- 1The First School of Clinical Medicine of Lanzhou University, Lanzhou, China
- 2Heart Center, The First Hospital of Lanzhou University, Lanzhou, China
- 3Gansu Key Laboratory for Cardiovascular Diseases of Gansu, Lanzhou, China
- 4Cardiovascular Clinical Research Center of Gansu, Lanzhou, China
Background: This study aimed to summarize and analyse the risk factors, clinical features, as well as prevention and treatment of limb ischemia complications in patients on veno-arterial extracorporeal membrane oxygenation (V-A ECMO).
Methods: We retrospectively analyzed 179 adult patients who had undergone V-A ECMO support in the Cardiac Care Unit of the First Hospital of Lanzhou University between March 2019 and December 2021. Patients were divided into the limb ischemia group (LI group) and the non-limb ischemia group (nLI group) according to whether limb ischemia occurred on the ipsilateral side of femoral artery cannulation. In the LI group, patients were salvaged with a distal perfusion cannula (DPC) according to each patient's clinical conditions. The baseline data and ECMO data were compared between the two groups, and risk factors for limb ischemia complications were screened using multiple logistic regression analysis.
Results: Overall, 19 patients (10.6%) had limb ischemia complications, of which 5 (2.8%) were improved after medication adjustment, 12 (8.4%) were salvaged with a DPC, and 2 had undergone surgical intervention. There were significant differences in terms of Extracorporeal Cardiopulmonary Resuscitation (ECPR), Intra-aortic balloon pump (IABP), peak vasoactive-inotropic score (VIS) within 24 h after ECMO (VIS-max), Left ventricular ejection fraction (LVEF), weaning from ECMO, and discharge rate between the two groups. ECPR, IABP, and VIS-max in the LI group were significantly higher than those in the nLI group, whereas weaning from ECMO, discharge rate, and LVEF were significantly lower in the LI group compared to those in the nLI group. Furthermore, multiple logistic regression analysis revealed that diabetes [odds ratio (OR) = 4.338, 95% confidence interval (CI): 1.193–15.772, P = 0.026], IABP (OR = 1.526, 95% CI: 1.038–22.026, P = 0.049) and VIS-max (OR = 1.054, 95% CI: 1.024–1.085, P < 0.001) were independent risk factors for limb ischemia complications in patients who underwent V-A ECMO.
Conclusion: Diabetes, prevalence of IABP and VIS-max value in analyzed groups were independent risk factors for predicting limb ischemia complications in patients who underwent V-A ECMO. The cannulation strategy should be optimized during the establishment of V-A ECMO, and limb ischemia should be systematically evaluated after ECMO establishment. A DPC can be used as a salvage intervention for the complications of critical limb ischemia.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a mechanical cardio-respiratory support for heart and lung failure in which conventional treatment is ineffective and is increasingly becoming a bridge to permanent solution such as recovery, transplantation, more durable device, or decision. Over the past decade, the indications for ECMO have expanded, and the use of ECMO has increased exponentially (1–3). Establishing veno-arterial (V-A) ECMO by percutaneous femoral artery and venous cannulation is easy to perform, minimally invasive, and widely used in clinically. However, femoral artery cannulation may result in acute limb ischemia (ALI) of the ipsilateral limb. Severe limb ischemia can render the arterial blood supply of the lower extremities unable to meet the basic physiological and metabolic needs at rest (4–8), which may require secondary surgical intervention, or amputation and even be life-threatening (9–11). In a systematic meta-analysis by Cheng et al., the cumulative incidence of limb ischemia in ECMO-assisted patients was 16.9% (12). Therefore, detection, prevention, and management of ECMO-related ALI are critical (12–14). Many prevention strategies have been proposed to avoid limb ischemia including the selection of small arterial return cannula and cannulation side selection, cannulation technique, and placement of a smaller cannula for anterograde or retrograde (ankle) distal perfusion (15). Recently, a new bidirectional femoral arterial cannula has been proposed and tested during cardiopulmonary bypass (16). With peripheral femoral cannulation, distal perfusion ipsilateral to the femoral artery cannulation is recommended by the ELSO Red Book and guidelines (1). Currently, the placement of the distal perfusion cannula (DPC) is the most common method for preventing and treating limb ischemia complications (17, 18). However, the timing of DPC application is not clearly defined. There is a lack of clear evidence-based medical recommendations as to whether the distal perfusion catheter should be placed prophylactically or only if acute limb ischemia develops (1, 18–21).
We reviewed the data of adult V-A ECMO-assisted patients at our hospital, as well as discussed the risk factors, clinical features, and summarized the prevention and treatment of limb ischemia complications in order to improve the prognosis of these patients.
Materials and Methods
Study Population
We collected the clinical data of all adult patients who required V-A ECMO support between March 2019 and December 2012 in the Department of Cardiology of the First Hospital of Lanzhou University.
The inclusion criteria
(1) Patients with cardiogenic shock due to various etiologies requiring V-A ECMO assistance.
(2) Patients undergone High-risk Percutaneous Coronary Interventions (HR-PCI) who need V-A ECMO intraoperative assistance.
(3) Extracorporeal Cardiopulmonary Resuscitation (ECPR).
(4) Age between 18–80 years old.
The exclusion criteria
(1) Poor vascular access conditions, including lower extremity arterial occlusion, vascular dissection, etc.
(2) Death or weaning from ECMO due to irreversible reasons within 24 h.
(3) Patients with assistance duration <4 h.
(4) Other types of shock.
(5) Incomplete baseline data.
