- Department of Clinical Laboratory, Affiliated Hangzhou First People's Hospital, Zhejiang University School of Medicine, Hangzhou, China
SARS-CoV-2 is a serious infectious respiratory virus that can cause lung, heart, kidney, and liver damage and even cause death. Early diagnosis of SARS-CoV-2 infection is vital for epidemic prevention and control. At present, the gold standard of COVID-19 diagnosis is nucleic acid detection of SARS-CoV-2. However, the nucleic acid detection of SARS-CoV-2 requires high site requirements and technology requirements, and the detection is time-consuming and cannot fully meet clinical needs. Although SARS-CoV-2 antigen test results cannot be directly used to diagnose COVID-19, positive results can be used for the early triage and rapid management of suspected populations. However, due to the limitations of the methodology itself, the SARS-CoV-2 antigen test is prone to produce false-positive and false-negative results in the process of detection. It is urgent to develop a batch of SARS-CoV-2 antigen reagents based on new detection technology and detection principles to overcome the defects of existing technologies.
Introduction
SARS-CoV-2 is a severe infectious respiratory virus that can cause injury to the lung, heart, kidney, and liver and even cause death (1–9). It is well-known that the basic principles of infectious disease control are controlling the source of infection, cutting off the route of transmission, and protecting the susceptible population. However, the existing vaccines and monoclonal antibodies are less effective because the virus mutates so quickly that immune escape is severe (10–15). In view of this situation, it is difficult to curb the COVID-19 epidemic solely from the perspective of patient prevention and treatment. It is necessary to control the source of infection further and cut off the transmission route of SARS-CoV-2 to control the epidemic situation thoroughly. To achieve these goals, timely, and accurate diagnosis and identification of COVID-19 patients are critical. At present, the gold standard of COVID-19 diagnosis is nucleic acid detection of SARS-CoV-2 (16, 17). However, the nucleic acid detection of SARS-CoV-2 requires professionals to perform in a PCR laboratory with level II biosafety (18, 19). Because of its high skill requirements and site requirements, it cannot be widely promoted, especially in some primary medical institutions. In addition, due to the complexity of the operation, standard SARS-CoV-2 nucleic acid detection takes 4–6 h to complete. Moreover, the report will be longer for limited fluxes and large specimens. This may lead to delays in identifying COVID-19 patients in medical institutions. The simultaneous gathering of COVID-19 patients and non-COVID-19 patients in health care facilities not only increases the risk of cross-infection but also occupies a large number of medical resources, putting great pressure on the prevention and control of COVID-19 in medical institutions.
