- Department of Critical Care Medicine, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
A 36-year-old previous healthy man presented with fever, cough, and dyspnea associated with adenovirus pneumonia. The patient developed left ventricular thrombus, pulmonary embolism and multisite embolism of undetermined etiology. Adenovirus is a rare cause of thrombotic events in immunocompetent individuals, calling for further studies for early diagnosis and management.
Introduction
Adenovirus is a one of the most common respiratory viruses. Human adenovirus pneumonia is notorious in immunosuppressed people and may cause outbreaks of acute lung injury (1). Among military trainees, patients with Acquired Immune Deficiency Syndrome (AIDS) and transplant recipients, life-threatening adenoviral pneumonia has been documented (2–4). In addition to lung involvement, patients may develop several extrapulmonary manifestations, such as retinitis, encephalitis, hepatitis, colitis, and cystitis (5).
In 2020, Corona Virus Disease 2019 (COVID-19) stands out as the leading cause of viral coagulopathy (6). A number of thrombotic and thromboembolic complications were reported and were associated with high mortality (7–10). In the course of viral infection, a severe inflammatory response and critical illness may predispose patients to thrombotic events (11, 12). In viral infection thrombosis was shown present in various sites, such as pulmonary, proximal deep-vein, coronary and intracranial vessels (13). Thrombosis, even cardiac thrombosis in viral infection, especially adenovirus pneumonia, is extremely rare. We present an unusual case of adenovirus pneumoniae infection presenting with left ventricular thrombus, pulmonary embolism and multisite embolism.
Case report
