- 1National Perinatal Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, Oxford, United Kingdom
- 2Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford, Oxford, United Kingdom
Background: Maternal HIV infection is associated with an increased risk of adverse perinatal outcomes. The World Health Organization (WHO) recommends immediate initiation of lifelong antiretroviral therapy (ART) for all people living with HIV, including pregnant women living with HIV (WLHIV). We aimed to assess the risk of adverse perinatal outcomes in WLHIV receiving ART compared to ART-naïve WLHIV and HIV-negative women.
Materials and methods: We conducted a systematic literature review by searching PubMed, CINAHL, Global Health, and EMBASE for studies published between Jan 1, 1980, and April 20, 2020. Two investigators independently selected relevant studies and extracted data from studies reporting on the association of pregnant WLHIV receiving ART with adverse perinatal outcomes. Perinatal outcomes examined were preterm birth (PTB), very PTB, spontaneous PTB (sPTB), low birth weight (LBW), very LBW (VLBW), term LBW, preterm LBW, small for gestational age (SGA), very SGA (VSGA), stillbirth, and neonatal death. Random-effects meta-analyses examined the risk of adverse perinatal outcomes in WLHIV receiving ART compared to ART-naïve WLHIV and HIV-negative women. Subgroup and sensitivity analyses were performed based on country income status and study quality, and adjustment for confounding factors assessed.
Results: Of 94,594 studies identified, 73 cohort studies, including 424,277 pregnant women, met the inclusion criteria. We found that WLHIV receiving ART are associated with a significantly decreased risk of PTB (relative risk 0.79, 95% CI 0.67–0.93), sPTB (0.46, 0.32–0.66), LBW (0.86, 0.79–0.93), and VLBW (0.62, 0.39–0.97) compared to ART-naïve WLHIV. However, WLHIV receiving ART are associated with a significantly increased risk of PTB (1.42, 1.28–1.57), sPTB (2.20, 1.32–3.67), LBW (1.58, 1.36–1.84), term LBW (1.88, 1.23–2.85), SGA (1.69, 1.32–2.17), and VSGA (1.22, 1.10–1.34) compared to HIV-negative women.
Conclusion: ART reduces the risk of adverse perinatal outcomes in pregnant WLHIV, but the risk remains higher than in HIV-negative women. Our findings support the WHO recommendation of immediate initiation of lifelong ART for all people living with HIV, including pregnant WLHIV.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021248987.
Introduction
37.7 million people globally were living with HIV in 2020, of whom 19.3 million are women over the age of 15 (1). An estimated 1.3 million women living with HIV (WLHIV) are pregnant each year, the vast majority residing in sub-Saharan Africa. This population is increasing, with women and girls accounting for 59% of new HIV infections in sub-Saharan Africa, a region that also has the highest neonatal and child mortality rates (2).
Pregnancies in WLHIV without antiretroviral therapy (ART) are associated with an increased risk of preterm birth (PTB), low birthweight (LBW), small for gestational age (SGA), and stillbirth, compared to HIV-negative women (3). PTB is the leading cause of neonatal and child mortality globally, with an estimated 14.8 million preterm births occurring each year (4). 23.3 million infants born SGA contribute to 21.9% of neonatal deaths in low- and middle-income countries (LMICs) (5). Both PTB and SGA contribute to the 18 million infants born annually with LBW (6), a perinatal outcome commonly used in LMICs, as gestational age at birth is often unknown.
ART is crucial for WLHIV to improve maternal health and to reduce perinatal HIV transmission. In the past, World Health Organization (WHO) guidelines included combination ART (cART) for pregnant WLHIV who required treatment for their own health, whereas zidovudine (ZDV) monotherapy was recommended for prevention of perinatal HIV transmission. From 2013, WHO recommended that all pregnant WLHIV should receive cART during pregnancy (7). This was updated in 2015 to a recommendation that all people living with HIV should initiate lifelong cART as soon as possible after diagnosis, irrespective of CD4 count, including pregnant WLHIV (8). As a result, the proportion of pregnant WLHIV receiving ART increased from 44 to 82% during 2010–2018. Whether ART use in pregnancy is associated with an increased risk of adverse perinatal outcomes has been controversial. A number of studies suggest adverse perinatal outcomes are associated with ART exposure during pregnancy, with conflicting results regarding regimen complexity, drug classes, and timing of ART initiation (9–14).
The United Nations’ Sustainable Development Goal 3 (SDG3) target 3.2 aims to end preventable deaths of new-borns and children under 5 years of age by 2030 and reduce neonatal and under-5 mortality to 12 and 25 per 1,000 live births, respectively (15). As the number of pregnant WLHIV receiving ART increases, a better understanding of the association of ART with perinatal outcomes is crucial. It is uncertain whether ART improves perinatal outcomes in WLHIV, and whether ART restores the risk of adverse perinatal outcomes to a level comparable with HIV-negative women. We conducted a systematic review and meta-analysis to examine the risk 11 specific perinatal outcomes in WLHIV receiving ART compared to WLHIV without ART and HIV-negative women.
Materials and methods
Search strategy
The systematic review and meta-analyses were conducted based on a protocol developed according to the Cochrane guidelines and registered online (PROSPERO, number CRD42021248987). Electronic literature databases PubMed, CINAHL (Ebscohost), Global Health (Ovid), EMBASE (Ovid) were searched for studies published between Jan 1, 1980, and April 20, 2020 using a comprehensive search strategy adapted for each database, developed by a specialist librarian (SK). Both free text and controlled vocabulary search terms for “pregnancy outcome,” “specific perinatal outcomes,” “HIV,” and “antiretroviral therapy” were used. No methodological, country, or language filters were applied, and both full-text articles and abstracts were considered. The full search terms can be found in Supplementary Appendix 1. Retrieved citations were imported into EndNote reference manager (EndNote X9; Clarivate Analytics, Philadelphia, PA, USA) and deduplicated.
