
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med., 20 June 2022
Sec. Gastroenterology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.939424
This article is part of the Research TopicRecent Advances in Enhancing Chemotherapeutic Efficacy of Colorectal CancerView all 10 articles
As the most common gastrointestinal malignancy, colorectal cancer (CRC) remains a leading cause of cancer death worldwide. Although multimodal chemotherapy has effectively improved the prognosis of patients with CRC in recent years, severe chemotherapy-associated side effects and chemoresistance still greatly impair efficacy and limit its clinical application. In response to these challenges, an increasing number of traditional Chinese medicines have been used as synergistic agents for CRC administration. In particular, ginseng, quercetin, and tea, three common dietary supplements, have been shown to possess the potent capacity of enhancing the sensitivity of various chemotherapy drugs and reducing their side effects. Ginseng, also named “the king of herbs”, contains a great variety of anti-cancer compounds, among which ginsenosides are the most abundant and major research objects of various anti-tumor studies. Quercetin is a flavonoid and has been detected in multiple common foods, which possesses a wide range of pharmacological properties, especially with stronger anti-cancer and anti-inflammatory effects. As one of the most consumed beverages, tea has become particularly prevalent in both West and East in recent years. Tea and its major extracts, such as catechins and various constituents, were capable of significantly improving life quality and exerting anti-cancer effects both in vivo and in vitro. In this review, we mainly focused on the adjunctive effects of the three herbs and their constituents on the chemotherapy process of CRC.
Colorectal cancer (CRC) is the third most common cancer globally and one of the leading causes of health burden on society (1). The latest epidemiological data show that the incidence of CRC is rapidly increasing year by year, and the number of young patients aged 20–40 years old has increased quickly (2). For early-stage CRC, surgical resection of the primary tumor is the main treatment method, and adjuvant chemotherapy can prolong the survival times of patients (3, 4). In terms of advanced CRC or metastatic CRC, the survival rate is less than 10%, and the primary treatment strategies include radiotherapy and chemotherapy. CRC represents a heterogeneous disease with distinct disease mechanisms and prognoses.
It has been confirmed that multiple factors were involved in CRC development and progression, such as genetic alterations, gut microbiota, chronic inflammation, environmental influence, and others (1, 3). However, the exact mechanisms underlying the onset of colorectal cancer are still unknown. With the development of precision medicine and personalized medicine, chemotherapy plays an increasingly important role in CRC administration. Especially for advanced patients, chemotherapy offers the only possibility of a cure. However, the clinical application of chemotherapeutic regimens is mainly limited by their side effects and toxicity. Therefore, urgent research is needed to discover more adjuvant chemotherapy compounds to enhance the tumoricidal effects at low doses (5).
Over the last decades, traditional herbal medicines have been widely utilized for modern drug development. More and more studies have indicated that a daily intake of these herbal products could improve the life quality of patients (6, 7). Notably, a growing body of research suggests that traditional Chinese herbal can be regarded as effective adjuvant chemotherapy agents for improving the efficacy of cancer chemotherapy. In this review, ginseng, quercetin, and tea are the main research objects. The reasons why we have focused on these herbs are described as follows. First, these herbs are the most widely used traditional herbal medicines both in the East and West, and their beneficial effects have been extensively advertised. Another reason that has led us to pick these phytochemicals is that they are common in dietary supplements and have been confirmed to improve the life quality of hosts. The last and most important reason is that their multiple pharmacological properties, such as anti-oxidant, anti-inflammatory, and anti-cancer properties, have been widely recognized. Based on the above findings, we reviewed a large body of literature and concluded that all these herbs could effectively improve the effects of CRC chemotherapy. It should be noted that the chemical and pharmacological properties of ginseng, quercetin, and tea are completely independent of each other. Therefore, to avoid confusion, we discussed their properties and functions in great detail separately, as shown in Figure 1.
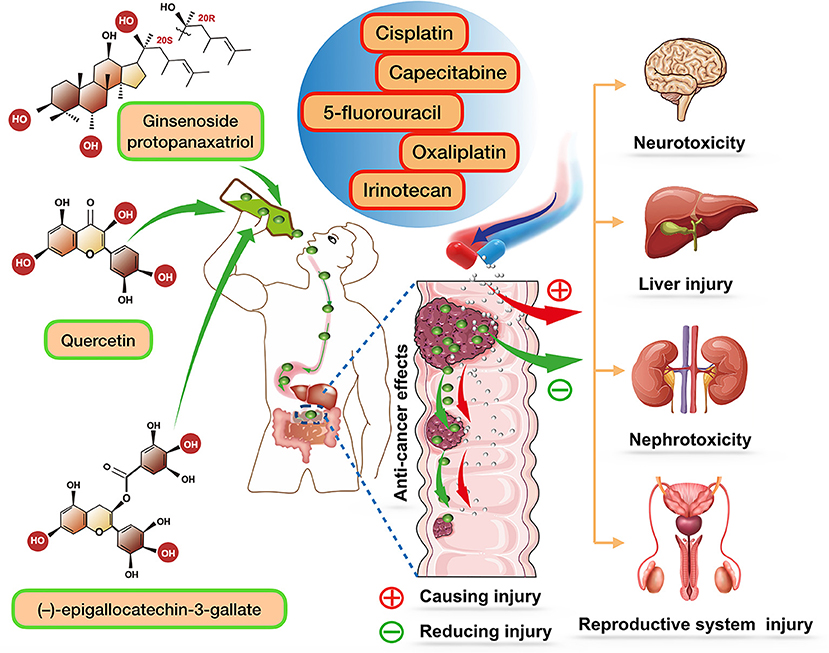
Figure 1. The role of ginsenoside protopanaxatriol (one major extract of ginseng), quercetin, and (–)-epigallocatechin-3-gallate (one major extract of tea) in enhancing chemotherapeutic efficacy of various chemotherapy drugs, and together reducing their side effects. After oral administration of these compounds, they can be biotransformed to stronger components and play a synergistic role with various chemotherapy drugs.
With improvements in CRC treatment, multiple chemotherapeutic agents have been used in routine clinical practice; the chemotherapeutic agents, mainly including 5-fluorouracil (5-FU), irinotecan, oxaliplatin, and capecitabine, can be used either alone or in combination with each other, (8). Among them, 5-FU has historically been considered the foundation of the therapy for CRC, which has been used in clinical treatment for more than 60 years (9). An increasing number of clinical trials have demonstrated that 5-FU administered alone or in combination with other chemotherapeutic agents can significantly improve the survival rate of patients with CRC (10, 11). The response rate of 5-FU administered alone is only approximately 10–15% (12). However, combining 5-FU with other chemotherapeutic agents can effectively enhance curative effects and has been regarded as the first routine clinical practice. For example, leucovorin, a folinic acid derivative, can enhance the therapeutic response rate to 37% by suppressing the activation of thymidylate synthase (13). However, like many other common chemotherapy drugs, 5-FU also has many side effects, mainly including leukopenia, nausea, vomiting, hematopoietic depression, bone marrow suppression, neurotoxicity, and cardiotoxicity (14). In particular, leukopenia has been reckoned major dose-limiting toxicity of 5-FU administration occurs in approximately 93% of patients (15). In recent years, with a deepening understanding of drug properties, 5-FU has also shown stronger anti-cancer efficacy in clinical combinations with new-generation chemotherapy drugs. In conclusion, although 5-FU is an essential agent for treating both advanced and early-stage patients with CRC, its side effects cannot be ignored. Therefore, it is necessary to overcome these therapeutic challenges.
