- 1Gastrointestinal and Liver Diseases Research Center, Iran University of Medical Sciences, Tehran, Iran
- 2Alimentary Tract Research Center, Clinical Sciences Research Institute, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
- 3Department of Social Medicine, Zanjan University of Medical Sciences, Zanjan, Iran
- 4Department of Virology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
- 5Centre for Intelligent Healthcare, Coventry University, Coventry, United Kingdom
- 6Department of Radiology, Firoozgar Hospital, Iran University of Medical Sciences, Tehran, Iran
The current study aimed to evaluate the efficacy of sitagliptin vs. placebo in treating non-alcoholic fatty liver disease (NAFLD). In a triple-blind randomized clinical trial, we assigned 120 eligible subjects with NAFLD to receive daily dosing of 50 mg sitagliptin (n = 60) or the placebo (n = 60) for 56 weeks and lifestyle modification in both groups. Laboratory and anthropometric outcomes were measured, and liver stiffness was assessed using a fibroscan. The primary outcome measures were changes from baseline in fibrosis scores and liver transferases. Out of 120 patients randomized into sitagliptin and placebo groups, 76 patients completed the trial, of whom 44 were in the sitagliptin and 32 in the placebo groups. Patients receiving sitagliptin showed a significant decrease in the fibrosis scores (P = 0.001). The reductions in the alanine aminotransferase (AST) (P = 0.036) and aspartate AST (P < 0.001) levels were also statistically significant. The effect of sitagliptin in reducing fibrosis scores was significantly greater in normal-weight and overweight individuals than in obese individuals (p = 0.036, and p = 0.018, respectively), whereas the effects of sitagliptin on AST levels were greater among overweight/obese patients (p = 0.028, and p = 0.016, respectively). Sitagliptin reduced fibrosis scores and liver enzymes in NAFLD patients after 56 weeks of therapy. The changes in fibrosis scores were more prominent in patients with normal weight and overweight than obese patients, whereas the effects on AST levels were greater among overweight/obese patients. Other randomized trials with larger sample sizes and longer treatment durations may be required before precise results can be reached.
Clinical Trial Registration: [https://www.irct.ir/trial/46140], identifier [IRCT20140430017505N2].
Introduction
The most common type of liver disease globally is a non-alcoholic fatty liver disease (NAFLD) (1), defined as hepatic steatosis without other causes of lipid accumulation in hepatocytes (2, 3). A meta-analysis in 2016 reported that the prevalence of NAFLD in adult populations in Europe, North America, and Asia was 23.7, 24.1, and 27.4%, respectively (4). According to research published in 2018, the prevalence of NAFLD in the adult population of Iran ranged from 20 to 40% (5).
NAFLD includes several stages, such as fatty steatosis to steatohepatitis, developing into liver fibrosis, and cirrhosis. NAFLD has been linked to metabolic syndrome, as the most well-known risk factor and several other metabolic diseases, including obesity, dyslipidemia, type 2 diabetes, and hypertension (6). NAFLD has recently been renamed metabolic-associated fatty liver disease (MAFLD) by an international expert consensus (7–9). Indeed, because insulin resistance (IR) and NAFLD are linked, diabetic people have a 4.7-fold higher prevalence of NAFLD than non-diabetic patients (10).
There is no definitive treatment for NAFLD, and no specific drug has been offered to treat it. The only therapeutic strategies are lifestyle modifications, including diet and physical activity/exercise, pointing to a weight loss of 5–7% (11, 12); although, IR modulation and various pharmacological approaches using existing drugs, including anti-obesity, anti-diabetic, antioxidants, and gastric cytoprotective agents, have been recently proposed as possible therapies in NAFLD and non-alcoholic steatohepatitis (NASH) (13, 14).
In the pathophysiology of NAFLD, metabolic abnormalities and insulin resistance play important roles (15, 16), such that several anti-diabetic therapies have been investigated in the treatment of NAFLD, with different results, including metformin (17, 18), tofogliflozin (19), empagliflozin (20), and liraglutide (21).
Dipeptidyl peptidase 4 (DDP-4) is one of the key molecules implicated in the pathogenesis of chronic liver illnesses, like NAFLD, even before diabetes develops (22). Sitagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor, increasing glucagon-like peptide-1 (GLP-1) levels and inhibiting glucagon release, enhancing insulin secretion, and ameliorating liver enzymes (23–26).
Clinical research studies have confirmed that sitagliptin significantly improves glycemic parameters, lipid profiles, and liver function of diabetic patients with NAFLD (27–29), although other studies reported contradictory results (30, 31).
In a study of 30 NAFLD patients, Iwasaki et al. noted that 4 months of treatment with 50 mg/day of sitagliptin significantly decreased liver transferases and improved diabetes parameters (27). In an open-label, single-arm, observational pilot study that included 15 patients, results showed that treatment for 1 year with sitagliptin was associated with a significant reduction in NASH scores and hepatic steatosis improvement (29).
However, there is limited evidence of the influence of sitagliptin on hepatic inflammation and fibrosis (30, 32, 33), and only one study has been done on patients without diabetes (34). Relevant randomized control trials and long-term studies are still needed to verify the efficacy and safety of sitagliptin (35). Therefore, the current work aimed to examine the effectiveness of sitagliptin in treating NAFLD compared to placebo in patients without diabetes. The inclusion of this group of NAFLD patients in our investigation was based on the assumption that, in the absence of simultaneous diabetes, several metabolic and other confounding factors could influence the response of the fatty liver disease to any recommended treatment.
Materials and methods
Study design, setting, and population
A randomized, triple-blind clinical trial was conducted in two tertiary referral teaching medical centers in Amol and Tehran, Iran. Adults diagnosed with NAFLD were enrolled in this study from January 2019 to May 2020. Inclusion criteria were patients aged ≥ 18 years old whose primary ultrasound was scored as grades 2 and 3 and whose liver enzyme levels based on ALT were above 20 in women and above 30 in men.
