
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 26 August 2022
Sec. Nephrology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.929880
Background: Acute oxalate nephropathy (AON) is an uncommon condition that causes acute kidney injury (AKI), characterized by the massive deposition of calcium oxalate crystals in the renal parenchyma. In previous studies, urinary oxalate excretion has been found to be increased in patients with diabetes mellitus (DM). Here, we report a case series of diabetic patients with AKI with biopsy-proven AON, aiming to alert physicians to the potential of AON as a trigger of AKI in diabetic patients in clinical practice.
Materials and methods: Cases with pathological diagnosis of AON who presented with AKI clinically and had DM between January 2016 and December 2020 were retrospectively enrolled. Their clinical and pathological manifestations, treatment, and prognosis were collected.
Results: Six male patients with biopsy-proven AON out of a total of 5,883 native kidney biopsies were identified, aged 58.3 ± 9.1 years at the time of kidney biopsy. Only one patient who had received Roux-en-Y gastric bypass surgery took oxalate-rich food before the onset of the disease. None of them had clinical features of enteric malabsorption. Three patients were currently on renin-angiotensin system inhibitor treatment for hypertension, and 5 of them received non-steroidal anti-inflammatory drugs. Three patients presented with oliguria and 4 patients needed dialysis at the beginning with none requiring dialysis at discharge. Four patients received a course of corticosteroid treatment empirically. Among them, two patients had estimated glomerular filtration rate (eGFR) recovered to over 60 ml/min/1.73 m2, while the other two patients remained with kidney dysfunction at the last follow-up. In two patients without corticosteroid treatment, one patient fully recovered with eGFR over 90 ml/min/1.73 m2 and the other patient remained with kidney dysfunction at the last follow-up.
Conclusion: AON might be a rare but potentially trigger of AKI in patients with DM. A kidney biopsy could help physicians to make the correct diagnosis. The proper treatment to alleviate oxalate-induced injury needs to be further studied.
Patients with diabetes mellitus (DM) may develop diabetic kidney disease (DKD), which develops progressively with a gradual decline in kidney function. Therefore, causes other than DKD should be differentiated when acute kidney injury (AKI) occurs in patients with DM. Acute oxalate nephropathy (AON) is diagnosed by the characteristic finding of widespread deposition of oxalate crystals in the kidney, which is a relatively uncommon cause of AKI in clinical practice (1, 2). The kidney serves as the main excretory organ of circulating oxalate. Patients with DM have been found to have increased urinary oxalate excretion (3–5). Furthermore, the prevalence of DM as comorbidity is approximately 40% in the reported cases of AON in the literature (6).
Elevation of plasma oxalate could lead to hyperoxaluria, which might induce oxalate crystal deposition in the kidney under certain circumstances. The causes of hyperoxaluria can be either primary or secondary (4). Primary hyperoxaluria refers to a group of autosomal recessive disorders with enzymatic defects in the glyoxylate pathway, which results in overproduction of oxalate and subsequent hyperoxaluria (7). Recurrent urolithiasis is the common clinical presentation of primary hyperoxaluria, which generally progresses to end-stage kidney disease (ESKD). Secondary hyperoxaluria is more common than primary hyperoxaluria. Oxalate may be derived exogenously from increased dietary oxalate intake and/or net availability of oxalate in the intestine (8). However, some patients have no evidence of exogenous oxalate derivatives, indicating the potential role of endogenous synthesis in the development of secondary hyperoxaluria. Interestingly, some oxalate precursors have been found with increased circulating levels in patients with DM, which are also identified as potential metabolite markers of DM in recent studies (9, 10). Here, we report 6 cases of AKI with biopsy-proven AON in patients with DM who were admitted to Peking University First Hospital, aiming to alert physicians about the potential of AON as a trigger of AKI in patients with DM in clinical practice.
This retrospective analysis enrolled 6 patients with biopsy-proven AON who underwent native kidney biopsy at Peking University First Hospital from January 2016 to December 2020. The inclusion criteria were as follows: (1) AKI (defined as an increase in serum creatinine to ≥1.5 times baseline, which is known to occur within the prior 7 days) or AKD (defined by > 50% increase in serum creatinine within 3 months) clinically; (2) extensive oxalate crystal deposition in the kidney associated with tubular injury, obstruction, interstitial inflammatory cell infiltration, and fibrosis; (3) exclusion of other causes of kidney disease apart from non-specific microvascular (nephrosclerosis) and diabetic nephropathy; and (4) DM. The study was approved by the ethics committee of Peking University First Hospital and performed in accordance with the Declaration of Helsinki. Informed consent was obtained from each patient at kidney biopsy.
