- Department of Anesthesiology, The First Hospital of Jilin University, Changchun, Jilin, China
Objectives: The prognostic impact of marital status on malignant pleural mesothelioma (MPM) is not investigated. This paper probes into the relationship between the prognosis of MPM and marital status.
Materials and methods: The Surveillance, Epidemiology, and End Results (SEER) database of American had been applied to choose eligible patients over the 2004–2015 periods. Moreover, cancer-specific survival (CSS) and overall survival (OS) of unmarried and married groups were compared.
Results: A total of 3,997 patients in total had been identified, including 2,735 (68.43%) married patients. In comparison to unmarried patients, married ones tended to be younger, male, white, and received active treatment (surgery, chemotherapy, or radiotherapy). In addition, the 1, 3, and 5-year CSS rates were 44.40, 12.09, and 6.88% in married patients, while 35.75, 12.12, and 6.37% in unmarried group (p = 0.0014). At the same time, the 1, 3, and 5-year OS rates were 41.84, 10.56, and 5.91% in married patients, while 33.67, 10.44, and 4.93%, respectively, in the unmarried group (p < 0.0001). As revealed by the multivariate analysis results, the marital status was an independent favorable prognostic factor, in which the married groups showed better CSS [hazard ratio (HR): 0.870; 95% confidence interval (CI): 0.808–0.938; p < 0.001] as well as OS (HR: 0.871; 95% CI: 0.810–0.936; p < 0.001). According to the results of subgroup analysis, the CSS and OS survival of married groups were better than the unmarried groups in almost all the subgroups.
Conclusion: Marital status is an independent favorable prognostic indicator of MPM. Poor prognosis in unmarried patients is likely to be related to insufficient treatments and socioeconomic and psychosocial factors.
Introduction
As an aggressive cancer, malignant pleural mesothelioma (MPM) is related to the previous exposure of asbestos, which has a long latency (1–5). Over the past few years, the MPM’s worldwide incidence has increased in a steady way, causing a global burden (1, 2). However, knowledge of MPM is currently limited, and the clinicopathological characteristics and outcome for this entity are not very clear (6).
Malignant pleural mesothelioma’s prognosis is poor, and its median survival is 8–14 months since diagnosis (1–3, 7). In fact, the prognostic factors of MPM have been reported by a lot of studies, and they primarily pay attention to the clinicopathological features, such as American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) stages, treatment, age, and gender (1, 2, 7). At present, the function of social determinant in the disease development is more stressed (8). As claimed by some researchers, the marital status refers to a prognostic factor in several cancers such as pancreatic cancer, breast cancer, lung cancer, melanoma, and prostate cancer (9–13). However, the effect of marital status on the MPM survival has not been studied previously.
The Surveillance, Epidemiology, and End Results (SEER) program includes 18 different cancer registries’ research data, and covers 30% of American population (14). What’s more, the data of SEER have been extensively applied to probe into the connection between survival outcome and marital status in cancer patients (8, 12, 15). This study will use the SEER database to explore the association of marital status with the MPM survival.
Materials and methods
Ethics statement
The SEER Research Data Agreement had been signed to acquire SEER information using the reference number of 19828-Nov 2018. Furthermore, we abided by guidelines and got data with the research approaches. In addition, the Human Research Protection Office considered that the data analysis was focused on non-human subjects and they were available. Hence, the approval from the institutional review board was not required.