Study selection and eligibility criteria
Studies that contained information on the association of pregnant WLHIV receiving ART with adverse perinatal outcomes were eligible. The titles and abstracts of citations retrieved by the literature searches were reviewed and full text manuscripts of selected citations were obtained and assessed against the eligibility criteria by at least two independent investigators (CP, HS, MK, and ZB). Inclusion criteria were study design (prospective and retrospective cohort studies), population (pregnant women), exposure (WLHIV with ART exposure) and comparators (WLHIV without ART exposure or HIV-negative women). ART exposure was defined as any number, class, and combination of antiretroviral drugs received during pregnancy. cART exposure was defined as exposure to ≥ 3 antiretroviral drugs. WLHIV were not considered to have been exposed to ART if they only received a single ART dose at delivery or received antenatal ART for < 30 days. Studies were not included if less than 95% of women in an exposure or comparator group conformed to the exposure/comparator definition (e.g., < 95% of WLHIV received ART) or if additional treatment was received by one exposure/comparator group only. Perinatal outcomes of interest were defined as follows: preterm birth (PTB, birth < 37+0 weeks gestation); (16) very PTB (VPTB, birth < 32+0 weeks gestation); (16) spontaneous PTB (sPTB, birth following spontaneous onset of labor < 37+0 weeks gestation); low birthweight (LBW, < 2,500 g); (6) very LBW (VLBW, < 1,500 g); (6) small for gestational age (SGA, birthweight for gestational age < 10th centile); (17) very SGA (VSGA, birthweight for gestational age < 3rd centile), (17) stillbirth (delivery of an infant without any signs of life with birthweight ≥ 1,000 g or gestational age ≥ 24+0 weeks or body length ≥ 35 cm); (18) and neonatal death (NND, death of an infant in the first 28 days of life) (18). Term and preterm LBW were defined according to definitions of PTB and LBW. Perinatal outcome data were not included if outcomes were not defined or if defined differently from our definitions. If a cohort was reported more than once, the study containing the most recent and complete data was included. If studies reported different perinatal outcomes for the same cohort, each study was included. References of included studies were assessed for additional relevant studies. Details of excluded papers are available upon request. Any ambiguities or disagreements regarding inclusion of studies were resolved through discussion with the senior investigator (JH).
Data extraction
Data on study and population characteristics, HIV/ART exposures and perinatal outcomes were independently extracted from eligible studies by at least two investigators (CP, HS, MK, and ZB) and reviewed by the senior investigator (JH). Outcome data according to HIV/ART exposure were extracted. Information on methods to adjust for confounders, including regression analysis (i.e., confounders corrected for), risk factor analysis (i.e., risk factors not significantly different between groups), and matching was extracted. Reported unadjusted and adjusted relative risks (RR), odds ratios (OR), and 95% confidence intervals (CIs) of perinatal outcomes according to HIV/ART exposure were also extracted.
Quality assessment
The quality of individual studies was assessed using an adapted Newcastle-Ottawa Scale by at least two investigators (CP, HS, MK, and ZB) and reviewed by the senior investigator (JH). Nine criteria were assessed in three groups: Selection of study participants (maximum 4 points), Comparability of comparator groups (maximum 2 points), and Assessment of outcomes of interest, including methods to assess gestational age at birth (maximum 3 points). Studies were defined as “good,” “average,” or “poor” quality according to predefined criteria (Supplementary Appendix 2).
Statistical analysis
Perinatal outcomes were compared between WLHIV receiving ART and either WLHIV without ART or HIV-negative women. Dichotomous outcome data according to HIV/ART exposure from individual studies were used to generate RRs and 95% CIs. Pairwise meta-analyses were carried out if two or more studies reported data for the same perinatal outcome for WLHIV receiving ART as well as WLHIV without ART or HIV-negative women. For all meta-analyses, a random-effects model was used to calculate a weighted summary effect estimate (RR) and 95% CI. Meta-analyses were represented in forest plots and the I2 statistic was used to quantify heterogeneity due to clinical and methodological variability between studies. The degree of heterogeneity was classified as none (< 25%), low (25–49%), moderate (50–74%), or high (≥ 75%). Prescribed subgroup analyses were carried out to assess the effects of country income status and sensitivity analyses were done to investigate whether study quality and the adjustment for confounders had an impact on the associations between HIV/ART exposure and perinatal outcomes. The Peters’ test was used to assess publication bias in meta-analyses containing ten or more studies. All statistical analyses were done with Stata version 13 (College Station, TX, USA). The systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
The literature search yielded 94,594 citations, of which 73 studies reported relevant data (Figure 1). The perinatal outcomes reported for WLHIV receiving ART compared to WLHIV without ART were PTB (32 studies), VPTB (3), sPTB (2), LBW (20), VLBW (4), SGA (9), and VSGA (1) (Figure 1). The perinatal outcomes reported for WLHIV receiving ART compared to HIV-negative women were PTB (32 studies), VPTB (5), sPTB (3), LBW (20), VLBW (6), term LBW (3), preterm LBW (1), SGA (21), VSGA (5), stillbirth (1), and NND (6) (Figure 1).
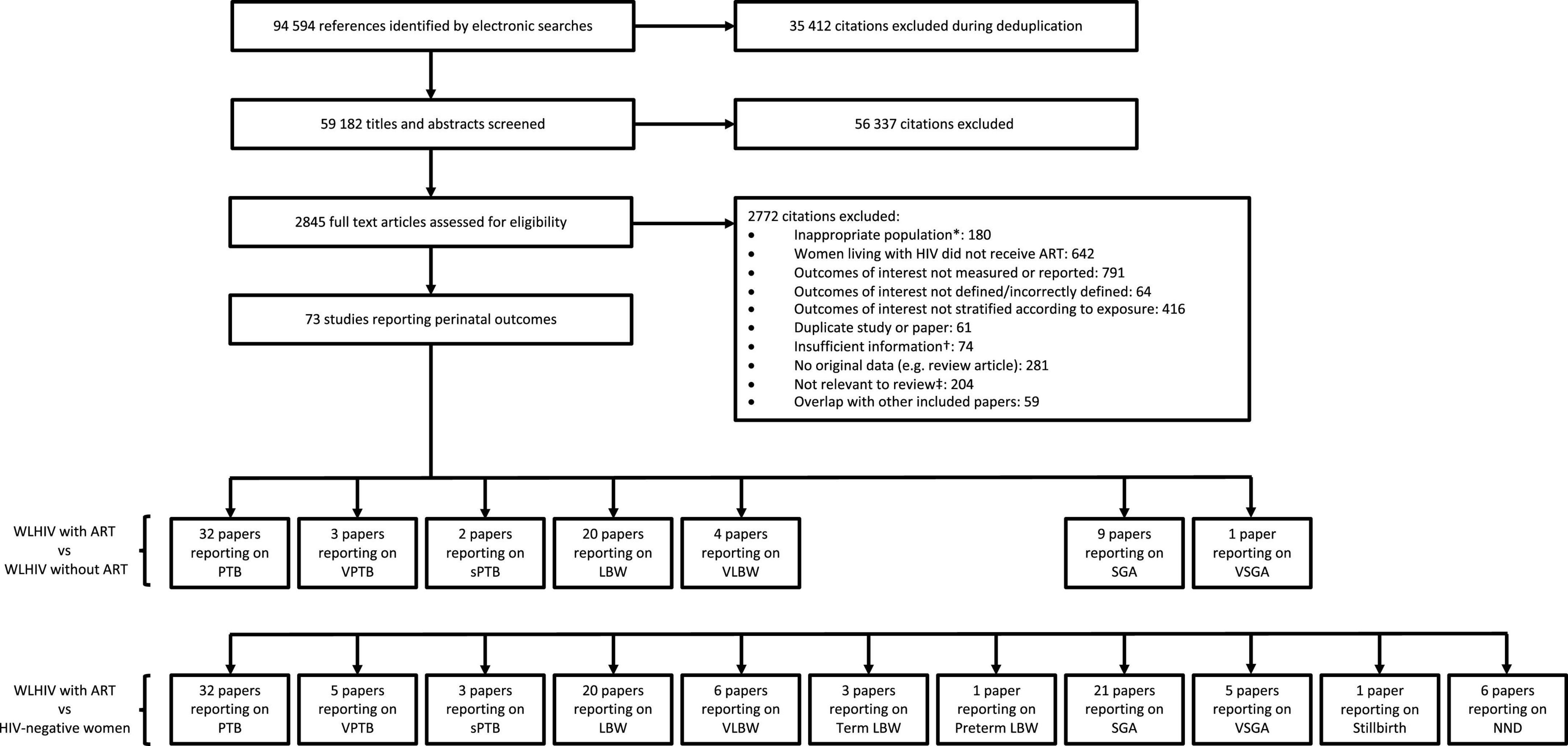
Figure 1. Study selection. *For example, women living with HIV were not pregnant. †For example, paper did not provide relevant outcome data. ‡For example, Assisted Reproductive Technology. ART, antiretroviral therapy; HIV, human immunodeficiency virus; LBW, low birthweight; NND, neonatal death; PTB, preterm birth; SGA, small for gestational age; sPTB, spontaneous preterm birth; VLBW, very low birthweight; VPTB, very preterm birth; VSGA, very small for gestational age; WLHIV, women living with HIV. See Materials and methods for definitions of perinatal outcomes.