Capecitabine, an oral 5-FU prodrug that has been used in treating CRC for 20 years, can be enzymatically transformed into 5-FU at colorectal tumor sites after oral administration (16). Moreover, it has been demonstrated that even along administration of capecitabine exerts stronger chemotherapy effects and lower incidence of side effects than combined administration of 5-FU and leucovorin (17). However, capecitabine also has deficiencies, in particular the significantly increased incidence of the hand-foot syndrome and hyperbilirubinemia (18). As the most commonly used chemotherapy drugs for various malignant diseases, platinum-based agents have also been used in CRC treatment. Oxaliplatin, a third-generation platinum anti-cancer agent, is also a novel first-line treatment for metastatic CRC. It can inhibit the growth of tumor cells by inducing the formation of platinum-DNA adducts and eliciting a DNA damage response (19). The typical side effects include hematologic toxicity, gastrointestinal symptoms, and peripheral neuropathy (20).
Irinotecan is approved as second-line therapy for treating advanced/metastatic CRC, especially for patients who do not respond to the first-line 5-FU therapy (21). Its active metabolite SN-38, a camptothecin-based agent, can promote DNA damage and tumor cell apoptosis by binding with topoisomerase I, an important mediator of DNA transcription (22). The most common side effects of irinotecan treatment include myelosuppression, delayed-type diarrhea, cholinergic syndrome, vomiting, constipation, and neutropenia (23, 24). As shown above, each chemotherapeutic agent has its own properties and side effects. Over the past decade, sequential combination therapy with multiple chemotherapeutics has been the most standard chemotherapeutic treatment for CRC management; this therapy can promote the synergy of different agents and improve chemotherapy resistance using different action mechanisms (8, 25). For instance, the combined administration of oxaliplatin and irinotecan can be used as a salvage therapy for patients failing to respond to single-agent 5-FU treatment and is a first-line sequential treatment option for advanced CRC (26, 27). Although chemotherapeutic therapies have greatly improved the outcomes of patients with CRC, serious side effects and drug resistance are still major clinical challenges. In recent years, more and more drugs, especially traditional Chinese medicines, have been used to alleviate various side effects and improve chemoresistance.
Ginseng is one of the most common traditional herbal medicines, which has been discovered in both East (Asian ginseng) and West (American ginseng) (28, 29). With the recent developments in the extraction process, multiple active components have been isolated from ginseng, mainly including ginsenosides, ginseng polysaccharides, flavonoids, polysaccharides, and ginseng polypeptides (30). Since ancient times, ginseng has been found to possess multiple pharmacological effects and has been used to treat various diseases, such as inflammation, cancers, metabolic syndromes, and autoimmune diseases. The anti-cancer effect of ginseng has attracted increasing interest and attention in the fields of various cancers, including ovarian cancer, CRC, breast cancer, lung cancer, prostate cancer, and liver cancer (31–34). Many in vitro and in vivo studies have demonstrated that ginseng or its extracts could significantly decrease the incidence of CRC and inhibit tumor growth (35). For example, Rg3, one of the most abundant and active ginsenosides can effectively inhibit the proliferation of CRC cells by suppressing the activity of the C/EBPβ/NF-κB signaling pathway (36). Similarly, another study also reported that ginsenoside Rg3 could inhibit the proliferation, migration and invasion of CRC cells and promote the apoptosis of these tumor cells by downregulating the expression of lncRNA CCAT1 (37). Other chemical compounds extracted from ginseng, such as flavonoids and polysaccharides, have been confirmed to have anti-CRC effects (38, 39). Recent studies further proposed that ginseng and its various constituents could improve the status of patients with CRC by increasing the efficiency of chemotherapy drugs (40, 41).
Ginseng and its extracts have great potential as chemotherapy adjuvant agents due to their low toxicity and strong anti-cancer properties (42). In particular, ginseng or its active components can enhance the sensitivity of chemotherapy and reduce its side effects. For instance, Fishbein et al. proposed that Asian ginseng could improve the anti-cancer function of 5-FU on HCT-116 human CRC cells (43). In addition, Panax notoginseng root extract, a remedy anti-cancer medicine, can also improve the chemopreventive functions of 5-FU and irinotecan in experiments in vitro (SW480) (44). These results are consistent with previous studies that notoginseng can enhance tumor radiosensitivity to the cytotoxic effect of ionizing radiation (45). In addition, another study reported that Panax notoginseng could increase the anti-proliferative ability of 5-FU on HCT-116 cells and significantly decrease the dosage of 5-FU required by CRC administration (46). Moreover, Li et al. reported that American ginseng berry extract could enhance the chemopreventive effect of 5-FU during CRC treatment both in vivo and in vitro (SW480, HCT-116 and HT-29), possibly by increasing cell arrest at S and G2/M phases (47).
Nausea and vomiting may be the most common adverse events in cancer chemotherapy treatment. For patients with oxaliplatin-based regimens, the incidence of nausea and vomiting is more than 70% (48). Previous studies reported that Korean red ginseng total extract could effectively attenuate cisplatin-induced nausea and vomiting in a ferret model (49). Further studies proposed that the anti-emetic effect of ginseng or its extracts was achieved by the antagonism of the 5-HT 3A receptor (50, 51). In a recent clinical trial, scholars investigated the curative effect of ginseng on nausea and vomiting induced by oxaliplatin-based regimens during CRC treatment, and they found that the administration of ginseng combined with some traditional medicines was capable of suppressing nausea and vomiting (52). In a randomized clinical phase III trial, Kim et al. proposed that Korean red ginseng administration could alleviate cancer-related fatigue in CRC patients with chemotherapy (53). Cancer-related fatigue, a common side effect of cancer chemotherapy treatment is a subjective physical feeling and can interfere with the sleep, mood, concentration, work, and daily life quality of patients (54). In this trial, 219 patients with mFOLFOX-6 administration chemotherapy were included in the Korean red ginseng treatment group, and other 219 patients treated with placebos were included in the control group. After 16-week administration, the results showed that Korean red ginseng treatment effectively improved fatigue, inhibited deterioration of fatigue-related life quality, and reduced the stress of these CRC patients receiving chemotherapy.
Panaxadiol (PD), a diol-type ginsenoside derived from Panax ginseng or Panax pseudoginseng can also enhance the anti-cancer effects of 5-FU on CRC (55). The results showed that the combined administration of 5-FU and PD significantly exerted stronger anti-proliferative and pro-apoptotic abilities in the HCT-116 human CRC cell line than treatment with 5-FU alone. These results are consistent with a previous clinical study (56). Moreover, another in vitro study (HCT-116 and SW480) showed that PD could also enhance the anti-cancer effects of irinotecan, which might be achieved via inducing tumor cell apoptosis (57). This synergistic administration can effectively reduce the dose of irinotecan and the rate of side effects, indicating that some natural products are beneficial for CRC chemoadjuvant treatment.
Ginsenoside Rg3, a tetracyclic triterpenoid saponin with strong anti-cancer properties can inhibit the proliferation, invasion and migration of various tumors (58). For instance, one study reported that Rg3 could block the progression of colon cancer and promote the apoptosis of HT-29 colon cells by inhibiting the stemness of cancer stem cells, reducing tumor angiogenesis, and upregulating the AMPK pathway (59). In recent studies, scholars further proposed that Rg3 administration could significantly enhance the anti-cancer function of 5-FU both in vivo and in vitro (SW620 and LOVO) (60). After treatment with Rg3 and 5-FU together, this synergistic therapy was found to effectively suppress the proliferation, development and metastasis of tumors by activating the PI3K/Akt signaling pathway.
