
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 26 September 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.979911
Objective: The aim of this study was to compare the efficacy and safety for particular regimen and dosage in venous thromboembolism (VTE) patients with renal insufficiency.
Methods: English language searches of PubMed, Embase, and Web of Science (inception to May 2021). RCTs evaluating anticoagulants for VTE treatment at acute phase, extension phase, and VTE prophylaxis in patients with renal insufficiency and reporting efficacy (death, recurrence, or occurrence of VTE) and safety (bleeding) outcomes were selected. The methodological quality of each study included was assessed at the outcome level using the risk-of-bias assessment tool developed by the Cochrane Bias Methods Group.
Results: Twenty-one trials that involved 76,574 participants and 8,972 (11.7%) patients with renal insufficiency were enrolled, including 10 trials on VTE treatment in acute phase (3–12 months), four trials on VTE treatment in extension phase (6–36 months), and seven trials for VTE prophylaxis. For acute VTE treatment, compared with dabigatran etexilate, apixaban (RR 5.90, 95%CI 1.00–34.60) and rivaroxaban (RR 6.18, 95%CI 1.17–32.75) were significantly associated with increased risk of death or recurrence. For extension treatment of VTE, aspirin had the highest probability of the most effective and safest treatment, followed by apixaban. For VTE prophylaxis, compared with enoxaparin, desirudin was associated with lower risk of VTE occurrence (RR 0.56, 95% CI 0.34–0.91), but had higher risk of bleeding than dabigatran etexilate.
Conclusion: The network meta-analysis informs the optimal choice of anticoagulants and their particular dosage for treatment and prophylaxis of VTE patients comorbid renal insufficiency.
Systematic review registration: www.crd.york.ac.uk/prospero/, identifier: CRD42021254086.
Chronic kidney disease (CKD)/renal insufficiency (RI) is associated with substantially increased risk for venous thromboembolism (VTE) (1, 2) and has been identified as an independent risk factor for short-term and long-term all-cause mortality and other adverse outcomes in VTE patients (3, 4). For example, compared with patients with normal renal function, patients with estimated glomerular filtration (eGFR) < 60 ml/min/1.73m2 had a 1.76-fold greater risk of short-term all-cause death, and patients with eGFR < 30 ml/min/1.73m2 had a 3.32-fold greater risk of short-term all-cause death and also higher rates of bleeding events (5).
Anticoagulation is the essential strategy for both VTE treatment and prophylaxis. Evidence-based guidelines recommended anticoagulation for the prevention of VTE in patients who have had major orthopedic or nonorthopedic surgery or hospitalized patients with acute illness (6), and for treatment of acute VTE (7). Unfractionated heparin (UFH), low molecular weight heparin (LMWH), and vitamin K antagonist (VKA) have been applied for decades. In recent years, direct oral anticoagulants (DOACs) addressed advantages over VKA and have been approved for both VTE treatment and prophylaxis worldwide.
Anticoagulation agents have proved their efficacy and safety by well-designed random controlled clinical trials (RCTs). Based on present evidence, dose adjustment recommendations for RI patients are for patients with creatinine clearance (CCr) ≤ 30 ml/min, LMWH is not recommended in European Society of Cardiology/European Respiratory Society (ESC/ERS) guideline, and anti-Xa levels need monitoring if LMWH was prescribed (7). Nevertheless, the usage and dose for DOACs for RI patients are incongruous in FDA recommendations and ESC/ERS guidelines (Supplementary Table 2). Since patients with severe RI were excluded in most clinical trials, drug/dose recommendation for those patients was insufficient. However, recent real-world studies revealed not only higher mortality rates, but also higher incidence of bleeding events in the treatment of VTE patients with RI (8–10), calling for an evaluation of the current administration of anticoagulation for those patients. Several meta-analyses have been conducted to compare different anticoagulation regimens among CKD/RI patients with both atrial fibrillation (AF) and VTE, but VTE patients with CKD/RI were seldom discussed. Particularly, recommended dosage of anticoagulants in treatment or prophylaxis of VTE was different from AF, especially in RI patients (Supplementary Table 2). Moreover, no meta-analysis emphasizing both categories and dosage of anticoagulants in VTE has been conducted. The aim of our systematic review and network meta-analysis was to evaluate the benefits and harms of different anticoagulants as well as their doses for treatment (both acute phase and extension use) and prophylaxis in VTE patients with RI.
This systematic review and network meta-analysis were conducted according to the PRISMA Extension Statement for Network Meta-analysis (Preferred Reporting Items for Systematic reviews and Meta-Analysis) (11, 12). The study was registered with PROSPERO (CRD42021254086).
We searched PubMed, Embase, and Web of Science from database inception up to 30 May 2021. We used keywords related to venous thromboembolism, kidney function, and anticoagulants in title and the full text of the articles. The full search strategies are provided in Supplementary Table 1. We hand searched reference lists from review articles and meta-analyses to identify any additional studies.
