- 1Faculty of Medicine, University of Belgrade, Belgrade, Serbia
- 2Clinic for Pulmonology, University Clinical Center of Serbia, Belgrade, Serbia
- 3COVID Hospital “Batajnica”, University Clinical Center of Serbia, Belgrade, Serbia
- 4Institute of Public Health of Serbia “Dr. Milan Jovanovic-Batut”, Belgrade, Serbia
- 5Faculty for Health and Business Studies, Singidunum University, Valjevo, Serbia
- 6Special Hospital for Pulmonary Diseases “Ozren”, Sokobanja, Serbia
- 7Clinic for Infective and Tropical Diseases, University Clinical Center of Serbia, Belgrade, Serbia
- 8Center for Anesthesiology, University Clinical Center of Serbia, Belgrade, Serbia
Introduction: COVID-19 and tuberculosis (TB) represent global threats to the public health system. The impact of COVID-19 on TB results in a reduction in the number of notified TB cases, delayed diagnosis and treatment, and increased case fatality and mortality rates. The aim of the study was to analyze the TB/COVID-19 co-infected cohort in Serbia as a low-burden country and compare it to the global TB/COVID-19 cohort.
Methods: A retrospective analysis was done on 53 TB and COVID-19 co-infected patients treated in COVID hospital “Batajnica” in Belgrade and Special Hospital for Pulmonary Diseases “Ozren” Sokobanja in the period from 6 March 2020 to 1 April 2022. A comparative analysis with the global cohort published recently was also performed.
Results: TB/COVID-19 cohort in Serbia included significantly fewer migrants and diabetes cases, but more cases with chronic respiratory diseases compared to the global. Descriptive analysis of TB cases in the Serbian TB/COVID-19 cohort showed fewer cases diagnosed with sputum smear and Gene Xpert/HAIN, fewer EPTB and mono-resistant cases, and more cases diagnosed with solid culture, unilateral pulmonary infiltrate (with bilateral cavity lesions), and bilateral pulmonary infiltrate (no cavities) compared to TB/COVID-19 cases worldwide. Nasal congestion and fever were more common COVID-19 symptoms in the global cohort. Radiology was more commonly used for the diagnosis of COVID-19 in Serbia. Typical bilateral ground opacities were less common among Serbian patients. Serbian patients spent fewer days in the hospital and achieved a higher PCR conversion rate and TB treatment success rate.
Conclusion: The Serbian TB/COVID-19 cohort achieved a higher treatment success rate compared to the global cohort. Encouraging vaccination against SARS-CoV-2 for people with a current or past TB disease, as well as rapid diagnosis and targeted treatment of TB in highly specialized pulmonology institutions, presents key points to avoid excessive morbidity and mortality.
Introduction
Tuberculosis (TB) presents a global public health threat with 10 million cases and 1.3 million deaths annually (1). Due to its rapid spread, clinical severity, and high mortality rate, with more than 6.4 million deaths in the past 2.5 years, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic represents the most contagious infectious disease worldwide (2). This epidemic had a great impact on all health systems in the world and the management of other diseases. The impact of COVID-19 on TB management is significant, especially in countries where the healthcare workforce has been reassigned to the COVID-19 emergency. This led to fewer TB diagnoses or diagnostic delays, and more TB deaths. However, not enough data on what actually happened are yet available. Diagnostic delays with more severe clinical presentations, worst outcomes, and missed follow-ups are only a few of the consequences and indirect effects of TB services disruption (3). A decrease in TB case notifications between 2019 and 2020 from 7.1 to 5.8 million cases was reported by the World Health Organization (WHO) (4). Results from the first pilot study of the Global Tuberculosis Network (GTN) on 49 TB/COVID-19 co-infected patients to the latest one of 767 patients from 34 countries showed that signs and symptoms are nearly the same (5, 6). Tuberculosis could be concomitantly diagnosed with COVID-19 and often presented with an increased case fatality rate. An estimated increase of ~20% in TB deaths is expected in the next 5 years (7). Due to the successful implementation of the WHO Directly Observed Treatment Short Course (DOTS) and STOP TB Strategy, the Republic of Serbia decreased its TB incidence rate from 37/100,000 to 9/100,000 in the past 17 years and now belongs to the low-burden TB country (8). The aim of the study was to analyze the TB/COVID-19 co-infected cohort in Serbia as a low-burden country and compare it to the published data on the global TB/COVID-19 cohort.