Protopanaxadiol (PPD), a secondary ginsenoside induced by a gut microbiome, can be bio-transformed by intestinal flora from ginseng extracts such as Rb1 and compound K (61, 62). According to a recent study, in addition to being able to inhibit tumor development directly, PPD can effectively enhance the effects of 5-FU on patients with CRC (62). It was found that the co-administration of PPD and 5-FU exerted stronger anti-proliferative and pro-apoptotic effects on HCT-116 human CRC cells than PPD or 5-FU alone treatment. A further in vivo experiment also confirmed that this co-administration could markedly reduce the tumor size in a dose-related manner.
Quercetin (3, 3′, 4′, 5, 7-pentahydroxyflavone), a well-studied flavonoid in various vegetables and fruits is easily dissolved in the glacial acetic acid and aqueous solution (63). Hydrophilic glycoside, one of the most common constituents of quercetin extracts, cannot be directly absorbed by the host body and has to be transformed into quercetin metabolites by interacting with intestinal flora and key enzymes in digestive systems (64). Multiple pharmacological effects, including anti-inflammatory, anti-oxidative, anti-atherosclerosis, and anti-cancer effects, have been discovered in quercetin or its extracts (65). Further study demonstrated that quercetin could exert anti-cancer effects through various mechanisms, including inhibiting the activity of tyrosine kinase, regulating pathways involved in tumorigenesis, and interacting with specific proteins or receptors (66). It was found that quercetin and its derivatives could effectively inhibit tumor initiation and progression in both in vivo and in vitro CRC models (67). The molecular mechanisms are very complex and incompletely understood. According to previous studies, multiple signaling pathways were involved in the anti-cancer processes, such as Wnt/β-catenin, MAPK/JNK, NF-κB, and other related pathways (67). For instance, quercetin was reported to suppress the growth of multiple CRC cell lines (such as HT-29, Caco-2, DLD-1, and HCT-15) by blocking the activity of the AKT pathway (68–70). Another study further indicated that 3, 4-dihydroxyphenylacetic acid, a major derivative of quercetin, was capable of exerting CRC protective effects by reducing reactive oxygen species responses (71). In addition, recent studies reported that quercetin could be used as an effectively adjuvant chemotherapy agent for various cancer administration (65, 72–74). Especially in CRC chemotherapy, the synergistic effect of polyphenols has achieved relatively good potentiating effects.
A study in 1994 first reported that the combined administration of quercetin and 5-FU could significantly inhibit the growth of CRC cell line COLO 320DM cells (75). Recent studies further indicated that quercetin could increase the bioavailability of drugs by regulating the expression of key proteins associated with the development of drug resistance (76). Based on the above findings, Atashpour et al. proposed that quercetin treatment could enhance the cytotoxicity and apoptosis induction of doxorubicin in CRC stem cells and HT-29 cells by arresting tumor cells at the G2/M phase (77). Moreover, Han et al. reported that quercetin pretreatment could significantly promote the apoptosis of HT-29 cells induced by cisplatin, thus improving the anti-cancer functions of cisplatin during CRC administration (78). Further studies found that the combination of quercetin and cisplatin could directly activate the NF-κB signaling pathway to suppress cell proliferation and induce apoptosis (78). A recent study suggested that the combination of quercetin and luteolin, a member of the flavone group of flavonoids, could effectively increase the anti-cancer functions of 5-FU in HT-29 cells (79). Compared with the control group, this combination exerted stronger anti-proliferative and pro-apoptotic effects. This phenomenon was caused by suppressing angiogenesis and vasculogenesis. This combination modulated the apoptotic pathways and minimized the toxic effects of 5-FU.
P-glycoprotein-mediated multidrug resistance has been considered one of the most fundamental factors of cancer chemotherapy. Quercetin has been regarded as an inhibitor of P-glycoprotein-mediated multidrug resistance, which can overcome CRC resistance to chemotherapy via molecular mechanisms. For example, Zhou et al. reported that quercetin could effectively increase the cytotoxicity of doxorubicin to P-glycoprotein-overexpressed SW620/Ad300 cells by blocking D-glutamate metabolism and reducing the solute carrier family 1 member 5 (80). The CRC with microsatellite instability is resistant to 5-FU administration, which remains a clinical challenge. Xavier et al. first reported that quercetin treatment could effectively enhance the sensitivity of 5-FU on CO-115 and HCT-15 cells (81). After treatment with quercetin and 5-FU together, they found that the ratio of apoptotic cells significantly increased, which might be caused by special activation of the mitochondrial pathway.
Quercetin has been recognized as the most representative drug of flavonoids. In addition to quercetin, other flavonoids, such as epigallocatechin-3 gallate and isoflavone genistein, also possess chemopreventive properties (82, 83). Howells et al. further investigated whether the chemical modification of flavonol structures could enhance the pharmacological and toxicological properties of other flavonoids. They hypothesized that a flavonol molecule had no hydroxyl group on the A ring and only methoxyl groups on the B ring, which might possess cancer chemopreventive efficacy (84). To test this hypothesis, they produced a new compound, 3′, 4′, 5′-trimethoxyflavonol, a quercetin analog. Then, they compared the preclinical cancer chemopreventive properties of the new compound with those of two naturally flavonol congeners, quercetin and fisetin, in vivo (human-derived HCT-116 adenocarcinoma-bearing nude mice) and in vitro (APC10.1 cells derived from adenomas of ApcMin mice). The result showed that the synthesized 3′, 4′, 5′-trimethoxyflavonol could significantly inhibit tumor proliferation and promote apoptosis by increasing wild-type p53 expression in two mouse models. The above studies also demonstrated that chemical modification might be an effective way to generate safe and efficacious cancer chemopreventive agents.
Moreover, one study reported that quercetin treatment could also effectively enhance the radio sensitivity of CRC in addition to improving the chemotherapy sensitivity. They found that the pretreatment of quercetin enabled colorectal cells to be more sensitive to radiotherapy by downregulating the ataxia–telangiectasia-mutated-related signaling pathways and promoting irradiation-induced γ-H2AX and 53BP1 focus formation (85). A recent study indicated that the combination of quercetin and ionizing radiation could have greater therapeutic potential for CRC, which is consistent with the above results (86). The detailed mechanism included directly suppressing the Notch-1 signaling pathway and targeting colon cancer stem cells, one group of rare immortal cells involved in radiation therapy resistance.
Tea is a commonly consumed beverage derived from the leaves and leaf buds of the Camellia sinensis. Tea has been studied extensively in health and disease fields, such as preventing hypertension and cardiovascular diseases, reducing obesity, treating metabolic syndromes, mediating gut microbiotas, and preventing and treating cancers (87). There are many types of tea, such as black tea, green tea, Pu-erh tea, white tea, yellow tea, oolong tea, and dark tea, all of which were produced via different methods (88). For instance, green tea, also named non-fermented tea is produced from dried green tea leaves. Black tea also named most or fully fermented tea is obtained from extensively solid-state fermentation involving microorganisms. The partially fermented tea is named oolong tea (89). In the last few years, various chemical components, mainly including catechin derivatives, polysaccharides, pigments, theophylline, glycosides, phenolic acids, and alkaloids have been isolated from tea. Catechins, such as (–)-epigallocatechin-3-gallate (EGCG), (–)-epigallocatechin (EGC), (–)-epicatechin-3-gallate (ECG), and (–)-epicatechin, have been studied extensively in cancer prevention and administration. Previous studies reported that tea and its components could exert anti-cancer effects through various signaling and metabolic mechanisms, such as inhibiting tumorigenesis, promoting apoptosis, regulating proliferation transformation, and targeting key transmembrane receptors or kinases (87). It has been confirmed that EGCG could inhibit CRC initiation and progression by reducing oxidative reaction and promoting tumor cell apoptosis (90). Some meta-analyses also showed that tea consumption was closely associated with CRC risk (91, 92). Based on these results, some scholars further proposed that tea and tea polyphenols could be used as promising chemopreventive agents for CRC treatment (93).
