- 1Department of Medicine, Rush University Medical Center, Chicago, IL, United States
- 2Department of Medicine, University of California, San Francisco, San Francisco, CA, United States
Background: Stress is common in patients with Systemic Lupus Erythematosus (SLE), and is associated with depression, fatigue, and disease flares. Stress may be modifiable and identifying those at high risk allows clinicians and allied health care professionals to develop a multidisciplinary management plan to direct appropriate resources. This study is aimed at identifying predictors of high stress over time among patients with SLE.
Methods: Longitudinal data from two interviews of the Lupus Outcomes Study 2 years apart from 726 patients with SLE were analyzed for stress, measured using the Perceived Stress Scale (PSS; high-stress PSS ≥6). T-test and Chi-square analyses compared patient characteristics by high-stress status. Logistic regressions were conducted with high stress as the dependent variable. Covariates included demographics, disease features, quality of life (QOL), health care utilization (HCU), and comorbidities. QoL was measured using the SF-36 form (Physical Component Score, PCS; Mental Component Score, MCS) and MOS Cognitive Functioning Scale (CFS). HCU indicated having established care with a rheumatologist, use of an emergency room or hospitalization, and quality of care. P ≤ 0.05 were considered significant.
Results: The mean age of the cohort was 50.6 (12.5) years, 92% were women and 68% were Caucasian. The mean (SD) PSS was 5.3 (3.6), and high stress (PSS >6) was noted in 253 participants. Those with high stress were more frequently below the poverty line and less commonly employed. They had a greater prevalence of comorbidities and HCU; and worse disease severity (activity, flare, damage) and QOL. In regression analyses, high stress (baseline) was associated with younger age, married status, worse QOL, and presence of diabetes. Better QOL (PCS, MCS) independently predicted decreased odds of high stress, while high stress (baseline) predicted high stress (OR 3.16, 95% CI 1.85, 5.37, p < 0.0001) at follow-up, after adjusting for demographics, disease features, HCU, and comorbidities.
Conclusion: Patients with SLE should be routinely screened for QOL and stress during their clinical care, to identify those at risk for poor health outcomes. This information can facilitate multidisciplinary management for those at risk for worse health outcomes.
Introduction
Quality of life (QOL) is adversely impacted in all domains in patients with Systemic Lupus Erythematosus (SLE) (1). The cumulative impact of SLE on patients QOL may exceed that of some of the other common chronic diseases (2). Adverse effects of SLE are also evident in their relationships and the QOL of partners and informal caregivers of persons with SLE (3). Fatigue is the most prevalent symptom in SLE and is noted to be disabling by 50%. In the LUMINA study, the prevalence of fatigue exceeded 80% across ethnic groups (4), and its severity was the highest among Caucasians (5). It is commonly perceived as an area of unmet needs by patients with SLE, with 54% identifying this to be a “moderate/high need” (6). No association was reported between fatigue and disease activity or damage in a study that included patients with and without fibromyalgia (5). In a study of 116 SLE patients without fibromyalgia, we did not find any association between fatigue and disease activity (7). However, we found a significant association of fatigue with stress, depression, and pain (7), where stress had the largest contribution toward fatigue (7). Stress and depression were correlated with fatigue in an analysis from the Lupus Outcomes Study that included 678 SLE patients. Stress and depression collectively accounted for 63% of the variance in fatigue, after adjusting for age, sex, disease duration, disease activity, damage, pain, fibromyalgia, and obesity (8). Stress had a greater contribution to fatigue than depression. Stress independently predicted fatigue over time, and the effects were mediated through depression (8). Any decline in stress was predictive of a clinically significant reduction in fatigue over time (8). We have also previously noted stress to be associated with cognitive dysfunction and poor body image in SLE (9, 10).
Stress is common among patients with SLE. Almost half of patients report major life stress in the past 6 months (11). Stress contributes to flares (12, 13) and disease activity (14). Furthermore, recent research validates the role of psychosocial trauma and associated stress responses in incidents (15) and prevalent SLE (16). Causes of stress in SLE may be multifactorial (17, 18), and some are modifiable (18). Biofeedback-assisted Cognitive Behavioral Therapy, Chronic Disease Self-Management Program, and Mindfulness-Based Stress Reduction Programs have each demonstrated improvements in stress (19–21), with an effect size of 0.41–0.49 (21).
We undertook this study to evaluate correlates and predictors of high stress among patients with SLE, with the goal of identifying variables that may be used in clinical practice to identify those at high risk and directing resources to them.
Methods
Data were obtained from the University of California, San Francisco (UCSF) Lupus Outcomes Study (LOS), a large observational cohort of patients with SLE. Enrolled patients met the 1997 American College of Rheumatology (ACR) classification criteria for SLE. Data were collected through structured telephone interviews conducted annually by trained survey workers beginning in 2002. Information on demographics, disease characteristics, medications, and healthcare use were collected. Patient-reported disease activity, damage, QOL and various other validated PROs were collected. The present study incorporated data from 726 LOS participants with data from wave 5 (the first year all relevant data elements for this analysis were collected; baseline for this analysis) and wave 7 (2-year follow-up) of the interviews. All study procedures were approved by the UCSF Committee on Human Research.