Any other cause of liver diseases, such as viral hepatitis, autoimmune hepatitis, hemochromatosis, Wilson disease, liver cirrhosis, and drug-induced liver injury, was ruled out. Patients would be excluded from the trial if they had one of the following conditions: Individuals on a specific dietary or physical activity regimen (due to a specific disease, weight loss, or professional exercise), taking Vitamin E supplement, history of excessive alcohol consumption (> 10 grams per day), history of taking drugs that cause hepatic steatosis (e.g., amiodarone, methotrexate, tamoxifen, glucocorticoids, valproate, anti-retroviral agents for HIV), diagnosed diabetes, known case of malignancy, non-cooperative patients, pregnant women, and breastfeeding mothers. All of the patients provided written informed consent prior to study participation.
Randomization and allocation concealment
In order to exclude confounding factors, randomization was stratified by age, sex, and ultrasound grade. Patients were divided into 12 groups, including subjects with grades 2 and 3 in three age categories: under 40 years, 40–59 years, and ≥ 60 years in both genders, separately. Then, randomization codes were assigned for each group, and patients were randomly allocated to one of the two treatment arms, including case (sitagliptin + lifestyle modification) and control (placebo + lifestyle modification) groups. Patients, researchers, and analyzers were blinded to allocation.
Primary and secondary outcome measurements
The primary outcome measures included hepatic fibrosis and liver enzymes, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), and alkaline phosphatase (ALP), were assessed using changes from baseline to week 56. Several secondary outcome measures included FBS, HOMA-IR, Insulin, and serum lipid profiles, including total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride.
Non-alcoholic fatty liver disease diagnosis
For hepatic steatosis, ultrasonography was utilized as a first-line investigation, providing a qualitative assessment of fatty infiltration of the liver. Patients were scanned in longitudinal, transverse, and oblique scanning planes while lying down and in the left posterior oblique posture. Fatty liver on ultrasonography was defined as normal, mild, and moderate to severe. This classification was based on the echogenicity of the liver during ultrasonography, liver-to-kidney contrast, and bright gallbladder and vessel walls definition (36).
Fibroscan evaluation was applied for all participants who had fatty liver confirmed by ultrasonography. Fibroscan assessment is considered a preferred non-invasive method to apply liver stiffness measurement (LSM) and steatosis level by control attenuated parameter (CAP). According to the standard protocol, two trained specialists, blinded to the treatment group in each research center, performed the fibro scan using a Fibroscan device (Fibro Scan; Supersonic Axiplore Ultimate Paris, France) (37).
Patients were considered to have NAFLD based on a fibroscan if the fibrosis score was more than 6 kpa with absent-mild, moderate, and severe fibrosis, defined as a fibrosis score lower than 6 kpa (F0-F1), between 6 and 9.1 kpa (F2), and more than 9.1 kpa (F3), respectively (38, 39).
Laboratory and anthropometric measurements
The following procedures were used to assess laboratory outcomes at baseline and after 56 weeks of intervention. At baseline and the end of the study, 5 ml of blood was drawn from all patients to measure serum insulin levels, fasting plasma glucose (FPG), lipid profiles, and liver biochemical tests (pars biochemical kits using the photometric method). HOMA-IR formula was calculated as [fasting plasma glucose (mg/dl) serum insulin level (mU/L)]/405 (40). Weights and heights were measured at baseline, and body mass index (BMI) was computed and classified according to the World Health Organization (WHO) classification into four groups: Less than 18.5 as underweight, 18.5–25 as normal, 25.0–30 as overweight, and 30.0 kg.m2 or over as obese (41).
Intervention protocol
Patients were randomly divided into case (sitagliptin + lifestyle modification) and control (placebo + lifestyle modification) groups. A computer-based technique was employed to use block randomization, with a block of 4, and subjects were randomized 1:1 to receive sitagliptin or placebo.
During the intervention period, patients in the sitagliptin group were given 50 mg of sitagliptin (Ziptin, Abidi, Iran), whereas those in the placebo group were given 1 mg of folic acid once daily. A trained person, who was blind to the medication and patients, distributed medication packages in the same shape packaging, without labeling, to participants. A total of 120 patients were randomly assigned to sitagliptin (n = 60) and placebo (n = 60) groups for 56 weeks, along with advising for lifestyle modifications for both groups (42).
At the end of the trial, patients’ adherence to their medicine use was examined using the pill counting method. Compliance was deemed appropriate if the leftover medications were less than 20%. Patients were contacted regularly and screened for side effects throughout and after the treatment to enhance adherence to the medication and follow-up examinations. The only reported side effect among patients in the sitagliptin arm was headache (n = 1).
Various patients’ information, such as demographic, anthropometric, and laboratory data, was obtained and recorded by a trained person who was blind to grouping. All individuals’ laboratory data were gathered at baseline and after 56 weeks of therapy. Finally, the two groups’ laboratory and fibrosis results were compared before and after the intervention.
Ethical issue
The Helsinki Declaration’s guidelines were followed in the design of this study. The Medical Ethics Committee of the Iran University of Medical Science reviewed, approved, and supervised this work (reference number IR.IUMS.REC.1397.1062). The Iranian Registry of Clinical Trials also authorized and registered this protocol (IRCT20140430017505N2, https://www.irct.ir/trial/46140). Written informed consent was obtained from participants before study commencement.
Sample size calculation and statistical analysis
According to an effect size (Cohen’s D) equal to 0.5 (based on 4 kpa changes in ultrasound grade), with an α of 0.05 and power of 80%, the required sample size was 60 per group (total of 120 participants) (43, 44). Continuous and categorical variables were presented as median (IQR) and n (%), respectively. The Wilcoxon rank-sum test, χ2-test, or Fisher’s exact test was used to compare differences between two independent groups. Furthermore, the Wilcoxon signed-rank test was used to compare differences between before and after interventions. A two-sided α of less than 0⋅05 was considered statistically significant. Statistical analyses were conducted using R statistical software, version 4.1.1 (2021-08-10).