Demographic (age, sex), medical history (hypertension, diabetes, hyperuricemia/gout, chronic kidney disease, urolithiasis, and surgery history), biological data [body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP)], and conditions known to predispose patients to hyperoxaluria were collected from electronic medical charts. Laboratory data of serum and urinalysis at the time of renal biopsy were also collected. Medication information, including renin-angiotensin system inhibitors (RASIs), diuretics, non-steroidal anti-inflammatory drugs (NSAIDs), oxalate precursors, and antibiotics, was recorded.
Frozen spot urine samples stored at –80°C were thawed overnight at 4°C. Then, the samples were centrifuged, and the supernatants were collected, acidified with hydrochloric acid, and further deproteinized by filtration through a Dionex OnGuard II RP 1 cc Cartridge (Thermo Fisher Scientific, United States) before measurement. Oxalate measurement was performed by Aquion Reagent-free Ion Chromatography (RFIC) (Thermo Fisher Scientific, United States).
Diabetes mellitus was defined as fasting plasma glucose ≥7.0 mmol/l, a self-reported history of diabetes, or receiving either insulin or oral antidiabetic drugs. Hypertension was defined as blood pressure (BP) higher than 140/90 mm Hg and/or the current use of antihypertensive medications. eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. Hematuria was defined as red blood cell (RBC) > 3/high-power field. Leukocyturia was defined as white blood cell (WBC) > 5/high-power field.
We identified 10 cases with pathological findings of extensive oxalate deposition out of a total of 5,883 native kidney biopsies performed between January 2016 and December 2020. Three patients were excluded for concurrent findings of other kidney diseases [one patient with crescentic immunoglobulin A (IgA) nephropathy, one patient with focal segmental glomerular sclerosis, and one patient with membranous nephropathy]. Another patient was further excluded for the absence of DM. Finally, six cases were included in the present study.
