- 1Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine, Tokyo, Japan
- 2Rare Disease Medical Affairs, Pfizer Japan Inc., Tokyo, Japan
- 3Division of Medical Education, Juntendo University School of Medicine, Tokyo, Japan
- 4Medical Technology Innovation Center, Juntendo University, Tokyo, Japan
- 5Department of Lillometrics, University of Lille, CHU Lille, Lille, France
- 6Center for Strategic Research Initiative, Nippon Medical School Foundation, Tokyo, Japan
- 7Department of Health Policy and Management, Nippon Medical School, Tokyo, Japan
- 8Department of Orthopedic Surgery, Juntendo University School of Medicine, Tokyo, Japan
- 9Faculty of Health Science, Juntendo University, Tokyo, Japan
- 10Clinical and Translational Research Center, Kobe University Hospital, Kobe, Japan
Introduction: Studies have not sufficiently clarified the differences in citation impact between funded and non-funded clinical research papers. Hence, this study seeks to evaluate the relation between research funding status and clinical research papers’ citation impact in different research fields using multiple evaluation indices.
Methods: In this cross-sectional bibliometric study, clinical research papers published by core clinical research hospitals in Japan were compared retrospectively in terms of times cited (TC), category normalized citation impact (CNCI), citation percentile (CP), journal impact factor (JIF), the Software to Identify, Manage, and Analyze Scientific Publications (SIGAPS) category, and whether they were the funded clinical research. The association between research funding status or the SIGAPS category and CNCI ≥ 2 was analyzed using logistic regression analysis.
Results: 11 core clinical research hospitals published 553 clinical research papers, of which 120 were non-funded and 433 were funded (public institution-funded and industry-funded). The study found that funded clinical research papers (public institution-funded and industry-funded) had significantly higher TC, CNCI, CP, and JIF than non-funded ones [TC: 8 (3–17) vs. 14 (8–31), p < 0.001; CNCI: 0.53 (0.19–0.97) vs. 0.87 (0.45–1.85), p < 0.001; CP: 51.9 (24.48–70.42) vs. 66.7 (40.53–88.01), p < 0.001; JIF: 2.59 (1.90–3.84) vs. 2.93 (2.09–4.20) p = 0.008], while the proportion of A or B rank clinical research papers of the SIGAPS category was not significantly different between the two groups (30.0 vs. 34.9%, p = 0.318). In the logistic regression analysis, having a CNCI ≥ 2 was significantly associated with research funding (public institution-funded and industry-funded) and publication in A or B rank journals of the SIGAPS category [research funding: Estimate 2.169, 95% confidence interval (CI) 1.153–4.083, p = 0.016; SIGAPS category A/B: Estimate 6.126, 95% CI 3.889–9.651, p < 0.001].
Conclusion: Analysis via multiple indicators including CNCI and the SIGAPS category, which allows for a comparison of the papers’ citation impact in different research fields, found a positive relation between research funding status and the citation impact of clinical research papers.
Introduction
It is important to explain the differences in citation impact between funded and non-funded clinical studies. Scholars have reported that industry-funded clinical studies tend to be published in journals with a higher journal impact factor (JIF) (1–4), which has been used to evaluate a research paper’s citation impact. However, the drawback to JIF, which is necessary to understand, is that its values cannot be compared across research fields because the frequency and tendency of citations differ depending on the field (5–7). In fact, for example, several journals of cardiac and cardiovascular systems in the Web of Science category tend to have a higher overall JIF than top journals in a particular category (8), and it is difficult to compare the JIF between journals in different clinical research disciplines.
The number of citations [or times cited (TC)] is also a well-known evaluation index for a research paper’s citation impact, but it is influenced by the research field, publication year, and paper category (5). Such a weakness is, therefore, compensated by citation percentile (CP) and category normalized citation impact (CNCI). Studies have reported that papers with different publication years and research fields can be compared (9–11), which highlights the importance of evaluating the relation between research funding status and clinical research papers’ citation impact using multiple evaluation indices.
The new evaluation index has been attracting attention and being used in recent years. The Software to Identify, Manage, and Analyze Scientific Publications (SIGAPS) scoring system is known as an evaluation index of citation impact for papers that compensate for the shortcomings of JIF (12, 13). The SIGAPS scoring system, which was developed in France, has been recognized by the French government as an extremely reliable tool (14). Hence, each research institute in France is officially funded based on an indicator calculated using the SIGAPS scoring system (15). The SIGAPS scoring system classifies each journal into specific fields (Web of Science categories) based on the Thomson Institute for Scientific Information, ranks them from high to low JIF, and assigns them to their own categories. Simply put, the SIGAPS category can be considered the relative JIF, which makes it possible to compare the impact factor of journals among different research fields in medical research (14–16). In SIGAPS, journals ranked A or B are overweighted because they correspond to the 25% of journals with the highest JIF in each category (Q1 journals in Journal Citation Reports) (14, 15, 17). In France, for a given institution, the percentage of articles published in A or B journals is used as an indicator of performance.