Materials and methods
A retrospective analysis of 53 patients with active TB co-infected with COVID-19 and treated in COVID hospital “Batajnica” in Belgrade and Special Hospital for Pulmonary Diseases “Ozren” Sokobanja from 6 March 2020 to 1 March 2022 was performed. During the study period, we enrolled all patients of any age who were diagnosed and notified in these hospitals with active TB and COVID-19 simultaneously.
The data were obtained from medical records and include demographic data, laboratory, radiological, and clinical status at TB diagnosis and COVID-19 diagnosis, treatment, and follow-up. Follow-ups were done every month for each patient during the treatment period. After the treatment period, follow-ups were performed every 3 and 6 months, and some patients are still being followed up. On average, six to seven follow-ups were made per patient.
All data were compared to global TB and COVID-19 cohort publicly available data. Serbia has given its contribution to the global cohort with the first six patients (6).
Statistical analysis includes descriptive statistical analysis of all patients with details of TB and COVID-19 in our cohort. Variables were summarized using frequencies and percentages. Continuous variables with normal distribution were compared using t-tests, and categorical variables were compared using chi-squared or Fisher exact test.
Result
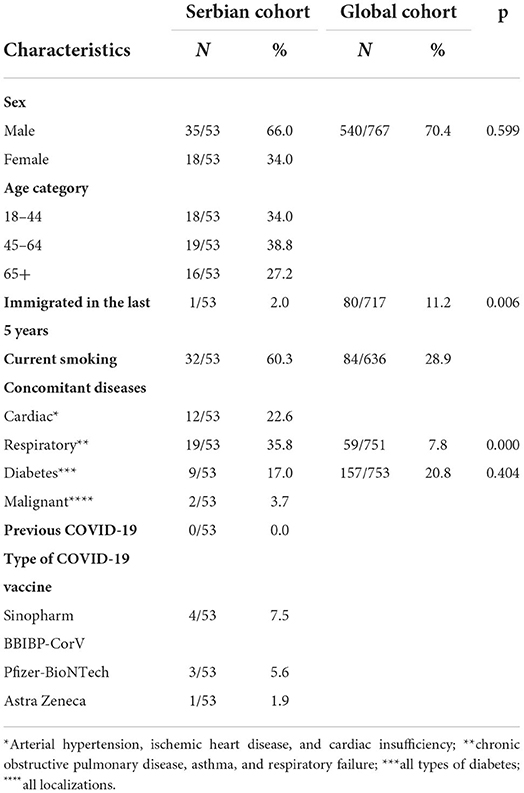
The demographical, epidemiological, and clinical characteristics of the 53 patients with TB/COVID-19 are shown in Table 1. Most patients were men (66%, 35/54) with a median age of 45–64 years in 19 (38.8%) patients. Only 1 (2.0%) had a history of migration in the last 5 years. Human immunodeficiency virus (HIV) co-infected patients were not identified. Cardiovascular diseases, mostly arterial hypertension and chronic obstructive pulmonary disease (COPD), were the most commonly observed co-morbidities as summarized in Table 1. The TB/COVID-19 cohort in Serbia is composed of significantly fewer migrants and diabetes cases but more cases with chronic respiratory diseases compared to the global. According to vaccine status, only 8/53 (15.3%) of the study participants were fully vaccinated, including booster doses, with Sinopharm BBIP-CorV in 4 (7.5%), Pfizer-BioNTech in 3 (5.6%), and Astra Zeneca in 1 (1.9%) case.
Among study participants, 9 patients (17.0%) had previously been treated for TB, while 19 (35.9%) had both diseases diagnosed at the same time and ~9 (16.9%) in the same week. As shown in Table 2, most of the patients had newly diagnosed TB (44/53, 83.0%). It was bacteriologically confirmed in 43/53 patients (81.1%), which was higher compared to the global cohort. Pulmonary localization of the disease was reported as well in more cases (52/53, 98%) in Serbia compared to global (612/732, 83.6). The majority of the patients (51/53, 96.2%) had pan-susceptible TB in Serbia. Mono-resistance to isoniazid was noticed in 2/53 (3.8%) of patients. Cavitary lesions were presented in statistically significantly more cases in Serbia compared to the global data, with 28/53 (52.8%) compared to 125/633 (19.7%), respectively.
