubMed was (((“meningitis, cryptococcal”[MeSH Terms] OR “cryptococcal”[Text Word] OR “cryptococcosis”[Text Word] OR “Cryptococcus neoformans”[Text Word] OR “cryptococcus gattii”[Text Word] OR “cryptococc*”[Text Word]) AND (“HIV”[MeSH Terms] OR “human immunodeficiency virus”[Text Word] OR “AIDS”[Text Word])) AND (“Death”[MeSH Terms] OR “Mortality”[MeSH Terms] OR “Mortality”[MeSH Subheading] OR “death*”[Text Word] OR “died”[Text Word] OR “non-survivor*”[Text Word] OR “non-survival”[All Fields] OR “poor outcome*”[Text Word] OR “fatal”[Text Word])) AND (“Africa”[MeSH Terms] OR Africa[Text Word]). A detailed search strategy for all databases is found in the Supplementary Appendix 1. Selected study references were checked for possible additional papers.
Study selection
Two independent reviewers (SGYM, DGA) performed the search selected articles by title and abstract and non-relevant articles were excluded. From the title and abstract of all publications, those that were duplicated or did not meet the inclusion criteria were excluded. The full articles of the remaining studies were further reviewed. Any disagreements between reviewers were solved through consensus.
Data extraction
The identified data were listed, and information was provided based on the study’s outcomes of interest. For the study on the primary outcome, thus the prevalence of CM, the data extraction format included the surname of the first author, year and country of publication, sample size, the total number of cases, and prevalence of CM with 95% CI. For studies on the secondary outcomes, the data extraction format included the surname of the first author, year and country of publication, study period, study design, sample size, demographic, and baseline characteristics of the participants, ART history, clinical and laboratory features at presentation and during admission, interventions, follow-up period, and outcomes.
Quality and risk of bias assessments
We used the Newcastle Ottawa Scale (NOS) for assessing the quality of included observational studies (33). This ROB tool can be used for cohort and cross-sectional studies, and it includes three main domains and eight subdomains covering selection, comparability, and outcome. Each subdomain receives 1 star with 2 stars being maximum for others and scores range from 0 to 9. The risk of bias for each trial was evaluated by two review authors independently using the Cochrane Collaboration’s tool for assessing the “Risk of bias.” This guidance was used to decrease the risk of bias amongst six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias. The risks were classified as high risk, unclear risk, and low risk (34). To assess the possibility of publication bias, a funnel plot for asymmetry (Egger’s test P < 0.05) was used.
Data management and statistical analysis
Selected studies were imported from databases using ENDNOTE X7 software. Data extracted from selected studies was first summarized in Microsoft Excel. To make the distribution of the data normal, we first transformed the data using the logit transformation. Meta-analysis was done using R-studio Version 1.4.1717 software to determine the pooled prevalence of HIV-related CM. We also conducted a trend of prevalence comparison for the period before and after 2010 taking into account the IDSA updated guideline on cryptococcal meningitis management which included PLWH as a risk group (35). A systematic narrative synthesis was done for mortality predictors and results were summarized using texts and tables. Studies were shown in forest plots in chronological order of the year the studies were published. A random-effects model was used since trials were done by different researchers operating independently, and it could be implausible that all trials had functional equivalence, with a common effect estimate.
Heterogeneity among the included studies was assessed by inspecting the forest plots and the Cochrane Q and I2 statistic used to measure heterogeneity among the trials in each analysis, the Chi2 test with a P < 0.10 to indicate statistical significance was used. The results were interpreted following Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (36). Subgroup analysis was done by dividing the studies into two groups based on the year when the studies were conducted. Meta-regression was used to investigate the association of study characteristics that cause heterogeneity with the prevalence. The covariates were Year the study was conducted. To assess the influence of small-study effects on the results of our meta-analysis, fixed-effect and random-effects estimates of the intervention effect were compared.
Statement of ethics compliance
This article was based on previously conducted studies and did not contain any studies with human participants or animals performed by any of the authors.
Results
Search results
After a systematic searching of databases and other sources, 364 studies were retrieved and sequentially screened for eligibility, after which 17 studies were included. Figure 1 summarizes the PRISMA flowchart of the study.
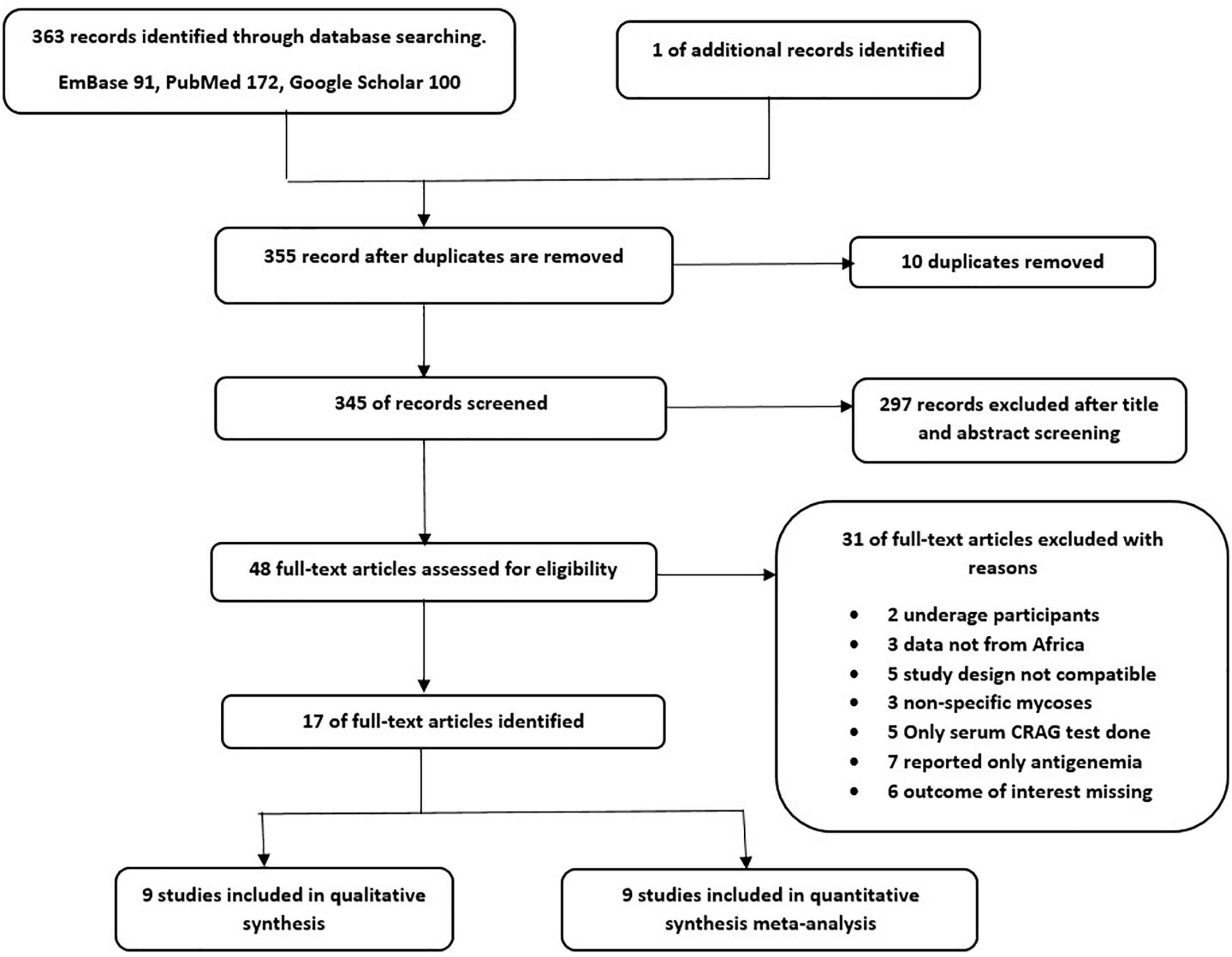
Figure 1. PRISMA Flow diagram of literature selection (1 study was used in both narrative synthesis and meta-analysis).
Study characteristics
A total of 17 studies were included in this review with 9 being used for the meta-analysis and 9 for the qualitative/narrative synthesis. One study (37) reporting both prevalence and mortality predictors was included in both the narrative review and meta-analysis.