Two reviewers (W.D and F.G) independently performed the review, and disagreements were resolved in a panel discussion with an additional reviewer (W.S). The inclusion criteria for our study included (1) randomized controlled trials; (2) adult patients (≥18 years old) diagnosed with DVT and/or PE or required VTE prophylaxis; (3) treatment with intravenous or subcutaneous or oral anticoagulants (including DOACs, UFH, LMWH, VKA, and fondaparinux) compared with one another or placebo; (4) enrolled participants with determined RI; and (5) efficacy, bleeding outcomes, or both were reported. We excluded observational studies, crossover trials, patients with dialysis-dependent end-stage renal disease (ESRD), studies published in non-English language, and conference abstracts.
The primary efficacy outcomes were recurrent VTE or death associated with VTE for treatment trials and asymptomatic or symptomatic VTE or VTE-related death for prophylaxis trials. The safety outcomes were major bleeding and/or clinically relevant nonmajor bleeding according to the criteria in the International Society of Thrombosis and Haemostatsis (ISTH) (13).
Data were extracted independently by two reviewers (W.D and F.G), and disagreements were resolved via consultation with another reviewer (W.S). A standardized form was used to extract the following data: study identifier, study design, location, length of follow-up, number of participants, age, sex, groups of renal function, intervention, and control details (drug name, dose, and timing); information relevant to the risk-of-bias assessment (including adherence to and withdrawal from randomized allocation); and definition of outcomes and number of events. The methodological quality of each included study was assessed at the outcome level independently by two reviewers (W.D and F.G) using the risk-of-bias assessment tool developed by the Cochrane Bias Methods Group (14) and checked by the third party (W.S). The risk-of-bias assessment tool includes seven aspects: random sequence generation (selection bias); allocation concealment (selection bias); blinding of participants and personnel (performance bias); blinding of outcome assessment (detection bias); incomplete outcome data (attrition bias); selective reporting (reporting bias); and other bias. Within each study, if one or more key domains were judged by reviewers as unknown risk or high risk, the study would be clarified as unknown risk or high risk. If the study was clarified as unknown risk and high risk at the same time, then high risk would be the final decision. As for the meta-analysis, whether studies at unknown or high risk of bias were sufficient to affect the interpretation of results depends on the proportion of these information.
As outcome data were acquired in different time points, we divided the studies into three categories (prophylaxis phase, acute phase, and extension phase). The data of each category were synthetized by standard meta-analysis and network meta-analyses within a frequentist environment, respectively, to simultaneously compare multiple regimens. Standard meta-analyses were used to analyze each pairwise direct comparison between interventions. Network meta-analysis combines evidence about treatments from direct head-to-head trials and indirectly from studies that used a common comparator for both treatments. The agreement between direct and indirect estimates in every closed loop of evidence using loop-specific and node-splitting approaches and global inconsistency test for the entire network using design-by-treatment interaction model was assessed. We assumed the same heterogeneity variance (τ2) for all comparisons in the network meta-analysis and compared its value with treatment-specific empirical distribution of variances to assess the magnitude of heterogeneity in the entire network. However, as the number of studies involved in each comparison was small, a fixed effect model was performed. Pooled relative risks (RRs) and 95% confidence (95% CIs) estimated for outcomes were binomial distribution using total number of patients randomized in each group as the denominator.
Network plots for comparisons were presented to visually describe the characteristics of involved studies. The nodes consisted in the network plots represent the treatments being compared, and edges represent the direct comparisons among different treatments. The sizes of nodes and the thicknesses of lines were weighted according to the sample size of specific comparison. Treatment strategies of DOACs, including doses and frequencies, for example, 2.5 mg twice daily or 5 mg once daily, were analyzed, respectively, and presented as independent dots in network plots. Studies without any events in all arms were excluded, and for those with at least one event in any arm, a constant of 0.5 was added to all cells in the table of that study. Cumulative rankograms for the network of adverse and safety outcomes were displayed to show the hierarchy for different interventions. The hierarchy was determined by the cumulative ranking probabilities estimated by network analysis for each treatment, which classified the competing treatments into first choice, second choice, …, etc.
Considering the competing treatments may be confusing, they were ranked according to their performance on one or more outcomes by clustered ranking plots. By using two-dimensional scaling approach, clustered ranking plots aimed to determine the optimal treatment for each efficacy/safety outcome group in each phase. Dendrograms of the hierarchical analysis were also displayed, which represent the dissimilarities between observations based on the cophenetic correlation coefficient and group the competing treatments into meaningful groups.
Publication bias was assessed using comparison-adjusted funnel plots for all active drugs against control. Statistical analyses were performed with Stata, version 16 (Stata, College Station, TX).
Of the 21 trials we reviewed, 8,972 patients of total 76,574 participants had RI (see Tables 1, 2 to for a summary and details of trial characteristics). Ten trials, including 3,200 VTE patients with RI, were involved in acute phase (follow-up 3 to 12 months) (15–24). Four trials, involving 488 VTE patients with RI who completed 6 to 12 months anticoagulation therapy, were designed for comparison of extension anticoagulation therapies (follow-up 6 to 36 months) (22, 25–27). Seven trials, including 5,284 RI patients who were medically ill or underwent joint replacement surgeries or with cancer, were involved for VTE prophylaxis network analysis (follow-up 8 to 180 days) (28–34) (Figure 1).