Limb Ischemia
The severity of limb ischemia was graded according to the Rutherford classification (22). The amount of vasopressor should be considered and eventually reduced or discontinued after the establishment of V-A ECMO. At the same time, vasodilators are used to increase perfusion. Moreover, in the absence of bleeding, high levels of anticoagulation were maintained. DPC is placed salvagely in patients who cannot be improved by medication adjustment and in patients with Rutherford class IIb.
Near-Infrared Spectroscopy (NIRS) Oximetry
NIRS has been used to assess limb ischemia since May 2021. The Cerebral/Somatic Oximeter (Covidien lic.,5,100 C, IRELAND) was used in all patients. The Oximeter pads for all patients were placed longitudinally on the medial aspect of the inner calf on both the cannulated and non-cannulated legs. Covidien has designated in their literature for the Cerebral/Somatic Oximeter that saturations below 40% require intervention and saturations from 40 to 50% is cautionary values for cerebral oximetry.
V-A ECMO Management
ECMO consists of a centrifugal pump, membrane oxygenator and heparinization tube (Maquet, PLS7050/2050, Germany or LivaNava, D905, Italy), femoral arteriovenous cannula (Medtronic, Bio-Medicus, United States), and air-oxygen mixer. V-A ECMO was established by percutaneous puncture of the femoral artery (Medtronic, Bio-Medicus, 15–19 Fr cannulation) and femoral vein (19–21 Fr) under the guidance of ultrasound. Femoral arterial and venous conditions were assessed by ultrasound before ECMO cannulation. The diameter of arterial cannulation was <80% of the diameter of blood vessels.
Anticoagulant Management
Unfractionated heparin (80–100 U/kg) was given before cannulation, and cannulation was started after activated clotting time (ACT) of whole blood > 200 s. During the period of ECMO assistance, activated partial thromboplastin time (APTT) and D-dimer were used to monitor. APTT was monitored every 4–6 h and maintained at 40–60 S, and D-dimer was maintained without an obvious increasing trend.
ECMO Weaning
If the flow was reduced to <20% of the ideal cardiac output, hemodynamic stability can be maintained with low dose or without positive inotropic drugs, blood oxygen saturation≥ 95%, and echocardiography indicated that the parameters of the left ventricle and right ventricle were up to the standard and LVEF ≥ 25%, these were the criteria for considering weaning in our study.
Distal Perfusion Cannula
For the distal perfusion cannulation, under ultrasound guidance, the superficial femoral artery was punctured in the opposite direction to the femoral artery cannulation, and an Avanti 6 F femoral sheath (Cordi, 504–606 X, Mexico) was routinely inserted and connected to the arterial end of ECMO.
We recommend the use of the “4S” scheme for the prevention and treatment of limb ischemia complications in patients on V-A ECMO.
Best Site Selection
The common femoral artery below the inguinal ligament and above the bifurcation is selected as the arterial puncture point.
Match Arterial Cannula Size
The selection of type and size of the arterial cannula should be based on a balance between the targeted flow rate and anatomical considerations, arterial cannula diameter was <80% of the diameter of blood vessels, while choosing a cannula as smaller as possible based on cardiac function.
Systematic Evaluation
Limb ischemia should be evaluated promptly after ECMO is established, including clinical symptoms, signs and NIRS.
Salvage Intervention
All patients with critical limb ischemic complications should be salvaged with a DPC placement rather than a routine placement.
Data Collection
Baseline Characteristics
Age; sex; body mass index (BMI); body surface area (BSA); left ventricular ejection fraction (LVEF); relevant comorbidities, including hypertension, diabetes mellitus, coronary artery disease (CAD), and chronic respiratory disease (CRD); ECMO assistance reason, including acute myocardial infarction (AMI), acute fulminant myocarditis (AFM), extracorporeal cardiopulmonary resuscitation (ECPR), high-risk percutaneous coronary intervention (HR-PCI), and other causes. Other interventions, including intra-aortic balloon pump (IABP), continuous renal replacement therapy (CRRT), and invasive mechanical ventilation (IMV).
ECMO-Related Characteristics
The size of arterial cannulation; peak vasoactive-inotropic score within 24 h after ECMO established (VIS-max); ECMO duration; location of ECMO; ECMO weaning rate.
Outcome Indicators
Cardic Care Unit (CCU) length of stay (LOS); Hospital LOS; Successful weaning from ECMO; Discharge rate.
For calculating the vasoactive-inotropic score, all vasoactive drugs were integrated with coefficients and assigned corresponding weights, and the integrated value was used as the vasoactive drug score (vasoactive-inotropic, VIS). The calculation was as follows: VIS = dopamine + dobutamine+ 10 × milrinone + 100 × epinephrine + 100 × norepinephrine + 10,000 × vasopressin [(U / (kg ·min))].
Statistical Analysis
All data were processed using SPSS version 24.0. Categorical and continuous variables were represented as counts (%) and medians (interquartile range). A Chi-square or Fisher's exact test was used for categorical variables, and a Student's t-test or Mann–Whitney U test was used for continuous variables. A multiple logistic regression analysis was used to analyze statistically significant variables to identify independent risk indicators related to neurological complications, which were summarized as odds ratios (OR) and 95% confidence intervals (95% CI). Statistical significance was set at p < 0.05.