Outcomes
The primary outcome was stress measured using the Perceived Stress Scale (PSS-4), a four-item measure that evaluates the degree of the burden regarding life demands and problems. PSS-4 Items are based on “feelings and thoughts during the last month” and responses are scored on a four-item Likert scale with 0 indicating “never” and 4 indicating “very often.” Scores range from 0 to 16, where higher scores indicate more stress. In our previous studies, PSS-4 has shown high internal consistency reliability among SLE patients, with a Cronbach's alpha of 0.97 (7).
Statistics
All analyses were completed using SAS software. We looked at cross-sectional correlates and longitudinal predictors of stress among LOS participants. Descriptive statistics were calculated for baseline demographics, disease characteristics, and health outcomes as mean ±SD for continuous variables and frequency and proportions for categorical variables. Chi-square and Student's t-tests were undertaken to identify factors associated with high stress in the baseline year (wave 5). Co-variates for regression analyses were selected based on the literature review of factors known to impact stress in SLE and the above analyses. Next, we performed univariate and multivariate binary logistic regression analyses with high stress at baseline (PSS >6) as the dependent variable (wave 5). Longitudinal logistic regression analyses were performed with high stress at follow-up (wave 7) as the dependent variable.
Five sequential models were tested. Step 1 included demographic covariates [age, marital status, and socioeconomic status (SES)]. SES was represented by household income. It was chosen over other SES surrogates (e.g., currently employed, below the poverty level (household income ≤125% of the Federal poverty limit) or education status) as it correlated most of these SES surrogate variables with PSS.
For step 2, we added SLE disease variables to step 1 model. These included SLE disease activity, flare severity, damage, and use of immunosuppressive medication (yes/no). Disease activity was measured using Systemic Lupus Activity Questionnaire (SLAQ) (22), a patient-reported assessment of disease activity modeled on the physician-reported disease activity index Systemic Lupus Activity Measure (SLAM). Patient-reported numeric rating scale (NRS) of their disease activity on a scale of 0–10 was recorded, which correlated highly with SLAQ scores. As SLAQ includes the symptom of depression, which may be correlated with stress, we chose to use the NRS to denote the disease activity. SLE damage was measured using the Brief Index of Lupus Damage (BILD) (23) which is also a patient-reported measure and contains 26 of the original Systemic Lupus International Collaborating Clinics (SLICC)/American College of Rheumatology (ACR) Damage Index (SDI) items. It is scored from 0 to 31, with higher scores representing more accrued damage.
Step 3 added patient-reported QOL variables to the step 2 model. These included Physical and Mental Component Scores (PCS, MCS) derived from the Medical Outcomes Study Short Form 36 (SF-36) which is validated for use in SLE. Each score ranges from 0 to 100, with higher values representing better QOL. Cognition was measured using the self-reported MOS Cognitive Function Scale. The 6-item MOS Cognitive function scale measures six aspects of cognitive functioning, including reasoning, concentration and thinking, confusion, memory, attention, and reaction time over the past 4 weeks. Scores range from 0 to 100, where higher scores represent better functioning. The internal consistency reliability of this scale in the LOS cohort was 0.93.
For Step 4, healthcare utilization variables were added to the step 3 model. These included established care with a rheumatologist, any emergency room visits or hospitalizations in the prior year, and quality of care (QOC) (24). The latter are processing quality measures, evaluated using thirteen quality indicators amenable to self-report. QOC was estimated as the proportion of indicators met of those for which the individual was eligible.
In Step 5, comorbidities commonly seen among SLE patients were added to the step 4 model. These included the presence or absence of fibromyalgia diagnosis, hypercholesterolemia, diabetes, hypertension, cardiovascular disease (myocardial infarction or transient ischemic attack), anxiety, and depression, evaluated by the Center for Epidemiological Studies-Depression scale (CES-D).
In addition, the Step 6 model was tested for the longitudinal data that further adjusted for baseline (Wave 5) PSS levels. All P ≤ 0.05 were significant on two-tailed tests.
