
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 04 November 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.973030
This article is part of the Research Topic Reviews on the Effect of COVID-19 on Kidney Diseases Diagnosis, Management, and Outcomes View all 8 articles
 Tauqeer Hussain Mallhi1*
Tauqeer Hussain Mallhi1* Yusra Habib Khan1,2*
Yusra Habib Khan1,2* Abdulaziz Ibrahim Alzarea1
Abdulaziz Ibrahim Alzarea1 Faiz Ullah Khan3
Faiz Ullah Khan3 Nasser Hadal Alotaibi1
Nasser Hadal Alotaibi1 Abdullah Salah Alanazi1,2
Abdullah Salah Alanazi1,2 Muhammad Hammad Butt4
Muhammad Hammad Butt4 Ahmed D. Alatawi1
Ahmed D. Alatawi1 Muhammad Salman5
Muhammad Salman5 Sami I. Alzarea6
Sami I. Alzarea6 Ziyad Saeed Almalki7
Ziyad Saeed Almalki7 Mansoor A. Alghazi8
Mansoor A. Alghazi8 Majed Ahmed Algarni9
Majed Ahmed Algarni9The COVID-19 associated acute kidney injury (CAKI) has emerged as a potential intricacy during the management of patients. Navigating the rapidly growing body of scientific literature on CAKI is challenging, and ongoing critical appraisal of this complication is essential. This study aimed to summarize and critically appraise the systematic reviews (SRs) on CAKI to inform the healthcare providers about its prevalence, risk factors and outcomes. All the SRs were searched in major databases (PubMed, EMBASE, Web of Science) from inception date to December 2021. This study followed SR of SRs methodology, all the records were screened, extracted and subjected to quality assessment by assessing the methodological quality of systematic reviews (AMSTAR-2). The extracted data were qualitatively synthesized and tabulated. This review protocol was registered in PROSPERO (CRD42022299444). Of 3,833 records identified; 42 SRs were included in this overview. The quality appraisal of the studies showed that 17 SRs were of low quality, while 8 moderate and 17 were of high-quality SRs. The incidence of CAKI ranged from 4.3% to 36.4% in overall COVID-19 patients, 36%–50% in kidney transplant recipients (KTRs), and up to 53% in severe or critical illness. Old age, male gender, cardiovascular disease, chronic kidney disease, diabetes mellitus and hypertension were frequently reported risk factors of CAKI. The need of renal replacement therapy (RRT) was up to 26.4% in overall COVID-19 patients, and 39% among those having CAKI. The occurrence of acute kidney injury (AKI) was found independent predictor of death, where mortality rate among CAKI patients ranged from 50% to 93%. This overview of SRs underscores that CAKI occurs frequently among COVID-19 patients and associated with high mortality, need of RRT and adverse outcomes. However, the confidence of these results is moderate to low which warrants the need of more SRs having established methodological standards.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=299444], identifier [CRD42022299444].
The clinical spectrum of COVID-19 is typically manifested by respiratory intricacies including dyspnea, alveolar injury and respiratory failure. However, the rapid geographical distribution of infection showed the involvement of various other organs during the disease course. Acute kidney injury (AKI) has emerged as one of the most common atypical complications of COVID-19 infection. Its pathogenesis is attributed to the cytokine storm, fluid loss associated with hypovolemia (due to high-grade pyrexia and tachypnea), acute respiratory distress syndrome (ARDS), and direct viral invasion specifically into intrinsic renal cells (1, 2). SARS-CoV-2 is a cytopathic virus that enters the renal cells through ACE2. Since spike protein on the viral surface is activated by a cellular transmembrane serine protease (TMPRSSs), ACE2 is expressed along with TMPRSSs in proximal straight tubule cells and podocytes, thereby exposing the kidney to SARS-CoV-2 (3).
The growing body of evidence suggests that COVID-19 associated acute kidney injury (CAKI) occurs in a considerable number of patients and is accompanied by the increased duration of hospital stay, healthcare cost, renal replacement therapy (RRT), and mortality (1, 2, 4). Researchers around the globe responded to this issue by publishing an impressive number of reports on the incidence, risk factors and clinical outcomes of CAKI. Despite an increasing number of studies relating to various aspects of CAKI and its prognosis; discrepancies in size, methodologies, and research focus as well as regional differences in these studies call for a comprehensive systematic review and analysis of reports to date. Moreover, the quality and reproducibility of these evidences have become an area of concern for healthcare professionals across the globe. Systematic reviews are considered a cornerstone of evidence-based medicine and reflect the current scientific knowledge. Systematic reviews hold a highest level in the hierarchy of evidence, aiding healthcare practitioners to have informed decisions on a specific topic (5). The large volume of studies on renal involvements in COVID-19 has encouraged the researchers to systematically synthesize the findings of these studies, resulting in a large number of systematic reviews on this topic. Navigating the rapidly growing body of scientific literature on CAKI is challenging, and ongoing critical appraisal of this complication is essential.
Systematic Review of Systematic Reviews (SR of SRs) is a subtype of Overview of Systematic Reviews, which allows performing a comprehensive review of the highest level of evidence from already synthesized SR-level data with the end product ready to be used by the clinicians and policy-makers (6, 7). To the best of our search, the incidence of CAKI, risk factors and clinical outcomes have been discussed in 42 SRs. Considering the high number of SRs, it is imperative to perform an overview so existing literature could be identified and organized to underscore the areas of priority in decision making. Moreover, this overview will provide a composite draft of recent advancements in AKI among COVID-19 patients. In this context, this study aimed to summarize and critically appraise the SRs on CAKI to ascertain its incidence, risk factors, need for RRT, associated mortality, and other adverse outcomes.
This study is exempted from ethical approval because it involves qualitative synthesis of publicly available data.
This SR of SRs was intended (1) to summarize and critically appraise the SRs on AKI during the COVID-19 infection, and (2) to ascertain the reported incidence of CAKI, contributing risk factors, need for RRT, and mortality among COVID-19 patients.
The protocol of the current review is registered in PROSPERO (Registration no. CRD42022299444).
This study followed the SR of SRs methodology, which is a subtype of an overview of SRs. This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (8), and Preferred Reporting Items for Overview of Systematic Reviews (PRIO-harms) (7). The results of existing SRs on incidence, risk factors, and clinical outcomes of CAKI are synthesized, without re-synthesizing the primary studies.
The primary outcomes evaluated were; (1) incidence of AKI in COVID-19, (2) risk factors or predictors of CAKI, (3) need for RRT among COVID-19 patients, with or without AKI (4) impact of CAKI on mortality, and (5) impact of CAKI on other adverse clinical outcomes i.e., admissions to intensive care units (ICU), the incidence of severe infections, and graft loss among transplant recipients. The incidence of CAKI was presented in proportion (%), risk factors or predictors of CAKI are indicated as odds ratio (OR), Q, or mean differences (SMD, WMD), need for RRT among COVID-19 patients in OR and proportion (%), and mortality in OR, risk ratio (RR), and proportion (%).
A systematic search was performed in databases (PubMed, Scopus, ProQuest, PMC), review registries (PROSPERO and CENTRAL) and Google scholar from the inception date to December 2021. The search strategy used in the current study is described in Table 1 and in Supplementary File. The PubMed function “related articles” was used to extend the search. PI (E) CO formula was considered for framing the research question and inclusion/exclusion criteria.
Two reviewers independently searched databases by adopting the search strategy (Table 1). The relevancy of each study was evaluated initially through screening of title and abstract. Only studies providing information on incidence, risk factors, and prognosis or outcomes of AKI among COVID-19 patients were considered for further screening. The full texts of the studies were extracted and subjected to inclusion criteria; systematic reviews and/or meta-analysis with extractable quantitative data on specified study outcomes. Narrative reviews, animal studies, and those published in languages other than English were excluded. The bibliography of eligible SRs was also checked for potentially eligible studies. The gray literature was searched using Google scholar and the search was continued up to 10 pages, or until the search does not reveal any article not captured by previous searches.
The studies fulfilling the inclusion criteria were subjected to the quality assessment by using the assessing the methodological quality of systematic reviews (AMSTAR-2) tool. This tool evaluates the quality of SRs through 16 domains on various aspects; appropriateness of study selection, risks of bias (RoB) in primary studies, sensitivity analyses, the correctness of methods used for meta-analyses, consideration of RoB and heterogeneity while interpreting or discussing the results, and protocol registration. An online web-supported estimation was used to classify the studies into four quality categories; high, moderate, low, and critically low (9). The quality assessment of the studies was performed by two reviewers independently, where disagreement among authors was resolved through discussion and consensus by considering the third opinion from the principal investigator. Moreover, the quality of the included SRs was also assessed visually by tabulating each SR with information on the type of study, search strategies used, inclusion and exclusion criteria, methods used to evaluate RoB in primary studies, registration of SR, and major comments on RoB and heterogeneity in studies.