Table 1 displays the demographic, clinical features, and potential hyperoxaluria enabling factors of the patients with AON in the present study. In brief, all the patients were male, and aged 58.3 ± 9.1 years at the time of kidney biopsy. No patient had a previous history of kidney disease, except one patient with a history of urolithiasis. Three patients were on RASI treatment for hypertension comorbidity. Five patients were found to have concurrent NSAID use at disease onset. Only one patient reported taking spinach in usual amounts before the onset of the disease, who also received Roux-en-Y gastric bypass surgery for gastric carcinoma 1 year ago and had acute diarrhea at the onset of the disease. No one patient reported chronic clinical steatorrhea that indicated malabsorption.
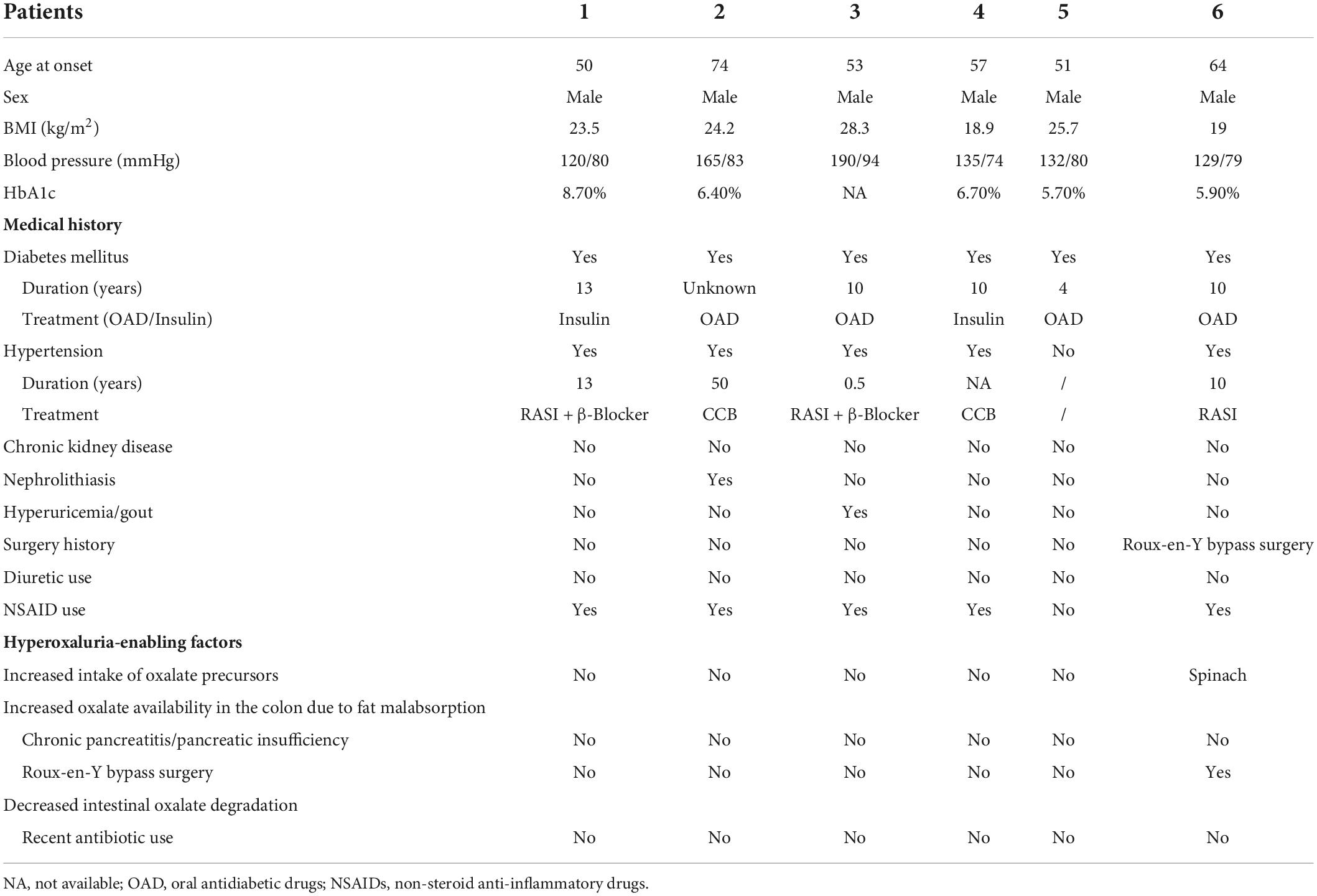
Table 1. Demographic, clinical features and potential hyperoxaluria enabling factors of 6 patients with acute oxalate nephropathy.
Regarding pathological findings, acute tubular epithelial injury with extensive oxalate deposition was the most prominent finding. The tubular cells presented with vacuolar and granular degeneration and effacement of the brush margin, with occasional atrophy found. The interstitium was edematous with the focal distribution of fibrosis. Focal infiltration of lymphocytes and monocytes was observed in all the specimens, with scattered eosinophil infiltration found in 4 cases. None of the cases were found with oxalate crystal deposition in the glomeruli. Instead, ischemic glomerular sclerosis and ischemic wrinkling of the glomerular basement membrane (GBM) to different degrees were observed in these cases. Some also had mild segmental mesangial cell proliferation and matrix accumulation, accompanied by a thickness of GBM observed under electron microscopy. Arteriosclerosis and hyalinization of arterioles were also observed. The typical pathological changes are shown in Figure 1. The polarized light microscopy image and periodic acid-Schiff (PAS) staining for each patient are shown in Supplementary Figure 1.
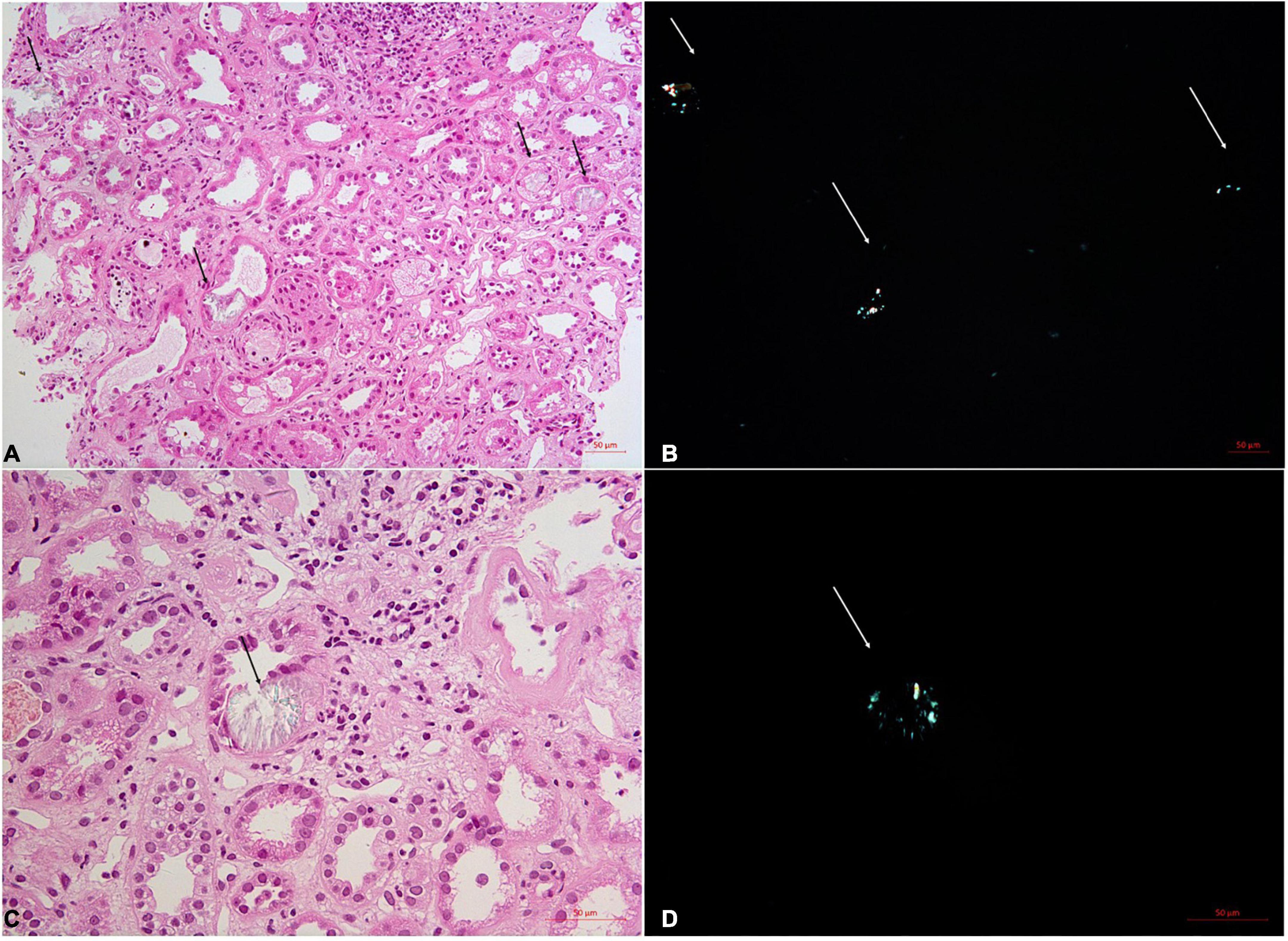
Figure 1. Renal biopsy of the No. 5 patients with acute oxalate nephropathy. On light microscopy, H&E staining shows acute tubular injuries with numerous intratubular oxalate crystals (A,C, arrows). These crystals demonstrate birefringence under polarized light (B,D, arrows). Original magnification 200X (A,B) and 400X (C,D).