A 36-year-old previously healthy male presented with fever, cough and expectoration for 9 days. He had no past medical history but had a history of smoking and alcohol consumption. He had a maximum temperature of 39.1°C. He revealed a blood pressure of 121/75 mmHg but with mild tachycardia and severe tachypnea of 111/min and 40/min, and oxygen saturation of 97% under 50% fraction of inspiration O2 (FiO2) supplied by a high flow nasal catheter. Chest radiograph and computed tomography (CT) scan demonstrated large area consolidation in the right lung (Figure 1). Intubation was performed, and mechanical ventilation was then administered. He was prescribed piperacillin/tazobactam, moxifloxacin and linezolid on the day of admission diagnosed as severe pneumonia.
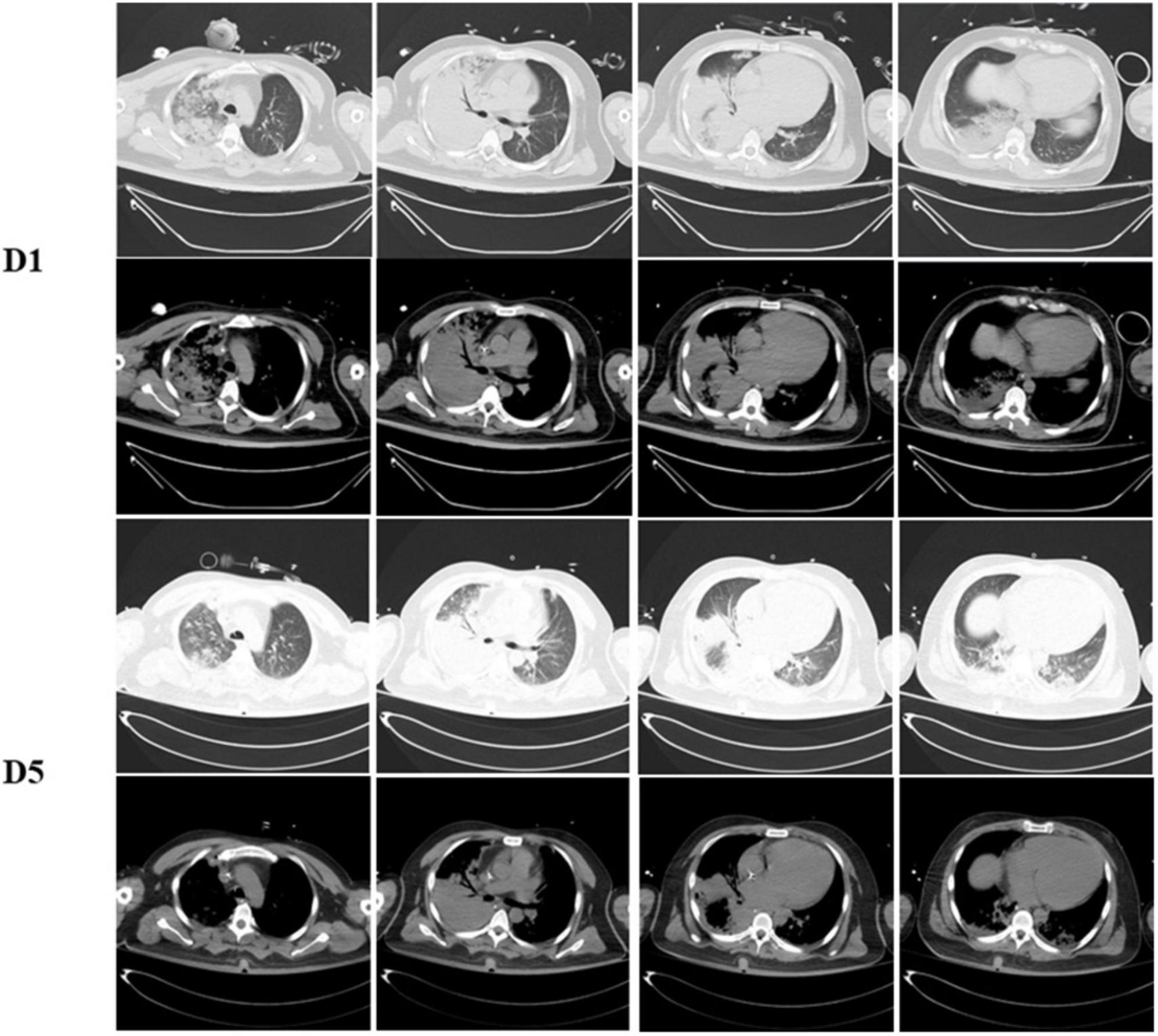
Figure 1. Dynamic change on computed tomography chest of the patient. Chest CT scans show with large area consolidation of the right lung on day 1 and improved after 5 days admitted.
After transferred to the intensive care unit, the patient exhibited onset right-sided weakness. He presented with remarkable left gaze and right-sided hemiplegia. An immediate CT scan of the head showed an acute infarction of the left frontal and temporal lobes, and computed tomography angiography (CTA) examination showed occlusion of the left middle cerebral artery (MCA) (Figure 2). D-dimer level was revealed markedly elevated at 52.26 mg/L FEU (<0.5 mg/L FEU, mg/L Forty-foot Equivalent Unit), and ultrasound of the lower extremity showed venous thrombosis of the right posterior tibial vein. His computed tomography pulmonary angiography (CTPA) showed embolism of the left pulmonary artery branches. Laboratory workup revealed slightly elevated cardiac troponin I (cTnI) at 0.29 μg/L (<0.017 μg/L) and N-terminal pro brain natriuretic peptide (NT-proBNP) at 1,461 pg/ml; his electrocardiogram (EKG) revealed non-specific manifestation. An echocardiogram revealed left ventricular (LV) thrombus with mildly reduced LV ejection fraction (36%), without concordant regional wall motion abnormality. A mural thrombus (measuring 2.8 × 1.8 cm) was identified attached to the posteromedial papillary muscle of the left ventricular apex (Figure 3).
