
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Med., 20 September 2022
Sec. Hematology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.986260
This article is part of the Research TopicNew Advances in the Treatment of Immune ThrombocytopeniaView all 4 articles
Immune thrombocytopenia (ITP) is a heterogeneous condition that comprehends forms with acute presentation, persistent disease course (lasting for more than 3 months from disease onset), and chronic disease course, with a duration longer than 12 months (1). While the management of acute ITP relies mostly on the administration of intravenous immunoglobulin (IVIG) and corticosteroids (2), the second-line therapeutic options used in patients with persistent and chronic ITP are numerous, and comprehend biologic agents (particularly, the anti-CD20 monoclonal antibody rituximab), thrombopoietin receptor agonists (TPO-RAS), such as eltrombopag and romiplostim, and immunosuppressive agents (mycophenolate mofetil, azathioprine, and others) (2, 3). Differently from the past decades, splenectomy is currently performed only in a limited number of patients, on the basis of the individual risk-benefit assessment (4).
Agents active on the immune system and TPO-RAS could be either used alone or in combination regimens, and current guidelines for the management of ITP do not strictly regulate the choice of the second-line treatment or the possibility of combining different agents (3). Therefore, the approaches used in daily clinical practice are markedly heterogeneous.
Despite the wide number of therapeutic options, a small percentage of the patients with chronic ITP show refractoriness to treatment, which is defined by the lack of hematological response (defined by a platelet count < 30,000/μl or relapses with bleeding) to immunosuppressive agents and TPO-RAS (5). Notably, patients with refractory ITP have a poor response rate following splenectomy (5, 6). Therefore, there is an urgent need for new therapeutic strategies against refractory ITP.
The biology of refractory ITP is largely unexplored. It is primarily recommended to reconsider the diagnosis, as refractory ITP could be the first sign of rheumatic diseases (mostly, systemic lupus erythematosus) or inborn errors of immunity (IEI) (2, 7). Among IEI, there is increasing interest on a subcategory of diseases called “immune dysregulation disorders” (IDD), which includes autoimmune lymphoproliferative syndrome (ALPS) and related conditions, disorders of regulatory T cells (Tregs), combined immunodeficiencies associated with autoimmunity and lymphoproliferation, and others (8, 9). Interestingly, immune thrombocytopenia is the leading sign of immune dysregulation in many of these immune disorders. The knowledge of the molecular pathogenesis underlying their phenotypic picture led to the identification of targeted therapies whose adoption (10) has significantly improved the disease management of IDD disorders, resulting in effectiveness in the treatment of their hematological manifestations (8, 10).
Moreover, the experience gained from the treatment of IDD-associated autoimmune cytopenia could provide new therapeutic strategies even for patients with refractory ITP in whom the IDD diagnosed has been excluded.
Autoimmune lymphoproliferative syndrome is one of the better-characterized diseases among the group of IDD. It is an inherited disease caused by mutations impairing the first apoptosis signal (FAS) molecular pathway, and particularly the FAS, FAS ligand (FASL), and caspase-10 (CASP-10) genes, with about 30% of the patients remaining without a molecular diagnosis (11). The ineffective apoptosis is associated with the accumulation of αβ double-negative T CD8 + cells (abDNT) and self-reactive T cells (12). At disease onset, the most frequently observed manifestations are features of lymphoproliferation (lymphadenopathy, splenomegaly, and hepatomegaly) and autoimmune cytopenia, with ITP and autoimmune hemolytic anemia being the most common cytopenia (11, 13). Since the mammalian target of rapamycin (mTOR) molecular pathway is central to the proliferation and expansion of abDNTs, the use of sirolimus in patients diagnosed with ALPS is part of routine clinical practice. Sirolimus has demonstrated efficacy in the management of lymphoproliferative features and autoimmune cytopenia in patients with ALPS (14). Sirolimus is also successfully administered in other IEI with ALPS-like presentation, such as activated phosphoinositide 3-kinase δ syndrome (APDS), RAG deficiency, and also disorders of Tregs (Tregopathies) (15).
Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome is the most widely nown disease among Tregopathies (16). It is caused by mutations impairing the function of forkhead box protein 3 (FoxP3) transcription factor, which is pivotal for the proliferation of Tregs (16). IPEX is featured by a clinical triad of autoimmune enteropathy, endocrinopathy (i.e., thyroiditis and type 1 diabetes), and early-onset severe eczematous dermatitis, but also other autoimmune features, such as autoimmune cytopenias, are frequently observed. Specifically, ITP is described in 5–10% of the patients diagnosed with IPEX (17).
Other Tregopathies associated with ITP include the cytotoxic T lymphocyte antigen-4 (CTLA-4) deficiency, lipopolysaccharide (LPS)-responsive, and beige-like anchor (LRBA) deficiency, and other disorders associated with mutations in signal transducer and activator of transcription (STAT) factors (16). The clinical phenotypes of CTLA-4 and LRBA deficiencies show significant clinical and pathogenic overlap. Indeed, CTLA-4 causes the reduction of the co-stimulatory molecules CD80 and CD86 on antigen-presenting cells, thus reducing the activation of T-dependent response, and LRBA acts through the inhibition of the lysosomal degradation of CTLA-4 (16, 18). Both diseases are associated with a complex phenotype that includes susceptibility to infections, autoimmunity, and lymphoproliferation. Autoimmune cytopenias (mostly ITP and autoimmune hemolytic anemia) are reported in about 70% of the patients with CTLA-4 and LRBA deficiency, often in association with lymphadenopathy and splenomegaly (19). The biologic agent abatacept, a fusion molecule containing the extracellular domain of CTLA-4, selectively enhances CTLA-4 dependent signaling and increases the CTLA-4/CD28 balance, thus reducing T cell activation. With the shared molecular pathogenesis between the two disorders, abatacept represents a valid therapeutic strategy in patients with LRBA deficiency (8, 20, 21). Literature data on the use of abatacept in these two conditions are still limited: a recent systematic review by Jamee et al. reported 15 cases of CTLA-4 deficiency and 60 patients with LRBA deficiency treated with abatacept, respectively (19).
Patients with disorders involving the JAK-STAT signaling pathways, and particularly STAT3 gain of function (GOF) and STAT1 GOF mutations, often present with an IPEX-like phenotype (16). The consequences of the molecular defect are better characterized for STAT3 GOF, in which patients show reduced proliferation of Tregs, decreased expression of CD25, and altered T helper type 17 cell proliferation, while the mechanism leading to altered Treg proliferation in STA1 GOF is less defined (22). In STAT3 GOF, more than 80% of the patients develop autoimmune cytopenia, with ITP being described in 50–60% of the patients (23). Differently, autoimmune cytopenia is less common (< 10%) in STAT1 GOF, in which the clinical spectrum is dominated by susceptibility to infections and endocrinopathy (24). Although the definitive treatment of STAT-related disorders is represented by hematopoietic stem cell transplantation (HSCT), the use of JAK inhibitors (ruxolitinib and baricitinib) has proven efficacy in the management of immune dysregulation in this condition (8, 25). However, the overall number of patients with STAT-related disorders treated with JAK inhibitors is still low. Indeed, the administration of JAK inhibitors has been reported only in 18 patients with STAT1 GOF and 13 patients with STAT3 GOF (27 receiving ruxolitinib, 3 tofactinib, and 1 baricitinib, respectively) with a high response rate but a reported short duration of follow-up (25).
Finally, as the activation of STAT3 is also triggered by interleukin (IL)-6, the anti-IL-6 antibody tocilizumab is a promising alternative for patients with severe disease and multisystemic involvement and its use has been described only in less than 10 patients with STAT3 GOF so far (25).