Two reviewers (YHK, MHB) extracted the required data independently using a standardized data collection form. Any conflict or deviation was solved through mutual consultation and concurrence with final approval from another reviewer (THM). Data were included according to the Cochrane recommendations for an overview of reviews. The following data were extracted: authors, year, type of review, the total number of primary studies, the total number of participants, objectives of the review, name, and the number of databases searched, the methodological quality of studies, limitations in SRs, the pooled prevalence of CAKI, risk factors of development of AKI during COVID-19, need for RRT among COVID-19 patients, an association of CAKI with mortality, and other relevant information on CAKI. In the case of missing information in SR, a gap in data reporting was described as not reported. Since the primary objective of this overview was to summarize the current body of available evidence on CAKI, an overlap analysis of primary studies was not performed. According to the Cochrane handbook for systematic reviews, it is acceptable to include the results of all relevant SRs regardless of overlapping of primary studies if the overview intends to describe the available evidence on a specified topic (10).
The extracted data were qualitatively synthesized and tabulated according to the aims of the study. Qualitative analysis of the findings from SRs was performed using narrative synthesis. Narrative summaries of the outcome data were presented within corresponding tables. The resulting data were also grouped according to study population i.e., adults, children, or kidney transplant recipients (KTRs). The pooled data from meta-analyses were presented and heterogeneity across the analyses was recorded as a minimum to maximum value for I2. The PRISMA flow diagram of this SR is shown in Figure 1. In addition, the PRISMA checklist has been provided in Supplementary Table 1.
This review has the following terms that need to be defined; COVID-19: confirmed COVID-19 cases, CAKI: acute kidney injury among COVID-19 patients, CAKI associated mortality: mortality rate among COVID-19 patients who developed AKI, Need for RRT: use of any modality of RRT among COVID-19 patients, risk factors of CAKI: factors or variables found to be associated with the development of AKI among COVID-19 patients. This review used various interchangeable terms which were originally used in included SRs e.g., RRT, CRRT, or dialysis are replaceable terms but they were used in this review as written in original SRs, as the definition of each term varied in SRs.
Initial search retrieved 3,833 articles from various databases and registers, where 276 were duplicates. Most of the SRs were excluded following abstract and title screening (n = 3,321) and 236 studies were subjected to full text screening. A total 42 reviews are included in this overview, where remaining studies were excluded due to the reasons described in Figure 1.
A total of 42 reviews (38 SRs with MA and 3 without MA) were included in this study (1, 2, 4, 11–49). The number of primary studies in the reviews ranged from 4 to 142 with confirmed COVID-19 cases ranging from n = 420 to n = 54,173. The primary studies in SRs were from various regions around the world, thereby covering all the global continents. None of the reviews included clinical trials (as they were not available on this topic), and three studies were published as research letters (12, 13, 27). Most of the reviews (n = 32, 76.2%) were reported according to PRISMA, MOOSE, and Cochrane checklists. However, nine studies (21.4%) did not include any information on reporting guidelines. The risk of bias (RoB) among primary studies was performed in 34 reviews (81%), while 8 studies (19%) did not appraise the primary studies. The New-Castel Ottawa Scale (NOS) was the most commonly (n = 14) used RoB tool in these reviews. Only eleven review protocols (26.2%) were registered before the synthesis of results. Six reviews (12, 16, 22, 24, 26, 27) which were published in 2020 did not provide details on specific inclusion and exclusion criteria. Most of the reviews excluded studies with < 10 patients, published in languages other than English, and on specialized populations i.e., children, pregnant women, and patients with cancer and CKD. One review was conducted on the pediatrics population (44), while five studies included KTRs in data synthesis (21, 31, 34, 36, 37). All the reviews did not include animal studies, and most of the reviews excluded case reports, except five studies (1, 11, 13, 21, 45). The heterogeneity across the primary studies was also observed by using I2-values from forest plots. Most of the reviews reported high heterogeneity with I2 > 90% during the analysis and sensitivity analysis was not considered in these studies. The methodological summary of included reviews is described in Table 2.
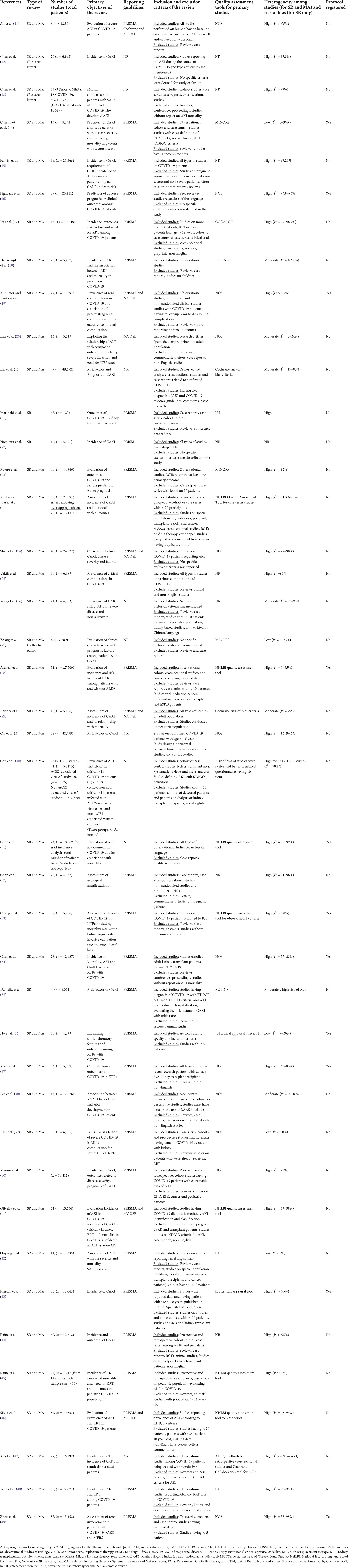
Table 2. Methodological summary of included reviews with reported heterogeneity across meta-analyses.
PubMed (n = 34), EMBASE (n = 29), Medline (n = 19), CENTRAL (n = 19), Preprints (n = 13), and SCOPUS (n = 12) were the most commonly used search databases in included SRs. Most of the studies used ≥ 2 databases with an average of 3.8 databases per review. However, two reviews only searched one database to retrieve the relevant studies (15, 25). Preprints and Chinese databases were also searched in various reviews (Figure 2).