Study population
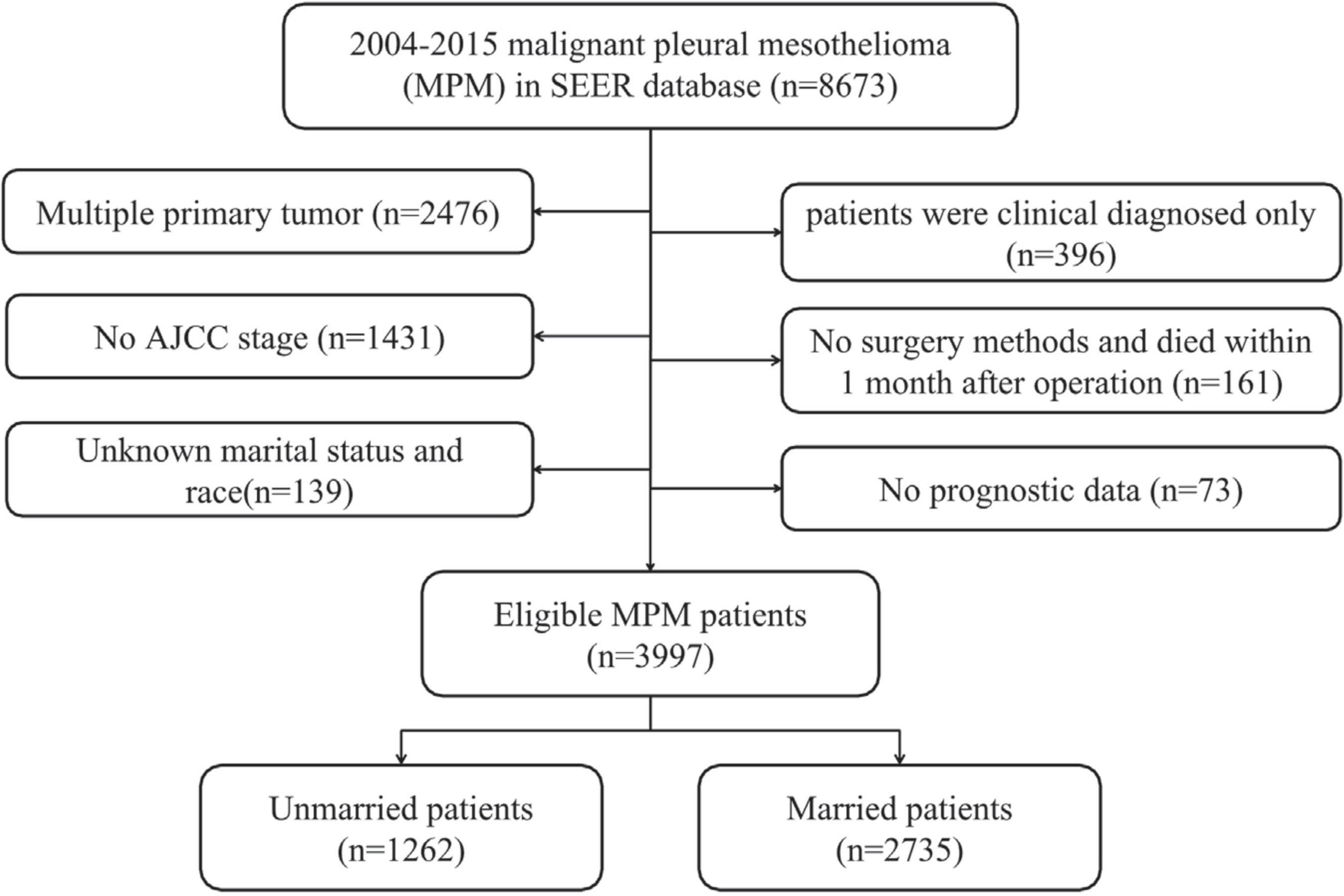
The tool of SEER*State v8.3.6 was adopted to choose the qualified subjects, including 18 SEER areas over the 1998–2015 periods (2018 submission). The standards for inclusion are as follows: (1) it should be primary MPM patients; and (2) MPM confirmed by pathology and diagnosed in line with the International Classification of Disease for Oncology, Third Edition (ICD-O-3; coded as 9050–9053) (16). The standards for exclusion are as follows: (1) patients had multiple primary tumors; (2) the diagnosis source of patients was from death certificate or autopsy, or they were just diagnosed in a clinical manner; (3) patients without survival and prognosis data; (4) patients had no AJCC stage; (5) patients had information of unknown race and marital status; and (6) patients had no surgery information or died within 1 month after surgery. Then, the remaining subjects were recruited as the initial groups of SEER.
Covariates and endpoint
The patients’ features had been examined through the factors, such as age, gender, histology, marriage status, race, grade, size of tumor, stage of AJCC, surgery, chemotherapy, and radiotherapy. We classified patients as married or unmarried (such as never married single, divorced, separated, and widowed) (9, 17). The grouping of the age referred to the published studies (<50, 50–69, and ≥70) (18). In terms of the race, patients were classified as black, white, and others (12). In terms of the histology, they were classified into biphasic, epithelioid, fibrous, and sarcomatoid type. As for the staging of cancer, all the qualified cases were reorganized based on the eighth AJCC TNM staging system (19).
The endpoint of this study was cancer-specific survival (CSS) and overall survival (OS). CSS was defined as the period from diagnosis to death attributed to MPM. OS was defined as the period from diagnosis to death from any cause. According to the 2018 Submission Database of SEER, the cut-off date was decided in advance, in which the data of death were included. Hence, the cut-off date was determined on November 31, 2018.
Statistical analyses
Kaplan–Meier (K–M) approach was used for univariate analysis. Meanwhile, the log-rank test was performed to measure the difference between CSS and OS. Beyond that, the variables with p-value lower than 0.1 were assessed in the Multivariate Cox Proportional Hazard Model. In the Cox regression analysis, the subgroup analysis was conducted. The SPSS software had been used to make statistical analysis. Additionally, the survival curves and forest plots were produced using the GraphPad Prism 5. It was considered that a two-sided p < 0.05 was statistically significant.
Results
Patient characteristics
In total, there were 8,673 patients with MPM from 2004 to 2015. In accordance with the standards of exclusion, 3,997 patients have been recruited after screening (see details in Figure 1). Overall, the median survival time was 9.0 months (range: 0–152 months). Then, the included patients were classified into unmarried (n = 1,262, 31.57%) and married groups (n = 2,735, 68.43%). As for the patients’ baseline features stratified by marital status, they had been showed in Table 1. Additionally, significant difference between unmarried and married groups in the age (p = 0.011), gender (p < 0.001), race (p < 0.001), AJCC stage (p = 0.048), surgery (p < 0.001), chemotherapy (p < 0.001), and radiotherapy (p < 0.001) could be observed. Besides, married patients were often younger, male, and white race and they had mostly received surgery, chemotherapy, and radiotherapy. In addition, among the married patients, there was a slightly less stage I/II patients compared to unmarried group (38.83 vs. 40.72%).
