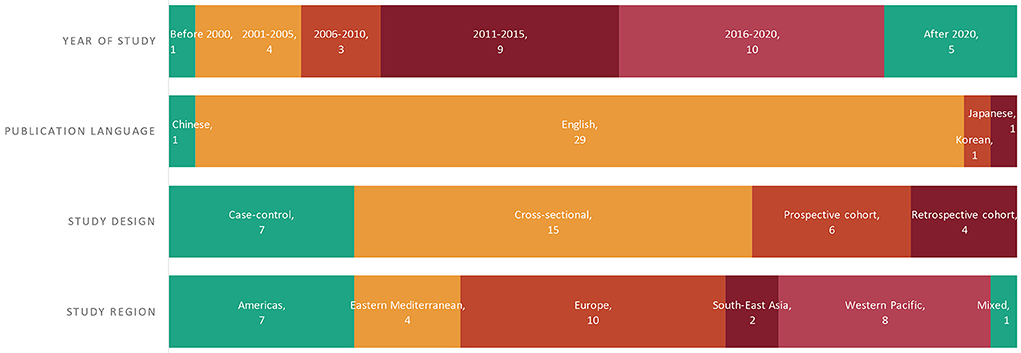- 1State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Institute of Social Medicine, School of Medicine, Zhejiang University, Hangzhou, China
Introduction: Non-alcoholic fatty liver disease (NAFLD) has become the most common liver disorder across the world, and non-invasive evaluation approaches are in need to assess NAFLD disease progression. Serum ferritin has been proposed as one of the biomarkers for NAFLD diagnosis in previous studies. This systematic review aims to identify, report, and synthesize studies that investigated the association of serum ferritin level with the various stages of NAFLD among the adult population.
Methods: Three databases – MEDLINE, EMBASE, and Scopus – were systematically searched to obtain potentially relevant publications before July 2022. No restrictions were applied to geographical region, study design, publication type and language. The association between serum ferritin level or different ferritin categories and the various stages of NAFLD was the primary outcome of interest. Title and abstract screenings, data extraction and coding, and quality assessment were independently completed by two authors with discrepancies resolved through discussion with a third author.
Results: Thirty-two studies were included and heterogeneity was considerable. The associations between serum ferritin level and the stages of hepatic steatosis, fibrosis, inflammation and ballooning and the occurrence of non-alcoholic steatohepatitis (NASH) were investigated but inconsistent associations were reported. Most studies identified serum ferritin to be a predictor of advanced NAFLD, while several revealed the opposite end.
Conclusions: Serum ferritin could be considered to act as a non-invasive biomarker for assessing various stages of NAFLD. Nevertheless, further studies are still in need to confirm its predictive value since this study reported inconsistent associations based on the qualitative synthesis.
Systematic Review Registration: http://www.crd.york.ac.uk/PROSPERO, identifier: CRD42021275630.
Introduction
Non-alcoholic fatty liver disease (NAFLD) represents a spectrum of hepatic pathology with fat excessively accumulating in the hepatic parenchyma in individuals who consume little or no alcohol (1, 2). It has become the most common liver disorder across the world, with a global prevalence estimated to be 25.24% (3) and still on the rise (4), heavy in both clinical and economic burdens. Noticeably, sex differences in NAFLD exist – NAFLD is more prevalent and more severe in men than in women during the reproductive age; the differences usually get smaller after menopause (5).
Generally, NAFLD consists of two subtypes: the first is simple steatosis (also termed as NAFL), which is nonprogressive; the second is non-alcoholic steatohepatitis (NASH), which has not only steatosis but also hepatocyte damage (6). NASH is progressive and may lead to end-stage liver diseases such as fibrosis, cirrhosis, and hepatocellular carcinoma, possibly resulting in liver-related mortality (Figure 1) (7, 8). In the United States, one of the major causes of adult cirrhosis is NASH, with NASH-related cirrhosis recognized as the second indication for liver transplantation (3). Hence, clinical evaluation of the disease progression in NAFLD patients is important for physicians to choose appropriate interventions and assess prognosis.
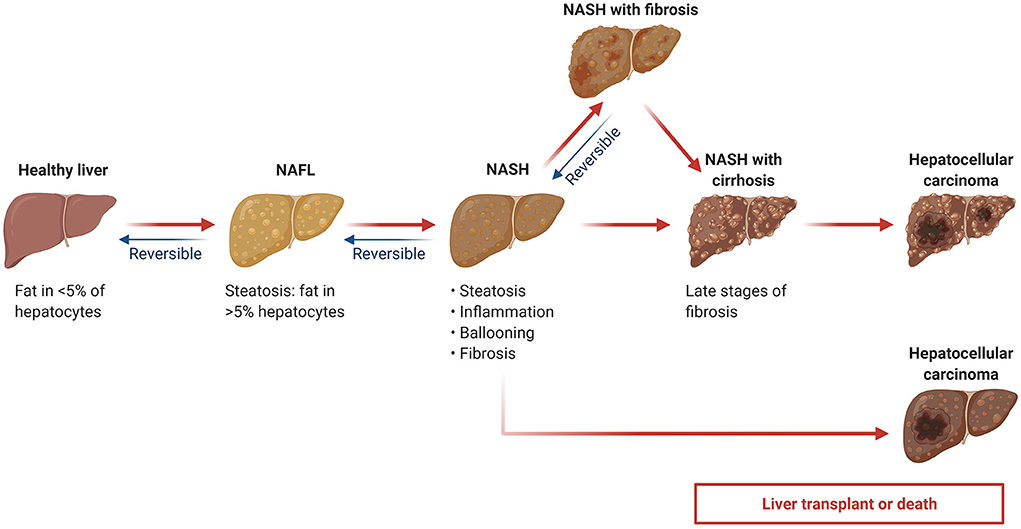
Figure 1. Non-alcoholic fatty liver disease (NAFLD) progression. NAFL: non-alcoholic fatty liver; NASH: non-alcoholic steatohepatitis. Adapted from “Non-Alcoholic Fatty Liver Disease (NAFLD) Spectrum”, by BioRender.com (2022). Retrieved from https://app.biorender.com/biorender-templates.