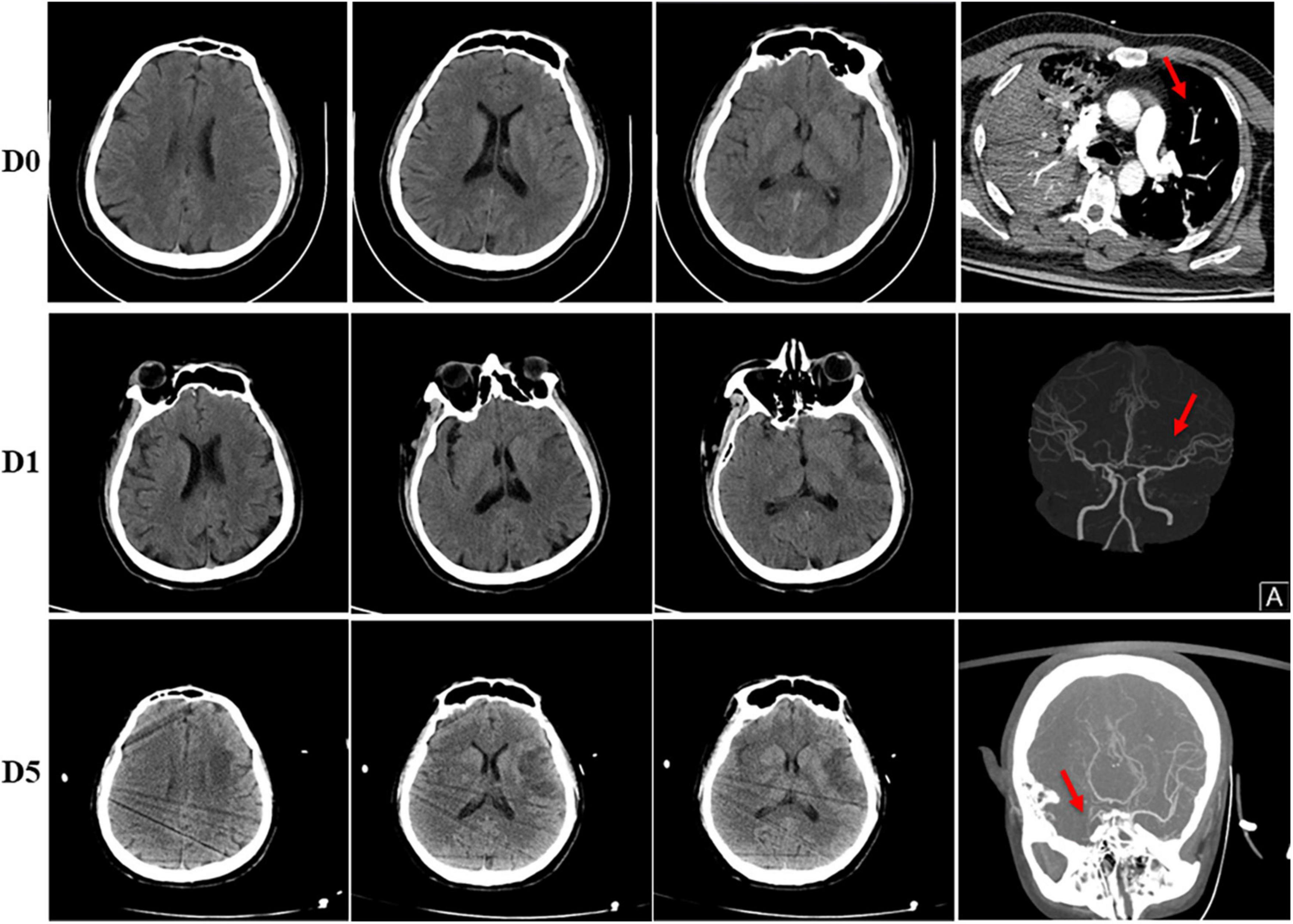
Figure 2. Head imaging of the patient. (D0) Normal head CT before admitted. His CTPA showed embolism of left pulmonary artery branches (red arrow). (D1) Ischemic infarction of left frontal and temporal lobes was shown in his head CT on day 1 after admitted, CTA examination showed stenosis of the left MCA (red arrow). (D5) CT scan of head on day 5 showed no obvious difference compared to day 1, however, CTA examination showed occlusion of right MCA (red arrow).