In our NOS risk of bias assessment, all our studies scored from 6 to 9 and hence had at least a moderate quality (Supplementary Appendix 2). For the two randomized controlled trials included, the risk of bias assessment results are summarized in Figure 2.
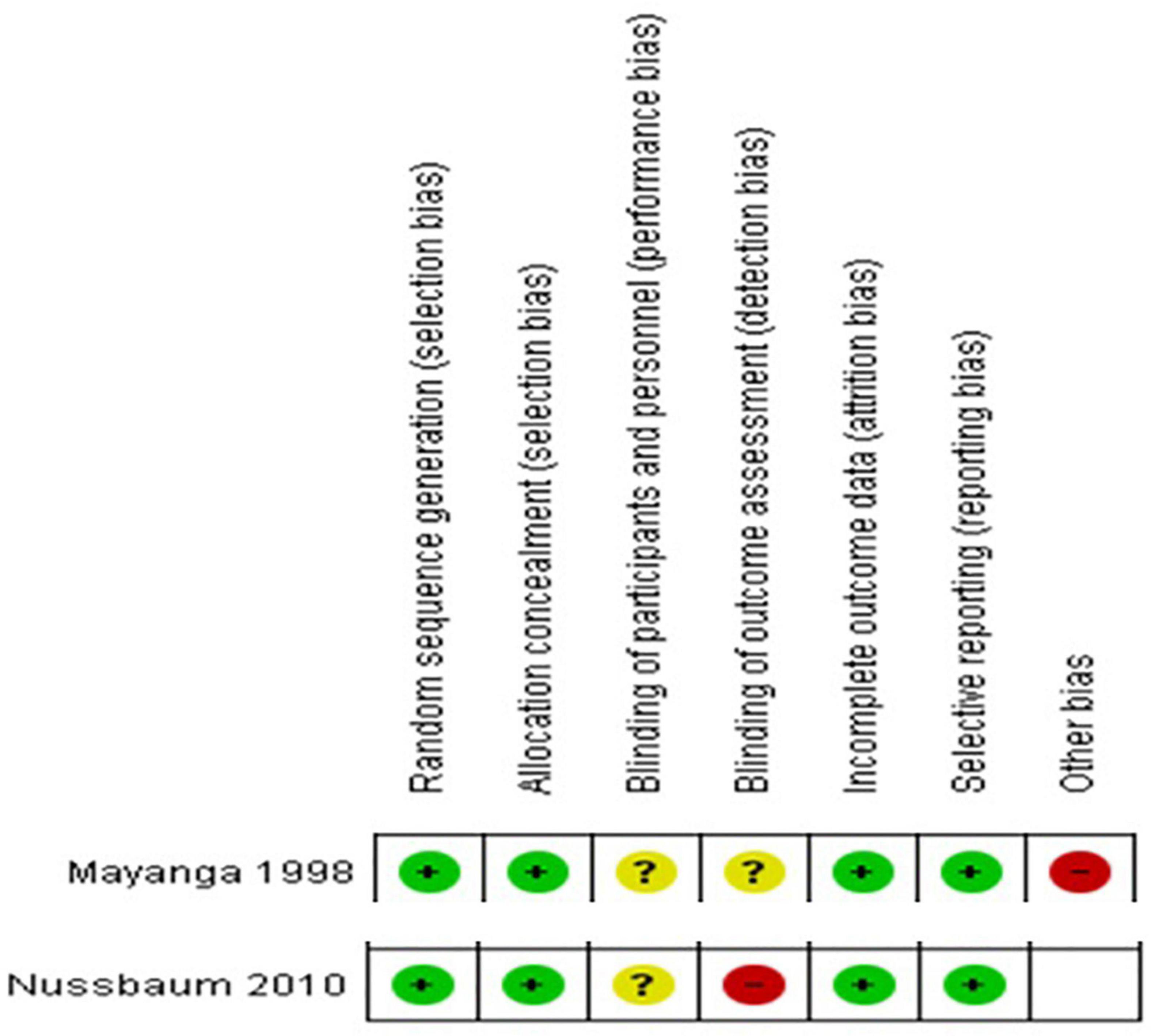
Figure 2. Risk of bias summary- review authors’ judgments about each risk of bias item for each included RCT.
Prevalence of cryptococcal meningitis among people living with HIV
Of the 17 studies included, 9 reported on the prevalence of CM among PLWH (Table 1).
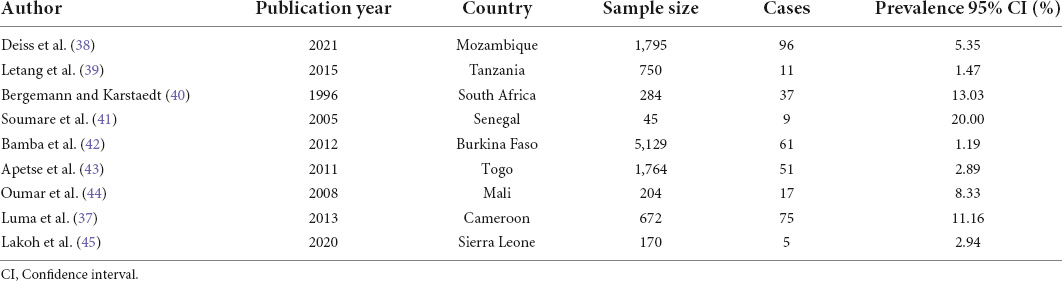
Table 1. Characteristics of studies included in the prevalence meta-analysis of CM among PLWH in Africa, 1995–2021.
The reported prevalence ranged from 1.2 to 20.00%. The prevalence of CM in PLWH in Africa was 5.11% (95% CI 2.71–9.43%; participants = 10,813; studies = 9; I2 = 97%) (Figure 3). There was high heterogeneity among included studies (P < 0.01).
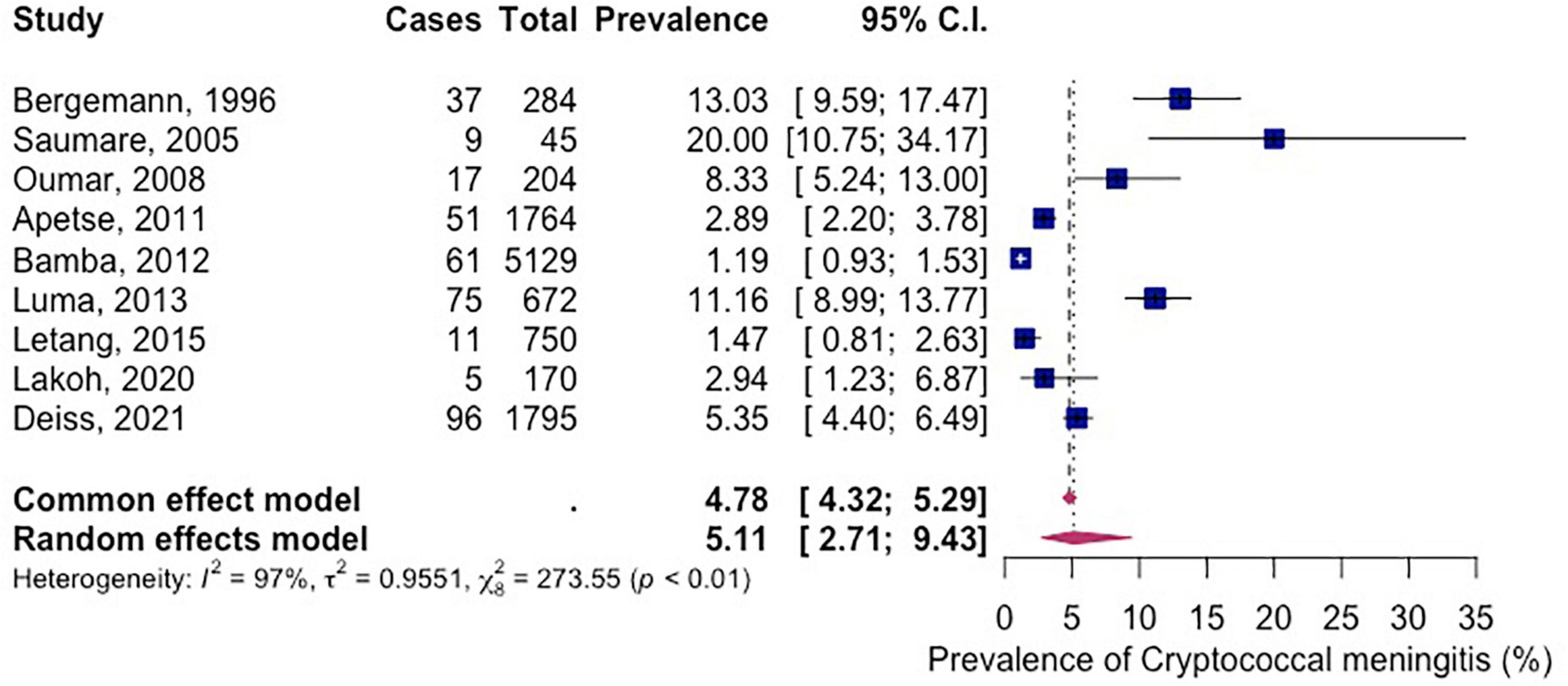
Figure 3. Forest plot of prevalence of HIV related cryptococcal meningitis in Africa (random effects), 1995–2021.
