
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 08 July 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.941647
This article is part of the Research TopicPrevention and Control of Human T Lymphotropic Viruses 1 and 2 (HTLV-1/2)View all 32 articles
 Rachael S. Barr1*†
Rachael S. Barr1*† Simon B. Drysdale2,3†
Simon B. Drysdale2,3† Mary Boullier2
Mary Boullier2 Hermione Lyall4
Hermione Lyall4 Lucy Cook5,6
Lucy Cook5,6 Graham P. Collins7
Graham P. Collins7 Dominic F. Kelly3,8
Dominic F. Kelly3,8 Lorna Phelan9
Lorna Phelan9 Graham P. Taylor6
Graham P. Taylor6Human T cell lymphotropic virus type 1 (HTLV-1) is a human retrovirus that is endemic in a number of regions across the world. There are an estimated 5–10 million people infected worldwide. Japan is currently the only country with a national antenatal screening programme in place. HTLV-1 is primarily transmitted sexually in adulthood, however it can be transmitted from mother-to-child perinatally. This can occur transplacentally, during the birth process or via breastmilk. If HTLV-1 is transmitted perinatally then the lifetime risk of adult T cell leukemia/lymphoma rises from 5 to 20%, therefore prevention of mother-to-child transmission of HTLV-1 is a public health priority. There are reliable immunological and molecular tests available for HTLV-1 diagnosis during pregnancy and screening should be considered on a country by country basis. Further research on best management is needed particularly for pregnancies in women with high HTLV-1 viral load. A first step would be to establish an international registry of cases and to monitor outcomes for neonates and mothers. We have summarized key risk factors for mother-to-child transmission of HTLV-1 and subsequently propose a pragmatic guideline for management of mothers and infants in pregnancy and the perinatal period to reduce the risk of transmission. This is clinically relevant in order to reduce mother-to-child transmission of HTLV-1 and it's complications.
Human T cell lymphotropic virus type 1 (HTLV-1) is a human retrovirus that was first identified in 1979 (1). HTLV-1 is widespread throughout the globe, being endemic in regions of Japan, West and Southern Africa, the Caribbean islands, Iran, some parts of South America, Central Australia and Melanesia (2, 3). It is also found in Europe, North America, India and China, with an estimated 5–10 million people infected worldwide (2). Japan has an estimated 800,000 carriers and is the only country with a national antenatal screening programme (4), although French Guiana also undertakes antenatal screening (5). Some countries, such as Brazil, are considering introduction of national screening (6). The recent technical report from the World Health Organization on the global health burden of HTLV-1 infection draws attention to the importance of incorporating HTLV-1 testing into antenatal care. Highlighting cessation of breast-feeding in a public health approach to the elimination of HTLV-1 mother-to-child transmission, whilst also drawing attention to the need for further research in mother to child transmission (3).
It has been estimated that there are 20,000-30,000 HTLV-1 seropositive people living in England and Wales (7). However, without a screening program in the UK, diagnosis of HTLV-1 infections is usually only made in relation to blood and tissue donor screening, related contact tracing, and rare cases of HTLV-1 associated diseases such as HTLV-1 associated myelopathy (HAM) and Adult T-cell leukemia/lymphoma (ATL) which usually have onset in adult life (7). Whilst rarely encountered in pediatric clinical practice, a recent series of ATL cases diagnosed during pregnancy in the UK have emphasized the importance of measures to prevent perinatal transmission in the setting of HTLV-1 associated diseases where due to higher proviral loads, transmission risks are greater than for asymptomatic individuals (8). Many clinicians including obstetricians, midwives, neonatologists and pediatricians, particularly those working in countries with a lower incidence, do not have clinical experience of this condition. This may result in missed opportunities for prevention of mother-to-child transmission (MTCT) of HTLV-1, and have significant implications for the health of the mother and infant in later life.
The majority of HTLV-1 infections are acquired via sexual transmission in adult life. Sexual transmission between serodiscordant heterosexual couples has been estimated at rates from 0.9 to 2.5 per 100 person-years (9, 10). Higher viral loads in the seropositive partner may be associated with an increased risk of transmission (11). HTLV-1 transmission can also occur by the same routes as other blood-borne viruses including unscreened donated blood, organ donation and injecting drug use (12). Transmission from mother-to-child may occur in the perinatal period via blood, the placenta, the birth canal or breastmilk. The primary route for vertical transmission is via breast milk (~ 80%) and the risk of perinatal transmission by other routes is low in comparison (13).