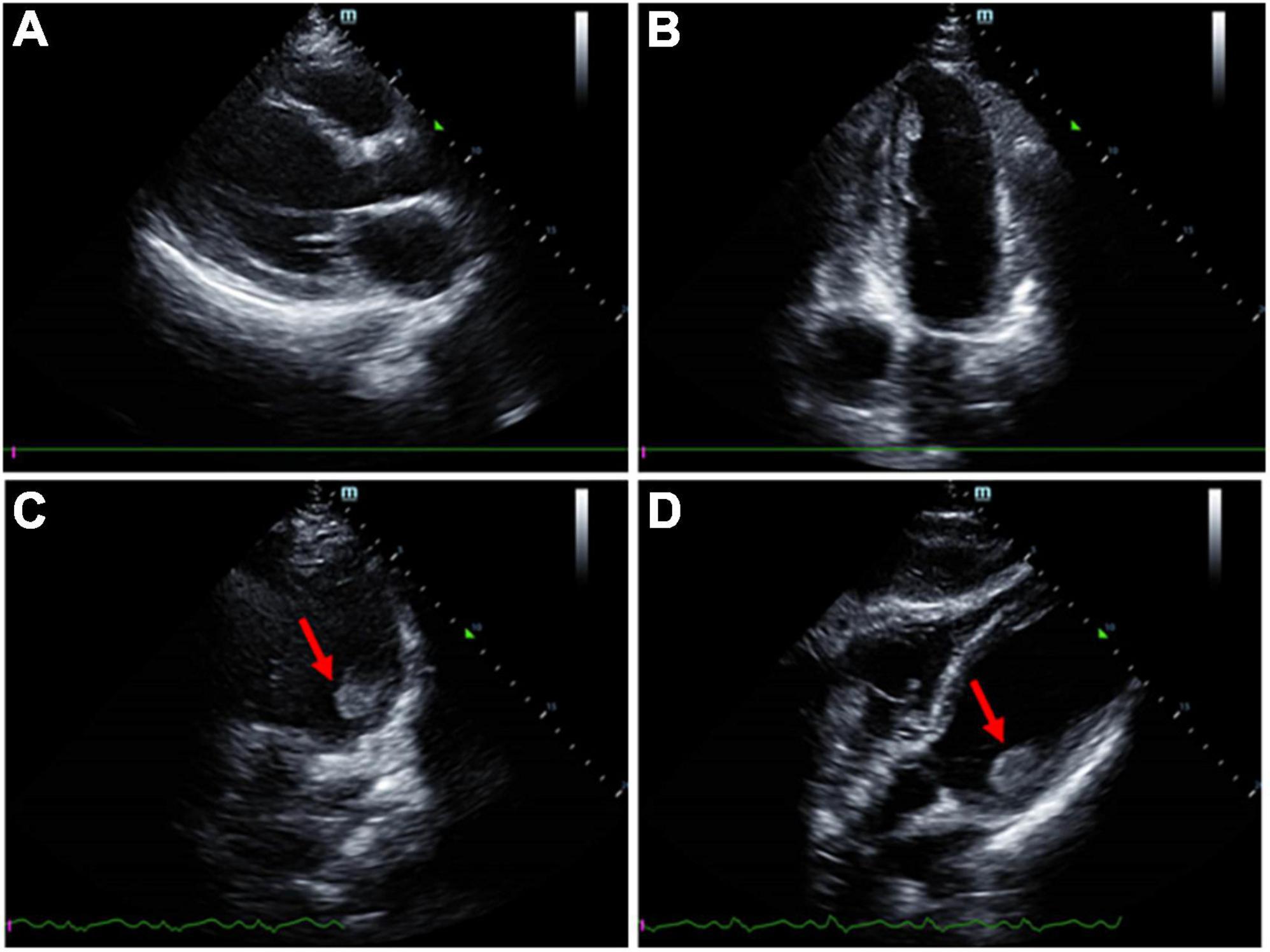
Figure 3. Transthoracic echocardiogram imaging of the patient. (A) Parasternal window parasternal long-axis view. (B) Apical window four-chamber view. (C,D) A mural thrombus was identified attached to the posteromedial papillary muscle.