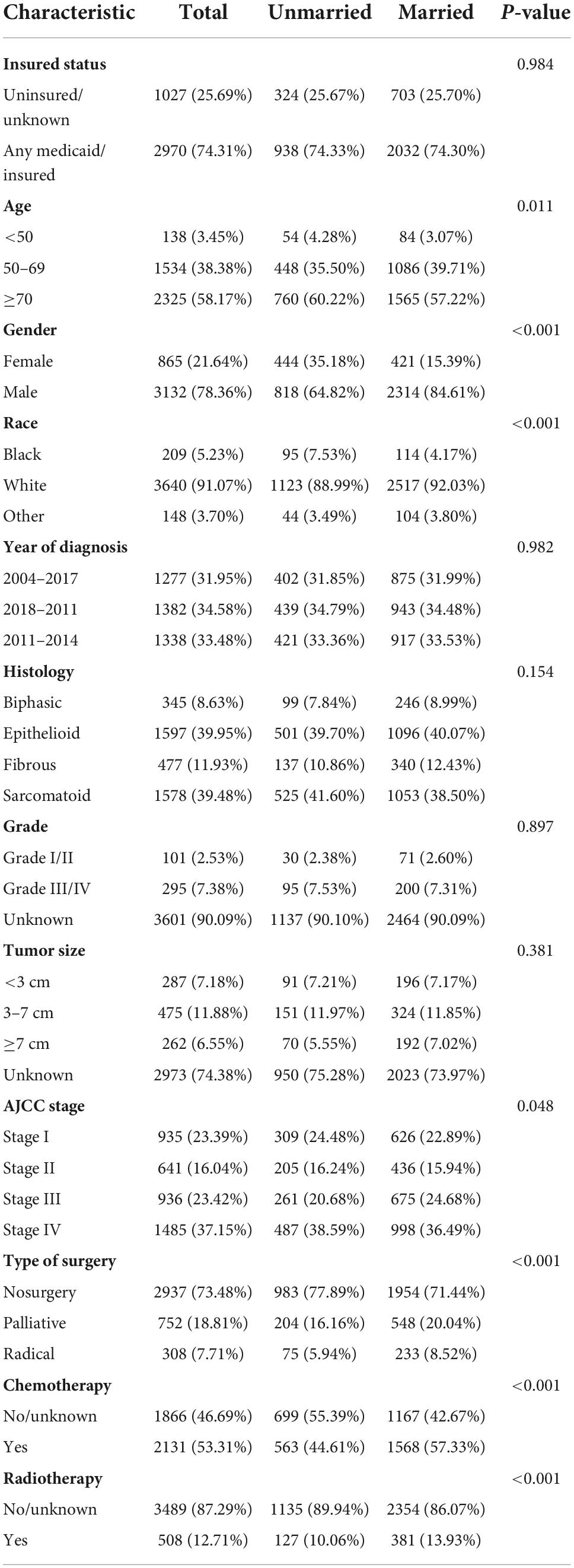
Table 1. The clinicopathological characteristics and treatments of the included 3,997 malignant pleural mesothelioma (MPM) patients.
Marital status and survival
In terms of the marital status, CSS and OS survival difference could be observed, as displayed in the Kaplan–Meier curves (Figure 2). To be specific, CSS and OS of married patients were better than those of the unmarried ones. The 1, 3, and 5-year CSS rates were 44.40, 12.09, and 6.88%, respectively, in married groups, while 35.75, 12.12, and 6.37%, respectively, in unmarried groups (p = 0.0014). Meanwhile, the 1, 3, and 5-year OS rates were 41.84, 10.56, and 5.91%, respectively, in married groups, while 33.67, 10.44, and 4.93%, respectively, in unmarried groups (p < 0.0001). The univariate log-rank test revealed that some covariates had significant association with CSS (p < 0.05), which included marital status, histology, gender, age, grade, AJCC stage, size of tumor, surgery methods, chemotherapy, and radiotherapy. Marital status remained a prognostic factor even after multivariate analysis adjustment. Compared with the unmarried groups, married ones had better CSS [hazard ratio (HR): 0.870; 95% confidence interval (CI): 0.808–0.938; p < 0.001]. Meanwhile, all included covariates were significantly associated with OS. According to the multivariate analysis, the marital status remained an independent prognostic factor of OS (HR: 0.871; 95% CI: 0.810–0.936; p < 0.001) (Table 2).