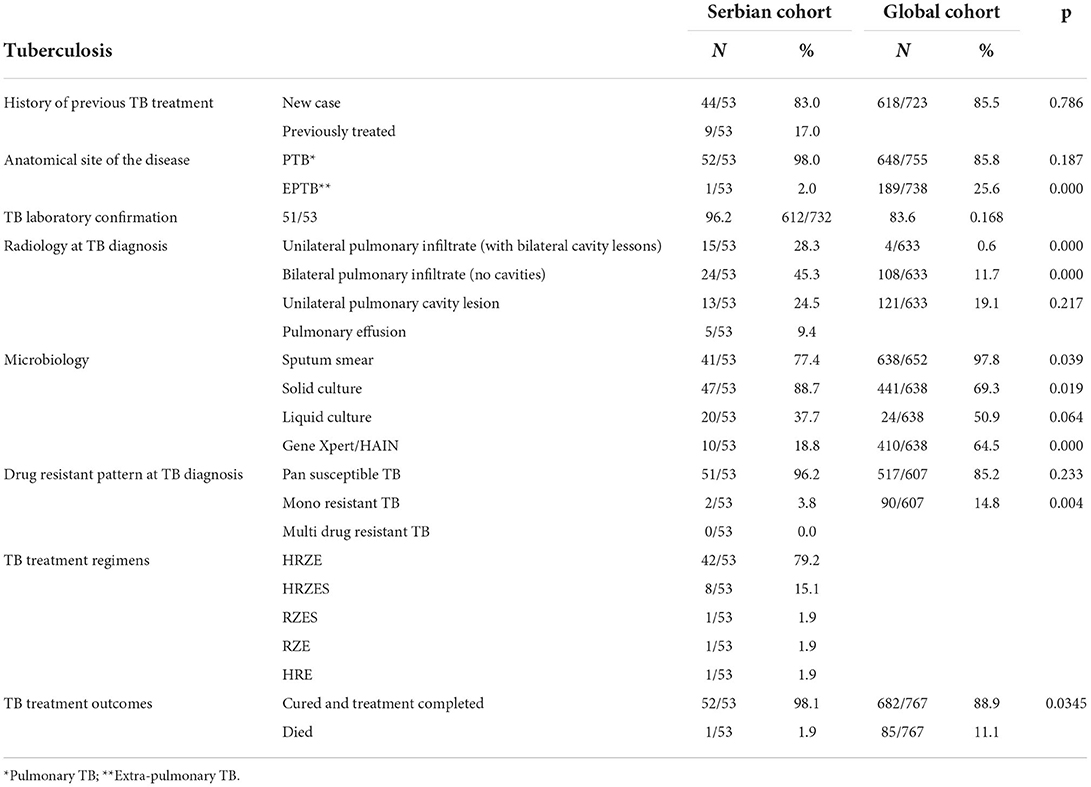
Table 2. Descriptive analysis of TB in the Serbian TB/COVID-19 cohort compared to the global cohort.
The SARS-CoV-2 laboratory confirmation was available in all Serbian patients in a higher percentage compared to global as shown in Table 3. Most of our COVID-19 patients reported signs and symptoms of dry cough (37/53, 69.8%). Fever and shortness of breath were presented in one-third of the study participants (16/53, 30.2%) and (16/53, 30.2%), respectively. A statistically significant number of patients in our study underwent computerized tomography (CT) compared to the global cohort. Among the 18 (32.1%) CT-scanned patients, typical or atypical “ground glass” opacities were found (Table 4). In our study, bilateral ground glass opacities were found in a statistically significantly less number of patients (15.1%) compared to the global study (47.4%). Interestingly, 45% (24/53) of patients had TB diagnosed before COVID-19 (including nine patients with the previous TB) and 18.8% (10/53) of patients had COVID-19 diagnosed first.