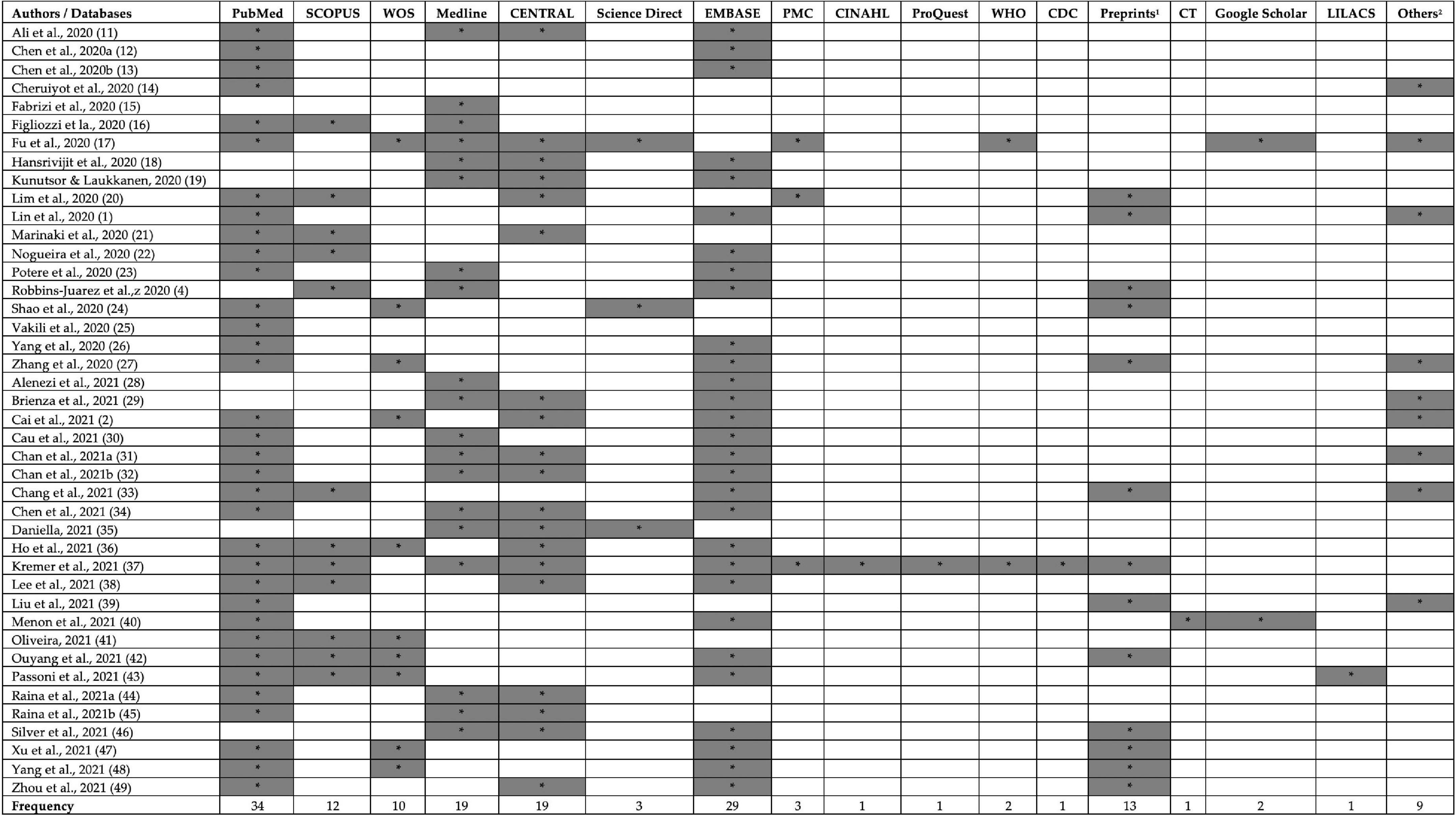
Figure 2. Search databases used in included reviews. CDC, Centers for Disease Control and Prevention database; CENTRAL, Cochrane Central Register of Controlled Trials; CINAHL, Cumulated Index to Nursing and Allied Health Literature; CT, ClinicalTrials.gov; PMC, PubMed Central; WHO, world health organization database; WOS, Web of Science. 1Preprints: BioRvix, MedRvix, and 2Other databases: Chinese databases, Intensive Care National Audit and Research Center (ICNARC) website, DARE database, CNKI, VIP, WanFang. The symbol * represents the presence database.
Approximately half of the reviews included (n = 17, 40.5%) were qualitatively judged as low (Low: 12, Critically low: 5). Only 17 (40.5%) reviews were judged as of high quality, while 8 (19.0%) studies were of moderate quality. None of the reviews provided the funding information for the primary studies (Figure 3). The quality appraisal indicates that most studies have critical flaws and may not provide accurate evidence synthesis. In this context, the major results were combined from studies having moderate to high methodological quality according to the AMSTAR-2 criteria. Seventeen reviews were excluded from the current overview due to poor methodological quality as reporting from low-quality reviews hinders concluding results for better understanding. However, all low-quality results were presented in Supplementary Table 2.
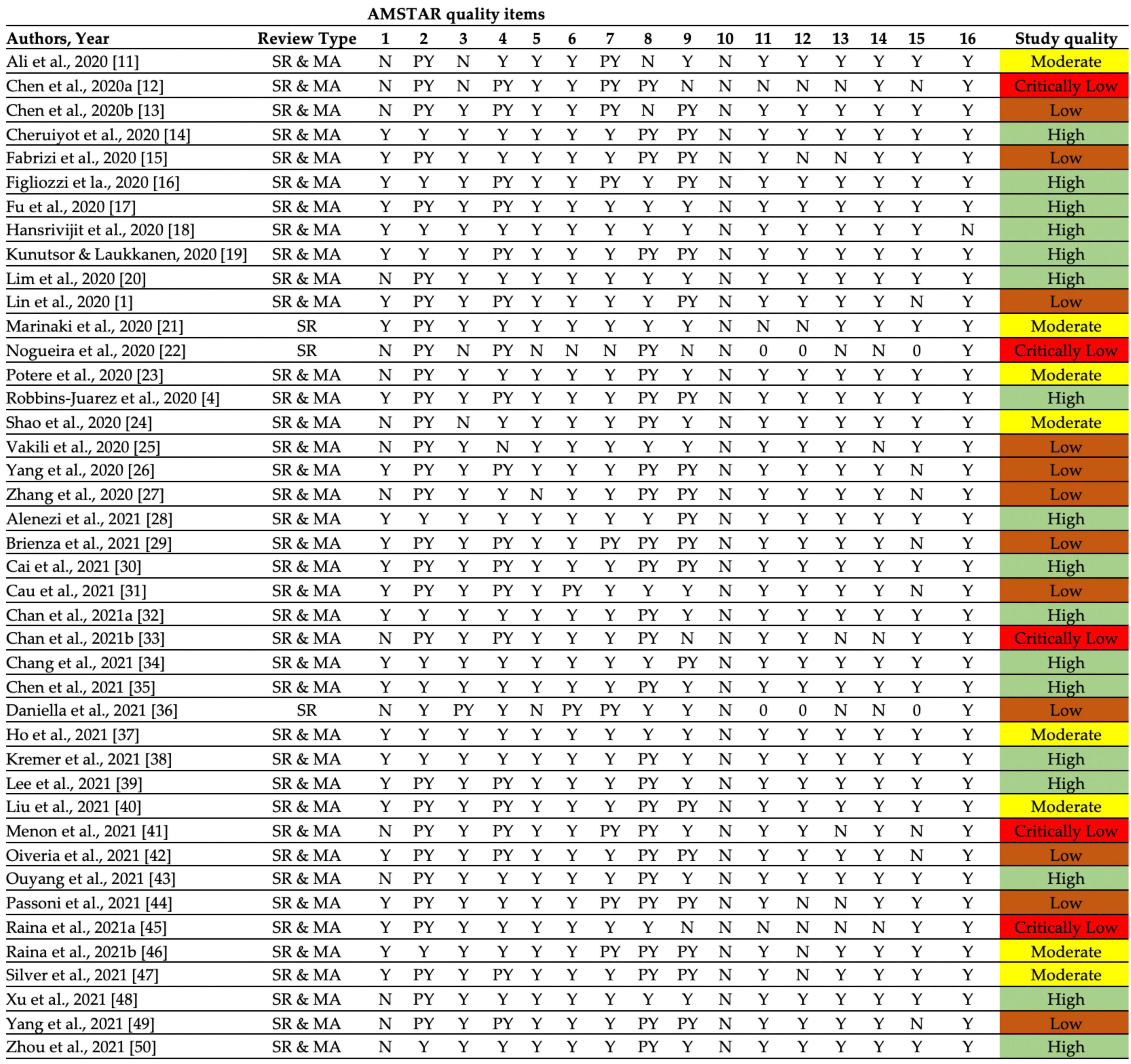
Figure 3. Quality assessment of included systematic reviews and meta-analyses according to AMSTAR-2 checklist. (High: Zero or one non-critical weakness, Moderate: More than one non-critical weakness, Low: One critical flaw with or without non-critical weaknesses, Critically low: More than one critical flaw with or without non-critical weaknesses).
Nineteen reviews reported the pooled incidence of AKI either in overall COVID-19 patients or in special populations (Table 3). The overall incidence of CAKI ranged from 4.3% (39) to 30.51% (45). However, the incidence of CAKI in specialized populations is given below;
Four reviews reported CAKI in KTRs ranging from 36% (31) to 50% (37). Fu et al. reported AKI in transplant patients ranging from 30% to 69%, but pooled prevalence was not estimated (17).
The definition of severe infection widely varied across the studies. Only one review reported pooled prevalence of CAKI as 21.1% in severe infection (39). However, six reviews (17, 18, 33, 34, 46, 47) pooled the incidence of CAKI from 19.9% (18) to 46% (46) among critically ill patients.
Only one study provided the estimation of CAKI as 12% among COVID-19 patients who were not admitted to ICU. None of the included SR reported the pooled prevalence of CAKI in non-severe, mild or moderate cases of infection. Such prevalence was pooled in two low quality reviews (1, 22). One SR indicated that AKI occurred in 59% of patients with ARDS and 6% among patients without ARDS (28).