According to current clinical guidelines, liver biopsy is heavily relied upon for the clinical evaluation of NAFLD, especially for the diagnosis of NASH (9). However, liver biopsy is an invasive procedure and may be accompanied by complications such as bleeding (10), and there might be underestimation of the disease progression, which is caused by sampling bias, since a biopsy specimen represents only ~1/50,000 of the liver volume (11). Therefore, it is suggested to develop and utilize accurate non-invasive evaluation approaches such as imaging and biomarkers, either to combine with liver biopsy for higher validity and reliability, or replace it to avoid invasive diagnostic procedures.
Serum ferritin has been widely studied to assist with disease diagnosis and progression, since it is an acute-phase reactant and a pro-inflammatory cytokine whose concentration is elevated in both infectious and non-infectious inflammation (12). Elevated serum ferritin is reported in about 30% patients diagnosed with NAFLD (13) and it has been proposed as one of the biomarkers for NAFLD diagnosis in previous studies (11, 14). For instance, one Iranian study proposed that the ferritin values of 150 ng/ml in females and 248 ng/ml in males as potential diagnostic cut-off points (15). Studies have also identified it as a potential indicator for the evaluation of NAFLD progression and prognosis, e.g., predicting liver fibrosis in NAFLD patients (16).
To the best of our knowledge, few studies have synthesized existing evidence on the association between serum ferritin and disease progression of NAFLD. This systematic review aims to address the research gap by identifying, reporting, and synthesizing studies that investigated the association of serum ferritin level or different ferritin categories with the various stages of NAFLD among the adult population.
Methods
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (17), and was prospectively registered with PROSPERO (protocol number CRD42021275630; http://www.crd.york.ac.uk/PROSPERO).
Search strategy and eligibility criteria
Three databases – MEDLINE, EMBASE, and Scopus – were systematically searched using a combination of the key terms “ferritin,” “fatty liver,” “hepatic steatosis,” “non-alcoholic steatohepatitis” and related syntax (title/abstract/keywords/MeSH) to obtain potentially relevant publications before July 2022. No restrictions were applied to geographical region, study design, publication type and language. Full search strategies are presented in Supplementary Table 1.
The inclusion criteria were as follows: (1) original and empirical human studies; (2) observational studies including cross-sectional, case-control, and cohort studies; (3) studies that enrolled adult NAFLD patients diagnosed with any approach; (4) studies that explored the association between serum ferritin and disease progression of NAFLD, with confounding factors either adjusted or not.
The exclusion criteria were as follows: (1) review, case-report, abstract, protocol, letter, commentary, meta-analysis and proceeding articles; (2) interventional studies such as clinical trials; (3) experiments performed in vitro or in animals; (4) studies that included pediatric patients or patients diagnosed with other chronic liver diseases, e.g., hepatitis B and C, autoimmune hepatitis, etc.; (5) studies not analyzing the association between serum ferritin and disease progression of NAFLD.
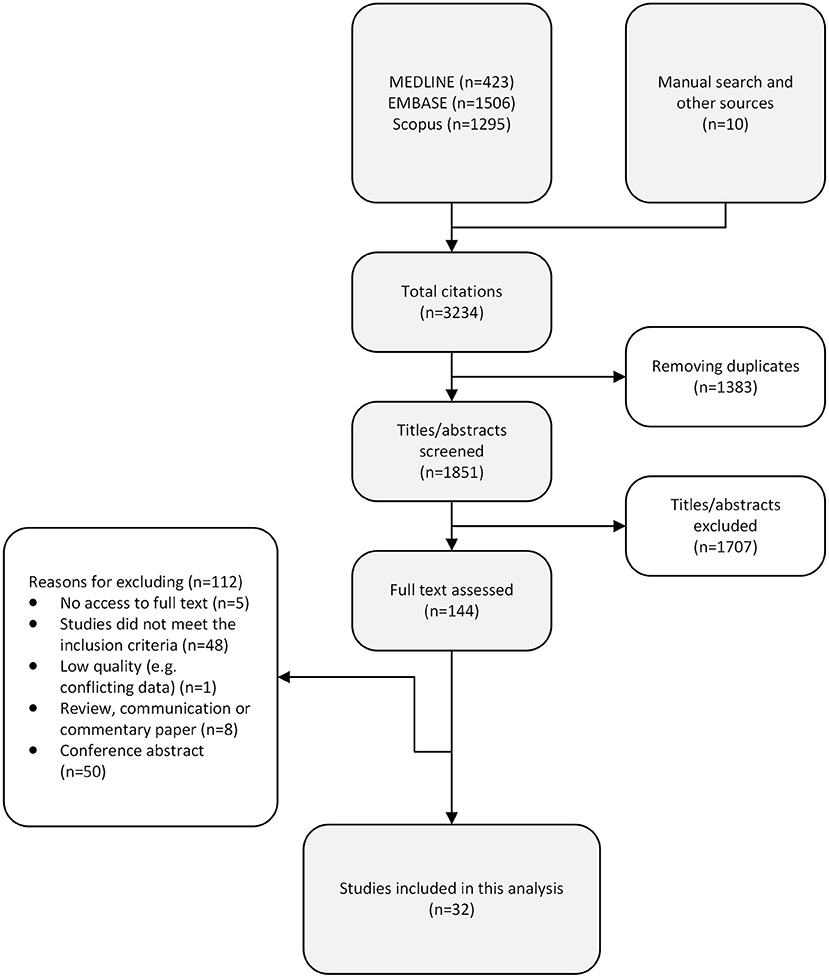
Results identified from the search were imported into a citation manager (Zotero), and duplicates were removed. Two authors (HW and RS) independently screened titles and abstracts against the eligibility criteria. Full texts were retrieved for evaluation when citations were considered relevant or with insufficient information for inclusion or exclusion during title/abstract screening. Manual searches were conducted in the reference lists of included studies to obtain additional relevant studies. Full-text evaluations were independently conducted by two authors (HW and RS). Disagreements between the two authors during screening and evaluation were discussed with a third author (CY) to reach consensus.