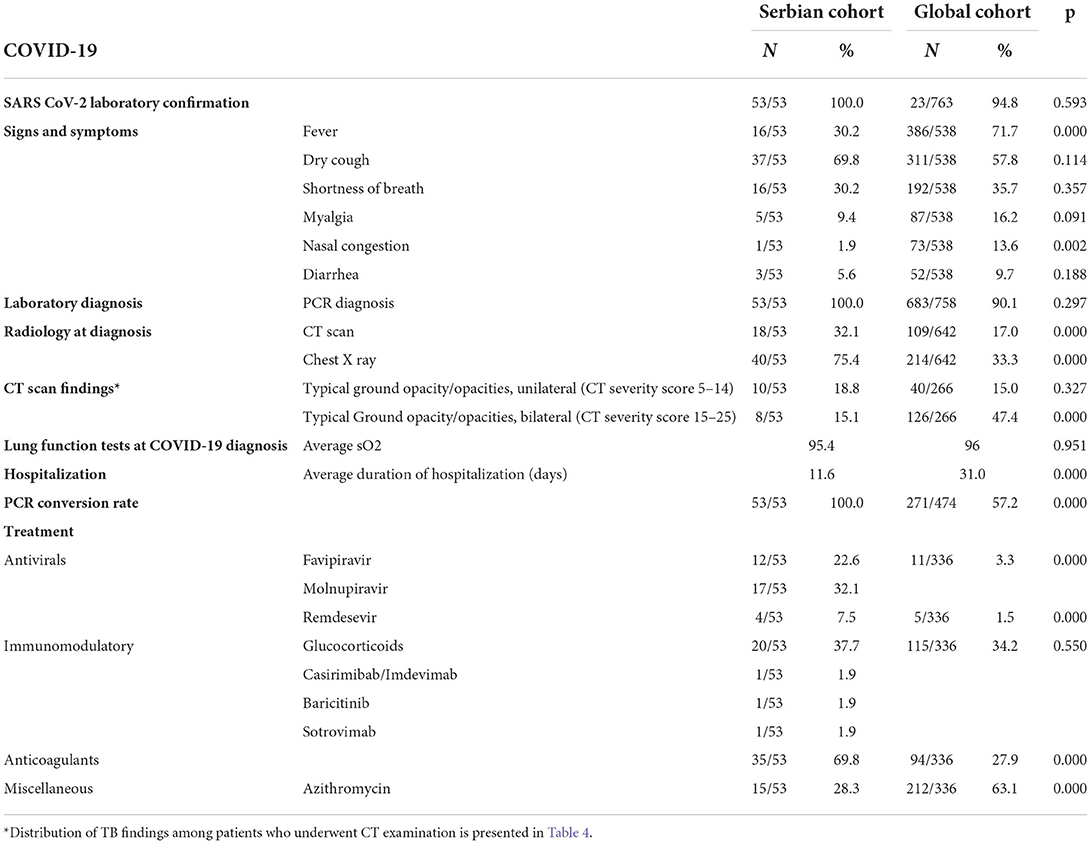
Table 3. Descriptive analysis of COVID-19 in the Serbian TB/COVID-19 cohort compared to the global cohort.
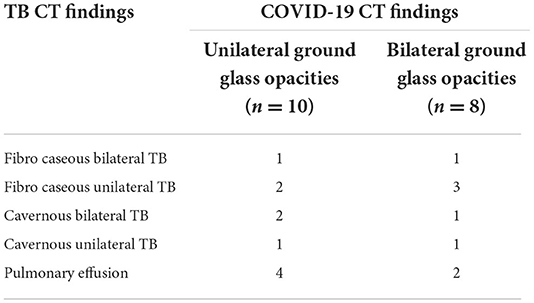
Table 4. Distribution of TB findings among patients who underwent CT examination in the Serbian TB/COVID-19 cohort (n = 18).
All the patients with COVID-19 and TB in Serbia were hospitalized during anti-COVID treatment. The average duration of hospitalization among our patients was statistically significantly lower (11.6 days) compared to the global cohort (31.0). Pulse oximetry was performed in all patients, with an average oxygen saturation of 95.4%. Oxygen support via high-flow nasal cannula and later noninvasive ventilation was applied in one patient (1/53, 1.9%). Antiviral drugs including favipiravir, molnupiravir, and remdesivir were used in 33/53 (62.2%) patients. Favipiravir (22.6% compared to 3.3% globally) and remdesivir (7.5% compared to 1.5% globally) were used in our study in statistically significantly more cases than among the patients from the global cohort. Immunomodulatory treatment was applied in 23/53 (43.4%) patients; anticoagulation therapy in statistically significantly more cases in our study (35/53, 69.8%) than in 94/336 (27.9%) globally; and azithromycin in statistically significantly fewer cases (15/53, 28.3%) in our study compared to global (212/336, 63.1%).
