- 1Yunnan Provincial Key Laboratory of Molecular Biology for Sinomedicine, Kunming, China
- 2First Clinical School of Medicine, Yunnan University of Chinese Medicine, Kunming, China
- 3Basic Medical School, Shanghai University of Chinese Medicine, Shanghai, China
Chronic obstructive pulmonary disease (COPD) is one of the most common pulmonary diseases. Evidence suggests that dysbiosis of pulmonary microbiota leads to the COPD pathological process. Yifei Sanjie Formula (YS) is widely used to treat diseases in respiratory systems, yet little is known about its mechanisms. In the present study, we first established the fingerprint of YS as the background for UHPLC-QTOF-MS. Components were detected, including alkaloids, amino acid derivatives, phenylpropanoids, flavonoids, terpenoids, organic acids, phenols, and the like. The therapeutic effect of YS on COPD was evaluated, and the pulmonary function and ventilatory dysfunction (EF50, TV, and MV) were improved after the administration of YS. Further, the influx of lymphocytes was inhibited in pulmonary parenchyma, accompanied by down-regulation of inflammation cytokines via the NLRP3/caspase-1/IL-1β signaling pathway. The severity of pulmonary pathological damage was reversed. Disturbed pulmonary microbiota was discovered to involve an increased relative abundance of Ralstonia and Mycoplasma and a decreased relative abundance of Lactobacillus and Bacteroides in COPD animals. However, the subversive effect was shown. The abundance and diversity of pulmonary microflora were remodeled, especially increasing beneficial genua Lactobacillus and Bacteroides, as well as downregulating pathogenic genua Ralstonia and Mycoplasma in the YS group. Environmental factor correlation analysis showed that growing pulmonary microbiota was positively correlated with the inflammatory factor, referring to Ralstonia and Mycoplasma, as well as negatively correlated with the inflammatory factor, referring to Lactobacillus and Bacteroides. These results suggest that the effects of YS involved remodeling lung microbes and anti-inflammatory signal pathways, revealing that intervention microbiota and an anti-inflammatory may be a potential therapeutic strategy for COPD.
Introduction
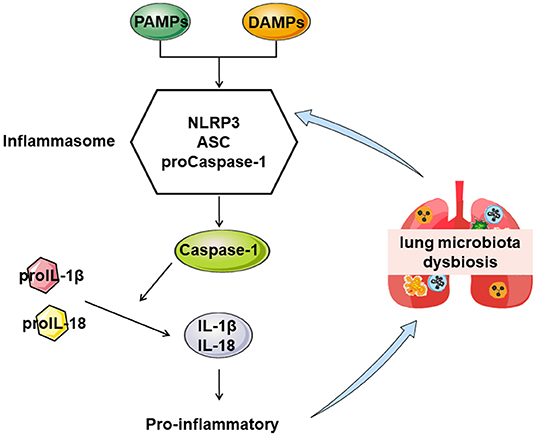
Chronic obstructive pulmonary disease (COPD) is a complex respiratory system disease characterizing continuous respiratory symptoms and airflow limitation followed by a progressive and irreversible decline in lung function. This disease has become the third-most-common life-threatening disease worldwide, leading to substantial social and economic burdens (1, 2). The complex pathogenesis of COPD includes inflammation, oxidative stress, and protease/antiprotease imbalance (3, 4). The inflammatory immune response runs throughout the whole disease process of COPD to reduce inflammation. It is beneficial in delaying the development and improving symptoms of COPD (5). Chronic inflammation in COPD is attributed to the infiltration of various immune cells, macrophages, lymphocytes, neutrophils, and the like, leading to epithelial cell injury and fibrocyte activation to remodel the airway structure (6). The nod-like receptor protein 3 (NLRP3) is known as a potent inducer of inflammation to process Interleukin-1β (IL-1β) and IL-18 (7, 8). NLRP3 inflammasome is essential for the development of COPD in animal or clinical research of human respiratory system samples (9, 10). Activating NLRP3 may be initiate the inflammatory response by binding to cytoplasmic pathogen-associated molecular patterns or damage-associated molecular patterns to aggravate COPD (11). Targeted anti-inflammatory therapy is likely to benefit COPD (12).
In the lungs and airways, there are enormous and complicated microbes that have a profound effect on the host's health, nutrition, and disease progression (13). Microbial community structure and distribution are crucial in maintaining a homeostasis environment (14). Research suggests that bacteria in the respiratory tract resist colonization by foreign pathogens, and cigarette smoking alters the composition of the pulmonary microbiota (15). Pulmonary microbiota dysbiosis is related to inflammation, pathological airway alterations, immune responses, and the aggravation of clinical symptoms in patients with COPD (16). Pathogens stimulate inflammatory cells to produce inflammatory media that often destroy the immune function of the airway and mucosa, leading to chronic inflammation and pulmonary microbiota dysbiosis, further aggravating COPD (17). Undeniably, remodeling pulmonary microbiota structure and proportion is a novel strategy for treating COPD.
Much attention has been paid to the development of Chinese herbal medicines to treat COPD (18). Yifei Sanjie Formula (YS), a traditional Chinese medicine, comprises eight medicinal herbs [Hedysarum Multijugum Maxim, Atractylodes Macrocephala Koidz, Saposhnikoviae Radix, Sinapis Semen, Fritillariae Thunbrgii Bulbus, Mori Cortex, Curcumae Rhizoma, and Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow] and has been shown to possess extensive pharmacological effects against pulmonary disease, including the reduction of lung injury and inflammatory responses (19, 20). Nevertheless, the pharmacological mechanisms of YS on COPD remain poorly understood. Therefore, we explored the efficacy mechanism of YS on COPD in alleviating lung injury and the ventilatory function and the relationship between the efficacy of YS and pulmonary microflora. We aimed to support YS's efficacy in correcting pulmonary microbiota dysbiosis (Figure 1).
Materials and Methods
Groups and Treatments
According to the “Guidelines for the Care and Use of Laboratory Animals” published by the National Institutes of Health, all rat experiments were approved by the Animal Ethics Experiment Committee of Yunnan University of Traditional Chinese Medicine and were conducted following the guidelines of the committee. SPF Wistar rats (n = 18; 200 ± 20 g; male) were provided by Chengdu Dossy Experimental Animals Co., Ltd. [Chengdu, China; license No. SCXK (Chuan)−2020-030]. The rats were randomly divided into the control (CT), COPD, and COPD + YS groups. Besides the CT group, the rat COPD model was replicated by using cigarette smoke exposure (Hongyun cigarette, tar 11 mg, nicotine 1.1 mg, CO12 mg) combined with airway instillation of lipopolysaccharide (LPS, Sigma) (21). 11.6 g/kg once per day of YS was administered daily by intragastric administration from days 57 to 84 (the same dosage is used clinically) (Figure 2A). Animal weight and behaviorism were monitored regularly and recorded. The composition of YS is shown in Table 1.
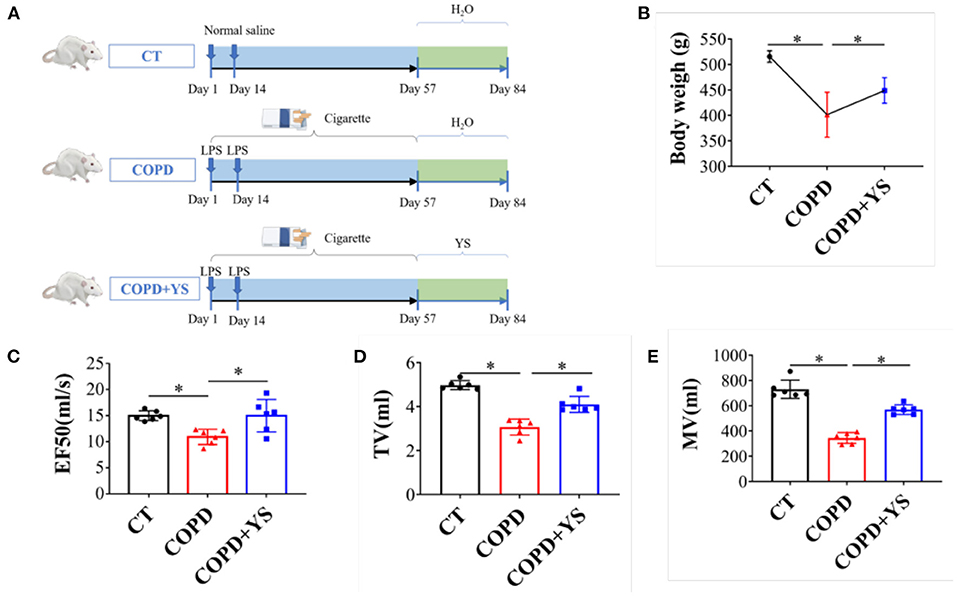
Figure 2. (A) Timeline diagrams of the experimental design (n = 6). (B) Body weight of the three groups including CT, COPD, and COPD + YS (n = 6). (C) EF50 of CT, COPD, and COPD + YS (n = 6). (D) TV of CT, COPD and COPD + YS (n = 6). (E) MV of CT, COPD and COPD+ YS (n = 6). The data are presented as the means ± SEM of three experiments (*P < 0.05).