Results
Comparison of Baseline Data
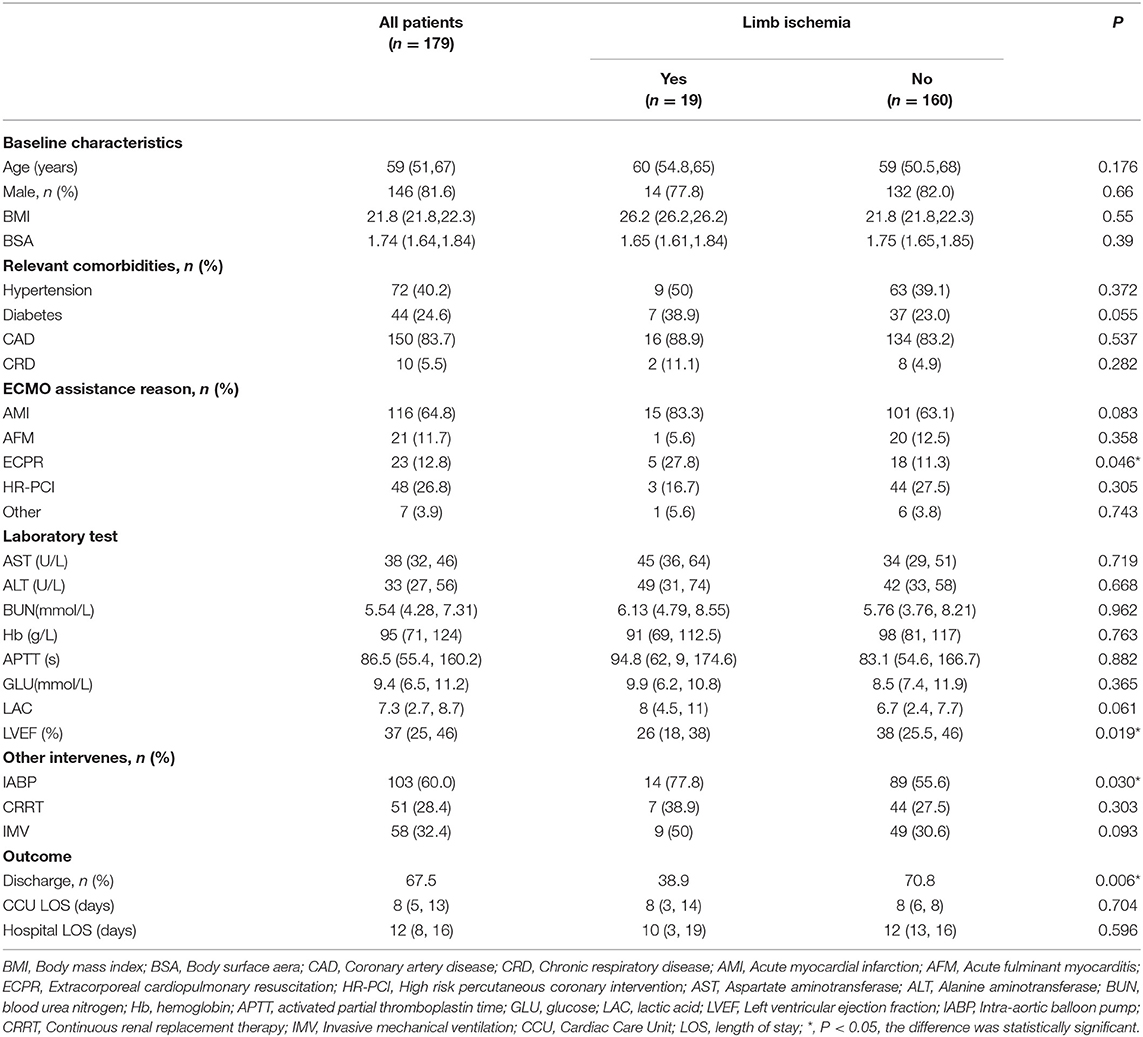
After excluding 15 patients, a total of 179 patients with V-A ECMO assistance were included. Patients were divided into the limb ischemia group (LI group) and a non-limb ischemia group (nLI group) based on the occurrence of limb ischemia complications (Figure 1). Among them, 146 cases were men (81.6%), 33 were women (18.4%), the average age was 59 (51, 67) years, ECMO duration was 82 (27, 120) h. 19 patients (10.6%) had limb ischemia complications, of which 5 (2.8%) improved after drug treatment, and 12 (8.4%) had salvage DPC placement. There were no statistically significant differences between the LI group and the nLI group in terms of age, sex, BMI, BSA and other basic conditions and laboratory tests. Patients were considered to be more likely to develop limb ischemia complications when the relevant comorbidities were diabetes (38.9 vs. 23.0%, P = 0.055), and when their etiologies for ECMO support were ECPR (27.8 vs. 11.3%, P < 0.046). Median LVEF values were lower in the LI group compared with the nLI group [26 (18, 23) vs. 38 (25.5, 46), P = 0.014]. Meanwhile, the results showed the proportion of IABP (77.8 vs. 55.6%, P = 0.03) was higher in the LI group (Table 1). The primary outcome indicators were CCU LOS, hospital LOS, ECMO weaning rate and discharge rate. The results showed no significant differences in CCU LOS and hospital LOS between the LI and nLI groups. However, we found that the ECMO weaning rate (50 vs. 79.5%, P = 0.005) and the and discharge rate (38.9 vs. 70.8%, P = 0.006) of the LI group were significantly lower than those of the nLI group (Table 1).

Figure 1. Flow chart of patient cohorts. V-A ECMO, veno-arterial extracorporeal membrane oxygenation; DPC, distal perfusion cannula; LI, limb ischemia complications group; nLI, non-limb ischemia complications group.