Table 2 shows the clinical presentation, treatment strategy, and disease course of the AON of the 6 patients. Three patients presented with oliguria at the onset of disease, and one patient reported approximately half daily urine output compared to usual, with peak creatinine ranging from 463 to 1,641 μmol/l. Neither leukocyturia nor urine crystals were found in the sediments of urine. Two patients without oliguria received hydration, and the other patients received renal replacement therapy, with none requiring dialysis at discharge. Alkali therapy with citrate was given to 4 patients. Four patients received a course of corticosteroid treatment, and two of them had eGFR recovery to over 60 ml/min/1.73 m2 at the last follow-up, while 2 patients remained with kidney dysfunction. Regarding the remaining 2 patients who did not receive corticosteroids, one patient experienced full recovery with eGFR over 90 ml/min/1.73 m2, while the other patient remained with kidney dysfunction at the last follow-up.
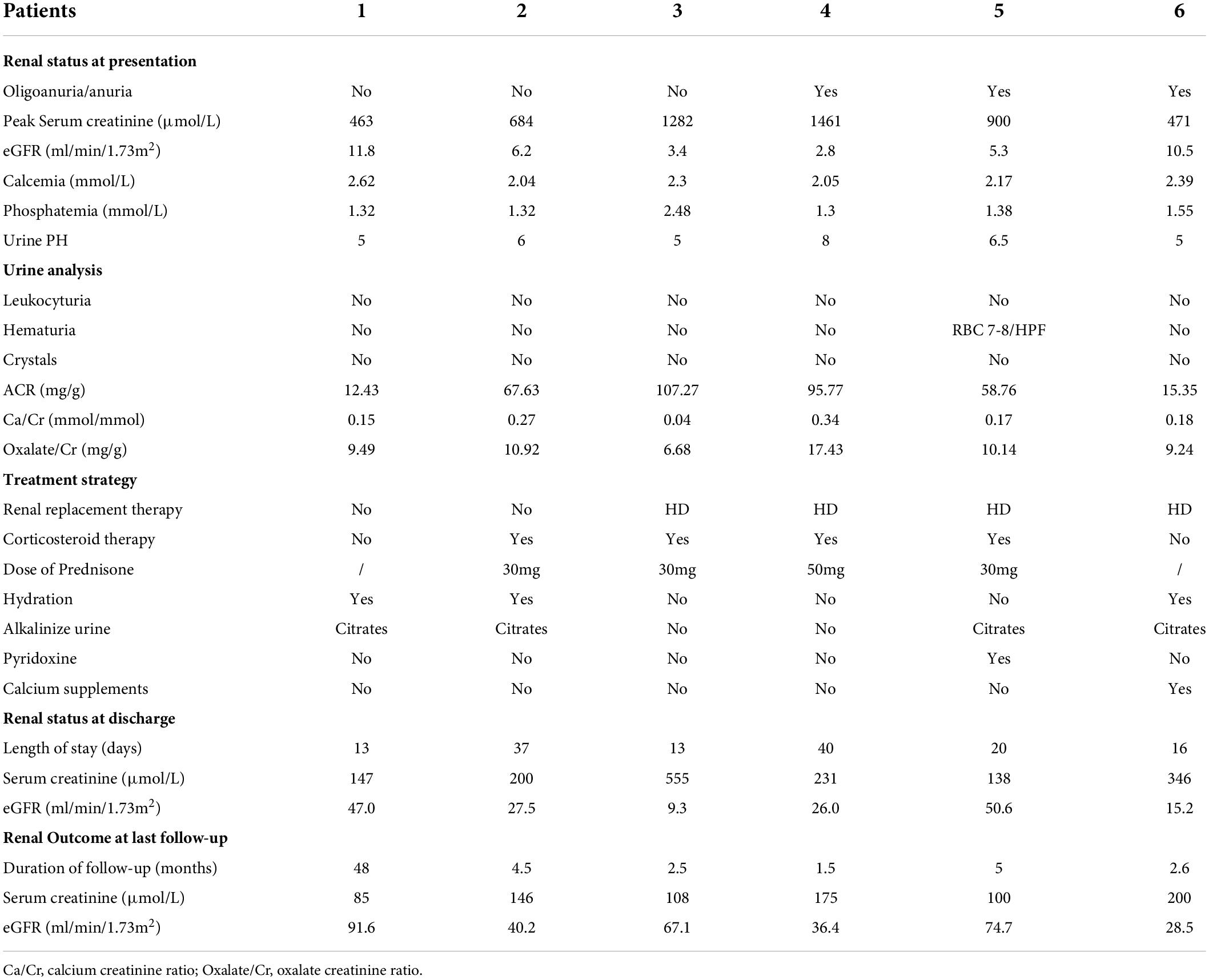
Table 2. Clinical presentation at admission, treatment strategy and disease course of the 6 patients with acute oxalate nephropathy.
In the present study, we reported 6 cases of biopsy-proven AON that presented with AKI in patients with DM. No one was considered to have oxalate nephropathy before the biopsy. Here, we described their clinical manifestations, natural history, and prognosis, aiming to trigger physicians’ alert about AON as a potential cause of AKI in the setting of DM.
Kidney excretion accounts for the majority of daily oxalate excretion from the body. Circulating oxalate is freely filtered at the glomerulus, reabsorbed, and secreted by the proximal tubule (10). Multiple studies have shown that the 24-h urine excretion of oxalate was higher in individuals with DM than in those patients without DM (3, 4). Nephrolithiasis is a common comorbidity of DM, with calcium oxalate as the most common composition of stones (11). In a retrospective analysis of 462 patients with nephrolithiasis, patients with DM excreted greater urinary oxalate than those patients without DM (3). In a recent report from the Chronic Renal