One study reported that funded phase 3 trials were associated with neither publication in journals with higher JIF nor citation (18), and other studies found that funded research was not necessarily associated with citation (19, 20). No reports have investigated the relation between research funding status and clinical research papers’ citation impact in different research fields of medical research from various aspects using multiple evaluation indices, including CNCI and the SIGAPS category. Therefore, we focused on the evaluation by multiple indices to clarify whether the research funding status influences the clinical research papers’ citation impact. Although JIF has some drawbacks, it is still one of the widely used bibliometric index, and in addition to the bibliometric indices, such as the CNCI and SIGAPS categories, we added JIF for this study. This study aims to (1) compare the bibliometric indexes of articles (TC, CNCI, CP, JIF, and the SIGAPS category) from funded and unfunded studies and (2) determine if it is possible to identify factors that could explain why an article is frequently cited.
Materials and methods
This study was a retrospective cross-sectional bibliometric study, and we focused on all clinical research papers in English published by all core clinical research hospitals in Japan approved by the Ministry of Health, Labor and Welfare (MHLW) in November 2018, and TC, CP, CNCI, JIF, and the SIGAPS category were aggregated retrospectively according to whether they constituted funded clinical research. In addition, the relation between research funding status or the SIGAPS category and CNCI was examined.
All core clinical research hospitals have submitted their all-clinical research papers to the MHLW since the last 3 years, which is one of the requirements for approval as a core clinical research hospital; all these papers were put in the fiscal year business report of the MHLW website. At the time of the data collection, the latest fiscal year business report publicly available on the MHLW website was for fiscal year 2017 (21). We collected data on all the clinical research papers of each hospital from the fiscal year 2017 business report on the MHLW website. All the papers had been published in journals from June 2013 to March 2017 (21). The following categories were noted: type of clinical trial (e.g., drug development or medical device), study design (e.g., randomized controlled trial or pilot study), and publishing options (e.g., open access, Web of Science). This information is summarized in Supplementary material.
Data on the research funding status for these papers were gathered based on their Acknowledgments and any funding information section. Research funding status was classified as (1) non-funded, (2) public institution-funded, and (3) industry-funded. The first category, non-funded, was defined as papers that did not mention any research funding. Meanwhile, the second and third categories comprised papers that had received funding. The public institution-funded category contained papers that mentioned having received research funding from a public institution, such as administrative agency, excluding private companies. The industry-funded category contained papers that mentioned having received research funding from a private company, such as a pharmaceutical agent. The bibliometric indices of each paper (TC, CNCI, CP, JIF, and the SIGAPS category) were obtained from the InCites Benchmarking & Analytics™ (Clarivate™) (22) and SIGAPS as of July 2021.
Core clinical research hospitals
Core clinical research hospitals in Japan are stipulated in the Medical Care Act, and hospitals that meet certain requirements, such as publishing 45 or more high-quality clinical trial papers in the last 3 years, are approved by the Minister of Health, Labor and Welfare. These hospitals play a pivotal role in international-standard clinical research and investigator-initiated clinical trials with the aim to develop innovative medicines and medical devices originating from Japan. When we planned this study, 11 medical institutions in Japan have been approved (eight national university hospitals, two national centers, and one private university hospital) since this system became effective in April 2015 (15, 21). Nine medical institutions are located in the 23 wards in Tokyo or 20 cities designated by the Ministry of Internal Affairs and Communications and two medical institutions are located in provincial cities (21). The median number of beds in each hospital was 1,044 (minimum–maximum: 425–1,275) (21). According to a Times Higher Education report, two of these core clinical research hospitals are among the global top 100 high-research-performance universities (23).
Times cited
The number of citations was obtained from the Web of Science Core Collection, considering the age of the article and its disciplinary field.
Citation percentile calculation method
A publication’s CP is calculated by sorting all the publications in a particular year and within the same disciplinary field (Web of Science category) in descending order of the number of citations and then computing their percentiles. If the CP is above 90, the paper is one of the top 10% most cited in this Web of Science category; accordingly, if the CP is above 99, the paper is a part of the top 1% most cited in this Web of Science category (9).
Category normalized citation impact calculation method
For a given publication, CNCI is calculated by dividing the observed number of citations by the expected number of citations (mean citation count of all papers of a document type published the same year in the same research field). If the paper belongs to more than one research field, the average of the actual-to-expected-citation ratio for each research field is used. A CNCI value of 1 indicates equivalence to the world average; if the CNCI value is more than 1, the paper is considered to be cited above the world average; and if the CNCI value is less than 1, the paper is considered to be cited below the world average. If the CNCI value is 2, the paper is considered to be cited twice the world average (9). The threshold of 2 is often used to identify high impact publications in terms of citations.
The software to identify, manage, and analyze scientific publications category calculation method
SIGAPS categories are based on the impact factor of journals provided by the Journal Citation Reports (Clarivate). For each disciplinary field (Web of Science categories), journals are ranked according to decreasing impact factor. The quartiles and the 90th percentile are then used to calculate the SIGAPS category (A to E) or NC (i.e., non-classified because it is not indexed in the Journal Citation Reports). The journal rankings according to the JIF percentile are as follows: the 90th and above percentile is the A rank, the 75th–90th percentile is the B rank, the 50th–75th percentile is the C rank, the 25th–50th percentile is the D rank, and the 25th and below percentile is the E rank (13–16). In the SIGAPS score, A rank journals are weighted 8 points, B rank journals 6 points, C rank journals 4 points, D rank journals 3 points, and E rank journals 2 points. Journals without JIF (NC category) are weighted 1 point (14, 15).