The workup for sepsis, including testing for bacterial and fungal cultures, mycoplasma, chlamydia, legionella, tubercle bacillus, virus like cytomegalovirus, Epstein-Barr virus, and novel coronavirus was negative. The patient was confirmed diagnosing adenovirus pneumonia through metagenomics next-generation sequencing. His blood and bronchoalveolar lavage (BAL)-fluid samples were obtained, cell-free DNA was extracted and sequencing library was constructed for pipeline of bioinformatics analysis, more details could be obtained from our previous study (14). High viral loads of HAdV-B were detected in both plasma and bronchoalveolar lavage (BAL)-fluid. In addition, our sample showed high average nucleotide sequence similarity with the genome GCA_006448055.1 of Human adenovirus B strain 55, and consistency in the coverage depth profiles of the genomes of HAdV-B55.
His lymphocyte count when admitted was normal at 850/μL (800–4,000/μL) but with a mildly decreased T lymphocyte count at 696/μL (1,185–1,901/μL), among which T4# was 494/μL (561–1,137/μL) and T8# was 186/μL (404–754/μL). Other test showed elevated level of C-reactive protein (CRP) at 172.1 mg/L (<8 mg/L) and high levels of inflammatory factors, IL-6 at 42 pg/ml (<5.9 pg/ml), IL-8 at 121 pg/ml (<62 pg/ml), IL-10 at 7.4 pg/ml (<9.2 pg/ml) and TNF α at 12.7 (<8.1 pg/ml). His platelet counts and coagulation function parameters, such as activated partial thromboplastin time and prothrombin time, were in the normal range. Tests of antiphospholipid antibodies, including anticardiolipin, anti-β2-glycoprotein lupus and anticoagulant were negative. Other thrombophilia indicators, such as antithrombin III, Protein-C and Protein-S, were within the normal range. The patient’s condition gradually improved, his fraction of inspired oxygen gradually improved to 30%, and successful weaning was carried out. Consolidation of the right lung on chest radiography and CT improved 5 days after admission (Figure 1).
Therapeutic heparin was administered once diagnosing acute stroke, LV thrombus and deep venous thrombosis (DVT), with decreased D-dimer from 52.26 to 5.34 mg/L FEU. However, on the fifth day after admission, the patient experienced an acute weak pulse of the right upper limb artery, and ultrasound verified onset thrombosis of the right brachial artery. Moreover, the patient gradually developed a progressive disturbance of consciousness. His CT scan of head showed no obvious difference compared to admitted; however, CTA examination showed new onset occlusion of right MCA and right internal carotid artery, likely due to dislodgement of the left ventricular thrombus (Figure 2). A multidisciplinary consultation was involved, including representatives from Cardiology, Neurology, Vascular surgery and Intervention Clinic. Recombinant tissue plasminogen activator or thrombectomy surgery could not be administered due to his LV thrombus for an unknown cause. The patient’s family refused further treatment, and the patient was discharged from the hospital.
Discussion
Thrombosis associated with adenovirus pneumoniae is extremely rare, especially among immunocompetent adults. Only a few cases showed extrapulmonary manifestation of adenovirus as disseminated intravascular coagulation (15) or thrombotic microangiopathy (16). We demonstrated a case of left ventricular thrombus, pulmonary embolism and multisite embolism, including cerebral embolism and artery embolism, in a patient with adenovirus pneumoniae.
