- 1The China Australia International Research Centre for Chinese Medicine, School of Health and Biomedical Sciences, STEM College, RMIT University, Bundoora, VIC, Australia
- 2Guangdong Provincial Hospital of Chinese Medicine and Guangdong Provincial Academy of Chinese Medical Sciences, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
Background: Migraine is a chronic neurological disease causing significant socioeconomic burden and impaired quality of life. Chinese medicine is commonly used for migraine in China. Clinical trials have generated evidence of the effectiveness of Chinese medicine therapies for migraine. However, little is known about how to use these therapies to treat migraine in real-world clinical settings.
Methods: In this retrospective study, we analyzed data from the electronic medical records (EMRs) of 2,023 migraine patients who attended the Guangdong Provincial Hospital of Chinese Medicine (GPHCM) between July 2018 and July 2020.
Results: More than three-quarters (77.21%) of the patients were female. Most (78.20%) of the patients were aged between 18 and 50 years, 18.49% were aged above 50 years, and the remaining 3.31% were under 18 years. Sleep disorders were the most documented comorbidity occurring in 27.29% of patients, and more common in females (29.77%) than male (18.87%). Fatigue was the most frequently reported trigger of migraine attacks among all patients (9.39%), while menstruation was the most common trigger for female patients (10.24%). Less than a quarter of patients (21.01%) reported a history of taking analgesic medication for their migraine. The median treatment duration reported by the patients was 10 days. Chinese herbal medicine (CHM) was the predominant treatment for migraine at the hospital (88.48%), while pharmacotherapies were prescribed to 28.97% of the patients. CHM was prescribed more often as a sole treatment (53.58% of patients) than combined with pharmacotherapies (27.39% of patients). Among patients who reported improvements after taking CHM, the most frequently used herbs were fu ling and chuan xiong, the most frequent patented CHM product was tong tian oral solution, and the main herbal formulae were chuan xiong cha tiao san and yi qi cong ming tang.
Conclusion: CHM formulae, such as chuan xiong cha tiao san and yi qi cong ming tang, patented CHM product tong tian oral solution, and some herbs are potentially effective treatments for migraine. As such, CHM can be used as an alternative to conventional pharmacotherapies for migraine and is worth further evaluation in randomized controlled trials.
Introduction
Migraine is a prevalent primary headache disorder characterized by recurrent, unilateral, moderate-to-severe pulsating headaches (1). The headache is usually associated with nausea, vomiting, phonophobia, and photophobia (1). Migraine is often accompanied by comorbidities, such as sleep disorders, anxiety, and depression (2–6), and can be triggered by common lifestyle factors, including stress, caffeine, and menstruation (7, 8). According to a systematic review of the Global Burden of Disease Study (9), migraine was estimated to affect 1.04 billion people with a global age-standardized prevalence of 14.4% and caused 45.1 million years of life with disabilities (YLDs). Both the prevalence and YLDs of migraine peaked between 35 and 39 years of age in both genders (9).
Current pharmacotherapies for migraine consist of acute treatments including triptans and ergots, and prophylactic therapies such as calcium channel blockers (CCBs), beta-blockers, and calcitonin gene-related peptide (CGRP) antibodies (10, 11). Patients are often unsatisfied with these medications due to their insufficient treatment effects, potential risk of causing comorbidities, and unwanted side effects (12–14). Moreover, acute pain-relief medications tend to be overused by migraine patients, resulting in the transformation from episodic migraine to chronic migraine (15, 16) as well as a higher risk of psychological comorbidities (17, 18), and hence increases the disease burden (19).
Due to these challenges, complementary and alternative medicine is popular among migraine patients (20, 21). Chinese medicine therapies, including Chinese herbal medicine (CHM) and acupuncture, were reported to be prescribed over 60% of migraine patients in China (22). Systematic reviews concluded that Chinese medicine therapies were comparable or superior to conventional pharmacotherapies, either being used solely or in combination with pharmacotherapies (23–33). However, the existing research evidence was obtained from randomized controlled trials (RCTs) that applied unified treatments to certain populations based on pre-defined selection criteria. There is a lack of real-world information about treatment patterns and first-hand clinicians’ experience. Moreover, the clinical characteristics and preferences of migraine patients seeking Chinese medicine therapies also remain unclear. Therefore, we conducted a retrospective analysis of electronic medical records (EMRs) from a large-sized Chinese medicine hospital to explore and summarize the real-world clinical evidence, patients’ characteristics, and clinicians’ experiences. The results of this research will be valuable for evidence-based clinical practice.
Materials and methods
This retrospective study collected clinical data from outpatient departments at the Guangdong Provincial Hospital of Chinese Medicine (GPHCM), the largest tertiary hospital that provides integrated Chinese medicine and conventional therapies for patients in China (34).
Ethics consideration
The study was approved by the Human Research Ethics Committee (HREC) of GPHCM (ZE2020-243-01) and registered with the HREC at RMIT University (no. 24235). Informed consent was waived since identifiable information, including names, identification numbers, dates of birth, phone numbers, and residential addresses of patients, had been deidentified in the dataset before data screening and analyses.
Data search and screening
The EMRs between July 2018 and July 2020 were retrieved by the Information Technology Department of GPHCM to locate migraine-related patient encounters (PEs). Only those PEs with migraine as the primary diagnosis were exported to an Excel dataset and then screened by a clinician who specialized in headache and neurology (S Lyu) for eligibility.
PE records were excluded if they met any of the following criteria: (1) incomplete records; (2) symptoms not consistent with migraine diagnostic criteria (1); (3) chief complaint is not related to migraine; and (4) reporting treatment effects in the first PE among a series of PEs. Uncertainties were resolved by consulting a senior headache/neurology specialist (J Sun).
Data extraction
Preliminary data extraction was conducted by H Weng using TNorm, a rule-based and pattern learning-based approach developed for automatic temporal expression extraction and normalization for data in Chinese text (35). Structured data, such as age (at the first visit), date of visits, gender, current and previous medical histories, diagnoses, and treatment details, were extracted from each PE into an Excel dataset at this stage. During this procedure, PEs sharing the same medical record number were merged as one EMR.
Further data extraction was manually conducted by S Lyu to identify unstructured data on migraine comorbidities, triggers, numbers of visits, total treatment duration, patients’ response to treatments, and in which visits patients reported improvements. Clinical conditions, such as depression, anxiety, sleep disorder, rhinitis, and dermatological conditions, were considered as comorbidities of migraine according to previous research (36–40). Factors including fatigue, menstruation, coldness or wind, emotion, crowded environment, poor sleep, weather changes, stress, diet, strong light exposure, exercise, washing hair, and odor, were classified as triggers of migraine episodes based on clinical guidelines and previous studies (41–48). Since migraine triggers vary across individuals, the trigger data were extracted based on patient-reported information. Treatment responses were briefly classified as “improved” and “no response or unclear,” based on patients’ self-reporting as recorded in EMRs. Where EMRs recorded a response consistent with the Diagnostic Criteria and Category of Treatment Response of Toufeng in China (49), including the reduction of migraine or other symptoms in general after certain treatments, the patients were marked as “improved” and the previous PE was marked as “patient encounter reporting improvements (PERI).” Data on the CHM prescribed in PERIs, including Chinese herbal decoctions and patented Chinese herbal medicine products (PCHMP), were extracted for further analyses. Acupuncture points were not extracted since they usually were not recorded in detail.
Logical data checking and correction were also conducted by S Lyu to identify inconsistencies between PEs from the same EMR.
Data standardization
Descriptions of the migraine-associated symptoms, comorbidities, triggers, and treatment responses in the EMRs were standardized using common medical terminologies. Chinese medicinal herbs in granule or decoction forms, which were processed in various ways were standardized as one herb using the herb name listed in the China Pharmacopoeia (version 2020) (50). For example, chao bai zhu (fried bai zhu) was standardized as bai zhu since they were not distinguished from each other in the China Pharmacopoeia. In contrast, sheng di huang remains distinguished from shu di huang because both names are listed in the China Pharmacopoeia with different therapeutic functions (50); this rule was also applied for gan cao and zhi gan cao, and ban xia and sheng ban xia.
Data analyses
Categorical variables were presented as frequency/percentage and compared via Chi-square or Fisher’s tests, where appropriate. Continuous variables were presented as mean with standard deviation and compared via the t-test when they were normally distributed. Otherwise, they were presented as median with interquartile range and compared via the Mann–Whitney U-test. SPSS software (version 20.0, SPSS Inc., Chicago, IL, U.S.A) was used for the descriptive analyses of patients’ characteristics and treatment information. Values of p < 0.05 were considered to indicate statistical significance.
Association rule construction based on the Apriori algorithm (51, 52) was conducted to identify high-frequency herb combinations and associations between triggers or comorbidities with herbs, using SPSS Modeler software (version 18.0, SPSS Inc., Chicago, IL, U.S.A). A network diagram was generated to visualize the co-occurrence between the frequently used herbs.
Generally, an Apriori algorithm is constructed based on the notion that the antecedent item sets and the consequent item set only co-occur in the dataset rather than due to a causal effect (53). Three parameters—support, confidence, and lift—are used to assess the associations of variables. Support is the prevalence of antecedent and corresponds to statistical significance (see formula A) (51). The minimum threshold of support is usually predefined to avoid occasional co-occurrence (51, 52). After iterative tests, we set the support level at 5% for herb pair and herb combination analyses to ensure only frequently used herbs appear in the antecedent. Confidence and lift indicate the strength of association, with higher values showing more robust connections between the consequent and antecedent. Confidence reflects the possibility of co-occurrences of consequent and antecedent in the datasets consisting of antecedent (see formula B), while the lift is a value that represents the likelihood of an increase in the consequent given a particular antecedent (see formula C). The thresholds of confidence in the association rules were determined according to individual situations.