Statistical analysis
First, descriptive analyses of each index (TC, CNCI, CP, JIF, and the SIGAPS category) were performed to examine and review the data. Assumption of normality was assessed using the Shaphiro-Wilk test. Bibliometric indexes were compared according to funding status. According to asymmetric distributions, numerical parameters were compared using non-parametric tests (Wilcoxon tests in the case of two groups, and Kruskall–Wallis test in the case of three groups). Multiple comparisons were performed using a Type I error correction using the Dwass, Steel and Critchlow-Fligner method. Two article subgroups were also defined: CNCI ≥ 2 and CNCI < 2. Moreover, they were compared according to funding status and the SIGAPS category (A or B vs. C, D, or E) using the Chi-square test or Fisher’s exact test as appropriate. Finally, a logistic regression was run with CNCI ≥ 2 as the dependent variable and research funding status (funded vs. non-funded) and the SIGAPS category (A or B vs. C, D, or E) as independent variables. A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using the Statistical Analysis System Software V9.4 (Cary, NC, USA).
Results
In the fiscal year 2017 business report on the MHLW website, it was reported that the Minister of Health, Labor and Welfare approved 11 core clinical research hospitals. These hospitals published 571 clinical research papers in English from June 2013 to March 2017, and the fields of study for the clinical research papers were oncology, gastroenterology and hepatology, pharmacology and pharmacy, urology and nephrology, medicine, research and experimental, cardiac and cardiovascular systems, clinical neurology, respiratory system, ophthalmology, peripheral vascular disease, and others. Among 571 clinical research papers, there were 419 (73.4%) drug clinical research, 244 (42.7%) randomized controlled trials, 64 (11.2%) pilot studies, and 422 (73.9%) published in open access journals. The characteristics of each clinical research shown by the status of research funding can be found in Supplementary Table 1.
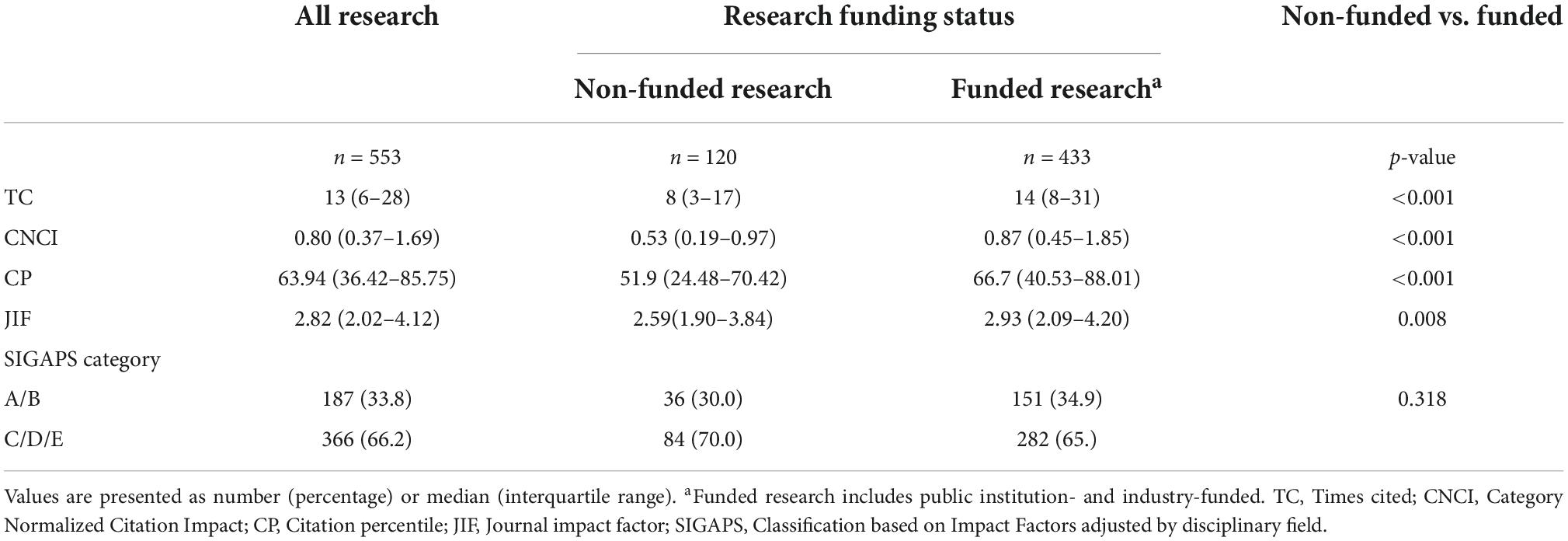
The 553 clinical research papers, excluding 18 papers that cannot be calculated or classified via the SIGAPS scoring system, were analyzed. The median number of clinical research papers published by each hospital was 48 (minimum–maximum: 41–65). There were a total of 120 non-funded clinical research papers, 225 public institution-funded clinical research papers, and 208 industry-funded clinical research papers. The median or interquartile range of each index in all clinical research papers is shown in Table 1. In the analysis of TC, CNCI, CP, and JIF in terms of whether or not they were funded clinical papers, a statistically significant difference was observed between funded clinical research papers (public institution-funded and industry-funded) and non-funded ones [TC: 8 (3–17) vs. 14 (8–31), p < 0.001; CNCI: 0.53 (0.19–0.97) vs. 0.87 (0.45–1.85), p < 0.001; CP: 51.9 (24.48–70.42) vs. 66.7 (40.53–88.01), p < 0.001; JIF: 2.59 (1.90–3.84) vs. 2.93 (2.09–4.20) p = 0.008]. Concerning the SIGAPS category, no statistically significant differences was found between the two groups in the proportion of A or B rank clinical research papers (30.0 vs. 34.9%, p = 0.318) (Table 1).