(–)-epigallocatechin-3-gallate is a major green tea polyphenol and is regarded as an important tumor inhibitor in various cancers (94). It has been shown that the combinations of EGCG and other catechins can exert relatively strong anti-cancer effects in both in vitro and in vivo experiments (95). Some studies indicated that EGCG could improve chemoresistance and reduce tumor recurrence. For instance, Toden et al. found that EGCG treatment could sensitize chemoresistant CRC cells (HCT-116 and SW480) to standard 5-FU administration. Specific chemopreventive activities include increasing 5-FU-induced cytotoxicity and suppressing the growth of tumor cells by triggering apoptosis and promoting cell cycle arrest (96). La et al. reported that EGCG administration could effectively increase the chemosensitivity of 5-FU in HCT-116 and DLD1 cell lines by suppressing tumor growth, promoting apoptosis, and causing DNA damage, which is consistent with the above results (97). According to further mechanistic studies, EGCG can upregulate the expression of NF-κB and miR-155-5p by blocking GRP78 activity, further suppressing the protein expression of MDR1 and increasing the 5-FU accumulation in CRC cells. In another study, Shimizu et al. proposed that EGCG could exert chemopreventive effects by inhibiting the activity of signaling pathways related to receptor tyrosine kinases, such as EGFR, IGF-1R, and VEGFR2 signaling pathways (98). Moreover, combining EGCG with cisplatin or oxaliplatin could significantly inhibit the proliferation of DLD-1 and HT-29 cells and reduce cytotoxic effects by regulating autophagy-related signaling pathways (93).
Irinotecan is a common DNA-damaging chemotherapeutic agent for CRC treatment, the use of which is limited by its low solubility and high toxicity. Combined with the previous studies, they found that the co-administration of EGCG and Gefitinib or Bleomycin could reduce their dose and resistance (99, 100). Wu et al. further investigated the synergy of EGCG and irinotecan on CRC treatment (101). They treated CRC cells RKO and HCT116 with EGCG and irinotecan together, and the results showed that the combined administration exerted relatively strong inhibitory effects on the proliferation, migration, and invasion of tumor cells. The specific molecular mechanism includes inducing S- or G2-phase arrest and causing more extensive DNA damage. Moreover, a study reported that EGCG and EGC could increase the chemosensitivity of low-dose doxorubicin both in vivo and in vitro (SW620) by blocking the activation of protein kinase C, a drug resistance-related protein (102). According to some studies, in addition to directly improving chemotherapy responses, tea nanoparticles can be used to deliver chemotherapeutic agents for cancer treatment. For instance, Wang et al. proposed that tea nanoparticles, a safe nanocarrier with good biocompatibility and low toxicity could load doxorubicin into tumors, thus enhancing its intertumoral accumulation and improving its chemotherapy efficacy in an animal study (103).
The view that drinking tea can prevent cancer has been proposed for many years. For instance, Shimizu et al. reported that consuming proper green tea every day could inhibit the recurrence of CRC (104). Another study showed that green tea catechins could prevent CRC through multiple molecular mechanisms, including decreasing detergent-insoluble membrane domain, inhibiting the activity of the specific receptor tyrosine kinases (such as EGFR, IGF-1R, and VEGFR-2), and reducing the expression of hypoxia-inducible factor 1a (HIF1a), IGF-1, IGF2, and EGF (98, 105). In a randomized controlled trial, Henning et al. proposed that tea polyphenols could be transformed into phenolic metabolites by the colonic microflora, thus playing a significant role in CRC prevention (106). Ku-jin tea, a very popular beverage in the world, is an essential anti-inflammatory and anti-oxidative regulator and can also play chemopreventive effects on CRC. In a CRC rat model induced by azoxymethane, Bi et al. found that long-term treatment with Ku-jin tea could significantly decrease the number of aberrant crypts, aberrant crypt foci (ACF), and crypts/foci in rats through regulating metabolism-associated pathways, further indicating that Ku-jin tea can be used as a promising chemopreventive agent for CRC chemoprevention (107).
The synergistic therapy of herbal medicines combined with chemotherapy may revolutionize cancer treatment. With the development of precision medicine, chemotherapy has played an increasingly important role in clinical cancer treatment (108, 109). Especially for CRC, various chemotherapeutic regimens have been proposed and have achieved remarkable clinical efficacy. For example, for lymph node-positive patients, the FOLFOX regimen (5-FU, leucovorin, and oxaliplatin) is recommended (110). In terms of locally advanced rectal cancer, neoadjuvant chemoradiation therapy with 5-FU and radiation therapy should be considered for the patients. For patients with metastatic CRC, FOLFOX, or FOLFIRI (5-FU, leucovorin, and irinotecan) regimens are recommended as standard first-line treatment choices (111).
Traditional herbal medicines, exerting huge therapeutic potential in various diseases, are promising adjuvant chemotherapy agents. In this review, to allow readers to quickly know this field, we selected the three most studied herbs (ginseng, quercetin, and tea) as representative drugs to conclude their synergies in CRC chemotherapy administration (Table 1). By summing up the points, we discovered that most studies were focused on investigating the synergistic effects of the three herbs on 5-FU, the most commonly used chemotherapy drug for CRC treatment. As expected, we found that all the three herbs and their major extracts could significantly enhance the chemopreventive functions of 5-Fu and reduce its side effects. In terms of ginseng, there are three most common species, including Asian ginseng, American ginseng, and Panax notoginseng, all of which have been confirmed to possess synergistic effects. Their major components, such as ginsenoside Rg3, PPT, and PPD, also possess stronger pharmacological and biological effects. In addition, the synergistic effects of them and other chemotherapy drugs, such as irinotecan, have also been studied both in vivo and in vitro. Quercitrin is commonly found in plant foods used to treat various diseases, especially, which has been identified to be an effectively antitumor agent. Herein, we have summarized that quercetin treatment could enhance the cytotoxicity of 5-FU, cisplatin, and doxorubicin by regulating different molecular mechanisms. The research on tea was mainly focused on EGCG, a major tea polyphenol, which can improve the chemotherapy efficacy of 5-FU, irinotecan, cisplatin, and oxaliplatin and play chemopreventive effects both in vivo and in vitro. However, its detailed mechanisms, mainly including promoting proliferation and inhibiting apoptosis by regulating related signaling pathways, are not fully understood.