DOACs were compared with VKAs (six trials, 2,079 renal-insufficient patients), LMWH (six trials, 2,431 renal-insufficient patients), placebo (four trials, 2,470 renal-insufficient patients), and aspirin (one trial, 1,299 renal-insufficient patients), respectively. LMWH was compared with VKAs (two trials, 293 renal-insufficient patients), UFH (one trial, 537 renal-insufficient patients), and desirudin (one trial, 1,006 renal-insufficient patients), respectively. The funding source was reported in all trials, and all of them were sponsored by pharmaceutical companies (Table 1).
The risk-of-bias assessment for studies contributing to the network analyses of each outcome is presented in Table 2, Supplementary Figure 1. Most studies were assessed to be low risk for sequence generation and at low risk of bias for allocation concealment. Several studies were open-label and were assessed to be at high risk of bias for blinding of participants and investigators. Most studies were assessed to be at low risk of bias for blinding of outcome assessment and for incomplete outcome data.
Among the 10 studies focusing on acute phase, a total of 3,200 VTE patients with RI were involved. Seven of 10 studies took warfarin (adjusted INR 2.0–3.0) as controls, and other treatments and doses were UFH (50I U/kg, twice daily), tinzaparin (1,75I U/kg, once daily), dalteparin (2,00I U/kg, once daily), rivaroxaban (20 mg, once daily), apixaban (10 mg, twice daily), edoxaban (60 mg, once daily for normal renal function and 30 mg, once daily for RI patients), and dabigatran etexilate (150 mg, twice daily) (Figures 2, 3). Compared with dabigatran etexilate, apixaban (RR 5.90, 95% CI 1.00–34.60) and rivaroxaban (RR 6.18, 95% CI 1.17–32.75) were significantly associated with increased risk of death or recurrence in acute phase. Instead, compared with tinzaparin and warfarin, patients administered dalteparin may have lower risk (RR 0.18, 95% CI 0.03–0.95 for tinzaparin; RR 0.16, 95% CI 0.04–0.67 for warfarin) and apixaban may also reduce the risk than UFH (RR 0.07, 95% CI 0.01–0.49) (Table 3, Supplementary Figure 2).
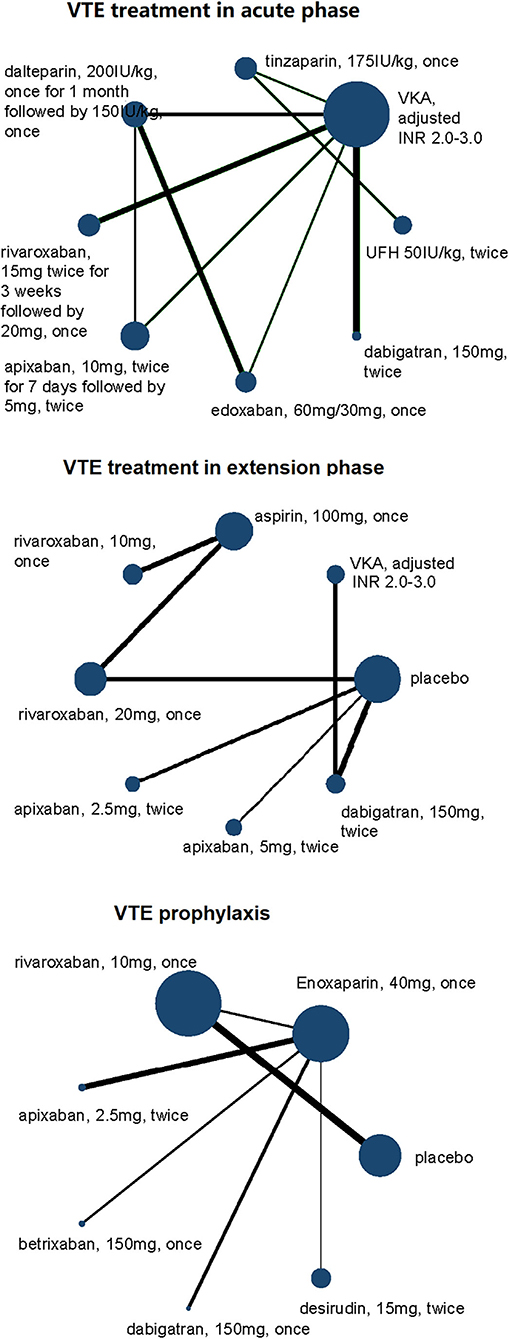
Figure 2. Network plots of VTE treatment network in acute phase, extension phase, and prophylaxis for efficacy outcomes of VTE patients with renal insufficiency. All drugs are present as daily dose and frequency. VTE, venous thrombus embolism; VKA, vitamin K antagonist.