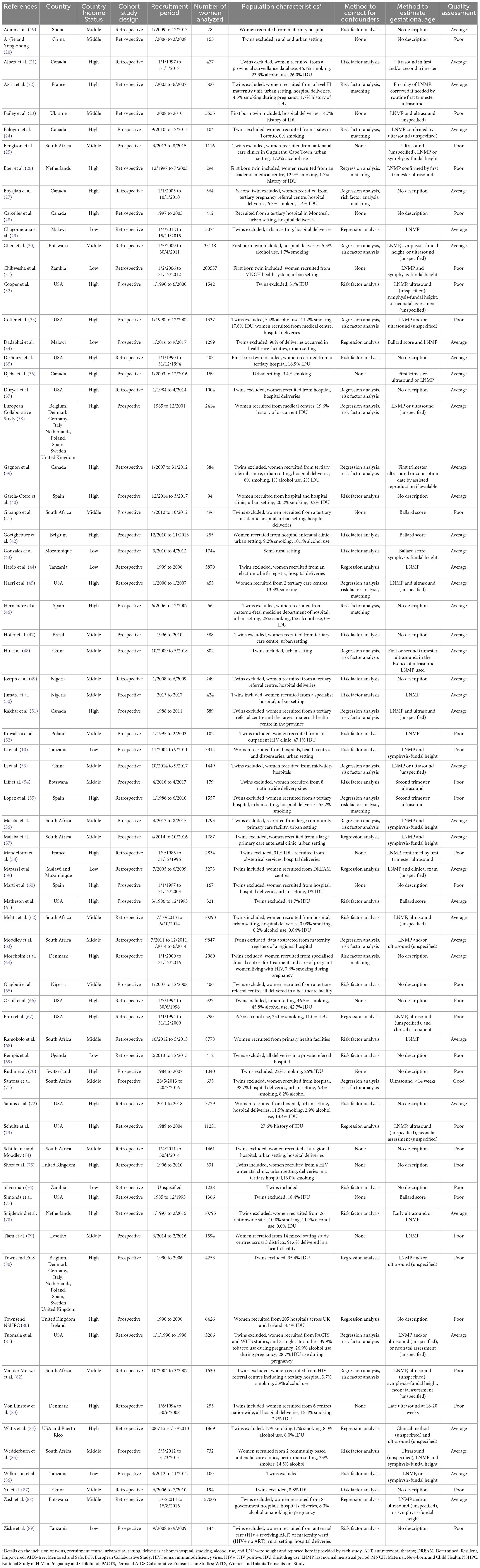
Characteristics of included studies are summarized in Table 1 (10, 19–89). 33 prospective (45%) and 40 retrospective (55%) cohort studies analyzed data from 424,277 women in 27 countries (Table 1). 36 studies (49%) with 64,778 women took place in high income countries (HICs), and 37 studies (51%) with 359,499 women took place in low- and middle-income countries (LMICs). 50 studies (68%) reported the methods used to determine gestational age, with six (8%) studies exclusively using, or confirming gestational age with, first trimester ultrasound, the most accurate method of establishing gestational age (12). 38 studies (52%) used last normal menstrual period (LNMP), 27 studies (37%) used second trimester or unspecified ultrasound, 12 studies (16%) used symphysis-fundal height measurements, and six studies (8%) used Ballard score to determine gestational age. Two studies (3%) used an unspecified “clinical method” to determine gestational age. 35 studies (48%) reported using > 1 method to determine gestational age. 23 (32%) studies did not report methods used to determine gestational age. 57 studies (78%) used methods to assess potential confounding factors. Regression analysis was conducted in 28 studies, risk factor analysis was carried out in 45 studies, and matching of participants was carried out in eight studies (Supplementary Appendix 2.4). Of the 41 comparisons which were adjusted for covariates in individual studies, only six resulted in a change of the effect estimate from significant to no significant difference in adverse perinatal outcomes between groups (Supplementary Appendix 4). Quality assessments classified 32 studies (44%) as poor quality, 40 (55%) as average quality and one (2%) as good quality (Table 1 and Supplementary Appendix 2.3). Studies from LMICs had quality ratings (3% good, 54% average, and 43% poor quality) comparable to studies from HICs (55% average, 44% poor quality).
The ART regimens taken by WLHIV receiving ART, exposure comparisons reported, and perinatal outcomes analyzed are displayed for each study in Table 2. 41 studies (56%) reported perinatal outcomes in WLHIV receiving ART compared to WLHIV without ART, and 38 studies (52%) compared perinatal outcomes in WLHIV receiving ART with HIV-negative women. Six studies (8%) reported on both comparisons. In 32 (44%) studies ≥ 95% of women received cART in the group of WLHIV who received ART. Only five studies (7%) included WLHIV solely exposed to ZDV monotherapy. The remaining 36 studies (49%) reported on WLHIV receiving a mixture of different ART regimens (Table 2).