Insufficiency Cohort (CRIC) Study with 3,123 established CKD, individuals with DM had 11% higher 24-h urinary oxalate excretion than those patients without diabetes. DM was found to be independently associated with higher urinary oxalate excretion after adjustment for body mass index, age, race, sex, medications, and laboratory tests (5). These results indicate that patients with DM are at risk for hyperoxaluria-enabling conditions.
Hyperoxaluria can develop from multiple causes. Urinary oxalate is derived either exogenously from dietary oxalate intake and net intestinal absorption or endogenously from oxalate synthesis mainly in the liver (10). Most reported AON cases in the literature have attributed the cause to either high intakes of oxalate-containing foods or oxalate precursors (12–15) or malabsorption induced by short-bowel syndrome (16), including celiac disease (17), gastric surgery (Roux-en-Y gastric bypass) (18), inflammatory bowel disease (19), chronic pancreatitis or pancreatic insufficiency, etc. (20, 21). In the present study, all the patients were further extensively questioned about the dietary source of oxalate or oxalate precursors such as ascorbic acid, fruit juice, and ketogenic diet after AON was confirmed pathologically. Only the patient who had a history of Roux-en-Y gastric bypass surgery reported taking spinach. Although diabetic patients may have undetected pancreatic exocrine insufficiency (22), no patients had clinical features of malabsorption in the present study. These 6 patients were all the diabetic patients. Glyoxylate is an immediate precursor of oxalate. In recent years, glyoxylate has been identified as a potential metabolite marker of type 2 DM (10). A retrospective study showed that the plasma level of glyoxylate was elevated in diabetic subjects, even up to 3 years before diabetes diagnosis (9). Glyoxal, another important precursor involved in endogenous oxalate synthesis in humans, has also been found to have higher plasma levels in diabetic patients than in controls, as well as increased urinary oxalate excretion (23). Therefore, the potential contribution of these metabolic components of diabetes to the development of hyperoxaluria in DM needs to be studied more.