Comparison of V-A ECMO-Related Data
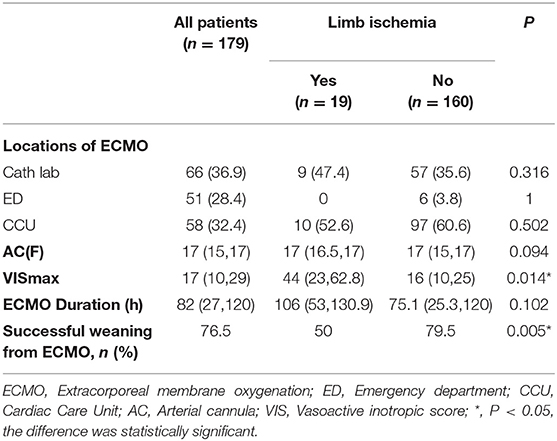
The locations of ECMO surgery included the cath lab (n = 66, 36.9%), emergency department (n = 51, 28.4%), and CCU (n = 58, 32.4%), and the median ECMO duration [106 (53, 130.9) vs. 75 (25.3, 120) days, P = 0.102]; the location of ECMO surgery and the ECMO duration were not significantly related to the development of limb ischemia complications. However, we found significant differences between the LI and nLI groups with respect to the median VIS-max [44 (23, 62.8) vs. 16 (10, 24), P = 0.014] within 24 h after ECMO established (Table 2).
Clinical Features of Limb Ischemia Complication
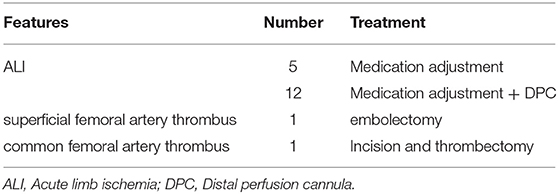
Among the 19 patients, 7 were treated with 15 Fr cannulation, 11 with 17 Fr cannulation, and 1 with 19 Fr cannulation; 5 cases improved after drug treatment, 12 cases were salvaged with a DPC, including 11 with a 6 F sheath and 1 was single lumen central venous catheter. One developed superficial femoral artery thrombosis and underwent interventional arterial thrombectomy and stent implantation. Femoral artery thrombosis occurred in one patient after weaning, and thrombectomy was performed. After the intervention, the lower limb ischemia gradually improved, the clinical symptoms were relieved, and there were no lower limb ulcers, gangrene, amputation, or osteofascial compartment syndrome (Table 3).
Multivariate Analysis of Limb Ischemia Complications in Patients Underwent V-A ECMO
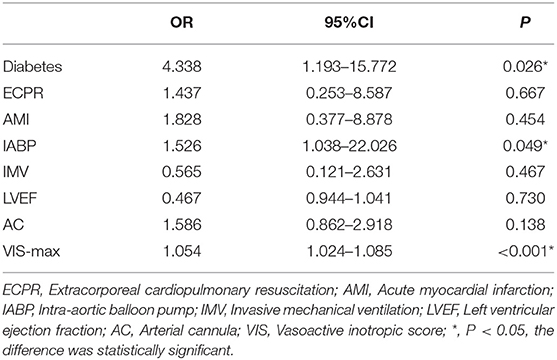
Result of the univariate analysis are shown in Tables 1, 2. Baseline variables that were considered clinically relevant or that showed a univariate relationship with outcome were entered into a multiple logistic regression model. Candidate variables with a P value < 0.1 in univariate analysis were included in the multivariable model. The association were checked in the multivariable model, and after adjustment for ECPR, AMI, IMV, LVEF, and arterial cannula size, multivariate analysis revealed that Diabetes (OR, 4.338; 95% CI, 1.193–15.772; P = 0.026), IABP (OR, 1.526, 95% CI, 1.038–22.026; P = 0.049) and VIS-max (OR, 1.054, 95% CI, 1.024–1.085; P < 0.00) were independent risk factors for limb ischemia complications in patients on V-A ECMO (Table 4).
Discussion
As a powerful life-support device, V-A ECMO is being increasingly used in clinical practice. This technique is an invasive procedure and may lead to related complications. Limb ischemia complications are often an important factor affecting the effect of ECMO. Previous studies reported that the incidence of distal limb ischemia on the ipsilateral femoral artery cannulation in patients with peripheral V-A ECMO ranges from 10 to 50% (25, 26). This may be related to differences in ECMO indications, disease distribution, baseline characteristics, implantation techniques, definition of limb ischemia, detection tools, and timing of DPC insertion. In a study of Rastan et al. (24), 5.4% of patients with femoral artery cannulation experienced limb ischemia, and the use of DPC reduced limb ischemia and fasciotomy to <40%. In a cohort study of 75 patients, the overall rate of limb ischemia was 14.7%, and the rate of limb ischemia remained 4.6% in patients treated with conventional DPC (21). Limb ischemia complications have been reported to be associated with unsuccessful ECMO weaning and have been shown to be independent predictors of in-hospital mortality (27, 28). We reviewed the incidence of limb ischemia complications, clinical features, and effectiveness of salvage DPC placement in adult patients assisted by V-A ECMO, as well as compared the effect of limb ischemia on ECMO weaning rate, discharge rate, and length of hospital stay. Our study found that the ECMO weaning and discharge rates in the LI group were significantly lower than those in the nLI group, which is consistent with previous reports in the literature. There was no significant difference in length of hospital stay between the two groups, which may be related to a higher rate of recovery and fewer serious complications. Our center is an adult heart center, with most patients with cardiogenic shock. The total incidence of limb ischemia was 10.3%, and the incidence of severe limb ischemia requiring salvage with a DPC placement was 6.5%. The incidence was relatively low, which may be related to disease distribution and treatment strategies.