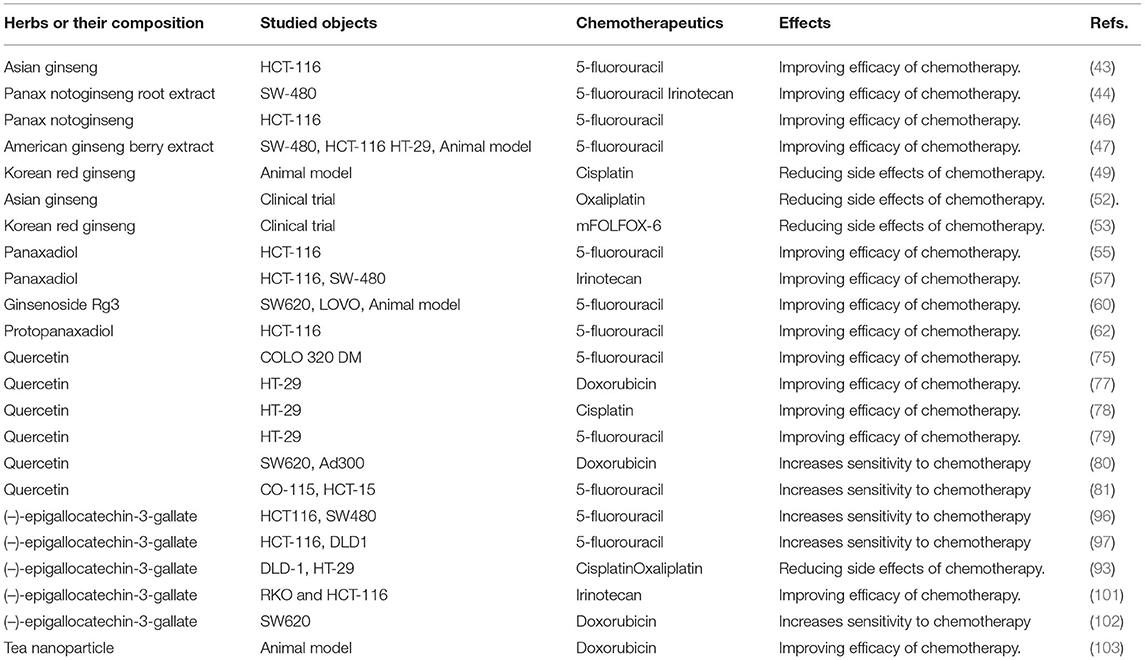
Table 1. The synergistic effects of ginseng, quercetin, and tea on chemotherapy treatment of colorectal cancer.
Although many preclinical studies have been performed, the clinical applications of these herbs are still limited due to too many unknown variables. In the future, an increasing number of studies should be performed to clarify specific mechanisms and develop more effective chemopreventive agents for CRC administration.
LZ drafted the review. HZ and MS generated the graphs. NL guided the construction of the manuscript. YZ edited the review. KZ, JL, and PL provided input on the scope and content of the review. All the authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRC, colorectal cancer; 5-FU, 5-fluorouracil; PD, panaxadiol; PPD, protopanaxadiol; EGCG, (–)-epigallocatechin-3-gallate; EGC, (–)-epigallocatechin; ECG, (–)-epicatechin-3-gallate.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. (2019) 68:1820–6. doi: 10.1136/gutjnl-2018-317592
3. Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. Bmj. (2021) 374:n1855. doi: 10.1136/bmj.n1855
4. Kim JH. Chemotherapy for colorectal cancer in the elderly. World J Gastroenterol. (2015) 21:5158–66. doi: 10.3748/wjg.v21.i17.5158
5. Printz C. Triple chemotherapy combination improves metastatic colorectal cancer outcomes. Cancer. (2021) 127:1547. doi: 10.1002/cncr.33608
6. Aiello A, Accardi G, Candore G, Carruba G, Davinelli S, Passarino G, et al. Nutrigerontology: a key for achieving successful ageing and longevity. Immun Ageing. (2016) 13:17. doi: 10.1186/s12979-016-0071-2
7. Corbi G, Conti V, Davinelli S, Scapagnini G, Filippelli A, Ferrara N. Dietary phytochemicals in neuroimmunoaging: a new therapeutic possibility for humans? Front Pharmacol. (2016) 7:364. doi: 10.3389/fphar.2016.00364
8. McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem. (2017) 24:1537–57. doi: 10.2174/0929867324666170111152436
9. Bertino JR. Chemotherapy of colorectal cancer: history and new themes. Semin Oncol. 1997;24(5 Suppl 18):S18-3-s-7.
10. Panettiere FJ, Goodman PJ, Costanzi JJ, Cruz AB, Vaitkevicius VK, McCracken JD, et al. Adjuvant therapy in large bowel adenocarcinoma: long-term results of a Southwest Oncology Group Study. J Clin Oncol. (1988) 6:947–54. doi: 10.1200/JCO.1988.6.6.947
11. Kishore C, Bhadra P. Current advancements and future perspectives of immunotherapy in colorectal cancer research. Eur J Pharmacol. (2021) 893:173819. doi: 10.1016/j.ejphar.2020.173819
12. O'Connell MJ. A phase III trial of 5-fluorouracil and leucovorin in the treatment of advanced colorectal cancer. A Mayo Clinic/North Central Cancer Treatment Group study. Cancer. (1989) 63:1026–30. doi: 10.1002/1097-0142(19890315)63:6+<1026::AID-CNCR2820631307>3.0.CO;2-R
13. Petrelli N, Herrera L, Rustum Y, Burke P, Creaven P, Stulc J, et al. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol. (1987) 5:1559–65. doi: 10.1200/JCO.1987.5.10.1559
14. Kennedy BJ. 5-fluorouracil toxicity: old or new? Cancer. (1999) 86:1099–100. doi: 10.1002/(SICI)1097-0142(19991001)86:7<1099::AID-CNCR1>3.0.CO;2-X
15. Petrelli N, Douglass HO, Herrera L, Russell D, Stablein DM, Bruckner HW, et al. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial Gastrointestinal Tumor Study Group. J Clin Oncol. (1989) 7:1419–26. doi: 10.1200/JCO.1989.7.10.1419
16. Schüller J, Cassidy J, Dumont E, Roos B, Durston S, Banken L, et al. Preferential activation of capecitabine in tumor following oral administration to colorectal cancer patients. Cancer Chemother Pharmacol. (2000) 45:291–7. doi: 10.1007/s002800050043
17. Van Cutsem E, Hoff PM, Harper P, Bukowski RM, Cunningham D, Dufour P, et al. Oral capecitabine vs intravenous 5-fluorouracil and leucovorin: integrated efficacy data and novel analyses from two large, randomised, phase III trials. Br J Cancer. (2004) 90:1190–7. doi: 10.1038/sj.bjc.6601676
18. Hoff PM, Ansari R, Batist G, Cox J, Kocha W, Kuperminc M, et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: results of a randomized phase III study. J Clin Oncol. (2001) 19:2282–92. doi: 10.1200/JCO.2001.19.8.2282
19. Bruno PM, Liu Y, Park GY, Murai J, Koch CE, Eisen TJ, et al. A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med. (2017) 23:461–71. doi: 10.1038/nm.4291
20. Amatu A, Mauri G, Tosi F, Bencardino K, Bonazzina E, Gori V, et al. Efficacy of retreatment with oxaliplatin-based regimens in metastatic colorectal cancer patients: the RETROX-CRC retrospective study. Cancers. (2022) 14:1548. doi: 10.3390/cancers14051197
21. Boven E, Massard C, Armand JP, Tillier C, Hartog V, Brega NM, et al. A phase I, dose-finding study of sunitinib in combination with irinotecan in patients with advanced solid tumours. Br J Cancer. (2010) 103:993–1000. doi: 10.1038/sj.bjc.6605852
22. Xu Y, Villalona-Calero MA. Irinotecan: mechanisms of tumor resistance and novel strategies for modulating its activity. Ann Oncol. (2002) 13:1841–51. doi: 10.1093/annonc/mdf337
23. Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol. (2004) 22:1382–8. doi: 10.1200/JCO.2004.07.173
24. Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, et al. Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet. (1998) 352:1407–12. doi: 10.1016/S0140-6736(98)03085-2
25. Folprecht G, Seymour MT, Saltz L, Douillard JY, Hecker H, Stephens RJ, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. (2008) 26:1443–51. doi: 10.1200/JCO.2007.14.0509
26. Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. (2000) 355:1041–7. doi: 10.1016/S0140-6736(00)02034-1
27. Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. (2004) 22:229–37. doi: 10.1200/JCO.2004.05.113
28. Baeg IH, So SH. The world ginseng market and the ginseng (Korea). J Ginseng Res. (2013) 37:1–7. doi: 10.5142/jgr.2013.37.1