Data extraction and quality assessment
Data was extracted from the included studies using a purposive-built data collection form in Excel. The following data was extracted and coded into the form: (1) publication information including first author's name, article title, year of publication; (2) study design including study type, study location, sample size, target population, and selection criteria for participant recruitment; (3) socio-demographic status and medical history of study participants; (4) results of liver imageology (ultrasound, CT, or MRT) and liver biopsy, including grades of steatosis, ballooning, inflammation, fibrosis, cirrhosis, etc.; (5) serum ferritin level, together with its testing methods; (6) approaches or standards employed for NAFLD diagnosis and grading; (7) proven associations between serum ferritin level and the various stages of NAFLD. Two authors (HW and RS) independently extracted and coded the data. Discrepancies during this process was discussed with a third author (CY) until consensus was reached.
Quality of the included studies was assessed using the quality control criteria for proteomic studies reporting potential biomarkers (18). Quality assessment was independently completed by two authors (HW and RS), and disagreements were resolved through discussion with a third author (CY).
Results
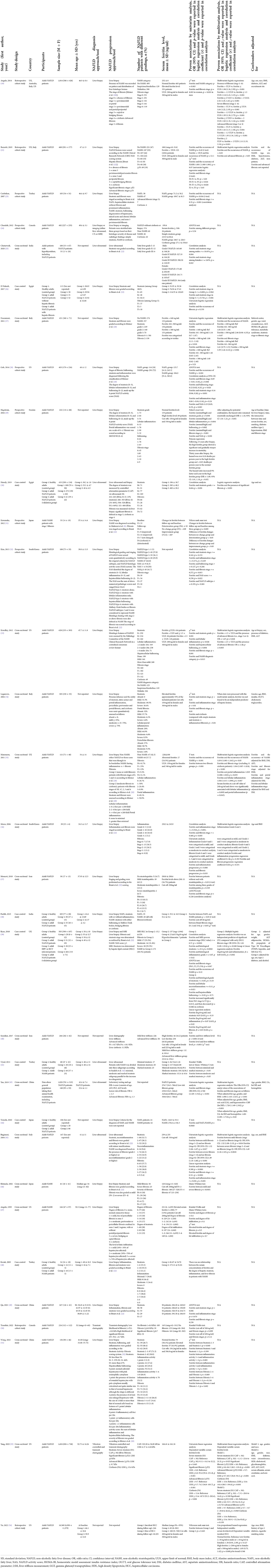
A total of 3,234 records were returned from the literature search, of which 1,383 duplicates were removed and 1,707 citations were excluded during title/abstract screenings (Figure 2). We assessed 144 full-text articles, and 32 studies met the predefined inclusion criteria. Figure 3 presents the characteristics of all included studies, categorized by year of study, publication language, study design, study region [the World Health Organization (WHO) regions], and participants. Nearly half of the included studies were published after 2016 (n = 15, 46.9%; Figure 3). Most of the studies were published in English (n = 29, 90.6%), with another one study published in Chinese, one study published in Japanese, and one study published in Korean. Fifteen studies employed cross-sectional design, 10 studies were cohort studies, and seven studies were case-control studies. The included studies covered a total of 28,261 participants, of whom 27,028 were NAFLD patients, including 2,376 NASH patients; one study explored the association of ferritin and the various stages of NAFLD in patients with hypothyroidism (Table 1).
Most studies utilized liver biopsy for NAFLD diagnosis and grading. The studies of Brunt et al. (22) and Kleiner et al. (20) were often referred to as the criteria for grading NAFLD progression, e.g., the grading of steatosis, inflammation and fibrosis stages. As the primary outcome of interest, the association of ferritin and various stages of NAFLD was proven by multivariate statistical analysis in 15 of the included studies, mostly adjusted for age, sex, BMI and other medical history variables. However, the other 17 studies only conducted univariate statistical analysis.
Serum ferritin level and hepatic steatosis stages
Altogether, 15 studies investigated the association of serum ferritin level and hepatic steatosis stages in NAFLD patients (25, 26, 28–30, 32–34, 39, 41, 45, 48, 49, 51, 53). Among the 15 studies, nine studies consistently reported that NAFLD patients with a higher serum ferritin level were more likely to have an advanced steatosis stage (25, 28–30, 33, 34, 41, 45, 49), usually analyzed by correlation analysis. An Indian study reported significant associations in both females and males (25). Three studies did not find any significant association between serum ferritin level and steatosis stage (32, 48, 53). The other three studies reported inconsistent associations: one Egyptian study identified ferritin as a predictor for steatosis among NAFLD patients with hepatic fibrosis, but the association was not significant among patients without fibrosis (26); one study from the UK reported ferritin to be a predictor in one group of NAFLD patients, while it was not significantly related to steatosis progression in another group of NAFLD patients (39); the other study from China revealed that ferritin could distinguish Stage 2 or 3 steatosis from Stage 1, but not Stage 3 from Stage 1 or 2 (51). Almost all of the above results were tested by univariate statistical analysis without further exploration via multivariate analysis, except from two studies – one study reporting the predictive role of serum ferritin for steatosis progression that became non-significant in the multivariate analysis (34), and the other showed consistent non-significant associations in both univariate and multivariate analysis (53).
Serum ferritin level and the occurrence of steatohepatitis
The association of serum ferritin level and the occurrence of steatohepatitis among NAFLD patients were investigated in 11 studies (19, 21, 23, 27, 28, 33, 35, 37–39, 44). Nine of the 11 studies compared the serum ferritin level in NAFL patients with it in NASH patients, among which five studies identified it to be a predictor for the occurrence of NASH (23, 27, 28, 35, 38, 44) yet two studies showed non-significant associations (21, 39). Data in these studies were usually analyzed by ANOVA test or t-test. Only three studies further included ferritin into a multivariate model and their result remained the same as it was in the univariate analysis (21, 27) except that in one study, the significant difference (p < 0.001) became borderline (p = 0.05) (35). Interestingly, one Italian study set 160 and 380 ng/ml as ferritin cut-offs and found both of them were predictive for the occurrence of NASH, with the cut-off of 380 ng/ml having a higher odds ratio in both univariate and multiple logistic regression analyses (27).