The formulae for these three metrics are listed as follows (53, 54):
Results
Summary of the research procedure
A total of 6,582 PEs with a primary diagnosis of migraine were identified and exported. After eligibility screening, 4,395 PEs from 2,023 EMRs (i.e., individual patient records) were included and analyzed for patient characteristics and treatment patterns. Among them, 1,812 PERIs contributed to the in-depth analyses of treatment patterns and therapeutic characteristics to identify the frequently used herbs and herb combinations. The procedure is illustrated in Figure 1.

Figure 1. Flow chart of the study procedure. CHM, Chinese herbal medicine; GPHCM, Guangdong Provincial Hospital of Chinese Medicine; PERI, Patient encounter reporting improvements; PCHMP, Patented Chinese herbal medicine products.
Clinical features of all patients
Demographics
Among the 2,023 patients (EMRs), 1,562 (77.21%) were female. The mean age of all patients was (37.89 ± 12.74) years. The mean age of female patients (38.11 ± 12.53 years) was similar to that of male patients (37.16 ± 13.42 years) (p = 0.16). More than three-quarters of the patients were adults under 50 years of age (n = 1,582, 78.20%), while 374 (18.49%) patients were over 50 years of age and the remaining 67 (3.31%) patients were under 18 years of age (Table 1).
Characteristics
The characteristics of migraine were insufficiently recorded in the EMRs. Only 140 (6.92%) EMRs contained information about aura, with 100 (4.94%) patients diagnosed as migraine with aura and 40 (1.98%) were diagnosed as migraine without aura. A total of 225 (11.12%) patients had a family history of migraine, 84 (4.15%) patients did not have such a family history, and the remaining 1,714 EMRs did not include this information. Acute medication-taking behavior was only reported by 432 (21.35%) patients. Among these patients, 425 (21.01%) had taken acute medication for migraine and 7 patients reported never taking any acute medication. The remaining 1,591 EMRs did not provide clear information on the history of acute medication usage history (Table 1).
In addition, 65 (4.32%) adult female patients reported the occurrence or aggregation of migraine after childbearing. However, the number of adult women whose migraine were not associated with childbearing was unavailable (Table 1).
Comorbidities
Common migraine comorbidities that patients reported were sleep disorders (n = 552, 27.29%), anxiety and/or depression (n = 117, 5.78%), rhinitis (n = 15, 0.74%), and dermatological conditions (n = 37, 1.83%).
As the most reported comorbidity, sleep disorders involved symptoms of insomnia, interrupted sleep, dreaminess, and so on. Around half (n = 890, 43.99%) of the patients were recorded with information on their sleep quality, with 338 (16.71%) reporting satisfactory sleep quality. Based on the available data, female patients seemed more likely to have sleep disorders than male patients (29.77% vs. 18.87%) (Table 1).
Triggers
Migraine triggers documented in the PEs for both genders were fatigue (n = 190, 9.39%), coldness (n = 99, 4.89%), emotion (n = 77, 3.81%), crowded environment (n = 76, 3.76%), poor sleep (n = 68, 3.36%), weather changes (n = 48, 2.37%), stress (n = 26, 1.29%), diet (n = 21, 1.04%), strong light exposure (n = 18, 0.89%), exercise (n = 11, 0.54%), washing hair (n = 8, 0.40%), and odor (n = 1, 0.05%). Among female patients, menstruation was the leading trigger (n = 160, 10.24% of all female patients) (Table 1).
Treatment patterns of all patients
Treatment categories
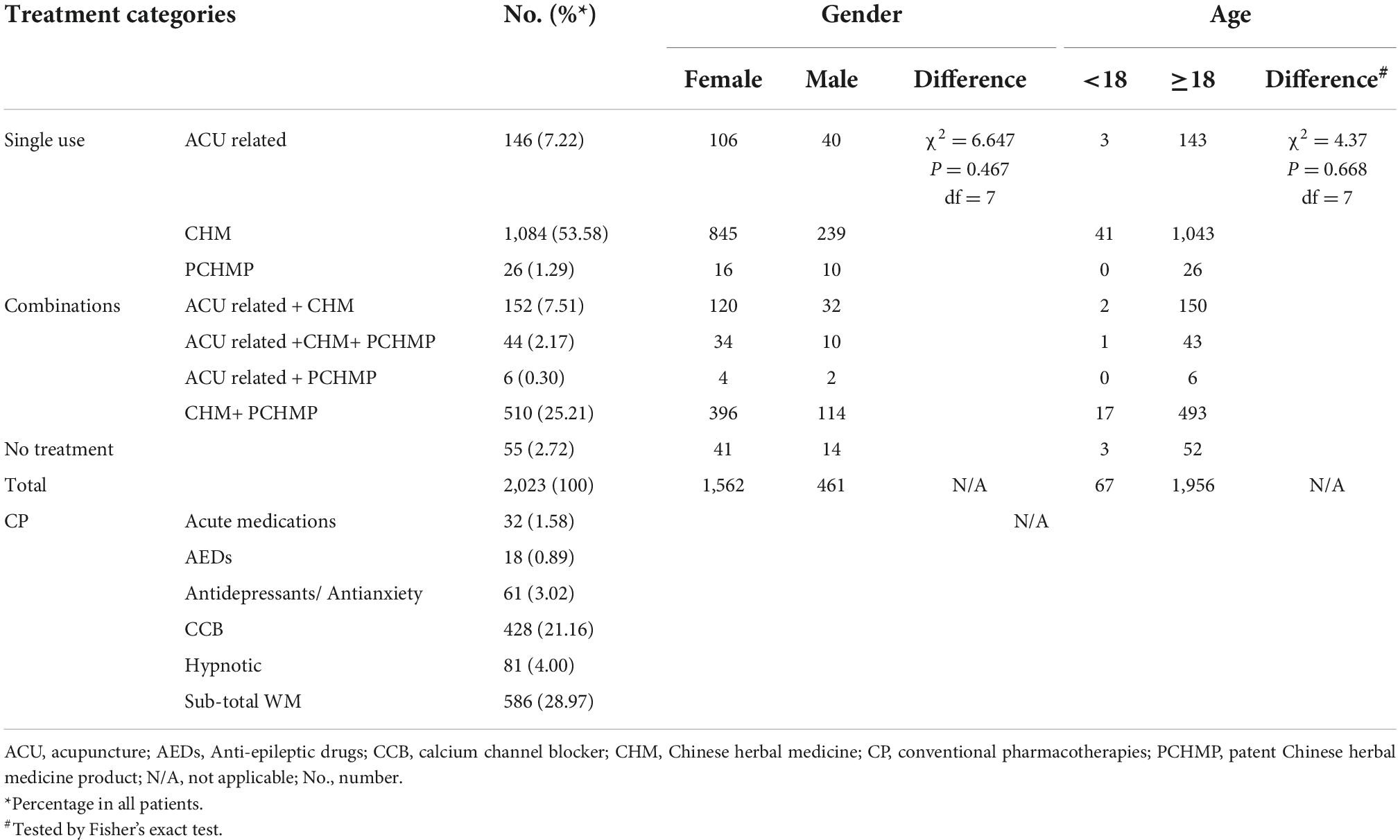
The treatment patterns among 2,023 EMRs are presented in Table 2. Most migraine patients (n = 1,790, 88.48%) had been prescribed CHM, followed by conventional pharmacotherapies (n = 586, 28.97%) and acupuncture (n = 348, 17.20%). CHM was prescribed more often as a sole treatment (n = 1,084) than in combination with conventional pharmacotherapies (n = 554). There was no significant statistical difference in treatment methods between genders (p = 0.467) nor between adult and non-adult groups (p = 0.668). During the treatment course, only 32 (1.58%) patients were newly prescribed acute medication, and 428 (21.16%) patients were prescribed CCBs (flunarizine or nimodipine tablets). Other pharmacotherapies included hypnotic drugs (n = 81, 4.00%), antidepressants/antianxiety drugs (n = 61, 3.02%), and anti-epileptic drugs (n = 18, 0.89%).