HTLV-1 RNA is rarely detected in plasma (14), which is deemed non-infectious. Ex vivo observations revealed that HTLV-1 transmission is through cell-to-cell transfer from infected to uninfected T lymphocytes. HTLV-1 integrates into the host cell DNA, mostly CD4+ T-lymphocytes, where it persists with a single copy per genome/cell (15) and is replicated during cell division. Over 90% of those infected are asymptomatic, however, ~10% develop the high morbidity, high mortality HTLV-1-associated diseases, mostly in adult life. There are two major disease associations, HAM and ATL. ATL is an aggressive CD4+ T-cell malignancy that follows decades of asymptomatic HTLV-1 infection and represents the malignant transformation of an infected CD4+ T cell clone (16). The median survival from diagnosis for the aggressive subtypes (acute and lymphoma) is 8 to 10 months despite intensive therapy (17). Despite novel chemotherapy based approaches, survival has remained unchanged in the ~40 years since HTLV-1 was first described. The overall lifetime risk of HTLV-1 carriers developing ATL is 5% (18). However, acquisition of HTLV-1 as an infant in the perinatal period increases the lifetime risk of developing ATL to 20%. (19) HAM is a progressive condition affecting the spinal cord and causing upper motor neurone symptoms predominantly in the lower limbs. The lifetime risk of developing this complication is estimated at 0.25–3.8% (20). Epidemiological studies have not identified a link between perinatal infection and an increased rate of development of HAM, but rare pediatric cases are reported (21). Looking beyond these known disease associations, a recent meta-analysis showed links between HTLV-1 and increased risk of a further 17 different diseases including dermatological, respiratory, rheumatological and oncological conditions (Figure 1) (22). In this meta- analysis HTLV-1 infection was associated with a 1.57 adjusted Hazard Ratio for mortality unexplained by the risk of ATL. Carriers of HTLV-1 infection are also suspected to be at risk of other opportunistic infections (23) and cardiovascular disease (24) (Figure 1).
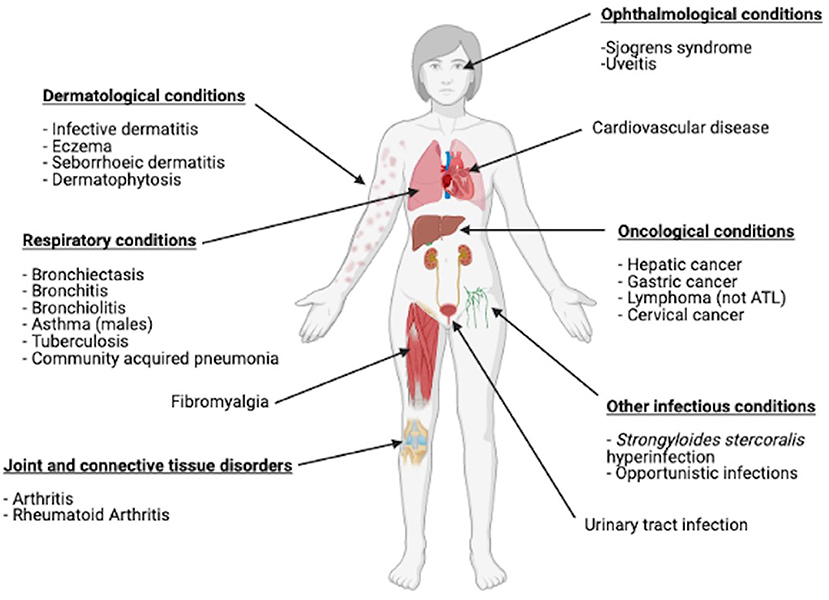
Figure 1. Conditions other than ATL and HAM that have been shown to be linked with HTLV-1 infection in a meta-analysis. Figure created with BioRender.com.
Given the link between perinatal transmission and increased lifetime risk of development of ATL, prevention of mother-to-child transmission is a public health priority. Despite this, there is currently no international consensus on the management of pregnant women with HTLV-1.
Timely and accurate diagnosis of HTLV-1 in women of child-bearing age or those who are pregnant is key in the prevention of mother-to-child transmission. Serological testing for detection of antibodies against HTLV-1 is the first line of testing. Options for serological testing include ELISA, particle agglutination, Western blots and chemiluminescence assays (25, 26). Of note, ELISA and particle agglutination tests are seen as screening tests with western blot, chemiluminescence assays and molecular testing being confirmatory.