The patient presented with an unusually large LV thrombus without past cardiac medical history or other positive thrombophilia indicators. The diagnosis of myocarditis could hardly be made either. A hypercoagulable state was described in the course of viral infection. Adenovirus vectors are most popular in basic vascular experiments (17). Adenoviruses seem to have the ability to directly infect the endothelium (18), and the vascular endothelium also presented specific anti-adenovirus reactions (17). Adenoviruses may also stimulate tissue factor expression to upregulate the extrinsic pathway of coagulopathy and induce procoagulant activity in infected endothelial cells (19). Direct viral endothelial injury and procoagulant activity might contribute the development of thrombosis during adenovirus infection. Moreover, proinflammatory cytokines, a condition found in COVID-19 patients, was also discussed (20). Coagulation pathways might be activated due to inflammatory cytokine release. A 17-year-old patient with COVID-19 showed improved clinical status, and the mural thrombus nearly resolved during the hospital stay after treating with tinzaparin (21). However, even when treated with anticoagulant, our patient still developed multiple site embolism repeatedly, which led to his abandonment of treatment. Because of the small number of cases, further exploration is still needed to determine the cause of the different prognoses.
Both arterial thromboembolism, including left ventricular thrombus, cerebral embolism, artery embolism, and venous thrombus, including pulmonary embolism and deep veinous thrombosis, were observed in our patient. In the critically ill patients with COVID-19, the rates of arterial and venous thromboembolism were 5 and 31% (22). A significantly high D-dimer level triggered us in an early stage to follow the clue of thrombosis and start anticoagulant therapy. D-dimer level is a sensitive indicator for identifying thrombus although it can rise in many conditions. In patients with COVID-19, D-dimer was also shown associated with the disease severity and mortality (23). The D-dimer level may also be an early warning indicator in patients with adenovirus pneumonia.
The patient in our case was previously healthy and provided no immunosuppressive background. His lymphocyte count was normal, with only a mildly decreased T lymphocyte count. Species of HAdV-55 were identified through NGS of his plasma and BALF samples. HAdV-55 was initially identified in emergent acute respiratory disease originating in China (24). Severe pneumonia has been reported in HAdV-55 compared to other serotypes, with high rates of oxygen therapy, mechanical ventilation, and mortality (25, 26). Moreover, immunocompetent adults seem to be susceptible (1). There is currently no description of thrombotic events in HAdV-55-infected patients. Further study is still needed to explore this relationship.
Some unexpected thrombotic events were developed after COVID-19 vaccinations (27). It seems that fewer thrombotic events were reported in vaccines using mRNA technology instead of adenovirus vectors (28). Direct interaction between adenovirus and blood components was hypothesized. Mechanism has been assumed recently that platelet-activating antibodies targeting the PF4–polyanion complex may account (29, 30). Overall, more research and clinical cases are still needed to clarify these findings.
Our study had several limitations. It is unfortunate that in our case the patient was discharged from the hospital after exacerbation, complete treatment and follow-up could not be performed. Second, only one patient was shown in our manuscript, more cases are still needed for further exploring occurrence rate, clinical features and prognoses of thrombotic events during adenovirus infection. Third, D-dimer level may be influenced by lots of factors, the appropriate level to identify thrombosis is still a challenge.
Conclusion
Adenovirus is a rare cause of thrombotic events in immunocompetent individuals. This case demonstrates unusual presentations of adenovirus infection with LV thrombus, pulmonary embolism and multisite thrombotic events. We hypothesize that it may be related to viral direct endothelial injury and procoagulant activity. We aim to raise awareness that thrombotic events are not unique in COVID-19, and more attention may be drawn to unusual extrapulmonary manifestations of adenovirus infection. D-dimer levels might be an indicator in early identification and intervention concerning thrombotic events in these patients.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NC revised the manuscript. J-YM and HZ collected the data and drafted the manuscript. All authors read the manuscript and approved of the version to be published.