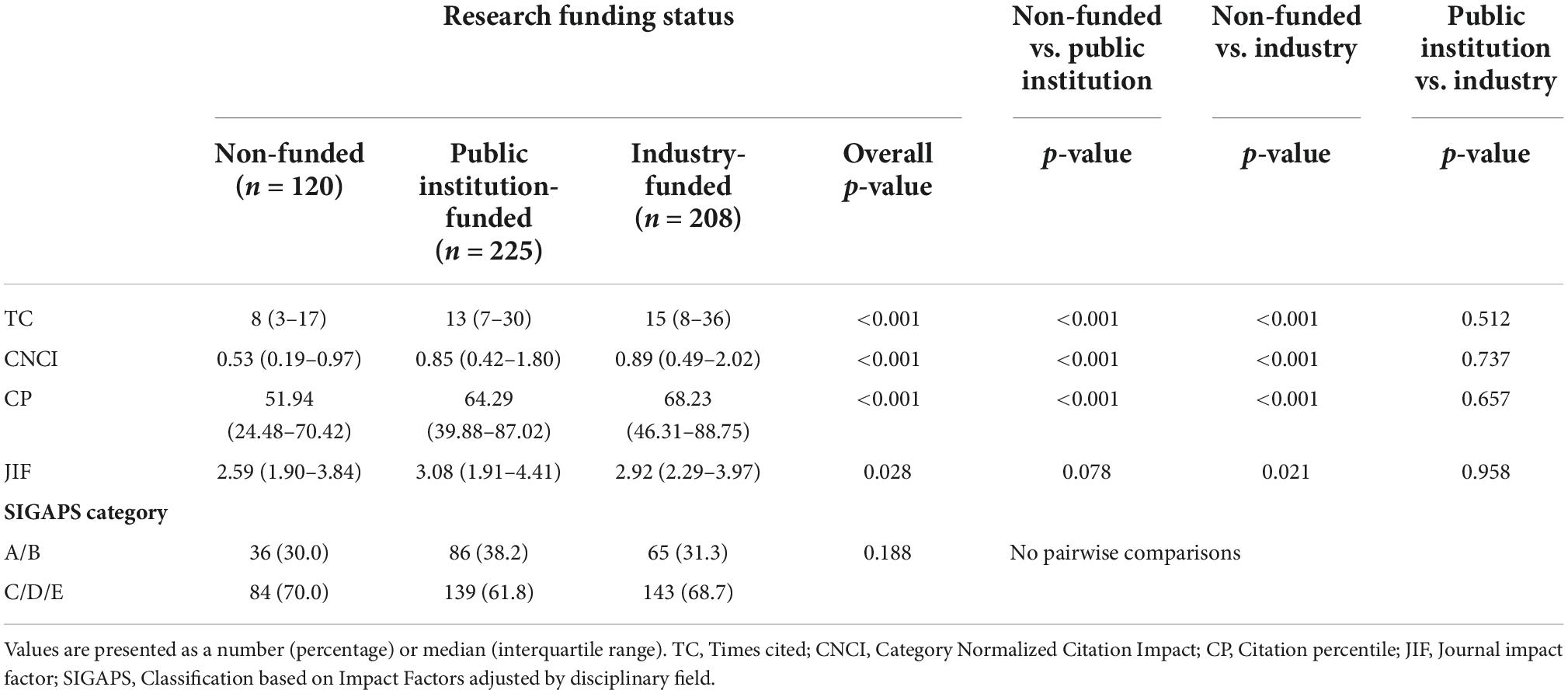
Table 2 summarizes the analysis of each bibliometric index according to the three research funding status groups. A statistically significant difference among the three groups was observed in the numerical indicators [TC: 8 (3–17) vs. 13 (7–30) vs. 15 (8–36), p < 0.001; CNCI: 0.53 (0.19–0.97) vs. 0.85 (0.42–1.80) vs. 0.89 (0.49–2.02), p < 0.001; CP: 51.94 (24.48–70.42) vs. 64.29 (39.88–87.02) vs. 68.23 (46.31–88.75), p < 0.001; JIF: 2.59 (1.90–3.84) vs. 3.08 (1.91–4.41) vs. 2.92 (2.29–3.97), p = 0.028]. Post hoc tests demonstrated that a statistically significant difference was observed between public institution-funded clinical research papers and non-funded ones [TC: 8 (3–17) vs. 13 (7–30), p < 0.001; CNCI: 0.53 (0.19–0.97) vs. 0.85 (0.42–1.80), p < 0.001; CP: 51.94 (24.48–70.42) vs. 64.29 (39.88–87.02), p < 0.001; JIF: 2.59 (1.90–3.84) vs. 3.08 (1.91–4.41), p = 0.078] and between industry-funded clinical research papers and non-funded ones [TC: 8 (3–17) vs. 15 (8–36), p < 0.001; CNCI: 0.53 (0.19–0.97) vs. 0.89 (0.49–2.02), p < 0.001; CP: 51.94 (24.48–70.42) vs. 68.23 (46.31–88.75), p < 0.001; JIF: 2.59 (1.90–3.84) vs. 2.92 (2.29–3.97), p = 0.021], but that no statistically significant differences existed between public institution-funded clinical research papers and industry-funded ones [TC: 13 (7–30) vs. 15 (8–36), p = 0.512; CNCI: 0.85 (0.42–1.80) vs. 0.89 (0.49–2.02), p = 0.737; CP: 64.29 (39.88–87.02) vs. 68.23 (46.31–88.75), p = 0.657; JIF: 3.08 (1.91–4.41) vs. 2.92 (2.29–3.97), p = 0.958]. Finally, no statistically significant difference among the three groups was observed in the proportion of A or B rank clinical research papers of the SIGAPS category [30.0 vs. 38.2 vs. 31.3%, p = 0.188].
Finally, concerning the predictive factors of highly cited clinical research papers (CNCI ≥ 2), two significant factors were identified in univariate analysis (Chi-square test): research funding (public institution-funded and industry-funded) (X2 = 6.749, p = 0.009) and publication in A or B rank journals of the SIGAPS category (X2 = 70.689, p < 0.001). The proportion of clinical research papers with a CNCI ≥ 2 did not differ between public institution-funded clinical research papers and industry-funded ones (20.0 vs. 25.0%, p = 0.213). Table 3 shows