In the present study, all of our patients presented with AKI with the finding of universal oxalate crystal deposition in tubules and/or the interstitia in kidney biopsy. The deposition of oxalate in the kidney is subjected to several factors, including supersaturation, precipitation, crystal aggregation, and adhesion (1). Decreased urine output with severe supersaturation may cause massive crystal deposition, renal epithelial cell damage, inflammation, and necrosis, resulting in AKI (24). The formation of highly concentrated urine in the dehydrated state is an essential prerequisite for crystal precipitation and deposition. Four of our cases presented with decreased urine output, indicating volume depletion to some extent. Notably, RASIs and/or NSAIDs were concurrently used in most of our patients at the onset of the disease. These drugs might enhance renal hypoperfusion at the glomerular level under the condition of volume depletion, contributing further to the urine concentration. Furthermore, in vitro studies showed that prostaglandins may decrease the adhesion ability of calcium oxalate to renal epithelial cells (25, 26). As NSAIDs downregulate prostaglandin production (27), it is feasible to speculate that NSAID use is predisposed to a greater likelihood of adhesion of oxalate crystals to the tubular epithelium. In this regard, we hypothesize that factors, which either decrease urinary flow in renal tubules or increase the adhesion ability of oxalate crystals to the epithelium, contribute convergently to the development of acute oxalate nephropathy when superimposed on hyperoxaluria in DM.
The reported outcomes of AON cases vary in the literature. Some patients experienced complete recovery of kidney function, while the other patients had residual kidney dysfunction with some remaining dialysis dependent (2). The recommended treatments include hydration, oral calcium supplements, alkalization, and correction of hyperoxaluria-enabling conditions. We generally followed these treatment strategies after AON diagnosis was established in our center. The role of alkalization is controversial, as oxalate is pH independent. Previous studies have reported inconsistent impacts on the recovery of kidney function by oral citrate supplements and intravenous fluids containing sodium bicarbonate (8, 28). We provided citrate supplementation not only for alkalization, but also to improve the solubility of oxalate. Many nephrologists also empirically use steroids for the management of AON, without confirming the effectiveness of steroids to date. We also administered a course of corticosteroid treatment to 4 patients with severely elevated serum creatinine levels in the present study. They were followed in the outpatient unit after discharge and instructed to taper corticosteroids gradually to a full stop. Although all the 4 patients showed improvement in kidney function to different degrees at the end of follow-up, 2 of them remained with kidney dysfunction. Of the two patients who did not receive corticosteroid treatment, one patient had eGFR recovered to over 90 ml/min/1.73 m2, while the other patient remained with kidney dysfunction. The recovery rate of kidney function was equal between patients with and without corticosteroid treatment. Given the small number of cases in the present study, it is hard for us to judge the effectiveness of corticosteroid treatment. A better understanding of the pathophysiology of oxalate nephropathy might be helpful to establish a more precise treatment for oxalate nephropathy. The inflammasome has been identified to play a critical role in the process of oxalate-induced epithelial injury in recent studies (4). Interleukin-1β (IL-1β) is released upon activation of the NLRP3 inflammasome (4, 29). Administration of an IL-1 receptor antagonist (IL-1ra) has been demonstrated to attenuate oxalate-induced AKI in an animal model (4). Whether IL-1ra treatment is effective in the treatment of AON in clinical practice needs to be studied further.
The strengths of our study are the comprehensive evaluation of the clinical and pathological features, natural history, and prognosis of a series of diabetic patients with biopsy-proven acute oxalate nephropathy. However, some important limitations should be noted. First, the study has inherent limitations of retrospective studies with limited cases, which weaken the strength of making “cause and effect” conclusions. Second, we did not perform special testing with labeled oxalate with urinary recovery to definitely rule out enteric sources at the time of diagnosis, which could not be supplemented given the retrospective nature of the study, and we did not examine blood glyoxalate or glyoxal levels. Third, we did not have data on 24-h urinary excretion of oxalate. We examine spot urine oxalate levels adjusted by urine creatinine, without significantly elevated levels found. One possibility is that the urine sample was collected several days after disease onset. Urinary oxalate excretion might have declined from the initial high level due to the alleviation of kidney dysfunction. In addition, no definite threshold of urinary oxalate concentration is established above which deposition in kidneys is inevitable. Coexisting factors might also play important roles in the process of deposition in addition to urinary oxalate excretion per se.