Results
Study flow
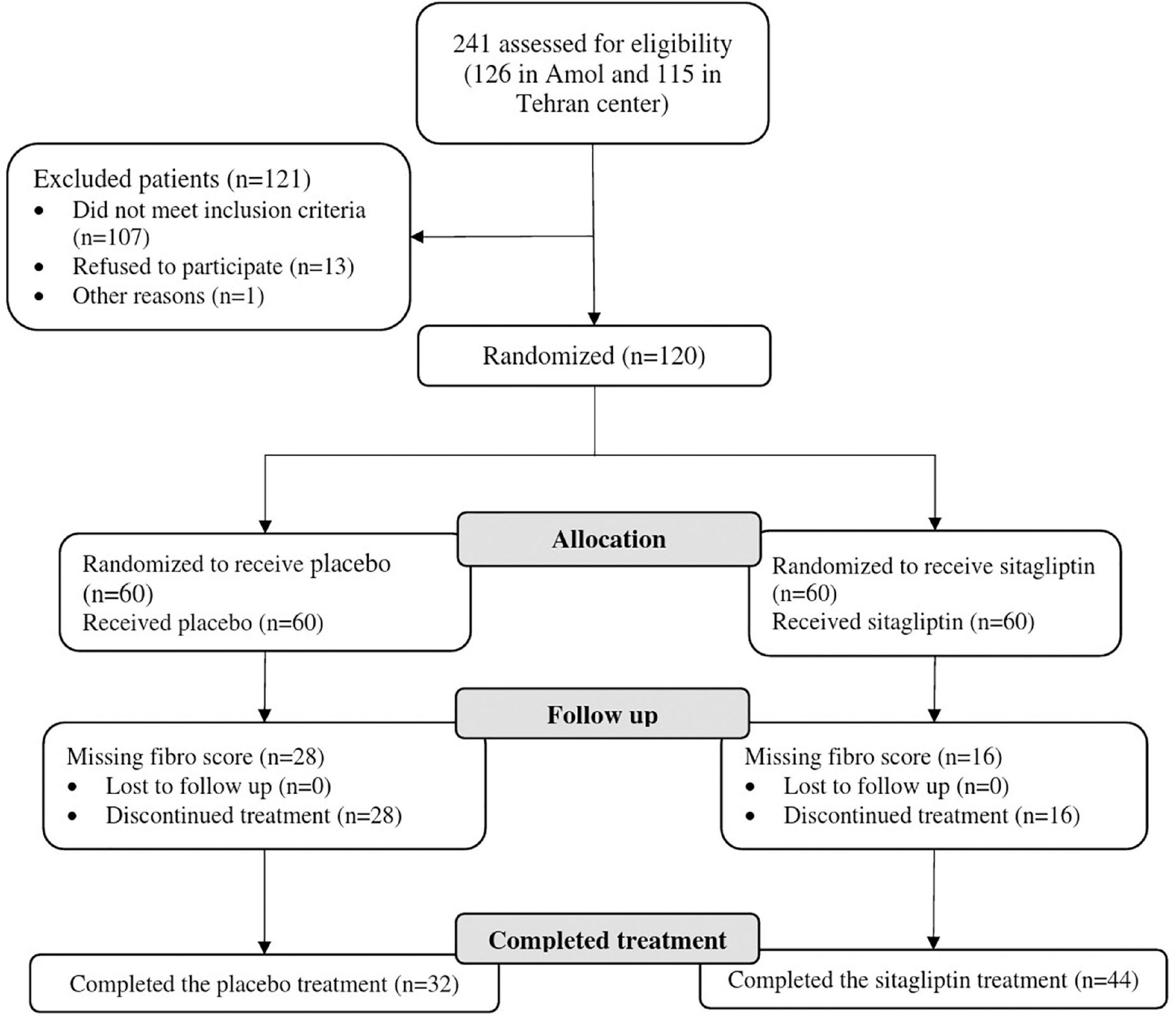
A total of 241 patients (126 in Amol and 115 in Tehran) were assessed for eligibility. One hundred twenty subjects were finally included in the study and were monitored from January 2019 to May 2020. Out of 120 patients randomized into sitagliptin (n = 60) and placebo groups (n = 60), 76 patients from both centers completed the trial, of whom 44 were in the sitagliptin and 32 in the placebo groups. Figure 1 depicts the trial patient’s assignment and flow chart based on the CONSORT flowchart (45). Dropouts from the placebo arm were not linked to any adverse events in the trial.
Baseline characteristics
The median (IQR) ages of sitagliptin and placebo groups were 45.0 (36.8, 53.2) and 39.0 (33.8, 44.2) years, respectively (P = 0.03). There were 6 (13.6%) normal weight, 18 (40.9%) overweight, and 20 (45.5%) obese patients in the sitagliptin group. In terms of demographic features and laboratory data, there was no statistically significant difference between the two groups, except for age, which was higher in the sitagliptin group than in the placebo group. The baseline demographic and laboratory characteristics of participants are shown in Table 1. Considering the sex dysmorphism of NAFLD (male predominance), a separate analysis for sex was also conducted and reported in Supplementary Tables 1–3. Demographic features, and clinical and laboratory characteristics of the study groups by sex at baseline are presented in Supplementary Table 1; females in the sitagliptin group were older than the placebo group (P = 0.003), and males in the sitagliptin group had higher fibrosis scores than the placebo group (P = 0.018), without significant differences for other variables.
Primary outcomes
Changes in hepatic fibrosis and liver enzymes
The decrease of the median fibrosis scores in patients receiving sitagliptin was statistically significant compared to individuals receiving placebo. The median (IQR) of fibrosis scores, before and after intervention with sitagliptin, were 6.25 (5.97, 7.30) and 6.00 (5.40, 6.85), respectively. The decrease of the median (IQR) fibrosis scores in patients receiving sitagliptin was statistically significant [median before 6.25 (5.97, 7.30) vs. median after 6.00 (5.40, 6.85), P = 0.001)], but not in individuals receiving placebo (P = 0.19).