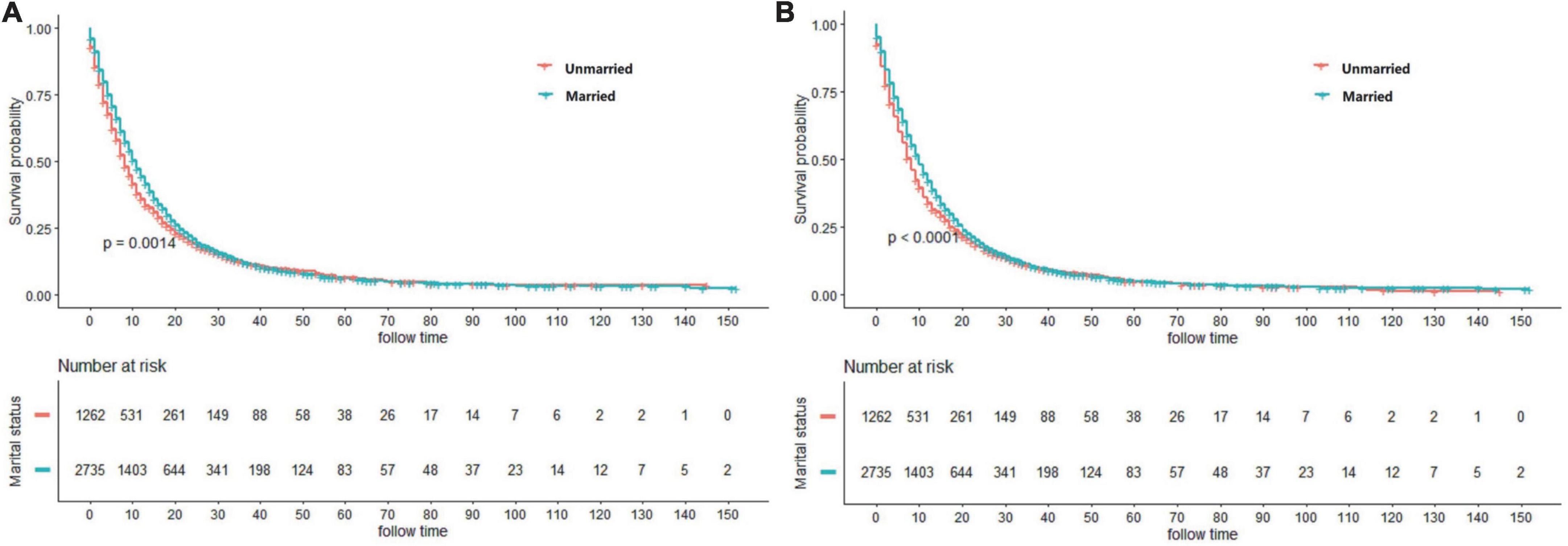
Figure 2. Kaplan–Meier curves showing cancer-specific survival (CSS) (A) and overall survival (OS) (B) between married and unmarried patients.
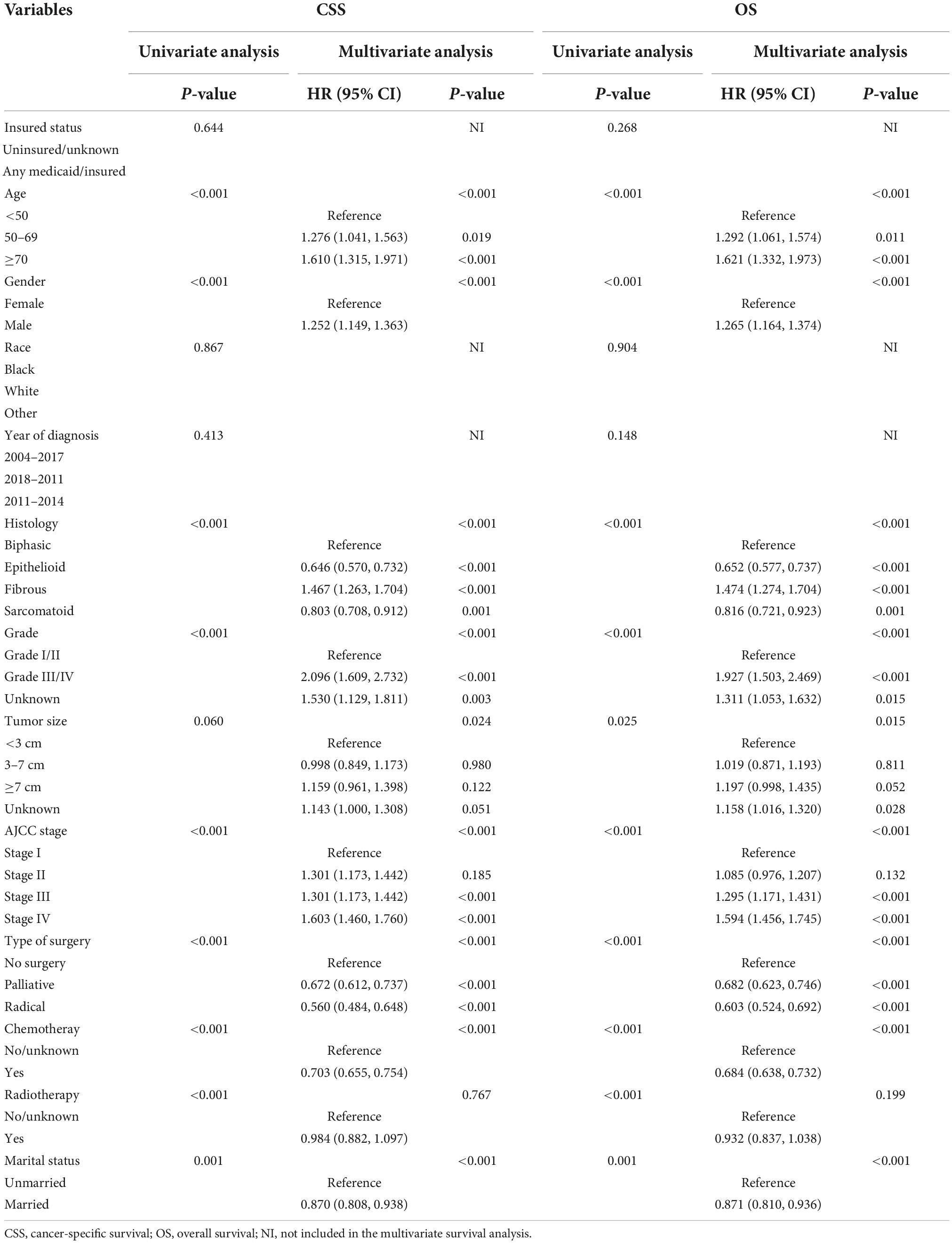
Table 2. Univariate and multivariate analyses of cancer special survival (CSS) and overall survival (OS) for patients with MPM.