ALI associated with V-A ECMO results from relative or absolute insufficiency of arterial blood flow to distal tissues, which is related to multiple factors. Larger size arterial cannulations may result in reduced lumen area and even vascular damage, resulting in limb ischemia (29). However, this relationship is not correlated, and ECMO cannula size depends on body surface area and ECMO mode, and is based on a match between target flow and vessel diameter (30). There was no significant difference in the size of arterial cannulation between the two groups in this study. Because of the lack of collateral circulation and the smaller diameter of the femoral artery, women and younger patients are prone to limb ischemia. The peripheral atherosclerotic disease also increases the risk of plaque displacement and injury during intubation and cannulation (7, 13). Diabetes and chronic respiratory disease are independent risk factors for limb ischemia during V-A ECMO (5, 31), which may be associated with chronic endothelial injury (32). In this study, the proportion of diabetes in the limb ischemia group was higher than that in the non-limb ischemia group, and multivariate analysis indicated that limb ischemia was independently associated with diabetes. Danial et al. (32) found that limb ischemia was independently associated with the Sequential Organ Failure Score (SOFA score). This also suggests that the patient's ability to compensate for hypoperfusion may affect vascular function. In addition, before the establishment of V-A ECMO, most patients are in a state of shock, requiring large doses of vasoactive drugs to maintain, especially in the ECPR state that causes vasoconstriction and acidic metabolites, which may affect distal perfusion. Our study also found that the LVEF in the ischemia group was significantly lower, the cardiac function was worse, the proportion of ECPR was higher than that in the non-ischemic group, and the VIS-max was significantly higher than that in the non-limb ischemia group. Therefore, after the establishment of ECMO, limb ischemia can be improved by drug adjustment (including reducing vasopressors, increasing vasodilators, and adequate anticoagulation) in some patients (9). For LV venting, patients with V-A ECMO-assisted cardiogenic shock often require a combined IABP, which may improve patient outcomes (33), but bilateral femoral arterial cannulation may lead to an increased risk of ALI (34). Multivariate regression analysis in this study also found that use of IABP in V-A ECMO patients was an independent risk factor for limb ischemia, and the application of dual distal perfusion catheters may be a strategy for the treatment of such patients (35).
Considering the hazards caused by limb ischemia complications, all the patient on peripheral V-A ECMO should be closely monitored, especially unconscious patients. Clinical symptoms and signs are widely used in clinical practice as the primary assessment method. The classical description of patients with limb ischemia is grouped into a mnemonic device known as the 6 Ps, including: pain, pallor, paralysis, pulseless, paraesthesia, and paralysis (36). However, these signs may be deceptive for various reasons. The continuous flow of ECMO may render patients less pulsatile and make distal pulses less detectable. In patients with cardiogenic shock, the extremities often become colder due to a high dose of inotropes and pressors despite adequate perfusion. In addition, patients with endotracheal intubation and sedation and analgesia are unable to make immediate judgments. In these cases, a more sensitive tool to detect early distal-limb ischemia is warranted. Near-infrared reflectance spectroscopy (NIRS) technology can reflect local oxygen saturation (RSO2), and tissue oxygen saturation below 50% for a duration of more than 4 min is strongly associated with hypoperfusion (37, 38). Our center is mainly for awake and non-tracheal cannula ECMO patients, and the early clinical situation is easier to monitor. However, in special populations, the combined use of NIRS to measure bilateral tissue oxygen saturation (SmO2) is helpful for early identification. The technical aspects of the various NIRS systems are not consistent. We have been using near-infrared reflectance spectroscopy as an auxiliary assessment tool for limb hypoperfusion since January 2021 but more samples are still needed to further validate its clinical relevance. Patients were also monitored to detect NIRS tissue saturation (StO2) differentials of 15% or more between the cannulated limb and the contralateral limb for the diagnosis of cannula-related obstruction to flow. The literature search for this paper found no references for intervention or cautionary values specific to distal limb saturations.
Prevention and treatment of limb ischemia should be individualized. Choosing a smaller size cannulation provides considerable clinical support and reduces complications, which is feasible in clinical practice (9, 23, 29, 39). In addition, evaluation of vascular access and selection of cannulation sites play an important role in preventing limb ischemia (1, 40–42). Proper anticoagulation, drug optimization and adequate perfusion of distal limb are necessary for patients who have occurred limb ischemia complications (9). At present, DPC is still the most commonly used invasive technique for the prevention and treatment of limb ischemia. In a meta-analysis of 1,850 patients, prophylactic DPC placement was found to reduce the incidence of limb ischemia (43). Rastan et al. (24) found that the use of DPC can reduce the risk of lower extremity ischemia and surgical intervention to <40%, so it is recommended to routinely place DPC in patients with peripheral V-A ECMO. However, there are corresponding risks associated with catheter placement, which may lead to lower extremity-related complications. In a retrospective study of 84 adult patients with V-A ECMO, Tanaka et al. found that even with prophylactic DPC placement, limb ischemic complications occurred in 12% of patients (13). Some studies suggested that DPC should be used as a salvage measure for limb ischemia after clinical assessment (21). In addition, the types of DPC used by different centers also vary greatly. The size of DPC ranges from 5 to 14 Fr and the most commonly used types are central venous catheters and vascular guide sheaths (usually 6–8 Fr) (44). In our study, we adopted the regimen of drug adjustment combined with DPC. For patients who cannot be improved by medication treatment or severe limb ischemia, DPC was placed as a salvage intervention. The limb ischemia in the whole group was improved, and no adverse events occurred, indicating DPC as a salvage intervention is feasible. Therefore, in order to prevent and treat V-A ECMO-related limb ischemia, we summarize it as a “4S” scheme (Best Site selection, Match arterial cannula Size, Systematic evaluation, Salvage intervention). During the establishment of V-A ECMO, a reasonable cannulation strategy can reduce the occurrence of limb ischemia complications to a certain extent. After ECMO was established, adequate timely evaluation and placement of DPC as a salvage intervention according to clinical conditions.