29. Ginseng. Drugs and Lactation Database (LactMed). Bethesda, MD: National Library of Medicine (US). (2006).
30. Choi KT. Botanical characteristics, pharmacological effects and medicinal components of Korean Panax ginseng C A Meyer. Acta Pharmacol Sin. (2008) 29:1109–18. doi: 10.1111/j.1745-7254.2008.00869.x
31. Song BK, Kim KM, Choi KD, Im WT. Production of the rare ginsenoside Rh2-MIX (20(S)-Rh2, 20(R)-Rh2, Rk2, and Rh3) by enzymatic conversion combined with acid treatment and evaluation of its anti-cancer activity. J Microbiol Biotechnol. (2017) 27:1233–41. doi: 10.4014/jmb.1701.01077
32. Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. (2007) 59:589–601. doi: 10.1007/s00280-006-0300-z
33. Sadeghian M, Rahmani S, Zendehdel M, Hosseini SA, Zare Javid A. Ginseng and cancer-related fatigue: a systematic review of clinical trials. Nutr Cancer. (2021) 73:1270–81. doi: 10.1080/01635581.2020.1795691
34. Hong H, Baatar D, Hwang SG. Anticancer activities of ginsenosides, the main active components of ginseng. Evid Based Complement Alternat Med. (2021) 2021:8858006. doi: 10.1155/2021/8858006
35. Dong J, Liang W, Wang T, Sui J, Wang J, Deng Z, et al. Saponins regulate intestinal inflammation in colon cancer and IBD. Pharmacol Res. (2019) 144:66–72. doi: 10.1016/j.phrs.2019.04.010
36. Yang X, Zou J, Cai H, Huang X, Yang X, Guo D, et al. Ginsenoside Rg3 inhibits colorectal tumor growth via down-regulation of C/EBPβ/NF-κB signaling. Biomed Pharmacother. (2017) 96:1240–5. doi: 10.1016/j.biopha.2017.11.092
37. Li J, Qi Y. Ginsenoside Rg3 inhibits cell growth, migration and invasion in Caco-2 cells by downregulation of lncRNA CCAT1. Exp Mol Pathol. (2019) 106:131–8. doi: 10.1016/j.yexmp.2019.01.003
38. Ji X, Peng Q, Wang M. Anti-colon-cancer effects of polysaccharides: a mini-review of the mechanisms. Int J Biol Macromol. (2018) 114:1127–33. doi: 10.1016/j.ijbiomac.2018.03.186
39. Chang H, Lei L, Zhou Y, Ye F, Zhao G. Dietary flavonoids and the risk of colorectal cancer: an updated meta-analysis of epidemiological studies. Nutrients. (2018) 10:950. doi: 10.3390/nu10070950
40. Choi MK, Song IS. Interactions of ginseng with therapeutic drugs. Arch Pharm Res. (2019) 42:862–78. doi: 10.1007/s12272-019-01184-3
41. Chen S, Wang Z, Huang Y, O'Barr SA, Wong RA, Yeung S, et al. Ginseng and anticancer drug combination to improve cancer chemotherapy: a critical review. Evid Based Complement Alternat Med. (2014) 2014:168940. doi: 10.1155/2014/168940
42. Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. (2007) 35:543–58. doi: 10.1142/S0192415X07005053
43. Fishbein AB, Wang CZ, Li XL, Mehendale SR, Sun S, Aung HH, et al. Asian ginseng enhances the anti-proliferative effect of 5-fluorouracil on human colorectal cancer: comparison between white and red ginseng. Arch Pharm Res. (2009) 32:505–13. doi: 10.1007/s12272-009-1405-9
44. Wang CZ, Xie JT, Zhang B, Ni M, Fishbein A, Aung HH, et al. Chemopreventive effects of Panax notoginseng and its major constituents on SW480 human colorectal cancer cells. Int J Oncol. (2007) 31:1149–56.
45. Chen FD, Wu MC, Wang HE, Hwang JJ, Hong CY, Huang YT, et al. Sensitization of a tumor, but not normal tissue, to the cytotoxic effect of ionizing radiation using Panax notoginseng extract. Am J Chin Med. (2001) 29:517–24. doi: 10.1142/S0192415X0100054X
46. Wang CZ, Luo X, Zhang B, Song WX Ni M, Mehendale S, et al. Notoginseng enhances anti-cancer effect of 5-fluorouracil on human colorectal cancer cells. Cancer Chemother Pharmacol. (2007) 60:69–79. doi: 10.1007/s00280-006-0350-2
47. Li XL, Wang CZ, Sun S, Mehendale SR, Du W, He TC, et al. American ginseng berry enhances chemopreventive effect of 5-FU on human colorectal cancer cells. Oncol Rep. (2009) 22:943–52. doi: 10.3892/or_00000521
48. Navari RM. Pharmacological management of chemotherapy-induced nausea and vomiting: focus on recent developments. Drugs. (2009) 69:515–33. doi: 10.2165/00003495-200969050-00002
49. Kim JH, Yoon IS, Lee BH, Choi SH, Lee JH, Lee JH, et al. Effects of Korean red ginseng extract on cisplatin-induced nausea and vomiting. Arch Pharm Res. (2005) 28:680–4. doi: 10.1007/BF02969358
50. Choi S, Lee JH, Oh S, Rhim H, Lee SM, Nah SY. Effects of ginsenoside Rg2 on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. Mol Cells. (2003) 15:108–13.
51. Lee BH, Jeong SM, Lee JH, Kim DH, Kim JH, Kim JI, et al. Differential effect of ginsenoside metabolites on the 5-HT3A receptor-mediated ion current in Xenopus oocytes. Mol Cells. (2004) 17:51–6.