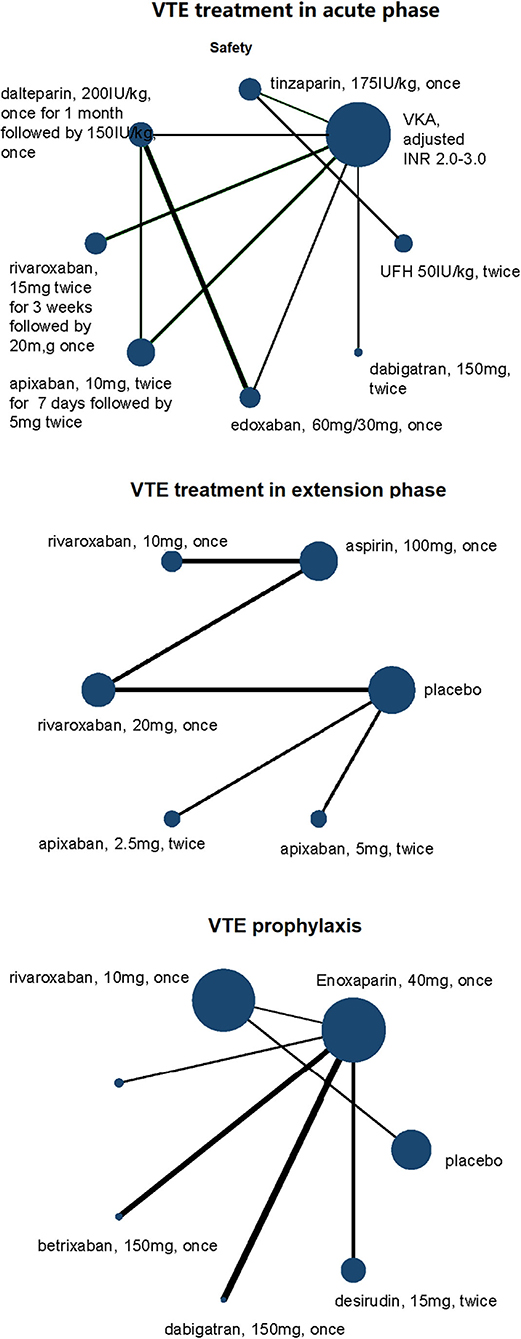
Figure 3. Network plots of VTE treatment network in acute phase, extension phase, and prophylaxis for safety outcomes of VTE patients with renal insufficiency. All drugs are present as daily dose and frequency. VTE, venous thrombus embolism; VKA, vitamin K antagonist.
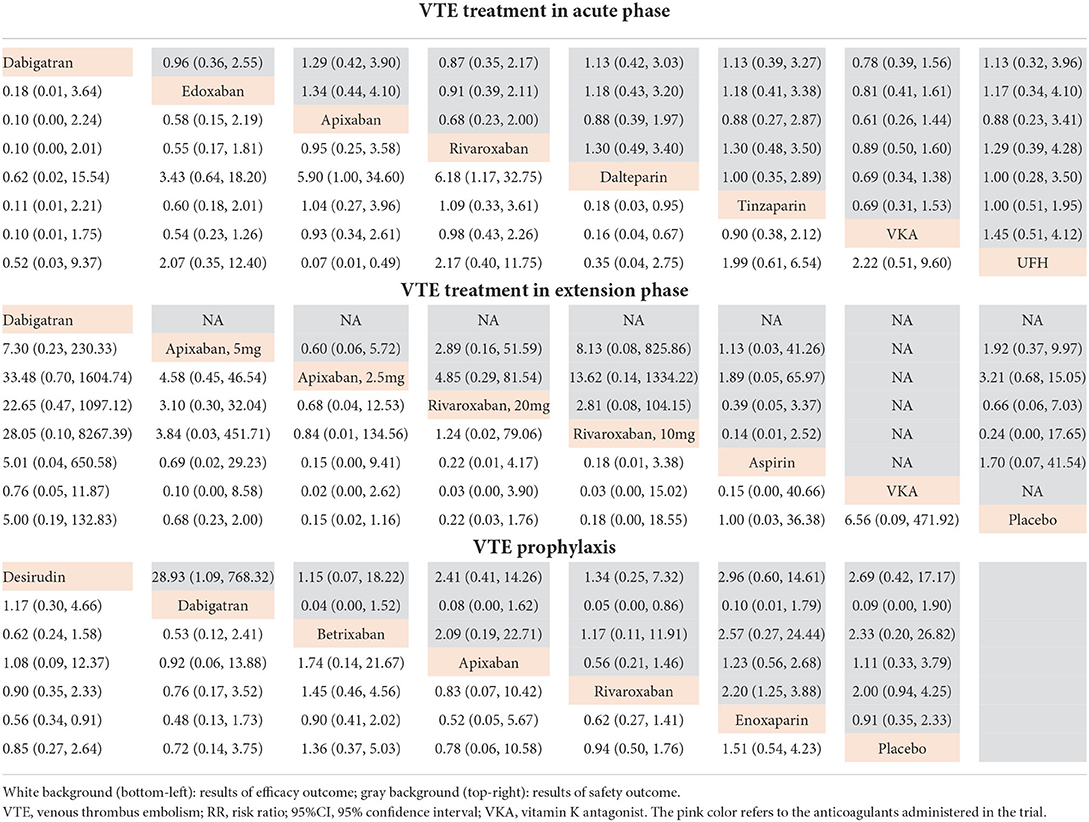
Table 3. Network RR and 95%CI between different treatments in acute phase, extension phase, and prophylaxis for efficacy and safety outcomes of VTE patients with renal insufficiency.
Clustered ranking plots indicated that warfarin had the highest probability of the most effective and safest treatment than the other treatments in acute phase, and the secondary treatment was rivaroxaban (Figure 4, Supplementary Figures 3, 4).