Times of visits and treatment duration
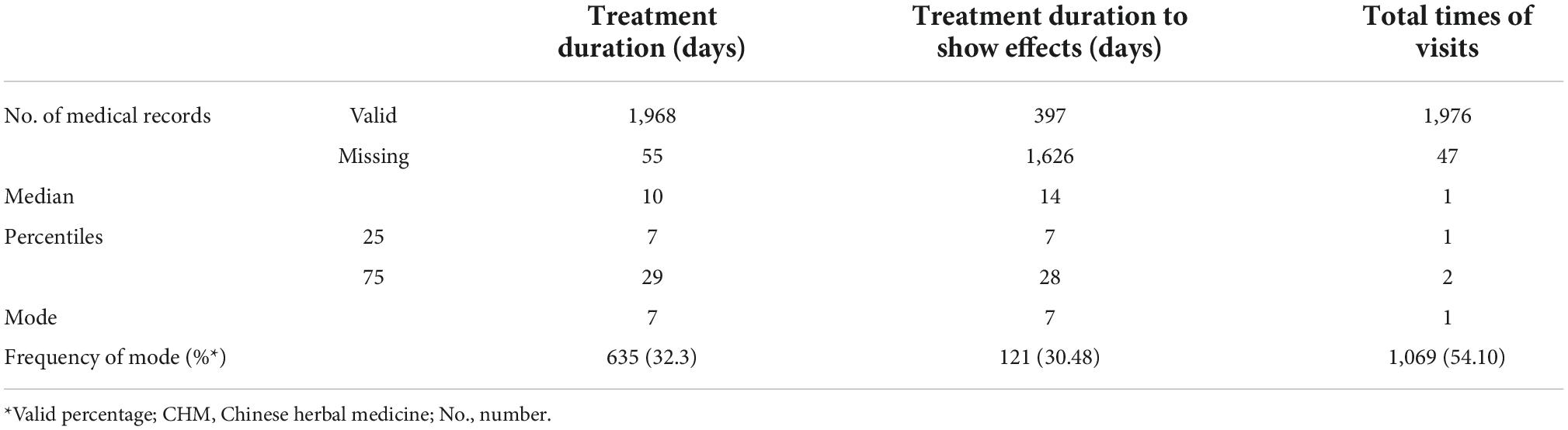
Fifty-five patients only visited the doctors for advice or examinations and did not receive any treatment. The total number of visits to the hospital for migraine was available from 1,976 patients. More than half (n = 1,069, 54.10%) only visited the hospital once for migraine, therefore, their responses to treatments were not available (Table 3).
According to 1,968 EMRs with treatment information, the median duration of migraine-specific treatments was 10 days. Of these patients, 72.41% (n = 1,425) of patients underwent treatment for less than 4 weeks, and nearly one-third of them (n = 635, 31.39%) only accepted treatments for 7 days. This is notably shorter than the recommendation of “at least 4 weeks treatment for migraine prophylaxis” in clinical guidelines (10, 11) (Table 3).
In addition, the duration of the treatment which achieved certain improvements was available from 397 EMRs. The most commonly reported duration was 7 days (n = 121, 30.48%), and the median treatment duration was 14 days (Table 3).
Therapeutic characteristics of Chinese herbal medicine
The therapeutic characteristics of CHM, as the predominant treatment method, were further summarized based on the 1,812 PERIs as follows, regardless of whether they were used alone or in combination with other treatments.
Chinese herbal decoction
Frequently used herb
In 1,506 PERIs, 258 individual herbs were prescribed 23,329 times in a decoction form. Herbs recorded by more than 15% of PERIs are listed in Table 4. The top 10 frequently used herbs were fu ling, chuan xiong, bai shao, ban xia, zhi gan cao, gui zhi, yan hu suo, bai zhu, chen pi, and chang pu.

Table 4. Frequently used herbs in Chinese herbal decoctions for which patients reported improvements.
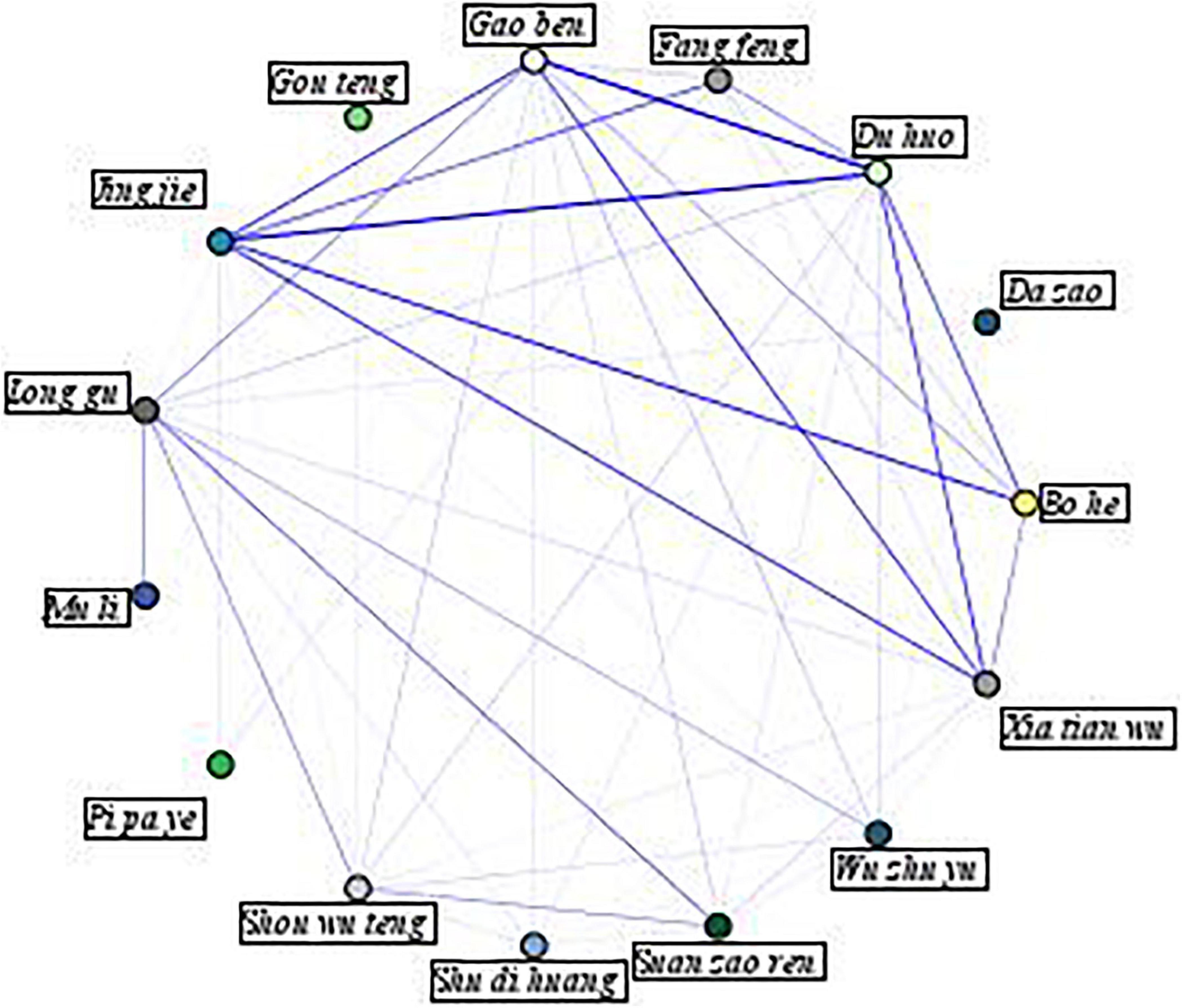
To visualize the associations between individual herbs, a network diagram was generated and presented in Figure 2. In this figure, a thicker line indicates a stronger association between two herbs, indicating that these herbs are more commonly prescribed together. As the figure illustrates, the top 10 strongest links are jing jie and du huo, gao ben, and du huo, bo he and jing jie, jing jie and xia tian wu, jing jie and gao ben, xia tian wu and du huo, xia tian wu and gao ben, bo he and du huo, fang feng and jing jie, and suan zao ren and long gu.
Core herb pairs
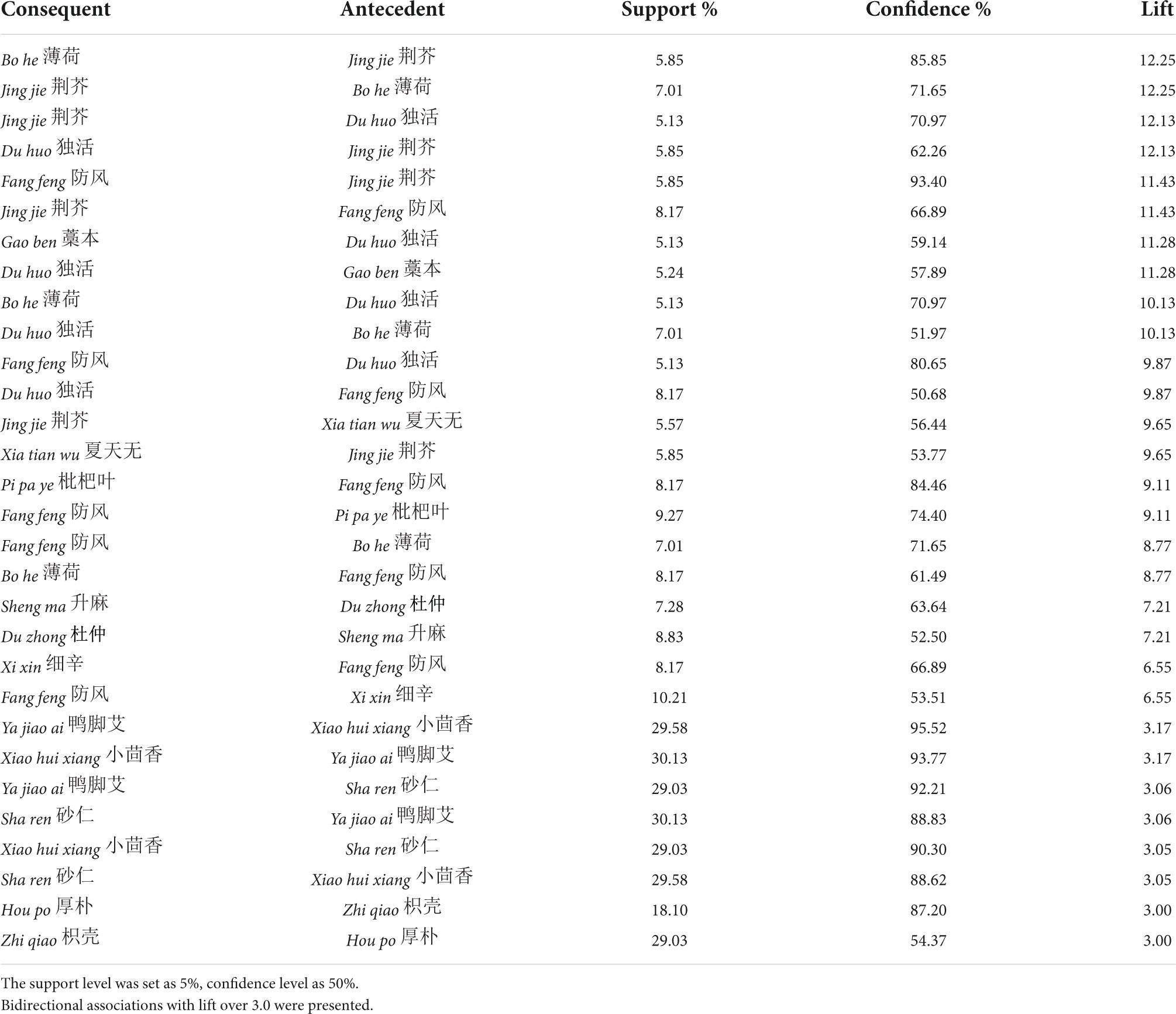
To identify core herb pairs, 491 association rules were constructed when support was set as 5% and confidence as 50%, with only one antecedent. Among them, 15 herb pairs shared bidirectional associations with lifts over three (Table 5). Bidirectional associations are considered mandatory relationships (55, 56). The herb pair with the highest lift (that is, these herbs are more commonly prescribed together) is bo he and jing jie, followed by jing jie and du huo, jing jie and fang feng, du huo and gao ben, du huo and bo he, du huo, and fang feng.