The phenotypic spectrum of IDD widely includes thrombocytopenia as the main or leading, sometimes unique sign. This strongly highlights the need to investigate patients with refractory ITP for an underlying IDD. Furthermore, the evolving knowledge of the mechanisms of lymphocyte proliferation and apoptosis, Treg function, contributing to the molecular aspects responsible for the immune dysregulation of these patients, led to the search for targeted therapies directed against mTOR and JAK-STAT pathways, serum cytokines, and influencing CTLA-4-dependent events (Table 1). The increasing positive experience with these therapeutic strategies on the management of the hematological manifestations of patients with IDD carries significant implications, providing a rationale for the treatment of ITP refractory patients even in absence of a defined diagnosis of IDD.
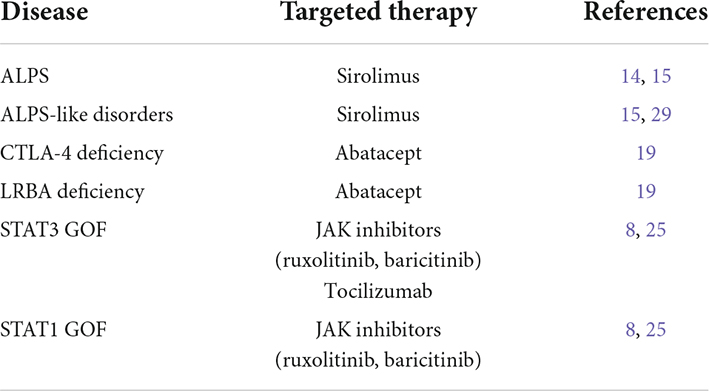
Table 1. Immune dysregulation disorders associated with refractory immune thrombocytopenia (ITP) and targeted therapies.
The differential diagnostic work-up of ITP refractory patients includes the search for an immune dysregulatory disorder. If that is the case, these patients could benefit from a specific molecular targeted therapy. Therefore, it is strongly recommended to perform an accurate personal and familial anamnesis and to search for other features of immune dysregulation (other autoimmune disorders, lymphoproliferation, and severe eczema) or susceptibility to infections. Additionally, a first-level immunological assessment (serum immunoglobulin levels and lymphocyte subpopulations) is mandatory in all patients with refractory ITP. In patients with clinical or laboratory features suggestive for IDD, second-level investigations (extended lymphocyte phenotyping, antibody response, and autoantibodies) and finally genetic testing, contribute to the formulation of a definitive diagnosis of immune disorder and lays the rationale for the adoption of targeted therapies.
There is evidence that dysregulation of the immune response, impaired Treg function, mTOR activation, and cytokine-dependent signaling participate in the complex pathogenic process leading to ITP also in patients without an underlying IDD (26–28). Therefore, drugs selectively targeting immune dysregulation could amplify the therapeutic armamentarium against ITP, particularly in refractory cases.
In this regard, the administration of sirolimus in patients with autoimmune cytopenia (such as ITP and Evans syndrome) has resulted in efficacy in different studies, and its use in the specific setting of refractory ITP has been analyzed recently with promising results (29–31). Sirolimus resulted in a complete response rate (CRR) of 40% and a partial response rate of 45% in a cohort of 86 patients described by Feng et al., with a higher likelihood to respond in patients younger than 40 years (30). Similarly, a multicentric study by Li et al. evidenced a long-term CRR of 45% and an overall response rate of 75% in a cohort of patients with refractory cytopenia (31). Overall, sirolimus has been well tolerated in the analyzed cohorts. These results strongly support the use of sirolimus as a therapeutic alternative in patients with refractory ITP. Notably, in one of the analyzed studies, the response to sirolimus was higher in patients showing other features of immune dysregulation associated with ITP (i.e., lymphoproliferation) (29), thus suggesting its application in patients with refractory ITP and high clinical suspicion of IDD, even in the absence of a defined molecular diagnosis.
On the other hand, the use of abatacept or JAK inhibitors in patients with refractory ITP, in the absence of demonstrated CTLA-4 or LRBA deficiency or STAT-associated disorders, has not yet been explored. Therefore, also considering their safety profile, it is reasonable to reserve these therapeutic options to patients who had undergone genetic analysis.