Subgroup analysis of the effect of marital status in cancer-specific survival and overall survival
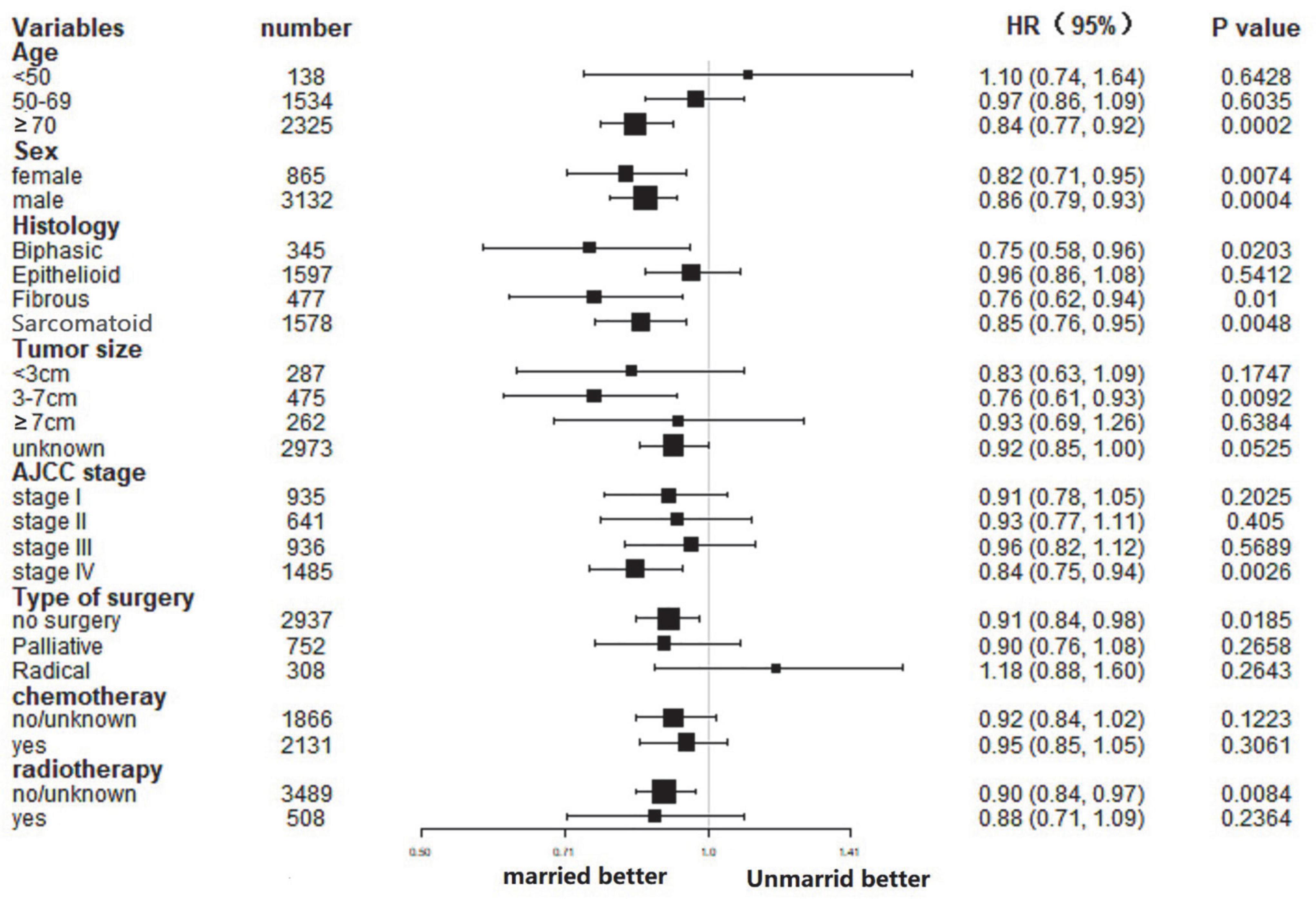
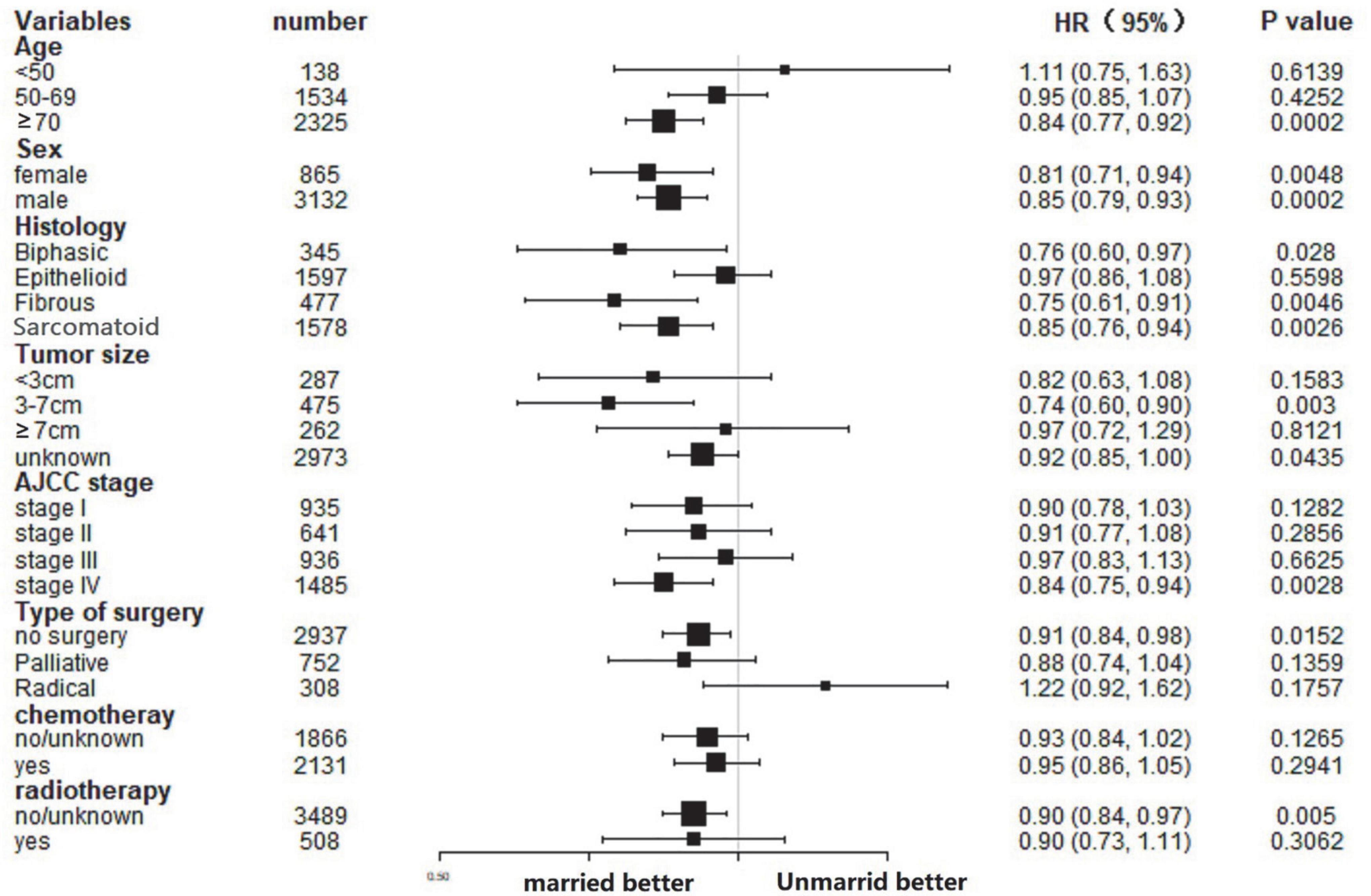
In this study, the influence of marital status on survival at different subgroups was analyzed. As demonstrated by the results of subgroup analysis, the married groups had better CSS and OS survival than the unmarried groups in nearly all study subgroups (Figures 3, 4). Specifically, the subgroups patients who aged ≥70, tumor size 3–7 cm, AJCC stage IV, and received no surgery or radiotherapy could significantly benefit from married status (all p < 0.05).
Discussion
Marital status could be considered an independent prognostic indicator of MPM patients. This research probes into the impact of marital status on the survival of MPM patients for the first time. In this study of 3,997 patients with MPM, we observed that married groups had lower risk of death compared with unmarried ones. After the demographic and tumor features were controlled, the married patients had a 13% lower risk of death in comparison to unmarried ones.
Most cases of MPM are caused by prior exposure to asbestos (2, 5). Other causes of MPM include erionite (a mineral found in Turkish rocks), chest wall radiation, and simian virus 40. The latter, an oncogenic virus that blocks tumor suppressor genes, may be a cofactor in the development of MPM, although evidence for causality is weak (2, 5, 20–22).
There is an interaction between gender and marital status among a general population. According to the previous research, the impact of marital status on survival would vary with gender (23, 24). Hence, the stratification by gender was taken into account for subgroup analysis. Nevertheless, in comparison to the unmarried groups, the married groups presented better survival results at each gender subgroup. That is to say, in addition to gender, there was other etiology for the marital status’s impact on prognosis.
Some studies had hypothesized that unmarried people’s poor prognosis was related to the delayed diagnosis in tumor stage. For instance, a study on laryngeal and oral cancers revealed that married people were diagnosed with earlier stage cancer (25). However, we found that there were a slightly more stages I and II patients among unmarried groups. Moreover, many researchers hold opposite opinions through study (26, 27). As shown by the subgroup analysis results, the married status was an independent prognostic factor of MPM in stage IV disease, but not in stage I–III disease. In other words, marital status play an important role in advanced stage diseases, and is scilicet a protective factor in patients with advanced disease particularly.