Pulmonary Function Testing
Pulmonary function testing was performed with a whole-body plethysmograph (EMMS, Alton, Hants, UK) after the experiment was over (12 weeks). According to the whole-body plethysmograph, data for EF50 (Expiratory Flow at 50%), tidal volume (TV), and minute volume (MV) were collected. All of the raw data were obtained from Supplementary Material S17.
Histological Evaluation
The upper lobe of the right lung of the rat was fixed with 4% formalin for 48 h and then embedded in paraffin, stained with hematoxylin and eosin (H&E) and Masson. Finally, it was evaluated histopathologically under an optical microscope (OLYMPUS VS200). All of the full scans of the entire original gels are displayed in Supplementary Materials S1–12.
Serum Cytokine/Protein Levels
Protein was extracted from lung tissue. Protein quantification was performed using the BCA protein quantification kit (P0012, Beyotime, Jiangsu, China) to calculate loading capacity. Samples were added to a precast SDS polyacrylamide gel for electrophoretic separation, and proteins in the gel were electrotransferred to a PVDF membrane using water bath electroblotting. The primary antibodies were added, anti-TGF-β1 (rabbit polyclonal antibody, Abcam, ab179695) and anti-NLRP3 (rabbit polyclonal antibody, Bioss, bs-10021R), overnight at 4°C. After washing the membrane, a secondary antibody was added, shook at 4°C, and incubated for 1 h. PVDF was dropped, and ECL luminescence liquid was developed and photographed (GeneGnome). The grayscale values of individual bands were analyzed using gene tools software. All of the full scans of the entire original gels are displayed in S13 to S16. ELISA kit (Jiangsu Enzyme-Linked Biotechnology Co., Ltd.) was used to detect the concentration of TNF-α and IL-6 in serum and the concentration of IL-1β, IL-18, and SIgA in the lung. The method and procedure were completed according to the kit operating instructions. Finally, read the absorbance at 450 nm after adding the stop solution within 15 min. All of the raw data were obtained from Supplementary Material S17.
16S rRNA Gene High-Throughput Sequencing to Detect the Pulmonary Microbiota
The total genome DNA from samples of the pulmonary microbiota was extracted using the CTAB/SDS method. DNA concentration and purity were monitored on 1% agarose gels. According to the concentration, DNA was diluted to 1 ng/μL using sterile water. 16S rRNA genes of distinct regions (16S V4/16S V3/16S V3-V4) were amplified using a specific primer (16S V4:515F-806R) with the barcode. PCR products were mixed in equidensity ratios. Then, the mixture of PCR products was purified with Qiagen Gel Extraction Kit (Qiagen, Germany). Sequencing libraries were generated using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina, USA) following the manufacturer's recommendations, and index codes were added. The library quality was assessed on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and AgilentBioanalyzer 2100 system. Finally, the library was sequenced on an Illumina NovaSeq platform, and 250 bp paired-end reads were generated. The data on pulmonary microbiota were obtained from the online tool-NovoMagic at https://magic.novogene.com via a personal account. All of the raw data were obtained from Supplementary Material S17.
UHPLC-QE-MS to Screen the Components of YS
We used UHPLC-QE-MS to detect and analyze the potential active components of YS to exert its medicinal effect and further clarify the components of YS. LC-MS/MS analysis was performed on a UHPLC system (Vanquish, Thermo Fisher Scientific) with a Waters UPLC BEH C18 column (1.7 μm 2.1 *100 mm). The flow rate was set at 0.4 mL/min, and the sample injection volume was set at 5 μL. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The multi-step linear elution gradient program was as follows: 0–3.5 min, 95–85% A; 3.5–6 min, 85–70% A; 6–6.5 min, 70–70% A; 6.5–12 min, 70–30% A; 12–12.5 min, 30–30% A; 12.5–18 min, 30–0% A; 18–25 min, 0–0% A; 25–26 min, 0–95% A; 26–30 min, 95–95% A.
An Orbitrap Exploris 120 mass spectrometer coupled with an Xcalibur software was employed to obtain the MS and MS/MS data based on the IDA acquisition mode. During each acquisition cycle, the mass range was from 100 to 1,500, and the top four of every cycle were screened. The corresponding MS/MS data were further acquired. Sheath gas flow rate: 30 Arb, Aux gas flow rate: 10 Arb, Ion Transfer Tube Temp: 350°C, Vaporizer Temp: 350°C, Full ms resolution: 60,000, MS/MS resolution: 15,000, Collision energy: 16/38/42 in NCE mode, Spray Voltage: 5.5 kV (positive) or−4 kV (negative).
Statistical Analysis
Data were expressed as means ± standard deviation ( ±s). The normal distribution of the data was assessed with the Shapiro-Wilk test. Depending on the data normality distribution, significant differences in the variance of parameters were evaluated either with ANOVA or the Kruskal-Wallis test in SPSS 22.0. All statistical tests were two-sided, and a P-value of < 0.05, or FDR adjusted Padj value < 0.05 was considered statistically significant. The analysis of pulmonary microbiota was performed using the online tool-NovoMagic at https://magic.novogene.com via a personal account. All raw data were obtained from S17 and the file of statistical programs (original).
Results
YS Improved Pulmonary Function in COPD Rats
The pulmonary function is an important index in evaluating COPD, including TV, MV, and EF50, reflecting a small airway obstruction. Studies found that COPD patients had decreased TV and MV. Our data showed that EF50, TV, and MV were significantly lower in COPD rats (P < 0.05), while EF50, TV, and MV were higher after YS intervention (P < 0.05) (Figures 2C–E). COPD rats exhibited hair withering, slow action, and shortness of breath. There were significant improvements in animal behaviors after treatment with YS. The body weights of COPD rats significantly decreased (P < 0.05). And, the body weights in the YS+ COPD group significantly increased (P < 0.05) (Figure 2B). These findings suggest that YS improves animal behaviors and pulmonary ventilatory functions in COPD rats.
Screening Components of YS With UHPLC-QTOF-MS
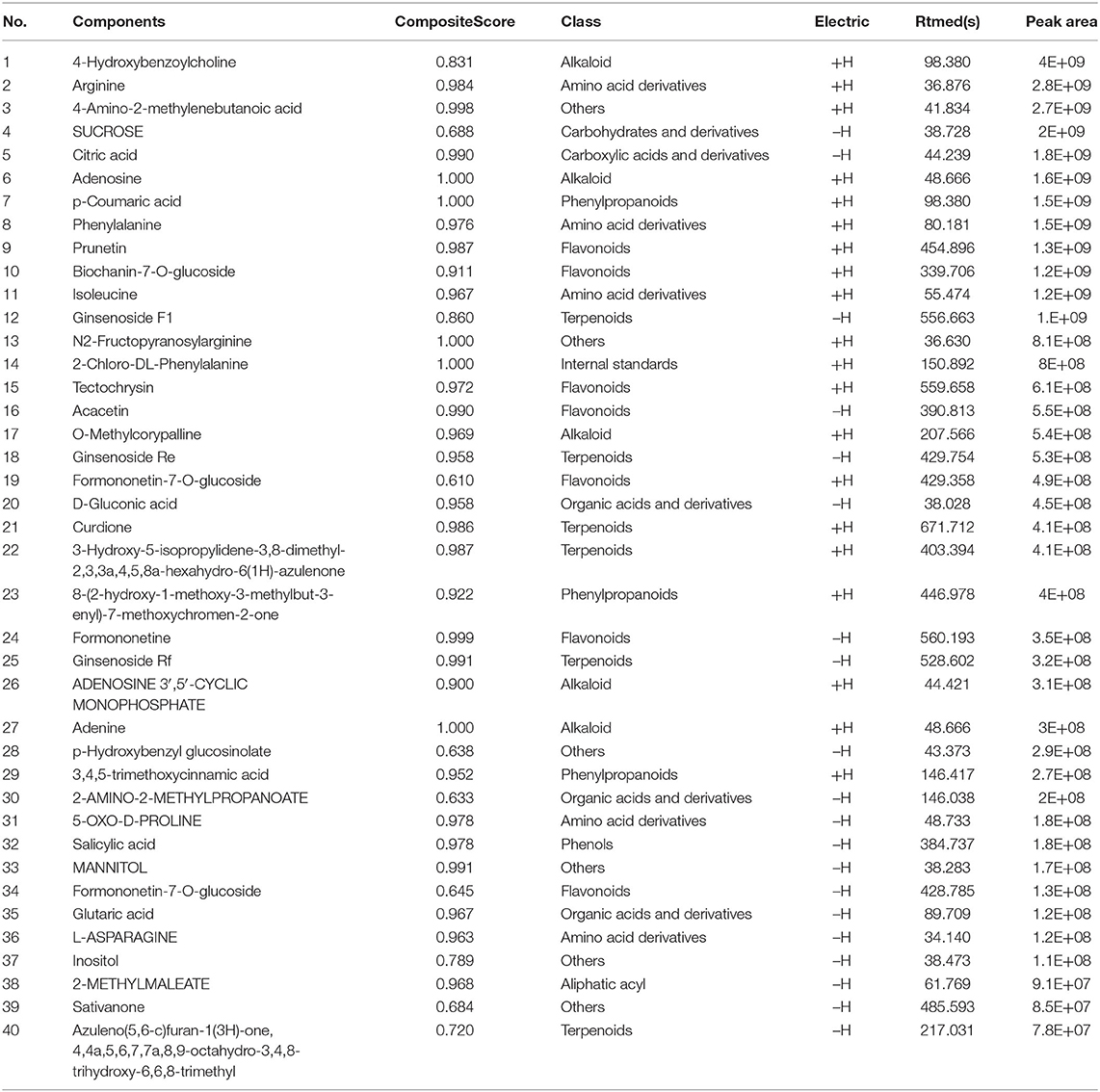
YS is a Chinese herbal medicine formula. It is composed of Hedysarum Multijugum Maxim, Atractylodes macrocephala Koidz, Saposhnikoviae Radix, Sinapis Semen, Fritillaria Thunbrgii Bulbus, Mori Cortex, Curcumae Rhizoma and Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow. We performed UHPLC-QTOF-MS to screen for the components of YS. We established the fingerprint of YS as the background for UHPLC-QTOF-MS, including the positive ion mode and negative ion mode. We analyzed the top 20 components by peak areas with the main peaks separated (Figures 3A,B). Among these, eight kinds of components were detected, including alkaloids, amino acid derivatives, carbohydrates and their derivatives, carboxylic acids and their derivatives, phenylpropanoids, flavonoids, terpenoids, organic acids and their derivatives, phenols, and aliphatic acyl based on a comparison with standard materials (Table 2). The main active products may be one or more of these components.