Patients receiving sitagliptin showed a significant decrease in ALT (P = 0.036) and AST level (P < 0.001). While in the placebo group, only AST decreased significantly (p = 0.019). However, the reduction in AST level was 2.4 times greater in sitagliptin-treated patients than in placebo-treated individuals, which was clinically significant. Table 2 shows the changes in laboratory data in both groups before and after treatment.
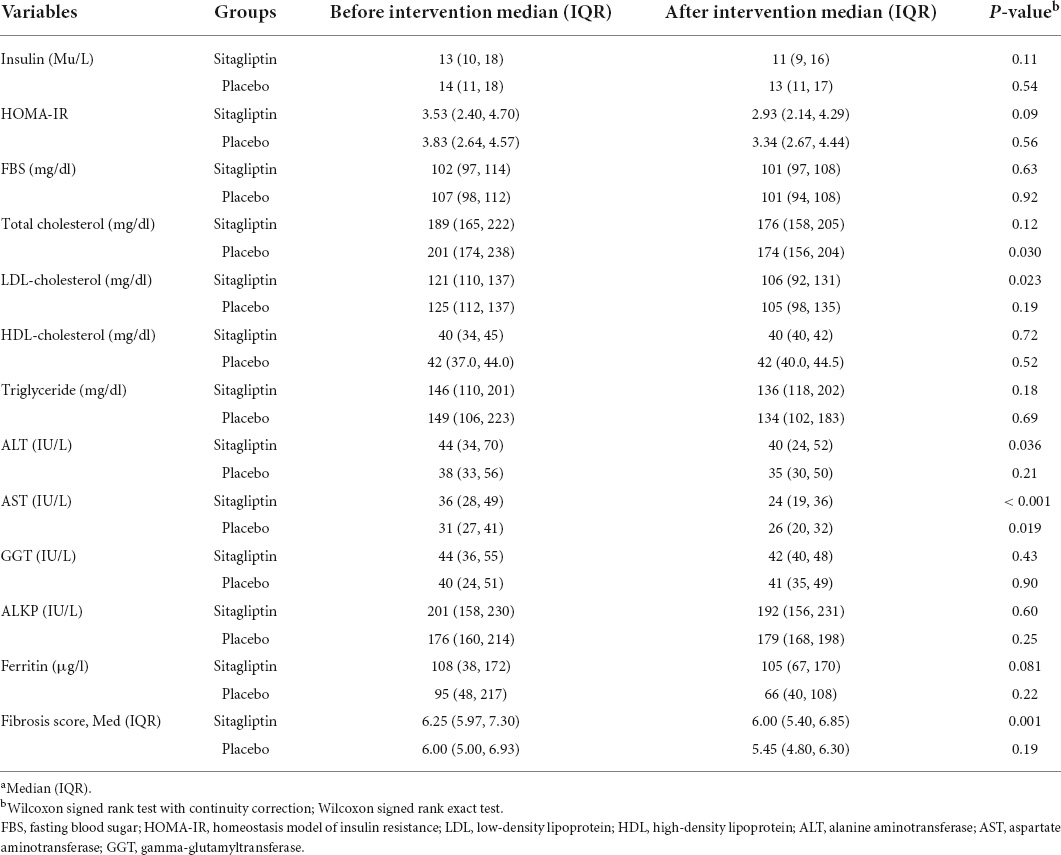
Table 2. Changes in fibrosis score and laboratory data in the sitagliptin group (n = 44) and the placebo group (n = 32) before and after the intervention.a
In male participants, there was a significant decrease in the level of ALT, AST, and fibrosis scores after treatment. There were no significant differences among females (see Supplementary Table 3).
Changes in hepatic fibrosis and liver enzymes by body mass index classification
Changes in fibrosis scores and liver enzyme levels according to the BMI classification in sitagliptin and placebo-treated patients are shown in Table 3. The effect of sitagliptin in reducing fibro scores was significantly greater in normal-weight and overweight individuals than in obese individuals (p = 0.036, and p = 0.018, respectively), whereas the effects on AST levels were higher among overweight/obese patients (p = 0.028, and p = 0.016, respectively). However, separate analysis for sex showed that the effect of sitagliptin in reducing fibro scores (p = 0.032) and AST level (p = 0.039 and p = 0.009, respectively) was significantly greater among overweight and obese males. The differences in these variables were not significant in females (Supplementary Table 3).