Two SRs (4, 46, 48) pooled the incidence of AKI stages; stage I: 15%–44%, stage II: 7%–19%, and stage III: 11%–34%. AKI stage I was most prevalent across the SRs included in this review.
One SR reported the pooled prevalence of CAKI among children as 16.1% (31). Fu et al. reported pooled prevalence of CAKI as 12% in patients with age > 60 years, while it was 6% among patients having age < 60 years (17).
One SR reported CAKI as of 30.72% (42) among deceased patients. The odds (OR) of AKI among deceased patients was reported as 77.48 (42) in the same review.
Some reviews stratified the analysis according to the geographical location of the primary studies. Two reviews reported the incidence of CAKI ranging from 8.2% (19) to 9% in China. Two reviews reported CAKI ranging from 5.5% (17) to 7% (47) in Asia. One SR indicated CAKI as 28.6% in the USA and Europe (17). The reported incidence in the USA alone was 19.9% (19).
Xu et al. reported 7% AKI among COVID-19 who were receiving remdesivir therapy (47). Many low-quality studies have reported pooled prevalence of CAKI among patients with early hospitalization, secondary infection, sepsis, septic shock and those infected with ACE2-associated viruses but the data was not presented here as these studies were of low quality.
Only five SRs reported the risk factors for the development of AKI during COVID-19 infection. The most commonly reported risk factors were diabetes mellitus, hypertension (2, 17, 18, 31), chronic kidney disease (2, 17, 31), cardiovascular disease (2, 17, 31), age (2, 17, 18), and male gender (2, 17, 31). Other predictors were coronary artery disease (31), smoking, obesity, cancer, pneumopathy, mechanical ventilation, vasopressor use (2), and blockade of renin-angiotensin aldosterone system (RAAS) (38). Most of these factors were reported through meta-regression analyses with odd ratios (Table 3).
Sixteen reviews reported the need for RRT among COVID-19 patients. However, the modalities of RRT were not adequately described in these reviews. The RRT was needed by 1% (28)–23% (21) of COVID-19 patients. However, the need for RRT was reported as 15.6% in one review (47). One review reported that 12.65% of kidney transplant patients needed RRT (31). Two reviews (17, 46) reported the need for RRT ranging from 19% (46) to 26.6% (17) among critically ill patients, while its need was only 1% in non-critical patients (46). One review (39) showed RRT needs among patients with severe infection, having an odds ratio of 23.63 (39). Chan et al. reported 5.54% of children needed RRT during the COVID-19 treatment (31). However, the RRT was needed in 31.51% of fatal cases (42). The pooled incidence of RRT was found to be comparatively high among COVID-19 patients with ARDS (20%) than those without ARDS (1%) (28) (Table 3).
Fourteen reviews reported the association of CAKI with increased mortality (Table 3). The CAKI-associated mortality rate ranged from 52% (4) to 74.3% (49). In addition, the prevalence of mortality with the urgent start of RRT was 74.2%, having an odds ratio of 3.04. Ten reviews (4, 11, 14, 16, 18, 24, 28, 31, 34, 45, 49) found CAKI to be an independent risk factor of mortality with odd ratios ranging from 2.55 (45) to 23.9 (14). However, the odds of mortality due to CAKI in severe COVID-19 infection ranged from 4.19 (11) to 14.05 (14). One review reported the relationship of AKI severity with mortality, where AKI stages 1, 2, and 3 had 7.45, 24.64, and 94.77 times higher odds of death as compared to patients who did not develop AKI (31) (Table 3). The critical cases portended higher mortality than non-critical cases (14.18% vs. 9.66%) (4). Likewise, COVID-19 patients with ARDs had higher mortality as compared to those without ARDS (60.3% vs. 34.2%) (28) (Figure 4).
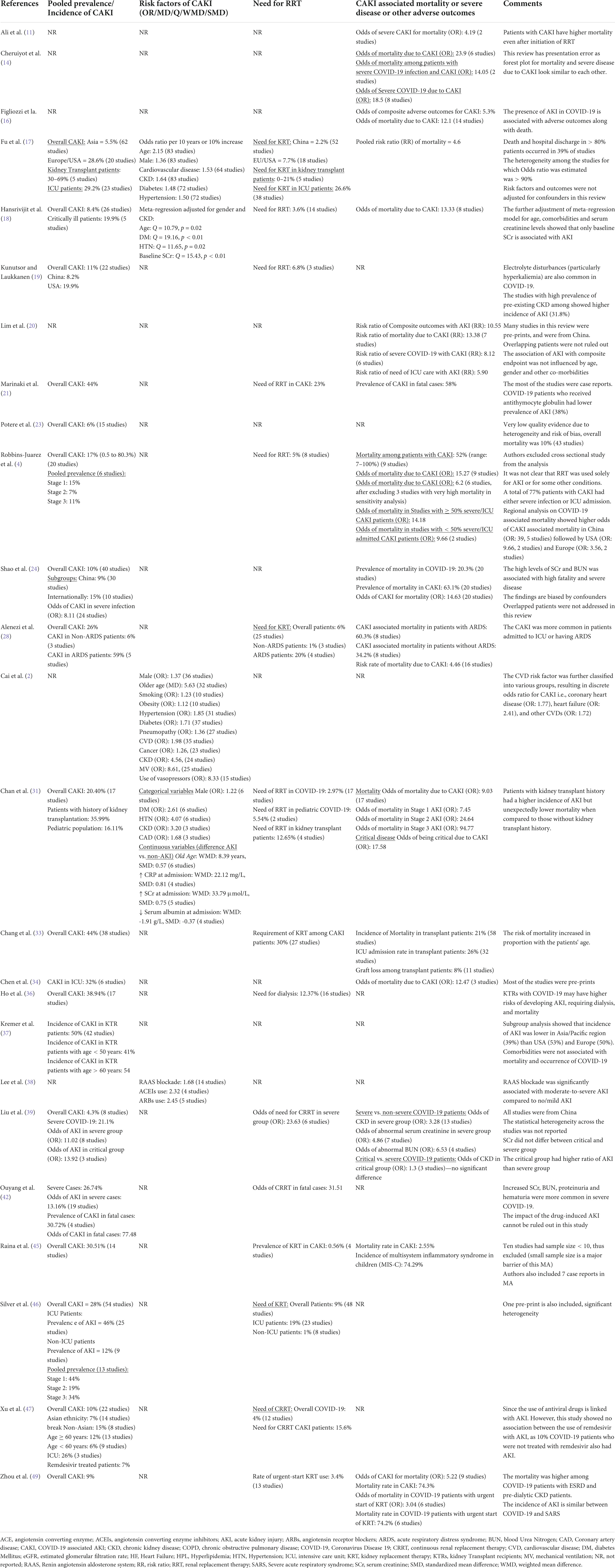
Table 3. Major findings extracted from the moderate and high-quality reviews (incidence, risk factors, need of RRT and outcomes of CAKI).