Core herb combinations
During the iterative tests of the association rule for core herb combinations, it was found that the overall confidence in the association rules was high. Therefore, the confidence was preset at 95%, while the support value remained at 5%. The maximum number of antecedents was limited to eight. Based on the predetermined values (see “Materials and methods” section), a total of 837,008 association rules were constructed, 87 of which had a lift over 10 (Table 6). Only 17 herbs were involved in these 87 combinations since the combinations shared the same or similar herbs. The first combination covering the most overlapping herbs is similar to the formula chuan xiong cha tiao san (CXCTS), whose ingredients include bo he, jing jie, fang feng, qiang huo, bai zhi, and chuan xiong. The second combination includes sheng ma, huang qi, man jing zi, ge gen, gan cao, bai zhu, fu ling, ze xie, and yuan zhi, which is similar to the CHM formula yi qi cong ming tang (YQCMT).

Table 6. Core herb combinations in Chinese herbal decoctions for which patients reported improvements.
Associations between comorbidities/triggers and herbs
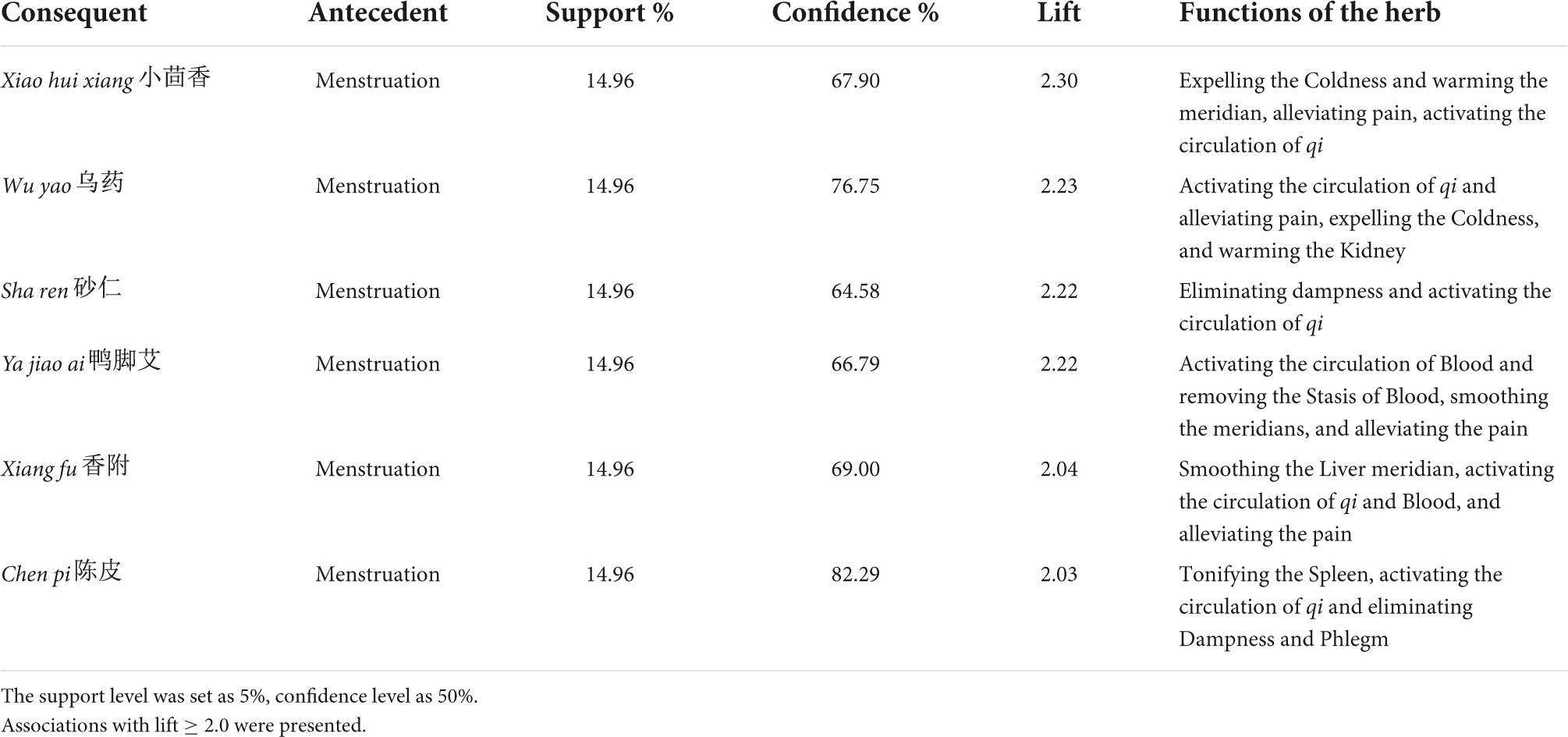
Triggers and comorbidities were set as antecedents, and frequently used herbs were put in the consequent sets. The support level was preset as 5% and confidence as 50%. Constructed associations with lift ≥ 2.0 are presented in Table 7. Menstruation was the only trigger successfully included in the associations and was usually associated with herbs that activate the circulation of Blood and qi, such as xiao hui xiang, wu yao, sha ren, and xiang fu.
Patented Chinese herbal medicine products
A total of 51 PCHMPs were identified from the 1,158 PERIs that used PCHMPs. The top 14 most frequently used PCHMPs are presented in Table 8. The leading PCHMP was tong tian oral solution, with a frequency of 762 (65.80%). It consists of chuan xiong, chi shao, tian ma, qiang huo, bai zhi, xi xin, ju hua, bo he, fang feng, cha, and gan cao. The other PCHMPs that contributed to more than 15% of PERIs were jian wei yu yang tablet (n = 208, 17.96%), tian shu tablet (n = 206, 17.79%), and er shi wu wei shan hu capsule (n = 196, 16.93%).