Result
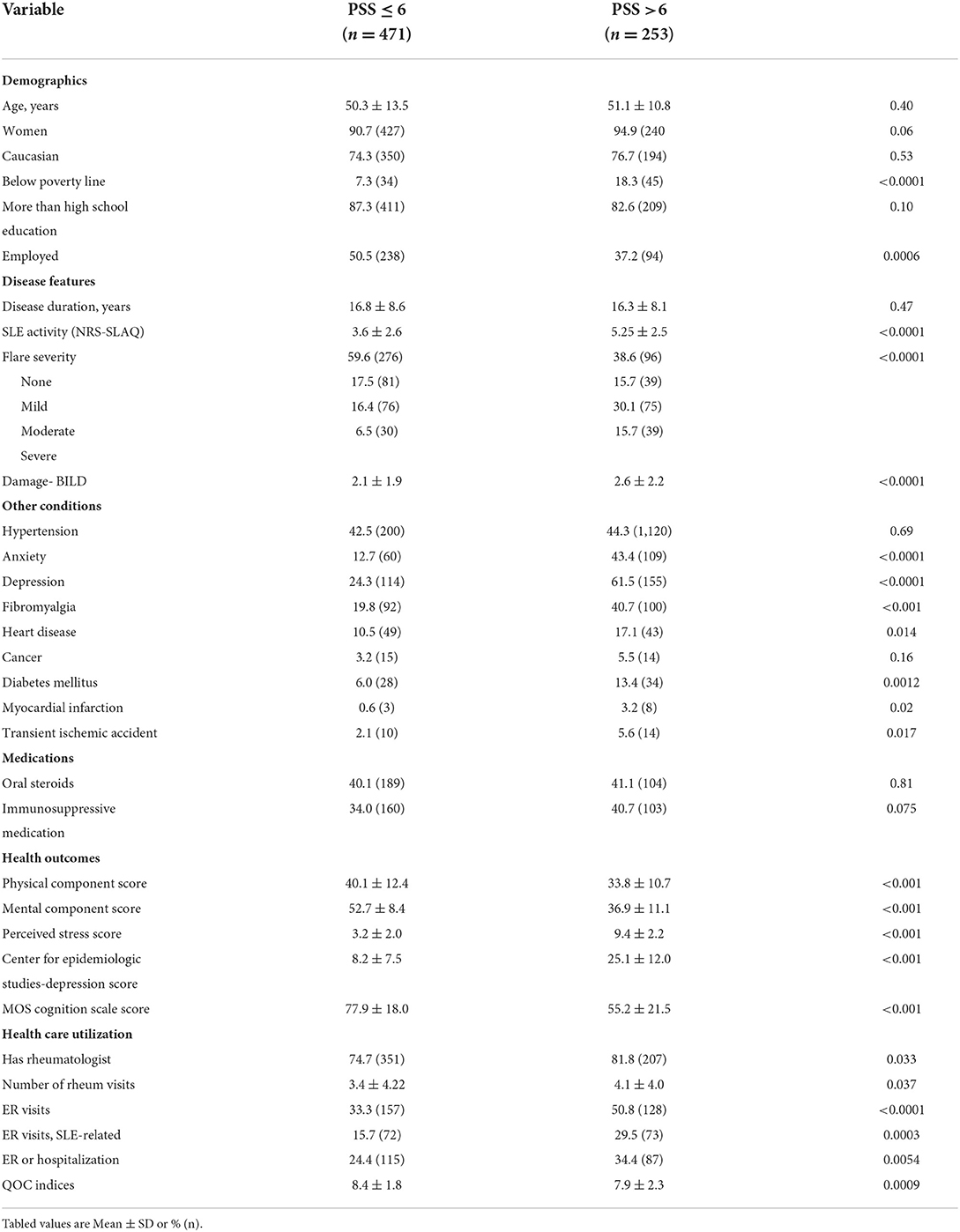
The mean age of the patients was 50.6 ± 12.6 years, and 92% of them were women (Table 1). Sixty-eight percent of patients were Caucasian, and most (85.5%) had greater than high school education. Less than half were currently employed, and 11% were below the poverty line. The most common comorbidity was hypertension (43%) followed by depression (37%).
The mean disease duration was 16.6 ± 8.4 years. The mean SLE disease activity on NRS and SLAQ was 4.2 ± 2.7 and 12.5 ± 7.9, respectively. Approximately 48% of patients reported having an SLE flare. Mean BILD damage was 2.2 ± 2.0. Forty percent of patients were taking oral corticosteroids, while 36% were on an immunosuppressive agent for their SLE. Over 75% of patients had established care with a rheumatologist. The mean number of visits to a rheumatologist was 3.7 ± 4.1 in the past year. The mean QOC indicators met were 8.2 (2.0).
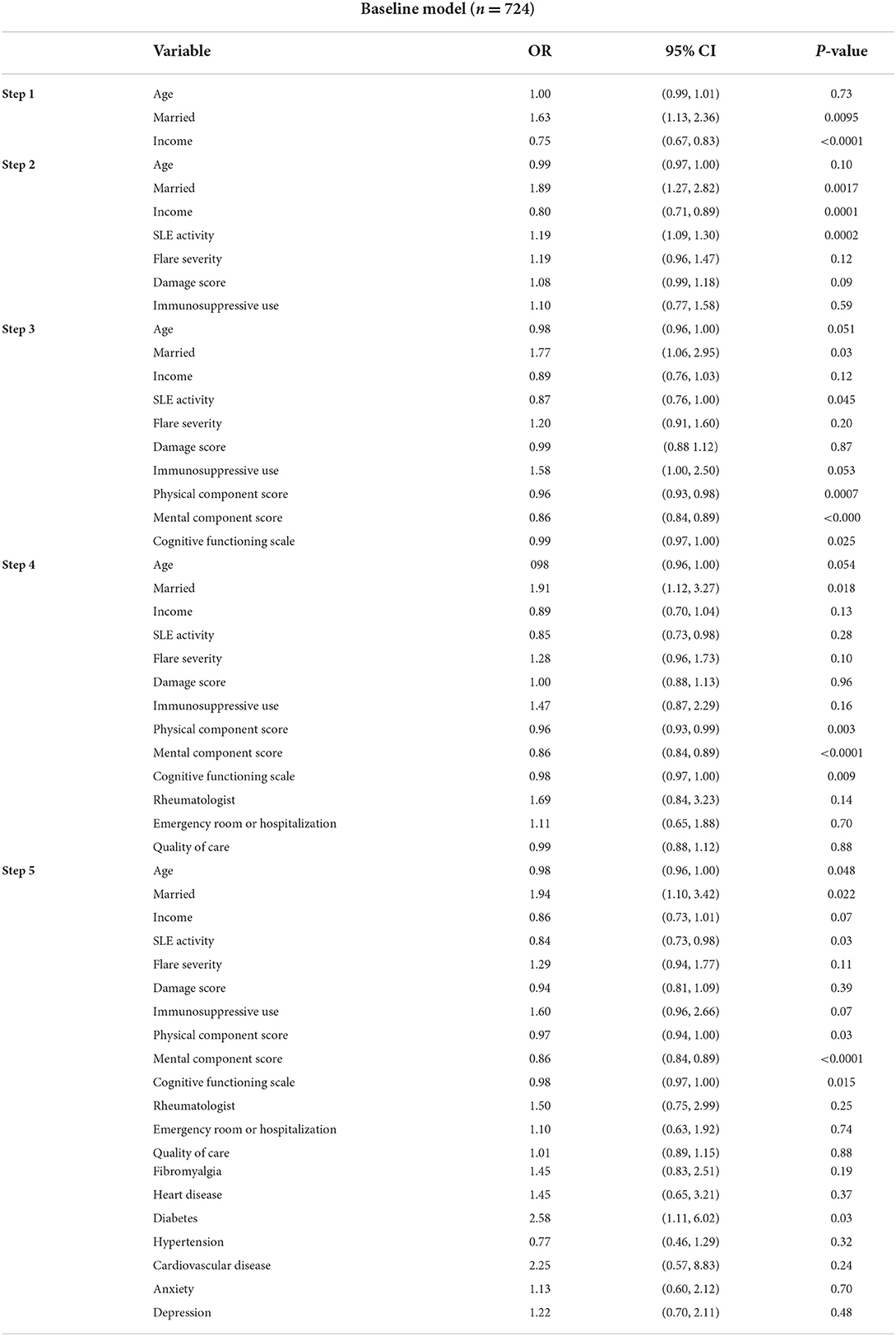
The mean stress score on the PSS was 5.3 ± 3.6. The mean PCS and MCS were 37.9 ± 12.2 and 47.2 ± 12.1, respectively. Mean Cognition Function Scale and CES-D scores were 69.8 ± 22.2, and 14.1 ±12.4, respectively. High stress (PSS >6) was noted among 253 individuals. Mean PSS scores among those with high stress were 9.4 ±2.2 compared to 3.2 ± 2.0 among those without (p <0.001). High-stress participants more had income below the poverty line, were less often employed, had greater disease activity and flare severity, and damage (Table 2). Patients with high stress have a greater prevalence of all the included comorbidities (except for hypertension and cancer) and had worse scores on PCS, MCS, cognition, and depression scales. Patients with greater stress more often had rheumatologist care and visited them more often, were seen at the emergency room more often (for non-SLE or SLE causes), were hospitalized more often and had worse QOC than those without high stress. In multiple logistic regression analyses, the covariates independently associated at baseline with high stress were younger age, being married (OR 1.94, 95% CI 1.10, 3.42, p 0.02), less disease activity (OR 0.84, 95% CI 0.73, 0.98, p 0.03), worse QOL (PCS, MCS and cognitive function), and having comorbid diabetes (OR 2.6, 95% CI 1.11, 6.02, p 0.03) (Table 3).
There were changes in stress over time (from baseline at wave 5 to follow-up at wave 7). Sixty-six percent of participants had PSS>6 at both baseline and follow-up. However, 34% of participants who had PSS>6 at follow-up did not have PSS >6 at baseline. Eighteen percent of participants who had PSS>6 at baseline did not have PSS>6 at follow-up (p < 0.0001).