The Serbian cohort achieved a statistically significantly better PCR conversion rate (100%) compared to the global cohort (57.2%), and TB treatment outcomes compared to the global cohort, namely, a treatment success rate of 98.1% compared to 88.9%.
Discussion
Our study described and compared the findings of TB/COVID-19 co-infected patients in the Serbian cohort with a global cohort of 767 patients from 172 centers in 34 countries.
We found similarities in the results of our study with the results of the global cohort in terms of the male sex that can be attributable to the global predomination of the male sex among patients with TB (1). In contrast, we found low TB/COVID-19 co-infection among migrants compared to a higher number in the global cohort as well as data shown in other EU countries (9). Patients with COVID-19/TB had a much higher rate of comorbidities than patients with COVID-19 (10). Comorbidities may present additional risk factors for COVID-19 and tuberculosis. To date, evidence suggests that COVID-19 patients with preexisting comorbidities such as diabetes; hypertension; and cardiovascular, immunological, and respiratory diseases are at a greater risk for death (11). In our study, 42/53 (79.2%) patients had comorbidities. Compared to the global TB/COVID-19 cohort, COPD was more frequently found among respiratory concomitant diseases in our study because a large number of active smokers was found in our study group. Therefore, due to the high prevalence of both infectious diseases and due to worse prognosis of co-infection, further investigations on COVID-TB cases should be conducted (12). Some immunological findings suggest that TB disease may be transiently immunosuppressive. The combined effect of TB and SARS-CoV-2 infection likely causes a pronounced lymphocytopenia and, consequently, a CD4 + cell decrease as a reliable indicator of the severity of COVID-19. Different mechanisms of lymphopenia have been speculated, including lymphocyte death due to direct infection through receptor ACE 2, direct damage of SARS-CoV-2 to lymphatic organs, and lymphocytes deficiency induced by pro-inflammatory cytokines, such as tumor necrosis factor (TNF) α and interleukin (IL)-6 (13). In addition, COPD may be a risk factor for disease progression (14). Lung parenchyma damage due to pulmonary remodeling because of persistent cavitation, fibrosis, or bronchiectasis is present in approximately 50% of cured TB patients and might be associated with increased susceptibility to COVID-19 and a higher mortality rate (15). Both dual lung damages after TB and COVID-19 necessitate the follow-up of patients with post-tuberculosis lung disease who had COVID-19 pneumonia (16). Extrapulmonary tuberculosis (EPTB) was less frequently found in the Serbian TB/COVID-19 cohort compared to the global TB/COVID-19 cohort, suggesting a delayed diagnosis of TB because of lockdown and severely restricted access to tuberculosis diagnosis and treatment. In our case, we found hematogenous forms of tuberculosis (osteoarticular, genitourinary, and gastrointestinal), which have not been seen for a long time as reported in some other countries (3). Although not in high coverage, we found some patients vaccinated in contrast to the global cohort. The Republic of Serbia was among the first countries to start applying COVID-19 vaccines. At the moment of data collection, the following four COVID-19 vaccines were available in the country: BNT162b2 mRNA (Pfizer-BioNTech), Sinopharm BBIBP-CorV (Vero Cell®), Gam-COVID-Vac (Sputnik V), and ChAdOk1 nCoV-19 (AstraZeneca) (17).
We found differences in microbiological confirmation of the TB cases among our patients and the global cohort. Although Gene Xpert/HAIN is widely used in other countries, according to national and international guidelines, culture is used as the gold standard for TB diagnosis in low-TB burden countries (8). For those reasons, sputum smear was significantly more positive in the global vs. our cohort, solid culture was highly positive in ours compared to the global cohort, and Gene Xpert/HAIN was much more used in the global cohort. Drug susceptible pulmonary TB was found as the most common pattern of TB disease among our patients in line with the general population TB data (8).
The Serbian TB cohort reached a high treatment success rate due to prompt diagnosis and therapy, as well as a low frequency of mono-resistant and an absence of multidrug-resistant TB. A positive trend in TB declining in the past decade can be reversed by COVID-19, resulting in the reduction of tuberculosis testing and access to tuberculosis-related health services. Reports show that TB deaths would return to those seen in 2013 and estimated TB mortality could increase up to 20% between 2020 and 20251 (7). Data from WHO and Stop TB Partnership predicted additional TB deaths between 2020 and 2025 (18). The impact of a pandemic on tuberculosis deaths during 2021 and 2022 is still unclear, probably worse because of fatal delta-driven surges and the current wave with an omicron variant. The COVID-19 pandemic affected tuberculosis preventive strategies, including a reduction of BCG vaccination by up to 60% in some parts of the world, treatment failure, and loss of follow-up (19).