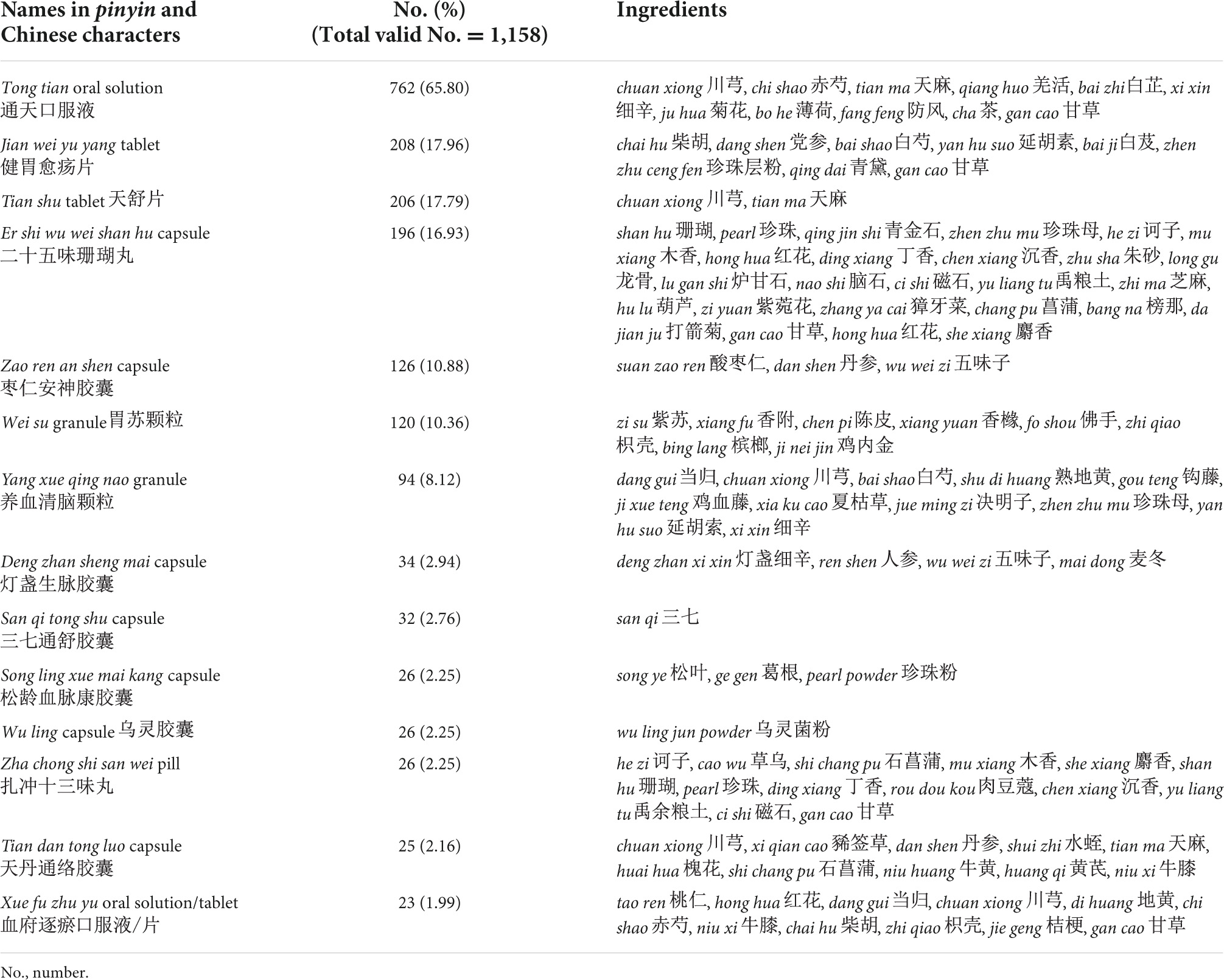
Table 8. Frequently used patented Chinese herbal medicine products for which patients reported improvements.
Mechanism of herbs and formula
The most frequently used herb, fu ling, tonifies Spleen and eliminates dampness (50). Patients in this study are primarily located in southern China, and as such, dampness would be the key Chinese medicine pathogenic factor in their cases (57, 58). It could be explained that fu ling was often used due to these patients’ general constitutions. Currently, there is no direct pre-clinical evidence to support fu ling’s mechanism for curing migraine or headaches. However, research illustrated that Poria cocos polysaccharide, an active compound of fu ling, could exert neuroprotective effects by alleviating oxidative stress, apoptosis, inflammation, and inhibiting the MAPK/NF-κB pathway in Alzheimer’s disease rats (59). Similar pathways were identified in migraine pathology (60–62).
The second frequently used herb chuan xiong activates qi and Blood, expels Wind, and alleviates pain (50). It has been widely used for migraine and headaches in historical and current clinical practice (23, 63–65). An RCT-based systematic review indicated that formulae containing chuan xiong were effective in migraine prophylaxis (24). The key active compound of chuan xiong, Senkyunolide I, exerts anti-migraine effects by adjusting monoamine neurotransmitters levels and turnover rates and decreases nitric oxide levels in the blood and brain (66). Also, the volatile oil from chuan xiong presents an analgesic effect by inhibiting the c-fos gene expression and plasma CGRP in nitroglycerin-induced headaches in rats (67). In addition, a chuan xiong extract, ligustrazine, showed potent activity against nitroglycerin-induced migraine in rats by inhibiting the c-fos/ERK signaling pathway (68).
The formula CXCTS has also been widely used in historical and current clinical practice (69, 70), and has been proven effective for migraine management (71). CXCTS exerts anti-migraine effects by reducing the CGRP level (72) and inhibiting the PI3K-AKT and HIF-1 signaling pathways (73).
The formula YQCMT was proved to be more effective than flunarizine in treating vestibular migraine with a Chinese medicine syndrome of qi deficiency (74). Mechanisms on the core herb pairs for migraine are yet to be discovered and require further bench research.
Discussion
Summary of the results
Migraine is a prevalent, disabling disease that causes significant burdens on patients and the health system. As current conventional migraine management, including pharmacotherapies and lifestyle changes, is not always effective, it is important to explore how complementary and alternative treatments for migraines can be used in real-world clinical practice. EMRs are invaluable sources of real-world data that can be used to generate clinical practice evidence (75). Hospital-based EMRs have the natural advantages of being reliable sources, large sample sizes, structured frameworks, and diverse levels (76). This real-world clinical data provides first-hand, convincing information about Chinese medicine clinicians’ experience (77), which can vitally contribute to evidence-based practice (78).
This retrospective study summarized migraine patients’ characteristics, comorbidities, and triggers based on EMRs from a tertiary Chinese medicine hospital with approximately seven million outpatient visits annually. The gender distribution of all EMRs (female to male ratio 3.4:1), among patients receiving CHM (1,395:395) and acupuncture-related treatments (264:84), are all consistent with previous epidemiological studies (79, 80). The age range of patients is also consistent with the Global Burden of Disease Study (9). The comorbidities (e.g., sleep disorders, anxiety, and depression) and triggers (e.g., fatigue, stress, diet, poor sleep, and menstruation) identified in this study have been commonly reported in previous research (2–5, 81–85). This study found that fatigue is the most commonly reported trigger of migraine attacks. However, fatigue might be a prodromal symptom (86) rather than a trigger, and migraine patients might not be able to differentiate between prodromal symptoms and triggers. In addition, it should be acknowledged that the information on triggers collected from EMRs was not as accurate as those recorded in patients’ migraine diaries used in clinical trials.
This study also summarized the treatment patterns in the hospital. It was found that CHM was more frequently prescribed to migraine patients than acupuncture and conventional pharmacotherapies, without significant differences between gender or age groups (adult group vs. non-adult group). In addition, we found that the rate of acute medication-taking history among migraine patients in the Chinese medicine hospital was relatively low (21.01%), especially when compared to the rate of acute medication usage in the U.S. [from 26% (12) to 95.1% (80)], Australia (80%) (87), and Japan (73%) (88). The proportion of patients being newly prescribed acute medication was also much lower (1.58%). While the proportion of patients being prescribed prophylactic medication (mainly CCBs, 21.16%) in the hospital is lower than that in the U.S. (30–60%) (89, 90). Such a low rate of conventional pharmacotherapies recorded in these EMRs could be due to (1) people purchasing over-the-counter acute medications and therefore these were not recorded in the EMRs; and/or (2) patients being given pharmacotherapies by other doctors before seeking additional Chinese medicine treatments. It is worth investigating whether taking CHM can reduce the usage of acute medication in rigorously designed RCTs in future because less acute medication usage can avoid the potential risk of medication overuse.
Our study briefly evaluated the overall improvement rate (50.42%). This was comparable to flunarizine, which has a commonly reported improvement rate ranging from 46.15 to 66.7% (91, 92). However, it should be noted that this was based on patient-reported improvements in all aspects, including the reduction of migraine or other symptoms in general. This was not accurately measured by the standard quantitative outcome measures used in clinical trials. It is also worth noting that improvements in comorbidities, such as insomnia and anxiety, were reported, although anti-depressant and hypnotic drugs were rarely used. Such findings could indicate the potential effects of Chinese medicine in managing various comorbidities and requires further exploration in future studies.
The treatment duration for migraine in the Chinese hospital (mean = 10 days) is shorter than the recommended duration of “at least 4 weeks” for conventional pharmacotherapies (10, 11). The duration for patients to report general improvements is also relatively short (median duration = 14 days), though not rigorously measured. This raised our curiosity about the optimal treatment duration and long-term effects of Chinese medicine. Whether adding Chinese medicine therapies to migraine management could shorten the required treatment duration is yet to be discovered.
Most patients were treated with a single type of treatment, mainly CHM (53.58%) or acupuncture (7.22%). Although both monotherapy and combination therapies of Chinese medicine with conventional pharmacotherapies have been proven effective for migraine in clinical trials (23–25), we suggest a pragmatic trial to compare the therapeutic effects and economic cost of Chinese medicine alone to that of Chinese medicine combined with conventional pharmacotherapies.
Furthermore, the study provided real-world clinicians’ experience of prescribing CHM for migraine. The frequency analysis results revealed that the most frequently used herbs are fu ling and chuan xiong, the most frequently used formula is CXCTS, and PCHMP is tong tian oral solution. These herbs and formulae have been proven to carry potential anti-migraine effects in previous bench or clinical research.
Identifying core CHM treatments is essential for selecting candidates for basic research, clinical trials, and daily practice (93, 94). Herb pair is the smallest compatible unit in CHM formulae, referring to two individual herbs repeatedly coexisting to enhance therapeutic effects or reduce toxicity (95). The top herb pair with a bidirectional association is bo he and jing jie. The core formulae found by herb combination construction include CXCTS and YQCMT, while CXCTS is also the basic formula for tong tian oral solution. These formulae are recommended for migraine treatment in the Guidelines for Diagnosis and Treatment of Common Internal Diseases in Chinese Medicine (96).