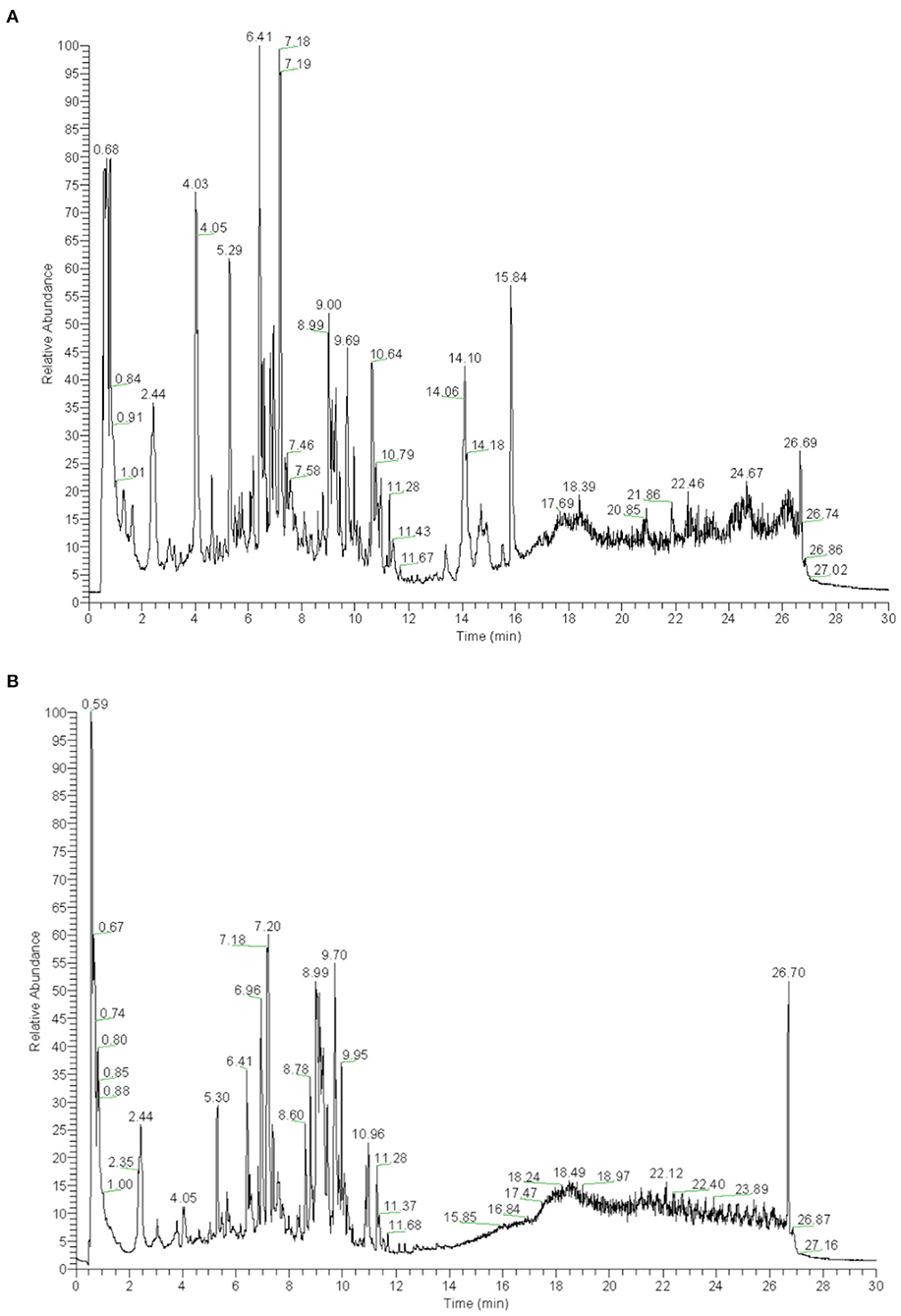
Figure 3. (A) UHPLC-QTOF-MS analysis base peak intensity chromatograms of YS in positive mode. (B) UHPLC-QTOF-MS analysis base peak intensity chromatograms of YS in negative mode.
YS Improved Immune Function in COPD Rats
The spleen and thymus are critical immune organs, and their organ indexes reflect the strength of immune function to a certain extent (22). Our results showed that the thymus index and spleen index were decreased significantly in COPD rats (P < 0.05); after being treated with YS, the thymus and spleen indexes significantly increased (P < 0.05) (Figures 4A,B). SIgA is a major effector molecule of the mucosal immune defense system against the colonization and adhesion of pathogenic microorganisms on mucosal surfaces (23). Local SIgA deficiency on small airway surfaces is associated with COPD progression (24). In this present study, the SIgA was decreased in COPD rats (P < 0.05), whereas the SIgA in the COPD + YS group was significantly increased (P < 0.05) (Figure 4C). These data suggest that YS could enhance the local mucosal immunity and improve immune function in COPD rats.
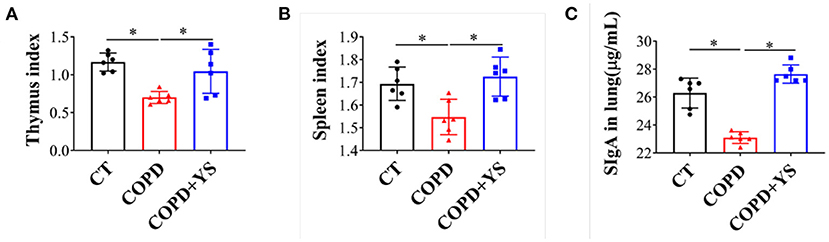
Figure 4. (A) Thymus index of CT, COPD, and COPD + YS (n = 6). (B) Spleen index of CT, COPD, and COPD + YS(n = 6). (C) The SIgA in the lung of CT, COPD, and COPD + YS (n = 6). The data are presented as the means ± SEM of three experiments (*P < 0.05).
YS Ameliorated Inflammation in COPD Rats
The inflammatory response of COPD primarily involves neutrophil infiltration. TNF-α activates neutrophils, and IL-6 inhibits apoptosis (25). HE staining showed that substantial amounts of neutrophil infiltration plugged the bronchi. The lumens were significantly narrowed, and the alveolar walls became thinner and fused in COPD rats. There were fewer pathological changes in the YS intervention group (Figure 5A). Masson staining exhibited that much collagen fiber deposition occurred around the trachea and pulmonary interstitium in COPD rats. Collagen deposition was less severe in COPD+YS rats than in COPD rats (Figure 5B). The levels of TNF-α and IL-6 were significantly higher in the serum of COPD rats (P < 0.05) with YS intervention (Figures 5C,D). TGF-β1 is a powerful profibrotic cytokine that participates in inflammatory repair (26). The expression of TGF-β1 was significantly elevated in lung tissue of COPD rats (P < 0.05). TGF-β1 in the COPD + YS groupwas significantly lower (P < 0.05) (Figure 5E). These findings suggest that YS ameliorates lung tissue damage via attenuating inflammatory responses and reducing collagen deposition in COPD rats.