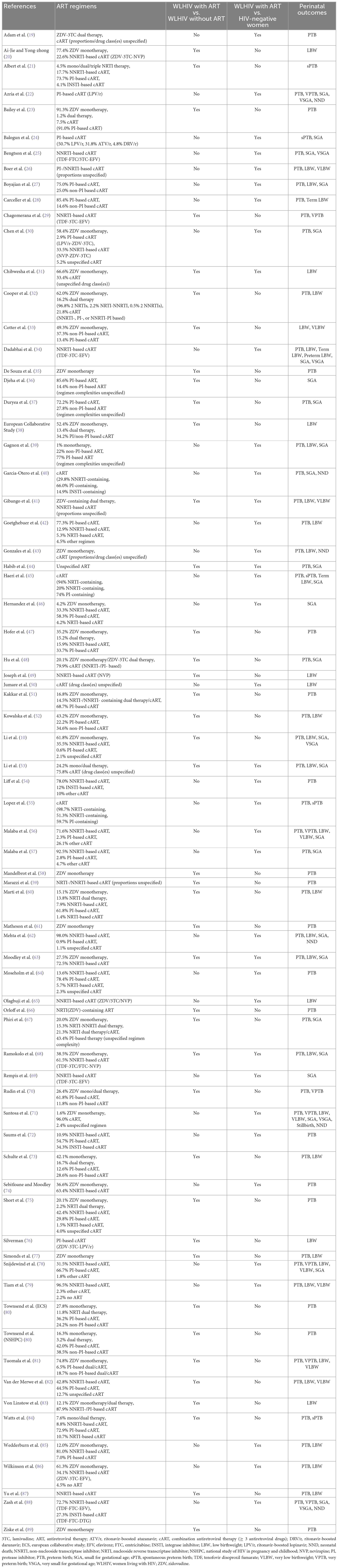
Table 2. Antiretroviral therapies, HIV/ART comparisons, and perinatal outcomes reported by studies included in the systematic review and meta-analysis.
Random-effects meta-analyses were conducted to compare perinatal outcomes in WLHIV receiving ART with WLHIV without ART and HIV-negative women. The summary effect estimates are presented in Figure 2 and the forest plots in Supplementary Appendix 3. Subgroup analyses were carried out according to country income status (Figures 3A, B, E, F), and study quality (Figures 3C, D, G–I).
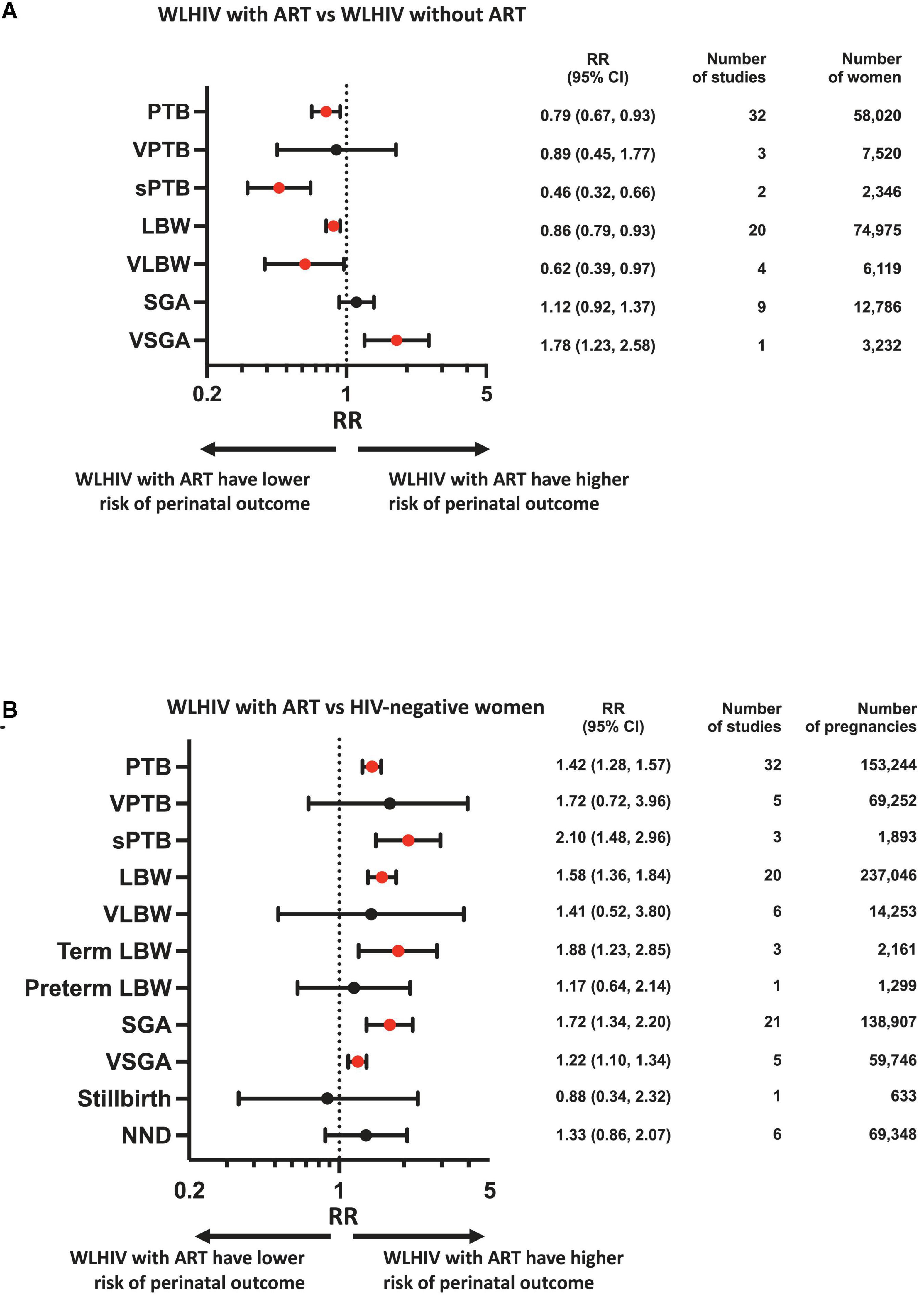
Figure 2. Perinatal outcomes of women living with HIV receiving ART compared to women living with HIV without ART and HIV-negative women. Random-effects meta-analysis results for perinatal outcomes associated with women living with HIV receiving ART compared to women living with HIV without ART (A) and HIV-negative women (B). Statistically significant effects are presented with red dots and non-significant effects with black dots. ART, antiretroviral therapy; HIV, human immunodeficiency virus; LBW, low birthweight; NND, neonatal death; PTB, preterm birth; RR, relative risk; SGA, small for gestational age; sPTB, spontaneous preterm birth; VLBW, very low birthweight; VPTB, very preterm birth; VSGA, very small for gestational age; WLHIV, women living with HIV; 95% CI, 95% confidence interval.