The subgroup analysis shows that the prevalence of CM among PLWH between the years 1996–2010 was 12.9% (95% CI 4.883–30.0; participants = 533; studies = 3; I2 = 63%) and between the years 2011–2021 was 3.18% (95% CI 1.54–6.45; participants = 10,280; studies = 6; I2 = 98%) (Figure 4). There is significant subgroup difference between the two groups (P = 0.02).
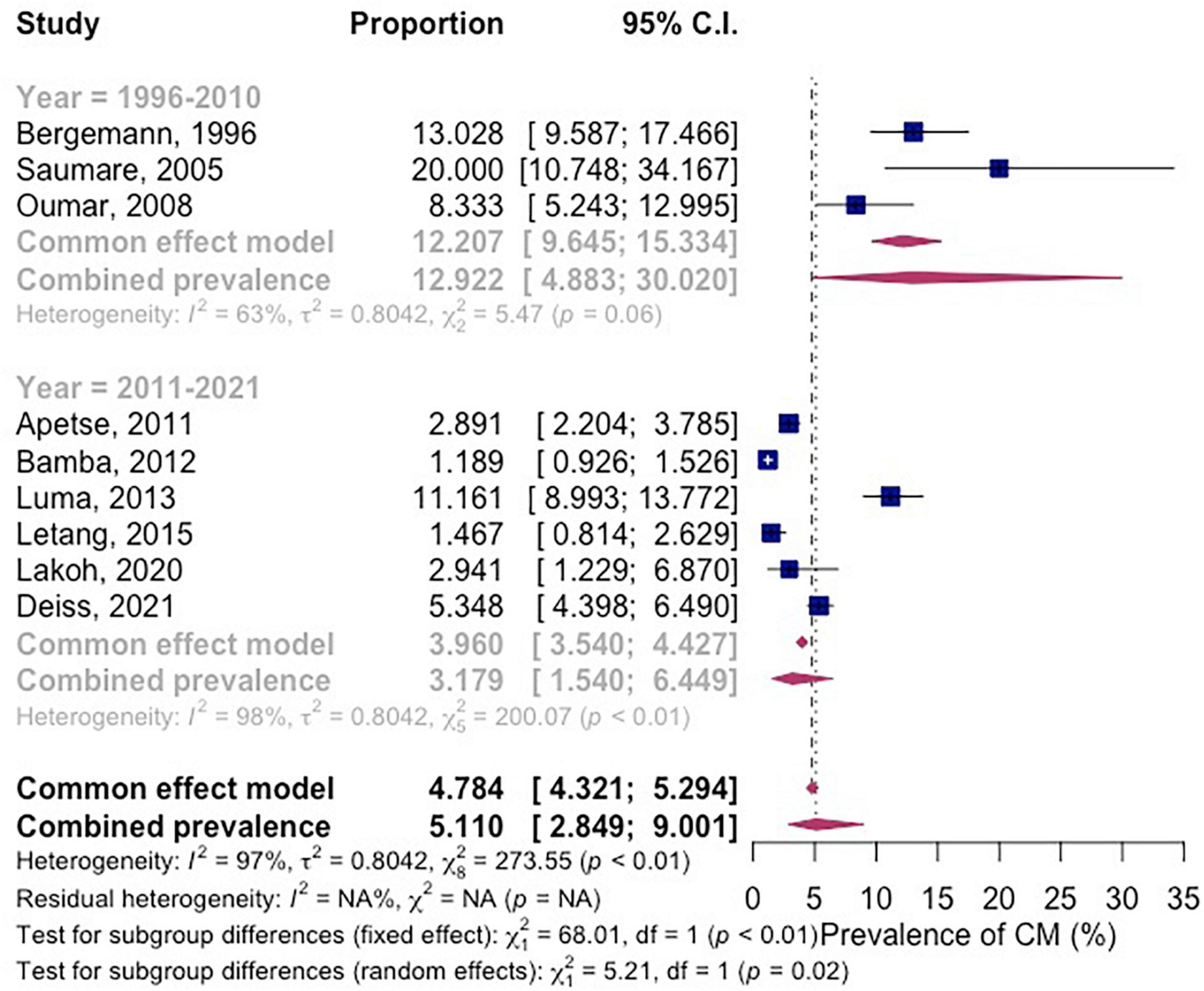
Figure 4. Forest plot of subgroup analysis by year of the studies were conducted for pooled prevalence in included studies.
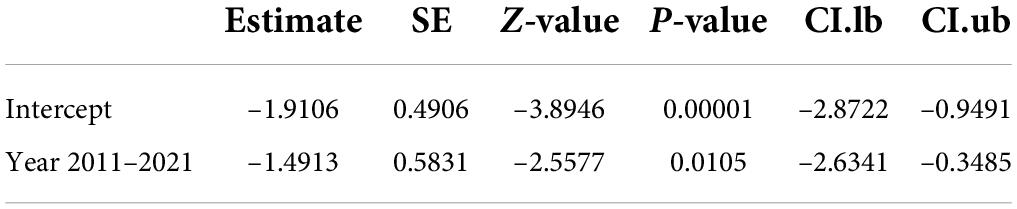
The meta-regression result shows that 96.59% of the observed variance about the regression line reflects variation in true effects rather than sampling error. The test for heterogeneity yields a Q-value of 205.53 with 7 degrees of freedom and a corresponding p-value of < 0.0001. We concluded that the model fully explains the variation in effects. The R2 analog is 15.80%, which tells us that the model can explain some 16% of the variance in prevalence. The coefficient was –1.5083 (95% CI –2.28 to 0.21). Between the years 2011–2021, the prevalence of CM in Africa decreased by 51% as compared to the year 1996–2010 (Table 2).