Colloidal gold immunochromatography is a rapid and straightforward method for detecting SARS-CoV-2 antigen. However, due to the factors of reagents and samples, some false positive or false negative results were caused (20, 21). This paper will analyze the common causes of false positives and false negatives in using the colloidal gold immunochromatography SARS-CoV-2 antigen assay to provide a reference for the interpretation of the results (Figure 1).
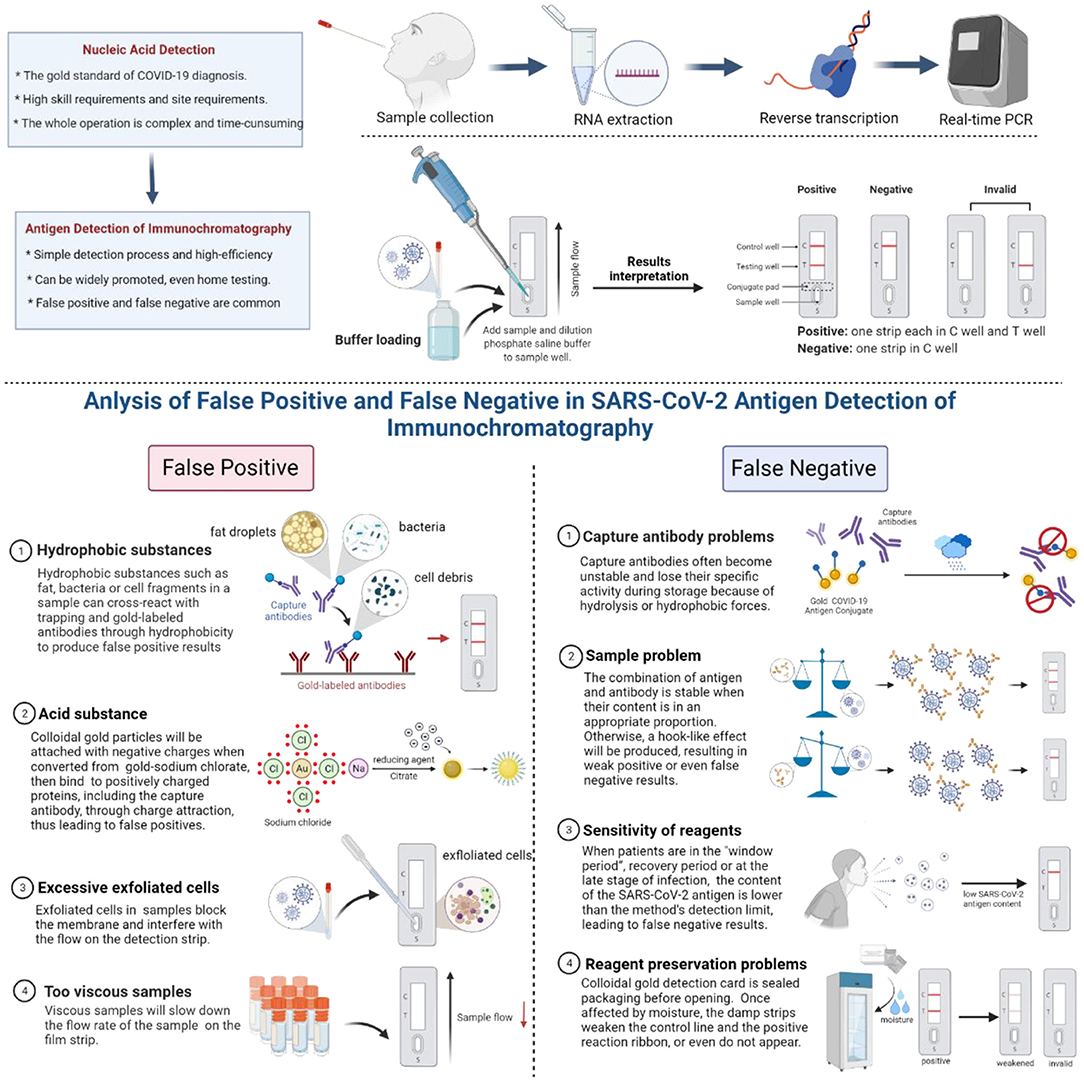
Figure 1. Common causes of false positives and false negatives of colloidal gold immunochromatography.
Common Causes of False Positives of Colloidal Gold Immunochromatography
Hydrophobic Substances
Proteins rich in non-polar amino acids, such as tryptophan, valine, leucine, isoleucine, or phenylalanine, will have a strong binding effect through hydrophobic forces once they are very close to each other (within a distance of <1 nm). Therefore, hydrophobic substances such as fat, bacteria, or cell fragments in a sample can cross-react with trapping and gold-labeled antibodies through hydrophobicity to produce false-positive results. These non-specific bonds can be decomposed by adding surfactants or hydrophilic polymers.
Acid Substance
When sodium chlorate is converted into colloidal gold, the reducing agent citrate is used with a layer of negative charge attached to the surface of colloidal gold particles, which gives the colloidal gold particles a negative charge. When the pH of the sample is less than the isoelectric point of zwitterions, the protonation of zwitterions is positively charged. In particular, protein regions rich in lysine and arginine are positively charged below the isoelectric point of lysine (pH 10.4) and arginine (pH 12.5). In this way, the negatively charged colloidal gold can bind non-specifically to positively charged proteins, including the capture antibody, through charge attraction, thus leading to false positives.
Excessive Exfoliated Cells
Some samples may contain a large number of exfoliated cells, which have the potential to block the membrane and interfere with the flow of gold standard solution on the detection strip.
Too Viscous Samples
Some samples may be very viscous, which will slow down the flow rate of the sample and sample diluent on the film strip. When there are not enough samples and sample dilutions in the detection system to move the gold-labeled antibody along the detection band, the colloidal gold particles will also adhere to the capture antibody band.
Reagent Problem
It is generally recommended that the reagent should be used immediately after unsealing, and the exposure time should not be too long. If the exposure time is too long, the reagent will deteriorate, and false-positive results will appear.
Common Causes of False Negatives of Colloidal Gold Immunochromatography
Problems of Capture Antibodies
Capture antibodies often become unstable and lose their specific activity during storage, which may be due to the destruction of hydrolysis due to a wet environment and insufficient drying or the destruction of hydrophobic forces. The former requires that reagents be stored in a dry environment, and the detection should be carried out in a controlled dry environment. The latter may be the characteristic of the antibody itself, which can be solved by replacing the antibody or removing the capture antibody layer and then treating the membrane with a surfactant.