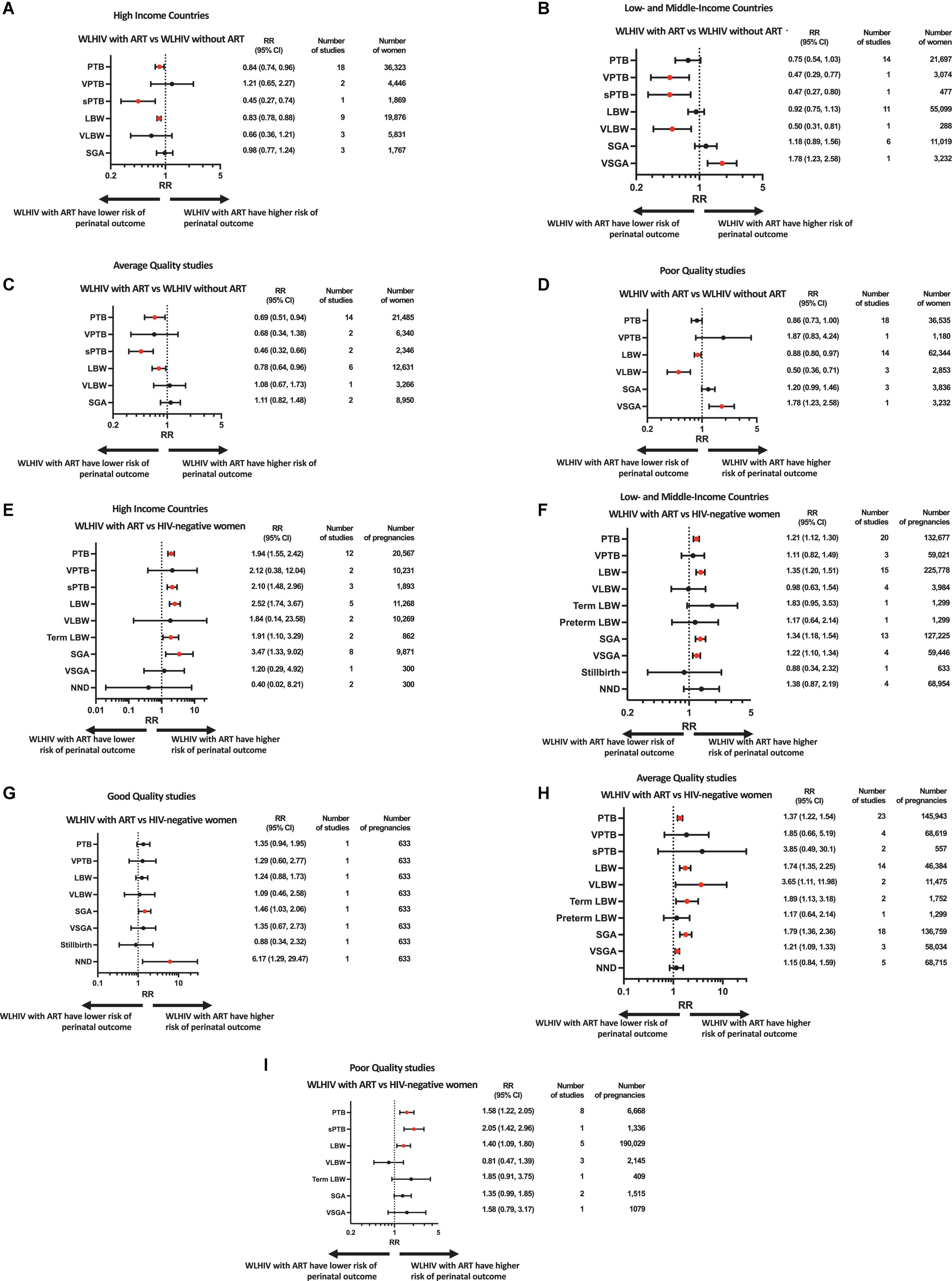
Figure 3. Subgroup and sensitivity analyses based on country income status and study quality. Random-effects meta-analysis results for perinatal outcomes associated with women living with HIV receiving ART compared to women living with HIV without ART (A–D) and HIV-negative women (E–I). Subgroups consisted of studies done in high income countries (A,E) or low- and middle-income countries (B,F). Sensitivity analysis was done for studies of good (G), average (C,H) or poor (D,I) quality. Statistically significant effects are presented with red dots and non-significant effects with black dots. ART, antiretroviral therapy; HIV, human immunodeficiency virus; LBW, low birthweight; NND, neonatal death; PTB, preterm birth; RR, relative risk; SGA, small for gestational age; sPTB, spontaneous preterm birth; VLBW, very low birthweight; VPTB, very preterm birth; VSGA, very small for gestational age; WLHIV, women living with HIV; 95% CI, 95% confidence interval.
WLHIV receiving ART vs. WLHIV without ART
41 studies, including 288,296 women, reported on seven perinatal outcomes in WLHIV receiving ART compared to WLHIV without ART.
In the analysis of 58,020 women from 32 studies, WLHIV receiving ART were associated with a significantly decreased risk of PTB compared to WLHIV without ART (RR 0.79, 95% CI 0.67–0.93) (Figure 2A). Heterogeneity between studies was high (I2 90.1%, Supplementary Appendix 3.1), but there was no evidence of publication bias (Peters’ test, p = 0.395). The significance of this association was retained in subgroup analyses of studies conducted in HICs (0.84, 0.74–0.96) (Figure 3A) and in average quality studies (0.69, 0.51–0.94) (Figure 3C), but not in studies from LMICs or poor quality studies (Figures 3B, D). One study adjusted for covariates, which did not result in a change in the significance of the effect estimate (Supplementary Appendix 4.3).
WLHIV receiving ART were not associated with VPTB compared to WLHIV without ART (Figure 2A). However, in the one study conducted in a LMIC, a significantly decreased risk of VPTB was observed for WLHIV receiving ART (0.47, 0.29–0.77) (Figure 3B), which was not seen in studies from HICs (Figure 3A).
In the analysis of 2,346 women from two average quality studies, a significant association between WLHIV receiving ART and decreased risk of sPTB was observed, compared to WLHIV without ART (0.46, 0.32–0.66) (Figure 2A). There was no heterogeneity (I2 0.0%, Supplementary Appendix 3.1). The significance of the association was retained in subgroup analyses of studies conducted in HICs (0.45, 0.27–0.74) and LMICs (0.47, 0.27–0.80) (Figures 3A, B).