that, in univariate analysis, a clinical research paper that received research funding (public institution-funded and industry-funded) was approximately twice as likely to be among the clinical research papers with a CNCI ≥ 2. A clinical research paper published in A or B rank journals of the SIGAPS category (journals ranked Q1 in Journal Citation Reports by Clarivate) was approximately six times more likely to be among the clinical research papers with CNCI ≥ 2.
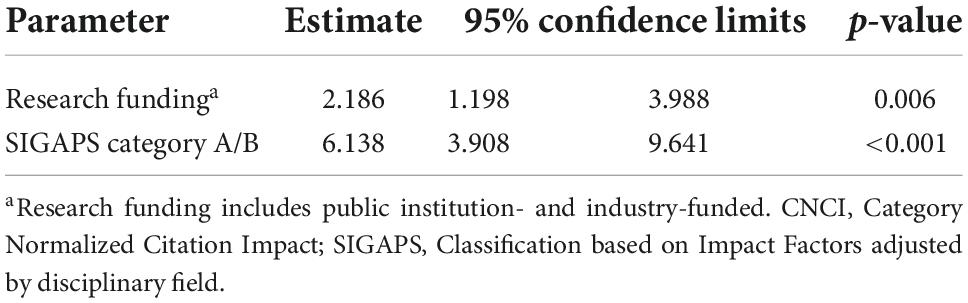
In multivariate logistic regression, the parameters of both research funding (public institution-funded and industry-funded) and publication in A or B rank journals of the SIGAPS category remained significantly associated with CNCI ≥ 2 [research funding: Estimate 2.169, 95% confidence interval (CI) 1.153–4.083, p = 0.016; SIGAPS category A/B: Estimate 6.126, 95% CI 3.889–9.651, p < 0.001]. This result was to be expected as the previous analysis had shown that there was no significant relationship between research funding (public institution-funded and industry-funded) and publication in A or B rank journals of the SIGAPS category (Table 4).
Discussion
The present research found that funded clinical research papers (public institution-funded and industry-funded) were high citation impact studies. This study used an unprecedented CNCI and the SIGAPS category alongside TC, CP, and JIF to evaluate clinical research papers’ citation impact. This is because JIF cannot be compared across fields, as the frequency and tendency of citations vary depending on the research area (5–7), a drawback shared by the TC (5). Therefore, we deemed it necessary to assess clinical research papers’ citation impact from various aspects using multiple evaluation indices such as CNCI and the SIGAPS category, which allows for the comparison of the papers’ citation impact in different research fields (9, 11, 14–16). While scholars have reported an association between research funding and JIF and between research funding and TC (1, 24, 25), to the best of our knowledge, no studies have examined the relation between research funding and multiple indices. Therefore, this study is the first to report a link between research funding and clinical research papers using multiple indices such as CNCI and the SIGAPS category.