The most common symptoms reported in both cohorts were dry cough, fever, and shortness of breath. Results correspond to global COVID-19 data1, while typical symptoms of COVID-19 like olfactory and taste disorders were not presented in our cohort. The mode of transmission of SARS-CoV2 and TB is the same, by small aerosol particles, but in COVID-19 also by large aerosol particles (droplets) (20). The initial signs and symptoms of COVID-19 and TB are like other respiratory infections, including influenza, and the main transmission route is through respiratory droplets, and the main targets are the lungs (5). Due to reported similar symptoms, for better screening, rapid molecular testing for TB and COVID-19 is suggested whenever it is possible (21).
Similar to symptoms, radiological findings overlapped in both COVID-19 and TB. The radiological findings of active tuberculosis and COVID-19 are often similar. Isolated upper-lobe pulmonary infiltrates are suggestive of TB, and lower infiltrates of other bacterial infections (22). Data showed that in almost half of patients co-infected with TB and COVID-19, CT findings were concluded only on COVID-19 (23, 24). COVID-19 radiological signs include typical or atypical ground-glass opacities. Existing evidence indicates that the features of lung imaging among COVID-19 patients include bilateral involvement, peripheral distribution, mixed ground glass opacities, and consolidations, while in COVID-19/TB pleural effusion, nodules and fibrosis could be present (25). We found the same distribution of unilateral while likely less frequent bilateral ground glass opacities since extensive bilateral CT findings including infiltrates and cavities accompanied by dyspnea are usually predictors of disease severity (9).
Due to the above-mentioned radiological overlapping in some of the patients, among some of the patients who had COVID-19 diagnosed before TB, we found cavities highly suspicious of TB, which were bacteriologically confirmed later. These data suggest that COVID-19 does not play a major role in advancing forms of TB (26). A potential connection between COVID-19 and active tuberculosis could be found in increased disease susceptibility and severity. Reduced frequency of M. tuberculosis-specific CD4+ T cells in peripheral blood was found in patients with COVID-19, suggesting increased susceptibility to progression to active tuberculosis (27). Diffuse lung parenchyma damage in patients with COVID-19/TB should be recognized in terms of invasive fungal infections, which are increasing problems in the long post-COVID period, especially in diabetic patients and patients on prolonged steroid use (28).
According to the COVID-19 treatment guidelines, treatment options include antiviral therapy, immunomodulatory therapy, and adjunctive therapy (29). Management of COVID-19 was similar in patients with both active tuberculosis and COVID-19 and COVID-19 alone. Antivirals including favipiravir, molnupiravir, and remdesivir were more frequently used in the Serbian TB/COVID-19 cohort compared to the global TB/COVID-19 cohort. Antiviral therapies may have a greater role in the early course of COVID-19 and influence good treatment outcomes of our patients, whereas immunomodulatory therapies are better in later stages (30). The need for steroids and oxygen support was confirmed due to oxygen saturation, partial pressure of oxygen, and clinical presentation. Glucocorticoids were used nearly the same in both cohorts. Multiple randomized trials suggest that systemic corticosteroid therapy improves clinical outcomes and reduces mortality in hospitalized patients with COVID-19 who require oxygen supplementation (31). According to the results of the same studies, larger samples are mandatory to explore the impact of corticosteroid therapy in patients with COVID-19/TB (32). If remdesivir is indicated for the treatment of COVID-19 in patients with active tuberculosis, clinicians should keep in mind that rifampicin could reduce the concentration of remdesivir (32).
The impact of COVID-19 and TB on long-term pulmonary sequelae and lung function impairment and the need for pulmonary rehabilitation should be determined in the next period (33).
The strength of our study is that it enrolled all diagnosed patients with TB/COVID-19 in our country. In addition, most of the variables are not collected in the routine COVID-19 and TB surveillance systems, and therefore the study gives a better insight into the characteristics of the patients with both diseases. The study limitation is that we were unable to perform adverse event analysis of therapies prescribed. Furthermore, it was not feasible to compare patients with TB/COVID-19 with the ones with a single disease alone.