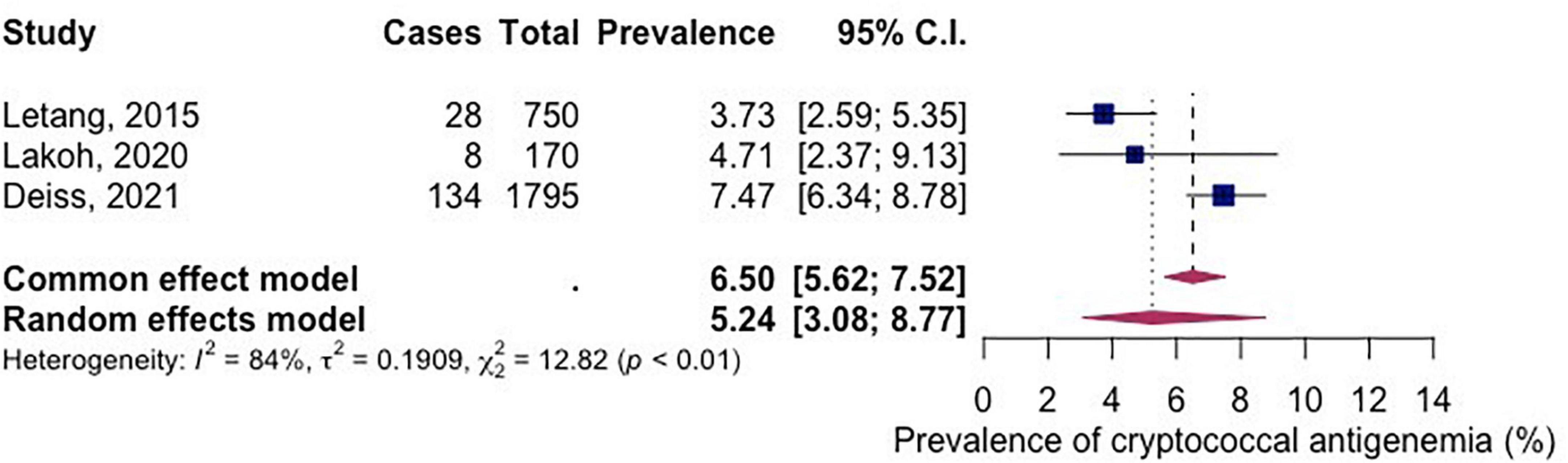
Three studies had reported the prevalence of cryptococcal antigenemia. The pooled prevalence of cryptococcal antigenemia (among included studies) in PLWH in Africa between the year 2015–2021 was 5.24% (95% CI 3.08-8.77; participants = 2,715; studies = 3; I2 = 84%) (Figure 5).
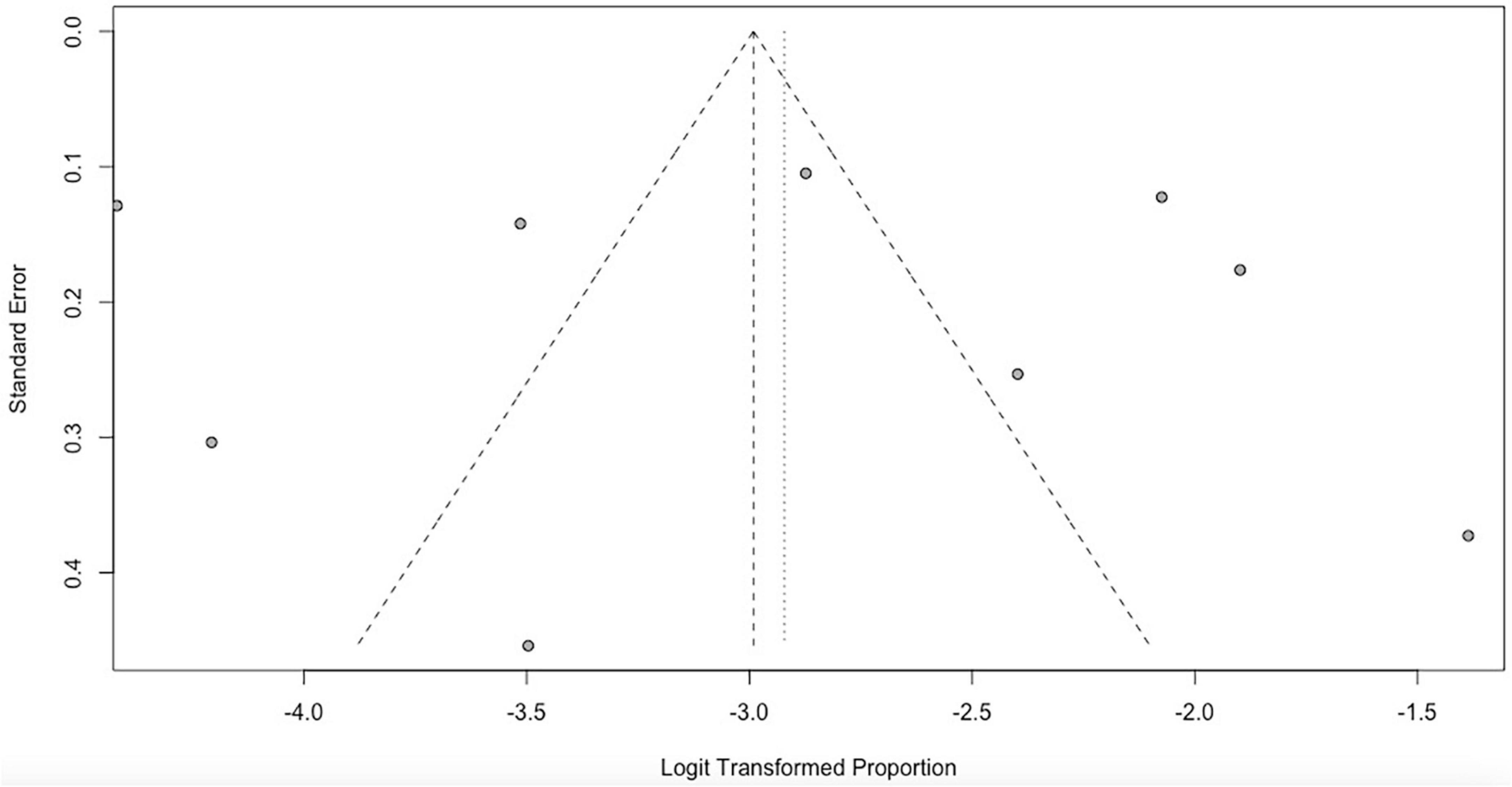
The funnel plot showed that all studies lay symmetrically around the pooled effect estimate implying that there was no publication bias (Figure 6).
Predictors of mortality
Of the 17 studies included, 9 reported the predictors of mortality among PLWH on CM induction therapy (Table 3). Majority of participants were male aged 32–36 years, with all studies reporting CD4 counts less than 100 cells/ml of which, eight of nine studies reported CD4 less than 50 cells/ml. Four studies reported some history of prior ART use among participants while only two studies mentioned that all participants were ART naïve. Furthermore, majority of the participants were treated with one or a combination of Amphotericin, Fluconazole, and Flucytosine.
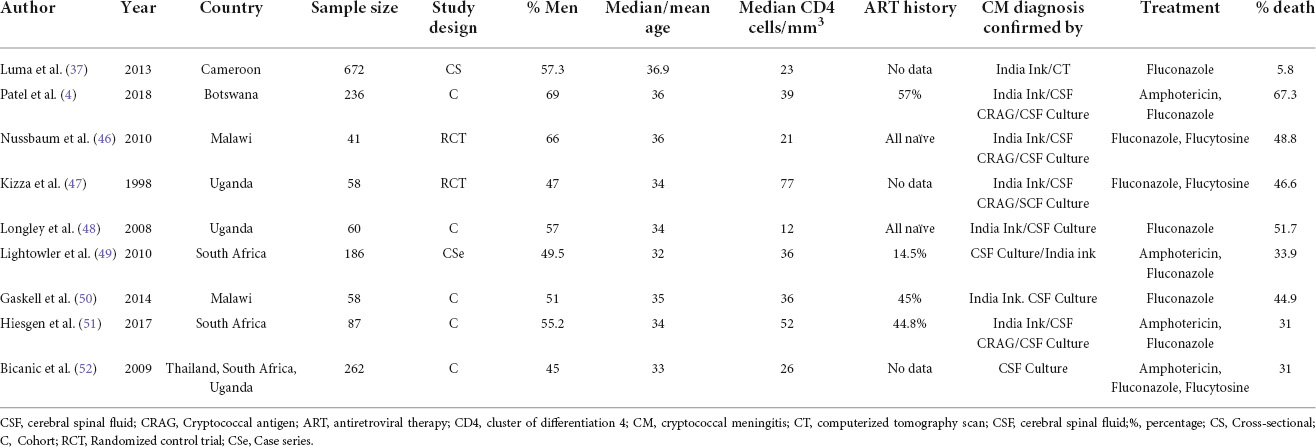
Table 3. Characteristics of included studies in the narrative synthesis of mortality predictors among PLWH on induction therapy in Africa, 1998–2018.