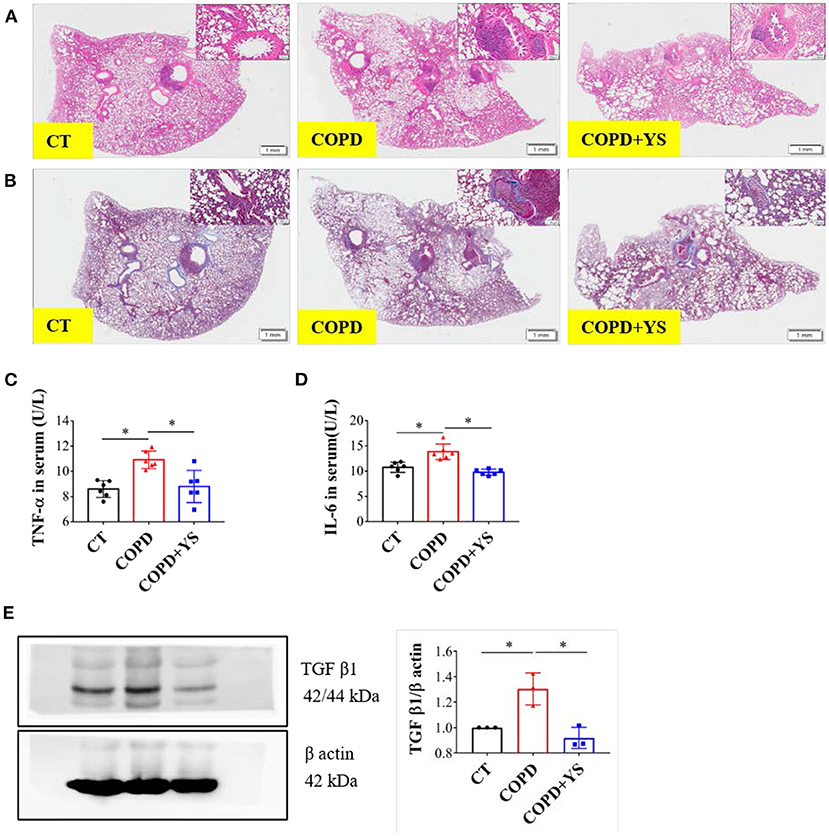
Figure 5. (A) H&E staining of lung tissues of CT, COPD, and COPD + YS. (B) Masson staining of lung tissues of CT, COPD, and COPD + YS. (C) TNF-α in serum of CT, COPD, and COPD + YS (n = 6). (D) IL-6 in serum of CT, COPD, and COPD + YS (n = 6). (E) The relative expression of TGF-β1 in the lung of CT, COPD, and COPD + YS (n = 3). The data are presented as the means ± SEM of three experiments (*P < 0.05).
YS Reduced NLRP3/Caspase-1/IL-1β Signaling Expression in COPD Rats
NLRP3 promotes the release of downstream inflammatory cytokines, inducing acute and chronic inflammatory responses. When NLRP3 is overactivated, ASC acts as an adaptor protein to recruit the precursor caspase-1, promotes IL-1β and IL-18 secreted outside the cell, and exerts proinflammatory effects (27). Compared with control, the protein levels of NLRP3 were significantly greater in lung tissues of COPD rats (P < 0.05). Caspase-1 and ASC were significantly increased in lung tissues (P < 0.05), and IL-1β and IL-18 were significantly higher in serum (P < 0.05). In the COPD + YS group, NLRP3 protein expression was lower in the lung (P > 0.05), caspase-1 and ASC were lower in lung tissue (P > 0.05), and IL-18 levels of serum were significantly lower (P < 0.05). These results suggest that YS inhibits the NLRP3/caspase-1/IL-1β signaling pathway (Figures 6A–E) to alleviate the inflammatory response.
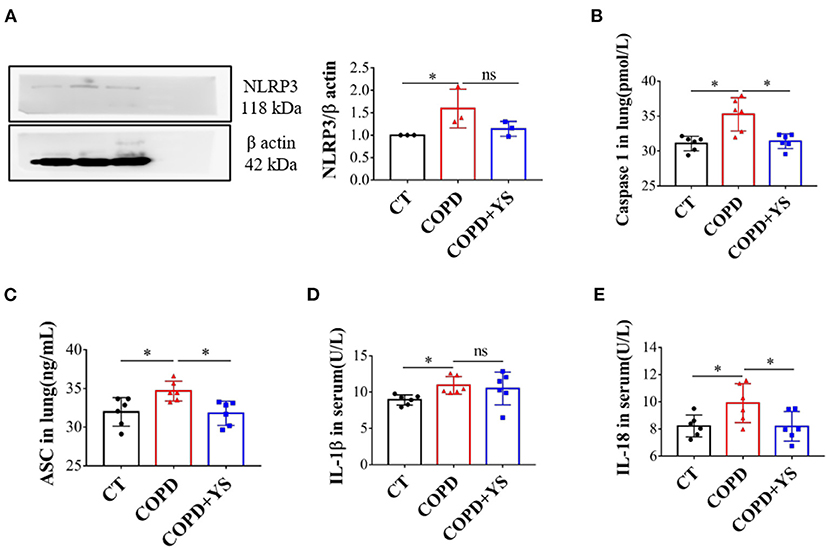
Figure 6. (A) NLRP3 in the lung of CT, COPD, and COPD + YS (n = 3). (B) Caspase1 in the lung of CT, COPD, and COPD + YS (n = 6). (C) ASC in the lung of CT, COPD, and COPD + YS (n = 6). (D) IL-1β in the serum of CT, COPD, and COPD+YS (n = 6). (E) IL-18 in the serum of CT, COPD, and COPD + YS (n=6). The data are presented as the means ± SEM of three experiments (ns, non-significant, *P < 0.05).
Effect of YS on Pulmonary Microbiota in COPD Rats
The pulmonary microbiota is strongly associated with COPD. 16s rDNA gene high-throughput sequencing was employed to analyze the pulmonary microbiota to explore the mechanisms. The Chao1 index reflects the relative abundance of pulmonary microbiota, and the Shannon index reflects the diversity of pulmonary microbiota. The relative abundance of pulmonary microbiota was decreased in COPD rats. After YS treatment, the relative abundance of pulmonary microbiota showed no significant change. Meanwhile, the diversity of the pulmonary microbiota was reduced in COPD rats. (P > 0.05), which was significantly greater after the YS intervention (P < 0.05) (Figure 7A).
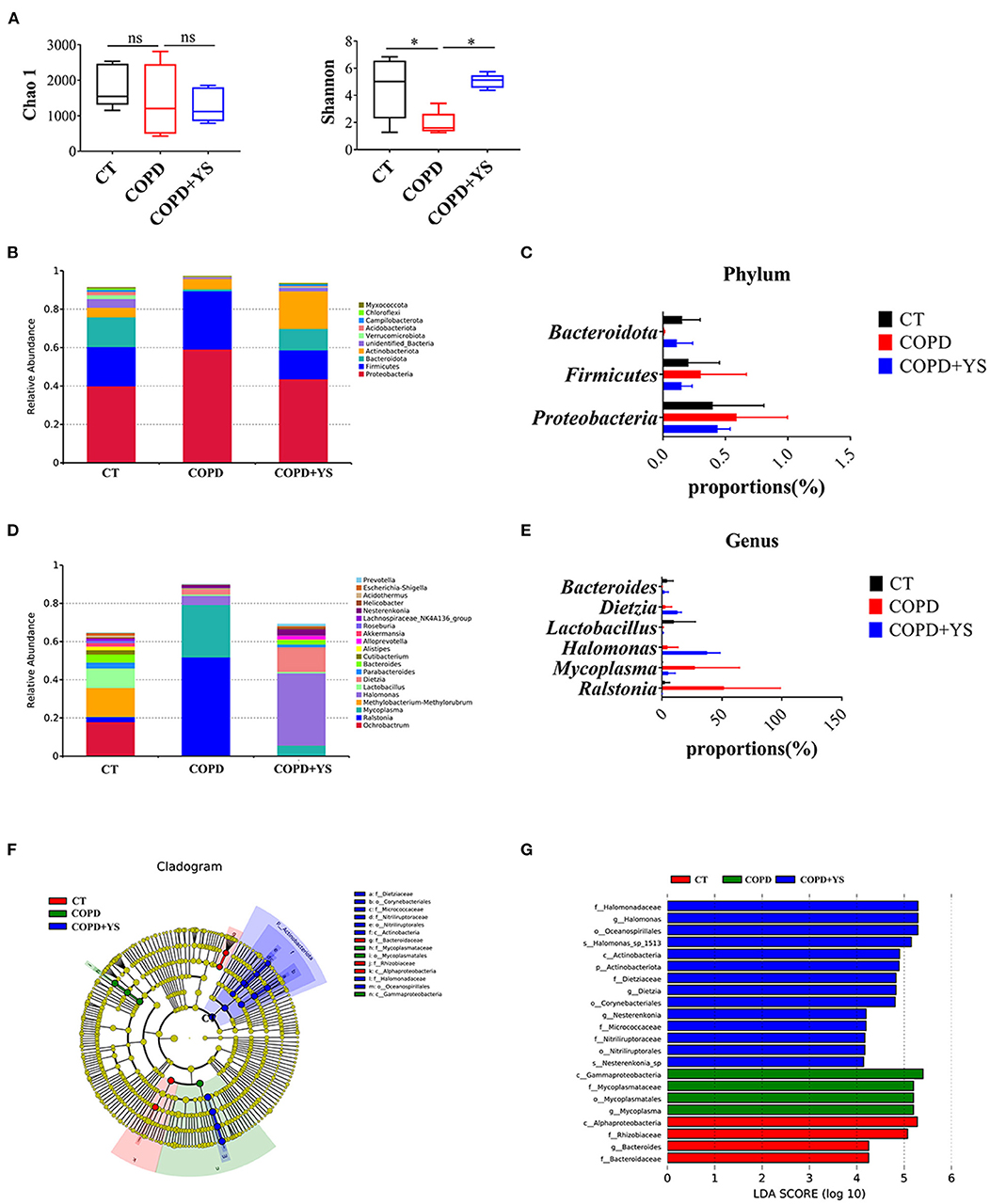
Figure 7. (A) Analysis of alpha diversity: Chao1 and Shannon index (n = 5). (B) The relative abundance of top ten pulmonary microbiota in the phylum (n = 5). (C) The advantage of pulmonary microbiota in the phylum (n = 5). (D) The relative abundance of the top twenty pulmonary microbiota in the genera (n = 5). (E) The advantage of pulmonary microbiota in the genera (n = 5). (F) Taxonomic differences of pulmonary microbiota among different groups. The taxonomic cladogram was obtained by LEfSe. Differences are represented by the color of the most abundant class. The diameter of each circle is proportional to the taxon's abundance (n = 5). (G) Linear discriminant analysis score of each group of gut microbiota (n = 5). The data are presented as the means ± SEM of three experiments (ns, non-significant, *P < 0.05). The analysis of pulmonary microbiota was performed using the online tool-NovoMagic at https://magic.novogene.com via a personal account.