An international study and a study from the US compared ferritin levels among patients with different NASH categories – no NASH, suspicious or borderline NASH and definitive NASH, and found a significant difference among the three groups of patients viaχ2 test, but no further analysis was conducted to identify the trend of serum ferritin level in NASH progression (19, 33). Another Iranian study had similar results; there was a difference of serum ferritin levels among patients with mild, moderate and severe steatohepatitis, but no further comparison was made (37).
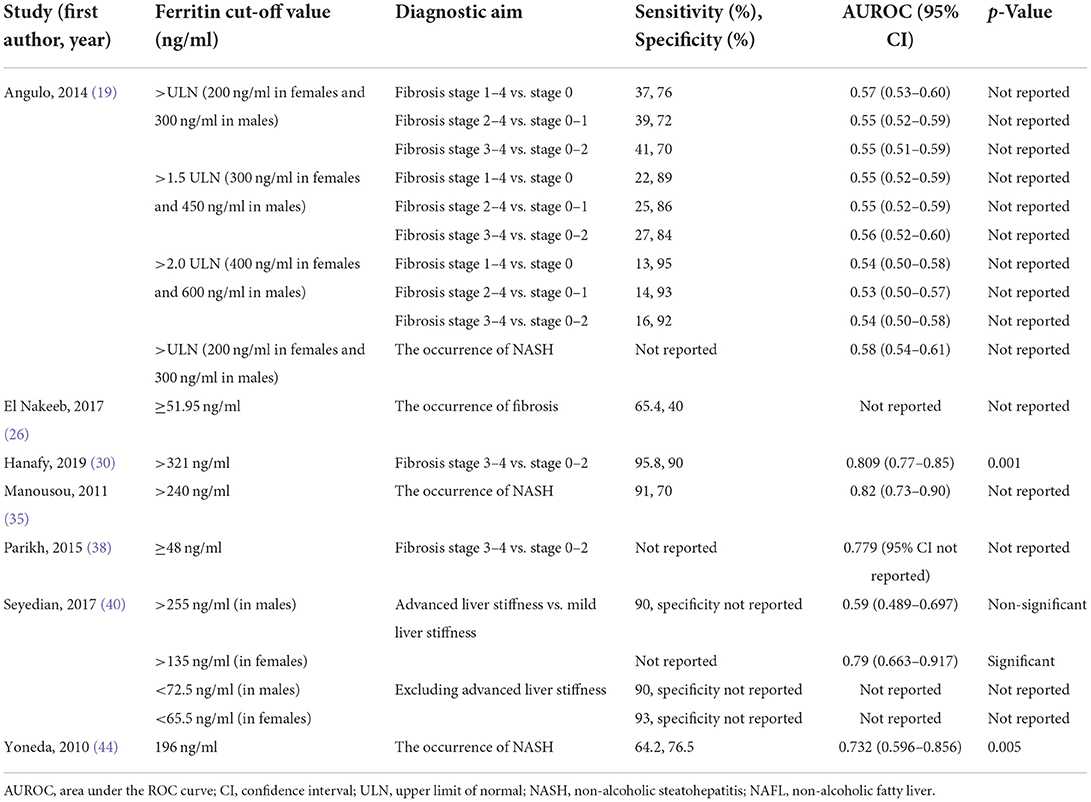
Five studies analyzed the accuracy of ferritin level for diagnosing NASH by conducting receiver operating characteristic (ROC) curve analysis (19, 28, 35, 38, 44) and reported inconsistent results (Table 2). Two suggested that ferritin had poor accuracy (19, 28), but three demonstrated the opposite end (35, 38, 44).
Serum ferritin level and hepatic fibrosis stages
There are 25 studies exploring the association of serum ferritin level and fibrosis stages in NAFLD patients (19, 21, 23, 26–29, 31–37, 39, 40, 43, 45–51, 53).
Ferritin and hepatic fibrosis stages graded from F0–F4 using Brunt et al.'s standards
Most of these studies employed the Brunt et al. (22) standards to grade fibrosis stages from F0 (absence of fibrosis) to F4 (cirrhosis). In an international study, ferritin was reported to be significantly different in NAFLD patients with different stages of fibrosis viaχ2 test, and further identified that serum ferritin levels higher than the upper limit of normal (ULN, which was 200 ng/ml in females and 300 ng/ml in males), higher than 1.5 ULN and higher than 2 ULN were predictors of presence of fibrosis (F1–F4), severe fibrosis (F2–F4) and advanced fibrosis (F3/F4), respectively, through multiple logistic regressions (19). Similarly, a study from the US reported significant results in univariate analysis, and identified both 1.5 ULN and 2.5 ULN ferritin levels to be predictors of advanced fibrosis (33). A study from two European countries reported that when comparing with the ferritin level of patients with F0–F1 fibrosis, that of F2 patients were non-significantly different, while that of F3 and F4 patients were significantly different; however, multiple analysis showed non-significant results (21).
Different adjusting confounders would influence the associations. One study from the US reported that ferritin could distinguish significant fibrosis (F2–F4), advanced fibrosis (F3–F4) and cirrhosis (F4) from less severe fibrosis; the associations remained significant when age, gender and race were adjusted, yet became non-significant when more variables were included, e.g., BMI, medical history of diabetes, waist circumference, laboratory analysis results including alanine aminotransferase (ALT), etc. (53).