Conclusion
The Serbian TB/COVID-19 cohort achieved a higher treatment success rate than the global. A possible explanation could be the fact that all the patients with COVID-19/TB were first hospitalized in COVID hospitals and then transferred to a special hospital for TB because of monitoring and targeted therapy. Clinicians should be aware of both diseases because of overlapping signs and symptoms. The radiological presence of ground-glass opacities and rapid molecular testing is mandatory for the diagnosis of COVID-19. Comorbidities (particularly cardiovascular and respiratory) and male gender have an important influence on the morbidity of COVID-19/TB co-infected persons. Encouraging vaccination against SARS-CoV-2 for people with a current or past TB disease, as well as rapid diagnosis and targeted treatment of TB in highly specialized pulmonology institutions, presents key points to avoid excessive morbidity and mortality.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Special Hospital for Pulmonary Diseases Ozren Sokobanja. The participants provided their written informed consent to participate in the study.
Author contributions
TA-V and MS: concept and design and interpretation of the data. MS: statistical analysis. TA-V: drafting the manuscript. TA-V, MS, GA, MJ, AR-S, and JV: critical revision of the manuscript for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. World Health Organization. Global Tuberculosis Report 2021. Geneva: World Health Organization (2021).
2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
3. Di Gennaro F, Gualano G, Timelli L, Vittozzi P, Di Bari V, Libertone R, et al. Increase in tuberculosis diagnostic delay during first wave of the COVID-19 pandemic: data from an italian infectious disease referral hospital. Antibiotics. (2021) 10:272. doi: 10.3390/antibiotics10030272
4. Impact of the COVID-19 Pandemic on TB Detection and Mortality in 2020. Geneva: World Health Organization (2021).
5. Tadolini M, Codecasa LR, Garcia-Garcia JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. (2020) 56:2001398. doi: 10.1183/13993003.01398-2020
6. TB/COVID-19 Global Study Group. Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. (2022) 59:2102538. doi: 10.1183/13993003.02538-2021
7. Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. (2020) 8:e132–41. doi: 10.1016/S2214-109X(20)30288-6
8. Institute of Public Health of Serbia. Report on Communicable Diseases for 2019. Belgrade: Institute of Public Health of Serbia (2021).
9. Motta I, Centis R, D'Ambrosio L, García-García JM, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants: preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. (2020) 26:233–40. doi: 10.1016/j.pulmoe.2020.05.002
10. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. (2020) 55:2000547. doi: 10.1183/13993003.00547-2020
11. Callender L, Curran M, Bates S, Mairesse M, Weigandt J, Betts CJ, et al. The impact of pre-existing comorbidities and theraoeutic intervention on COVID-19. Front Immunol. (2020) 11:1991. doi: 10.3389/fimmu.2020.01991
12. Song W-M, Zhao J-Y, Zhang Q-Y, Liu S-Q, Zhu X-H, Q-Q, et al. COVID 19 and tuberculosis coinfection: an overview and case reports/Case series and meta-analysis. Front in Med. (2021) 8:657006. doi: 10.3389/fmed.2021.657006
13. Musso M, Di Gennaro F, Gualano G, Mosti S, Cerva C, Fard SN, et al. Concurrent cavitary pulmonary tuberculosis and COVID-19 pneumonia with in vitro immune cell anergy. Infection. (2021) 49:1061–4. doi: 10.1007/s15010-021-01576-y
14. Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, et al. Risk factors of critical and mortal COVID-19 cases: a systemic literature review and meta analysis. J Infect. (2020) 81:e16–25. doi: 10.1016/j.jinf.2020.04.021
15. Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases. Risk factors for coronavirus disease 2019 (COVID-19) death in population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. (2021) 73:e2005–15. doi: 10.1093/cid/ciaa1198