Next, we observed the bacteria in phylum and genera levels among the CT, COPD, and COPD + YS groups. At the phylum level, the pulmonary microbiota was dominated by Proteobacteria, Firmicutes, and Bacteroidota. It's shown that Proteobacteria and Firmicutes increased in COPD rats, while Bacteroidota decreased in COPD rats. After YS intervention, Proteobacteria and Firmicutes decreased while Bacteroidota increased (Figures 7B,C). At the genera level, the pulmonary microbiota was dominated by Ralstonia, Mycoplasma, Halomonas, Lactobacillus, Dizetzia, and Bacteroides. The COPD group showed an increased relative abundance of Ralstonia, Mycoplasma, Halomonas, and Dizetzia over the CT group. Meanwhile, there was a decreased relative abundance of Lactobacillus and Bacteroides. The relative abundance of Halomonas, Lactobacillus, Dizetzia, and Bacteroides were increased, and there was a lower relative abundance of Ralstonia and Mycoplasma after being treated with YS (Figures 7D,E).
Linear discriminant analysis of effect size showed that the iconic microorganisms in each group contributed significantly to differences in microbial structures. Mycoplasma was more abundant in the pulmonary microbiota of the COPD group. Mycoplasma was significantly increased in COPD patients. Meanwhile, Halomonas, Dietzia, and Nesterenkonia were enriched after the YS intervention. These results suggest that YS changes the relative abundance of specific bacteria and modulates the pulmonary microbiota in COPD rats (Figures 7F,G).
Environmental Factor Correlation Analysis
Correlation heatmap plots were used to assess the top 35 genera and correlations with 12 environmental factors (EF50, TV, MV, SIgA, TGF-β1, IL-6, TNF-α, Caspase1, IL-18, IL-1β, ASC, and NLRP3). As shown in the Figure 8, EF50 was positively correlated with Clostridium_sensu_stricto_1 and Klebsiella (P < 0.05). TV was positively correlated with Pseudolabrys (P < 0.05). MV was negatively related with Mycoplasma (P < 0.05) and positively related with Clostridium_sensu_stricto_1 (P < 0.05). SIgA had a positive correlation with Methylobacterium, Methylorubrum, Halomonas, Dietzia, Parabacteroides, Bacteroides, Roseburia, Clostridium_sensu_stricto_1, Blautia, Pseudolabrys and Klebsiella (P < 0.05). TGF-β1 was negatively correlated with Bacteroides, Clostridium_sensu_stricto_1 and Blautia (P < 0.05). IL-6 was negatively associated with Clostridium_sensu_stricto_1 (P < 0.01). TNF-α had a negative correlation with Clostridium_sensu_stricto_1 and Pseudolabrys (P < 0.05). Caspase1 negatively correlated with Methylobacterium, Methylorubrum, Parabacteroides, Escherichia, Shigella, Clostridium_sensu_stricto_1, Blautia and Coriobacteriaceae_UCG.002 (P < 0.05). IL-18 had a positive correlation with Ralstonia (P < 0.05) and a negative correlation with Staphylococcus, Pseudolabrys (P < 0.05). IL-1β was negatively correlated with Lactobacillus (P < 0.05). NLRP3 was positively correlated with Mycoplasma (P < 0.05) and negatively correlated with Bacteroides, Clostridium_sensu_stricto_1 (P < 0.05) (Figure 8). Among them, Clostridium_sensu_stricto_1 was associated with more than half of the environmental factors, which may be the potential target bacteria of pulmonary microbiota for COPD progression and YS therapy. At the same time, SIgA in the lung was more correlated with pulmonary microbiota.
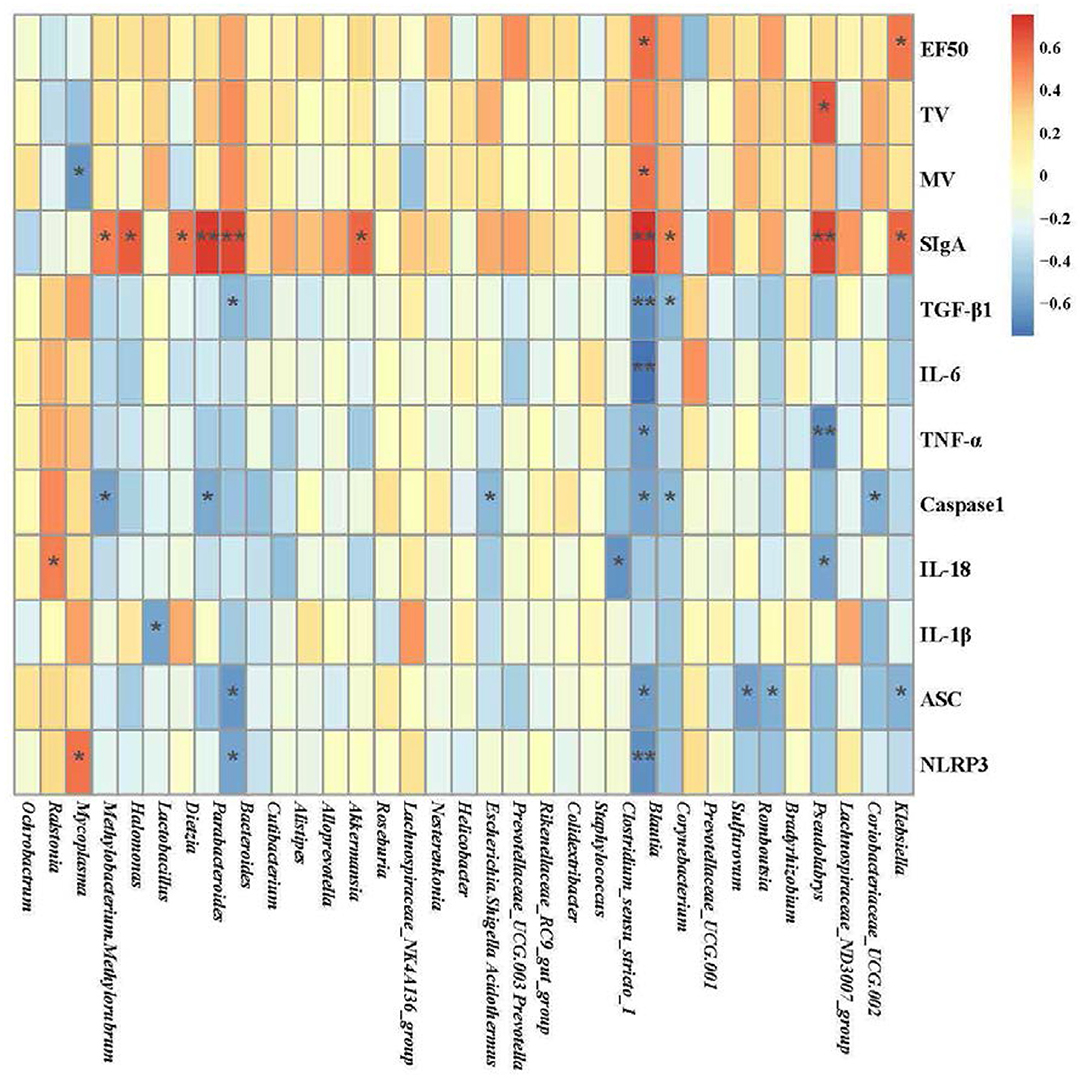
Figure 8. Heatmap of the correlation among the pulmonary microbiota related to environmental factors (*P < 0.05, **P < 0.01; blue represents a negative correlation; red represents a positive correlation). The analysis of pulmonary microbiota was performed using the online tool of NovoMagic at https://magic.novogene.com via a personal account.