Different associations of ferritin and hepatic fibrosis stages
Ten studies simply reported patients with more advanced fibrosis were more likely to have a higher serum ferritin level (23, 29, 32, 34, 35, 38, 40, 43, 45, 46), tested by univariate analysis. Four of the 10 studies further included ferritin into multivariate analysis models, and three studies had results that remained the same (35, 43, 45) yet one study showed non-significant association (36). One study only had results from multivariate analysis and reported the significant association that a higher ferritin level predicts the presence of significant fibrosis (30). Another seven studies showed non-significant results in univariate analysis (28, 37, 47–51). Further, one study found non-significant association between ferritin and the occurrence of fibrosis in NAFLD patients (26). Additionally, one study reported inconsistent results from two different groups of NAFLD patients (39), and one study reported that ferritin was higher in patients with cirrhosis when comparing with patients with simple steatosis and steatosis plus inflammation or fibrosis (34).
Interestingly, when ferritin cut-offs were set as 160 ng/ml and 380 ng/ml, the differences of ferritin levels among patients with different fibrosis were non-significant for both two cut-offs in univariate analysis, but the association of ferritin level and fibrosis stages became significant when ferritin cut-off was 380 ng/ml in multiple logistic regression (27).
Ferritin and hepatic fibrosis progression in longitudinal study
A Japanese study using longitudinal data followed a group of NAFLD patients with F3 fibrosis at baseline, and categorized them into deterioration group (F4), no-change group (F3) and improvement group (F1/F2) according to their fibrosis stage at follow-up after 1–10 year(s) (31). This study showed that changes of ferritin levels in these patients were significantly different among the three groups, with significant differences in both between no-change group and deterioration group and between no-change group and improvement group (31).
Accuracy of ferritin for predicting hepatic fibrosis
Three studies further explored the accuracy of ferritin for predicting fibrosis stages (19, 30, 40). One study suggested it had poor accuracy among males yet had high accuracy among females (40), one study reported poor accuracy generally (19), and one study demonstrated it was a good predictor (30).
Serum ferritin level and hepatic inflammation stages
Eleven studies explored the association serum ferritin level and inflammation stages among NAFLD patients (28, 29, 32–36, 39, 45, 48, 49). Ten of the 11 studies conducted univariate analysis: four studies demonstrated that NAFLD patients with a higher ferritin level were more likely to have more advanced hepatic inflammation (29, 33, 34, 36); five studies showed non-significant results (28, 32, 45, 48, 49); and one study reported inconsistent associations from two different groups of patients (39). Only three studies explored the association of ferritin and inflammation progression via multivariate analysis: one study identified ferritin as a predictor for more advanced portal and lobular inflammation status, with a significant cut-off value of 240 ng/ml (35); another study found non-significant associations of ferritin between patients with mild (Grade 0 and 1) and moderate (Grade 2 and 3) inflammation (36); the other study found significant association of ferritin (log 10 ng/ml) and inflammation stages by multiple linear regression analysis (39).
Serum ferritin level and hepatic ballooning stages
The association of serum ferritin level and hepatic ballooning were investigated in five studies (28, 29, 32, 33, 39), all tested by univariate analysis. Three of them suggested that NALFD patients with higher ferritin were more likely to have a more advanced ballooning stage (28, 29, 33), and the other two reported non-significant results (32, 39).
Another one study from China combined inflammation and ballooning score as the inflammation activity score (1–4, the higher the more severe), and found that ferritin levels were different between patients with 4 points and 1–3 points, but not 3–4 points and 1–2 points (51).
Serum ferritin level and integrated NAFLD progression including incident HCC and mortality
Three studies reported that serum ferritin level was positively correlated with NAFLD activity score (NAS) (28, 29, 32).
One study from the US explored the role of ferritin in predicting future incident hepatocellular carcinoma (HCC), with an average follow-up of 4.34 years. The authors reported non-significant associations both in univariate analysis and multivariate Cox proportional hazard regression analysis (54).
There is one study investigating the association of serum ferritin level and mortality (29). It suggested that following 15 years after liver biopsy, patients with elevated ferritin (>350 ng/ml in males and >150 ng/ml in females) showed a significant and gradually steeper increase in mortality compared with those with normal ferritin levels at biopsy; following 30 years after biopsy, the hazard ratio increased 9% faster per year in patients with elevated ferritin, and the significance remained when potential confounders were adjusted.
Discussion
This systematic literature review identified 32 studies reporting the association between serum ferritin level or different ferritin categories and various stages of NAFLD, including the occurrence of NASH, hepatic steatosis stages, fibrosis stages, inflammation stages, ballooning stages, incident HCC and mortality. Most studies suggested that serum ferritin was a predictor for more advanced NAFLD and could relate to higher mortality. However, non-significant association was also reported by a few included studies. The accuracy of ferritin as a predictor for NAFLD progression was also reported inconsistently.
This study not only synthesized current evidence on the association of ferritin and NAFLD progressions, but also identified certain research gaps in this field. First, more than half of the included studies only employed univariate statistical analysis. Under these circumstances, the reliability of the association was not high due to the potential influences exerted by confounders such as age, sex, ATL levels, etc. Future studies should apply a rigorous study design. Second, although many studies employed a cohort design, only three of them used longitudinal data for analysis (29, 31, 54). Two of them investigated the association of ferritin and future incident HCC (54) and mortality (29), respectively; the other revealed the association of ferritin and changes of fibrosis stages (31). This calls for more studies to explore the predictive value of ferritin for NAFLD prognosis. Third, when categorizing the included studies according to the WHO regions, we found that none of the studies were from the African Region, indicating a research gap among African populations. Fourth, heterogeneity was high among the included studies and it prevented further data synthesis via meta-analysis. The included studies used different grading standards and various statistical analysis approaches. Future studies could apply consistent study design for better homogeneity to assist data synthesis on this topic. Fifth, many studies did not evaluate the diagnosis accuracy, specificity, or sensitivity of serum ferritin level, without which the predictive value of ferritin for evaluating various stages of NAFLD would not be clear. Sixth, many studies used the same ferritin cut-off values for all participants, thus failed to observe the potential sex differences in the associations of ferritin and NAFLD stages between the two populations, since in addition to the sex differences in NAFLD prevalence, there are also differences in ferritin cut-off values as a result of different iron status between females and males (55). Future studies are supposed to take sex differences into consideration.