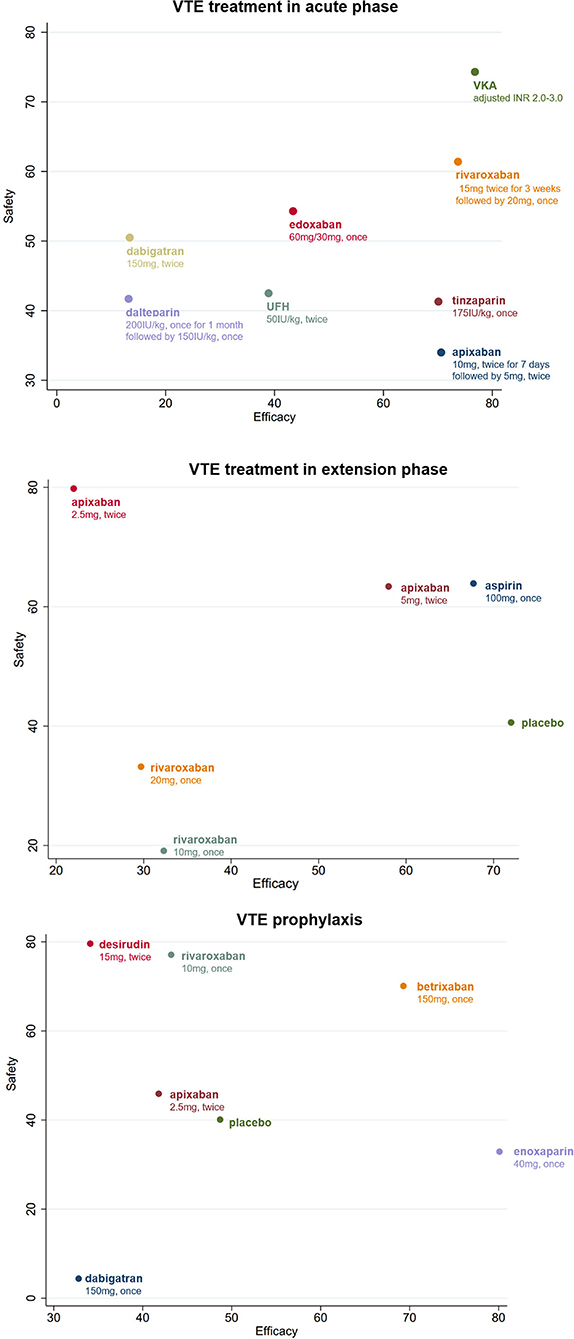
Figure 4. Clustered ranking plot of the different treatments based on cluster analysis for efficacy and safety for VTE patients with renal insufficiency. All drugs are present as daily dose and frequency. VTE, venous thrombus embolism; VKA, vitamin K antagonist.
A total of eight different treatments from four studies with 488 VTE patients with RI involved in the extension phase network analysis and the direct comparisons of efficacy and safety are shown in Figures 2, 3. However, no evidence in direct comparison or network comparison indicated that any treatment would reduce or increase VTE risk in this phase (Tables 2, 3, Supplementary Figure 2). According to the clustered ranking plots, aspirin had the highest probability of the most effective and safest treatment than the other treatments in extension phase, followed by apixaban (Figure 4, Supplementary Figures 3, 4).
The network analysis of VTE prophylaxis phase contains seven different treatments from seven studies involving 5,284 VTE patients with RI. The direct comparisons of efficacy and safety are shown in Figures 2, 3. Compared with enoxaparin, desirudin was associated with lower risk of VTE occurrence (RR 0.56, 95% CI 0.34–0.91); however, it significantly increased the risk of bleeding compared with dabigatran etexilate (RR 28.93, 95% CI 1.09–768.32). The risk of bleeding in VTE prophylaxis was lower for patients administered with dabigatran etexilate than those with rivaroxaban (RR 0.05, 95% CI 0.00–0.86) and was higher for those with rivaroxaban than with enoxaparin (RR 2.20, 95% CI 1.25–3.88), although the efficacy was comparable among these treatments (Tables 2, 3, Supplementary Figure 2). The clustered ranking plots show that betrixaban may have the highest probability of the most effective and safest treatment than the other treatments in prophylaxis phase (Figure 4, Supplementary Figures 3, 4).
Asymmetries of the funnel plots of network analysis for VTE patients with RI were acceptable and suggested few presence of small-study effects (Supplementary Figure 5).
Considering their treatment which may differ from VTE patients with renal insufficiency, those without RI were also involved in another independent network analysis. For VTE patients without RI, seven, eight, and five treatments were found in the network analyses of acute phase, extension phase, and prophylaxis phase, respectively (Supplementary Figure 6). The treatment with highest probability of the most effective and safest treatment than the other treatments in acute phase was warfarin (adjusted INR 2.0–3.0), in extension phase was rivaroxaban (20 mg, once daily), and in prophylaxis phase was enoxaparin (40 mg, once daily). More details and results are presented in Supplementary Figures 7–11.
Our analysis indicates that for patients with RI, currently available anticoagulant with the highest probability of the most effective and safest treatment was VKAs in VTE treatment during acute phase, aspirin in VTE extension treatment, followed by apixaban, and betrixaban in VTE prophylaxis, followed by enoxaparin. To the best of our knowledge, this is the first and most comprehensive network meta-analysis focusing on the efficacy and safety of anticoagulation regimens and their particular dosage on VTE patients comorbid RI.