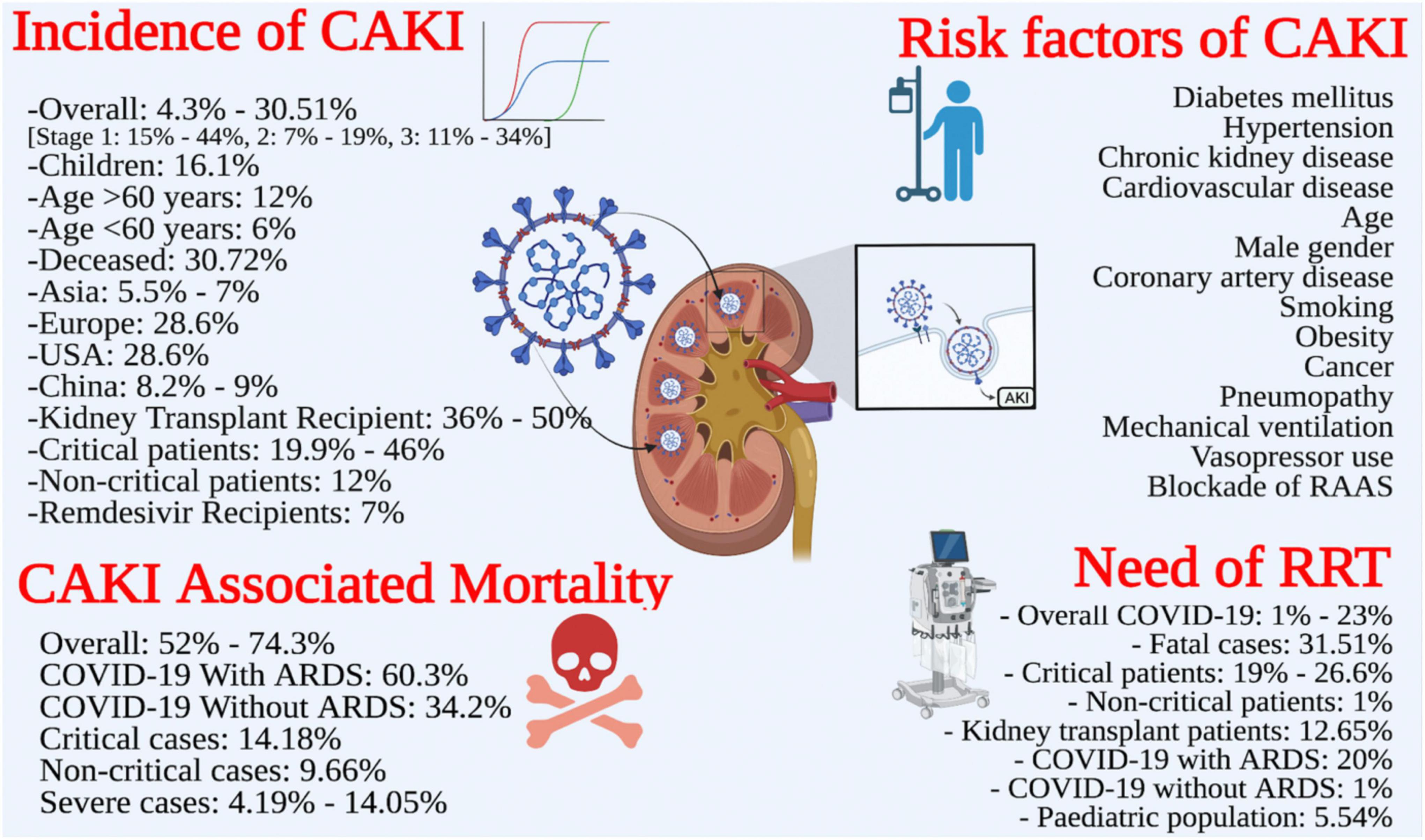
Figure 4. Review summary: incidence, risk factors, need for renal replacement therapy (RRT), outcomes of CAKI.
This is the first study of its own kind overviewing the existing data on CAKI. The large volume and variable quality of published work on COVID-19 highlight an overwhelming need to organize and summarize findings so that the most current and accurate information can easily be accessed. Though evidence in the form of SRs relating to the development of AKI during the COVID-19 infection exists but is conflicting. In this context, bringing together reviews transparently and systematically and aiding informed decision-making by gathering, appraising, and systematically analyzing the evidence have a valuable role during this ongoing pandemic. The findings originated from this SR generate pivotal insights and understanding of CAKI, thereby summarizing and enhancing the accessibility of existing evidence.
The kidney involvement during the COVID-19 infection ranges from mild proteinuria to an advanced AKI. The pathogenesis of SARS-CoV-2 mediated kidney injury is found to be complex and multifactorial. SARS-CoV-2 mainly binds with ACE2, which is expressed in kidneys on the brush border of the apical membrane of proximal tubules and to a lesser extent in podocytes. The receptor-binding domain (RBD) of the viral spike protein and transmembrane protease serine 2 (TMPRSS2) facilitates the entry of SARS-CoV-2 into renal cells (50). In addition, the upregulation of the NRP-1 receptor (due to arterial injury in severe COVID-19 patients) is also associated with viral invasion into the renal cells (51). Other mechanisms underlying the renal injury during the COVID-19 infection are cytokine storm, Angiotensin II pathway activation, dysregulation of complement, hypercoagulation, and microangiopathy (52, 53).
It is pertinent to mention that some of the currently approved drugs for the treatment of COVID-19 have nephrotoxic potential, their role in perpetuating renal damage cannot be disregarded. On the other hand, many drugs being used for the treatment of chronic ailments may also portend nephrotoxicity among COVID-19 patients. However, very few studies have investigated the impact of drugs on kidney functions among COVID-19 patients. Xu et al. quantitively synthesized the impact of remdesivir on kidney functions from five observational studies (n = 972 patients). The pooled incidence of AKI among patients who were using remdesivir was 7%, while it was 10% in patients who were not receiving remdesivir. Their findings indicate that remdesivir does not induce AKI among COVID-19 patients (47). Another study reported no association of remdesivir with drug-induced AKI even in patients who had a baseline eCrCl < 30 mL/min (54). In contrast, Lee et al. reported two times higher risks of developing moderate to severe AKI among ACEIs and ARBs‘ users compared to no/mild AKI. These findings underscored the relationship of RAAS blockade with the development of AKI among COVID-19 patients (38). The possible mechanism may relate to the significance of ACE2 during the pathogenesis of COVID-19. However, these hypothetical risks require more investigations to make a firm conclusion. The contribution of drugs in synergizing the renal damage among COVID-19 patients can only be assessed through large-scale studies. Likewise, the role of chronic diseases such as diabetes mellitus should also be considered while ascertaining the pathogenesis of kidney injury due to SARS-CoV-2.
The existing evidence indicates that the renal intricacies during COVID-19 infection are common and may require RRT. This study found a great disparity in the incidence of CAKI where its occurrence varied from 4.3% (39) to 30.51% (44) in overall COVID-19 patients. The wide variations in the incidence of CAKI across these SRs might be attributed to various factors i.e., disease severity, sample size, risk of bias in primary studies, demographic variations among patients (comorbidities, pre-existing renal impairment, use of nephrotoxic drugs), study design, diagnostic bias, inappropriate methods for statistical combination of results leading to under- or over-estimation of actual incidence, variation in methods used to estimate the baseline serum creatinine and variable operational definitions such as diagnostic criteria of AKI used in the primary studies. It is pertinent to mention that the reviews reporting the lowest (23, 26, 39) and highest (28, 35, 42, 45, 46) incidence of CAKI did not explain the reasons for such variations. However, the disease severity was reported as the most common contributor to the variable incidence of CAKI among COVID-19 patients. It can also be explained by the methodological quality status of the SR, as a low quality review reported the pooled incidence of CAKI among critically ill patients as 80.6% (41). The quality appraisal must be accounted for while interpreting the results from the reviews as poorly designed review methods may lead to erroneous results. Taken together, the findings of this overview indicate that CAKI is a commonly occurring atypical complication among COVID-19 patients. However, careful evaluation of these SRs indicated that various patient-related factors contributed to the higher incidence i.e., severe or critical illness, pre-existing renal anomalies, and renal transplants.
Interestingly, the incidence of AKI among COVID-19 patients was also compared with that reported in ACE2 (SARS-CoV-1, influenza H1N1, H7N9) and non-ACE2 (MERS-CoV, other influenza strains) viruses associated respiratory infections (30). The subgroup analysis showed that the frequency of AKI occurrence among COVID-19 patients (51%) was equivalent to that reported in infections associated with other ACE2 viruses (52%), while lower than that reported in non-ACE2 viruses associated infections (63%). Similarly, another study estimated the pooled prevalence of AKI in three coronavirus-related infections and found the highest prevalence of AKI in MERS (42%), followed by SARS (9.6%) and COVID-19 (9%) (49). Additionally, the need for RRT was also highest among MERS patients (30). It has been hypothesized that coronaviruses share common biological mechanisms to induce AKI among patients. However, the variation in the prevalence of AKI lends support to distinct pathophysiological processes among coronaviruses (30). For example, high renal expression of dipeptidyl peptidase-4 (receptor for MERS-CoV) might be attributed to the high prevalence of AKI and RRT need among MERS patients (55). Another study pooled the mortality rate due to AKI among coronavirus infections (SARS, MERS, COVID-19) at 77.4%, where the highest mortality rate was observed among patients with SARS (86.6%), followed by COVID-19 (76.5%) and MERS (68.5%) (13). However, these findings must be considered in light of various demerits of this study i.e., low quality, published as a research letter, and a small number of primary studies related to SARS and MERS. Another rapid review reported the mortality rate ranging from 10% to 15% in SARS, up to 35% in MERS, and 13% in COVID-19 (56). The low mortality rate among COVID-19 patients as compared to SARS and MERS has also been reported by other studies (30) and might be attributed to the improvement in medical services and facilities through the years, particularly during this pandemic. However, more studies comparing the coronavirus infections are of significant importance during this pandemic.