Several previous reviews have narratively summarized existing evidence on this topic, which mostly elaborated the association of serum ferritin and the occurrence of NASH (11, 16, 56, 57) or fibrosis (16, 56, 57). One meta-analysis identified that ferritin was independently associated with NAFLD and NASH diagnosis (58). Our study included a broader body of evidence and added insights into the associations of ferritin and stages of steatosis, inflammation and ballooning, indicating the potential value of ferritin as a biomarker for clinical assessment of NAFLD progression and prognosis. The inconsistent associations reported by the included studies might result from inadequate sample sizes in some of the included studies. Gene variants or polymorphisms that are relevant to iron metabolism could influence serum ferritin levels in NAFLD patients, which might provide an explanation for the non-significant role of serum ferritin in predicting NAFLD progression in certain studies (21). In addition, previous studies recommended ferritin to be a component of non-invasive integrated scoring system for NAFLD assessment (59, 60), since the diagnosis accuracy improved under this circumstance (61).
Studies have explored the mechanisms of the elevated serum ferritin and how it is related to disease various disease stages in NAFLD patients. On the one hand, NAFLD disease progression process can upregulate serum ferritin: one mechanism is that the excessive ferritin is released by damaged hepatocytes and/or systematic inflammation; inflammation can also upregulate hepcidin levels and result in dysmetabolic iron overload syndrome (DIOS), consequently raising up serum ferritin level; the p.C282Y homozygote HFE mutation in NAFLD patients is related to elevated transferrin saturation, accompanied by abnormally higher ferritin (13); further, signals that mediate NASH pathogenesis (e.g., TNF-α, IL-1β) can elevate ferritin level (33). On the other hand, ferritin is involved in NASH pathogenesis by promoting apoptosis and inducing signaling cascades related to inflammation, oxidative stress, lipid transport, and fibrogenesis (33).
To the best of our knowledge, this is the first study that has comprehensively synthesized and reported the existing evidence on this topic, including articles published in three languages and from various countries, and shedding light on the diagnostic and predictive value of ferritin for NAFLD assessment. However, some limitations should be noted. First, meta-analysis was not conducted due to the heterogeneity of NAFLD grading standards and statistical analysis methods among the included studies. Moreover, the absence of necessary data in some included studies for conducting meta-analysis prevented us from further analysis. Second, five studies were not incorporated in this study since we did not have access to their full texts. This might result in a certain level of bias.
In conclusion, serum ferritin level could be considered to act as a non-invasive biomarker for NAFLD progression assessment owing to its associations with the occurrence of NASH, the stages of steatosis, inflammation, ballooning, fibrosis, general NAFLD progression and mortality. Nevertheless, further studies are still in need to confirm its predictive value since this study reported inconsistent associations based on the qualitative synthesis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CY conceptualized the study, supervised the project, and was acting as the submission's guarantor. HW and RS searched the literature, extracted, and coded the data, completed the visualization, and interpreted the results. HW prepared the original draft with important contributions from RS. SY, XM, and CY commented on drafts, and provided edits and feedback. All authors had full access to all the data and have approved the final version of the paper.
Funding
The work was supported by grants from the National Scientific and Technological Major Project of China (No. 2017ZX10105001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.934989/full#supplementary-material
References
1. Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. (2002) 122:1649–57. doi: 10.1053/gast.2002.33573
2. Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. (2013) 10:686–90. doi: 10.1038/nrgastro.2013.171
3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatol Baltim Md. (2016) 64:73–84. doi: 10.1002/hep.28431
4. Zhu J-Z, Dai Y-N, Wang Y-M, Zhou Q-Y, Yu C-H, Li Y-M. Prevalence of nonalcoholic fatty liver disease and economy. Dig Dis Sci. (2015) 60:3194–202. doi: 10.1007/s10620-015-3728-3
5. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, et al. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatol Baltim Md. (2019) 70:1457–69. doi: 10.1002/hep.30626
6. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatol Baltim Md. (2012) 55:2005–23. doi: 10.1002/hep.25762
7. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. (2005) 129:113–21. doi: 10.1053/j.gastro.2005.04.014
8. Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. (1999) 116:1413–9. doi: 10.1016/S0016-5085(99)70506-8
9. Zhu J-Z, Hollis-Hansen K, Wan X-Y, Fei S-J, Pang X-L, Meng F-D, et al. Clinical guidelines of non-alcoholic fatty liver disease: a systematic review. World J Gastroenterol. (2016) 22:8226–33. doi: 10.3748/wjg.v22.i36.8226
10. Grant A, Neuberger J. Guidelines on the use of liver biopsy in clinical practice. Gut. (1999) 45:IV1–11. doi: 10.1136/gut.45.2008.iv1
11. Wong VW-S, Adams LA, de Lédinghen V, Wong GL-H, Sookoian S. Noninvasive biomarkers in NAFLD and NASH - current progress and future promise. Nat Rev Gastroenterol Hepatol. (2018) 15:461–78. doi: 10.1038/s41575-018-0014-9
12. Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. (2017) 29:401–9. doi: 10.1093/intimm/dxx031
13. Moris W, Verhaegh P, Jonkers D, Deursen CV, Koek G. Hyperferritinemia in nonalcoholic fatty liver disease: iron accumulation or inflammation? Semin Liver Dis. (2019) 39:476–82. doi: 10.1055/s-0039-1693114
14. Hu K-C, Wang H-Y, Liu S-C, Liu C-C, Hung C-L, Bair M-J, et al. Nonalcoholic fatty liver disease: updates in noninvasive diagnosis and correlation with cardiovascular disease. World J Gastroenterol. (2014) 20:7718–29. doi: 10.3748/wjg.v20.i24.7718
15. Etminani R, Manaf ZA, Shahar S, Azadbakht L, Adibi P. Predictors of nonalcoholic fatty liver disease among middle-aged Iranians. Int J Prev Med. (2020) 11:113. doi: 10.4103/ijpvm.IJPVM_274_19
16. Pearce SG, Thosani NC, Pan J-J. Noninvasive biomarkers for the diagnosis of steatohepatitis and advanced fibrosis in NAFLD. Biomark Res. (2013) 1:7. doi: 10.1186/2050-7771-1-7
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
18. Mischak H, Allmaier G, Apweiler R, Attwood T, Baumann M, Benigni A, et al. Recommendations for biomarker identification and qualification in clinical proteomics. Sci Transl Med. (2010) 2:46ps42. doi: 10.1126/scitranslmed.3001249
19. Angulo P, George J, Day CP, Vanni E, Russell L, De la Cruz AC, et al. Serum ferritin levels lack diagnostic accuracy for liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. (2014) 12:1163–9.e1. doi: 10.1016/j.cgh.2013.11.035
20. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatol Baltim Md. (2005) 41:1313–21. doi: 10.1002/hep.20701
21. Buzzetti E, Petta S, Manuguerra R, Luong TV, Cabibi D, Corradini E, et al. Evaluating the association of serum ferritin and hepatic iron with disease severity in non-alcoholic fatty liver disease. Liver Int. (2019) 39:1325–34. doi: 10.1111/liv.14096
22. Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA, NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatol Baltim Md. (2011) 53:810–20. doi: 10.1002/hep.24127
23. Canbakan B, Senturk H, Tahan V, Hatemi I, Balci H, Toptas T, et al. Clinical, biochemical and histological correlations in a group of non-drinker subjects with non-alcoholic fatty liver disease. Acta Gastro-Enterol Belg. (2007) 70:277–84.