Two potential mechanisms may explain the relationship between survival and marital status. First, after a diagnosis of cancer, the distress of married groups is less than that of unmarried ones, since a partner can help offer suitable support and share the stress (28). Moreover, distress and loneliness will trigger angiogenesis of tumor, cause down-regulation of cellular immune response (29), and lift invasiveness of tumor (30–32). Second, the married patients who gain support from their children or spouses could better comply with advice of doctors (33, 34). In this way, they are more likely to obtain active treatment. Likewise, our research discovered that married patients were more possibly to receive treatments. Hence, it is essential to provide social support services and psychological interventions that may help to lower the great survival differences between unmarried and married patients with cancer.
Nevertheless, owing to the SEER database’s limited nature, this study has a few limitations. Firstly, the marital status in this study was recorded at diagnosis. Hence, whether the changed marital status was unknown. Secondly, the detailed quality of marriage was not offered in SEER database, which would affect the survival results as well (35). Thirdly, there was a lack of more detailed data on education, insurance, and income, which may had a certain impact on the interpretation of the results. In order to verify these findings, prospective cohort studies are needed in the future. In spite of the above limitations, our research reveals that the marital status could significantly affect the survival of MPM. This study is meaningful, as it stresses the great impact of marriage, especially social support, on survival of cancer. At the same time, this study puts forward that offering social support interventions targeted at vulnerable people could help improve the possibility of being cured to a great extent. In the future research, this intervention may be confirmed a cost-effective way to improve outcomes among unmarried cancer patients.
Conclusion
To sum up, the marital status is an independent prognostic indicator of MPM patients. Compared with unmarried patients, married ones are better in CSS and OS. Indeed, unmarried patients’ poor prognosis may be related to deficient treatment, socioeconomic and psychosocial factors. Further study is needed to confirm the finding of the existing study.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The Human Research Protection Office considered that the data analysis is focused on non-human subjects, and they were available. Hence, the approval from the institutional review board was not required. Written informed consent from the (patients/participants OR patients/participants legal guardian/next of kin) was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
ZL conceived the study and revised the manuscript. SP searched the database and literature and wrote the manuscript. NY and YZ discussed and analyzed the data. All authors approved the final version.
Funding
This research was supported by the National Natural Science Foundation of China (ID: 82072206).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
MPM, malignant pleural mesothelioma; CSS, cancer-specific survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; AJCC, American joint committee on cancer; TNM, tumor–node–metastasis; SEER, surveillance epidemiology, and end results; ICD-O-3, international classification of disease for oncology, third edition; K–M, Kaplan–Meier.
References
1. British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax. (2007) 62(Suppl. 2):ii1–19. doi: 10.1136/thx.2007.087619
2. Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. (2010) 35:479–95.
3. Yates DH, Corrin B, Stidolph PN, Browne K. Malignant mesothelioma in South East England: clinicopathological experience of 272 cases. Thorax. (1997) 52:507–12. doi: 10.1136/thx.52.6.507
4. Howel D, Arblaster L, Swinburne L, Schweiger M, Renvoize E, Hatton P. Routes of asbestos exposure and the development of mesothelioma in an English region. Occup Environ Med. (1997) 54:403–9.
6. Bibby AC, Tsim S, Kanellakis N, Ball H, Talbot DC, Blyth KG, et al. Malignant pleural mesothelioma: an update on investigation, diagnosis and treatment. Eur Respir Rev. (2016) 25:472–86.
7. Beckett P, Edwards J, Fennell D, Hubbard R, Woolhouse I, Peake MD. Demographics, management and survival of patients with malignant pleural mesothelioma in the national lung cancer audit in England and Wales. Lung Cancer. (2015) 88:344–8. doi: 10.1016/j.lungcan.2015.03.005
8. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. (2013) 31:3869–76.
9. Liu YL, Wang DW, Yang ZC, Ma R, Li Z, Suo W, et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: an analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res Treat. (2019) 178:379–88.
10. Wu Y, Ai Z, Xu G. Marital status and survival in patients with non-small cell lung cancer: an analysis of 70006 patients in the SEER database. Oncotarget. (2017) 8:103518–34. doi: 10.18632/oncotarget.21568
11. Khan S, Nepple KG, Kibel AS, Sandhu G, Kallogjeri D, Strope S, et al. The association of marital status and mortality among men with early-stage prostate cancer treated with radical prostatectomy: insight into post-prostatectomy survival strategies. Cancer Causes Control. (2019) 30:871–6. doi: 10.1007/s10552-019-01194-y
12. Zhou H, Zhang Y, Song Y, Tan W, Qiu Z, Li S, et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: an analysis of the surveillance, epidemiology, and end results (SEER) database. Clin Res Hepatol Gastroenterol. (2017) 41:476–86.
13. Buja A, Lago L, Lago S, Vinelli A, Zanardo C, Baldo V. Marital status and stage of cancer at diagnosis: a systematic review. Eur J Cancer Care. (2018) 27.
14. Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol. (2016) 40:e94–102. doi: 10.1097/PAS.0000000000000749
15. Chen Z, Cui J, Dai W, Yang H, He Y, Song X. Influence of marital status on small intestinal adenocarcinoma survival: an analysis of the surveillance, epidemiology, and end results (SEER) database. Cancer Manag Res. (2018) 10:5667–76. doi: 10.2147/CMAR.S177430
16. Zhuo M, Zheng Q, Chi Y, Jia B, Zhao J, Wu M, et al. Survival analysis via nomogram of surgical patients with malignant pleural mesothelioma in the surveillance, epidemiology, and end results database. Thorac Cancer. (2019) 10:1193–202. doi: 10.1111/1759-7714.13063
17. Feng L, Yang YJ, Du J, Yu YJ, Diao JD. Marital status and survival of patients with colorectal signet ring cell carcinoma: a population-based study. Sci Rep. (2020) 10:17881.
18. Shao F, Qi W, Meng FT, Qiu L, Huang Q. Role of palliative radiotherapy in unresectable intrahepatic cholangiocarcinoma: population-based analysis with propensity score matching. Cancer Manag Res. (2018) 10:1497–506. doi: 10.2147/CMAR.S160680
19. Cheng R, Du Q, Ye J, Wang B, Chen Y. Prognostic value of site-specific metastases for patients with advanced intrahepatic cholangiocarcinoma: a SEER database analysis. Medicine. (2019) 98:e18191. doi: 10.1097/MD.0000000000018191
20. Carbone M, Emri S, Dogan AU, Steele I, Tuncer M, Pass HI, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer. (2007) 7:147–54. doi: 10.1038/nrc2068
21. López-Ríos F, Illei PB, Rusch V, Ladanyi M. Evidence against a role for SV40 infection in human mesotheliomas and high risk of false-positive PCR results owing to presence of SV40 sequences in common laboratory plasmids. Lancet. (2004) 364:1157–66. doi: 10.1016/S0140-6736(04)17102-X
22. Proietti L, Migliore M, Polosa R, Comba P, Circo C, Di Maria GU. [Malignant pleural mesothelioma in housewives in the province of Catania]. Recenti Prog Med. (2004) 95:365–8.
23. Buckman JEJ, Saunders R, Stott J, Arundell LL, O’Driscoll C, Davies MR, et al. Role of age, gender and marital status in prognosis for adults with depression: an individual patient data meta-analysis. Epidemiol Psychiatr Sci. (2021) 30:e42. doi: 10.1017/S2045796021000342
24. Kaplan RM, Kronick RG. Marital status and longevity in the United States population. J Epidemiol Community Health. (2006) 60:760–5.
25. Inverso G, Mahal BA, Aizer AA, Donoff RB, Chau NG, Haddad RI. Marital status and head and neck cancer outcomes. Cancer. (2015) 121:1273–8.
26. Mwaka AD, Garimoi CO, Were EM, Roland M, Wabinga H, Lyratzopoulos G. Social, demographic and healthcare factors associated with stage at diagnosis of cervical cancer: cross-sectional study in a tertiary hospital in Northern Uganda. BMJ Open. (2016) 6:e007690. doi: 10.1136/bmjopen-2015-007690
27. Li M, Dai CY, Wang YN, Chen T, Wang L, Yang P, et al. Marital status is an independent prognostic factor for tracheal cancer patients: an analysis of the SEER database. Oncotarget. (2016) 7:77152–62. doi: 10.18632/oncotarget.12809
28. Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. (1996) 276:293–9.
29. Pike JL, Irwin MR. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain Behav Immunity. (2006) 20:169–74. doi: 10.1016/j.bbi.2005.05.004
30. Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. (2006) 6:240–8. doi: 10.1038/nrc1820
31. Jaremka LM, Peng J, Bornstein R, Alfano CM, Andridge RR, Povoski SP, et al. Cognitive problems among breast cancer survivors: loneliness enhances risk. Psychooncology. (2014) 23:1356–64. doi: 10.1002/pon.3544
32. Kissane DW. Unrecognised and untreated depression in cancer care. Lancet Psychiatry. (2014) 1:320–1.
33. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. (2000) 160:2101–7.
34. Anderson JC, Fortinsky RH, Kleppinger A, Merz-Beyus AB, Huntington CG III, Lagarde S. Predictors of compliance with free endoscopic colorectal cancer screening in uninsured adults. J Gen Intern Med. (2011) 26:875–80. doi: 10.1007/s11606-011-1716-7
Keywords: malignant pleural mesothelioma, marital status, SEER, survival, cancer specific survival, overall survival
Citation: Pan S, Yan N, Zhao Yy and Li Zw (2022) Marital status as an independent prognostic factor for patients of malignant pleural mesothelioma. Front. Med. 9:955619. doi: 10.3389/fmed.2022.955619
Received: 29 May 2022; Accepted: 30 September 2022;
Published: 21 October 2022.
Edited by:
Federica Meloni, University of Pavia, ItalyReviewed by:
Sara Lettieri, San Matteo Hospital Foundation (IRCCS), ItalyMarcello Migliore, University of Catania, Italy
Copyright © 2022 Pan, Yan, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiwen Li, bGl6aGl3ZW5Aamx1LmVkdS5jbg==
 Shu Pan
Shu Pan Zhiwen Li
Zhiwen Li