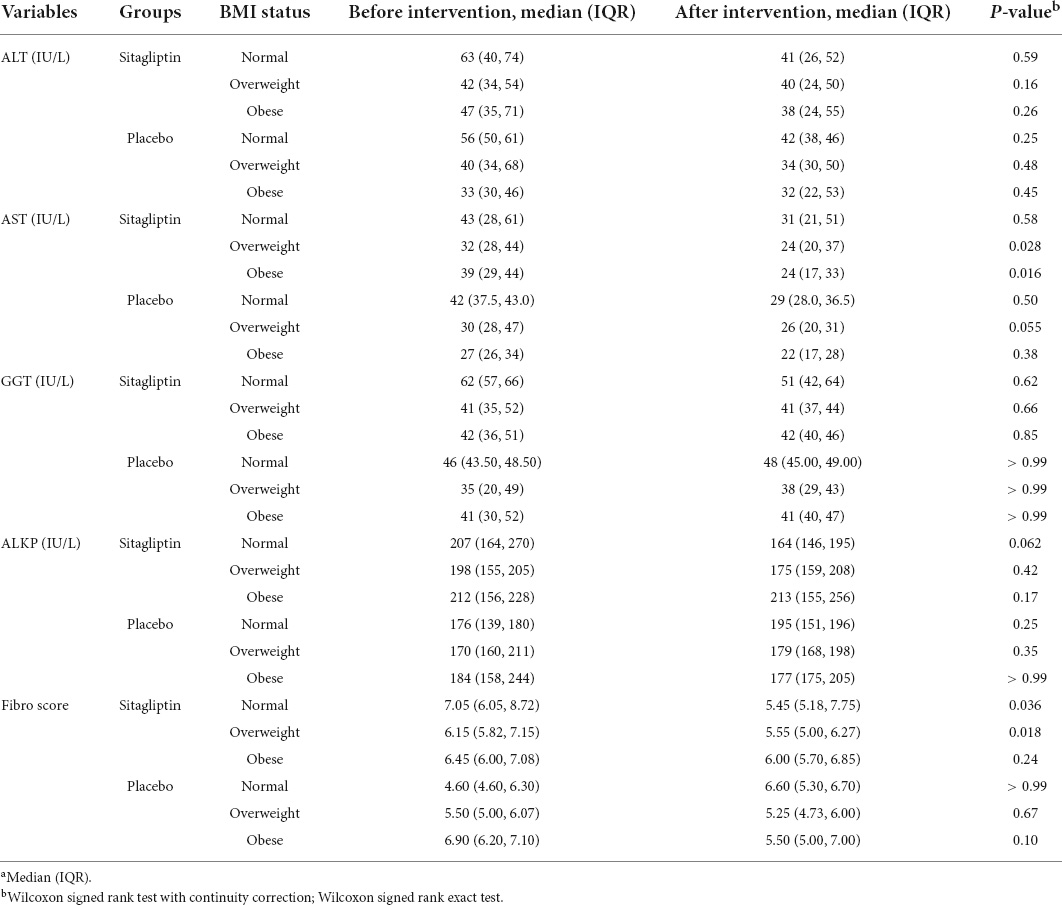
Table 3. Changes in outcome in the treatment group (n = 44) and placebo group (n = 32) based on the BMI status.a
Secondary outcomes: Changes in ferritin, lipid profile, and glucose parameters
LDL-cholesterol was significantly decreased in patients receiving sitagliptin compared to the placebo group (P = 0.023), while the effect of sitagliptin on the reduction of the other secondary outcomes was not statistically significant. A significant increase in serum ferritin level was observed in the sitagliptin group among females, after the intervention (P = 0.01). There were no significant differences for other variables (Supplementary Table 2).
Discussion
To our knowledge, this is the first multicenter randomized, triple-blind, placebo-controlled clinical trial to evaluate the effect of sitagliptin in NAFLD treatment. Our findings showed that sitagliptin, combined with lifestyle modification, reduced fibrosis score, ALT, and AST levels, over 56 weeks of treatment.
Existing evidence evaluating the effects of sitagliptin on fibrosis score and liver transferases in NAFLD is equivocal. Nevertheless, consistent with our results, Alam et al. (34), in an open-label randomized control trial on 40 NASH patients diagnosed with dual-pass liver biopsy, found that intervention with sitagliptin, daily for 1 year, combined with lifestyle modification, improved hepatic histological and fibrosis of non-alcoholic steatosis patients, as compared with lifestyle modification only. Our results also agreed with Sayari et al. (46), who reported that treatment with sitagliptin in NAFLD patients for 16 weeks significantly reduced ALT and AST levels.
The results of several small-scale, placebo-controlled RCTs conducted in patients with NAFLD indicated that 24 weeks of sitagliptin therapy might not have a beneficial effect on liver fibrosis and transferases (21, 30, 47). Indeed, it has been posited that long-term treatment periods of 1 year or more may be needed to observe such effects (34).
Empirical evidence suggests that sitagliptin might improve steatosis by suppressing lipogenic and gluconeogenic pathways through the inhibitation of DPP-4 and increasing levels of biological activity of GLP-1 and GIP (48, 49). Indeed, researchers have concluded that sitagliptin may have more robust efficacy than weight loss in improving non-alcoholic steatohepatitis, irrespective of diabetes (34).
In the current study, the fibrosis changes were significantly more prominent in patients with normal weight and overweight at baseline, whereas the effects of sitagliptin on AST level were greater among overweight/obese patients. To the best of our knowledge, the role of baseline BMI as a predictor of NAFLD patients’ response to sitagliptin has not been previously reported. Our results showed that lower baseline BMI may lead to better fibrosis scores in patients receiving sitagliptin. This pattern was reversed in the effect of sitagliptin on the AST level. In fact, the extant literature suggests that lifestyle modifications have a greater impact on downgrading fibroscan values and ALT than other NAFLD biomarkers (50).
Obesity may attenuate the liver fibrosis-lowering effect of sitagliptin in NAFLD patients. In some studies (51, 52), GLP-1 levels in response to increased carbohydrate intake in obese patients have been reduced, leading to sitagliptin exerting a less potent effect on the decrease in fibrosis score with increasing BMI. In addition, obese patients may not adhere to their lifestyle modification as well as normal-weight patients, although we have no objective data to support this.
Among the secondary outcome measures, sitagliptin significantly reduced LDL-cholesterol in the sitagliptin group and did not affect other lipid profiles, IR parameters, and ferritin. Concordant with our study, Derosa et al. reported that patients receiving sitagliptin, after 7 years of therapy, experienced a greater decrease in total cholesterol and LDL-cholesterol compared to baseline (53). In contrast, in other studies, sitagliptin elicited no significant change in serum level of LDL-cholesterol, despite a reduction in TG, total cholesterol, and HDL cholesterol (54).
A retrospective study by Horton et al. indicated that treatment with sitagliptin for 90–365 days substantially decreased serum levels of TG, total cholesterol, and LDL-cholesterol, except for HDL cholesterol, in individuals with type 2 diabetes (55). Overall, either alone or in combination, sitagliptin resulted in a better lipid profile. The favorable effects of sitagliptin on serum lipid profiles might contribute to its protective effects on gastric inhibitory polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) in improving lipid metabolism. On the other hand, the other effects of sitagliptin on glycemic control and insulin resistance, weight reduction, or delayed stomach emptying can putatively exhibit beneficial effects on lipoprotein metabolism (56).