There is no current guideline or recommendation that indicates whether one anticoagulant is preferred for VTE patients with RI over another. Previous meta-analysis on applications of anticoagulants among CKD patients: Harel et al. compared DOACS and VKA in 4 RCTs and found that neither efficacy nor safety outcomes among VTE patients with CKD were different between the two specimens of anticoagulants (35). Ha et al. evaluated the benefits and harms of VKAs and DOACs in patients with CKD stages 3–5, including those with dialysis-dependent ESRD reported no significant difference in either efficacy or safety outcomes, between DOACs and LMWH, DOACs and VKAs, and VKAs and LMWH (35, 36). They also showed that in patients with AF and early-stage CKD, DOACs were superior to VKAs, with significant reduction of systemic embolism and bleeding events. However, for VTE patients with CKD, the advantages of DOACs compared with VKAs were uncertain and controversial (37). In our network meta-analysis, we sorted anticoagulant regimens as particular species and dosage, other than by pharmacological mechanisms (DOACs and LMWH), for the purpose of an identification of more exact recommendation of anticoagulation for either VTE treatment or prophylaxis among patients with RI. In real-world settings, patients with RI (especially severe RI) or CKD, the complexity of comorbidity and co-medication increases the difficulty of adjustment and monitoring the dose of VKA, which might be a reason of the result from some observational studies that a higher risk of bleeding occurred among CKD patients administered with VKA (38).
CKD was observed 10.7% in the Chinese population and has been increasing along with the high prevalence of diabetes and hypertension (39, 40). Folsom et, al revealed a 1.6- to 1.7-fold risk for CKD stage 3–4 patients to develop VTE (41), and the prevalence of RI among PE patients was around 27% to 49% (42) 43, 44. Recent evidence indicates higher rates of bleeding events among those patients (10, 42). Lim et, al revealed a significantly higher risk of major bleeding in patients with a CCr ≤ 30 ml/min than those with a CCr >30 ml/min in a meta-analysis. The risk of major bleeding was increased when a standard therapeutic dose of enoxaparin was used, but not when an empirically adjusted dose of enoxaparin was used [45]. Researchers recently rose the point of the influence of anticoagulation therapy to bleeding events in patients with CKD or RI [9, 46]. Pharmacokinetic studies also suggested an association between creatinine clearance and levels of antifactor Xa heparin, and guidelines recommended the Xa level should be monitored during LMWH application in RI patients, which needed wide application in clinical practice [47].
In our study, we also conducted network analysis among patients with normal renal function. The results of preference were different from RI patients, either in acute, extension phase, or VTE prophylaxis. These results emphasized the importance of different considerations in choosing appropriate anticoagulation regimens according to the renal function of VTE patients. During the analysis, we recognized that the number of RI patients was far less than those without RI, and those with severe RI (e.g., CCr or eGFR < 15 or 30 ml/min) were excluded from the design of the RCTs. Thus, recommendations of certain dosage for RI patients would be weak, and even absent for patients with severe RI. The small sample size of patients was one of the reasons of potential heterogeneity of the results. Two highly anticipated ongoing studies were focused on patients with AF and ESRD, RENAL-AF (RENal Hemodialysis Patients ALlocated Apixaban Versus Warfarin in Atrial Fibrillation) (Clinical Trials.gov: NCT02942407), and AXADIA (ClinicalTrials.gov: NCT02933697), but for VTE patients with CKD/RI, there still requires studies providing stronger evidence for certain recommendation of anticoagulation therapy.
The study has several limitations. First, VTE patients with RI in each study were much less in number than those without RI and were different in comorbidities, like cancer, and post-hip or knee replacement, which inevitably became the possible source of heterogeneity. The variability in definition of RI among involved studies, which varies from CCr < 50 ml/min to CCr < 80 ml/min, also impacted the potential variability in source populations. Both sample size and various definitions of RI may lead to the inaccurate estimation of pooled RRs, which were noted by wide confidence intervals and extreme effect estimates. Second, the network may be incomplete in some network analyses for the competing treatments which was classified by their regimens and dosages. For example, there was only one study focused on aspirin and UFH. Therefore, the global inconsistency test for those entire networks would be inaccurate, and only consistency models were performable. Third, the heterogeneity between the studies might affect the results of our study. For example, some studies that focused on cancer patients would have higher rates of all-cause mortality than other studies. Similarly, for the prophylaxis purpose, different patients were under different risks of VTE (e.g., medically ill and surgery), thus leading to difference of occurrence. Analysis for specific group of patients is required after more studies published in future. Fourth, even though the funnel plot was symmetrical, there were some studies focused on anticoagulation treatment and VTE patients of various renal functions were excluded due to unavailable data in patients with RI. These articles may contribute some publication bias to our study. Fifth, the grades of ROBs differ among different included trials, because in some studies, the anticoagulants were administered by i.v. or oral delivery. Therefore, it would be impossible to blind. However, our study highlights the absence of evident in patients with RI, especially for patients who require treatment or prevention of VTE. The potential benefit of anticoagulation needs to be weighed against the risk for bleeding in this population.