52. Chen MH, May BH, Zhou IW, Zhang AL, Xue CC. Integrative medicine for relief of nausea and vomiting in the treatment of colorectal cancer using oxaliplatin-based chemotherapy: a systematic review and meta-analysis. Phytother Res. (2016) 30:741–53. doi: 10.1002/ptr.5586
53. Kim JW, Han SW, Cho JY, Chung IJ, Kim JG, Lee KH, et al. Korean red ginseng for cancer-related fatigue in colorectal cancer patients with chemotherapy: a randomised phase III trial. Eur J Cancer. (2020) 130:51–62. doi: 10.1016/j.ejca.2020.02.018
54. Wang XS, Woodruff JF. Cancer-related and treatment-related fatigue. Gynecol Oncol. (2015) 136:446–52. doi: 10.1016/j.ygyno.2014.10.013
55. Li XL, Wang CZ, Mehendale SR, Sun S, Wang Q, Yuan CS. Panaxadiol, a purified ginseng component, enhances the anti-cancer effects of 5-fluorouracil in human colorectal cancer cells. Cancer Chemother Pharmacol. (2009) 64:1097–104. doi: 10.1007/s00280-009-0966-0
56. Meregalli M, Martignoni G, Frontini L, Zonato S, Pavia G, Beretta G. Increasing doses of 5-fluorouracil and high-dose folinic acid in the treatment of metastatic colorectal cancer. Tumori. (1998) 84:662–5. doi: 10.1177/030089169808400609
57. Du GJ, Wang CZ, Zhang ZY, Wen XD, Somogyi J, Calway T, et al. Caspase-mediated pro-apoptotic interaction of panaxadiol and irinotecan in human colorectal cancer cells. J Pharm Pharmacol. (2012) 64:727–34. doi: 10.1111/j.2042-7158.2012.01463.x
58. Sun M, Ye Y, Xiao L, Duan X, Zhang Y, Zhang H. Anticancer effects of ginsenoside Rg3 (Review). Int J Mol Med. (2017) 39:507–18. doi: 10.3892/ijmm.2017.2857
59. Yuan HD, Quan HY, Zhang Y, Kim SH, Chung SH. 20(S)-Ginsenoside Rg3-induced apoptosis in HT-29 colon cancer cells is associated with AMPK signaling pathway. Mol Med Rep. (2010) 3:825–31. doi: 10.3892/mmr.2010.328
60. Hong S, Cai W, Huang Z, Wang Y, Mi X, Huang Y, et al. Ginsenoside Rg3 enhances the anticancer effect of 5-FU in colon cancer cells via the PI3K/AKT pathway. Oncol Rep. (2020) 44:1333–42. doi: 10.3892/or.2020.7728
61. Bae EA, Park SY, Kim DH. Constitutive beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human intestinal bacteria. Biol Pharm Bull. (2000) 23:1481–5. doi: 10.1248/bpb.23.1481
62. Wang CZ, Zhang Z, Wan JY, Zhang CF, Anderson S, He X, et al. Protopanaxadiol, an active ginseng metabolite, significantly enhances the effects of fluorouracil on colon cancer. Nutrients. (2015) 7:799–814. doi: 10.3390/nu7020799
63. Parvaresh A, Razavi R, Rafie N, Ghiasvand R, Pourmasoumi M, Miraghajani M. Quercetin and ovarian cancer: an evaluation based on a systematic review. J Res Med Sci. (2016) 21:34. doi: 10.4103/1735-1995.181994
64. Brito AF, Ribeiro M, Abrantes AM, Pires AS, Teixo RJ, Tralhão JG, et al. Quercetin in cancer treatment, alone or in combination with conventional therapeutics? Curr Med Chem. (2015) 22:3025–39. doi: 10.2174/0929867322666150812145435
65. Darband SG, Kaviani M, Yousefi B, Sadighparvar S, Pakdel FG, Attari JA, et al. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J Cell Physiol. (2018) 233:6544–60. doi: 10.1002/jcp.26595
66. Khan F, Niaz K, Maqbool F, Ismail Hassan F, Abdollahi M, Nagulapalli Venkata KC, et al. Molecular targets underlying the anticancer effects of quercetin: an update. Nutrients. (2016) 8:529. doi: 10.3390/nu8090529
67. Neamtu AA, Maghiar TA, Alaya A, Olah NK, Turcus V, Pelea D, et al. A comprehensive view on the quercetin impact on colorectal cancer. Molecules. (2022) 27:1873. doi: 10.3390/molecules27061873
68. Yang L, Liu Y, Wang M, Qian Y, Dong X, Gu H, et al. Quercetin-induced apoptosis of HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc signaling axis. Mol Med Rep. (2016) 14:4559–66. doi: 10.3892/mmr.2016.5818
69. Refolo MG, D'Alessandro R, Malerba N, Laezza C, Bifulco M, Messa C, et al. Anti proliferative and pro apoptotic effects of flavonoid quercetin are mediated by CB1 receptor in human colon cancer cell lines. J Cell Physiol. (2015) 230:2973–80. doi: 10.1002/jcp.25026
70. Raja SB, Rajendiran V, Kasinathan NK, P A, Venkatabalasubramanian S, Murali MR, et al. Differential cytotoxic activity of Quercetin on colonic cancer cells depends on ROS generation through COX-2 expression. Food Chem Toxicol. (2017) 106:92–106. doi: 10.1016/j.fct.2017.05.006
71. Catalán M, Ferreira J, Carrasco-Pozo C. The microbiota-derived metabolite of quercetin, 3,4-dihydroxyphenylacetic acid prevents malignant transformation and mitochondrial dysfunction induced by hemin in colon cancer and normal colon epithelia cell lines. Molecules. (2020) 25:4138. doi: 10.3390/molecules25184138
72. Aires V, Limagne E, Cotte AK, Latruffe N, Ghiringhelli F, Delmas D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol Nutr Food Res. (2013) 57:1170–81. doi: 10.1002/mnfr.201200766
73. Shakibaei M, Mobasheri A, Lueders C, Busch F, Shayan P, Goel A. Curcumin enhances the effect of chemotherapy against colorectal cancer cells by inhibition of NF-κB and Src protein kinase signaling pathways. PLoS ONE. (2013) 8:e57218. doi: 10.1371/journal.pone.0057218
74. Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem Biophys Res Commun. (2005) 332:433–40. doi: 10.1016/j.bbrc.2005.04.143
75. Boersma HH, Woerdenbag HJ, Bauer J, Scheithauer W, Kampinga HH, Konings AW. Interaction between the cytostatic effects of quercetin and 5-fluorouracil in two human colorectal cancer cell lines. Phytomedicine. (1994) 1:239–44. doi: 10.1016/S0944-7113(11)80071-1
76. Chen C, Zhou J, Ji C. Quercetin: a potential drug to reverse multidrug resistance. Life Sci. (2010) 87:333–8. doi: 10.1016/j.lfs.2010.07.004
77. Atashpour S, Fouladdel S, Movahhed TK, Barzegar E, Ghahremani MH, Ostad SN, et al. Quercetin induces cell cycle arrest and apoptosis in CD133(+) cancer stem cells of human colorectal HT-29 cancer cell line and enhances anticancer effects of doxorubicin. Iran J Basic Med Sci. (2015) 18:635–43.
78. Jin W, Han H, Ma L, Zhou H, Zhao C. The chemosensitization effect of quercetin on cisplatin induces the apoptosis of human colon cancer HT-29 cell line. Int J Clin Exp Med. (2016) 9:2285–92.