Discussion
A well-known inflammation and airway remodeling cause airflow limitation, the primary pathological features of COPD (28). The infiltration of lymphocytes, neutrophils, and monocytes into the lung tissue or airway contributes to chronic inflammation (6). Clinically, neutrophilia of sputum and blood is a characteristic feature of all COPD patients (29), which is derived from chemokines including IL-1β and CXCL (30). The NLRP3 inflammasome is involved in developing diseases by promoting the release of downstream inflammatory cytokines, inducing acute and chronic inflammatory responses (31). In response to endogenous or exogenous stimuli, NLRP3 is activated. Further, ASC acts as an adaptor protein to recruit the precursor caspase-1, which has an enzymatic activity after cleaving pro-interleukin-1β and IL-18 precursor, producing mature IL-1β and IL-18, exerting proinflammatory effects (32). Superfluous inflammasome activation is a feature of numerous lung diseases, including infections (33). Excessive inflammatory factors are proven to be involved in tissue injuries and remodeling in mouse models (34). However, transgenic animal knockout IL-1β and TNF-α receptor mice are protected from developing airway remodeling (35). In the present study, the levels of NLRP3, caspase-1, ASC, IL-18, and IL-1β were decreased in YS administration animals. Insufficiently, we did not evaluate COPD sensitivity and the therapeutic effect of YS in transgenic animals of NLRP3.
Bronchodilators, anti-inflammatory agents, and hormones may improve symptoms in patients with COPD (36). The four main types (Anticholinergics, β2-Agonists, inhaled corticosteroids, and combination bronchodilator therapy) are found in GOLD reports to reduce dynamic hyperinflation at rest and during exercise and improve exercise performance (37). Antioxidant and anti-inflammatory agents were applied to treat COPD in vitro and in vivo (38). Anti-inflammatory and entire modeling effects of muscarinic receptors and antagonists were confirmed in COPD (39). All of them are important for improving symptoms. The contribution is limited in halting the progressive deterioration of pulmonary function and the progression of the disease. YS, as traditional Chinese medicine, is a clinically effective empirical formula for the treatment of lung disease, which is composed of Hedysarum Multijugum Maxim, Atractylodes Macrocephala Koidz, Saposhnikoviae Radix, Sinapis Semen, Fritillariae Thunbrgii Bulbus, Mori Cortex, Curcumae Rhizoma, and Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow (19, 40). Pharmacological studies indicate that the main components of “Hedysarum Multijugum Maxim,” include astragalus polysaccharides (APS), flavonoids, saponins, alkaloids, and the like. Astragalus polysaccharide is the most important natural active ingredient in Hedysarum Multijugum Maxim, with immunomodulatory, antiviral, antifibrotic, and other pharmacological effects (41). Astragaloside IV (AS-IV) is one of the main compounds in the water extract of Hedysarum Multijugum Maxim. Studies have shown that AS-IV has effective protective effects on cardiovascular, lung, kidney, and brain, which may be related to its antioxidant, anti-inflammatory, and anti-apoptotic effects. It is closely related to the enhancement of immunity, the attenuation of migrations, the invasion of cancer cells, and the improvement of chemosensitivity to chemotherapeutic drugs (42). The main chemical components of Atractylodes Macrocephala Koidz are volatile oils, polysaccharides, lactones, and the like. Atractylodes macrocephala polysaccharide (PAMK) can improve animal immunity (43) and reduce inflammatory damage and oxidative stress in mice (44). As the main ingredient of Saposhnikoviae Radix (SR), SR can inhibit the release of histamine for an anti-allergic effect (45). Further, the water-soluble polysaccharides extracted and purified from SR may be one of its main anti-allergic active ingredients (46). Prim-O-glucosylcimifugin Attenuates, one of the active ingredients of Saposhnikoviae Radix, can attenuate endotoxin-induced inflammatory response in macrophages (47). Likewise, Sinapis Semen shows anti-inflammatory properties (48). Fritillaria thunbergii Miq and its bulbs are mainly composed of alkaloids, essential oils, diterpenoids, carbohydrates, sterols, amino acids, nucleotides, fatty acids, and lignans. Among them, Nepeta and its bulbs have a wide range of biological activities, such as anti-inflammatory, anticancer, antitussive, expectorant, antiulcer, antibacterial, antioxidant, antithyroid, regulating blood rheology, antidiarrheal, neuroprotective, and sedative pain effects (49). Curcumae Rhizoma has anti-platelet aggregation and antithrombotic, hepatoprotective, antioxidant, antibacterial, and antiviral activities, in which antitumor activity research is the most extensive and focused (50). Among them, curcumin, as one of the main active components of Curcumae Rhizoma, is a highly effective antibacterial agent effective against various types of cancer, diabetes, obesity, cardiovascular disease, lung disease, nervous system disease, and autoimmune disease (51). Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow can reduce reactive oxygen species, lysozyme, prostaglandins, leukotrienes, and inflammatory cytokines, such as IL-1, IL-12, and TNF-α, to reduce potential damage to surrounding tissues, exerting anti-inflammatory effects (52). The main active ingredient of Panax Notoginseng (Burk.) F. H. Chen Ex C. Chow has been widely studied for its applications such as anti-inflammatory effects (53, 54). Pharmacologically, the function of YS has been confirmed to improve pulmonary function and ventilatory dysfunction and played an important role in repairing inflammatory damage in COPD animals.
The study of the airway microbiota is in its infancy. Still, it has been gradually recognized that the microbiota of the respiratory tract is associated with pulmonary disease (55). Abundant microbial diversity is regarded as beneficial in development and immune system domestication, affecting the susceptibility to allergic asthma, cystic fibrosis, respiratory infection, and lung cancer (56–58). Current reports suggest that the development of COPD is primarily achieved through microbiota. However, risk factors for COPD, including cigarette smoke and inhaling contaminated air, can affect the composition of the pulmonary microbiota (59). In contrast to healthy individuals, the influence of the lung microenvironment on the microbiome in COPD could be obvious (60). The diversity of the sputum microbiome is decreased in COPD patients (61), such as Haemophilis influenzae increasing at the exacerbation of COPD (62). We observed lower microbial alpha diversity in the COPD group than in the control group. The lung microbial alpha diversity of mice in the COPD+YS groups differed from that of the COPD group. Analysis of flora diversity showed that YS prevented pulmonary microbiota dysbiosis in COPD rats. Consistent with this finding, linear discriminant analysis of effect size showed different lung microbial compositions in these groups. Mycoplasma belongs to Firmicutes, which accelerates disease processes by promoting chronic inflammation (63). Halomonas are gram-positive bacteria. Various oligotypes of Christensenella and Clostridium were detected in lung tissue and a few oligotypes of Halomonas in both airway fluid and lung tissue from pulmonary fibrosis and lung cancer patients, suggesting the involvement of these microbial communities in fibrotic diseases (64). Nesterenkonia belongs to Actinobacteria and Lactobacillaceae. The microbiome might play a significant role in maintaining normal lung function, including structural and immune barriers (65). These findings suggest that in COPD treatment, the relative abundance of Halomonas and Nesterenkonia are increased by decreasing the relative abundance of Mycoplasma, potentially inhibiting the inflammatory factors IL-1β, TNF-α, IL-6, IL-1β, and TGF-β1. This further increases SIgA expression, which enhances immunity, alleviates collagen deposition and improves lung function. We used the correlation heatmap plots to assess the top 35 genera and correlations with 12 environmental factors (EF50, TV, MV, SIgA, TGF-β1, IL-6, TNF-α, Caspase1, IL-18, IL-1β, ASC, and NLRP3) to further explore the relationship between pulmonary microbiota and pulmonary ventilation function, inflammatory factors, and immunity. Clostridium_sensu_stricto_1 may be the potential target bacteria of pulmonary microbiota for COPD progression and YS therapy. Besides, SIgA in the lung may be an important factor affecting the pulmonary microbiota.
Summary and Prospects
In this work, we confirmed the function of YS in improving the pulmonary ventilatory function in COPD rats referring to EF50, TV, and MV. Histopathological findings showed that inflammatory injury associated with COPD was weakened. The inflammatory mediators of NLRP3/caspase-1/IL-1βsignaling were involved in pulmonary pathological damage. Furthermore, disturbed pulmonary microbiota was discovered in COPD animals. However, after administration with YS, the abundance and diversity of pulmonary microflora were remodeled, especially increasing the beneficial genua. The replacement of flora is associated with inflammation by environmental factor correlation analysis. In general, we provided some evidence that dysbiosis of pulmonary microbiota leads to the COPD pathological process. The function of the Yifei Sanjie Formula was involved in remodeling lung microbes and anti-inflammatory signal pathways. To evaluate the sensitivity of COPD or pharmacodynamic tests is positive in animals with germ-free lungs, which is verified for further study in the future. It is certain targeting intervention microbiota to anti-inflammatory may be a potential therapeutic strategy for COPD.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by the Animal Ethics Experiment Committee of Yunnan University of Traditional Chinese Medicine. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
ZY and JY: study design. YW: data collection. YW, HM, and BQ: statistical analysis and data interpretation. NL, QZ, WJ, HX, and YL: manuscript preparation. ZY, JY, and YW: funds collection. All authors contributed to the article and approved the submitted version.