We also showed a significant association between research funding (public institution-funded and industry-funded) and CNCI ≥ 2. Because a paper with a CNCI of 2 is considered to be cited twice the world average, we analyzed the funding types for highly cited papers with CNCI ≥ 2. Industry-funded research is often introduced at academic conferences and symposiums, and the increase in citations may be caused by these public-relations efforts to disseminate clinical research findings (1). In addition, these projects have generally been widely evaluated, and only the best ones receive funding. This may also explain why they are cited more frequently. Additionally, since this study focused on clinical research papers reported by core clinical research hospitals, it also included physician-led clinical trials of pharmaceutical agents. In fact, three-quarters of industry-funded research papers in this study were clinical trials funded by pharmaceutical agents, and it is possible that sufficient funding has been directed to high-quality clinical trials. Moreover, these clinical trials are conducted in compliance with Good Clinical Practice, so the research may be high-quality and its results highly reliable when compared to research that is not conducted in compliance with Good Clinical Practice. Therefore, these papers may attract attention and may be cited in many papers consequently.
The CNCI and the impact factor (thus the SIGAPS category) are directly related to the number of citations of clinical research papers. It is therefore normal to find a relationship between CNCI ≥ 2 and the fact that a clinical research paper is published in A or B rank journals of the SIGAPS category. More surprisingly, we showed that even after adjusting for this parameter, the difference between funded (public institution-funded and industry-funded) and non-funded clinical research papers remained significant. This means that funded clinical research papers, even if they are not published in high impact journals, can be highly cited (26).
We believe that research funding improves the citation impact of clinical research papers as follows. Conducting clinical research requires a wide range of tasks such as support for the preparation of documents for submission to the institutional review board, patient enrollment, data management, and statistical analysis, which are costly and time-consuming (27). Because investigators are usually busy, they often find it difficult to perform these tasks by themselves, which necessitates the establishment of a research implementation system consisting of clinical research support including a research secretariat, a clinical research coordinator (CRC), and a data manager. In phase 3 clinical trials, labor accounted for 37% of total costs, and outsourcing accounted for 22% (28). Clinical trials encounter challenges such as cost and staff shortages (29, 30), and these barriers are the same for investigator- and industry-initiated clinical trials (29). In addition, the electronic data capture (EDC) system may reduce data collection time using a paper case report form (CRF) (31), and it is further suggested that the EDC will improve data collection accuracy (32). However, implementing the EDC system requires a high initial investment cost and technical support (33). Substantial expense is also needed to establish the research implementation system, which includes employing a staff and operating the data management system, and we believe that research funding is significantly linked to the improvement of the citation impact of clinical research.
Patient enrollment is also a vital aspect in the success of clinical research; however, only 31% of clinical trials have achieved their target patient count. In addition, approximately 45% of clinical trials have enrolled less than 80% of their target number of patients (34), making patient enrollment a barrier to clinical trial success (29, 30). Collaboration with clinical research assistants such as CRCs is indispensable for efficient patient enrollment and requires funding. Simply put, research funding and the improvement of clinical research papers’ citation impact are closely related.
This study has several limitations. First, open-access journals may have influenced the findings. Because of the spread of open-access journals in recent years, subscription journals and open-access journals may be different in terms of number of views and citations (35). Here, the influence of subscription journals and open-access journals has not been addressed, and the increase in the number of open-access journals may have influenced each index. Second, this study did not address self-citation issues, and self-citation may have influenced the citation impact of clinical research papers. Third, this study did not consider the study design of each clinical research, i.e., whether they are single-centered or multicentered, RCTs or non-RCTs and so on, which may influence the adopted journals and the citation count. Fourth, this study did not consider the causal relationship. In particular, we did not consider that the most impactful work is selected for funding and funding makes impactful work possible because of retrospective cross-sectional study design. Fifth, in this study, non-funded was defined as not having received research funding; i.e., there was no mention of funding in the Acknowledgments or a funding information section. Therefore, even if the research funding status of the papers was not mentioned directly, the research may have received funding from an entity other than a public institution or industry. Sixth, this study did not investigate useful evaluation indices, such as collaboration impact, relative citation ratio (RCR), and h-index. In the future, we will try to conduct another research project that uses these evaluation indices. The final issue concerns generalizability; as this study included only 553 clinical research papers published by 11 core clinical research hospitals in Japan, the results cannot be applied to clinical research in general.
Conclusion
This study found a positive association between research funding status and clinical research papers’ citation impact after an evaluation that used multiple indicators including CNCI and the SIGAPS category, which allows for a comparison of the papers’ citation impact in different research fields.
Data availability statement
The data that support the findings of this study are available from Clarivate (https://clarivate.com/); however, restrictions apply regarding the availability of these data, which were used under license for the current study and are not publicly available.
Author contributions
FM and YN contributed to conceptualization. FM, YN, PD, and RU contributed to data curation and investigation. FM, YN, PD, and NY contributed to formal analysis. SS contributed to funding acquisition. YN contributed to project administration. PD contributed to resources. HD, TM, and SS contributed to supervision. FM, YN, and PD contributed to original draft preparation. All authors contributed to review, editing, and reviewed and approved the final manuscript for submission.