Acute oxalate nephropathy is a rare but potentially devastating trigger of AKI in patients with DM. Physicians should be more alert about this condition, especially in the setting of oxalate precipitation/attachment-enabling conditions, and perform the renal biopsy in time to establish the diagnosis. How to properly treat patients to alleviate oxalate-induced injury needs to be studied further.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Peking University First Hospital, approval number: 2017[1333]. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DB and YW analyzed and interpreted the patient data and were major contributors to writing the manuscript. XY performed interpretation of pathological data. MZ interpreted the clinical data and substantively revised it. All authors have contributed to the article and approved the submitted version of the manuscript.
This study was supported by the National Natural Science Foundation of China (Nos. 81770671 and 82090021). The funders had a role in the data collection, analysis, and writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.929880/full#supplementary-material
Supplementary Figure 1 | Polarized light microscopy image (A) and PAS staining (B) of the renal biopsy of the six patients with acute oxalate nephropathy. Original magnification 200X (A) and 100X (B).
1. Geraghty R, Wood K, Sayer JA. Calcium oxalate crystal deposition in the kidney: Identification, causes and consequences. Urolithiasis. (2020) 48:377–84. doi: 10.1007/s00240-020-01202-w
2. Buysschaert B, Aydin S, Morelle J, Gillion V, Jadoul M, Demoulin N, et al. Etiologies, clinical features, and outcome of oxalate nephropathy. Kidney Int Rep. (2020) 5:1503–9. doi: 10.1016/j.ekir.2020.06.021
3. Eisner BH, Porten SP, Bechis SK, Stoller ML. Diabetic kidney stone formers excrete more oxalate and have lower urine pH than nondiabetic stone formers. J Urol. (2010) 183:2244–8. doi: 10.1016/j.juro.2010.02.007
4. Ermer T, Eckardt KU, Aronson PS, Knauf F. Oxalate, inflammasome, and progression of kidney disease. Curr Opin Nephrol Hypertens. (2016) 25:363–71. doi: 10.1097/MNH.0000000000000229
5. Waikar SS, Srivastava A, Palsson R, Shafi T, Hsu CY, Sharma K, et al. Association of urinary oxalate excretion with the risk of chronic kidney disease progression. JAMA Intern Med. (2019) 179:542–51. doi: 10.1001/jamainternmed.2018.7980
6. Wijayaratne DR, Bavanthan V, de Silva MVC, Nazar ALM, Wijewickrama ES. Star fruit nephrotoxicity: A case series and literature review. BMC Nephrol. (2018) 19:288. doi: 10.1186/s12882-018-1084-1
7. Cochat P, Rumsby G. Primary hyperoxaluria. N Engl J Med. (2013) 369:649–58. doi: 10.1056/NEJMra1301564
8. Lumlertgul N, Siribamrungwong M, Jaber BL, Susantitaphong P. Secondary oxalate nephropathy: A systematic review. Kidney Int Rep. (2018) 3:1363–72. doi: 10.1016/j.ekir.2018.07.020
9. Nikiforova VJ, Giesbertz P, Wiemer J, Bethan B, Looser R, Liebenberg V, et al. Glyoxylate, a new marker metabolite of type 2 diabetes. J Diabetes Res. (2014) 2014:685204. doi: 10.1155/2014/685204
10. Efe O, Verma A, Waikar SS. Urinary oxalate as a potential mediator of kidney disease in diabetes mellitus and obesity. Curr Opin Nephrol Hypertens. (2019) 28:316–20. doi: 10.1097/MNH.0000000000000515
11. Patel PM, Kandabarow AM, Druck A, Hart S, Blackwell RH, Kadlec A, et al. Association of impaired renal function with changes in urinary mineral excretion and stone composition. Urology. (2020) 141:45–9. doi: 10.1016/j.urology.2020.03.023
12. Getting JE, Gregoire JR, Phul A, Kasten MJ. Oxalate nephropathy due to ‘juicing’: Case report and review. Am J Med. (2013) 126:768–72. doi: 10.1016/j.amjmed.2013.03.019