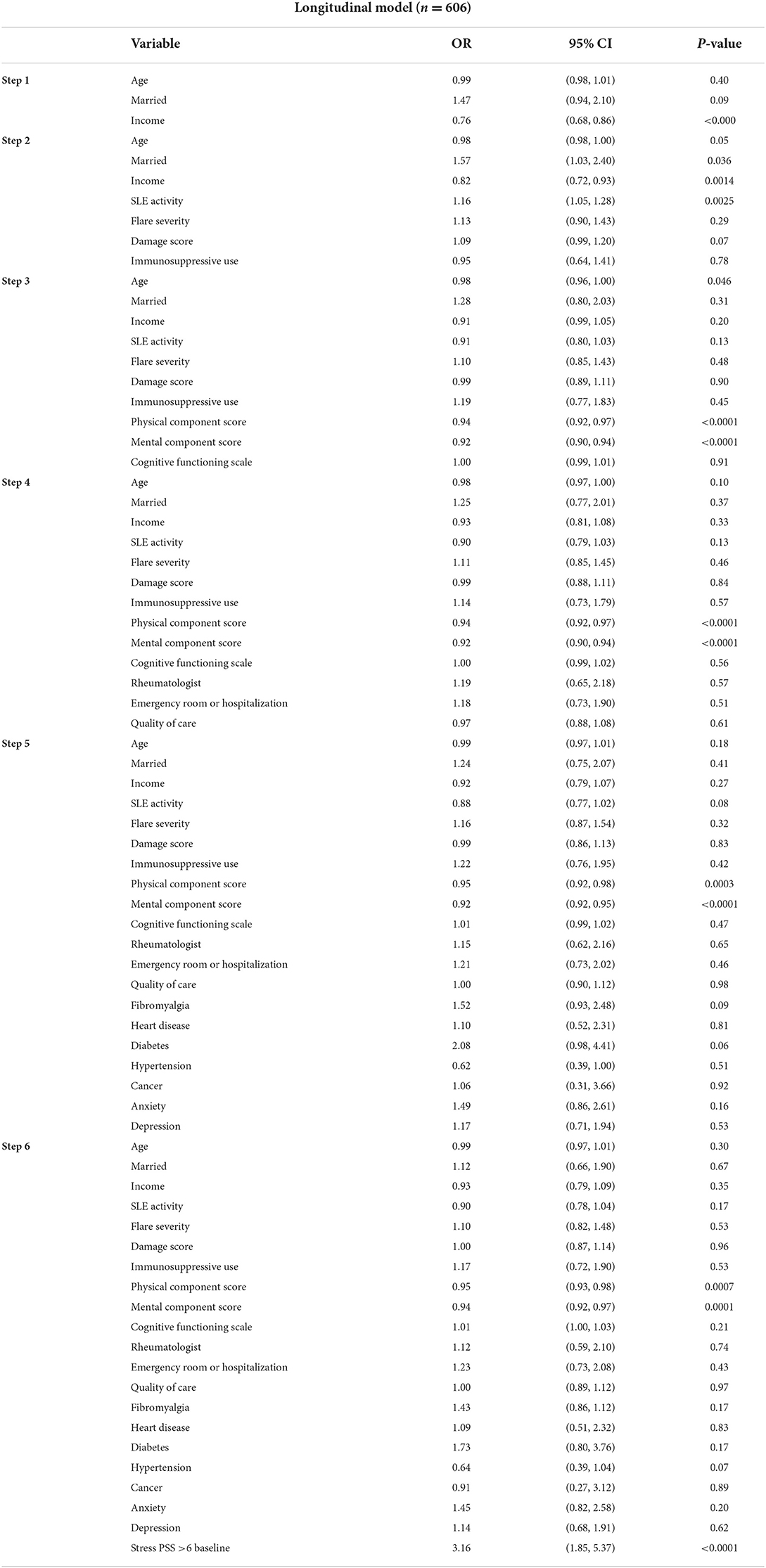
Regression analyses of the longitudinal data revealed lower QOL (PCS, MCS), and baseline stress (PSS>6) to be independent predictors of high stress at follow up (Table 4). Patients with high stress (PSS>6) at baseline were three times more likely to have high stress (PSS>6) at follow-up.
Discussion
The impact of SLE or its treatment on a patient's psycho-social functioning and QOL may lead to implications for long-term health behaviors and downstream health outcomes. Accepting the disease or the need for ongoing medical care, medications with adverse side effect profile, impact on personal (including relationships) and vocational growth, independence, the strain on financial and internal resources, changes in appearance or function, the unpredictability of flares, pain, poor sleep, loss of control on self, may all contribute to stress. We know the importance of the mind-body connection in health and that stress can result in disease flares in SLE (12, 13, 17, 25, 26). Alexithymia has been noted in patients with SLE (27). Concerns about reproductive and sexual health (28, 29) from the disease or its medications may add to the stress. Negative effects of SLE on relationships and the health of their informal caregivers have been reported (3, 30). Stress along with the number of symptoms, anxiety, and depression were correlated with QOL in SLE (31). As noted earlier, stress is an independent and largest contributing predictor of fatigue in SLE, the effects of which are mediated through depression (8). In our previous analyses, any reduction in stress is associated with a large and significant reduction in fatigue (8). If stress can be improved, patients with SLE may be able to cope better with the disease, flares, their impact on their daily lives, their medical care, and possibly resultant urgent and emergent health care utilization.
In our study, SLE patients with high stress had low incomes and were less likely to be employed. Correlations of stress with education, income, poverty, and number of symptoms have been previously reported (17, 31). We did not find an association between stress and education. Part of the reason for the lack of association with education may have been a lack of variability in educational status, as over 80% of patients had more than a high school education. Younger participants had higher stress, and this could potentially be from the challenges imposed by the chronic disease diagnosis at a young age, the need for frequent and long-term management, adverse effects on relationships, procreation, vocational training, and limited internal or external resources (including financial independence or reserves to cope with the disease or its management). Surprisingly, we noted in our study that being married is associated with greater stress. It is known that less stress and better mental health are seen among married people than single (32, 33), especially among men (34). It is plausible that we noted greater stress among married participants in our study first because majority of participants were women, and second SLE impacts not only patients' health and daily life but also the quality of their relationship and intimacy with their partner (3), procreation, and vocation.