The strengths of this study include the randomized controlled design, which facilitates a low probability of selection bias and residual confounding. The implementation of a well-validated outcome assessment, such as a fibroscan, is another strength of the study. Further, the present study was conducted through multicenter tertiary care, thus favorably supporting the generalizability of our work. However, there are limitations to the present study that should be considered. The major limitation of this study is the relatively small sample size recruited and the high dropout rate. Indeed, the second 6 months of the trial coincided with the COVID-19 pandemic, which had significant effects on the dropout of participants. This limitation reduces the power of the study to identify significant effects; however, even with the limited sample, significant improvements in several outcomes were observed. This suggests that the effect of the sitagliptin on these measures may be more potent than estimated in prior power calculations. The other main limitations of the study included the lack of assessment of abdominal obesity by WC or WHR; indeed, although visceral fat excess is the main mechanism of NAFLD, lifestyle modifications, including dietary intake and physical activity, there was no strict protocol in this study, and weight status was not evaluated at the end of the treatment. One of the potential limitations in all clinical trials is the contamination across groups. Contamination of control participants has two related effects. It reduces the point estimate of an intervention’s effectiveness, and this apparent reduction may lead to a type II error- rejection of an effective intervention as ineffective because the observed effect size was neither statistically nor clinically significant (57). However, this issue was mitigated by the triple-blind design in the current study. Further, the effect of social desirability due to the cultural context of our society was another limitation that could affect how the study participants responded to the question of alcohol consumption history. However, we tried to mitigate this bias effect by reassuring patients about the confidentiality of their information and active and ongoing communication with participants. Furthermore, shear-wave elastography was used to determine NAFLD severity rather than liver biopsy, which is the gold-standard procedure (58). Since liver biopsy is an invasive procedure carrying potential risks of several complications (59), newer commercially available equipment for liver elastography, such as shear-wave elastography, gives enhanced diagnostic accuracy compared to other elastographic techniques and minimizes different physical limits of the approach, such as the presence of obesity (60, 61). Finally, we used additional glycemic control markers, such as FBS, serum insulin, and HOMA-IR, instead of measuring HbA1C, which is an index for overall glycemia.
Conclusion
Sitagliptin reduced fibrosis scores and liver enzymes in NAFLD patients after 56 weeks of therapy. The changes in fibrosis scores were more prominent in patients with normal weight and overweight than obese patients, whereas the effects on AST levels were greater among overweight/obese patients. Further randomized trials with larger sample sizes and longer treatment durations may be required before consensus can be reached.
Data availability statement
The raw data supporting the conclusions of this article will be available from the corresponding author on reasonable request. Requests to access these data sets should be directed to FZ, emFtYW5pLmZhcmhhZEBnbWFpbC5jb20=.
Ethics statement
The studies involving human participants were reviewed and approved by the Iran University of Medical Sciences (IUMS) ethical committee (No. IR.IUMS.REC.1397.1062). The patients/participants provided their written informed consent to participate in this study.
Author contributions
FZ, AN, NM, HA, MN, AF, ES, and RE were responsible for the study concept and design. FZ and AD had full access to all data and took responsibility for the integrity of the data and the accuracy of the data analysis. MM, FS, and MS involved in data collection. NM, CC, and MF analyzed and interpreted the data. AD and AN wrote the initial draft of the manuscript. FZ was the guarantor and takes responsibility for the manuscript as a whole. All authors revised the manuscript critically for important intellectual content and approved the final manuscript.
Funding
This work was funded by the Gastrointestinal and liver Diseases Research Center (GILDRC), Iran University of Medical Sciences (IUMS) (grant no. 97-3-30-13227).
Acknowledgments
We greatly appreciate the participants, healthcare executives in public health centers in Amol, and the GILDRC staff (https://gildrc.iums.ac.ir), without whom the study would not have been possible.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.937554/full#supplementary-material
References
1. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. (2019) 69:2672–82.
2. Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: definitions, risk factors, and workup. Clin Liver Dis. (2012) 1:99.
3. Burt AD, Lackner C, Tiniakos DG. Diagnosis and assessment of NAFLD: definitions and histopathological classification. Semin Liver Dis. (2015) 35:207–20.
4. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—meta−analytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84.
5. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. (2018) 15:11–20.
6. Rinaldi L, Pafundi PC, Galiero R, Caturano A, Morone MV, Silvestri C, et al. Mechanisms of non-alcoholic fatty liver disease in the metabolic syndrome. A narrative review. Antioxidants. (2021) 10:270.
7. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. (2020) 73:202–9.
8. Shiha G, Korenjak M, Eskridge W, Casanovas T, Velez-Moller P, Högström S, et al. Redefining fatty liver disease: an international patient perspective. Lancet Gastroenterol Hepatol. (2021) 6:73–9. doi: 10.1016/S2468-1253(20)30294-6
9. Xian Y-X, Weng J-P, Xu F. MAFLD vs. NAFLD: shared features and potential changes in epidemiology, pathophysiology, diagnosis, and pharmacotherapy. Chin Med J. (2021) 134:8–19. doi: 10.1097/CM9.0000000000001263
10. Almahmoud MH, Al Khawaja NM, Alkinani A, Khader Y, Ajlouni KM. Prevalence of fatty liver disease and its associated factors among Jordanian patients with type 2 diabetes mellitus: a cross-sectional study. Ann Med Surg. (2021) 68:102677. doi: 10.1016/j.amsu.2021.102677
11. Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. (2021) 160:912–8. doi: 10.1053/j.gastro.2020.11.051
12. Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. (2012) 55:885–904. doi: 10.1007/s00125-011-2446-4
13. Negi CK, Babica P, Bajard L, Bienertova-Vasku J, Tarantino G. Insights into the molecular targets and emerging pharmacotherapeutic interventions for nonalcoholic fatty liver disease. Metabolism. (2022) 126:154925.
14. Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. (2019) 70:711–24.
15. Perticone M, Cimellaro A, Maio R, Caroleo B, Sciacqua A, Sesti G, et al. Additive effect of non-alcoholic fatty liver disease on metabolic syndrome-related endothelial dysfunction in hypertensive patients. Int J Mol Sci. (2016) 17:456. doi: 10.3390/ijms17040456
16. Foroughi M, Maghsoudi Z, Khayyatzadeh S, Ghiasvand R, Askari G, Iraj B. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. (2016) 5:28.