Funding
This study was supported by the Japan Agency for Medical Research and Development (AMED) (Grant no. JP181k1903001) from April 01, 2018 to March 31, 2019.
Acknowledgments
We would like to thank Enago (www.enago.jp) for the English language review.
Conflict of interest
FM was employed by the Pfizer Japan Inc. However, Pfizer Japan Inc. did not fund and had no involvement in conducting this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.978174/full#supplementary-material
References
1. Jefferson T, Di Pietrantonj C, Debalini MG, Rivetti A, Demicheli V. Relation of study quality, concordance, take home message, funding, and impact in studies of influenza vaccines: systematic review. BMJ. (2009). 338:b354. doi: 10.1136/bmj.b354
2. Khan NA, Lombeida JI, Singh M, Spencer HJ, Torralba KD. Association of industry funding with the outcome and quality of randomized controlled trials of drug therapy for rheumatoid arthritis. Arthr Rheum. (2012) 64:2059–67. doi: 10.1002/art.34393
3. Venincasa MJ, Kuriyan AE, Sridhar J. Effect of funding source on reporting bias in studies of intravitreal anti-vascular endothelial growth factor therapy for retinal vein occlusion. Acta Ophthal. (2019) 97:e296–302. doi: 10.1111/aos.13917
4. Fundytus A, Wells JC, Sharma S, Hopman WM, Del Paggio JC, Gyawali B, et al. Industry funding of oncology randomised controlled trials: implications for design, results and interpretation. Clin Oncol. (2022) 34:28–35. doi: 10.1016/j.clon.2021.08.003
5. Garfield E. The history and meaning of the journal impact factor. JAMA. (2006) 295:90–3. doi: 10.1001/jama.295.1.90
6. Seglen PO. Why the impact factor of journals should not be used for evaluating research. BMJ. (1997) 314:498–502. doi: 10.1136/bmj.314.7079.497
7. DORA. The San Francisco declaration on research assessment. (2020). Available online at: https://sfdora.org/read/. (accessed December 2, 2020).
8. Clarivate. (2022). Available online at: https://jcr.clarivate.com/jcr/browse-journals. (accessed May 15, 2022).
9. Clarivate. Indicators handbook. (2021). Available from: https://incites.help.clarivate.com/Content/Indicators-Handbook/ih-normalized-indicators.htm#.%20Published%202020.%20Accessed%20August%209,%202021. (accessed August 9, 2021).
10. Devos P, Ménard J. Trends in worldwide research in hypertension over the period 1999-2018: a bibliometric study. Hypertension. (2020) 76:1649–55. doi: 10.1161/HYPERTENSIONAHA.120.15711
11. Grubbs JC, Glass RI, Kilmarx PH. Coauthor country affiliations in international collaborative research funded by the US national institutes of health, 2009 to 2017. JAMA Netw Open. (2019) 2:e1915989. doi: 10.1001/jamanetworkopen.2019.15989
12. Devos P, Lefranc H, Dufresne E, Beuscart R. From bibliometric analysis to research policy: the use of SIGAPS in lille university hospital. Stud Health Technol Inform. (2006) 124:543–8.
13. Devos P. De la bibliométrie au financement: le logiciel SIGAPS [from the bibliometry to the financing: the SIGAPS software]. [article in French]. J Neuroradiol. (2008) 35:31–3. doi: 10.1016/j.neurad.2008.01.003
14. Rouvillain JL, Derancourt C, Moore N, Devos P. Scoring of medical publications with SIGAPS software: application to orthopedics. Orthop Traumatol Surg Res. (2014) 100:821–5. doi: 10.1016/j.otsr.2014.06.020
15. Ueda R, Nishizaki Y, Homma Y, Sanada S, Otsuka T, Yasuno S, et al. Importance of quality assessment in clinical research in Japan. Front Pharmacol. (2019) 10:1228. doi: 10.3389/fphar.2019.01228
16. Devos P, Dufresne E, Renard JM, Beuscart R. SIGAPS. A prototype of bibliographic tool for medical research evaluation. Stud Health Technol Inform. (2003) 95:721–6. doi: 10.3233/978-1-60750-939-4-721
17. Clarivate. Quartile rankings and other metrics. (2022). Available online at: https://support.clarivate.com/ScientificandAcademicResearch/s/article/Journal-Citation-Reports-Quartile-rankings-and-other-metrics?language=en_US (accessed March 2, 2022).
18. Abi Jaoude J, Kouzy R, Rooney M, Thompson P, Patel R, Turner MC, et al. Impact factor and citation metrics in phase III cancer trials. Oncotarget. (2021) 12:1780–6. doi: 10.18632/oncotarget.28044
19. Kolkailah AA, Fugar S, Vondee N, Hirji SA, Okoh AK, ayoub a, et al. bibliometric analysis of the top 100 most cited articles in the first 50 years of heart transplantation. Am J Cardiol. (2019) 123:175–86. doi: 10.1016/j.amjcard.2018.09.010
20. Alkhawtani RHM, Kwee TC, Kwee RM. Funding of radiology research: frequency and association with citation rate. AJR Am J Roentgenol. (2020) 215:1286–9. doi: 10.2214/AJR.20.22786
21. Ministry of Health Labour and Welfare [MHLW]. MHLW core clinical research hospitals. (2021). Available online at: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/tyukaku.html (accessed June 5, 2021).