Limitation
This retrospective case-control study yielded some meaningful results for clinical guidance. However, there are still some limitations. First, this was a single-center retrospective study, and the distribution of disease are relatively single, which may cause selection bias. Second, no more objective indicators have been recorded and NIRS was not used in all patients to assess limb ischemia. In addition, there is a lack of short-and long term follow up on prognosis. Therefore, it is necessary to conduct a randomized controlled study to confirm the effectiveness of the placement of DPC as a salvage intervention.
Conclusion
In this study, limb ischemia complications were uncommon in patients receiving V-A ECMO assistance, but their occurrence may affect the ECMO weaning rate and survival rate. Diabetes, IABP, and VIS-max were independent risk factors for predicting limb ischemia complications in patients with V-A ECMO. It should be adequately evaluated during and after the establishment of V-A ECMO. DPC can be used as a measure of salvage for critical limb ischemic complications.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of Affiliated the First Hospital of Lanzhou University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SH, AL, and MB: contributed to study concept and design. YW and WQ: acquisition and analysis of data. SH and AL: writing of the original manuscript and statistical analysis. MB and AL: revision and editing of the manuscript. BZ and CP: material, administrative support, and supervision. All authors approved the final version of the manuscript and agree to be responsible for all aspects of the work.
Funding
This work was supported by the Key Science and Technology Foundation of Gansu Province (No. 21YF5FA118) and the Natural Science Foundation of Gansu Province (No. 21JR7RA385).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to cardiac care unit physicians (Weiting Cai, Yurun Su, Lina Wan, Shanshan Zhang, and Zhijie Yan), cardiac care unit nursing team who participated in the VA-ECMO treatment, and interventional cardiologists (Zheng Zhang, Ming Pan, Youqi Zhu, Xiaoxue Meng, Zhongyuan Mu, Cunrui Zhao, Peng Lei, Xinghu Zhou, Yiming Zhang, Gaxue Jiang, Jinglei Niu, Xiaowei Niu, and Dongdong Yan).
Abbreviations
V-A ECMO, veno-arterial extracorporeal membrane oxygenation; LI, limb ischemia; nLI, non-limb ischemia; ALI, acute Limb ischemia; CCU, cardiac care unit; DPC, distal perfusion cannula; BMI, body mass index; BSA, body surface area; CAD, coronary artery disease; CRD, chronic respiratory disease; AMI, acute myocardial infarction; AFM, acute fulminant myocarditis; ECPR, extracorporeal cardiopulmonary resuscitation; HR-PCI, high-risk percutaneous coronary intervention; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BNU, blood urea nitrogen; HB, hemoglobin; APTT, activated partial thromboplastin time; GLU, glucose; LAC, lactic acid; IABP, intra-aortic balloon pump; CRRT, continuous renal replacement therapy; IMV, invasive mechanical ventilation; ED, emergency department; AC, arterial cannula; VIS, vasoactive inotropic score; LVEF, left ventricular ejection fraction; LOS, length of stay; NIRS, near-infrared spectroscopy; OR, odds ratios; CI, confidence intervals.
References
1. Lorusso R, Shekar K, MacLaren G, Schmidt M, Pellegrino V, Meyns B, et al. Elso interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. (2021) 67:827–44. doi: 10.1097/MAT.0000000000001510
2. Swol J, Belohlavek J, Brodie D, Bellezzo J, Weingart SD, Shinar Z, et al. Extracorporeal life support in the emergency department: a narrative review for the emergency physician. Resuscitation. (2018) 133:108–17. doi: 10.1016/j.resuscitation.2018.10.014
3. Lorusso R, Centofanti P, Gelsomino S, Barili F, Di Mauro M, Orlando P, et al. Venoarterial extracorporeal membrane oxygenation for acute fulminant myocarditis in adult patients: a 5-year multi-institutional experience. Ann Thorac Surg. (2016) 101:919–26. doi: 10.1016/j.athoracsur.2015.08.014
4. Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg. (2016) 151:1070–7. doi: 10.1001/jamasurg.2016.2018
5. Yau P, Xia Y, Shariff S, Jakobleff WA, Forest S, Lipsitz EC, et al. Factors associated with ipsilateral limb ischemia in patients undergoing femoral cannulation extracorporeal membrane oxygenation. Ann Vasc Surg. (2019) 54:60–5. doi: 10.1016/j.avsg.2018.08.073
6. Yen CC, Kao CH, Tsai CS, Tsai SH. Identifying the risk factor and prevention of limb ischemia in extracorporeal membrane oxygenation with femoral artery cannulation. Heart Surg Forum. (2018) 21:E018–22. doi: 10.1532/hsf.1824