Funding
This work was funded in part by a grant from the National Natural Science Foundation of China (81860646, 81760819), Yunnan Provincial Science and Technology Department (202201AS070084, 202005AC160058, 202101AZ070001-012, 202005AF150063, 202102AE090031), the Science Research Foundation of Yunnan Provincial Department of Education (2020Y0204), Yunnan Science and Technology Planning Project-Joint Major Project of Traditional Chinese Medicine (2019FF002[-002]), and the Provincial Innovation Team of the Yunnan University of Chinese Medicine for Traditional Chinese Medicine to Regulate Human Microecology (No. 2018HC011).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are very grateful to Prof. Xu Ou of Fuwai Cardiovascular Hospital Yunnan Province in the guidance for the pulmonary function experiment.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.927607/full#supplementary-material
References
1. Agustí A, Vogelmeier C, Faner R, COPD. 2020: changes and challenges. Lung cellular and molecular physiology. Am J Physiol. (2020) 319:L879–83. doi: 10.1152/ajplung.00429.2020
2. Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: Systematic review and meta-analysis. J Glob Health. (2015) 5:020415. doi: 10.7189/jogh.05.020415
3. Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Aller Clin Immunol. (2016) 138:6–27. doi: 10.1016/j.jaci.2016.05.011
4. McGuinness AJ, Sapey E. Oxidative stress in COPD: sources, markers, and potential mechanisms. J Clin Med. (2017) 6:2. doi: 10.3390/jcm6020021
5. Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. (2018) 391:1706–17. doi: 10.1016/S0140-6736(18)30841-9
6. Wang Y, Xu J, Meng Y, Adcock IM, Yao X. Role of inflammatory cells in airway remodeling in COPD. Int J Chronic Obstr. (2018) 13:3341–8. doi: 10.2147/COPD.S176122
7. Rotta Detto Loria J, Rohmann K, Droemann D, Kujath P, Rupp J, Goldmann T, et al. Nontypeable haemophilus influenzae infection upregulates the nlrp3 inflammasome and leads to caspase-1-dependent secretion of Interleukin-1β - a possible pathway of exacerbations in COPD. PLoS ONE. (2013) 8:e66818. doi: 10.1371/journal.pone.0066818
8. Wang H, Lv C, Wang S, Ying H, Weng Y, Yu W. NLRP3 inflammasome involves in the acute exacerbation of patients with chronic obstructive pulmonary disease. Inflammation. (2018) 41:1321–33. doi: 10.1007/s10753-018-0780-0
9. Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, et al. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke-induced pulmonary inflammation and COPD. Eur Respir J. (2011) 38:1019–28. doi: 10.1183/09031936.00158110
10. Di Stefano A, Caramori G, Barczyk A, Vicari C, Brun P, Zanini A, et al. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax. (2014) 69:516–24. doi: 10.1136/thoraxjnl-2012-203062
11. Nachmias N, Langier S, Brzezinski RY, Siterman M, Bar-Shai A. NLRP3 inflammasome activity is upregulated in an in-vitro model of COPD exacerbation. PLoS ONE. (2019) 14:e0214622. doi: 10.1371/journal.pone.0214622
12. Markelić I, Hlapčić I, Ceri A, Radić Antolic M, SamarŽija M, Popović-Grle S, et al. Activation of NLRP3 inflammasome in stable chronic obstructive pulmonary disease. Sci Rep. (2022) 12:7544. doi: 10.1038/s41598-022-11164-1
13. Invernizzi R, Lloyd CM, Molyneaux PL. Respiratory microbiome and epithelial interactions shape immunity in the lungs. Immunology. (2020) 160:171–82. doi: 10.1111/imm.13195
14. Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. (2019) 12:843–50. doi: 10.1038/s41385-019-0160-6
15. Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol. (2019) 20:1279–90. doi: 10.1038/s41590-019-0451-9
16. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. (2014) 157:121–41. doi: 10.1016/j.cell.2014.03.011
17. Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, et al. Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS ONE. (2019) 6:e16384. doi: 10.1371/journal.pone.0016384
18. Li J, Zhao P, Li Y, Tian Y, Wang Y. Systems pharmacology-based dissection of mechanisms of Chinese medicinal formula Bufei Yishen as an effective treatment for chronic obstructive pulmonary disease. Sci Rep. (2015) 5:15290. doi: 10.1038/srep15290
19. Yu JZ, Ying Y, Liu Y, Sun CB Dai C, Zhao S, et al. Antifibrotic action of Yifei Sanjie formula enhanced autophagy via PI3K-AKT-mTOR signaling pathway in mouse model of pulmonary fibrosis. Biomed Pharmacother. (2019) 118:109293. doi: 10.1016/j.biopha.2019.109293
20. Sun CB, Ying Y, Wu QY, Liu Y, Yu JZ, Xing HJ, et al. The main active components of Curcuma zedoaria reduces collagen deposition in human lung fibroblast via autophagy. Mol Immunol. (2020) 124:109–16. doi: 10.1016/j.molimm.2020.05.017
21. Song YP, Cui DJ, Mao PY, A. new way of establishing a rat chronic obstructive pulmonary disease model and the effects of drugs on them. Acad J Pla Postgrad Med Sch. (2001). 39:51–52+73–74.
22. Li Y, Chen J, Zhang L, Wei W. Research advance of tumor necrosis factor receptor-associated factors in inflammatory immune regulation. Chin Pharmacol Bull. (2015) 31:1206–11. doi: 10.3969/j.issn.1001-1978.2015.09.006
23. Li Y, Jin L, Chen T. The effects of secretory IgA in the mucosal immune system. Biomed Res Int. (2020) 2020:2032057. doi: 10.1155/2020/2032057
24. Du RH, Richmond BW, Blackwell TS, Cates JM, Massion PP, Ware LB, et al. Secretory IgA from submucosal glands does not compensate for its airway surface deficiency in chronic obstructive pulmonary disease. Virch Arch. (2015) 467:657–65. doi: 10.1007/s00428-015-1854-0
25. Cheng Q, Fang L, Feng D, Tang S, Yue S, Huang Y, et al. Memantine ameliorates pulmonary inflammation in a mice model of COPD induced by cigarette smoke combined with LPS. Biomed Pharmacother. (2019) 109:2005–13. doi: 10.1016/j.biopha.2018.11.002
26. Wang L, Meng J, Wang C, Yang C, Li Y. Hydrogen sulfide alleviates cigarette smoke-induced COPD through inhibition of the TGF- β 1/smad pathway. Exp Biol Med. (2020) 245:153537022090434. doi: 10.1177/1535370220904342
27. Peng Z, Zhang W, Qiao J, He B. Melatonin attenuates airway inflammation via SIRT1 dependent inhibition of NLRP3 inflammasome and IL-1β in rats with COPD. Int Immunopharmacol. (2018) 62:23–8. doi: 10.1016/j.intimp.2018.06.033
28. Berg K, Wright JL. The pathology of chronic obstructive pulmonary disease: progress in the 20th and 21st centuries. Arch Pathol Lab Med. (2016) 140:1423–8. doi: 10.5858/arpa.2015-0455-RS
29. Dima E, Rovina N, Gerassimou C, Roussos C, Gratziou C. Pulmonary function tests, sputum induction, and bronchial provocation tests: diagnostic tools in the challenge of distinguishing asthma and COPD phenotypes in clinical practice. Int J Chronic Obstr. (2010) 5:287–96. doi: 10.2147/COPD.S9055
30. Baek KJ, Cho JY, Rosenthal P, Alexander L, Nizet V, Broide DH. Hypoxia potentiates allergen induction of HIF-1α, chemokines, airway inflammation, TGF-β1, and airway remodeling in a mouse model. Clin Immunol. (2013) 147:27–37. doi: 10.1016/j.clim.2013.02.004
31. Mangan M, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. (2018) 17:588–606. doi: 10.1038/nrd.2018.97
32. Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. (2020) 76:100889. doi: 10.1016/j.mam.2020.100889
33. Kim RY, Pinkerton JW, Gibson PG, Cooper MA, Horvat JC, Hansbro PM. Inflammasomes in COPD and neutrophilic asthma. Thorax. (2015) 70:1199–201. doi: 10.1136/thoraxjnl-2014-206736
34. Yang Y, Di TT, Zhang ZX, Liu JX, Fu CL, Wu Y, et al. Dynamic evolution of emphysema and airway remodeling in two mouse models of COPD. BMC Pulm Med. (2021) 21:134. doi: 10.1186/s12890-021-01456-z