Conclusively, refractory ITP still represents a considerable therapeutic challenge in daily clinical practice. Recognizing in these patients of an underlying IDD could provide the rationale for the adoption of targeted treatments. Moreover, the knowledge of the clinical spectrum and molecular pathogenetic mechanisms underlying ITP in IDD, together with the increasing experience gained with drugs targeting immune dysregulation, could open new perspectives for the treatment of patients with refractory primary ITP.
GC and RC conceptualized the manuscript. GC drafted the manuscript. RC critically revised the manuscript. Both authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. (2009) 113:2386–93. doi: 10.1182/blood-2008-07-162503
2. Consolini R, Costagliola G, Spatafora D. The centenary of immune thrombocytopenia-part 2: revising diagnostic and therapeutic approach. Front Pediatr. (2017) 5:179. doi: 10.3389/fped.2017.00179
3. Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. (2019) 3:3780–817. doi: 10.1182/bloodadvances.2019000812
4. Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood. (2018) 131:1172–82. doi: 10.1182/blood-2017-09-742353
5. Miltiadous O, Hou M, Bussel JB. Identifying and treating refractory ITP: difficulty in diagnosis and role of combination treatment. Blood. (2020) 135:472–90. doi: 10.1182/blood.2019003599
6. Kim DJ, Chung JH. Long-term results of laparoscopic splenectomy in pediatric chronic immune thrombocytopenic purpura. Ann Surg Treat Res. (2014) 86:314–8. doi: 10.4174/astr.2014.86.6.314
7. Zhao S, Ma J, Zhu X, Zhang J, Wu R. Chronic refractory immune thrombocytopenia is associated with variants in immune genes. Clin Appl Thromb Hemost. (2021) 27:10760296211059813. doi: 10.1177/10760296211059813
8. Delmonte OM, Castagnoli R, Calzoni E, Notarangelo LD. Inborn errors of immunity with immune dysregulation: from bench to bedside. Front Pediatr. (2019) 7:353. doi: 10.3389/fped.2019.00353
9. Bousfiha A, Jeddane L, Picard C, Al-Herz W, Ailal F, Chatila T, et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. (2020) 40:66–81. doi: 10.1007/s10875-020-00758-x
10. Costagliola G, Cappelli S, Consolini R. Autoimmunity in primary immunodeficiency disorders: an updated review on pathogenic and clinical implications. J Clin Med. (2021) 10:4729. doi: 10.3390/jcm10204729
11. Bride K, Teachey D. Autoimmune lymphoproliferative syndrome: more than a fascinating disease. F1000Res. (2017) 6:1928. doi: 10.12688/f1000research.11545.1
12. Consonni F, Gambineri E, Favre C. ALPS, FAS, and beyond: from inborn errors of immunity to acquired immunodeficiencies. Ann Hematol. (2022) 101:469–84. doi: 10.1007/s00277-022-04761-7
13. Matson DR, Yang DT. Autoimmune lymphoproliferative syndrome: an overview. Arch Pathol Lab Med. (2020) 144:245–51. doi: 10.5858/arpa.2018-0190-RS
14. Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. (2011) 118:5741–51. doi: 10.1182/blood-2011-07-325217
15. Hafezi N, Zaki-Dizaji M, Nirouei M, Asadi G, Sharifinejad N, Jamee M, et al. Clinical, immunological, and genetic features in 780 patients with autoimmune lymphoproliferative syndrome (ALPS) and ALPS-like diseases: a systematic review. Pediatr Allergy Immunol. (2021) 32:1519–32. doi: 10.1111/pai.13535
16. Cepika AM, Sato Y, Liu JM, Uyeda MJ, Bacchetta R, Roncarolo MG. Tregopathies: monogenic diseases resulting in regulatory T-cell deficiency. J Allergy Clin Immunol. (2018) 142:1679–95. doi: 10.1016/j.jaci.2018.10.026