17. Li Y, Liu L, Wang B, Wang J, Chen D. Metformin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Biomed Rep. (2013) 1:57–64.
18. Haukeland JW, Konopski Z, Eggesbø HB, von Volkmann HL, Raschpichler G, Bjøro K, et al. Metformin in patients with non-alcoholic fatty liver disease: a randomized, controlled trial. Scand J Gastroenterol. (2009) 44:853–60.
19. Yoneda M, Honda Y, Ogawa Y, Kessoku T, Kobayashi T, Imajo K, et al. Comparing the effects of tofogliflozin and pioglitazone in non-alcoholic fatty liver disease patients with type 2 diabetes mellitus (ToPiND study): a randomized prospective open-label controlled trial. BMJ Open Diabetes Res Care. (2021) 9:e001990. doi: 10.1136/bmjdrc-2020-001990
20. Kuchay MS, Krishan S, Mishra SK, Farooqui KJ, Singh MK, Wasir JS, et al. Effect of empagliflozin on liver fat in patients with type 2 diabetes and nonalcoholic fatty liver disease: a randomized controlled trial (E-LIFT Trial). Diabetes Care. (2018) 41:1801–8. doi: 10.2337/dc18-0165
21. Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. (2016) 387:679–90. doi: 10.1016/S0140-6736(15)00803-X
22. Sell H, Blüher M, Klöting N, Schlich R, Willems M, Ruppe F, et al. Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care. (2013) 36:4083–90. doi: 10.2337/dc13-0496
23. Itou M, Kawaguchi T, Taniguchi E, Sata M. Dipeptidyl peptidase-4: a key player in chronic liver disease. World J Gastroenterol. (2013) 19:2298. doi: 10.3748/wjg.v19.i15.2298
24. Hussain H, Abbas G, Green IR, Ali I. Dipeptidyl peptidase IV inhibitors as a potential target for diabetes: patent review (2015-2018). Expert Opin Ther Patents. (2019) 29:535–53. doi: 10.1080/13543776.2019.1632290
25. Liu Y, Wei R, Hong T-P. Potential roles of glucagon-like peptide-1-based therapies in treating non-alcoholic fatty liver disease. World J Gastroenterol. (2014) 20:9090. doi: 10.3748/wjg.v20.i27.9090
26. Ideta T, Shirakami Y, Miyazaki T, Kochi T, Sakai H, Moriwaki H, et al. The dipeptidyl peptidase-4 inhibitor teneligliptin attenuates hepatic lipogenesis via AMPK activation in non-alcoholic fatty liver disease model mice. Int J Mol Sci. (2015) 16:29207–18. doi: 10.3390/ijms161226156
27. Iwasaki T, Tomeno W, Yoneda M, Inamori M, Shirakawa J, Imajo K, et al. Non-alcoholic fatty liver disease adversely affects the glycemic control afforded by sitagliptin. Hepato Gastroenterol. (2012) 59:1522–5. doi: 10.5754/hge11689
28. Díaz EG, Guagnozzi D, Gutiérrez V, Mendoza C, Maza C, Larrañaga Y, et al. Effect of incretin therapies compared to pioglitazone and gliclazide in non-alcoholic fatty liver disease in diabetic patients not controlled on metformin alone: an observational, pilot study. Endocrinol Nutr. (2016) 63:194–201. doi: 10.1016/j.endonu.2016.01.006
29. Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastro Enterol Belg. (2012) 75:240–4.
30. Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: a randomized controlled trial. J Hepatol. (2016) 65:369–76.
31. Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan H, Naeshiro N, et al. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepato Gastroenterol. (2014) 61:323–8.
32. Onoyama T, Koda M, Okamoto T, Kishina M, Matono T, Sugihara T, et al. Therapeutic effects of the dipeptidyl peptidase-IV inhibitor, sitagliptin, on non-alcoholic steatohepatitis in FLS-ob/ob male mice. Mol Med Rep. (2015) 12:6895–902. doi: 10.3892/mmr.2015.4329
33. Ren J, Wang X, Yee C, Gorrell MD, McLennan SV, Twigg SM. Sitagliptin is more effective than gliclazide in preventing pro-fibrotic and pro-inflammatory changes in a rodent model of diet-induced non-alcoholic fatty liver disease. Molecules. (2022) 27:727. doi: 10.3390/molecules27030727
34. Alam S, Ghosh J, Mustafa G, Kamal M, Ahmad N. Effect of sitagliptin on hepatic histological activity and fibrosis of nonalcoholic steatohepatitis patients: a 1-year randomized control trial. Hepat Med Evid Res. (2018) 10:23. doi: 10.2147/HMER.S158053
35. Lian J, Fu J. Pioglitazone for NAFLD patients with prediabetes or type 2 diabetes mellitus: a meta-analysis. Front Endocrinol. (2021) 12:615409. doi: 10.3389/fendo.2021.615409
36. Mahale AR, Prabhu SD, Nachiappan M, Fernandes M, Ullal S. Clinical relevance of reporting fatty liver on ultrasound in asymptomatic patients during routine health checkups. J Int Med Res. (2018) 46:4447–54. doi: 10.1177/0300060518793039
37. Wong VW-S, Petta S, Hiriart J-B, Cammà C, Wong GL-H, Marra F, et al. Validity criteria for the diagnosis of fatty liver by M probe-based controlled attenuation parameter. J Hepatol. (2017) 67:577–84. doi: 10.1016/j.jhep.2017.05.005
38. Hashemi S-A, Alavian S-M, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Caspian J Int Med. (2016) 7:242.
39. Karlas T, Petroff D, Garnov N, Böhm S, Tenckhoff H, Wittekind C, et al. Non-invasive assessment of hepatic steatosis in patients with NAFLD using controlled attenuation parameter and 1H-MR spectroscopy. PLoS One. (2014) 9:e91987. doi: 10.1371/journal.pone.0091987
40. Matthews DR, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
41. WHO Expert Committee. Physical Status: the Use and Interpretation of Anthropometry. WHO Technical Report Series No. 854. Geneva: World Health Organization (1995). p. 1–452.
42. Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American heart association nutrition committee. Circulation. (2006) 114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158
43. Patel J, Bettencourt R, Cui J, Salotti J, Hooker J, Bhatt A, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. (2016) 9:692–701. doi: 10.1177/1756283X16656735
44. Machin D, Campbell MJ, Tan SB, Tan SH. Sample Sizes for Clinical, Laboratory and Epidemiology Studies. 4th ed. Hoboken, NJ: Wiley (2018). p. 232.
45. Bian Z-X, Shang H-C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Int Med. (2011) 154:290–1.
46. Sayari S, Neishaboori H, Jameshorani M. Combined effects of synbiotic and sitagliptin versus sitagliptin alone in patients with nonalcoholic fatty liver disease. Clin Mol Hepatol. (2018) 24:331. doi: 10.3350/cmh.2018.0006
47. Joy TR, McKenzie CA, Tirona RG, Summers K, Seney S, Chakrabarti S, et al. Sitagliptin in patients with non-alcoholic steatohepatitis: a randomized, placebo-controlled trial. World J Gastroenterol. (2017) 23:141.
48. Akaslan SB, Degertekin CK, Yilmaz G, Cakir N, Arslan M, Toruner FB. Effects of sitagliptin on nonalcoholic fatty liver disease in diet-induced obese rats. Metab Syndr Relat Disord. (2013) 11:243–50. doi: 10.1089/met.2012.0128
49. Nargis T, Chakrabarti P. Significance of circulatory DPP4 activity in metabolic diseases. IUBMB Life. (2018) 70:112–9.
50. Paul J, Venugopal RV, Peter L, Hussain S, Naresh Kumar Shetty K, Shetti MP. Effects of lifestyle modification on liver enzyme and Fibroscan in Indian patients with non-alcoholic fatty liver disease. Gastroenterol Rep. (2018) 6:49–53. doi: 10.1093/gastro/gox020
51. Muscelli E, Mari A, Casolaro A, Camastra S, Seghieri G, Gastaldelli A, et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. (2008) 57:1340–8. doi: 10.2337/db07-1315
52. Ranganath L, Beety J, Morgan L, Wright J, Howland R, Marks V. Attenuated GLP-1 secretion in obesity: cause or consequence? Gut. (1996) 38:916–9.
53. Derosa G, Tritto I, Romano D, D’Angelo A, Catena G, Maffioli P. Effects of sitagliptin on lipid profile in patients with type 2 diabetes mellitus after 7 years of therapy. J Clin Pharmacol. (2019) 59:1391–9.
54. Fan M, Li Y, Zhang S. Effects of sitagliptin on lipid profiles in patients with type 2 diabetes mellitus: a meta-analysis of randomized clinical trials. Medicine (Baltimore). (2016) 95:e2386. doi: 10.1097/MD.0000000000002386
55. Horton ES, Silberman C, Davis KL, Berria R. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. (2010) 33:1759–65.
56. Dhir G, Cusi K. Glucagon like peptide-1 receptor agonists for the management of obesity and non-alcoholic fatty liver disease: a novel therapeutic option. J Investig Med. (2018) 66:7–10. doi: 10.1136/jim-2017-000554
57. Torgerson DJ. Contamination in trials: is cluster randomisation the answer? BMJ. (2001) 322:355–7.
58. Sebastiani G, Halfon P, Castera L, Pol S, Thomas DL, Mangia A, et al. SAFE biopsy: a validated method for large−scale staging of liver fibrosis in chronic hepatitis C. Hepatology. (2009) 49:1821–7. doi: 10.1002/hep.22859
59. Seeff LB, Everson GT, Morgan TR, Curto TM, Lee WM, Ghany MG, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. (2010) 8:877–83. doi: 10.1016/j.cgh.2010.03.025
60. Roulot D, Costes J-L, Buyck J-F, Warzocha U, Gambier N, Czernichow S, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. (2011) 60:977–84. doi: 10.1136/gut.2010.221382
Keywords: sitagliptin, NAFLD, clinical trial design, fibrosis scores, liver enzymes
Citation: Doustmohammadian A, Nezhadisalami A, Safarnezhad Tameshke F, Motamed N, Maadi M, Farahmand M, Sohrabi M, Clark CCT, Ajdarkosh H, Faraji AH, Nikkhah M, Sobhrakhshankhah E, Ebrahimi R and Zamani F (2022) A randomized triple-blind controlled clinical trial evaluation of sitagliptin in the treatment of patients with non-alcoholic fatty liver diseases without diabetes. Front. Med. 9:937554. doi: 10.3389/fmed.2022.937554
Received: 06 May 2022; Accepted: 07 July 2022;
Published: 28 July 2022.
Edited by:
Mohammad Siddiqui, Virginia Commonwealth University, United StatesReviewed by:
Gabriella Garruti, University of Bari Aldo Moro, ItalyGiovanni Tarantino, University of Naples Federico II, Italy
Copyright © 2022 Doustmohammadian, Nezhadisalami, Safarnezhad Tameshke, Motamed, Maadi, Farahmand, Sohrabi, Clark, Ajdarkosh, Faraji, Nikkhah, Sobhrakhshankhah, Ebrahimi and Zamani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farhad Zamani, emFtYW5pLmZhcmhhZEBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Azam Doustmohammadian
Azam Doustmohammadian Ahmad Nezhadisalami2†
Ahmad Nezhadisalami2† Nima Motamed
Nima Motamed Mansooreh Maadi
Mansooreh Maadi Mohammad Farahmand
Mohammad Farahmand Masoudreza Sohrabi
Masoudreza Sohrabi Cain C. T. Clark
Cain C. T. Clark Elham Sobhrakhshankhah
Elham Sobhrakhshankhah Farhad Zamani
Farhad Zamani