At least two studies reported FLU monotherapy, focal neurological signs, low GCS and delayed diagnosis of CM as predictors of mortality regardless of time point. FLU monotherapy was reported by at least two studies while focal neurological symptoms, DBP < 60 mmHg, and current TB coinfection (TB) were reported as significant predictors of mortality by a single study at both 2 and 10 weeks timepoints. Other significant predictors reported by at least one study at 2 weeks timepoint includes prior ART use and high serum sodium level. Additionally, low CD4 count, altered level of consciousness, high white blood cell count, low CSF white cell count and high serum sodium were reported as predictors of mortality at 10 weeks timepoint (Table 4).
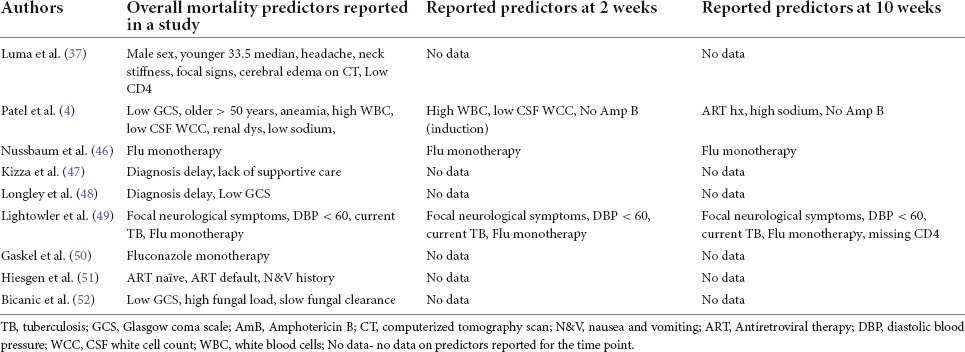
Table 4. Summary of reported predictors of mortality for HIV-related CM for each study included in the narrative synthesis.
Discussion
CM is an important cause of adult meningitis in PLWH presenting with severe immunosuppression (3). Though ART access has improved via the WHO test and treat program, CM continues to be perpetuated by failures in the ART systems apparent in Africa (2). Our systematic review and meta-analysis of 12,473 participants aimed to establish the prevalence of CM in HIV-positive individuals and its predictors of mortality.
The majority of the studies in our review confirmed CM diagnosis by India ink/CSF culture. CSF fungal culture is the gold standard and is often preferred over Indian ink for its higher sensitivity (53). However, culture has drawbacks which include being more expensive and delaying diagnosis by 3–7 days (54). The use of serum cryptococcal antigen (CRAG) testing is indicated as a screening method for likely CM in advanced HIV and is only used to commence prophylactic therapy among CRAG-positive individuals (14, 55).
In this review, over 80% of the studies included reported a CD4 count of less than 50 cells/mm3 at baseline. Unlike most studies that suggest that the occurrence of CM is common in those with CD4 count less than 200, our study found an even lower CD4 count as a common finding (23, 56). This could explain why the majority of our studies had a mortality percentage greater than 30% which was comparable to 44% (short-term mortality) reported by Tenforde et al. (31) and 36.7% by Majumder et al. (57). As the majority of the included studies reported less than 50% prior ART use or none at all, we infer that our population had advanced immunosuppression and hence explaining the much lower than usual CD4 count. Both low CD4 count and delayed ART initiation are risks for opportunistic infections such as CM among PLWH.
Our review results showed that the prevalence of CM among PLWH in Africa ranged between 1.4 and 20.0% with a pooled prevalence of 5.11% (95% CI 2.71–9.43%; participants = 10,813; studies = 9; I2 = 97%). This finding was not similar to the global percentage of CM in AIDS-related deaths which stands at 15% (3). The result was comparable to a global burden of disease study from 2009 which estimated the burden of CM among PLWH between 0.04 and 12% with SSA leading in annual numbers (58). In contrast, our result was much lower than that from a western Indian study that reported a 10-year prevalence ranging between 49 and 100%. In that study, only 23.3% of its participants were HIV positive and just over half of them tested positive for CM (59). This implies that the overall CM prevalence among PLWH in the study could have been much lower or even closer to our African estimate. A systematic review by Derbie et al. found the prevalence of cryptococcal antigenemia to be 8% (95% CI 6–10) which was almost double our prevalence for CM which was logical since not all cases of cryptococcal antigenemia result in invasive cryptococcal disease (55). Although cryptococcal antigenemia is not equivalent to a diagnosis of CM, it has been thought of as an important proxy measure for CM in PLWH (60).
Meta-regression of prevalence comparing the period from 1996 to 2010 and that from 2011 to 2021 demonstrated a significant reduction of HIV-related CM of about 51%. This observation could be credited to the evolved WHO ART treatment guideline that included triple therapy for all HIV-positive patients regardless of CD4 count (61). Early ART initiation prevents the occurrence of opportunistic infections (62).
Biases may have been introduced in our calculation of pooled prevalence since included studies only focused on patients who presented to the health facility. This may have introduced participant selection bias and undermined our disease prevalence. Additionally, patient populations from the studies included had inert variations that could have influenced our synthesized prevalence. Our prevalence conclusion may have been overestimated, as our study utilized data for PLWH with CD4 counts less than 100 cells/ml which could be more representative of an annual incidence for persons with low CD4 count in Africa.
This study found that low GCS (< 15/15), focal neurological signs, delay in CM diagnosis, and FLU monotherapy were significant predictors of mortality for CM in PLWH. Low GCS, defined as a score < 15/15 or a reduced level of consciousness, was supported as a mortality predictor by studies looking at both short-term and long-term mortality predictors which applied cox regression models to establish associations (57, 63). Another study done in Uganda linked low GCS to seizure occurrence and these were associated with higher 10-week mortality (64). Delayed CM diagnosis results in an increase of fungal load which in turn accelerates the occurrence of raised intracranial pressures causing a lower GCS and focal neurological symptoms (65–67). Interventions such as serial CSF drainage via lumbar puncture in addition to antifungal therapy, continue to prove invaluable to patient survival (68). In 2018, WHO introduced a strategy to reduce death from HIV-related CM with the use of combined antifungal therapy and routine serum CRAG screening. Our study found that FLU monotherapy, though not a standard of care regimen, had continued to be majorly used in Africa and this was according to Loyse et al. (69). This was possibly perpetuated by the high cost, need for in-hospital administration and need for frequent laboratory monitoring of AmB and 5FC (61, 70). FLU monotherapy even at high doses continues to be a sub-optimal treatment option for HIV-related CM due to its fungistatic nature that results in a slow fungal clearance rate and consequently a higher 2 and 10-week mortality rate (54, 71, 72).
Possible predictors for mortality at 2 weeks were found to be low CSF white cell count (WCC), focal neurological symptoms and FLU monotherapy. Low CSF white cell count was also reported as a mortality predictor at day-14 by a study done in Thailand and United States (73). Patients diagnosed with CM in HIV usually have a CD4 count < 200 cells/mm3 which translates to an inability to mount an adequate immune response, which could explain why low WCC was found to be a significant risk factor for early mortality (74). Focal neurological signs in CM occur as a result of raised intracranial pressure or the presence of space-occupying lesions. A study from Thailand documented focal deficits in 12% of participants while an Indian study found it in 13% of their participants (70, 75). Only 50% of our studies reported a raised intracranial pressure while others mentioned that measuring this was not possible due to lack of equipment or that it was not routinely done. Delayed fungal clearance that is associated with monotherapy could explain why FLU monotherapy was significant at as early as 2 weeks of therapy.
In this study, FLU monotherapy was found to be the only risk factor reported by at least 2 of the included articles as a predictor of mortality at 10 weeks. This finding consolidates evidence that though an inferior treatment option, FLU monotherapy remains largely used in Africa due to the high cost of AmB and unavailability of 5FC (69).
Interestingly history of ART use was indicated by one article as a predictor of mortality at 10 weeks. It is well known that the timing of ART in those found to have CM is key, due to the risk of immune reconstitution syndrome (IRIS). It is therefore logical to expect that those already on ART before CM diagnosis would have a higher CD4 count and hence better outcomes (14). Our result though seemingly contradictory, was similar to results from a study done by the Caribbean, Central and Southern American Network for HIV Epidemiology. They found that among 340 adult patients with HIV, mortality was high among those that had started ART 2–3 years before CM diagnosis (p-value = 0.03) (76). This may be due to the presence of virological failure (secondary to poor adherence) coupled with immunological failure resulting in CM infection. It may also have been due to a reactivation of “dormant” prior CM infection or IRIS (77, 78). Another study done in Uganda stratified 605 participants as ART naïve or not and also by duration on ART before CM diagnosis. They found no difference in the 2-week mortality by ART groups but those who received ART ≤ 14 days prior to CM diagnosis had higher mortality of about 47%. This agrees with our study that found no association with ART history at 2 weeks.