Unlike other studies (17, 31), lower disease activity was correlated with higher stress. We have previously reported greater disease activity to be associated with higher patient satisfaction with care (35). This might reflect patients' expectations anchored around acute illness or a result of greater interactions with the health care systems and providers. The presence of most comorbidities as expected was associated with greater stress but the use of steroids or immunosuppressive medications was not. In the multivariate models, only diabetes out of other comorbidities was independently associated with high stress (Table 3). Lack of association with immunosuppressive medications could potentially be because the use of these medications may have been confounded with either disease activity or health care use. We found patients with high stress to have worse QOL (PCS, MCS), depression (CES-D), and cognitive functioning (MOS cognitive function scale). The association of stress with QOL, depression, and cognition is known (7–10, 31). Cognitive deficits may occur in SLE as part of neuropsychiatric involvement, effects of dealing with a chronic disease, comorbidities, and side effects of medications. SLE patients have shown poor decision-making, lower cognitive flexibility, and greater vulnerability to stress, some of which may be attributable to the use of corticosteroids (36). Patients with high stress in our study had greater access to rheumatologist care and had greater health care utilization indicated by a greater number of visits annually to the rheumatologist, or to the emergency room and hospitalization. This is plausible as patients with high stress had greater disease activity, the severity of flares, damage, and comorbidities. Quality of care indices met was significantly higher among those with less stress (PSS<6).
Longitudinal data models showed worse QOL (PCS, MCS) and having high stress at baseline to be the independent predictors for high stress. Being in a high-stress state at baseline had the biggest impact (OR 3.2, 95% CI 1.9, 5.4, p < 0.0001) on having high stress over time in our study. Stress has been found to be modifiable in some studies. Biofeedback-assisted Cognitive Behavioral Therapy, Chronic Disease Self-Management Programs, mindfulness-based stress reduction programs may lead to improvements in stress (19–21, 37, 38), with moderate to large effect sizes (21, 37). However, in a randomized clinical trial involving brief supportive-expressive psychotherapy of 133 women with SLE across 9 clinics in Canada, no improvements in psychological distress, stress, or coping were noted among the intervention and control group (39). Another study with an aerobic exercise program intervention did not result in a reduction in psychological stress in SLE (40).
There are several limitations to this study. First, the cohort was predominantly Caucasian. We did not have physician assessed disease activity and damage measures, although SLAQ and BILD are both validated measures for activity and damage and are widely used in research. Most patients had an education level of high school and greater, which may not be generalizable to other groups. We did not have detailed information on various immunosuppressive medications. Multiple comparisons in bivariate analyses were undertaken without applying Bonferroni correction. The study also has some strengths. This is the first study evaluating stress as the primary outcome in a longitudinal setting with a large cohort of patients with SLE, which includes a large number of relevant covariates. The relevant findings of QOL and high stress being independent predictors were observed after accounting for a wide variety of pertinent variables. Applying Bonferroni correction to the longitudinal analysis model 6, QOL (PCS, MCS) and baseline high stress were independent and significant predictors of high stress at follow-up.
The importance of evaluating and addressing stress in SLE patients cannot be over-emphasized. More studies are needed addressing interventions to modify stress in SLE and to systematically evaluate the downstream effects on health outcomes and health care utilization. A care coordination model such as ambulatory integration of the medical and social (AIMS) model (41) could be embedded using the Biopsychosocial model for chronic disease care for SLE patients. In conclusion, SLE patients should be routinely screened for QOL and Stress during clinical care, to identify those at risk for poor health outcomes and to facilitate multidisciplinary management plans.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Permission from UCSF is needed for use of any data related to Lupus Outcomes Study. Requests to access these datasets should be directed to Lupus Outcomes Study UGF0dGkuS2F0ekB1Y3NmLmVkdQ==.
Ethics statement
The studies involving human participants were reviewed and approved by UCSF. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The Lupus Outcomes Study was funded by NIH grant P60 AR053308.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jolly M, Pickard SA, Mikolaitis RA, Rodby RA, Sequeira W, Block JA. LupusQoL-US benchmarks for US patients with systemic lupus erythematosus. J Rheumatol. 37:1828–33. doi: 10.3899/jrheum.091443
2. Jolly M. How does quality of life of patients with systemic lupus erythematosus compare with that of other common chronic illnesses? J Rheumatol. (2005) 32:1706–8.