The network meta-analysis informs the optimal choice of anticoagulants and their particular dosage for treatment and prophylaxis of VTE patients comorbid RI. Studies designed for patients with RI are required for more sufficient evidence for establishing benefits and harms for anticoagulants.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
GF, DW, and SW designed the study and drafted the manuscript. GF and DW searched the database and screened the involved studies. GF, DW, and XL analyzed the data. GF, DW, MZ, XL, ZZ, and SW revised the manuscript and approved it for submission. All authors contributed to the article and approved the submitted version.
This study was funded by the National High Level Hospital Clinical Research Funding (2022-NHLHCRFLX-01-01-01), Fundamental Research Funds for the Central Universities (3332021086), and China-Japan Friendship Hospital Grant (2019-2-QN-78).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.979911/full#supplementary-material
1. Tveit DP, Hypolite IO, Hshieh P, Cruess D, Agodoa LY, Welch PG, et al. Chronic dialysis patients have high risk for pulmonary embolism. Am J Kidney Dis. (2002) 39:1011–7. doi: 10.1053/ajkd.2002.32774
2. Sirolli V, Ballone E, Garofalo D, Merciaro G, Settefrati N, Di Mascio R, et al. Platelet activation markers in patients with nephrotic syndrome. A comparative study of different platelet function tests. Nephron. (2002) 91:424–30. doi: 10.1159/000064282
3. Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. (1999) 353:1386–9. doi: 10.1016/S0140-6736(98)07534-5
4. Monreal M, Falgá C, Valle R, Barba R, Bosco J, Beato JL, et al. Venous thromboembolism in patients with renal insufficiency: findings from the RIETE Registry. Am J Med. (2006) 119:1073–9. doi: 10.1016/j.amjmed.2006.04.028
5. Wang D, Fan G, Liu X, Wu S, Zhai Z. Renal insufficiency and short-term outcomes of acute pulmonary embolism: a systemic review and meta-analysis. Thromb Haemost. (2020) 120:1025–34. doi: 10.1055/s-0040-1712459
6. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149:315–52. doi: 10.1016/j.chest.2015.11.026
7. Jiménez D, Bikdeli B, Barrios D, Morillo R, Nieto R, Guerassimova I, et al. Management appropriateness and outcomes of patients with acute pulmonary embolism. Eur Respir J. (2018) 51:1800445. doi: 10.1183/13993003.00445-2018
8. Chopard R, Piazza G, Falvo N, Ecarnot F, Besutti M, Capellier G, et al. An original risk score to predict early major bleeding in acute pulmonary embolism: the syncope, anemia, renal dysfunction (PE-SARD) bleeding score. Chest. (2021) 160:1832–43. doi: 10.1016/j.chest.2021.06.048
9. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
10. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
11. Schulman S, U A, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. (2010) 8:202–4. doi: 10.1111/j.1538-7836.2009.03678.x
12. Higgins JP, Altman Dg Fau, Gøtzsche PC, Gøtzsche Pc Fau, Jüni P, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343. doi: 10.1136/bmj.d5928
13. Goldhaber SZ, Schulman S, Eriksson H, Feuring M, Fraessdorf M, Kreuzer J, et al. Dabigatran versus warfarin for acute venous thromboembolism in elderly or impaired renal function patients: pooled analysis of RE-COVER and RE-COVER II. Thromb Haemost. (2017) 117:2045–52. doi: 10.1160/TH17-03-0176
14. Bauersachs R, Lee AYY, Kamphuisen PW, Meyer G, Janas MS, Jarner MF, et al. Renal impairment, recurrent venous thromboembolism and bleeding in cancer patients with acute venous thromboembolism-analysis of the CATCH study. Thromb Haemost. (2018) 118:914–21. doi: 10.1055/s-0038-1641150
15. Hokusai-VTE, Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. (2013) 369:1406–15. doi: 10.1056/NEJMoa1306638
16. Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. (2018) 378:615–24. doi: 10.1056/NEJMoa1711948
17. Woodruff S, Feugère G, Abreu P, Heissler J, Ruiz MT, Jen F, et al. Post hoc analysis of dalteparin versus oral anticoagulant (VKA) therapy for the prevention of recurrent venous thromboembolism (rVTE) in patients with cancer and renal impairment. J Thromb Thrombolysis. (2016) 42:494–504. doi: 10.1007/s11239-016-1386-8
18. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. (2013) 369:799–808. doi: 10.1056/NEJMoa1302507
19. Agnelli G, Becattini C, Meyer G, Muñoz A, Huisman MV, Connors JM, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. (2020) 382:1599–607. doi: 10.1056/NEJMoa1915103
20. EINSTEIN Investigators, Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. (2010) 363:2499–510. doi: 10.1056/NEJMoa1007903
21. EINSTEIN–PE Investigators, Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. (2012) 366:1287–97. doi: 10.1056/NEJMoa1113572
22. Leizorovicz A, Siguret V, Mottier D. Safety profile of tinzaparin versus subcutaneous unfractionated heparin in elderly patients with impaired renal function treated for acute deep vein thrombosis: The Innohep® in Renal Insufficiency Study (IRIS). Thromb Res. (2011) 128:27–34. doi: 10.1016/j.thromres.2011.03.002