In the analysis of 74,975 women from 20 studies, WLHIV receiving ART were associated with a significantly decreased risk of LBW compared to WLHIV without ART (0.86, 0.79–0.93) (Figure 2A). A moderate level of heterogeneity was observed between studies (I2 56.1%, Supplementary Appendix 3.1), and there was no evidence of publication bias (Peters’ test, p = 0.109). The significance of the association was retained in subgroup analyses of studies conducted in HICs (0.83, 0.78–0.88) (Figure 3A), but not LMICs (Figure 3B), and in average (0.78, 0.64–0.96) and poor quality studies (0.88, 0.80–0.97) (Figures 3C, D). One study adjusted for covariates, which did not result in a change in the significance of the effect estimate (Supplementary Appendix 4.3).
In the analysis of 6,119 women from four studies, WLHIV receiving ART were associated with a significantly decreased risk of VLBW, compared to WLHIV without ART (0.62, 0.39–0.97) (Figure 2A). A moderate level of heterogeneity was observed (I2 61.9%) (Supplementary Appendix 3.1). The significance of the association was retained in subgroup analyses of studies conducted in LMICs (0.50, 0.31–0.81) (Figure 3B) and in poor quality studies (0.50, 0.36–0.71) (Figure 3D), but not in studies from HICs or average quality studies (Figures 3A, C).
In the analysis of 12,786 women from nine studies, WLHIV receiving ART were not associated with SGA compared to WLHIV without ART (Figure 2A). There was a moderate level of heterogeneity (I2 49.9%) (Supplementary Appendix 3.1) and no significant associations were seen in the subgroup analyses (Figure 3).
In the analysis of 3,232 women from one poor quality study conducted in a LMIC, WLHIV receiving ART were associated with a significantly increased risk of VSGA compared to WLHIV without ART (1.78, 1.23–2.58) (Figures 2A, 3B, D).
No data was found for WLHIV receiving ART compared to WLHIV without ART for term and preterm LBW, stillbirth, and NND.
WLHIV receiving ART vs. HIV-negative women
38 studies, including 362,978 women, reported on 11 perinatal outcomes of WLHIV receiving ART compared to HIV-negative women.
In the analysis of 153,244 women from 32 studies, WLHIV receiving ART were associated with a significantly increased risk of PTB, compared to HIV-negative women (1.42, 1.28–1.57) (Figure 2B). Heterogeneity was high (I2 86.5%, Supplementary Appendix 3.2), but there was no evidence of publication bias (Peters’ test, p = 0.371). The significant association was retained in subgroup analyses by country income status, with a higher relative risk estimate in HICs (1.94, 1.55–2.42) (Figure 3E) than LMICs (1.21, 1.12–1.30) (Figure 3F). The association was significant in average and poor quality studies (Figures 3H, I), but not the single good quality study (Figure 3G). Of the 11 studies which adjusted for covariates, only one resulted in a change in the significance of the effect estimate (Supplementary Appendix 4.1).
WLHIV receiving ART were not associated with VPTB, compared to HIV-negative women (Figure 2B). There was a high level of heterogeneity (I2 92.0%) (Supplementary Appendix 3.2).
In the analysis of 1,893 women from three studies conducted in HICs, WLHIV receiving ART were associated with a significantly increased risk of sPTB (2.10, 1.48–2.96), compared to HIV-negative women (Figures 2B, 3E). There was no heterogeneity (I2 12.5%, Supplementary Appendix 3.2). The significance of this association was retained in subgroup analyses of the single poor quality study (2.05, 1.42–2.96) (Figure 3I), but not in the average quality studies (Figure 3H). One study adjusted for covariates, which did not result in a change in the significance of the effect estimate (Supplementary Appendix 4.1).
In the analysis of 237,046 women from 20 studies, WLHIV receiving ART were associated with a significantly increased risk of LBW compared to HIV-negative women (1.58, 1.36–1.84) (Figure 2B). Heterogeneity was high (I2 90.1%, Supplementary Appendix 3.2), but there was no evidence of publication bias (Peters’ test, p = 0.407). The significant association was retained in subgroup analyses by country income status, with a higher relative risk in HICs (2.52, 1.74–3.67) (Figure 3E) than LMICs (1.35, 1.20–1.51) (Figure 3F). The association was significant in average and poor quality studies (Figures 3H, I), but not the single good quality study (Figure 3G). Of the seven studies which adjusted for covariates, this resulted in a change in the significance of the effect estimate in two studies (Supplementary Appendix 4.1).
WLHIV receiving ART were not associated with VLBW, compared to HIV-negative women (Figure 2B). There was a high level of heterogeneity (I2 91.1%, Supplementary Appendix 3.2). The association was significant for two average quality studies (3.65, 1.11–11.98) (Figure 3H).
In the analysis of 2,161 women from three studies, WLHIV receiving ART were associated with a significantly increased risk of term LBW compared to HIV-negative women (1.88, 1.23–2.85) (Figure 2B). There was no heterogeneity (I2 0.0%, Supplementary Appendix 3.2). The significance of the association was retained in subgroup analyses of average quality studies (1.89, 1.13–3.18) (Figure 3H), but not in the single poor quality study (Figure 3I). There was no significant association in subgroup analyses by country income status (Figures 3E, F).
WLHIV receiving ART were not associated with preterm LBW, compared to HIV-negative women (Figure 2B).
In the analysis of 138,907 women from 21 studies, WLHIV receiving ART were associated with a significantly increased risk of SGA compared to HIV-negative women (1.72, 1.34–2.20) (Figure 2B). Heterogeneity was high (I2 97.1%, Supplementary Appendix 3.2), but there was no evidence of publication bias (Peters’ test, p = 0.692). The significant association was retained in subgroup analyses by country income status, with a higher RR in HICs (3.47, 1.33–9.02) (Figure 3E) than LMICs (1.34, 1.18–1.54) (Figure 3F), and in good and average quality studies (Figures 3G, H), but not poor quality studies (Figure 3I). Of the ten studies which adjusted for covariates, this resulted in a change in the significance of the effect estimate in three studies (Supplementary Appendix 4.2).
In the analysis of 59,746 women from five studies, WLHIV receiving ART were associated with a significantly increased risk of VSGA, compared to HIV-negative women (1.22, 1.10–1.34) (Figure 2B). There was no heterogeneity (I2 0.0%, Supplementary Appendix 3.2). The significant association was retained in subgroup analyses of studies conducted in LMICs (1.22, 1.10–1.34) (Figure 3F), but not in the single study from a HIC (Figure 3E). The significant association was retained in subgroup analyses of average quality studies (Figure 3H), but not poor or high quality studies (Figures 3G, I). Two studies adjusted for covariates, which did not result in a change in the significance of the effect estimate (Supplementary Appendix 4.2).