3. Jolly M, Thakkar A, Mikolaitis RA, Block JA. Caregiving, dyadic quality of life and dyadic relationships in lupus. Lupus. (2015) 24:918–26. doi: 10.1177/0961203314567749
4. Zonana-Nacach A, Roseman JM, McGwin G Jr, Friedman AW, Baethge BA, Reveille JD, et al. Systemic lupus erythematosus in three ethnic groups VI: factors associated with fatigue within 5 years of criteria diagnosis LUMINA study group LUpus in MInority populations: NAture vs Nurture. Lupus. (2000) 9:101–9. doi: 10.1191/096120300678828046
5. Burgos PI, Alarcon GS, McGwin G Jr, Crews KQ, Reveille JD, Vila LM. Disease activity and damage are not associated with increased levels of fatigue in systemic lupus erythematosus patients from a multiethnic cohort: LXVII. Arthritis Rheum. (2009) 61:1179–86. doi: 10.1002/art.24649
6. Moses N, Wiggers J, Nicholas C, Cockburn J. Prevalence and correlates of perceived unmet needs of people with systemic lupus erythematosus. Patient Educ Couns. (2005) 57:30–8. doi: 10.1016/j.pec.2004.03.015
7. Azizoddin DR, Gandhi N, Weinberg S, Sengupta M, Nicassio PM, Jolly M. Fatigue in systemic lupus: the role of disease activity and its correlates. Lupus. (2019) 28:163–73. doi: 10.1177/0961203318817826
8. Azizoddin DR, Jolly M, Arora S, Yelin E, Katz P. Longitudinal study of fatigue, stress, and depression: role of reduction in stress toward improvement in fatigue. Arthritis Care Res. (2020) 72:1440–8. doi: 10.1002/acr.24052
9. Lillis TA, Tirone V, Gandhi N, Weinberg S, Nika A, Sequeira W, et al. Sleep disturbance and depression symptoms mediate the relationship between pain and cognitive dysfunction in lupus patients. Arthritis Care Res. (2019) 71:406–12. doi: 10.1002/acr.23593
10. Weinberg SL, Gandhi N, Arora S, Sequeira W, Sengupta M, Block JA, Jolly M. Body image in lupus: is it disease activity, physical function, depression, pain, fatigue, sleep, fibromyalgia or stress? Int J Clin Rheumtol. (2018) 13:250–7. doi: 10.4172/1758-4272.1000194
11. Kozora E, Ellison MC, Waxmonsky JA, Wamboldt FS, Patterson TL. Major life stress, coping styles, and social support in relation to psychological distress in patients with systemic lupus erythematosus. Lupus. (2005) 14:363–72. doi: 10.1191/0961203305lu2094oa
12. Roussou E, Iacovou C, Weerakoon A, Ahmed K. Stress as a trigger of disease flares in SLE. Rheumatol Int. (2013) 33:1367–70. doi: 10.1007/s00296-011-2292-1
13. Pawlak CR, Witte T, Heiken H, Hundt M, Schubert J, Wiese B, et al. Flares in patients with systemic lupus erythematosus are associated with daily psychological stress. Psychother Psychosom. (2003) 72:159–65. doi: 10.1159/000069735
14. Spears EC, Allen AM, Chung KW, Martz CD, Hunter EA, Fuller-Rowell TE, et al. Anticipatory racism stress, smoking and disease activity: the Black women's experiences living with lupus (BeWELL) study. J Behav Med. (2021) 44:760–71. doi: 10.1007/s10865-021-00235-9
15. Roberts AL, Malspeis S, Kubzansky LD, Feldman CH, Chang SC, Koenen KC, et al. Association of trauma and posttraumatic stress disorder with incident systemic lupus erythematosus in a longitudinal cohort of women. Arthritis Rheumatol. (2017) 69:2162–9. doi: 10.1002/art.40222
16. Case SM, Feldman CH, Guan H, Stevens E, Kubzansky LD, Koenen KC, et al. Post-traumatic stress disorder (PTSD) and risk of systemic lupus erythematosus (SLE) among medicaid recipients. Arthritis Care Res. (2021) 30:1405–14. doi: 10.1002/acr.24758
17. Yelin E, Trupin L, Bunde J, Yazdany J. Poverty, neighborhoods, persistent stress, and systemic lupus erythematosus outcomes: a qualitative study of the patients' perspective. Arthritis Care Res. (2019) 71:398–405. doi: 10.1002/acr.23599
18. Case S, Sinnette C, Phillip C, Grosgogeat C, Costenbader KH, Leatherwood C, et al. Patient experiences and strategies for coping with SLE: a qualitative study. Lupus. (2021) 30:1405–14. doi: 10.1177/09612033211016097
19. Taub R, Horesh D, Rubin N, Glick I, Reem O, Shriqui G, et al. Mindfulness-based stress reduction for systemic lupus erythematosus: a mixed-methods pilot randomized controlled trial of an adapted protocol. J Clin Med. (2021) 10:4450. doi: 10.3390/jcm10194450
20. Williams EM, Penfield M, Kamen D, Oates JC. An intervention to reduce psychosocial and biological indicators of stress in African American lupus patients: the balancing lupus experiences with stress strategies study. Open J Prev Med. (2014) 4:22–31. doi: 10.4236/ojpm.2014.41005
21. Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis Rheumatol. (2004) 51:625–34. doi: 10.1002/art.20533
22. Karlson EW, Daltroy LH, Rivest C, Ramsey-Goldman R, Wright EA, Partridge AJ, et al. Validation of a systemic lupus activity questionnaire (SLAQ) for population studies. Lupus. (2003) 12:280–6. doi: 10.1191/0961203303lu332oa