Rosadas et al. found that both immunological and molecular testing available for HTLV-1 remains reliable during pregnancy (27).
In the UK, HTLV-1 pro-viral load (PVL) testing is undertaken by contacting the National Center for Human Retrovirology (https://www.imperial.ac.uk/medicine/molecular-diagnostic-unit/diagnostic-services/htlv-load-testing-and-genot-yping/). In the proposed management algorithm we suggest antibody testing is performed in all infants of HTLV-1 positive mothers at 18 months of age. This is because by 18 months maternal antibody will have waned and the results are likely to be representative of the child's own antibody response (28). Japan currently recommend this testing is done at 3 years of age however there is a large loss to follow up at 3 years (29).
A number of factors influence the risk of transmission of HTLV-1 perinatally and each provides an opportunity to decrease the risk to the infant if considered and managed appropriately. A high maternal HTLV-1 PVL (defined as HTLV-1 DNA copies/100 peripheral blood mononuclear cells [PBMCs] expressed as a percentage of cells infected given there is a single copy per cell) has been found to be an independent risk factor for increased transmission to the infant (30). Hisada et al. (30) showed a PVL of >3.0 log10 per 105 cells (equivalent to >1%) was associated with a significant increase in the risk of transmission. Transmission risk went from 6.4% when the PVL was <1 to 36.1% when the PVL was 1% or greater. In addition, the same study (30) showed the risk of transmission was negligible when the mother's HTLV-1 antibody titer was <2.0 log10 but increased significantly when ≥2.0 log10. Symptomatic patients (e.g. those with HAM / ATL) have been shown to have a greatly increased PVL when compared with asymptomatic carriers (31).
We, therefore, recommend stratifying pregnant women who are not symptomatic due to HTLV-1 infection, into low risk (PVL < 1%) and high risk (PVL ≥ 1%) based on their PVL. Women who are symptomatic with ATL should always be considered high-risk, but those with other symptomatic conditions (e.g., dermatitis) may be stratified according to their PVL.
Other risk factors for mother-to-child transmission of HTLV-1 infection include women who have had a previous child with HTLV-1 infection (32), women who are infected with Strongyloides stercoralis (33) and genetic risk factors such as an increased risk of transmission with increased concordance in HLA class 1 alleles between mother and child (28, 33, 34). We suggest screening for Strongyloides stercoralis in pregnant women newly diagnosed with HTLV-1 infection in pregnancy, or those who are known to have HTLV-1 infection who have not been previously screened for Strongyloides stercoralis as it is a treatable risk factor.
Breast milk transmission has been reported to occur in as many as 77% of exclusively breastfed infants compared with 3.3% of non-breastfed infants (35), although other studies have reported lower transmission rates (6). Data from Japan established that there is a direct correlation between duration of breastfeeding and risk of transmission, with longer durations associated with increased transmission (6). The risk of transmission in an infant breastfed for less than 3 months is <2.5% (29, 36). Some studies suggest the risk of MTCT with short term (≤3 month) breastfeeding is not significantly higher than that of infants exclusively formula fed (29, 37). Infants breastfed for less than or equal to 6 months had a transmission risk of 3.9% compared with 20.3% for those breastfed for longer than 6 months (36). Breastfeeding should therefore be avoided if feasible (according to AFASS (Acceptable, Feasible, Affordable, Sustainable and Safe) criteria) (38), or limited to as short a time as possible (e.g., for less than 3 months). However, this should only be applied to populations where the risk of transmitting HTLV-1 infection outweighs the risk of stopping breastfeeding. This decision must be balanced against the known benefits to both infant and mother of breastfeeding and the disadvantages (e.g., cost and need for a clean water supply) associated with formula milk. If a mother decides to breastfeed, or is unable to formula feed safely, then measurement of proviral load in breastmilk should be considered if this is available. In this circumstance, we recommend undertaking HTLV-1 PCR on breastmilk at 1 week and 3 months and every 3 months thereafter as long as breastfeeding continues, done via real-time PCR (39). If the breastmilk HTLV-1 PVL is ≥1%, or if the mother breastfeeds for more than 3 months, there should be further encouragement for breastfeeding to cease if possible, and if it continues then the infant should be considered high risk for developing HTLV-1 infection (6). There is some evidence that freeze-thawing of breast milk provides some benefit in reducing mother-to-child transmission of HTLV-1. Freezing has been shown to affect HTLV-1 infected cells in vitro (40) and small studies have shown a reduction in mother-to-child transmission (41). However, this method is not always possible for mothers to employ as it involves freezing milk at −20°C for at least 12 h (40). There are also limited data on pasteurization of breastmilk to reduce HTLV-1 transmission (42).