16. Harries AD Ade S, Burney P, Hoa NB, Schluger NW, Castro JL, et al. Successfully treated but not fit for purpose: paying attention to chronic lung impairment after TB treatment. Int J Tuberc Lung dis. (2016) 20:1010–14. doi: 10.5588/ijtld.16.0277
17. Stosic M, Milic M, Markovic M, Kelic I, Bukumiric Z, Veljkovic M, et al. Immunogenicity and reactogenicity of the booster dose of COVID-19 vaccines and related factors: a panel study from the general population in Serbia. Vaccines. (2022) 10:838. doi: 10.3390/vaccines10060838
18. Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. (2020) 8:914–24. doi: 10.1016/S2213-2600(20)30323-4
19. Shaikh N, Pelzer PT, Thysen SM, Roy P, Harris RC, White RG, et al. Impact of COVID-19 disruptions on global BCG coverage and pediatric TB mortality: a modelling study. Vaccines. (2021) 9:1228. doi: 10.3390/vaccines9111228
20. Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. AJR Am J Roentgenol. (2020) 214:1072–7. doi: 10.2214/AJR.20.22976
21. Guan W-j, Ni Z-y, Hu Y, Liang w-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
22. Zaini J, Fadhillah M, Reisa T, Isbaniyah F, Handayani RRD. Tuberculosis and COVID-19 coinfection: a report of two cases at the tertiary refferal in Indonesia. J Infect Dev Ctries. (2022) 16:478–83. doi: 10.3855/jidc.15481
23. Riou C, du Bruyn E, Stek C, Daroowala R, Goliath RT, Abrahams F, et al. Relationship of SARS-CoV 2 specific CD4 response to COVID-19 severity and impact of HIV-1 and tuberculosis coinfection. J Clin Invest. (2021) 131:e149125. doi: 10.1172/JCI149125
24. Koehler P, Bassetti M, Chakrabarti A, Chen SCA, Colombo AL, Hoenigl M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. (2021) 21:e149–62. doi: 10.1016/S1473-3099(20)30847-1
25. Dheda K, Jaumdally S, Davids M, Chang JW, Gina P, Pooran A, et al. Diagnosis of COVID-19: Considerations, controversies and challenges. Afr J Thorac Crit Care Med. (2020) 26:36. doi: 10.7196/AJTCCM.2020.v26i2.099
26. Dheda K, Perumal T, Moultrie H, Perumal R, Esmail A, Scott AJ, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. (2022) 10:603–22. doi: 10.1016/S2213-2600(22)00092-3
27. Protocol for management of COVID-19 patients version 3. Government of the Republic of Serbia (2020). Available online at: https://www.vladars.net/sr-SP-Cyrl/Vlada/Ministarstva/MZSZ/Documents/Kratki%20protokol%20za%20COVID-19.pdf
28. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19-final report. N Engl J Med. (2020) 383:1813–26. doi: 10.1056/NEJMoa2007764
29. Li H, Yan B, Gao R, Ren J, Yang J. Effectiveness of corticosteroids to treat severe COVID-19: a systematic review and meta-analysis of prospective studies. Int Immunopharmacol. (2021) 100:108121. doi: 10.1016/j.intimp.2021.108121
30. Gopalswamy R, Subbian S. Corticosteroids for COVID-19 therapy. Potentional implication on tuberculosis. Int J Mol Sci. (2021) 22:3773. doi: 10.3390/ijms22073773
31. Yang K. What do we know about remdesivir drug interactions? Clin Transl Sci. (2020) 13:842–44. doi: 10.1111/cts.12815
32. Cilloni L, Fu H, Vesga JF, Dowdy D, Pretorius C, Ahmedov S, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. Clin Medicine. (2020) 28:100603. doi: 10.1016/j.eclinm.2020.100603
Keywords: tuberculosis, COVID-19, co-infection, Serbia, pandemic
Citation: Adzic-Vukicevic T, Stosic M, Antonijevic G, Jevtic M, Radovanovic-Spurnic A and Velickovic J (2022) Tuberculosis and COVID-19 co-infection in Serbia: Pandemic challenge in a low-burden country. Front. Med. 9:971008. doi: 10.3389/fmed.2022.971008
Received: 16 June 2022; Accepted: 17 October 2022;
Published: 16 November 2022.
Edited by:
Alfonso J. Rodriguez-Morales, Fundacion Universitaria Autónoma de las Américas, ColombiaReviewed by:
Ludovica Capitelli, University of Naples Federico II, ItalyAmer Hayat Khan, Universiti Sains Malaysia (USM), Malaysia
Copyright © 2022 Adzic-Vukicevic, Stosic, Antonijevic, Jevtic, Radovanovic-Spurnic and Velickovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maja Stosic, bWFqYV9zdG9zaWNAYmF0dXQub3JnLnJz
 Tatjana Adzic-Vukicevic1,2,3
Tatjana Adzic-Vukicevic1,2,3 Maja Stosic
Maja Stosic Jelena Velickovic
Jelena Velickovic