23. Yazdany J, Trupin L, Gansky SA, Dall'era M, Yelin EH, Criswell LA, Katz PP. Brief index of lupus damage: a patient-reported measure of damage in systemic lupus erythematosus. Arthritis Care Res. (2011) 63:1170–7. doi: 10.1002/acr.20503
24. Yazdany J, Panopalis P, Gillis JZ, Schmajuk G, MacLean CH, Wofsy D, et al. Quality indicator set for systemic lupus erythematosus. Arthritis Rheum. (2009) 61:370–7. doi: 10.1002/art.24356
25. Adams SG Jr, Dammers PM, Saia TL, Brantley PJ, Gaydos GR. Stress, depression, and anxiety predict average symptom severity and daily symptom fluctuation in systemic lupus erythematosus. J Behav Med. (1994) 17:459–77. doi: 10.1007/BF01857920
26. Azizoddin DR, Jolly M, Arora S, Yelin E, Katz P. Patient reported outcomes predict mortality in lupus. Arthritis Care Res. (2019) 71:1028–35. doi: 10.1002/acr.23734
27. Barbosa F, Mota C, Patrício P, Alcântara C, Ferreira C, Barbosa A. The relationship between alexithymia and psychological factors in systemic lupus erythematosus. Compr Psychiatry. (2011) 52:754–62. doi: 10.1016/j.comppsych.2010.11.004
28. Jolly M, Sequeira W, Block JA, Toloza S, Bertoli A, Blazevic I, et al. Sex differences in quality of life in patients with systemic lupus erythematosus. Arthritis Care Res. (2019) 71:1647–52. doi: 10.1002/acr.23588
29. Moghadam ZB, Rezaei E, Faezi ST, Zareian A, Ibrahim FM, Ibrahim MM. Prevalence of sexual dysfunction in women with systemic lupus erythematosus and its related factors. Reumatologia. (2019) 57:19–26. doi: 10.5114/reum.2019.83235
30. Uzuner S, Durcan G, Sahin S, Bahali K, Barut K, Kilicoglu AG, et al. Caregiver burden and related factors in caregivers of patients with childhood-onset systemic lupus erythematosus. Clin Rheumatol. (2021) 40:5025–32. doi: 10.1007/s10067-021-05867-5
31. Ratanasiripong NT, Ratanasiripong P. Predictive factors of quality of life among systemic lupus erythematosus patients in Thailand: a web-based cross-sectional study. Qual Life Res. (2020) 29:2415–23. doi: 10.1007/s11136-020-02494-6
32. Coombs RH, Fawzy FI. The effect of marital status on stress in medical school. Am J Psychiatry. (1982) 139:1490–3. doi: 10.1176/ajp.139.11.1490
33. Ta VP, Gesselman AN, Perry BL, Fisher HE, Garcia JR. Stress of singlehood: marital status, domain-specific stress, and anxiety in a national US sample. J Soc Clin Psychol. (2017) 36:461–85. doi: 10.1521/jscp.2017.36.6.461
34. Grundström J, Konttinen H, Berg N, Kiviruusu O. Associations between relationship status and mental well-being in different life phases from young to middle adulthood. SSM Popul Health. (2021) 14:100774. doi: 10.1016/j.ssmph.2021.100774
35. Jolly M, Sethi B, O'Brien C, Sequeira W, Block JA, Toloza S, et al. Drivers of satisfaction with care for patients with lupus. ACR Open Rheumatol. (2019) 1:649–56. doi: 10.1002/acr2.11085
36. Montero-López E, Santos-Ruiz A, Navarrete-Navarrete N, Ortego-Centeno N, Pérez-García M, Peralta-Ramírez MI. The effects of corticosteroids on cognitive flexibility and decision-making in women with lupus. Lupus. (2016) 25:1470–8. doi: 10.1177/0961203316642313
37. Navarrete-Navarrete N, Peralta-Ramírez MI, Sabio-Sánchez JM, Coín MA, Robles-Ortega H, Hidalgo-Tenorio C, et al. Efficacy of cognitive behavioural therapy for the treatment of chronic stress in patients with lupus erythematosus: a randomized controlled trial. Psychother Psychosom. (2010) 79:107–15. doi: 10.1159/000276370
38. Kim HA, Seo L, Jung JY, Kim YW, Lee E, Cho SM, et al. Mindfulness-based cognitive therapy in Korean patients with systemic lupus erythematosus: a pilot study. Complement Ther Clin Pract. (2019) 35:18–21. doi: 10.1016/j.ctcp.2019.01.009
39. Dobkin PL, Da Costa D, Joseph L, Fortin PR, Edworthy S, Barr S, et al. Counterbalancing patient demands with evidence: results from a pan-Canadian randomized clinical trial of brief supportive-expressive group psychotherapy for women with systemic lupus erythematosus. Ann Behav Med. (2002) 24:88–99. doi: 10.1207/S15324796ABM2402_05
40. Gavilán-Carrera B, Vargas-Hitos JA, Morillas-de-Laguno P, Rosales-Castillo A, Sola-Rodríguez S, Callejas-Rubio JL, et al. Effects of 12-week aerobic exercise on patient-reported outcomes in women with systemic lupus erythematosus. Disabil Rehabil. (2022) 44:1863–71. doi: 10.1080/09638288.2020.1808904
Keywords: SLE, stress, quality of life, longitudinal analysis, biopsychosocial model
Citation: Jolly M and Katz P (2022) Predictors of stress in patients with Lupus. Front. Med. 9:986968. doi: 10.3389/fmed.2022.986968
Received: 05 July 2022; Accepted: 22 August 2022;
Published: 29 September 2022.
Edited by:
Maurizio Cutolo, Università degli Studi di Genova, ItalyReviewed by:
Filipa Farinha, University College London, United KingdomMatteo Piga, University of Cagliari, Italy
Copyright © 2022 Jolly and Katz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meenakshi Jolly, bWVlbmFrc2hpX2pvbGx5QHJ1c2guZWR1
 Meenakshi Jolly
Meenakshi Jolly Patricia Katz
Patricia Katz