13. Park H, Eom M, Won Yang J, Han BG, Choi SO, Kim JS, et al. Peanut-induced acute oxalate nephropathy with acute kidney injury. Kidney Res Clin Pract. (2014) 33:109–11. doi: 10.1016/j.krcp.2014.03.003
14. Makkapati S, D’Agati VD, Balsam L. “Green smoothie cleanse” causing acute oxalate nephropathy. Am J Kidney Dis. (2018) 71:281–6. doi: 10.1053/j.ajkd.2017.08.002
15. Fijen L, Weijmer M. Acute oxalate nephropathy due to high vitamin C doses and exocrine pancreatic insufficiency. BMJ Case Rep. (2019) 12:e231504. doi: 10.1136/bcr-2019-231504
16. Khneizer G, Al-Taee A, Mallick MS, Bastani B. Chronic dietary oxalate nephropathy after intensive dietary weight loss regimen. J Nephropathol. (2017) 6:126–9. doi: 10.15171/jnp.2017.21
17. Singh A, Sarkar SR, Gaber LW, Perazella MA. Acute oxalate nephropathy associated with orlistat, a gastrointestinal lipase inhibitor. Am J Kidney Dis. (2007) 49:153–7. doi: 10.1053/j.ajkd.2006.10.004
18. Moreland AM, Santa Ana CA, Asplin JR, Kuhn JA, Holmes RP, Cole JA, et al. Steatorrhea and hyperoxaluria in severely obese patients before and after Roux-en-Y gastric bypass. Gastroenterology. (2017) 152:1055–67.e1053. doi: 10.1053/j.gastro.2017.01.004
19. Hueppelshaeuser R, von Unruh GE, Habbig S, Beck BB, Buderus S, Hesse A, et al. Enteric hyperoxaluria, recurrent urolithiasis, and systemic oxalosis in patients with Crohn’s disease. Pediatr Nephrol. (2012) 27:1103–9. doi: 10.1007/s00467-012-2126-8
20. Cartery C, Faguer S, Karras A, Cointault O, Buscail L, Modesto A, et al. Oxalate nephropathy associated with chronic pancreatitis. Clin J Am Soc Nephrol. (2011) 6:1895–902. doi: 10.2215/CJN.00010111
21. Ye J, Hodgson T, Putt T, Schollum J, Walker R. Oxalate nephropathy presenting in exocrine pancreatic insufficiency. Nephrology (Carlton). (2017) 22:658. doi: 10.1111/nep.12858
22. Muji A, Moll S, Saudan P. Oxalate nephropathy: A new entity of acute kidney injury in diabetic patients? Rev Med Suisse. (2015) 11:493–4, 496–8.
23. Lange JN, Wood KD, Knight J, Assimos DG, Holmes RP. Glyoxal formation and its role in endogenous oxalate synthesis. Adv Urol. (2012) 2012:819202. doi: 10.1155/2012/819202
24. Bargagli M, Tio MC, Waikar SS, Ferraro PM. Dietary oxalate intake and kidney outcomes. Nutrients. (2020) 12:2673. doi: 10.3390/nu12092673
25. Glew RH, Sun Y, Horowitz BL, Konstantinov KN, Barry M, Fair JR, et al. Nephropathy in dietary hyperoxaluria: A potentially preventable acute or chronic kidney disease. World J Nephrol. (2014) 3:122–42. doi: 10.5527/wjn.v3.i4.122
26. Mulay SR, Anders HJ. Crystallopathies. N Engl J Med. (2016) 374:2465–76. doi: 10.1056/NEJMra1601611
27. Fox B, Saxena N, Schuppener L, Maursetter L. Combining acute kidney injury with gastrointestinal pathology: A clue to acute oxalate nephropathy. Case Rep Nephrol. (2018) 2018:8641893. doi: 10.1155/2018/8641893
28. Cohen-Bucay A, Garimella P, Ezeokonkwo C, Bijol V, Strom JA, Jaber BL, et al. Acute oxalate nephropathy associated with Clostridium difficile colitis. Am J Kidney Dis. (2014) 63:113–8. doi: 10.1053/j.ajkd.2013.09.010
Keywords: oxalate nephropathy, diabetes mellitus, acute kidney injury, hyperoxaluria, inflammasome, prognosis
Citation: Bao D, Wang Y, Yu X and Zhao M (2022) Acute oxalate nephropathy: A potential cause of acute kidney injury in diabetes mellitus—A case series from a single center. Front. Med. 9:929880. doi: 10.3389/fmed.2022.929880
Received: 27 April 2022; Accepted: 05 August 2022;
Published: 26 August 2022.
Edited by:
Nan Zhang, Zhejiang University, ChinaReviewed by:
Xiaoyuan Qian, Huazhong University of Science and Technology, ChinaCopyright © 2022 Bao, Wang, Yu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Wang, ZGR3YW5neXVAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.