23. Weitz JI, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, Bounameaux H, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. (2017) 376:1211–22. doi: 10.1056/NEJMoa1700518
24. Schulman S, Kearon C, Kakkar AK, Schellong S, Eriksson CH, Baanstra CD, et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. New England Journal of Medicine. (2013) 368:709–18. doi: 10.1056/NEJMoa1113697
25. Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, Johnson M, et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. (2013) 368:699–708. doi: 10.1056/NEJMoa1207541
26. Weitz JI, Raskob GE, Spyropoulos AC, Spiro TE, De Sanctis Y, Xu J, et al. Thromboprophylaxis with Rivaroxaban in Acutely Ill Medical Patients with Renal Impairment: Insights from the MAGELLAN and MARINER Trials. Thromb Haemost. (2020) 120:515–24. doi: 10.1055/s-0039-1701009
27. Spyropoulos AC, Ageno W, Albers GW, Elliott CG, Halperin JL, Hiatt WR, et al. Rivaroxaban for Thromboprophylaxis after Hospitalization for Medical Illness. N Engl J Med. (2018) 379:1118–27. doi: 10.1056/NEJMoa1805090
28. Shorr AF, Eriksson BI, Jaffer AK, Smith J. Impact of stage 3B chronic kidney disease on thrombosis and bleeding outcomes after orthopedic surgery in patients treated with desirudin or enoxaparin: insights from a randomized trial. J Thrombosis and Haemostasis. (2012) 10:1515–20. doi: 10.1111/j.1538-7836.2012.04803.x
29. Dahl OE, Kurth AA, Rosencher N, Noack H, Clemens A, Eriksson BI. Thromboprophylaxis with dabigatran etexilate in patients over seventy-five years of age with moderate renal impairment undergoing or knee replacement. Int Orthop. (2011) 36:741–8. doi: 10.1007/s00264-011-1393-5
30. Pineo GF, Gallus AS, Raskob GE, Chen D, Ramirez LM, Ramacciotti E, et al. Apixaban after hip or knee arthroplasty versus enoxaparin: efficacy and safety in key clinical subgroups. J Thromb Haemost. (2013) 11:444–51. doi: 10.1111/jth.12109
31. Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer. N Engl J Med. (2019) 380:720–8. doi: 10.1056/NEJMoa1814630
32. Harel Z, Sood MM, Perl J. Comparison of novel oral anticoagulants versus vitamin K antagonists in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. (2015) 24:183–92. doi: 10.1097/MNH.0000000000000098
33. Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med. (2019) 171:181–9. doi: 10.7326/M19-0087
34. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. (2011) 6:2662–8. doi: 10.2215/CJN.04550511
35. Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. (2012) 379:815–22. doi: 10.1016/S0140-6736(12)60033-6
36. Wang F, Yang C, Long J, Zhao X, Tang W, Zhang D, et al. Executive summary for the 2015 Annual Data Report of the China Kidney Disease Network (CK-NET). Kidney Int. (2019) 95:501–5. doi: 10.1016/j.kint.2018.11.011
37. Folsom AR, Lutsey PL, Astor BC, Wattanakit K, Heckbert SR, Cushman M, et al. Chronic kidney disease and venous thromboembolism: a prospective study. Nephrol Dial Transplant. (2010) 25:3296–301. doi: 10.1093/ndt/gfq179
38. Kostrubiec M, Pływaczewska M, Jiménez D, Lankeit M, Ciurzynski M, Konstantinides S, et al. The prognostic value of renal function in acute pulmonary embolism-a multi-centre cohort study. Thromb Haemost. (2019) 119:140–8. doi: 10.1055/s-0038-1676522
39. Trimaille A, Marchandot B, Girardey M, Muller C, Lim HS, Trinh A, et al. Assessment of renal dysfunction improves the simplified pulmonary embolism severity index (sPESI) for risk stratification in patients with acute pulmonary embolism. J Clin Med. (2019) 8:160. doi: 10.3390/jcm8020160
40. Lim W, Dentali F, Eikelboom JW, Crowther MA. Meta-analysis: low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Int Med. (2006) 11. doi: 10.1182/blood.V106.11.907.907
41. Farooq V, Hegarty J, Chandrasekar T, Lamerton EH, Mitra S, Houghton JB, et al. Serious adverse incidents with the usage of low molecular weight heparins in patients with chronic kidney disease. Am J Kidney Dis. (2004) 43:531–7. doi: 10.1053/j.ajkd.2003.11.012
42. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. (2020) 41:543–603. doi: 10.1093/eurheartj/ehz405
Keywords: anticoagulant agents, efficacy and safety, venous thromboembolism, renal insufficiency, network meta-analysis
Citation: Fan G, Wang D, Zhang M, Luo X, Zhai Z and Wu S (2022) Anticoagulant for treatment and prophylaxis of venous thromboembolism patients with renal dysfunction: A systematic review and network meta-analysis. Front. Med. 9:979911. doi: 10.3389/fmed.2022.979911
Received: 28 June 2022; Accepted: 15 August 2022;
Published: 26 September 2022.
Edited by:
Geun Hee Seol, Korea University, South KoreaReviewed by:
Cristiano Bortoluzzi, Azienda Ulss 12 Veneziana, ItalyCopyright © 2022 Fan, Wang, Zhang, Luo, Zhai and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sinan Wu, dGtzMDQyM0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.