Using retrospective studies to predict the outcome of HIV-related CM is difficult due to variabilities in patient’s treatment regimens, routine care such as serial LP and comorbidities. Further, not all studies used regression and survival analysis in addition to the linear correlation between factors assessed. The result was inadequate accounting for possible overlapping effects that may have confounded and overestimated the strength of associations.
This systematic review only included studies that were published or translated into the English language, and this may have resulted in the exclusion of possibly relevant studies, hence reducing our precision. Included studies did not have a standardized data collection method with relation to outcome measurements and their time points. This affected our capacity to conclude using more inferential statistical methods rather than simple narrative analysis. We were also unable to quantitatively estimate the excess risk of identified risk factors which unadvertently decreased statistical power or usefulness of our conclusion on mortality risk. Sparse data were available for inclusion in our review as the majority of studies undertaken in Africa looked at cryptococcal antigenemia rather than CM prevalence. Cryptococcal antigenemia is not a proxy for CM prevalence as they are very different disease states, but it remains useful for generating data on CM screening and early treatment policies. Our study was, however, one of the first to attempt to systematically review the pooled prevalence of CM for PLWH in Africa and its mortality predictors.
Conclusion
The findings indicate that CM remains of concern in Africa despite the increase in ART coverage among PLWH. The subgroup analysis shows that the prevalence of CM has significantly decreased from 1996–2010 to 2011–2021 among PLWH on induction therapy living in Africa. FLU monotherapy, focal neurological symptoms, diastolic blood pressure (DBP) < 60 mmHg, and current tuberculosis coinfection were significant predictors of mortality at 2- and 10-weeks time points. Efforts must be made to increase the availability of standard antifungal therapy for implementing early combined induction therapy in Africa.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
SM and DA developed the protocol, reviewed the reference list, extracted data, and entered it into R-studio for analysis, performed the analysis, constructed the summary tables, and extracted result figures. SM, DA, CP, TG, SS, GY, and TM were responsible for ensuring quality of the data reported and manuscript review. All authors have read and approved the final manuscript.
Acknowledgments
We would like to express our gratitude to the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, for funding the study. This review was supported by Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), Addis Ababa University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.989265/full#supplementary-material
Abbreviations
ACTA, Advancing Cryptococcal Meningitis Treatment for Africa; AIDS, Acquired Immuno-Deficiency Virus; AmB, Amphotericin B; ART, Antiretroviral Therapy; ATT, Anti-Tuberculous Therapy; CD4, cluster of differentiation 4; CM, Cryptococcal Meningitis; CRAG, Cryptococcal Antigen; CSF, Cerebral Spinal Fluid; CT, computerized tomography scan; DBP, Diastolic blood pressure, GCS, Glasgow coma scale; 5FU, Flucytosine; FLU, Fluconazole; Hgb, Hemoglobin; HIV, Human Immuno-deficiency Virus; ICP, Intracranial Pressure, LMIC, Low and Middle-Income Countries, LP, Lumbar Puncture; PEP, Post Exposure Prophylaxis; PICO, Population intervention, comparison and outcome; PLWH, People Living With HIV; PrEP, Pre-exposure Prophylaxis; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PTB, Pulmonary Tuberculosis; RCT, Randomized controlled trial; SBP, Systolic blood pressure; SSA, Sub-Saharan Africa; TB, Tuberculosis; WBC, White blood cells; WCC, White cell count, IDSA, Infectious Disease Society of America.
References
1. Britz E, Perovic O, Von Mollendorf C, Von Gottberg A, Iyaloo S, Quan V, et al. The epidemiology of meningitis among adults in a South African province with a high HIV prevalence, 2009-2012. PLoS One. (2016) 11:e0163036. doi: 10.1371/journal.pone.0163036
2. Molloy SF, Chiller T, Greene GS, Burry J, Govender NP, Kanyama C, et al. Cryptococcal meningitis: a neglected NTD? PLoS Negl Trop Dis. (2017) 11:e0005575. doi: 10.1371/journal.pntd.0005575
3. Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV-associated cryptococcal meningitis: an updated analysis. Lancet Infect Dis. (2017) 17:873–81. doi: 10.1016/S1473-3099(17)30243-8
4. Patel RKK, Leeme T, Azzo C, Tlhako N, Tsholo K, Tawanana EO, et al. High mortality in HIV-Associated cryptococcal meningitis patients treated with amphotericin B-Based Therapy Under Routine Care Conditions in Africa. Open Forum Infect Dis. (2018) 5:ofy267. doi: 10.1093/ofid/ofy267
5. Collaborators GH. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. (2019) 6:e831–59. doi: 10.1016/S2352-3018(19)30196-1
6. Pinheiro SB, Sousa ES, Cortez ACA, da Silva Rocha DF, Menescal LSF, Chagas VS, et al. Cryptococcal meningitis in non-HIV patients in the State of Amazonas, Northern Brazil. Braz J Microbiol. (2020) 52:279–88. doi: 10.1007/s42770-020-00383-1
7. Motoa G, Pate A, Chastain D, Mann S, Canfield GS, Franco-Paredes C, et al. Increased cryptococcal meningitis mortality among HIV negative, non-transplant patients: a single US center cohort study. Ther Adv Infect Dis. (2020) 7:2049936120940881. doi: 10.1177/2049936120940881
8. Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet. (2015) 385:2173–82. doi: 10.1016/S0140-6736(15)60164-7
9. Lawrence DS, Boyer-Chammard T, Jarvis JN. Emerging concepts in HIV-associated cryptococcal meningitis. Curr Opin Infect Dis. (2019) 32:16–23. doi: 10.1097/QCO.0000000000000514
10. Thakur R, Sarma S, Kushwaha S. Prevalence of HIV-associated cryptococcal meningitis and utility of microbiological determinants for its diagnosis in a tertiary care center. Indian J Pathol Microbiol. (2008) 51:212–4. doi: 10.4103/0377-4929.41689
11. Jarvis JN, Harrison TS. HIV-associated cryptococcal meningitis. Aids. (2007) 21:2119–29. doi: 10.1097/QAD.0b013e3282a4a64d
12. Li M, Liu J, Deng X, Gan Q, Wang Y, Xu X, et al. Triple therapy combined with ventriculoperitoneal shunts can improve neurological function and shorten hospitalization time in non-HIV cryptococcal meningitis patients with increased intracranial pressure. BMC Infect Dis. (2020) 20:844. doi: 10.1186/s12879-020-05510-9
13. Boyer-Chammard T, Temfack E, Alanio A, Jarvis JN, Harrison TS, Lortholary O. Recent advances in managing HIV-associated cryptococcal meningitis. F1000Research. (2019) 8:F1000. doi: 10.12688/f1000research.17673.1
14. World Health Organization.Guidelines for the diagnosis prevention and management of cryptococcal disease in hiv-infected adults adolescents and children. Geneva: World Health Organization (2018). p. 1–65.
15. Migone C, Ford N, Garner P, Eshun-Wilson I. Updating guidance for preventing and treating cryptococcal disease in low and middle income countries: how evidence and decisions interface. Cochrane Database Syst Rev. (2018) 30:ED000130. doi: 10.1002/14651858.ED000130
16. Shroufi A, Govender NP, Meintjes G, Black J, Nel J, Moosa M-YS, et al. Time to embrace access programmes for medicines: lessons from the South African flucytosine access programme. Int J Infect Dis. (2020) 95:459–61. doi: 10.1016/j.ijid.2020.02.057
17. Meiring S, Fortuin-de Smidt M, Kularatne R, Dawood H, Govender NP, Germs SA. Prevalence and hospital management of amphotericin b deoxycholate-related toxicities during treatment of HIV-associated cryptococcal meningitis in South Africa. PLoS Negl Trop Dis. (2016) 10:e0004865. doi: 10.1371/journal.pntd.0004865
18. Martinez MG, Villeret F, Testoni B, Zoulim F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. (2020) 40(Suppl 1):27–34. doi: 10.1111/liv.14364
19. Berhe T, Melkamu Y, Amare A. The pattern and predictors of mortality of HIV/AIDS patients with neurologic manifestation in Ethiopia: a retrospective study. AIDS Res Ther. (2012) 9:1–7. doi: 10.1186/1742-6405-9-11
20. Kobayashi T, Pitisuttithum P, Kaewkungwal J, Phuphuakrat A, Sungkanuparph S. Clinical outcomes of cryptococcal meningitis among HIV-infected patients in the era of antiretroviral therapy. Southeast Asian J Trop Med Public Health. (2017) 48:56–64.