24. Chandok N, Minuk G, Wengiel M, Uhanova J. Serum ferritin levels do not predict the stage of underlying non-alcoholic fatty liver disease. J Gastrointestin Liver Dis. (2012) 21:53–8.
25. Chaturvedi M, Pal K, Verma R, Paramjeet. Prevalance of non-alcoholic fatty liver disease in hypothyroid patients and its correlation with serum ferritin levels. J Indian Acad Clin Med. (2020) 21:43–5.
26. El Nakeeb N, Saleh SA, Massoud YM, Hussein A, Hamed R. Serum ferritin as a non-invasive marker in the prediction of hepatic fibrosis among Egyptian patients with non-alcoholic fatty liver disease. JGH Open. (2017) 1:112–9. doi: 10.1002/jgh3.12019
27. Fracanzani AL, Valenti L, Bugianesi E, Vanni E, Grieco A, Miele L, et al. Risk of nonalcoholic steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease and low visceral adiposity. J Hepatol. (2011) 54:1244–9. doi: 10.1016/j.jhep.2010.09.037
28. Goh GB, Issa D, Lopez R, Dasarathy S, Dasarathy J, Sargent R, et al. The development of a non-invasive model to predict the presence of non-alcoholic steatohepatitis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol Aust. (2016) 31:995–1000. doi: 10.1111/jgh.13235
29. Hagström H, Nasr P, Bottai M, Ekstedt M, Kechagias S, Hultcrantz R, et al. Elevated serum ferritin is associated with increased mortality in non-alcoholic fatty liver disease after 16 years of follow-up. Liver Int. (2016) 36:1688–95. doi: 10.1111/liv.13144
30. Hanafy AS, Seleem WM. El-kalla F, AbdAlkhalik Basha M, Abd-Elsalam S. Efficacy of a non-invasive model in predicting the cardiovascular morbidity and histological severity in non-alcoholic fatty liver disease. Diabetes Metab Syndr Clin Res Rev. (2019) 13:2272–8. doi: 10.1016/j.dsx.2019.05.032
31. Kawanaka M, Oka T, Urata N, Kimura T, Nakamura J, Goto D, et al. Clinical characteristics of non-alcoholic steatohepatitis (NASH) patients who progressed from F3 stage fibrosis to cirrhotic NASH. J Jpn Soc Gastroenterol. (2012) 109:2042–8.
32. Kim YS, Jung ES, Hur W, Bae SH, Choi JY, Song MJ, et al. Noninvasive predictors of nonalcoholic steatohepatitis in Korean patients with histologically proven nonalcoholic fatty liver disease. Clin Mol Hepatol. (2013) 19:120–30. doi: 10.3350/cmh.2013.19.2.120
33. Kowdley KV, Belt P, Wilson LA, Yeh MM, Neuschwander-Tetri BA, Chalasani N, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. (2012) 55:77–85. doi: 10.1002/hep.24706
34. Loguercio C, De Simone T, D'Auria MV, de Sio I, Federico A, Tuccillo C, et al. Non-alcoholic fatty liver disease: a multicentre clinical study by the Italian Association for the Study of the Liver. Dig Liver Dis. (2004) 36:398–405. doi: 10.1016/S1590-8658(04)00094-5
35. Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int. (2011) 31:730–9. doi: 10.1111/j.1478-3231.2011.02488.x
36. Moon JH, Park SH, Oh KC, Jung JO, Shin WG, Kim JP, et al. Association of hepatic iron deposition and serum iron indices with hepatic inflammation and fibrosis stage in nonalcoholic fatty liver disease. Korean J Gastroenterol Taehan Sohwagi Hakhoe Chi. (2006) 47:432–9.