The route of transmission in infants who are not breastfed is not completely clear, however it is likely to be at the time of delivery. Transplacental infection may occur, but even when infected lymphocytes are detected in cord blood, not all infants become infected (43, 44).
Healthcare professionals should, therefore, consider options to reduce direct transfer of body fluids, particularly blood, at the time of delivery. Currently there are few studies investigating the most effective method of delivery of infants of women who are HTLV-1 carriers to reduce transmission, although (pre-labor) cesarean section may reduce the risk (28).
As in management of HIV-1, antiretroviral medications could be used to reduce HTLV-1 transmission rates in high-risk infants. However, there are few data regarding the use of antiretrovirals for peri-exposure prophylaxis for neonates. We have published a case series (8) of four mothers from the United Kingdom, all with a diagnosis of ATL, where the mothers were treated, and the infants received post-exposure prophylaxis (PEP), with antiretroviral medications. One infant became seropositive at 15 months, the other three remained negative at 6 weeks, 3 months and 6 months of follow up respectively. We are not aware of any other cases in the literature of infants receiving PEP to try to prevent HTLV-1 infection. There are sporadic case reports of women who are carriers of HTLV-1 infection receiving antiretroviral medications to try to prevent MTCT (45). Potential side effects from ARV's also need to be considered when using them in both neonatal and maternal populations.
There are currently no clinical trials of anti-retroviral (ARV) treatment for HTLV-1 infection in pregnancy or for prevention of MTCT of HTLV-1 that we are aware of, although limited in vitro data suggest their use may be beneficial (28). Several nucleoside analogs have been demonstrated to have anti-HTLV-1 reverse transcriptase activity in vitro, including zidovudine (46). Zidovudine (ZDV) has also been shown to prevent transmission in a rabbit model and has activity against ATL. (47) ZDV with interferon-alpha (IFNα) is an established therapy in leukemic ATL (where available) (48) and is often used as an adjunct to chemotherapy in lymphoma subtype ATL (49). Both ZDV / IFNα are known to be relatively safe in pregnancy, and in cases of ATL have the additional advantage of reducing the risk of mother to infant transmission. Raltegravir (and other integrase inhibitors), also have anti-HTLV-1 integrase activity, and may be used as an adjunct to ZDV (50). While studies (51–53) of antiretroviral therapy have repeatedly failed to show activity in vivo in established infection due to the dominance of cell proliferation in the maintenance of the proviral burden, a role in prevention of transmission when given peri-exposure is biologically plausible. As transmission of HTLV-1 is mainly due to cell-to-cell transmission rather than via free virions in plasma, and maternal lymphocytes in the newborn circulation have a longer half-life (54), it is important to note that infant PEP for HTLV-1 should be for a longer duration (6 weeks) than for HIV which is most likely and most often acquired from free virus in plasma (55). Of note, future treatments may include use of neutralizing monoclonal antibodies. None have been used in clinical trials to date however there has been some initial work in animal models (56).
Drawing together the available evidence on HTLV-1 transmission, and our experience of managing women and their infants, and bearing in mind the potential role of cesarean section, maternal and neonatal antiretroviral therapy, and avoidance of breastfeeding in preventing mother-to-child transmission of HIV, a similar retrovirus, we propose the following perinatal algorithm (Figure 2) for managing pregnant women with HTLV-1 and their infants.

Figure 2. *If a mother decides to breastfeed, consider undertaking breastmilk diagnostics; HTLV-1 PCR at week 1 and 3 months and every 3 months thereafter as long as breastfeeding continues. If the breastmilk HTLV-1 PVL is ≥1% or if the mother breastfeeds for more than 3 months then the infant should have testing for HTLV-1 as per the “high-risk of transmission” arm of the algorithm. **For antiretroviral therapy use same dosing as for treatment (mother) / prevention (infant) of HIV infection (use local guidelines). +Women with leukaemic ATL are theoretically at ultra-high risk of transmission simply because they have higher absolute white cell counts. ++If an infant is shown to be infected at any point, there should be HTLV-1 antibody and PVL by PCR testing at 12–18 months of age and then annual quantitative HTLV-1 PVL testing thereafter. Key: ARV, Antiretroviral; ATL, adult T cell Leukemia/Lymphoma; ZDV, Zidovudine; INSTI, Integrase strand transfer inhibitor (e.g. raltegravir); PCR, Polymerase chain reaction; BF, Breastfeeding; PEP, Post-exposure prophylaxis.