The constructed association rules between comorbidities/triggers and herbs indicate that females with menstruation-triggered migraine are more likely to be prescribed herbs that warm the meridian and activate the circulation of Blood and qi. This is consistent with recently published data-mining results (97). These results provide insight into the management of menstrual migraine and menstrually related migraine. We did not conduct association rule construction between other health conditions (e.g., hypertension and diabetes) and herbs, because (1) these conditions are not closely associated with migraine; and (2) they were not detailed in the EMRs for patients who visited the hospital to seek migraine treatment.
Limitations
The findings of this study should be interpreted with several limitations in mind. First, the patients’ characteristics, triggers, and comorbidities were collected from the EMRs, then there is a lack of consistency in depth and detail across EMRs. Second, the treatment response was defined and extracted based on the text recorded in the EMRs. It was not feasible for us to rigorously evaluate the effectiveness, because clinicians did not record treatment effects assessed by standard outcome measures as is done in clinical trials. Third, this study was conducted in one Chinese medicine hospital, so the results may be restricted in generality and more valuable for Chinese medicine clinical practice in southern China. Multi-center, prospective registry studies based on different geographic locations will provide more accurate and applicable information about Chinese medicine treatments for migraine.
Conclusion
This study presented the clinical features of 2,023 migraine patients and their treatment patterns, based on their EMRs in a Chinese medicine hospital. CHM can be used as an alternative to conventional pharmacotherapies, given that CHM was taking predominant treatment for migraine management while acute medication and prophylactic medicine were only prescribed to a small proportion of the migraine patients in the hospital. CHM formulae, such as chuan xiong cha tiao san and yi qi cong ming tang, patented CHM product tong tian oral solution, and some herb ingredients are potentially effective for migraine and are worth further evaluation. The optimal treatment duration, long-term effects, and treatment-effect curve of Chinese medicine for migraine need further exploration.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
SL and CZ planned and drafted the article. CZ, AZ, JS, XG, and CX provided the informative and critical comments on the manuscript revision. SL and HW extracted and screened the data. SL conducted the data analyses. SL, XG, and AZ undertook the final proofing of the manuscript and are responsible for its accuracy. All authors critically revised the manuscript and approved the final version.
Funding
This study was supported by the China-Australia International Research Centre for Chinese Medicine and funded by the Guangzhou University of Chinese Medicine “Double First-Class” and High-level University Discipline Collaborative Innovation Team (No. 2021xk84).
Acknowledgments
We appreciate the assistance provided by the Information Technology Department of GPHCM with data extraction, Genghang Chen for his guidance on statistical analyses, and Louise Pobjoy for proofreading this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CCB, calcium channel blocker; CGRP, calcitonin gene-related peptide; CHM, Chinese herbal medicine; CXCTS, formula of chuan xiong cha tiao san; PE, patient encounter; EMR, electronic medical record; GPHCM, Guangdong Provincial Hospital of Chinese Medicine; PERI, patient encounters reporting improvement; PCHMP, Patented Chinese herbal medicine products; YLD, years of life with disability; YQCMT, formula of yi qi cong ming tang.
References
1. Cephalalgia. Headache classification committee of the international headache society (IHS). The international classification of headache disorders 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
2. Jette N, Patten S, Williams J, Becker W, Wiebe S. Comorbidity of migraine and psychiatric disorders–a national population-based study. Headache. (2008) 48:501–16. doi: 10.1111/j.1526-4610.2007.00993.x
3. Kim J, Cho SJ, Kim WJ, Yang KI, Yun CH, Chu MK. Insufficient sleep is prevalent among migraineurs: a population-based study. J Headache Pain. (2017) 18:50. doi: 10.1186/s10194-017-0756-8
4. Kozak HH, Boysan M, Uca AU, Aydı A, Kılıc̨ İ, Genç E, et al. Sleep quality, morningness-eveningness preference, mood profile, and levels of serum melatonin in migraine patients: a case-control study. Acta Neurol Belg. (2017) 117:111–9. doi: 10.1007/s13760-016-0723-1
5. Song TJ, Yun CH, Cho SJ, Kim WJ, Yang KI, Chu MK. Short sleep duration and poor sleep quality among migraineurs: a population-based study. Cephalalgia. (2018) 38:855–64. doi: 10.1177/0333102417716936
6. Torta R, Ieraci V. Migraine and depression comorbidity: antidepressant options. Neurol Sci. (2012) 33(Suppl. 1):S117–8. doi: 10.1007/s10072-012-1055-4
7. Hagen K, Åsberg AN, Stovner L, Linde M, Zwart JA, Winsvold BS, et al. Lifestyle factors and risk of migraine and tension-type headache. Follow-up data from the nord-trøndelag health surveys 1995-1997 and 2006-2008. Cephalalgia. (2018) 38:1919–26. doi: 10.1177/0333102418764888
8. Holsteen KK, Hittle M, Barad M, Nelson LM. Development and internal validation of a multivariable prediction model for individual episodic migraine attacks based on daily trigger exposures. Headache. (2020) 60:2364–79. doi: 10.1111/head.13960
9. Gbd 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global burden of disease study 2016. Lancet Neurol. (2018) 17:954–76. doi: 10.1016/s1474-4422(18)30322-3
11. Chinese Medical Association Group. Guide to the prevention and treatment of migraine in China [in Chinese]. Chin J Pain Med. (2016) 22:721–7.
12. Lipton RB, Munjal S, Buse DC, Alam A, Fanning KM, Reed ML, et al. Unmet acute treatment needs from the 2017 migraine in america symptoms and treatment study. Headache. (2019) 59:1310–23. doi: 10.1111/head.13588
13. Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. (2013) 53:1300–11. doi: 10.1111/head.12154
14. Kim BK, Chu MK, Yu SJ, Dell’Agnello G, Han JH, Cho SJ. Burden of migraine and unmet needs from the patients’ perspective: a survey across 11 specialized headache clinics in Korea. J Headache Pain. (2021) 22:45. doi: 10.1186/s10194-021-01250-6
15. Sun-Edelstein C, Rapoport AM, Rattanawong W, Srikiatkhachorn A. The evolution of medication overuse headache: history, pathophysiology and clinical update. CNS Drugs. (2021) 35:545–65. doi: 10.1007/s40263-021-00818-9
16. Bigal ME, Lipton RB. Excessive opioid use and the development of chronic migraine. Pain. (2009) 142:179–82. doi: 10.1016/j.pain.2009.01.013
17. Schwedt TJ, Alam A, Reed ML, Fanning KM, Munjal S, Buse DC, et al. Factors associated with acute medication overuse in people with migraine: results from the 2017 migraine in America symptoms and treatment (MAST) study. J Headache Pain. (2018) 19:38–38. doi: 10.1186/s10194-018-0865-z
18. Radat F, Creac’h C, Swendsen JD, Lafittau M, Irachabal S, Dousset V, et al. Psychiatric comorbidity in the evolution from migraine to medication overuse headache. Cephalalgia. (2005) 25:519–22. doi: 10.1111/j.1468-2982.2005.00910.x
19. Schwedt TJ, Buse DC, Argoff CE, Reed ML, Fanning KM, Hussar CR, et al. Medication overuse and headache burden: results from the CaMEO study. Neurol Clin Pract. (2021) 11:216–26. doi: 10.1212/cpj.0000000000001037
20. Rhee TG, Harris IM. Reasons for and perceived benefits of utilizing complementary and alternative medicine in U.S. adults with migraines/severe headaches. Complement Ther Clin Pract. (2018) 30:44–9. doi: 10.1016/j.ctcp.2017.12.003
21. Wells RE, Bertisch SM, Buettner C, Phillips RS, McCarthy EP. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. (2011) 51:1087–97. doi: 10.1111/j.1526-4610.2011.01917.x
22. Yu S, Zhang Y, Yao Y, Cao H. Migraine treatment and healthcare costs: retrospective analysis of the China health insurance research association (CHIRA) database. J Headache Pain. (2020) 21:53. doi: 10.1186/s10194-020-01117-2
23. Lyu S, Zhang CS, Guo X, Zhang AL, Sun J, Lu C, et al. Oral Chinese herbal medicine as prophylactic treatment for episodic migraine in adults: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2020) 2020:5181587. doi: 10.1155/2020/5181587
24. Shan CS, Xu QQ, Shi YH, Wang Y, He ZX, Zheng GQ. Chuanxiong formulae for migraine: a systematic review and meta-analysis of high-quality randomized controlled trials. Front Pharmacol. (2018) 9:589. doi: 10.3389/fphar.2018.00589
25. Zhou L, Chen P, Liu L, Zhang Y, Liu X, Wu Y, et al. Systematic review and meta-analysis of traditional Chinese medicine in the treatment of migraines. Am J Chin Med. (2013) 41:1011–25. doi: 10.1142/s0192415x13500687
26. Giovanardi CM, Cinquini M, Aguggia M, Allais G, Campesato M, Cevoli S, et al. Acupuncture vs. pharmacological prophylaxis of migraine: a systematic review of randomized controlled trials. Front Neurol. (2020) 11:576272. doi: 10.3389/fneur.2020.576272
27. Li X, Dai Q, Shi Z, Chen H, Hu Y, Wang X, et al. Clinical efficacy and safety of electroacupuncture in migraine treatment: a systematic review and network meta-analysis. Am J Chin Med. (2019) 47:1755–80. doi: 10.1142/s0192415x19500897
28. Ou MQ, Fan WH, Sun FR, Jie WX, Lin MJ, Cai YJ, et al. Systematic review and meta-analysis of the therapeutic effect of acupuncture on migraine. Front Neurol. (2020) 11:596. doi: 10.3389/fneur.2020.00596
29. Seo J, Chu H, Kim CH, Sung KK, Lee S. Cupping therapy for migraine: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2021) 2021:7582581. doi: 10.1155/2021/7582581
30. Trinh KV, Diep D, Chen KJQ. Systematic review of episodic migraine prophylaxis: efficacy of conventional treatments used in comparisons with acupuncture. Med Acupunct. (2019) 31:85–97. doi: 10.1089/acu.2019.1337
31. Xu J, Zhang FQ, Pei J, Ji J. Acupuncture for migraine without aura: a systematic review and meta-analysis. J Integr Med. (2018) 16:312–21. doi: 10.1016/j.joim.2018.06.002
32. Yang M, Du T, Long H, Sun M, Liang F, Lao L. Acupuncture for menstrual migraine: a systematic review. BMJ Support Palliat Care. (2020) 1–11. doi: 10.1136/bmjspcare-2019-002024
33. Zhang N, Houle T, Hindiyeh N, Aurora SK. Systematic review: acupuncture vs standard pharmacological therapy for migraine prevention. Headache. (2020) 60:309–17. doi: 10.1111/head.13723
34. Guangdong Provincial Hospital of Chinese Medicine. Introduction to Guangdong Provincial Hospital of Chinese Medicine. (2021). Available online at: http://www.gdhtcm.com/index.html (accessed November 9, 2021).