37. Mousavi SRM, Geramizadeh B, Anushiravani A, Ejtehadi F, Anbardar MH, Moini M. Correlation between serum ferritin level and histopathological disease severity in non-alcoholic fatty liver disease. Middle East J Dig Dis. (2018) 10:90–5. doi: 10.15171/mejdd.2018.96
38. Parikh P, Patel J, Ingle M, Sawant P. Serum ferritin levels predict histological severity in patients with nonalcoholic fatty liver disease in India. Indian J Gastroenterol. (2015) 34:200–8. doi: 10.1007/s12664-015-0572-5
39. Ryan JD, Armitage AE, Cobbold JF, Banerjee R, Borsani O, Dongiovanni P, et al. Hepatic iron is the major determinant of serum ferritin in NAFLD patients. Liver Int. (2018) 38:164–73. doi: 10.1111/liv.13513
40. Seyedian SS, Hajiani E, Hashemi SJ, Masjedizadeh A, Shayesteh AA, Alavinejad P, et al. Relationship between serum ferritin level and transient elastography findings among patients with nonalcoholic fatty liver disease. J Fam Med Prim Care. (2017) 6:750–4. doi: 10.4103/jfmpc.jfmpc_158_17
41. Uysal S, Armutcu F, Aydogan T, Akin K, Ikizek M, Yigitoglu MR. Some inflammatory cytokine levels, iron metabolism and oxidant stress markers in subjects with nonalcoholic steatohepatitis. Clin Biochem. (2011) 44:1375–9. doi: 10.1016/j.clinbiochem.2011.09.017
42. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. (2002) 123:745–50. doi: 10.1053/gast.2002.35354
43. Yao J, Dai Y, Zhang J, Zhang X, Zheng R. Association between serum ferritin level and nonalcoholic fatty liver disease in a non-obese Chinese population: a cross-sectional study. Clin Lab. (2019) 65:1075–80. doi: 10.7754/Clin.Lab.2019.181250
44. Yoneda M, Nozaki Y, Endo H, Mawatari H, Iida H, Fujita K, et al. Serum ferritin is a clinical biomarker in Japanese patients with nonalcoholic steatohepatitis (NASH) independent of HFE gene mutation. Dig Dis Sci. (2010) 55:808–14. doi: 10.1007/s10620-009-0771-y
45. Bugianesi E, Manzini P, D'Antico S, Vanni E, Longo F, Leone N, et al. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatol Baltim Md. (2004) 39:179–87. doi: 10.1002/hep.20023
46. Shimada M, Hashimoto E, Kaneda H, Noguchi S, Hayashi N. Nonalcoholic steatohepatitis: risk factors for liver fibrosis. Hepatol Res. (2002) 24:429–38. doi: 10.1016/S1386-6346(02)00246-2
47. Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatol Baltim Md. (1999) 30:1356–62. doi: 10.1002/hep.510300604
48. Koruk M, Tayşi S, Savaş MC, Yilmaz O, Akçay F, Karakök M. Serum levels of acute phase proteins in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol. (2003) 14:12−7.
49. Qu HJ, Wang L, Zhuang ZJ, Yang WJ, Ding JP, Shi JP. [Studying the correlation between ferritin and non-alcoholic fatty liver disease]. Zhonghua Gan Zang Bing Za Zhi. (2021) 29:1089–94.
50. Trasolini R, Cox B, Galts C, Yoshida EM, Marquez V. Elevated serum ferritin in non-alcoholic fatty liver disease is not predictive of fibrosis. Can Liver J. (2022) 5:152–9. doi: 10.3138/canlivj-2021-0002
51. Wang Q, Zhu M, Li H, Chen P, Wang M, Gu L, et al. Hyperferritinemia correlates to metabolic dysregulation and steatosis in Chinese biopsy-proven nonalcoholic fatty liver disease patients. Diabetes Metab Syndr Obes Targets Ther. (2022) 15:1543–52. doi: 10.2147/DMSO.S361187
52. Bedossa P, Poitou C, Veyrie N, Bouillot J-L, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. (2012) 56:1751–9. doi: 10.1002/hep.25889
53. Yang N, Lu Y, Cao L, Lu M. The association between non-alcoholic fatty liver disease and serum ferritin levels in American adults. J Clin Lab Anal. (2022) 36:e24225. doi: 10.1002/jcla.24225
54. Yu Y-C, Luu HN, Wang R, Thomas CE, Glynn NW, Youk AO, et al. Serum biomarkers of iron status and risk of hepatocellular carcinoma development in patients with nonalcoholic fatty liver disease. Cancer Epidemiol Biomark Prev. (2022) 31:230–5. doi: 10.1158/1055-9965.EPI-21-0754
55. Rushton DH, Barth JH. What is the evidence for gender differences in ferritin and haemoglobin? Crit Rev Oncol Hematol. (2010) 73:1–9. doi: 10.1016/j.critrevonc.2009.03.010
56. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. (2019) 156:1264–81.e4. doi: 10.1053/j.gastro.2018.12.036
57. Barros RK, Cotrim HP, Daltro CH, Oliveira YA. Hyperferritinemia in patients with nonalcoholic fatty liver disease. Rev Assoc Medica Bras. (2017) 63:284–9. doi: 10.1590/1806-9282.63.03.284
58. Du S-X, Lu L-L, Geng N, Victor DW, Chen L-Z, Wang C, et al. Association of serum ferritin with non-alcoholic fatty liver disease: a meta-analysis. Lipids Health Dis. (2017) 16:228. doi: 10.1186/s12944-017-0613-4
59. Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. (2011) 46:257–68. doi: 10.1007/s00535-010-0305-6
60. Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. (2015) 21:11567–83. doi: 10.3748/wjg.v21.i41.11567
Keywords: non-invasive predictor, clinical evaluation, hepatic fibrosis, hepatic steatosis, hepatic inflammation, non-alcoholic steatohepatitis
Citation: Wang H, Sun R, Yang S, Ma X and Yu C (2022) Association between serum ferritin level and the various stages of non-alcoholic fatty liver disease: A systematic review. Front. Med. 9:934989. doi: 10.3389/fmed.2022.934989
Received: 03 May 2022; Accepted: 18 July 2022;
Published: 03 August 2022.
Edited by:
Daniel Q. Huang, National University of Singapore, SingaporeReviewed by:
Xinting Pan, Mengchao Hepatobiliary Hospital, ChinaMargaret Teng, National University Hospital, Singapore
Copyright © 2022 Wang, Sun, Yang, Ma and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengbo Yu, eXVjaGVuZ2JvMTk3NEB6anUuZWR1LmNu
 Huanqiu Wang1
Huanqiu Wang1 Chengbo Yu
Chengbo Yu