22. Clarivate, Clarivate InCites. Benchmarking & analytics. (2021). Available online at: https://clarivate.com/webofsciencegroup/solutions/incites/ (accessed July 20, 2021).
23. Times Higher Education. World university rankings. (2021). Available online at: https://www.timeshighereducation.com/world-university-rankings/2021/world-ranking#!/page/0/length/25/sort_by/rank/sort_order/asc/cols/stats (accessed July 9, 2021).
24. Ahmed Ali U, Reiber BMM, Ten Hove JR, van der Sluis PC, Gooszen HG, Boermeester MA, et al. Journal impact factor and methodological quality of surgical randomized controlled trials: an empirical study. Langenbecks Arch Surg. (2017) 402:1015–22. doi: 10.1007/s00423-017-1593-6
25. Froud R, Bjørkli T, Bright P, Rajendran D, Buchbinder R, Underwood M, et al. The effect of journal impact factor, reporting conflicts, and reporting funding sources, on standardized effect sizes in back pain trials: a systematic review and meta-regression. BMC Musculoskelet Disord. (2015) 16:370. doi: 10.1186/s12891-015-0825-6
26. Hutchins BI, Yuan X, Anderson JM, Santangelo GM. Relative citation ratio (RCR): a new metric that uses citation rates to measure influence at the article level. PLoS Biol. (2016) 14:e1002541. doi: 10.1371/journal.pbio.1002541
27. Emanuel EJ, Schnipper LE, Kamin DY, Levinson J, Lichter AS. The costs of conducting clinical research. J Clin Oncol. (2003) 21:4145–50.
28. Martin L, Hutchens M, Hawkins C, Radnov A. How much do clinical trials cost? Nat Rev Drug Discov. (2017) 16:381–2. doi: 10.1038/nrd.2017.70
29. Varse F, Janani L, Moradi Y, Solaymani-Dodaran M, Baradaran HR, Rimaz S. Challenges in the design, conduct, analysis, and reporting in randomized clinical trial studies: a systematic review. Med J Islam Repub Iran. (2019) 33:37. doi: 10.34171/mjiri.33.37
30. Alemayehu C, Mitchell G, Nikles J. Barriers for conducting clinical trials in developing countries- a systematic review. Int J Equity Health. (2018) 17:37. doi: 10.1186/s12939-018-0748-6
31. Fleischmann R, Decker AM, Kraft A, Mai K, Schmidt S. Mobile electronic versus paper case report forms in clinical trials: a randomized controlled trial. BMC Med Res Methodol. (2017) 17:153. doi: 10.1186/s12874-017-0429-y
32. Dillon DG, Pirie F, Rice S, Pomilla C, Sandhu MS, Motala AA, et al. Open-source electronic data capture system offered increased accuracy and cost-effectiveness compared with paper methods in Africa. J Clin Epidemiol. (2014) 67:1358–63. doi: 10.1016/j.jclinepi.2014.06.012
33. Rorie DA, Flynn RWV, Grieve K, Doney A, Mackenzie I, MacDonald TM, et al. Electronic case report forms and electronic data capture within clinical trials and pharmacoepidemiology. Br J Clin Pharmacol. (2017) 83:1880–95. doi: 10.1111/bcp.13285
34. McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, et al. What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials. (2006) 7:9. doi: 10.1186/1745-6215-7-9
Keywords: clinical research, research funding, citation impact, SIGAPS, category normalized citation impact, bibliometrics
Citation: Morisawa F, Nishizaki Y, Devos P, Yanagisawa N, Matsuyama K, Homma Y, Ueda R, Sekine M, Daida H, Minamino T and Sanada S (2022) The association between research funding status and clinical research papers’ citation impact in Japan: A cross-sectional bibliometric study. Front. Med. 9:978174. doi: 10.3389/fmed.2022.978174
Received: 25 June 2022; Accepted: 30 September 2022;
Published: 19 October 2022.
Edited by:
Hubert G. Leufkens, Utrecht University, NetherlandsReviewed by:
M. Ahmed, Phcog.Net, IndiaGaurav Dave, University of North Carolina at Chapel Hill, United States
Copyright © 2022 Morisawa, Nishizaki, Devos, Yanagisawa, Matsuyama, Homma, Ueda, Sekine, Daida, Minamino and Sanada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuji Nishizaki, eW5pc2hpemFAanVudGVuZG8uYWMuanA=
 Fumito Morisawa
Fumito Morisawa Yuji Nishizaki
Yuji Nishizaki Patrick Devos
Patrick Devos Naotake Yanagisawa4
Naotake Yanagisawa4 Kotone Matsuyama
Kotone Matsuyama Rieko Ueda
Rieko Ueda Miwa Sekine
Miwa Sekine Hiroyuki Daida
Hiroyuki Daida