21. Qu J, Zhou T, Zhong C, Deng R, Lü X. Comparison of clinical features and prognostic factors in HIV-negative adults with cryptococcal meningitis and tuberculous meningitis: a retrospective study. BMC Infect Dis. (2017) 17:51. doi: 10.1186/s12879-016-2126-6
22. Shih CC, Chen YC, Chang SC, Luh KT, Hsieh WC. Cryptococcal meningitis in non-HIV-infected patients. QJM. (2000) 93:245–51. doi: 10.1093/qjmed/93.4.245
23. Wykowski J, Galagan SR, Govere S, Wallis CL, Moosa M-Y, Celum C, et al. Cryptococcal antigenemia is associated with meningitis or death in HIV-infected adults with CD4 100–200 cells/mm 3. BMC Infect Dis. (2020) 20:61. doi: 10.1186/s12879-020-4798-1
24. Tugume L, Morawski BM, Abassi M, Bahr NC, Kiggundu R, Nabeta HW, et al. Prognostic implications of baseline anaemia and changes in haemoglobin concentrations with amphotericin B therapy for cryptococcal meningitis. HIV Med. (2017) 18:13–20. doi: 10.1111/hiv.12387
25. Kanyama C, Molloy SF, Chan AK, Lupiya D, Chawinga C, Adams J, et al. One-year mortality outcomes from the advancing cryptococcal meningitis treatment for Africa Trial of cryptococcal meningitis treatment in Malawi. Clin Infect Dis. (2020) 70:521–4. doi: 10.1093/cid/ciz454
26. Molloy SF, Kanyama C, Heyderman RS, Loyse A, Kouanfack C, Chanda D, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. New Engl J Med. (2018) 378:1004–17. doi: 10.1056/NEJMoa1710922
27. Anekthananon T, Manosuthi W, Chetchotisakd P, Kiertiburanakul S, Supparatpinyo K, Ratanasuwan W, et al. Predictors of poor clinical outcome of cryptococcal meningitis in HIV-infected patients. Int J STD AIDS. (2011) 22:665–70. doi: 10.1258/ijsa.2011.010538
28. Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, et al. Determinants of mortality in a combined cohort of 501 patients with HIV-associated Cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis. (2014) 58:736–45. doi: 10.1093/cid/cit794
29. Pasquier E, Kunda J, De Beaudrap P, Loyse A, Temfack E, Molloy SF, et al. Long-term mortality and disability in cryptococcal meningitis: a systematic literature review. Clin Infect Dis. (2018) 66:1122–32. doi: 10.1093/cid/cix870
30. Ramakrishnan M, Ulland AJ, Steinhardt LC, Moïsi JC, Were F, Levine OS. Sequelae due to bacterial meningitis among African children: a systematic literature review. BMC Med. (2009) 7:47. doi: 10.1186/1741-7015-7-47
31. Tenforde MW, Gertz AM, Lawrence DS, Wills NK, Guthrie BL, Farquhar C, et al. Mortality from HIV-associated meningitis in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc. (2020) 23:e25416. doi: 10.1002/jia2.25416
32. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
33. Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. Ottawa: Ottawa Hospital Research Institute (2011). p. 1–12.
34. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
35. Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. (2010) 50:291–322. doi: 10.1086/649858
36. Manyazewal T, Woldeamanuel Y, Holland DP, Fekadu A, Blumberg HM, Marconi VC. Electronic pillbox-enabled self-administered therapy versus standard directly observed therapy for tuberculosis medication adherence and treatment outcomes in Ethiopia (SELFTB): protocol for a multicenter randomized controlled trial. Trials. (2020) 21:383. doi: 10.1186/s13063-020-04324-z
37. Luma HN, Temfack E, Halle MP, Tchaleu BCN, Mapoure YN, Koulla-Shiro S. Cryptococcal meningoencephalitis in human immunodeficiency virus/acquired immunodeficiency syndrome in douala, cameroon: a cross sectional study. N Am J Med Sci. (2013) 5:486–91. doi: 10.4103/1947-2714.117318
38. Deiss R, Loreti CV, Gutierrez AG, Filipe E, Tatia M, Issufo S, et al. High burden of cryptococcal antigenemia and meningitis among patients presenting at an emergency department in Maputo, Mozambique. PLoS One. (2021) 16:e0250195. doi: 10.1371/journal.pone.0250195
39. Letang E, Ntamatungiro AJ, Kimera N, Faini D, Furrer H, Battegay M, et al. Cryptococcal antigenemia in immunocompromised HIV patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis. (2015) 2:ofv046. doi: 10.1093/ofid/ofv046
40. Bergemann A, Karstaedt A. The spectrum of meningitis in a population with high prevalence of HIV disease. QJM Int J Med. (1996) 89:499–504. doi: 10.1093/qjmed/89.7.499
41. Soumare M, Seydi M, Ndour C, Dieng Y, Diouf A, Diop B. Update on neuromeningeal cryptococcosis in Dakar. Med Trop. (2005) 65:559–62.
42. Bamba S, Barro-Traoré F, Sawadogo E, Millogo A, Guiguemdé R. Retrospective study of cases of neuromeningeal cryptococcosis at the University Hospital of Bobo Dioulasso since accessibility to antiretroviral in Burkina Faso. J Mycol Med. (2012) 22:30–4. doi: 10.1016/j.mycmed.2011.12.074
43. Apetse K, Assogba K, Kevi K, Balogou A, Pitche P, Grunitzky E. Opportunistic infections of the HIV/AIDS in adults in hospital settings in Togo. Bull Soc Pathol Exot. (2011) 104:352–4. doi: 10.1007/s13149-011-0139-3
44. Oumar A, Dao S, Ba M, Poudiougou B, Diallo A. Epidemiological, clinical and prognostic aspects of cryptococcal meningitis in hospital area of Bamako, Mali. Rev Med Brux. (2008) 29:149–52.
45. Lakoh S, Rickman H, Sesay M, Kenneh S, Burke R, Baldeh M, et al. Prevalence and mortality of cryptococcal disease in adults with advanced HIV in an urban tertiary hospital in Sierra Leone: a prospective study. BMC Infect Dis. (2020) 20:141. doi: 10.1186/s12879-020-4862-x
46. Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, et al. Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clin Infect Dis. (2010) 50:338–44. doi: 10.1086/649861
47. Kizza HM, Oishi K, Mitarai S, Yamashita H, Nalongo K, Watanabe K, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clin Infect Dis. (1998) 26:1362–6. doi: 10.1086/516372
48. Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high-dose fluconazole for HIV-associated cryptococcal meningitis in southwestern Uganda. Clin Infect Dis. (2008) 47:1556–61. doi: 10.1086/593194
49. Lightowler JV, Cooke GS, Mutevedzi P, Lessells RJ, Newell M-L, Dedicoat M. Treatment of cryptococcal meningitis in KwaZulu-Natal, South Africa. PLoS One. (2010) 5:e8630. doi: 10.1371/journal.pone.0008630
50. Gaskell KM, Rothe C, Gnanadurai R, Goodson P, Jassi C, Heyderman RS, et al. A prospective study of mortality from cryptococcal meningitis following treatment induction with 1200mg oral fluconazole in Blantyre, Malawi. PLoS One. (2014) 9:e110285. doi: 10.1371/journal.pone.0110285
51. Hiesgen J, Schutte C, Olorunju S, Retief J. Cryptococcal meningitis in a tertiary hospital in Pretoria, mortality and risk factors–A retrospective cohort study. Int J STD AIDS. (2017) 28:480–5. doi: 10.1177/0956462416653559
52. Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, et al. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clin Infect Dis. (2009) 49:702–9. doi: 10.1086/604716
53. Dominic RS, Prashanth H, Shenoy S, Baliga S. Diagnostic value of latex agglutination in cryptococcal meningitis. J Lab Phys. (2009) 1:67–8. doi: 10.4103/0974-2727.59702
54. Abassi M, Boulware DR, Rhein J. Cryptococcal meningitis: diagnosis and management update. Curr Trop Med Rep. (2015) 2:90–9. doi: 10.1007/s40475-015-0046-y
55. Derbie A, Mekonnen D, Woldeamanuel Y, Abebe T. Cryptococcal antigenemia and its predictors among HIV infected patients in resource limited settings: a systematic review. BMC Infect Dis. (2020) 20:407. doi: 10.1186/s12879-020-05129-w
56. Touma M, Rasmussen LD, Martin-Iguacel R, Engsig FN, Stærke NB, Stærkind M, et al. incidence, clinical presentation, and outcome of hiV-1-associated cryptococcal meningitis during the highly active antiretroviral therapy era: a nationwide cohort study. Clin Epidemiol. (2017) 9:385. doi: 10.2147/CLEP.S135309
57. Majumder S, Mandal S, Bandyopadhyay D. Prognostic markers in AIDS-related cryptococcal meningitis. J Assoc Phys India. (2011) 59:152–4.
58. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids. (2009) 23:525–30. doi: 10.1097/QAD.0b013e328322ffac
59. Satpute MG, Telang NV, Litake GM, Niphadkar KB, Joshi SG. Prevalence of cryptococcal meningitis at a tertiary care centre in Western India (1996 2005). J Med Microbiol. (2006) 55:1301–2. doi: 10.1099/jmm.0.46657-0
60. Greene G, Lawrence DS, Jordan A, Chiller T, Jarvis JN. Cryptococcal meningitis: a review of cryptococcal antigen screening programs in Africa. Expert Rev Anti Infect Ther. (2021) 19:233–44. doi: 10.1080/14787210.2020.1785871
61. Bigna JJR, Plottel CS, Koulla-Shiro S. Challenges in initiating antiretroviral therapy for all HIV-infected people regardless of CD4 cell count. Infect Dis Poverty. (2016) 5:85. doi: 10.1186/s40249-016-0179-9
62. Low A, Gavriilidis G, Larke N, B-Lajoie M-R, Drouin O, Stover J, et al. Incidence of opportunistic infections and the impact of antiretroviral therapy among HIV-infected adults in low-and middle-income countries: a systematic review and meta-analysis. Clin Infect Dis. (2016) 62:1595–603. doi: 10.1093/cid/ciw125
63. Kitonsa J, Nsubuga R, Mayanja Y, Kiwanuka J, Nikweri Y, Onyango M, et al. Determinants of two-year mortality among HIV positive patients with Cryptococcal meningitis initiating standard antifungal treatment with or without adjunctive dexamethasone in Uganda. PLoS Negl Trop Dis. (2020) 14:e0008823. doi: 10.1371/journal.pntd.0008823
64. Pastick KA, Bangdiwala AS, Abassi M, Flynn AG, Morawski BM, Musubire AK, et al. Seizures in human immunodeficiency virus-associated Cryptococcal meningitis: predictors and outcomes. Open Forum Infect Dis. (2019) 6:ofz478. doi: 10.1093/ofid/ofz478
65. Aye C, Henderson A, Yu H, Norton R. Cryptococcosis—the impact of delay to diagnosis. Clin Microbiol Infect. (2016) 22:632–5. doi: 10.1016/j.cmi.2016.04.022
66. Bollela V, Frigieri G, Vilar F, Spavieri D, Tallarico F, Tallarico G, et al. Noninvasive intracranial pressure monitoring for HIV-associated cryptococcal meningitis. Braz J Med Biol Res. (2017) 50:e6392. doi: 10.1590/1414-431X20176392
67. Denning DW, Armstrong RW, Lewis BH, Stevens DA. Elevated cerebrospinal fluid pressures in patients with cryptococcal meningitis and acquired immunodeficiency syndrome. Am J Med. (1991) 91:267–72. doi: 10.1016/0002-9343(91)90126-I
68. Bicanic T, Brouwer AE, Meintjes G, Rebe K, Limmathurotsakul D, Chierakul W, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. Aids. (2009) 23:701–6. doi: 10.1097/QAD.0b013e32832605fe
69. Loyse A, Burry J, Cohn J, Ford N, Chiller T, Ribeiro I, et al. Leave no one behind: response to new evidence and guidelines for the management of cryptococcal meningitis in low-income and middle-income countries. Lancet Infect Dis. (2019) 19:e143–7. doi: 10.1016/S1473-3099(18)30493-6
70. Nimitvilai S, Banlengchit S. 1593 Predictors of Mortality for HIV-associated Cryptococcal Meningitis. Open Forum Infect Dis. (2014) 1(Suppl 1):S424. doi: 10.1093/ofid/ofu052.1139
71. Hope W, Stone NR, Johnson A, McEntee L, Farrington N, Santoro-Castelazo A, et al. Fluconazole monotherapy is a suboptimal option for initial treatment of cryptococcal meningitis because of emergence of resistance. MBio. (2019) 10:e2575–2519. doi: 10.1128/mBio.02575-19
72. Farlow AW. The challenges and possibilities of reducing deaths from cryptococcal meningitis in sub-Saharan Africa. PLoS Med. (2012) 9:e1001318. doi: 10.1371/journal.pmed.1001318
73. Team B-S. Predictors of poor clinical outcome of cryptococcal meningitis in HIV-infected patients. Int J STD AIDS. (2011) 22:665–70.
74. Ferreira-Paim K, Andrade-Silva L, Fonseca FM, Ferreira TB, Mora DJ, Andrade-Silva J, et al. MLST-based population genetic analysis in a global context reveals clonality amongst Cryptococcus neoformans var. grubii VNI isolates from HIV patients in Southeastern Brazil. PLoS Negl Trop Dis. (2017) 11:e0005223. doi: 10.1371/journal.pntd.0005223
75. Nimitvilai S, Banlengchit S. Predictors of Mortality for HIV-associated Cryptococcal Meningitis. Seizure. (1908) 7:135.
76. Crabtree Ramírez B, Caro Vega Y, Shepherd BE, Le C, Turner M, Frola C, et al. Outcomes of HIV-positive patients with cryptococcal meningitis in the Americas. Int J Infect Dis. (2017) 63:57–63. doi: 10.1016/j.ijid.2017.08.004
77. Leeme T, Patel R, Azzo C. Mortality due to HIV-associated cryptococcal meningitis in Botswana in the ART era. in Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI). Seattle, WA: (2017).
Keywords: HIV, cryptococcal meningitis, antifungal therapy, amphotericin B, flucytosine, fluconazole, systematic review, meta-analysis
Citation: Muzazu SGY, Assefa DG, Phiri C, Getinet T, Solomon S, Yismaw G and Manyazewal T (2022) Prevalence of cryptococcal meningitis among people living with human immuno-deficiency virus and predictors of mortality in adults on induction therapy in Africa: A systematic review and meta-analysis. Front. Med. 9:989265. doi: 10.3389/fmed.2022.989265
Received: 08 July 2022; Accepted: 08 August 2022;
Published: 08 September 2022.
Edited by:
Andres Felipe Henao, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Lei Zhang, Shaanxi Provincial People’s Hospital, ChinaKennio Ferreira-Paim, Universidade Federal do Triângulo Mineiro, Brazil
Copyright © 2022 Muzazu, Assefa, Phiri, Getinet, Solomon, Yismaw and Manyazewal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seke G. Y. Muzazu, c2VrZW11emF6dUBnbWFpbC5jb20=
 Seke G. Y. Muzazu
Seke G. Y. Muzazu Dawit Getachew Assefa
Dawit Getachew Assefa Christabel Phiri4
Christabel Phiri4 Tewodros Getinet
Tewodros Getinet Tsegahun Manyazewal
Tsegahun Manyazewal