7. Bisdas T, Beutel G, Warnecke G, Hoeper MM, Kuehn C, Haverich A, et al. Vascular complications in patients undergoing femoral cannulation for extracorporeal membrane oxygenation support. Ann Thorac Surg. (2011) 92:626–31. doi: 10.1016/j.athoracsur.2011.02.018
8. Russo CF, Cannata A, Vitali E, Lanfranconi M. Prevention of limb ischemia and edema during peripheral venoarterial extracorporeal membrane oxygenation in adults. J Card Surg. (2009) 24:185–7. doi: 10.1111/j.1540-8191.2009.00829.x
9. Bonicolini E, Martucci G, Simons J, Raffa GM, Spina C, Lo Coco V, et al. Limb ischemia in peripheral veno-arterial extracorporeal membrane oxygenation: a narrative review of incidence, prevention, monitoring, and treatment. Crit Care. (2019) 23:266. doi: 10.1186/s13054-019-2541-3
10. Chanan EL, Bingham N, Smith DE, Nunnally ME. Early detection, prevention, and management of acute limb ischemia in adults supported with venoarterial extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth. (2020) 34:3125–32. doi: 10.1053/j.jvca.2020.02.020
11. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute Limb Ischemia The New England journal of medicine. (2012) 366:2198–206. doi: 10.1056/NEJMcp1006054
12. Cheng R, Hachamovitch R, Kittleson M, Patel J, Arabia F, Moriguchi J, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg. (2014) 97:610–6. doi: 10.1016/j.athoracsur.2013.09.008
13. Tanaka D, Hirose H, Cavarocchi N, Entwistle JW. The impact of vascular complications on survival of patients on venoarterial extracorporeal membrane oxygenation. Ann Thorac Surg. (2016) 101:1729–34. doi: 10.1016/j.athoracsur.2015.10.095
14. Chen KH, Chen YT, Yeh SL, Weng LC, Tsai FC. Changes in quality of life and health status in patients with extracorporeal life support: a prospective longitudinal study. PLoS One. (2018) 13:e0196778. doi: 10.1371/journal.pone.0196778
15. Babu A. Techniques for venoarterial extracorporeal membrane oxygenation support and conversion to temporary left ventricular assist device. Oper Tech Thorac Cardiovasc Surg. (2014) 19:365–79. doi: 10.1053/j.optechstcvs.2014.11.003
16. Marasco SF, Tutungi E, Vallance SA, Udy AA, Negri JC, Zimmet AD, et al. A phase 1 study of a novel bidirectional perfusion cannula in patients undergoing femoral cannulation for cardiac surgery. Innovations. (2018) 13:97–103. doi: 10.1177/155698451801300204
17. Rao AS, Pellegrini RV, Speziali G, Marone LK. A novel percutaneous solution to limb ischemia due to arterial occlusion from a femoral artery ecmo cannula. J Endovasc Ther. (2010) 17:51–4. doi: 10.1583/09-2845.1
18. Richardson ASC, Tonna JE, Nanjayya V, Nixon P, Abrams DC, Raman L, et al. Extracorporeal cardiopulmonary resuscitation in adults. Interim guideline consensus statement from the extracorporeal life support organization. ASAIO J. (2021) 67:221–8. doi: 10.1097/MAT.0000000000001344
19. Schad CA, Fallon BP, Monteagudo J, Okochi S, Cheung EW, Morrissey NJ, et al. Routine use of distal arterial perfusion in pediatric femoral venoarterial extracorporeal membrane oxygenation. Artif Organs. (2017) 41:11–6. doi: 10.1111/aor.12861
20. Jia D, Yang IX, Ling RR, Syn N, Poon WH, Murughan K, et al. Vascular complications of extracorporeal membrane oxygenation: a systematic review and meta-regression analysis. Crit Care Med. (2020) 48:e1269–e77. doi: 10.1097/CCM.0000000000004688
21. Elmously A, Bobka T, Khin S, Afzal A, de Biasi AR, DeBois WJ, et al. Distal perfusion cannulation and limb complications in venoarterial extracorporeal membrane oxygenation. J Extra Corpor Technol. (2018) 50:155–60. Available online at: https://pubmed.ncbi.nlm.nih.gov/30250341/
22. Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. (1997) 26:517–38. doi: 10.1016/S0741-5214(97)70045-4
23. Takayama H, Landes E, Truby L, Fujita K, Kirtane AJ, Mongero L, et al. Feasibility of smaller arterial cannulas in venoarterial extracorporeal membrane oxygenation. J Thorac Cardiovasc Surg. (2015) 149:1428–33. doi: 10.1016/j.jtcvs.2015.01.042
24. Rastan AJ, Dege A, Mohr M, Doll N, Falk V, Walther T, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. (2010) 139:302–11. doi: 10.1016/j.jtcvs.2009.10.043
25. Yang F, Hou D, Wang J, Cui Y, Wang X, Xing Z, et al. Vascular complications in adult postcardiotomy cardiogenic shock patients receiving venoarterial extracorporeal membrane oxygenation. Ann Intensive Care. (2018) 8:72. doi: 10.1186/s13613-018-0417-3
26. Pozzi M, Koffel C, Djaref C, Grinberg D, Fellahi JL, Hugon-Vallet E, et al. High rate of arterial complications in patients supported with extracorporeal life support for drug intoxication-induced refractory cardiogenic shock or cardiac arrest. J Thorac Dis. (2017) 9:1988–96. doi: 10.21037/jtd.2017.06.81