The algorithm is based on assessment of transmission risk according to clinical disease and PVL: women with no symptoms and PVL < 1%, follow the green path with normal pregnancy, birth and postnatal care, avoiding breast feeding if feasible; women with ATL (whatever the PVL), follow the red path with antiretroviral treatment, elective cesarean section, avoidance of breast feeding and PEP for the infant; women with no symptoms / mild symptoms and PVL >1% follow the yellow path and should be considered for antiretroviral treatment, elective cesarean section, avoidance of breast feeding and PEP for the infant. Clinicians are welcome to contact the National Center for Human Retrovirology (http://www.htlv.eu/) for advice in the management of pregnant women with HTLV-1 infection.
Data on prevention of mother-to-child transmission of HTLV-1 have been available since the 1980s and are robust, all demonstrating the effectiveness of avoidance of breast-feeding or at least limiting to less than 3 months duration. Mother-to-child transmission is associated with a high risk of subsequent HTLV-1 associated disease especially adult T-cell leukemia. Data on reducing the risk of peripartum transmission are limited, therefore, we propose a pragmatic perinatal algorithm to aid management of these cases, highlighting transmission risks, perinatal diagnostics, and antiretroviral and obstetric interventions. However, further study on best management for the prevention of mother-to-child transmission of HTLV-1 is needed, especially for high-risk pregnancies. A first step would be to establish an international registry of cases and to monitor outcomes for neonates and mothers.
RB: writing—original draft and writing-review and editing. SD: conceptualisation, writing—original draft, and writing—review and editing. MB, HL, LC, GC, DK, LP, and GT: conceptualisation and writing—review and editing. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gallo RC. The discovery of the first human retrovirus: HTLV-1 and HTLV-2. Retrovirology. (2005) 2:17. doi: 10.1186/1742-4690-2-17
2. Gessain A, Cassar O. Geographical distribution of areas with a high prevalence of HTLV-1 infection. Eur Centre Dis Prevent Control. (2015). p. 1–15. doi: 10.2900/047633
3. Bajis S, Bull R, Causer L, Kaldor J, Legrand N, Matinello M, et al. Human T-Lymphotropic Virus Type 1: Technical Report. World Health Organization. (2021). p. 1–37.
4. Itabashi K, Miyazawa T, Sekizawa A, Tokita A, Saito S, Moriuchi H, et al. A nationwide antenatal human T-cell leukemia virus type-1 antibody screening in Japan. Front Microbiol. (2020) 11:595. doi: 10.3389/fmicb.2020.00595
5. Ramassamy JL, Tortevoye P, Ntab B, Seve B, Carles G, Gaquiere D, et al. Adult T-cell leukemia/lymphoma incidence rate in French Guiana: a prospective cohort of women infected with HTLV-1. Blood Adv. (2020) 4:2044–8. doi: 10.1182/bloodadvances.2020001628
6. Rosadas C, Malik B, Taylor GP, Puccioni-Sohler M. Estimation of HTLV-1 vertical transmission cases in Brazil per annum. PLoS Negl Trop Dis. (2018) 12:e0006913. doi: 10.1371/journal.pntd.0006913
7. Tosswill JHC, Taylor GP, Tedder RS, Mortimer PP. HTLV-I/II associated disease in England and Wales, 1993-7: Retrospective review of serology requests. BMJ. (2000) 320:611–2. doi: 10.1136/bmj.320.7235.611
8. Motedayen Aval L, Boullier M, Lyall H, Collins GP, Ayto R, Kelly DE, et al. Adult T cell leukaemia/lymphoma (ATL) in pregnancy: a UK case series. eJHaem. (2020) 2:131–5. doi: 10.1002/jha2.142