Only two SRs estimated the pooled incidence of AKI severity stages (4, 46). The variation in the prevalence of AKI stages is attributed to the small number of studies in the synthesis of results. The limited number of primary studies on AKI stages warrants an urgent need for more investigations so severity-based prognosis could be ascertained.
Only 5 studies reported the risk factors for the development of CAKI. Diabetes mellitus and hypertension were most commonly reported risk factors of CAKI in the literature (2, 17, 18, 31), followed by chronic kidney disease (2, 17, 31), cardiovascular disease (2, 17, 31), age (2, 17, 18), and male gender (2, 17, 31). Previous studies (2, 57) have reported that elderly patients with COVID-19 have a more severe or critical illness, comorbid conditions, immunodeficiencies, and secondary infections, rendering them more susceptible to renal injury as compared to younger adults. Moreover, a decrease in the proliferative ability of stem cells in this age group also increases the odds of renal injury (58). Another review also found that patients with age > 60 years had higher odds of developing CAKI as compared to those with age < 60 years (1). Some patients with comorbid conditions such as hypertension and diabetes mellitus are treated with ACE inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs). These drugs upregulate ACE2, thereby increasing the risks of severe or critical COVID-19 infection and the disease severity is well known to be associated with the development of AKI. Elderly patients frequently use these drugs which might be another factor leading to a high incidence of CAKI among them. Lee et al. in their systematic review also reported the RAAS blockade as an independent risk factor of CAKI among COVID-19 patients (38). Available evidence also indicates a great disparity in gender and ethnic distribution of CAKI among patients. Four reviews have identified the male gender as a predictor of CAKI. Recent investigations suggested the role of hormones in the gender-wise pathogenesis of CAKI. The high levels of androgen upregulate the expression of ACE2 and TMPRSSs in renal tissues, as well as modulate the immune response and reduce the antibody response resulting in severe COVID-19 infection. These mechanisms explain the greater risk of CAKI in males (59). Moreover, the expression of ACE2 and TMPRSSs is also higher among elderly people and smokers, making this population more vulnerable to kidney damage (59). The estrogen and progesterone play a protective role by inhibiting SARS-CoV-2 from entering host cells through the downregulation of ACE2 and TMPRSSs, providing a theoretical basis for the lower incidence of CAKI in females (60). These findings indicate that gender differences must be considered while investigating or managing COVID-19 patients. However, the confounding effect of various factors must be adjusted before performing any predictive analysis, i.e., the use of drugs is associated with comorbidities that may be further linked to old age.
This overview found that COVID-19 patients with AKI portend adverse clinical outcomes as compared to those without AKI. The substantial need for RRT has been reported in 16 reviews. These findings indicate that COVID-19 patients with or without AKI require RRT during their management. However, the need for RRT is more among patients with CAKI (47), severe disease (39), admitted to ICU (17), and those with respiratory distress syndrome (ARDS) (28). Furthermore, the patients who died of COVID-19 displayed a higher application rate of RRT than the survival cases (42). It has been reported that critical COVID-19 patients needed RRT within 4 days of their ICU admission. The RRT modalities used were continuous RRT, intermittent hemodialysis and peritoneal dialysis (61). It is important to note that RRT is not only needed to compensate for the renal damage but also required to remove inflammatory factors, thus blocking cytokine storm syndrome among COVID-19 patients and ultimately reducing the damage inflicted on multiple organs (62). However, the indication of RRT, choice of modality, timing of initiation, and duration of use are still some unanswered questions that warrant a dire need for further investigations.
The data pertaining to the mortality due to CAKI were reported in 14 reviews. The AKI-associated mortality in COVID-19 occurred in approximately half of the overall patients and 3/4 of the severe cases and those receiving RRT. It has been noted that CAKI is associated with high mortality even after initiating RRT (11). Cheruiyot et al. reported mortality up to 1/4th of the COVID-19 patients, where severe cases had fourteen times higher chances of death than non-severe cases (14). Despite a wide disparity in fatality rate attributed to CAKI, the results of these reviews explicitly indicate that patients with CAKI had higher odds of mortality than those without kidney injury. It might be due to initiating and perpetuating the cardiac and lung damage due to AKI, underlining the potential multifaceted impact of lung-kidney crosstalk. In this context, the monitoring of kidney functions should be emphasized even among patients with mild illness and particular attention should be paid to those with the compromised renal profile.
Despite a growing body of evidence on CAKI from systematic reviews, the existing literature does not answer some research questions. There is a dearth of investigations presenting the data on AKI staging and its relationship with prognosis, involvement of medications in the pathogenesis of CAKI, and risk factors of CAKI-associated mortality. Moreover, the use of variable definitions of AKI and COVID-19 severity, and the unavailability of baseline serum creatinine may also under- or over- estimate the prevalence of CAKI. In addition, the post-AKI recovery pattern is not assessed in these studies. The data on the management of CAKI and its additional burden on the healthcare budget is not revealed in included SRs. These reviews also did not provide detailed data on CAKI in special populations, i.e., pregnant, cancer, immunodeficient, and pediatric patients.
Since this is a large study intended to summarize and critically appraise the SRs on CAKI, the findings must be interpreted in light of a few shortcomings. This study was not subjected to overlap analysis and some included reviews share various primary studies. However, it is admissible if the purpose of the overview is to summarize the evidence on a specific topic (10). The systematic reviews in this overview may have had some issues as primary studies of these SRs were not checked for the accuracy of data. Some reviews were published as short reports or rapid reviews, thereby providing limited information on search methodology, which may impact the quality assessment of SRs in this overview. However, it is not necessary that the low quality of these SRs is due to poor methodology but might be associated with the lack of reporting. Various SRs in this overview included case series and case reports, thereby impacting the quality of evidence. Furthermore, some SRs also included non-peer-reviewed research studies which were available as preprints at the time of the review process that encourages the critical evaluation of their findings while interpreting the results of this overview. In addition, the results of this overview are combined from moderate to high-quality SRs, thereby indicating the high validity and implications of findings in the clinical practice. However, the use of other quality appraisal tools for systematic reviews may result in variable findings due to the inclusion of studies that we had excluded from this overview. It is important to note that some of the reviews included primary studies classifying AKI with various definitions. The use of different criteria for AKI stratification may result in over- or under-estimation of AKI, thereby, it is suggested to firmly consider the use of the KDIGO criterion to define AKI in future studies. Last but not least, this overview may not cover some studies published after the search date. Nevertheless, this overview carries particular importance and provides an insight into CAKI in a diverse population to policymakers, healthcare professionals, and researchers. This study also revealed some evidence gaps which could help future researchers.
This overview underscored that AKI is a commonly occurring intricacy among COVID-19 patients. CAKI is associated with higher mortality, severe or critical illness, need for RRT, and poor prognosis. Male gender, old age, certain comorbid conditions (diabetes mellitus, hypertension), smoking, obesity, mechanical ventilation, preexisting renal anomalies, and nephrotoxic drugs are independent risk factors for the development of CAKI. However, this evidence is generated from moderate quality reviews of studies with considerable methodological differences. Despite a large number of SRs, the data on long-term outcomes of CAKI and its association with the severity of renal injury are still scarce. Further investigations should consider these research questions along with the limitations of available evidence so that more firm conclusions can be made. Early detection, timely management including blood purification therapies, adequate hemodynamic support, nephrology consultation, risk assessment through predictive models and avoidance of nephrotoxic drugs may help to improve the vital prognosis of COVID-19 patients with AKI. Since COVID-19 will continue to evolve, it is an impetus to design and implement renal care guidelines for COVID-19 patients.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
TM, YK, AIA, and NA: conceptualization. TM, FK, ASA, MB, and ADA: methodology. YK, ASA, MB, MS, SA, and ZA: software. TM, AIA, NA, and ManA: validation. TM, YK, FK, MB, ADA, MS, and MajA: writing—original draft preparation. TM, YK, AIA, NA, ASA, ZA, and ManA: writing—review and editing. MB: visualization. TM and YK: supervision. TM: project administration. All authors have read and agreed to the published version of the manuscript.