35. Pan X, Chen B, Weng H, Gong Y, Qu Y. Temporal expression classification and normalization from chinese narrative clinical texts: pattern learning approach. JMIR Med Inform. (2020) 8:e17652. doi: 10.2196/17652
36. Buse DC, Reed ML, Fanning KM, Bostic R, Dodick DW, Schwedt TJ, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. (2020) 21:23. doi: 10.1186/s10194-020-1084-y
37. Caponnetto V, Deodato M, Robotti M, Koutsokera M, Pozzilli V, Galati C, et al. Comorbidities of primary headache disorders: a literature review with meta-analysis. J Headache Pain. (2021) 22:71–71. doi: 10.1186/s10194-021-01281-z
38. Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. (2005) 45:904–10. doi: 10.1111/j.1526-4610.2005.05159.x
39. Ku M, Silverman B, Prifti N, Ying W, Persaud Y, Schneider A. Prevalence of migraine headaches in patients with allergic rhinitis. Ann Allergy Asthma Immunol. (2006) 97:226–30. doi: 10.1016/s1081-1206(10)60018-x
40. Egeberg A, Mallbris L, Hilmar Gislason G, Skov L, Riis Hansen P. Increased risk of migraine in patients with psoriasis: a Danish nationwide cohort study. J Am Acad Dermatol. (2015) 73:829–35. doi: 10.1016/j.jaad.2015.08.039
41. Hindiyeh NA, Zhang N, Farrar M, Banerjee P, Lombard L, Aurora SK. The role of diet and nutrition in migraine triggers and treatment: a systematic literature review. Headache. (2020) 60:1300–16. doi: 10.1111/head.13836
42. Casanova A, Vives-Mestres M, Donoghue S, Mian A, Martin PR. An observational study of self-reported migraine triggers and prospective evaluation of the relationships with occurrence of attacks enabled by a smartphone application (App). Headache. (2022) 1–10. doi: 10.1111/head.14328
43. van Casteren DS, Verhagen IE, Onderwater GL, MaassenVanDenBrink A, Terwindt GM. Sex differences in prevalence of migraine trigger factors: a cross-sectional study. Cephalalgia. (2021) 41:643–8. doi: 10.1177/0333102420974362
44. Tanik N, Saçmaci H, Aktürk T. The relationship between exposure to hot/cold weather and the clinical features of headaches in patients with migraine and tension-type headaches. Neurol Res. (2020) 42:239–43. doi: 10.1080/01616412.2020.1723300
45. Albanês Oliveira Bernardo A, Lys Medeiros F, Sampaio Rocha-Filho PA. Osmophobia and odor-triggered headaches in children and adolescents: prevalence, associated factors, and importance in the diagnosis of migraine. Headache. (2020) 60:954–66. doi: 10.1111/head.13806
46. Steiner T, MacGregor EA, Davies PTG. Guidelines for All Healthcare Professionals in the Diagnosis and Management of Migraine, Tension-Type, Cluster and Medication-Overuse Headache. Hull (2010).
47. Pringsheim T, Davenport W, Mackie G, Worthington I, Aube M, Christie SN, et al. Canadian headache society guideline for migraine prophylaxis. Can J Neurol Sci. (2012) 39(Suppl. 2):S1–59.
48. Worthington I, Pringsheim T, Gawel MJ, Gladstone J, Cooper P, Dilli E, et al. Canadian headache society guideline: acute drug therapy for migraine headache. Can J Neurol Sci. (2013) 40(5 Suppl. 3):S1–80.
49. National Administration of Traditional Chinese Medicine. Diagnostic criteria and category of treatment response of toufeng 头风诊断与疗效评定标准 [in Chinese]. J Beijing Univ Tradit Chin Med. (1993) 16:69.
50. State Pharmacopoeia Committee of China. Chinese Pharmacopoeia. China Medical Science and Technology Press: Beijing (2020).
51. Agrawal R, Imieliński T, Swami A. Mining association rules between sets of items in large databases. Proceedings of the 1993 ACM SIGMOD International Conference on Management of Data. Washington, DC: Association for Computing Machinery (1993). p. 207–16. doi: 10.1145/170035.170072
52. Xiong H. Association Analysis: Basic Concepts and Algorithms. (2006). Available online at: http://www.columbia.edu/~jwp2128/Teaching/W4721/Spring2017/slides/lecture_4-11-17.pdf (accessed March 8, 2022).
53. Lu P-H, Keng J-L, Kuo K-L, Wang Y-F, Tai Y-C, Kuo C-Y. An apriori algorithm-based association rule analysis to identify herb combinations for treating uremic pruritus using Chinese herbal bath therapy. Evid Based Complement Alternat Med. (2020) 2020:8854772. doi: 10.1155/2020/8854772
56. Lora-Michiels A, Salinesi C, Mazo R. A method based on association rules to construct product line model. Proceedings of the 4th International Workshop on Variability Modelling of Software-Intensive Systems (VaMos). Essen: (2010). 50 p.
57. Zeng L, Yang W, Liang G, Luo M, Chen H, Guo D, et al. Roles exploration of chronic disease control involving TCM damp syndrome in Ling-nan region [in Chinese] 岭南中医湿证与慢性病防治创新模式探讨. Chin J Tradit Chine Med. (2019) 34:2345–9.
58. Lu M. The difference of dampness and the corresponding treatments between Lingnan region and Jiangnan region [in Chinese] 江南与岭南地区湿邪的特性及证治差异. Zhejiang J Tradit Chine Med. (2002) 37:6–7.
59. Zhou X, Zhang Y, Jiang Y, Zhou C, Ling Y. Poria cocos polysaccharide attenuates damage of nervus in Alzheimer’s disease rat model induced by D-galactose and aluminum trichloride. Neuroreport. (2021) 32:727–37. doi: 10.1097/wnr.0000000000001648
60. Lai T, Chen L, Chen X, He J, Lv P, Ge H. Rhynchophylline attenuates migraine in trigeminal nucleus caudalis in nitroglycerin-induced rat model by inhibiting MAPK/NF-êB signaling. Mol Cell Biochem. (2019) 461:205–12. doi: 10.1007/s11010-019-03603-x
61. Spekker E, Tanaka M, Szabó Á, Vécsei L. Neurogenic inflammation: the participant in migraine and recent advancements in translational research. Biomedicines. (2021) 10:76. doi: 10.3390/biomedicines10010076
62. Yazǧan Y, Nazıroǧlu M. Involvement of TRPM2 in the neurobiology of experimental migraine: focus on oxidative stress and apoptosis. Mol Neurobiol. (2021) 58:5581–601. doi: 10.1007/s12035-021-02503-w
63. Huang Y, Ni N, Hong Y, Lin X, Feng Y, Shen L. Progress in traditional chinese medicine for the treatment of migraine. Am J Chin Med. (2020) 48:1731–48. doi: 10.1142/s0192415x2050086x
64. Wang W. The Literature and Data Mining of Migraine [in Chinese] 基于古今医案数据分析的偏头痛病证治规律研究 Doctoral Thesis. Jichang: Guangzhou University of Chinese Medicine (2017).
65. Fan R. Based on Data Mining of the Jin ad Yuan Dynasty in the Treatment of Headache Prescription Medication Law [in Chinese] 基于数据挖掘的金元时期治头痛方用药规律研究 Master Thesis. Jinan: Shandong Traditional Chinese Medicine University (2014).