Funding
This work was supported by the National High Level Hospital Clinical Research Funding (No. 2022-PUMCH-A-219), National Natural Science Foundation of China (No. 82072226), Beijing Municipal Science and Technology Commission (No. Z201100005520049), and CAMS Innovation Fund for Medical Sciences (CIFMS) 2021-I2M-1-062 from Chinese Academy of Medical Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AIDS, Acquired Immune Deficiency Syndrome; COVID-19, Corona Virus Disease 2019; FiO2, fraction of inspiration O2; CT, computed tomography; CTA, computed tomography angiography; FEU, Forty-foot Equivalent Unit; MCA, middle cerebral artery; CTPA, computed tomography pulmonary angiography; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro brain natriuretic peptide; EKG, electrocardiogram; LV, left ventricular; BAL, bronchoalveolar lavage; CRP, C-reactive protein; DVT, deep venous thrombosis.
References
1. Sun B, He H, Wang Z, Qu J, Li X, Ban C, et al. Emergent severe acute respiratory distress syndrome caused by adenovirus type 55 in immunocompetent adults in 2013: a prospective observational study. Crit Care. (2014) 18:456. doi: 10.1186/s13054-014-0456-6
2. Journal of the American Medical Association. From the centers for disease control and prevention. Civilian outbreak of adenovirus acute respiratory disease–South Dakota, 1997. JAMA. (1998) 280:596. doi: 10.1001/jama.280.7.596-JWR0819-2-1
3. Hwang SM, Park DE, Yang YI, Park SJ, Lee HK, Kim MJ, et al. Outbreak of febrile respiratory illness caused by adenovirus at a South Korean military training facility: clinical and radiological characteristics of adenovirus pneumonia. Jpn J Infect Dis.. (2013) 66:359–65. doi: 10.7883/yoken.66.359
4. Klinger JR, Sanchez MP, Curtin LA, Durkin M, Matyas B. Multiple cases of life-threatening adenovirus pneumonia in a mental health care center. Am J Respir Crit Care Med. (1998) 157:645–9. doi: 10.1164/ajrccm.157.2.9608057
5. Walter JM. Other respiratory viruses as a cause of community-acquired pneumonia. Semin Respir Crit Care Med. (2020) 41:579–91. doi: 10.1055/s-0040-1710537
6. Merrill JT, Erkan D, Winakur J, James JA. Emerging evidence of a COVID-19 thrombotic syndrome has treatment implications. Nat Rev Rheumatol. (2020) 16:581–9. doi: 10.1038/s41584-020-0474-5
7. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:2950–73. doi: 10.1016/j.jacc.2020.04.031
8. Garg A, Hakeem H, Chennu G, Saeed Q, Vucic E, Kats Y, et al. Left ventricular mural thrombi with multisystem thrombosis in patients with COVID-19 and myocardial injury: a case series. Eur Heart J Case Rep. (2021) 5:ytab239. doi: 10.1093/ehjcr/ytab239
9. Wong RS, Wu A, To KF, Lee N, Lam CW, Wong CK, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. (2003) 326:1358–62. doi: 10.1136/bmj.326.7403.1358
10. Tsui KL, Leung TC, Yam LY, So LK, Poon E, Lung KC, et al. Coronary plaque instability in severe acute respiratory syndrome. Int J Cardiol. (2005) 99:471–2. doi: 10.1016/j.ijcard.2003.11.052
11. Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, et al. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. (2003) 290:374–80. doi: 10.1001/jama.290.3.374
12. Madjid M, Aboshady I, Awan I, Litovsky S, Casscells SW. Influenza and cardiovascular disease: is there a causal relationship? Tex Heart Inst J. (2004) 31:4–13.
13. Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. (2020) 191:145–7. doi: 10.1016/j.thromres.2020.04.013
14. Li D, Gai W, Zhang J, Cheng W, Cui N, Wang H. Metagenomic next-generation sequencing for the microbiological diagnosis of abdominal sepsis patients. Front Microbiol. (2022) 13:816631. doi: 10.3389/fmicb.2022.816631
15. Hussain SA, Zafar A, Faisal H, Vasylyeva O, Imran F. Adenovirus-associated disseminated intravascular coagulation. Cureus. (2021) 13:e14194. doi: 10.7759/cureus.14194
16. Sanathkumar HT, Kurien AA, Raj YT, Fernando EM. Adenovirus-associated thrombotic microangiopathy and necrotizing interstitial nephritis in a renal transplant recipient: a case report and review. Indian J Nephrol. (2021) 31:314–8. doi: 10.4103/ijn.IJN_344_19
17. Murata T, Hori M, Lee S, Nakamura A, Kohama K, Karaki H, et al. Vascular endothelium has a local anti-adenovirus vector system and glucocorticoid optimizes its gene transduction. Arterioscler Thromb Vasc Biol. (2005) 25:1796–803. doi: 10.1161/01.ATV.0000174130.75958.b7
18. Pham TT, Burchette JL Jr., Hale LP. Fatal disseminated adenovirus infections in immunocompromised patients. Am J Clin Pathol. (2003) 120:575–83. doi: 10.1309/AWXDGNC5D70EN7YT
19. Visseren FL, Bouwman JJ, Bouter KP, Diepersloot RJ, de Groot PH, Erkelens DW. Procoagulant activity of endothelial cells after infection with respiratory viruses. Thromb Haemost. (2000) 84:319–24. doi: 10.1055/s-0037-1614014
20. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. (2020) 8:e46–7. doi: 10.1016/S2213-2600(20)30216-2
21. Schroder J, Lund MAV, Vejlstrup N, Juul K, Nygaard U. Left ventricular thrombus in multisystem inflammatory syndrome in children associated with COVID-19. Cardiol Young. (2022) 32:138–41. doi: 10.1017/S1047951121002456
22. Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. (2020) 29:100639. doi: 10.1016/j.eclinm.2020.100639
23. Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. (2020) 18:1324–9. doi: 10.1111/jth.14859
24. Hang J, Kajon AE, Graf PCF, Berry IM, Yang Y, Sanborn MA, et al. Human adenovirus type 55 distribution, regional persistence, and genetic variability. Emerg Infect Dis. (2020) 26:1497–505. doi: 10.3201/eid2607.191707
25. Cao B, Huang GH, Pu ZH, Qu JX, Yu XM, Zhu Z, et al. Emergence of community-acquired adenovirus type 55 as a cause of community-onset pneumonia. Chest. (2014) 145:79–86. doi: 10.1378/chest.13-1186
26. Zhu Q, Chen S, Gu L, Qu J. Comparative analyses of clinical features reveal the severity of human adenovirus type 55 and type 7 in acute respiratory tract infections. J Med Microbiol. (2021) 70. doi: 10.1099/jmm.0.001445
27. Bilotta C, Perrone G, Adelfio V, Spatola GF, Uzzo ML, Argo A, et al. COVID-19 vaccine-related thrombosis: a systematic review and exploratory analysis. Front Immunol. (2021) 12:729251. doi: 10.3389/fimmu.2021.729251
28. Iba T, Levy JH, Warkentin TE. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. (2022) 50:e80–6. doi: 10.1097/CCM.0000000000005211
29. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. (2021) 384:2092–101. doi: 10.1056/NEJMoa2104840
Keywords: adenovirus, pneumonia, left ventricular thrombus, cerebral embolism, multisite embolism
Citation: Mao JY, Zhao H and Cui N (2022) Case report: An unusual case of multisite embolism in a patient with adenovirus pneumoniae. Front. Med. 9:939102. doi: 10.3389/fmed.2022.939102
Received: 08 May 2022; Accepted: 16 August 2022;
Published: 06 September 2022.
Edited by:
Kumari Asha, Rosalind Franklin University of Medicine and Science, United StatesReviewed by:
Yash Gupta, Mayo Clinic Florida, United StatesMangesh Vasant Suryavanshi, Cleveland Clinic, United States
Copyright © 2022 Mao, Zhao and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Cui, cHVtY2hjbkAxNjMuY29t
 Jia-Yu Mao
Jia-Yu Mao Hua Zhao
Hua Zhao Na Cui
Na Cui