This work was funded by the Deanship of Scientific Research at Jouf University under (grant no. DSR-2021-01-0338).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.973030/full#supplementary-material
1. Lin L, Wang X, Ren J, Sun Y, Yu R, Li K, et al. Risk factors and prognosis for COVID-19-induced acute kidney injury: a meta-analysis. BMJ Open. (2020) 10:e042573. doi: 10.1136/bmjopen-2020-042573
2. Cai X, Wu G, Zhangand J, Yang L. Risk factors for acute kidney injury in adult patients with COVID-19: a systematic review and meta-analysis. Front Med. (2021) 8:719472. doi: 10.3389/fmed.2021.719472
3. Yalameha B, Roshan B, Vks Bhaskarand L, Mohmoodnia L. Perspectives on the relationship of renal disease and coronavirus disease 2019. J Nephropharmacol. (2020) 9:e22. doi: 10.34172/npj.2020.22
4. Robbins-Juarez SY, Qian L, King KL, Stevens JS, Husain SA, Radhakrishnan J, et al. Outcomes for patients with COVID-19 and acute kidney injury: a systematic review and meta-analysis. Kidney Int Rep. (2020) 5:1149–60. doi: 10.1016/j.ekir.2020.06.013
5. Lunny C, Brennan SE, McDonaldand S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 2—risk of bias assessment; synthesis, presentation and summary of the findings; and assessment of the certainty of the evidence. Syst Rev. (2018) 7:1–31. doi: 10.1186/s13643-018-0784-8
6. Aromataris E, Pearson A. The systematic review: an overview. AJN Am J Nurs. (2014) 114:53–8. doi: 10.1097/01.NAJ.0000444496.24228.2c
7. Bougioukas KI, Liakos A, Tsapas A, Ntzaniand E, Haidich A-B. Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol. (2018) 93:9–24. doi: 10.1016/j.jclinepi.2017.10.002
8. Mallhi TH, Ijaz I, Khan YH, Javed A, Saleem U-N, Aftab RA, et al. Relationship between the use of drugs and changes in body weight among patients: a systematic review and meta-analysis. Trop J Pharm Res. (2022) 21:899–908. doi: 10.4314/tjpr.v21i4.30
9. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. Bmj. (2017) 358:j4008. doi: 10.1136/bmj.j4008
10. Pollock M, Fernandes RM, Becker LA, Pieperand D, Hartling L. Chapter V: overviews of reviews. In: Cochrane editor. Cochrane Handbook for Systematic Reviews of Interventions Version. (Vol. 6), Hoboken, NJ: Cochrane Library (2020).
11. Ali H, Daoud A, Mohamed MM, Salim SA, Yessayan L, Baharani J, et al. Survival rate in acute kidney injury superimposed COVID-19 patients: a systematic review and meta-analysis. Renal Fail. (2020) 42:393–7. doi: 10.1080/0886022X.2020.1756323
12. Chen Y-T, Shao S-C, Hsu C-K, Wu IW, Hungand M-J, Chen Y-C. Incidence of acute kidney injury in COVID-19 infection: a systematic review and meta-analysis. Crit Care. (2020) 24:1–4. doi: 10.1186/s13054-020-03009-y
13. Chen Y-T, Shao S-C, Lai EC-C, Hungand M-J, Chen Y-C. Mortality rate of acute kidney injury in SARS, MERS, and COVID-19 infection: a systematic review and meta-analysis. Crit Care. (2020) 24:439. doi: 10.1186/s13054-020-03134-8
14. Cheruiyot I, Kipkorir V, Ngure B, Misiani M, Munguti J, Henry B, et al. Acute kidney injury is associated with worse prognosis in COVID-19 patients: a systematic review and meta-analysis. Acta Bio Med. (2020) 91:e2020029.
15. Fabrizi F, Alfieri CM, Cerutti R, Lunghiand G, Messa P. COVID-19 and acute kidney injury: a systematic review and meta-analysis. Pathogens. (2020) 9:1052. doi: 10.3390/pathogens9121052
16. Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, et al. Predictors of adverse prognosis in COVID−19: a systematic review and meta−analysis. Eur J Clin Investig. (2020) 50:e13362. doi: 10.1111/eci.13362
17. Fu EL, Janse RJ, de Jong Y, Endt VHW, Milders J, van der Willik EM, et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin Kidney J. (2020) 13:550–63. doi: 10.1093/ckj/sfaa160
18. Hansrivijit P, Qian C, Boonpheng B, Thongprayoon C, Vallabhajosyula S, Cheungpasitporn W, et al. Incidence of acute kidney injury and its association with mortality in patients with COVID-19: a meta-analysis. J Investig Med. (2020) 68:1261–70. doi: 10.1136/jim-2020-001407
19. Kunutsor SK, Laukkanen JA. Renal complications in COVID-19: a systematic review and meta-analysis. Ann Med. (2020) 52:345–53. doi: 10.1080/07853890.2020.1790643
20. Lim MA, Pranata R, Huang I, Yonas E, Soerotoand AY, Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Health Dis. (2020) 7:2054358120938573. doi: 10.1177/2054358120938573
21. Marinaki S, Tsiakas S, Korogiannou M, Grigorakos K, Papaloisand V, Boletis I. A systematic review of COVID-19 infection in kidney transplant recipients: a universal effort to preserve patients’ lives and allografts. J Clin Med. (2020) 9:2986. doi: 10.3390/jcm9092986
22. Nogueira SÁR, Oliveira SCS, Carvalho AFM, Neves JMC, Silva LSV, Silva GB, et al. Renal changes and acute kidney injury in covid-19: a systematic review. Rev Assoc Méd Bras. (2020) 66:112–7. doi: 10.1590/1806-9282.66.s2.112
23. Potere N, Valeriani E, Candeloro M, Tana M, Porreca E, Abbate A, et al. Acute complications and mortality in hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Crit Care. (2020) 24:1–12. doi: 10.1186/s13054-020-03022-1
24. Shao M, Li X, Liu F, Tian T, Luoand J, Yang Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: a systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol Res. (2020) 161:105107. doi: 10.1016/j.phrs.2020.105107
25. Vakili K, Fathi M, Pezeshgi A, Mohamadkhani A, Hajiesmaeili M, Rezaei-Tavirani M, et al. Critical complications of COVID-19: a descriptive meta-analysis study. Rev Cardiovasc Med. (2020) 21:433–42. doi: 10.31083/j.rcm.2020.03.129
26. Yang X, Jin Y, Li R, Zhang Z, Sunand R, Chen D. Prevalence and impact of acute renal impairment on COVID-19: a systematic review and meta-analysis. Crit Care. (2020) 24:1–8. doi: 10.1186/s13054-020-03065-4
27. Zhang Z, Zhang L, Zha D, Huand C, Wu X. Clinical characteristics and risks of Chinàs 2019 novel coronavirus patients with AKI: a systematic review and meta-analysis. Renal Fail. (2020) 42:926–31. doi: 10.1080/0886022X.2020.1812401
28. Alenezi FK, Almeshari MA, Mahida R, Bangash MN, Thickettand DR, Patel JM. Incidence and risk factors of acute kidney injury in COVID-19 patients with and without acute respiratory distress syndrome (ARDS) during the first wave of COVID-19: a systematic review and Meta-Analysis. Renal Fail. (2021) 43:1621–33. doi: 10.1080/0886022X.2021.2011747
29. Brienza N, Puntillo F, Romagnoliand S, Tritapepe L. Acute kidney injury in coronavirus disease 2019 infected patients: a meta-analytic study. Blood Purif. (2021) 50:35–41. doi: 10.1159/000509274
30. Cau AA-O, Cheng MP, Lee T, Levin A, Lee TC, Vinh DC, et al. Acute kidney injury and renal replacement therapy in COVID-19 versus other respiratory viruses: a systematic review and meta-analysis. Can J Kidney Health Dis. (2021) 8:20543581211052185. doi: 10.1177/20543581211052185
31. Chan KW, Yu KY, Lee PW, Laiand KN, Tang SC-W. Global Renal Involvement of CORonavirus Disease 2019 (RECORD): a systematic review and meta-analysis of incidence, risk factors, and clinical outcomes. Front Med. (2021) 8:678200. doi: 10.3389/fmed.2021.678200
32. Chan VW-S, Chiu PK-F, Yee C-H, Yuan Y, Ngand C-F, Teoh JY-C. A systematic review on COVID-19: urological manifestations, viral RNA detection and special considerations in urological conditions. World J Urol. (2021) 39:3127–38. doi: 10.1007/s00345-020-03246-4