66. Wang YH, Liang S, Xu DS, Lin X, He CY, Feng Y, et al. Effect and mechanism of senkyunolide I as an anti-migraine compound from Ligusticum chuanxiong. J Pharm Pharmacol. (2011) 63:261–6. doi: 10.1111/j.2042-7158.2010.01191.x
67. Peng C, Xie X, Wang L, Guo L, Hu T. Pharmacodynamic action and mechanism of volatile oil from Rhizoma Ligustici Chuanxiong Hort. on treating headache. Phytomedicine. (2009) 16:25–34. doi: 10.1016/j.phymed.2008.10.010
68. Li H, Bai F, Cong C, Chen B, Xie W, Li S, et al. Effects of ligustrazine on the expression of neurotransmitters in the trigeminal ganglion of a rat migraine model. Ann Transl Med. (2021) 9:1318. doi: 10.21037/atm-21-3423
69. Chang YY, Tsai YT, Lai JN, Yeh CH, Lin SK. The traditional Chinese medicine prescription patterns for migraine patients in Taiwan: a population-based study. J Ethnopharmacol. (2014) 151:1209–17. doi: 10.1016/j.jep.2013.12.040
70. Wang L. The Research of Theory of Ancient Prescription Treating Headache Characteristics of Medication and Rule of Prescription Compatibility [in Chinese] 古代治头痛方用药特点及方剂配伍规律研究 Master Thesis. Jinshui: Henan College of Traditional Chinese Medicine (2015).
71. Wang Y, Shi Y, Zhang X, Zou J, Liang Y, Tai J, et al. A Chinese prescription Chuanxiong Chatiao san for migraine: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2019) 2019:2301680. doi: 10.1155/2019/2301680
72. Li D, Yao H, Sun K. Effects of micro Chuanxiong chatiao san on β-EP, CGRP of migraine mice [in Chinese] 超微川芎茶调散对偏头痛模型大鼠β-EP、CGRP的影响. Modern Med Health Res. (2020) 4:1–3.
73. Li J, Zhang Y, Sun F, Fan H, Shou Z. Mechanism of Chuanxiong chatiao san for migraine: a study based on network pharmacology analysis [in Chinese] 基于中药网络药理学分析川芎茶调散治疗偏头痛的作用机制. Chine J Hosp Pharm. (2020) 40:2406–13.
74. Gu J. Effectiveness of the Method of Tonifying qi and Yang for Vastibular Migraine with a Syndrome of qi Deficiency: A Clinical Trial [in Chinese] 益气升阳法治疗气虚型前庭性偏头痛的临床观察 Master Thesis. Jinan: Shandong Traditional Chinese Medicine University (2019).
75. Food and Drugs Administration. Framework for FDA’s Real-World Evidence Program. Silver Spring, MD: Food and Drugs Administration (2018).
76. Zhuang Y, Xie B, Weng S, Xie Y. Designs and thoughts of real world integrated data warehouse from HIS on re-evaluation of post-maketing traditional Chinese medicine [in Chinese] 中药上市后再评价 HIS ““真实世界” 集成数据” HIS 仓库的设计方法探讨 China. J Chine Mater Med. (2011) 36:2880–2.
77. Zhou X, Chen S, Liu B, Zhang R, Wang Y, Li P, et al. Development of traditional Chinese medicine clinical data warehouse for medical knowledge discovery and decision support. Artif Intell Med. (2010) 48:139–52. doi: 10.1016/j.artmed.2009.07.012
78. Dawes M, Summerskill W, Glasziou P, Cartabellotta A, Martin J, Hopayian K, et al. Sicily statement on evidence-based practice. BMC Med Educ. (2005) 5:1. doi: 10.1186/1472-6920-5-1
79. Renjith V, Pai MS, Castelino F, Pai A, George A. Clinical profile and functional disability of patients with migraine. J Neurosci Rural Pract. (2016) 7:250–6. doi: 10.4103/0976-3147.176188
80. Lipton RB, Munjal S, Alam A, Buse DC, Fanning KM, Reed ML, et al. Migraine in America symptoms and treatment (MAST) study: baseline study methods, treatment patterns, and gender differences. Headache. (2018) 58:1408–26. doi: 10.1111/head.13407
81. Hajjarzadeh S, Mahdavi R, Shalilahmadi D, Nikniaz Z. The association of dietary patterns with migraine attack frequency in migrainous women. Nutr Neurosci. (2020) 23:724–30. doi: 10.1080/1028415x.2018.1550890
82. Hajjarzadeh S, Nikniaz Z, Shalilahmadi D, Mahdavi R, Behrouz M. Comparison of diet quality between women with chronic and episodic migraine. Headache. (2019) 59:1221–8. doi: 10.1111/head.13623
83. Aljaafari D, Aldossary N, Almuaigel MF, Alsulaiman FA, Nazish S, Zafar A, et al. Migraine prevalence, characteristics, triggers, and coping strategies among medical students in Saudi Arabia. Prim Care Companion CNS Disord. (2021) 23:20m02859. doi: 10.4088/PCC.20m02859
84. Javaid Q. Prevalence, triggers and presentation of migraine among the college and university students: review of the available literature. J Pak Med Assoc. (2021) 71:2617–22. doi: 10.47391/jpma.011148
85. Silva-Néto RP, de Almeida Soares A, Augusto Carvalho de Vasconcelos C, da Silva Lopes L. Watermelon and others plant foods that trigger headache in migraine patients. Postgrad Med. (2021) 133:760–4. doi: 10.1080/00325481.2021.1922211
86. Cuvellier JC. Pediatric vs. Adult prodrome and postdrome: a window on migraine pathophysiology? Front Neurol. (2019) 10:199. doi: 10.3389/fneur.2019.00199
87. Stark RJ, Valenti L, Miller GC. Management of migraine in Australian general practice. Med J Aust. (2007) 187:142–6. doi: 10.5694/j.1326-5377.2007.tb01170.x
88. Meyers JL, Davis KL, Lenz RA, Sakai F, Xue F. Treatment patterns and characteristics of patients with migraine in Japan: a retrospective analysis of health insurance claims data. Cephalalgia. (2019) 39:1518–34. doi: 10.1177/0333102419851855
89. Takaki H, Onozuka D, Hagihara A. Migraine-preventive prescription patterns by physician specialty in ambulatory care settings in the United States. Prev Med Rep. (2018) 9:62–7. doi: 10.1016/j.pmedr.2017.12.009
90. Jackson JL, Kay C, Scholcoff C, Nickoloff S, Fletcher K. Migraine prophylactic management in neurology and primary care (2006-2015). J Neurol. (2018) 265:3019–21. doi: 10.1007/s00415-018-9066-6
91. Jiang L, Yuan DL, Li M, Liu C, Liu Q, Zhang Y, et al. Combination of flunarizine and transcutaneous supraorbital neurostimulation improves migraine prophylaxis. Acta Neurol Scand. (2019) 139:276–83. doi: 10.1111/ane.13050
92. Luo N, Di W, Zhang A, Wang Y, Ding M, Qi W, et al. A randomized, one-year clinical trial comparing the efficacy of topiramate, flunarizine, and a combination of flunarizine and topiramate in migraine prophylaxis. Pain Med. (2012) 13:80–6. doi: 10.1111/j.1526-4637.2011.01295.x
93. Chen HY, Lin YH, Chen YC. Identifying Chinese herbal medicine network for treating acne: implications from a nationwide database. J Ethnopharmacol. (2016) 179:1–8. doi: 10.1016/j.jep.2015.12.032
94. Chen HY, Lin YH, Thien PF, Chang SC, Chen YC, Lo SS, et al. Identifying core herbal treatments for children with asthma: implication from a chinese herbal medicine database in taiwan. Evid Based Complement Alternat Med. (2013) 2013:125943. doi: 10.1155/2013/125943
95. Zhong G. Chinese Materia Medica 中药学 (全国中医药行业高等教育 “十三五” 规划教材). 1st ed. Beijing: Traditional Chinese Medicine publishing co (2016).
96. Ren Y, Li H, Wang Y, Chen Y. Report of guidelines for diagnosis and treatment of common internal diseases in Chinese medicine: Headache. J Evid Based Med. (2020) 13:70–80. doi: 10.1111/jebm.12378
Keywords: migraine, electronic medical records (EMR), Chinese medicine, Chinese herbal medicine (CHM), real-world, clinical features, treatment patterns, therapeutic characteristics
Citation: Lyu S, Zhang CS, Sun J, Weng H, Xue CC, Guo X and Zhang AL (2022) Chinese herbal medicine for migraine management: A hospital-based retrospective analysis of electronic medical records. Front. Med. 9:936234. doi: 10.3389/fmed.2022.936234
Received: 05 May 2022; Accepted: 13 October 2022;
Published: 10 November 2022.
Edited by:
Magda Tsolaki, Aristotle University of Thessaloniki, GreeceReviewed by:
Weizhen Dong, University of Waterloo, CanadaYao Jie Xie, Hong Kong Polytechnic University, Hong Kong SAR, China
Copyright © 2022 Lyu, Zhang, Sun, Weng, Xue, Guo and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinfeng Guo, Z3VveGluZmVuZ0BnenVjbS5lZHUuY24=; Anthony Lin Zhang, dG9ueS56aGFuZ0BybWl0LmVkdS5hdQ==
 Shaohua Lyu
Shaohua Lyu Claire Shuiqing Zhang
Claire Shuiqing Zhang Jingbo Sun
Jingbo Sun Heng Weng
Heng Weng Charlie Changli Xue
Charlie Changli Xue Xinfeng Guo
Xinfeng Guo Anthony Lin Zhang
Anthony Lin Zhang