27. Hei F, Lou S, Li J, Yu K, Liu J, Feng Z, et al. Five-year results of 121 consecutive patients treated with extracorporeal membrane oxygenation at fu wai hospital. Artif Organs. (2011) 35:572–8. doi: 10.1111/j.1525-1594.2010.01151.x
28. Aubron C, Cheng AC, Pilcher D, Leong T, Magrin G, Cooper DJ, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care. (2013) 17:R73. doi: 10.1186/cc12681
29. Kim J, Cho YH, Sung K, Park TK, Lee GY, Lee JM, et al. Impact of cannula size on clinical outcomes in peripheral venoarterial extracorporeal membrane oxygenation. ASAIO J. (2019) 65:573–9. doi: 10.1097/MAT.0000000000000858
30. Kohler K, Valchanov K, Nias G, Vuylsteke A. Ecmo cannula review. Perfusion. (2013) 28:114–24. doi: 10.1177/0267659112468014
31. Madonna R, De Caterina R. Cellular and molecular mechanisms of vascular injury in diabetes–part i: pathways of vascular disease in diabetes. Vascul Pharmacol. (2011) 54:68–74. doi: 10.1016/j.vph.2011.03.005
32. Brusselle G, Bracke K, De Pauw M. Peripheral artery disease in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2017) 195:148–50. doi: 10.1164/rccm.201608-1712ED
33. Gass A, Palaniswamy C, Aronow WS, Kolte D, Khera S, Ahmad H, et al. Peripheral venoarterial extracorporeal membrane oxygenation in combination with intra-aortic balloon counterpulsation in patients with cardiovascular compromise. Cardiology. (2014) 129:137–43. doi: 10.1159/000365138
34. Lin LY, Liao CW, Wang CH, Chi NH Yu HY, Chou NK, et al. Effects of additional intra-aortic balloon counter-pulsation therapy to cardiogenic shock patients supported by extra-corporeal membranous oxygenation. Sci Rep. (2016) 6:23838. doi: 10.1038/srep23838
35. Xin M, Wang L, Tian X, Hou D, Wang H, Wang J, et al. Double distal perfusion catheters for severe limb ischemia on the iabp side in patients who received femoro-femoral va-ecmo with iabp. Front Med. (2021) 8:692399. doi: 10.3389/fmed.2021.692399
36. Callum K, Bradbury A. Abc of arterial and venous disease: acute limb ischaemia. BMJ. (2000) 320:764–7. doi: 10.1136/bmj.320.7237.764
37. Patton-Rivera K, Beck J, Fung K, Chan C, Beck M, Takayama H, et al. Using near-infrared reflectance spectroscopy (Nirs) to assess distal-limb perfusion on venoarterial (V-a) extracorporeal membrane oxygenation (Ecmo) patients with femoral cannulation. Perfusion. (2018) 33:618–23. doi: 10.1177/0267659118777670
38. Marin T, Moore J. Understanding near-infrared spectroscopy. Adv Neonatal Care. (2011) 11:382–8. doi: 10.1097/ANC.0b013e3182337ebb
39. Stephens AF, Wickramarachchi A, Burrell AJC, Bellomo R, Raman J, Gregory SD. Hemodynamics of small arterial return cannulae for venoarterial extracorporeal membrane oxygenation. Artif Organs. (2022) 46:1068–76. doi: 10.1111/aor.14179
40. Lamb KM, Hirose H, Cavarocchi NC. Preparation and technical considerations for percutaneous cannulation for veno-arterial extracorporeal membrane oxygenation. J Card Surg. (2013) 28:190–2. doi: 10.1111/jocs.12058
41. Rupprecht L, Lunz D, Philipp A, Lubnow M, Schmid C. Pitfalls in percutaneous ecmo cannulation. Heart Lung Vessel. (2015)7:320–6. Available online at: https://pubmed.ncbi.nlm.nih.gov/26811838/
42. Lamperti M, Bodenham AR, Pittiruti M, Blaivas M, Augoustides JG, Elbarbary M, et al. International evidence-based recommendations on ultrasound-guided vascular access. Intensive Care Med. (2012) 38:1105–17. doi: 10.1007/s00134-012-2597-x
43. Zhang X, Fang Q, Chen K. [Distal Perfusion Cannulae in Preventing Limb Ischemia in Venous-Arterial Extracorporeal Membrane Oxygenation: A Meta-Analysis]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. (2020) 32:1361–6. doi: 10.3760/cma.j.cn121430–20200920–00639
Keywords: V-A ECMO, limb ischemia, risk factors, distal perfusion, salvage intervention
Citation: Hu S, Lu A, Pan C, Zhang B, Wa Yl, Qu W and Bai M (2022) Limb Ischemia Complications of Veno-Arterial Extracorporeal Membrane Oxygenation. Front. Med. 9:938634. doi: 10.3389/fmed.2022.938634
Received: 07 May 2022; Accepted: 23 June 2022;
Published: 15 July 2022.
Edited by:
Guo-wei Tu, Fudan University, ChinaReviewed by:
Silver Heinsar, Critical Care Research Group (CCRG), AustraliaGionata Fragomeni, Magna Græcia University, Italy
Bartlomiej Zych, Harefield Hospital, United Kingdom
Copyright © 2022 Hu, Lu, Pan, Zhang, Wa, Qu and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andong Lu, bGFkMTIyMzA5MDRAMTI2LmNvbQ==; Ming Bai, YmFpbUBsenUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Sixiong Hu
Sixiong Hu Andong Lu
Andong Lu Chenliang Pan
Chenliang Pan Bo Zhang1,2,3,4
Bo Zhang1,2,3,4 Yong ling Wa
Yong ling Wa Wenjing Qu
Wenjing Qu Ming Bai
Ming Bai