9. Roucoux DF, Wang B, Smith D, Nass C, Smith J, Hutching ST, et al. A Prospective Study of Sexual Transmission of Human T Lymphotropic Virus (HTLV)–I and HTLV-II. J Infect Dis. (2005) 191:1490–7. doi: 10.1086/429410
10. Stuver SO, Taehibana N, Okayama A, Shioiri S, Tsunetoshi Y, Tsuda K, et al. Heterosexual transmission of human T cell leukemia/lymphoma virus type I among married couples in Southwestern Japan: an initial report from the miyazaki cohort study. J Infect Dis. (1993) 167:57–65. doi: 10.1093/infdis/167.1.57
11. Kaplan J, Khabbaz R, Murphy E, Hermansen D, Roberts C, Lal R, et al. Male-to-Female Transmission of Human T-Cell Lymphotropic Virus Types I and II: association with viral load. J Acquir Immune Defic Syndr Hum Retrovirol. (1996) 12:193–201. doi: 10.1097/00042560-199606010-00014
12. Seydel J, Krämer A. Transmission and population dynamics of HTLV-1 infection. Int J Cancer. (1996) 66:197–200. doi: 10.1002/(SICI)1097-0215(19960410)66:2<197::AID-IJC10>3.0.CO;2-A
13. Percher F, Jeannin P, Martin-Latil S, Gessain A, Afonso P, Vidy-Roche A, et al. Mother-to-child transmission of HTLV-1 epidemiological aspects, mechanisms and determinants of mother-to-child transmission. Viruses. (2016) 8:40. doi: 10.3390/v8020040
14. Demontis MA, Sadiq MT, Golz S, Taylor GP. HTLV-1 viral RNA is detected rarely in plasma of HTLV-1 infected subjects. J Med Virol. (2015) 87:2130–4. doi: 10.1002/jmv.24264
15. Cook LB, Rowan AG, Melamed A, Taylor GP, Bangham CRM. HTLV-1-infected T cells contain a single integrated provirus in natural infection. Blood. (2012) 120:3488–90. doi: 10.1182/blood-2012-07-445593
16. World Health Organization. IARC Monographs on the evaluation of carcinogenic risks to humans - Hepatitis viruses. IARC Monogr Eval Carcinog Risks Humans. (1994) 59:1–255.
17. Katsuya H, Ishitsuka K, Utsunomiya A, Hanada S, Eto T, Moriuchi Y, et al. Treatment and survival among 1594 patients with ATL. Blood. (2015) 126:2570–7. doi: 10.1182/blood-2015-03-632489
18. Tajima K. The 4th nation-wide study of adult T-cell leukemia/lymphoma (ATL) in Japan: Estimates of risk of ATL and its geographical and clinical features. Int J Cancer. (1990) 45:237–43. doi: 10.1002/ijc.2910450206
19. Malik B, Taylor GP. Can we reduce the incidence of adult T-cell leukaemia/lymphoma? Cost-effectiveness of human T-lymphotropic virus type 1 (HTLV-1) antenatal screening in the United Kingdom. Br J Haematol. (2019) 184:1040–3. doi: 10.1111/bjh.15234
20. Maloney EM, Cleghorn FR, Morgan OSC, Rodgers-Johnson P, Cranston B, Jack N, et al. Incidence of HTLV-I-Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP) in Jamaica and Trinidad. J Acquir Immune Defic Syndr Hum Retrovirol. (1998) 17:167–70. doi: 10.1097/00042560-199802010-00011
21. Bartholomew C, Jack N, Edwards J, Charles W, Corbin D, Cleghorn FR, et al. HTLV-I serostatus of mothers of patients with adult T-cell leukemia and HTLV-I-associated myelopathy/tropical spastic paraparesis. J Hum Virol. (1998) 1:302–5.
22. Schierhout G, McGregor S, Gessain A, Einsiedel L, Martinello M, Kaldor J. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis. (2020) 20:133–43. doi: 10.1016/S1473-3099(19)30402-5
23. Kawano N, Nagahiro Y, Yoshida S, Tahara Y, Himeji D, Kuriyama T, et al. Clinical features and treatment outcomes of opportunistic infections among human T-lymphotrophic virus type 1 (HTLV-1) carriers and patients with adult T-cell leukemia-lymphoma (ATL) at a single institution from 2006 to 2016. J Clin Exp Hematop. (2019) 59:156–67. doi: 10.3960/jslrt.18032