WLHIV receiving ART were not associated with stillbirth, compared to HIV-negative women (Figure 2B).
WLHIV receiving ART were not associated with NND, compared to HIV-negative women (Figure 2B). However, in the one good quality study a significantly increased risk of NND was observed for WLHIV receiving ART (6.17, 1.29, 29.47) (Figure 3G).
Discussion
This meta-analysis shows that WLHIV receiving ART are associated with a significantly decreased risk of PTB, sPTB, LBW, and VLBW compared to WLHIV without ART. However, WLHIV receiving ART are associated with a significantly increased risk of PTB, sPTB, LBW, term LBW, SGA, and VSGA compared to HIV-negative women. Therefore, ART reduces the risk of adverse perinatal outcomes in pregnant WLHIV, but perinatal outcomes remain higher than in HIV-negative women.
As the proportion of pregnant WLHIV that receive ART during pregnancy continues to increase, it is an important finding that ART not only improves maternal health and reduces perinatal HIV transmission, but also improves perinatal outcomes in WLHIV. The decreased risk of PTB and LBW in WLHIV receiving ART was observed in HICs, but not in LMICs. This suggests that the benefits of ART in pregnancy may be diminished in LMIC settings, which may be attributable to initiation of ART late in pregnancy, which remains common in LMICs (14). As more WLHIV in LMICs initiate life-long ART from before pregnancy, this may further improve the perinatal outcomes of WLHIV in LMICs.
Our findings agree with a smaller meta-analysis by Shinar et al. which reported that WLHIV receiving ART are associated with a higher risk of PTB, LBW, and SGA compared to HIV-negative women (90). Our analysis includes 73 studies and examines 11 outcomes in contrast to the 27 studies and 4 outcomes examined in Shinar et al. Furthermore, our analysis examines whether ART improves perinatal outcomes in WLHIV. Our finding that WLHIV receiving ART are at increased risk of adverse perinatal outcomes compared to HIV-negative women also aligns with a previous meta-analyses reporting increased risks of adverse perinatal outcomes in WLHIV without ART (3). Importantly, the effect estimates for WLHIV receiving ART compared to HIV-negative women in the current analysis were smaller than those previously reported for WLHIV without ART compared to HIV-negative women: the relative risk of PTB for WLHIV on ART was 1.42 (1.28–1.57) compared to a relative risk of 1.63 (1.37–1.93) for WLHIV without ART; relative risk of LBW of 1.58 (1.36–1.84) for WLHIV on ART compared to 1.75 (1.52–2.02) for WLHIV without ART, and relative risk of stillbirth of 0.88 (0.34–2.32) for WLHIV on ART compared to 1.67 (1.05–2.66) for WLHIV without ART (3). This is consistent with our finding that ART improves perinatal outcomes in pregnant WLHIV women. It is noteworthy, however, that the reductions in relative risk estimates are modest and that the risks of adverse perinatal outcomes remain high in WLHIV receiving ART compared to HIV-negative women.
The increased risk for WLHIV receiving ART, compared to HIV-negative women, was found in both HICs and LMICs, and the relative risk estimates of PTB, LBW, and SGA were higher in HICs than in LMICs. This is despite the improvements of perinatal outcomes with ART in WLHIV, compared to WLHIV without ART, which were observed in HICs, but not LMICs. This may in part be due to the levels of adverse perinatal outcomes in HIV-negative women, which are low in HICs, but very high in some LMICs (71, 88).
This study has several strengths. It is the largest study to date reporting on a comprehensive range of adverse perinatal outcomes associated with WLHIV receiving ART, including 424,277 women from 73 studies. Importantly, the significant findings for PTB, LBW, and SGA were each powered by ≥20 studies with > 58,000 participants, thereby providing strong evidence for the associations found. The study was conducted according to Cochrane guidelines, with exposures and outcomes clearly defined at the outset to reduce misclassification bias and ensure consistency across studies.
This study has a number of limitations. All studies included are observational and are therefore associated with an increased risk of bias, which was extensively assessed for each study. Indeed, in studies that corrected for covariates using regression analysis, only 6 comparisons (15%) resulted in a change in significance of the effect estimate. Additionally, cohort studies may be more representative of events in the real world, compared to trials in which ART is initiated during pregnancy, often in the second or third trimester (i.e. 12, 91, 92). There were few studies (< 5) reporting on comparisons for several perinatal outcomes, including VPTB, sPTB, VLBW, term LBW, preterm LBW, VSGA, and stillbirth, which renders the results for these outcomes less reliable. 23 studies did not describe a method to estimate gestational age, and only six used first trimester ultrasound, which is the most accurate method to determine gestational age (93). Lack of accurate gestational age estimation may lead to misclassification bias for outcomes that rely on gestational age, such as PTB and SGA. Consequently, only one study was classified as “good” quality.
We included studies in which WLHIV receiving ART were exposed to any ART regimen in an effort to capture the overall effect of ART on perinatal outcomes since ART use in pregnancy was introduced. The evidence of the association of different ART regimens with adverse perinatal outcomes is conflicting (9, 13, 94). Some studies have shown an increased risk of PTB with antenatal initiation of cART compared to ZDV monotherapy (30), but this was not seen in other studies (10, 95). A recent meta-analysis suggested that ZDV monotherapy decreases the risk of PTB and LBW compared to ART-naïve WLHIV, while cART does not (96). Similarly, protease inhibitor containing cART was associated with an increased risk of PTB in a number of studies (11), but not in others (97). Preconception initiation of ART may be associated with increased risk of adverse outcomes compared to ART initiation during pregnancy, although this is disputed by others (13, 14). Differential ART regimens, as well as differences in the populations, settings, and methods to estimate gestational age between included studies, may have contributed to the heterogeneity observed in our analyses.
There is a need to determine the optimal ART regimen for use in pregnancy. Current WHO guidance recommends dolutegravir (DTG)-containing regimens as preferred first-line ART, including for women of childbearing potential and pregnant women (98). A retrospective cohort study from Botswana showed that perinatal outcomes were comparable between WLHIV receiving DTG-based and efavirenz (EFV)-based ART (88). Recent randomized controlled trials of ART regimens initiated during pregnancy showed that DTG-containing regimens had superior virological efficacy compared to EFV-based ART (91, 92), and that a regimen containing DTG, emtricitabine and tenofovir alafenamide fumarate had the lowest rate of adverse pregnancy outcomes (92).