33. Chang R, Elhusseiny KM, Yehand Y-C, Sun W-Z. COVID-19 ICU and mechanical ventilation patient characteristics and outcomes—A systematic review and meta-analysis. PLoS One. (2021) 16:e0246318. doi: 10.1371/journal.pone.0246318
34. Chen J-J, Kuo G, Lee TH, Yang H-Y, Wu HH, Tu K-H, et al. Incidence of mortality, acute kidney injury and graft loss in adult kidney transplant recipients with coronavirus disease 2019: systematic review and meta-analysis. J Clin Med. (2021) 10:5162. doi: 10.3390/jcm10215162
35. Daniella D, Kandariniand Y, Mahadita GW. Risk factors for acute kidney injury in COVID-19 patients: a systematic review. Open Access Macedonian J Med Sci. (2021) 9:118–23. doi: 10.3889/oamjms.2021.5846
36. Ho QY, Sultana R, Lee TL, Thangaraju S, Keeand T, Htay H. Coronavirus disease 2019 in kidney transplant recipients: a systematic review and meta-analysis. Singapore Med J. (2021) [Online ahead of print]. doi: 10.11622/smedj.2021171
37. Kremer D, Pieters TT, Verhaar MC, Berger SP, Bakker SJL, van Zuilen AD, et al. A systematic review and meta−analysis of COVID−19 in kidney transplant recipients: lessons to be learned. Am J Transpl. (2021) 21:3936–45. doi: 10.1111/ajt.16742
38. Lee SA, Park R, Yang JH, Min IK, Park JT, Han SH, et al. Increased risk of acute kidney injury in coronavirus disease patients with renin–angiotensin–aldosterone-system blockade use: a systematic review and meta-analysis. Sci Rep. (2021) 11:1–8. doi: 10.1038/s41598-021-92323-8
39. Liu Y-F, Zhang Z, Pan X-L, Xing G-L, Zhang Y, Liu Z-S, et al. The chronic kidney disease and acute kidney injury involvement in COVID-19 pandemic: a systematic review and meta-analysis. PLoS One. (2021) 16:e0244779. doi: 10.1371/journal.pone.0244779
40. Menon T, Sharma R, Kataria S, Sardar S, Adhikari R, Tousif S, et al. The association of acute kidney injury with disease severity and mortality in COVID-19: a systematic review and meta-analysis. Cureus. (2021) 13:e13894. doi: 10.7759/cureus.13894
41. Oliveira CB, Lima CAD, Vajgel G, Coelhoand AVC, Sandrin-Garcia P. High burden of acute kidney injury in COVID-19 pandemic: systematic review and meta-analysis. J Clin Pathol. (2021) 74:796–803. doi: 10.1136/jclinpath-2020-207023
42. Ouyang L, Gong Y, Zhuand Y, Gong J. Association of acute kidney injury with the severity and mortality of SARS-CoV-2 infection: a meta-analysis. Am J Emerg Med. (2021) 43:149–57. doi: 10.1016/j.ajem.2020.08.089
43. Passoni R, Lordani TVA, Peresand LAB, da Silva Carvalho AR. Occurrence of acute kidney injury in adult patients hospitalized with COVID-19: a systematic review and meta-analysis. Nefrología. (2021) 42:404–14. doi: 10.1016/j.nefro.2021.09.002
44. Raina R, Chakraborty R, Mawby I, Agarwal N, Sethiand S, Forbes M. Critical analysis of acute kidney injury in pediatric COVID-19 patients in the intensive care unit. Pediatr Nephrol. (2021) 36:2627–38. doi: 10.1007/s00467-021-05084-x
45. Raina R, Mahajan ZA, Vasistha P, Chakraborty R, Mukunda K, Tibrewal A, et al. Incidence and outcomes of acute kidney injury in COVID-19: a systematic review. Blood Purif. (2021) 51:199–212. doi: 10.1159/000514940
46. Silver SA, Beaubien-Souligny W, Shah PS, Harel S, Blum D, Kishibe T, et al. The prevalence of acute kidney injury in patients hospitalized with COVID-19 infection: a systematic review and meta-analysis. Kidney Med. (2021) 3:83–98. doi: 10.1016/j.xkme.2020.11.008
47. Xu Z, Tang Y, Huang Q, Fu S, Li X, Lin B, et al. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. (2021) 22:52. doi: 10.1186/s12882-021-02244-x
48. Yang X, Tianand S, Guo H. Acute kidney injury and renal replacement therapy in COVID-19 patients: a systematic review and meta-analysis. Int Immunopharmacol. (2021) 90:107159. doi: 10.1016/j.intimp.2020.107159
49. Zhou S, Xu J, Xue C, Yang B, Maoand Z, Ong ACM. Coronavirus-associated kidney outcomes in COVID-19, SARS, and MERS: a meta-analysis and systematic review. Renal Fail. (2021) 43:1–15. doi: 10.1080/0886022X.2020.1847724
50. Faour WH, Choaib A, Issa E, Choueiry FE, Shbaklo K, Alhajj M, et al. Mechanisms of COVID-19-induced kidney injury and current pharmacotherapies. Inflamm Res. (2021) 71:39–56. doi: 10.1007/s00011-021-01520-8
51. Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. (2020) 370:856–60. doi: 10.1126/science.abd2985
52. Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, Welling PA, et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. (2020) 31:1380–3. doi: 10.1681/ASN.2020040419
53. Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, et al. Covid−19 and kidney injury: pathophysiology and molecular mechanisms. Rev Med Virol. (2021) 31:e2176. doi: 10.1002/rmv.2176
54. Ackley TW, McManus D, Topal JE, Cicaliand B, Shah S. A valid warning or clinical Lore: an evaluation of safety outcomes of Remdesivir in patients with impaired renal function from a multicenter matched cohort. Antimicrob Agents Chemother. (2021) 65:e2290–320. doi: 10.1128/AAC.02290-20
55. Yin Y, Wunderink RG. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology. (2018) 23:130–7. doi: 10.1111/resp.13196
56. Abdelghany TM, Ganash M, Bakri MM, Qanash H, Al-Rajhiand AMH, Elhussieny NI. SARS-CoV-2, the other face to SARS-CoV and MERS-CoV: future predictions. Biomed J. (2021) 44:86–93. doi: 10.1016/j.bj.2020.10.008
57. Zhao M, Wang M, Zhang J, Gu J, Zhang P, Xu Y, et al. Comparison of clinical characteristics and outcomes of patients with coronavirus disease 2019 at different ages. Aging. (2020) 12:10070. doi: 10.18632/aging.103298
58. Min YD, Quan MT, Chen N, Chen Y, Xiaoand X, Li WW. Meta-analysis of risk factors for acute renal injury in patients with septic shock. Hebei Med. (2020) 26:463–8.
59. He W, Liu X, Hu B, Li D, Chen L, Li Y, et al. Gender and ethnic disparities of acute kidney injury in COVID-19 infected patients: a literature review. Front Cell Infect Microbiol. (2022) 11:778636. doi: 10.3389/fcimb.2021.778636
60. O’Brien J, Duand KY, Peng C. Incidence, clinical features, and outcomes of COVID-19 in Canada: impact of sex and age. J Ovar Res. (2020) 13:1–12. doi: 10.1186/s13048-020-00734-4
61. Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. (2021) 32:161–76.
Keywords: COVID-19, SARS-CoV-2, coronavirus, acute kidney injury, complications, mortality, risk factors
Citation: Mallhi TH, Khan YH, Alzarea AI, Khan FU, Alotaibi NH, Alanazi AS, Butt MH, Alatawi AD, Salman M, Alzarea SI, Almalki ZS, Alghazi MA and Algarni MA (2022) Incidence, risk factors and outcomes of acute kidney injury among COVID-19 patients: A systematic review of systematic reviews. Front. Med. 9:973030. doi: 10.3389/fmed.2022.973030
Received: 19 June 2022; Accepted: 13 September 2022;
Published: 04 November 2022.
Edited by:
Nianqiao Gong, Huazhong University of Science and Technology, ChinaCopyright © 2022 Mallhi, Khan, Alzarea, Khan, Alotaibi, Alanazi, Butt, Alatawi, Salman, Alzarea, Almalki, Alghazi and Algarni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tauqeer Hussain Mallhi, dGhodXNzYWluQGp1LmVkdS5zYQ==, dGF1cWVlci5odXNzYWluLm1hbGxoaUBob3RtYWlsLmNvbQ==; Yusra Habib Khan, eWhraGFuQGp1LmVkdS5zYQ==, eXVzcmFoYWJpYkB5bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.