24. Abolbashari S, Ghayour-Mobarhan M, Ebrahimi M, Meshkat Z. The role of human T-lymphotropic virus (HTLV) in cardiovascular diseases: a review of literature. ARYA Atheroscler. (2018) 14:183–7. doi: 10.22122/arya.v14i4.1608
25. Verdier M, Denis F, Leonard G, Sangare A, Patillaud S, Prince-David M, et al. Comparison of immunofluorescence, particle agglutination, and enzyme immunoassays for detection of human T-cell leukemia virus type I antibody in African sera. J Clin Microbiol. (1990) 28:1988–93. doi: 10.1128/jcm.28.9.1988-1993.1990
26. Thorstensson R, Albert J, Andersson S. Strategies for diagnosis of HTLV-I and -II. Transfusion. (2002) 42:780–91. doi: 10.1046/j.1537-2995.2002.00114.x
27. Rosadas C, Tosswill JH, Tedder R, Taylor GP. Pregnancy does not adversely impact diagnostic tests for HTLV-1/2 infection. PLoS Negl Trop Dis. (2019) 13:e0007736. doi: 10.1371/journal.pntd.0007736
28. Rosadas C, Taylor GP. Mother-to-child HTLV-1 transmission: unmet research needs. Front Microbiol. (2019) 10:999. doi: 10.3389/fmicb.2019.00999
29. Itabashi K, Miyazawa T, Nerome Y, Sekizawa A, Moriuchi H, Saito S, et al. Issues of infant feeding for postnatal prevention of human T-cell leukemia/lymphoma virus type-1 mother-to-child transmission. Pediatr Int. (2021) 63:284–9. doi: 10.1111/ped.14356
30. Hisada M, Maloney EM, Sawada T, Miley W, Palmer P, Hanchard B, et al. Virus Markers Associated with Vertical Transmission of Human T Lymphotropic Virus Type 1 in Jamaica. Clin Infect Dis. (2002) 34:1551–7. doi: 10.1086/340537
31. Demontis MA, Hilburn S, Taylor GP. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retroviruses. (2013) 29:359–64. doi: 10.1089/aid.2012.0132
32. Paiva AM, Assone T, Haziot MEJ, Smid J, Fonseca LAM, Luiz ODC, et al. Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Sci Rep. (2018) 8:7742. doi: 10.1038/s41598-018-25939-y
33. Gotuzzo E, Moody J, Verdonck K, Cabada M, Gonzalez E, Van Dooren S, et al. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1-associated myelopathy/tropical spastic paraparesis or strongyloidiasis. Rev Panam Salud Publica/Pan Am J Public Heal. (2007) 22:223–30. doi: 10.1590/S1020-49892007000900001
34. Biggar RJ, Ng J, Kim N, Hisada M, Li H, Cranston B, et al. Human leukocyte antigen concordance and the transmission risk via breast-feeding of human T cell lymphotropic virus type I. J Infect Dis. (2006) 193:277–82. doi: 10.1086/498910
35. Ando Y, Saito K, Nakano S, Kakimoto K, Furuki K, Tanigawa T, et al. Bottle-feeding can prevent transmission of htlv-1 from mothers to their babies. J Infect. (1989) 19:25–9. doi: 10.1016/S0163-4453(89)94772-5
36. Takezaki T. Short-term breast-feeding may reduce the risk of vertical transmission of HTLV-I. Leukemia. (1997) 11:60–2.
37. Itabashi K, Miyazawa T. Mother-to-child transmission of human T-cell leukemia virus type 1: mechanisms and nutritional strategies for prevention. Cancers. (2021) 13:4100. doi: 10.3390/cancers13164100
38. World Health Organization, Guideline: Updates on HIV and Infant Feeding (2016). p. 1–57. Available online at: https://apps.who.int/iris/bitstream/handle/10665/246260/9789241549707-eng.pdf
39. Rosadas C, Woo T, Haddow J, Rowan A, Taylor GP. Anti-HTLV-1/2 IgG antibodies in the breastmilk of seropositive mothers. Microorg. (2021) 9:1413. doi: 10.3390/microorganisms9071413
40. Ando Y, Nakano S, Saito K, Shimamoto I, Ichijo M, Toyama T, et al. Prevention of HTLV-I transmission through the breast milk by a freeze-thawing process. NCBI Japanese J Cancer Res. (1986) 77:974–7.