35. Churg A, Zhou S, Wang X, Wang R, Wright JL. The role of interleukin-1beta in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Resp Cell Mol. (2009) 40:482–90. doi: 10.1165/rcmb.2008-0038OC
36. Tashkin DP. Smoking cessation in chronic obstructive pulmonary disease. Semin Resp Crit Care. (2015) 36:491–507. doi: 10.1055/s-0035-1555610
37. Terry PD, Dhand R. Inhalation therapy for stable COPD: 20 years of GOLD reports. Adv Ther. (2020) 37:1812–28. doi: 10.1007/s12325-020-01289-y
38. Sadowska AM. Manuel-Y-Keenoy B, De Backer WA. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: discordant in vitro and in vivo dose-effects: a review. Pulm Pharmacol Ther. (2007) 20:9–22. doi: 10.1016/j.pupt.2005.12.007
39. Karakiulakis G, Roth M. Muscarinic receptors and their antagonists in COPD: anti-inflammatory and antiremodeling effects. Mediat Inflamm. (2012) 2012:409580. doi: 10.1155/2012/409580
40. Wei N, Wang XX, Wang YQ Li N, Cheng Y, Tian SZ, et al. Effect of Yupingfengsan improved decoction on airway inflammatory microenvironment in rats with COPD. Mod J Integr Trad Chin West Med. (2018) 27:457–61. doi: 10.3969/j.issn.1008-8849.2018.05.001
41. Zheng YJ, Ren WY, Zhang LN, Zhang YM, Liu DL, Liu YQ, et al. Review of the pharmacological action of astragalus polysaccharide. Front Pharmacol. (2020) 11:349. doi: 10.3389/fphar.2020.00349
42. Zhang JQ, Wu CX, Gao L, Du GH, Qin XM. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv Pharmacol. (2020) 87:89–112. doi: 10.1016/bs.apha.2019.08.002
43. Lin Z, Liu YF, Qu Y, Shi LY, Dou DQ, Kuang HX. Characterisation of oligosaccharides from Baizhu by HILIC-MS. Nat Prod Res. (2015) 29:1194–200. doi: 10.1080/14786419.2014.995652
44. Guo SX Li WY, Chen FY, Yang SZ, Huang YM, Tian YB, et al. Polysaccharide of Atractylodes macrocephala Koidz regulates LPS-mediated mouse hepatitis through the TLR4-MyD88-NFκB signaling pathway. Int Immunopharmacol. (2021) 98:107692. doi: 10.1016/j.intimp.2021.107692
45. Jia QQ, Sun W, Zhang LY, Fu J, Lv YN, Lin YY, et al. Screening the anti-allergic components in Saposhnikoviae Radix using high-expression Mas-related G protein-coupled receptor X2 cell membrane chromatography online coupled with liquid chromatography and mass spectrometry. J Sep Sci. (2019) 42:2351–9. doi: 10.1002/jssc.201900114
46. Gao YY, Jiang Y, Chen GC Li SS, Yang F, Ma Q. Saposhnikoviae RadixA sensitive and rapid UPLC-MS/MS method for determination of monosaccharides and anti-allergic effect of the polysaccharides extracted from. Molecules. (2018) 23:1924. doi: 10.3390/molecules23081924
47. Zhou J, Sun YY, Sun MY, Mao WA, Wang L, Zhang J, et al. Prim-O-glucosylcimifugin attenuates lipopolysaccharideinduced inflammatory response in RAW 2647 macrophages. Pharmacogn Mag. (2017) 13:378–84. doi: 10.4103/pm.pm_323_16
48. Xian YF, Hu Z, Ip SP, Chen JN, Su ZR, Lai XP, et al. Comparison of the anti-inflammatory effects of Sinapis alba and Brassica juncea in mouse models of inflammation. Phytomedicine. (2018) 50:196–204. doi: 10.1016/j.phymed.2018.05.010
49. Nile SH, Su J, Wu D, Wang L, Hu J, Sieniawska E, et al. Fritillaria thunbergii Miq (Zhe Beimu): a review on its traditional uses, phytochemical profile and pharmacological properties. Food Chem Toxicol. (2021) 153:112289. doi: 10.1016/j.fct.2021.112289
50. Zhou Y, Xie M, Song Y, Wang WP, Zhao HR, Tian YX, et al. Two Traditional Chinese Medicines Curcumae Radix and Curcumae Rhizoma: an ethnopharmacology, phytochemistry, and pharmacology review. Evid Based Comp Altern Med. (2016) 2016:4973128. doi: 10.1155/2016/4973128
51. Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, Prasad S, et al. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br J Pharmacol. (2017) 174:1325–48. doi: 10.1111/bph.13621
52. Xu Y, Tan HY Li S, Wang N, Feng Y. Panax notoginseng for inflammation-related chronic diseases: a review on the modulations of multiple pathways. Am J Chin Med. (2018) 46:971–96. doi: 10.1142/S0192415X18500519
53. Ruan JY, Zhang Y, Zhao W, Sun F, Han LF Yu HY, et al. Panax notoginsengNew 12,23-epoxydammarane type saponins obtained from leaves and their anti-inflammatory activity. Molecules. (2020) 25:3784. doi: 10.3390/molecules25173784
54. Zheng YR, Fan CL, Chen Y, Quan JY, Shi LZ, Tian CY, et al. Anti-inflammatory, anti-angiogenetic and antiviral activities of dammarane-type triterpenoid saponins from the roots of Panax notoginseng. Food Funct. (2022) 13:3590–602. doi: 10.1039/D1FO04089H
55. O'Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Resp Crit Care. (2019) 199:1127–38. doi: 10.1164/rccm.201809-1650OC
56. Daley D. The evolution of the hygiene hypothesis: the role of early-life exposures to viruses and microbes and their relationship to asthma and allergic diseases. Curr Opin Allergy Clin. (2014) 14:390–6. doi: 10.1097/ACI.0000000000000101
57. Gray J, Oehrle K, Worthen G, Alenghat T, Whitsett J, Deshmukh H. Intestinal commensal bacteria mediate lung mucosal immunity and promote resistance of newborn mice to infection. Sci Transl Med. (2017) 9:eaaf9412. doi: 10.1126/scitranslmed.aaf9412
58. Dayama G, Priya S, Niccum DE, Khoruts A, Blekhman R. Interactions between the gut microbiome and host gene regulation in cystic fibrosis. Genome Med. (2020) 12:12. doi: 10.1186/s13073-020-0710-2
59. Bassis CM, Erb-Downward JR, Dickson RP, Freeman CM, Schmidt TM, Young VB, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. MBio. (2015) 6:e00037. doi: 10.1128/mBio.00037-15
60. Mammen MJ, Sethi S. COPD and the microbiome. Respirology. (2016) 21:590–9. doi: 10.1111/resp.12732
61. Galiana A, Aguirre E, Rodriguez JC, Mira A, Santibañez M, Candela I, et al. Sputum microbiota in moderate versus severe patients with COPD. Eur Respir J. (2014) 43:1787–90. doi: 10.1183/09031936.00191513
62. Short B, Carson S, Devlin AC, Reihill JA, Crilly A, MacKay W, et al. Haemophilus influenzaeNon-typeable chronic colonization in chronic obstructive pulmonary disease (COPD). Crit Rev Microbiol. (2021) 47:192–205. doi: 10.1080/1040841X.2020.1863330
63. Chaudhry R, Ghosh A, Chandolia A. Pathogenesis of mycoplasma pneumoniae: an update. Indian J Med Microbiol. (2016) 34:7–16. doi: 10.4103/0255-0857.174112
64. D'Alessandro-Gabazza CN, Méndez-García C, Hataji O, Westergaard S, Watanabe F, Yasuma T, et al. Identification of halophilic microbes in lung fibrotic tissue by oligotyping. Front Microbiol. (2018) 9:1892. doi: 10.3389/fmicb.2018.01892
Keywords: chronic obstructive pulmonary disease, pulmonary microbiota, inflammation, Yifei Sanjie Formula, NLRP3/caspase-1/IL-1β signaling pathway
Citation: Wu Y, Meng H, Qiao B, Li N, Zhang Q, Jia W, Xing H, Li Y, Yuan J and Yang Z (2022) Yifei Sanjie Formula Treats Chronic Obstructive Pulmonary Disease by Remodeling Pulmonary Microbiota. Front. Med. 9:927607. doi: 10.3389/fmed.2022.927607
Received: 24 April 2022; Accepted: 10 June 2022;
Published: 29 June 2022.
Edited by:
I-Shiang Tzeng, National Taipei University, TaiwanReviewed by:
Marcos Edgar Herkenhoff, University of São Paulo, BrazilZhou Wang, Macau University of Science and Technology, Macao SAR, China
Copyright © 2022 Wu, Meng, Qiao, Li, Zhang, Jia, Xing, Li, Yuan and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongshan Yang, eWFuZ3pob25nc2hhbkB5bnV0Y20uZWR1LmNu; Jiali Yuan, Mjc0ODEzMjgwMEBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Yueying Wu
Yueying Wu Hui Meng1†
Hui Meng1† Ning Li
Ning Li Qiang Zhang
Qiang Zhang Wenqing Jia
Wenqing Jia Zhongshan Yang
Zhongshan Yang