79. Erdogan MK, Agca CA, Aşkin H. Quercetin and luteolin improve the anticancer effects of 5-fluorouracil in human colorectal adenocarcinoma in vitro model: a mechanistic insight. Nutr Cancer. (2022) 74:660–76. doi: 10.1080/01635581.2021.1900301
80. Zhou Y, Zhang J, Wang K, Han W, Wang X, Gao M, et al. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur J Pharmacol. (2020) 881:173185. doi: 10.1016/j.ejphar.2020.173185
81. Xavier CP, Lima CF, Rohde M, Pereira-Wilson C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother Pharmacol. (2011) 68:1449–57. doi: 10.1007/s00280-011-1641-9
82. Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. (2001) 98:10350–5. doi: 10.1073/pnas.171326098
83. Lamartiniere CA, Moore JB, Brown NM, Thompson R, Hardin MJ, Barnes S. Genistein suppresses mammary cancer in rats. Carcinogenesis. (1995) 16:2833–40. doi: 10.1093/carcin/16.11.2833
84. Howells LM, Britton RG, Mazzoletti M, Greaves P, Broggini M, Brown K, et al. Preclinical colorectal cancer chemopreventive efficacy and p53-modulating activity of 3',4',5'-trimethoxyflavonol, a quercetin analogue. Cancer Prev Res (Phila). (2010) 3:929–39. doi: 10.1158/1940-6207.CAPR-09-0236
85. Lin C, Yu Y, Zhao HG, Yang A, Yan H, Cui Y. Combination of quercetin with radiotherapy enhances tumor radiosensitivity in vitro and in vivo. Radiother Oncol. (2012) 104:395–400. doi: 10.1016/j.radonc.2011.10.023
86. Li Y, Wang Z, Jin J, Zhu SX, He GQ Li SH, et al. Quercetin pretreatment enhances the radiosensitivity of colon cancer cells by targeting Notch-1 pathway. Biochem Biophys Res Commun. (2020) 523:947–53. doi: 10.1016/j.bbrc.2020.01.048
87. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. (2009) 9:429–39. doi: 10.1038/nrc2641
88. Zhu MZ Li N, Zhou F, Ouyang J, Lu DM, Xu W, et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. (2020) 312:126043. doi: 10.1016/j.foodchem.2019.126043
89. Xu J, Wang M, Zhao J, Wang YH, Tang Q, Khan IA. Yellow tea (Camellia sinensis L.), a promising Chinese tea: Processing, chemical constituents and health benefits. Food Res Int. (2018) 107:567–77. doi: 10.1016/j.foodres.2018.01.063
90. Ju J, Lu G, Lambert JD, Yang CS. Inhibition of carcinogenesis by tea constituents. Semin Cancer Biol. (2007) 17:395–402. doi: 10.1016/j.semcancer.2007.06.013
91. Chen Y, Wu Y, Du M, Chu H, Zhu L, Tong N, et al. An inverse association between tea consumption and colorectal cancer risk. Oncotarget. (2017) 8:37367–76. doi: 10.18632/oncotarget.16959
92. Zhu MZ, Lu DM, Ouyang J, Zhou F, Huang PF, Gu BZ, et al. Tea consumption and colorectal cancer risk: a meta-analysis of prospective cohort studies. Eur J Nutr. (2020) 59:3603–15. doi: 10.1007/s00394-020-02195-3
93. Hu F, Wei F, Wang Y, Wu B, Fang Y, Xiong B, et al. synergizes the therapeutic effect of cisplatin and oxaliplatin through autophagic pathway in human colorectal cancer cells. J Pharmacol Sci. (2015) 128:27–34. doi: 10.1016/j.jphs.2015.04.003
94. Yang CS, Wang X. Green tea and cancer prevention. Nutr Cancer. (2010) 62:931–7. doi: 10.1080/01635581.2010.509536
95. Fujiki H, Sueoka E, Watanabe T, Suganuma M. Synergistic enhancement of anticancer effects on numerous human cancer cell lines treated with the combination of EGCG, other green tea catechins, and anticancer compounds. J Cancer Res Clin Oncol. (2015) 141:1511–22. doi: 10.1007/s00432-014-1899-5
96. Toden S, Tran HM, Tovar-Camargo OA, Okugawa Y, Goel A. Epigallocatechin-3-gallate targets cancer stem-like cells and enhances 5-fluorouracil chemosensitivity in colorectal cancer. Oncotarget. (2016) 7:16158–71. doi: 10.18632/oncotarget.7567
97. La X, Zhang L, Li Z, Li H, Yang Y. (-)-Epigallocatechin Gallate (EGCG) Enhances the Sensitivity of Colorectal Cancer Cells to 5-FU by Inhibiting GRP78/NF-κB/miR-155-5p/MDR1 Pathway. J Agric Food Chem. (2019) 67:2510–8. doi: 10.1021/acs.jafc.8b06665
98. Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol Nutr Food Res. (2011) 55:832–43. doi: 10.1002/mnfr.201000622
99. Abe O, Ono T, Sato H, Müller F, Ogata H, Miura I, et al. Role of (-)-epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur J Clin Pharmacol. (2018) 74:775–83. doi: 10.1007/s00228-018-2436-2
100. Alshatwi AA, Periasamy VS, Athinarayanan J, Elango R. Synergistic anticancer activity of dietary tea polyphenols and bleomycin hydrochloride in human cervical cancer cell: Caspase-dependent and independent apoptotic pathways. Chem Biol Interact. (2016) 247:1–10. doi: 10.1016/j.cbi.2016.01.012
101. Wu W, Dong J, Gou H, Geng R, Yang X, Chen D, et al. EGCG synergizes the therapeutic effect of irinotecan through enhanced DNA damage in human colorectal cancer cells. J Cell Mol Med. (2021) 25:7913–21. doi: 10.1111/jcmm.16718
102. Stammler G, Volm M. Green tea catechins (EGCG and EGC) have modulating effects on the activity of doxorubicin in drug-resistant cell lines. Anticancer Drugs. (1997) 8:265–8. doi: 10.1097/00001813-199703000-00007
103. Wang YJ, Huang Y, Anreddy N, Zhang GN, Zhang YK, Xie M, et al. Tea nanoparticle, a safe and biocompatible nanocarrier, greatly potentiates the anticancer activity of doxorubicin. Oncotarget. (2016) 7:5877–91. doi: 10.18632/oncotarget.6711
104. Shimizu M, Fukutomi Y, Ninomiya M, Nagura K, Kato T, Araki H, et al. Green tea extracts for the prevention of metachronous colorectal adenomas: a pilot study. Cancer Epidemiol Biomarkers Prev. (2008) 17:3020–5. doi: 10.1158/1055-9965.EPI-08-0528
105. Fujiki H, Suganuma M. Green tea: an effective synergist with anticancer drugs for tertiary cancer prevention. Cancer Lett. (2012) 324:119–25. doi: 10.1016/j.canlet.2012.05.012
106. Henning SM, Wang P, Abgaryan N, Vicinanza R, de Oliveira DM, Zhang Y, et al. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol Nutr Food Res. (2013) 57:483–93. doi: 10.1002/mnfr.201200646
107. Bi W, Liu H, Shen J, Zhang LH Li P, Peng B, et al. Chemopreventive effects of Ku-jin tea against AOM-induced precancerous colorectal lesions in rats and metabolomic analysis. Sci Rep. (2017) 7:15893. doi: 10.1038/s41598-017-16237-0
108. Fujiwara K. Gynecologic Cancer Chemotherapy for Gynecologic Cancer 2020. Gan To Kagaku Ryoho. (2020) 47:232.
109. Mitsuma A, Ando Y. Chemotherapy for older patients with cancer. Gan To Kagaku Ryoho. (2022) 49:13–8.
110. Yamaguchi K, Sakai M, Shimokawa T, Yamada Y, Nakamura Y, Furukawa Y. C20orf20 (MRG-binding protein) as a potential therapeutic target for colorectal cancer. Br J Cancer. (2010) 102:325–31. doi: 10.1038/sj.bjc.6605500
Keywords: colorectal cancer, ginseng, quercetin, tea, chemotherapy, chemoresistance
Citation: Zhao L, Zhao H, Zhao Y, Sui M, Liu J, Li P, Liu N and Zhang K (2022) Role of Ginseng, Quercetin, and Tea in Enhancing Chemotherapeutic Efficacy of Colorectal Cancer. Front. Med. 9:939424. doi: 10.3389/fmed.2022.939424
Received: 09 May 2022; Accepted: 24 May 2022;
Published: 20 June 2022.
Edited by:
Chunyi Li, Changchun University of Science and Technology, ChinaReviewed by:
Jian-lin Wu, Macau University of Science and Technology, Macao SAR, ChinaCopyright © 2022 Zhao, Zhao, Zhao, Sui, Liu, Li, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Liu, bGl1X25pbmdAamx1LmVkdS5jbg==; Kai Zhang, emhhbmdfa2FpQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.