Sample Problem
The combination of antigen and corresponding antibody is the most stable when the content of antigen and corresponding antibody is in a certain appropriate proportion during the detection process. If the concentration of SARS-CoV-2 antigen is too high, a hook-like effect will be produced, making the ratio of antigen and antibody imbalanced and resulting in weak positive or even false-negative results. In particular, the hook effect is more likely to occur in the detection based on the one-step principle. At this point, more accurate results can be obtained if the sample is diluted properly.
Sensitivity of Reagents
One of the main reasons for the false-negative detection of SARS-CoV-2 antigen by the colloidal gold immunochromatography is the limited sensitivity of the reagent itself. When the content of the SARS-CoV-2 antigen is lower than the method's detection limit, false negatives may occur. Therefore, patients are prone to have false-negative results in the “window period” with low SARS-CoV-2 antigen content, in the recovery period, or at the late stage of infection (22–25).
Reagent Preservation Problems
Colloidal gold detection card is sealed packaging before opening. It is easily affected by moisture when the detection card is stored in a 4°C refrigerator after opening. The damp strips weaken the control line, and the positive reaction ribbon or even do not appear. Therefore, the sensitivity of the detection will decrease or be invalid, affecting the accuracy of the test results.
Immune Escape
The SARS-CoV-2 mutant reduces the detection rate of the SARS-CoV-2 antigen reagent, especially the vaccine escaping the SARS-CoV-2 mutant (26–29).
Summary and Prospects
The immunochromatographic colloidal gold method has the advantages of rapid and straightforward operation. Nevertheless, due to the limitation of the methodology itself, it is easy to produce false positive and false negative results in the application process. It is urgent to develop a batch of SARS-CoV-2 antigen reagents based on new detection technology and detection principles to overcome the defects of existing technologies.
Author Contributions
WS contributed to the content, writing, and critical review of the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen Z, Wang B, Mao S, Ye Q. Assessment of global asymptomatic SARS-CoV-2 infection and management practices from China. Int J Biol Sci. (2021) 17:1119–24. doi: 10.7150/ijbs.59374
2. Han X, Ye Q. Kidney involvement in COVID-19 and its treatments. J Med Virol. (2021) 93:1387–95. doi: 10.1002/jmv.26653
3. Tian D, Ye Q. Hepatic complications of COVID-19 and its treatment. J Med Virol. (2020) 92:1818–24. doi: 10.1002/jmv.26036
4. Ye Q, Lai EY, Luft FC, Persson PB, Mao J. SARS-CoV-2 effects on the renin-angiotensin-aldosterone system, therapeutic implications. Acta Physiol. (2021) 231:e13608. doi: 10.1111/apha.13608
5. Ye Q, Lu D, Shang S, Fu J, Gong F, Shu Q, et al. Crosstalk between coronavirus disease 2019 and cardiovascular disease and its treatment. ESC Heart Fail. (2020) 7:3464–72. doi: 10.1002/ehf2.12960
6. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ’cytokine storm' in COVID-19. J Infect. (2020) 80:607–13. doi: 10.1016/j.jinf.2020.03.037
7. Ye Q, Wang B, Mao J, Fu J, Shang S, Shu Q, et al. Epidemiological analysis of COVID-19 and practical experience from China. J Med Virol. (2020) 92:755–69. doi: 10.1002/jmv.25813
8. Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. (2020) 319:G245–52. doi: 10.1152/ajpgi.00148.2020
9. Zhou X, Ye Q. Cellular immune response to COVID-19 and potential immune modulators. Front Immunol. (2021) 12:646333. doi: 10.3389/fimmu.2021.646333
10. Han X, Xu P, Ye Q. Analysis of COVID-19 vaccines: types, thoughts, and application. J Clin Lab Anal. (2021) 35:e23937. doi: 10.1002/jcla.23937
11. Han X, Ye Q. The variants of SARS-CoV-2 and the challenges of vaccines. J Med Virol. (2022) 94:1366–72. doi: 10.1002/jmv.27513
12. Meng H, Mao J, Ye Q. Booster vaccination strategy: necessity, immunization objectives, immunization strategy, and safety. J Med Virol. (2022) 94:2369–75. doi: 10.1002/jmv.27590
13. Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. (2022) 94:2376–83. doi: 10.1002/jmv.27643
14. Tian D, Sun Y, Zhou J, Ye Q. The global epidemic of the SARS-CoV-2 delta variant, key spike mutations and immune escape. Front Immunol. (2021) 12:751778. doi: 10.3389/fimmu.2021.751778
15. Tian D, Sun Y, Zhou J, Ye Q. The global epidemic of SARS-CoV-2 variants and their mutational immune escape. J Med Virol. (2021) 94:847–57. doi: 10.1002/jmv.27376
16. Ye Q, Zhang T, Lu D. Potential false-positive reasons for SARS-CoV-2 antibody testing and its solution. J Med Virol. (2021) 93:4242–46. doi: 10.1002/jmv.26937
17. Zhang X, Meng H, Liu H, Ye Q. Advances in laboratory detection methods and technology application of SARS-CoV-2. J Med Virol. (2022) 94:1357–65. doi: 10.1002/jmv.27494
18. Ye Q, Lu D, Zhang T, Mao J, Shang S. Application experience of a rapid nucleic acid detection system for COVID-19. Microbes Infect. (2022) 24:104945. doi: 10.1016/j.micinf.2022.104945
19. Ye Q, Lu D, Zhang T, Mao J, Shang S. Recent advances and clinical application in point-of-care testing of SARS-CoV-2. J Med Virol. (2022) 94:1866–75. doi: 10.1002/jmv.27617
20. Itoh K, Kawamitsu T, Osaka Y, Sato K, Suzuki Y, Kiriba C, et al. False positive results in severe acute respiratory coronavirus 2 (SARS-CoV-2) rapid antigen tests for inpatients. J Infect Chemother. (2021) 27:1089–91. doi: 10.1016/j.jiac.2021.03.011
21. Ye Q, Shao W, Meng H. Performance and application evaluation of SARS-CoV-2 antigen assay. J Med Virol. (2022) 92:548–51. doi: 10.1002/jmv.27798
22. Dinnes J, Deeks JJ, Berhane S, Taylor M, Adriano A, Davenport C, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. (2021) 3:CD013705. doi: 10.1002/14651858.CD013705.pub2
23. Pray W, Ford L, Cole D, Lee C, Bigouette JP, Abedi GR, et al. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep. (2021) 69:1642–7. doi: 10.15585/mmwr.mm695152a3
24. Okoye NC, Barker AP, Curtis K, Orlandi RR, Snavely EA, Wright C, et al. Performance characteristics of BinaxNOW COVID-19 antigen card for screening asymptomatic individuals in a university setting. J Clin Microbiol. (2021) 59:e03282–20. doi: 10.1128/JCM.03282-20
25. Ferté T, Ramel V, Cazanave C, Lafon ME, Bébéar C, Malvy D, et al. Accuracy of COVID-19 rapid antigenic tests compared to RT-PCR in a student population: the StudyCov study. J Clin Virol. (2021) 141:104878. doi: 10.1016/j.jcv.2021.104878
26. Bekliz M, Perez-Rodriguez F, Puhach O, Adea K, Melancia SM, Baggio S, et al. Sensitivity of SARS-CoV-2 antigen-detecting rapid tests for omicron variant. medRxiv [Preprint]. (2022). doi: 10.1101/2021.12.18.21268018
27. Barrera-Avalos C, Luraschi R, Vallejos-Vidal E, Mella-Torres A, Hernández F, Figueroa M, et al. The rapid antigen detection test for SARS-CoV-2 underestimates the identification of Covid-19 positive cases and compromises the diagnosis of the SARS-CoV-2 (K417N/T, E484K, N501Y) variants. Front Public Health. (2021) 9:780801. doi: 10.3389/fpubh.2021.780801
28. Albert E, Torres I, Bueno F, Huntley D, Molla E, Fernández-Fuentes M, et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin Microbiol Infect. (2021) 27:472.e7–10. doi: 10.1016/j.cmi.2020.11.004
29. de Michelena P, Torres I, Ramos-García Á, Gozalbes V, Ruiz N, Sanmartín A, et al. Real-life performance of a COVID-19 rapid antigen detection test targeting the SARS-CoV-2 nucleoprotein for diagnosis of COVID-19 due to the omicron variant. J Infect. (2022) 84:e64–6. doi: 10.1101/2022.02.02.22270295
Keywords: COVID-19, SARS-CoV-2, immunochromatography, antigen, false positive, false negative
Citation: Shao W (2022) Accurate Interpretation of SARS-CoV-2 Antigen Detection by Immunochromatography. Front. Med. 9:949554. doi: 10.3389/fmed.2022.949554
Received: 24 May 2022; Accepted: 13 June 2022;
Published: 29 June 2022.
Edited by:
Svetlana Khaiboullina, University of Nevada, Reno, United StatesReviewed by:
Qing Ye, Zhejiang University School of Medicine, ChinaTing Zhang, Zhejiang Chinese Medical University, China
Copyright © 2022 Shao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxia Shao, d3g1MzY2JiN4MDAwNDA7MTYzLmNvbQ==
 Wenxia Shao
Wenxia Shao