41. Ando Y, Ekuni Y, Matsumoto Y, Nakano S, Saito K, Kakimoto K, et al. Long-term serological outcome of infants who received frozen-thawed milk from human T-lymphotropic virus type-I positive mothers. J Obstet Gynaecol Res. (2004) 30:436–8. doi: 10.1111/j.1447-0756.2004.00227.x
42. Eusebio-Ponce E, Candel FJ, Anguita E. Human T-Cell Lymphotropic Virus Type 1 and associated diseases in Latin America. Trop Med Int Heal. (2019) 24:934–53. doi: 10.1111/tmi.13278
43. Bittencourt AL. Vertical transmission of HTLV-I/II: a review. Rev Inst Med Trop Sào Paulo. (1998) 40:245–51. doi: 10.1590/S0036-46651998000400008
44. Tezuka K, Fuchi N, Okuma K, Tsukiyama T, Miura S, Hasegawa Y, et al. HTLV-1 targets human placental trophoblasts in seropositive pregnant women. J Clin Invest. (2020) 130:6171–86. doi: 10.1172/JCI135525
45. Bohiltea R, Turcan N, Berceanu C, Munteanu O, Georgescu T, Ducu I, et al. Implications of human T-lymphotropic virus in pregnancy: A case report and a review of the diagnostic criteria and management proposal. Exp Ther Med. (2020) 21:1–1. doi: 10.3892/etm.2020.9514
46. Matsushita S, Mitsuya H, Reitz MS, Broder S. Pharmacological inhibition of in vitro infectivity of human T lymphotropic virus type I. J Clin Invest. (1987) 80:394–400. doi: 10.1172/JCI113085
47. Isono T, Ogawa K, Seto A. Antiviral effect of zidovudine in the experimental model of adult T cell leukemia in rabbits. Leuk Res. (1990) 14:841–7. doi: 10.1016/0145-2126(90)90172-6
48. Cook LB, Fuji S, Hermine O, Bazarbachi A, Ramos JC, Ratner L, et al. Revised adult T-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol. (2019) 37:677–87. doi: 10.1200/JCO.18.00501
49. Dearden CE, Johnson R, Pettengell R, Devereux S, Cwynarski K, Whittaker S, et al. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma). Br J Haematol. (2011) 153:451–85. doi: 10.1111/j.1365-2141.2011.08651.x
50. Seegulam ME, Ratner L. Integrase inhibitors effective against human T-cell leukemia virus type 1. Antimicrob Agents Chemother. (2011) 55:2011–7. doi: 10.1128/AAC.01413-10
51. Machuca A, Rodés B, Soriano V. The effect of antiretroviral therapy on HTLV infection. Virus Res. (2001) 78:93–100. doi: 10.1016/S0168-1702(01)00287-8
52. Pasquier A, Alais S, Roux L, Thoulouze MI, Alvarez K, Journo C, et al. How to control HTLV-1-associated diseases: Preventing de novo cellular infection using antiviral therapy. Front Microbiol. (2018). 9:278. doi: 10.3389/fmicb.2018.00278
53. Trevino A, Parra P, Bar-Magen T, Garrido C, de Mendoza C, Soriano V. Antiviral effect of raltegravir on HTLV-1 carriers. J Antimicrob Chemother. (2012) 67:218–21. doi: 10.1093/jac/dkr404
54. Derse D, Hill SA, Lloyd PA, Chung H, Morse BA. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J Virol. (2001) 75:8461–8. doi: 10.1128/JVI.75.18.8461-8468.2001
55. Taylor GP, Hall SE, Navarrete S, Michie CA, Davis R, Witkover AD, et al. Effect of lamivudine on human T-cell leukemia virus type 1 (HTLV-1) DNA copy number, T-cell phenotype, and anti-tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J Virol. (1999) 73:10289–95. doi: 10.1128/JVI.73.12.10289-10295.1999
56. Fujii H, Shimizu M, Miyagi T, Kunihiro M, Tanaka R, Takahashi Y, et al. A potential of an Anti-HTLV-I gp46 neutralizing monoclonal antibody (LAT-27) for passive immunization against both horizontal and mother-to-child vertical infection with human T cell leukemia virus type-I. Viruses. (2016) 8:41. doi: 10.3390/v8020041
Keywords: HTLV-1, pregnancy, neonate, antiretrovirals, adult T cell lymphoma/leukemia, prevention of mother-to-child transmission
Citation: Barr RS, Drysdale SB, Boullier M, Lyall H, Cook L, Collins GP, Kelly DF, Phelan L and Taylor GP (2022) A Review of the Prevention of Mother-to-Child Transmission of Human T-Cell Lymphotrophic Virus Type 1 (HTLV-1) With a Proposed Management Algorithm. Front. Med. 9:941647. doi: 10.3389/fmed.2022.941647
Received: 11 May 2022; Accepted: 10 June 2022;
Published: 08 July 2022.
Edited by:
Juarez Antonio Simões Quaresma, Universidade Do Estado Do Pará, BrazilReviewed by:
Masakazu Tanaka, Kagoshima University, JapanCopyright © 2022 Barr, Drysdale, Boullier, Lyall, Cook, Collins, Kelly, Phelan and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rachael S. Barr, UmFjaGFlbC5iYXJyQGJyaXN0b2wuYWMudWs=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.