17. Park JH, Lee KH, Jeon B, Ochs HD, Lee JS, Gee HY, et al. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome: a systematic review. Autoimmun Rev. (2020) 19:102526. doi: 10.1016/j.autrev.2020.102526
18. Costagliola G, Consolini R. Lymphadenopathy at the crossroad between immunodeficiency and autoinflammation: an intriguing challenge. Clin Exp Immunol. (2021) 205:288–305. doi: 10.1111/cei.13620
19. Jamee M, Hosseinzadeh S, Sharifinejad N, Zaki-Dizaji M, Matloubi M, Hasani M, et al. Comprehensive comparison between 222 CTLA-4 haploinsufficiency and 212 LRBA deficiency patients: a systematic review. Clin Exp Immunol. (2021) 205:28–43. doi: 10.1111/cei.13600
20. Egg D, Rump IC, Mitsuiki N, Rojas-Restrepo J, Maccari ME, Schwab C, et al. Therapeutic options for CTLA-4 insufficiency. J Allergy Clin Immunol. (2022) 149:736–46. doi: 10.1016/j.jaci.2021.04.039
21. Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. (2015) 349:436–40. doi: 10.1126/science.aaa1663
22. Lorenzini T, Dotta L, Giacomelli M, Vairo D, Badolato R. STAT mutations as program switchers: turning primary immunodeficiencies into autoimmune diseases. J Leukoc Biol. (2017) 101:29–38. doi: 10.1189/jlb.5RI0516-237RR
23. Fabre A, Marchal S, Barlogis V, Mari B, Barbry P, Rohrlich PS, et al. Clinical aspects of STAT3 gain-of-function germline mutations: a systematic review. J Allergy Clin Immunol Pract. (2019) 7:1958–69.e9. doi: 10.1016/j.jaip.2019.02.018
24. Zhang W, Chen X, Gao G, Xing S, Zhou L, Tang X, et al. Clinical relevance of gain- and loss-of-function germline mutations in STAT1: a systematic review. Front Immunol. (2021) 12:654406. doi: 10.3389/fimmu.2021.654406
25. Hadjadj J, Frémond ML, Neven B. Emerging place of JAK inhibitors in the treatment of inborn errors of immunity. Front Immunol. (2021) 12:717388. doi: 10.3389/fimmu.2021.717388
26. Consolini R, Legitimo A, Caparello MC. The centenary of immune thrombocytopenia - part 1: revising nomenclature and pathogenesis. Front Pediatr. (2016) 4:102. doi: 10.3389/fped.2016.00102
27. Jaime-Pérez JC, Ramos-Dávila EM, Meléndez-Flores JD, Gómez-De León A, Gómez-Almaguer D. Insights on chronic immune thrombocytopenia pathogenesis: a bench to bedside update. Blood Rev. (2021) 49:100827. doi: 10.1016/j.blre.2021.100827
28. Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. (2017) 16:620–32. doi: 10.1016/j.autrev.2017.04.012
29. Miano M, Rotulo GA, Palmisani E, Giaimo M, Fioredda F, Pierri F, et al. Sirolimus as a rescue therapy in children with immune thrombocytopenia refractory to mycophenolate mofetil. Am J Hematol. (2018) 93:E175–7. doi: 10.1002/ajh.25119
30. Feng Y, Xiao Y, Yan H, Wang P, Zhu W, Cassady K, et al. Sirolimus as rescue therapy for refractory/relapsed immune thrombocytopenia: results of a single-center, prospective, single-arm study. Front Med. (2020) 7:110. doi: 10.3389/fmed.2020.00110
Keywords: abatacept, autoimmune lymphoproliferative syndrome, eltrombopag, immunodeficiency, inborn errors of immunity, rituximab, sirolimus
Citation: Costagliola G and Consolini R (2022) Refractory immune thrombocytopenia: Lessons from immune dysregulation disorders. Front. Med. 09:986260. doi: 10.3389/fmed.2022.986260
Received: 04 July 2022; Accepted: 24 August 2022;
Published: 20 September 2022.
Edited by:
Lalitha Nayak, Case Western Reserve University, United StatesReviewed by:
Ahmet Emre Eskazan, Istanbul University-Cerrahpasa, TurkeyCopyright © 2022 Costagliola and Consolini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Consolini, cml0YS5jb25zb2xpbmlAbWVkLnVuaXBpLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.