The biological mechanisms contributing to the associations between HIV status, antenatal ART and adverse perinatal outcomes remain unclear. The pathogenesis underlying adverse perinatal outcomes is multifaceted, and the cause is often unknown (99). Our data indicate that perinatal outcomes in WLHIV receiving ART remain higher than in HIV-negative women, suggesting that adverse perinatal outcomes may be related to physiological changes resulting from HIV infection which are not reversed by ART. HIV-infection is associated with depletion of CD4+ T cells and chronic immune activation (100), which may interfere with the immunological processes of pregnancy. However, despite the success of ART in suppressing viral load, some people living with HIV never achieve full CD4+ T cell recovery (101). ART may promote a shift toward pro-inflammatory Th1 activity, counteracting the Th1 to Th2 shift required to support pregnancy (102). A number of innate immune cells, including innate lymphoid cells and mucosal associated invariant T cells, are rapidly depleted early after HIV infection, which is irreversible by institution of ART and may be associated with an increased risk of PTB (103, 104). It was reported that WLHIV receiving protease inhibitors have lower plasma progesterone levels, which was proposed as a potential mediator of adverse outcomes in WLHIV. Interestingly, a recent RCT of progesterone supplementation in pregnant WLHIV on ART (mostly NNRTI-ART, only 3% PI-ART), showed that administration of 17-alpha-hydroxyprogesterone had no effect on the primary outcomes of PTB or stillbirth, but was instead associated with a reduction in the risk of VSGA (105).
We have shown that ART reduces the risk of adverse perinatal outcomes in pregnant WLHIV, thereby supporting the WHO policy of initiation of ART at diagnosis for all people living with HIV, including pregnant women (98). However, the risk of adverse perinatal outcomes remains high in the increasing number of WLHIV who receive ART, compared to HIV-negative women, which continues to contribute to the global burden of adverse perinatal outcomes and limit progress toward achieving Sustainable Development Goal 3 (15). Further studies are urgently needed to determine the optimal ART regimen(s) in pregnancy to minimize adverse perinatal outcomes in WLHIV, elucidate the mechanism underlying adverse perinatal outcomes in WLHIV, and develop preventative and therapeutic interventions to improve perinatal outcomes in WLHIV.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CP, HS, MK, and ZB screened the literature search results for relevant manuscripts and assessed their eligibility, extracted data, and conducted methodological quality assessments. CP conducted the meta-analyses, subgroup and sensitivity analyses, interpreted the data, and wrote the first draft of the manuscript. SK designed and conducted the literature search. JH conceived, designed, coordinated the study, developed the systematic review protocol, assisted with the literature search, assessment of eligibility of manuscripts, data extraction, and methodological quality assessment, designed the meta-analysis plan, interpreted the data, wrote the manuscript, had full access to all the data in the study, and had final responsibility for the decision to submit the manuscript for publication. All authors read and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.924593/full#supplementary-material
References
2. Wang H, Bhutta Z, Coates M, Coggeshall M, Dandona L, Diallo K, et al. Global, regional, national, and selected subnational levels of stillbirths, neonatal, infant, and under-5 mortality, 1980–2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1725–74.
3. Wedi C, Kirtley S, Hopewell S, Corrigan R, Kennedy S, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. (2016) 3:e33–48. doi: 10.1016/S2352-3018(15)00207-6
4. Chawanpaiboon S, Vogel J, Moller A, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7:e37–46.
5. Lee A, Kozuki N, Cousens S, Stevens G, Blencowe H, Silveira M, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21 st standard: analysis of CHERG datasets. BMJ. (2017) 358:j3677.
6. Lee A, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel J, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. (2013) 1:e26–36. doi: 10.1016/S2214-109X(13)70006-8
7. World Health Organization. Consolidated Guidelines on the use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Geneva: World Health Organization (2013).
8. World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Preexposure Prophylaxis for HIV. Geneva: World Health Organization (2015).
9. Mofenson L. Antiretroviral therapy and adverse pregnancy outcome: the elephant in the room? J Infect Dis. (2016) 213:1051–4. doi: 10.1093/infdis/jiv390
10. Li N, Sando M, Spiegelman D, Hertzmark E, Liu E, Sando D, et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis. (2016) 213:1057–64. doi: 10.1093/infdis/jiv389
11. Kourtis A, Schmid C, Jamieson D, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS. (2007) 21:607–15. doi: 10.1097/QAD.0b013e32802ef2f6
12. Tshivuila-Matala C, Honeyman S, Nesbitt C, Kirtley S, Kennedy S, Hemelaar J. Adverse perinatal outcomes associated with antiretroviral therapy regimens: systematic review and network meta-analysis. AIDS. (2020) 34:1643–56. doi: 10.1097/QAD.0000000000002593
13. Uthman O, Nachega J, Anderson J, Kanters S, Mills E, Renaud F, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV. (2017) 4:e21–30. doi: 10.1016/S2352-3018(16)30195-3
14. Stringer J, Stoner M, Kasaro M, Vwalika B, Cole S. Preconception ART and preterm birth: real effect or selection bias? Lancet HIV. (2017) 4:e150. doi: 10.1016/S2352-3018(17)30046-2
15. United Nation. Transforming our World: the 2030 Agenda for Sustainable Development Department of Economic and Social Affair. (2020). Available online at: https://sdgs.un.org/2030agenda (Accessed December 29, 2020).
16. Blencowe H, Cousens S, Oestergaard M, Chou D, Moller A, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. (2012) 379:2162–72. doi: 10.1016/S0140-6736(12)60820-4
17. Villar J, Ismail L, Victora C, Ohuma E, Bertino E, Altman D, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. (2014) 384:857–68. doi: 10.1016/S0140-6736(14)60932-6
18. Lawn J, Cousens S, Zupan J. 4 Million neonatal deaths: when? Where? Why? Lancet. (2005) 365:891–900. doi: 10.1016/S0140-6736(05)71048-5
19. Adam G, Ahmed M, Ali A. Human immune deficiency virus (HIV) infection during pregnancy at Gadarif hospital, Eastern Sudan. J Obstet Gynaecol (Lahore). (2016) 36:962–3. doi: 10.1080/01443615.2016.1174838
20. Ai-jie L, Yong-zhong W. Study on low birth weight and correlates of infants born by HIV positive woman. Chine J Dermatovenereol. (2013) 27:161–3.
21. Albert A, Elwood C, Wagner E, Pakzad Z, Chaworth-Musters T, Berg K, et al. Investigation of factors associated with spontaneous preterm birth in pregnant women living with HIV. AIDS. (2020) 34:719–27. doi: 10.1097/QAD.0000000000002464
22. Azria E, Moutafoff C, Schmitz T, Le Meaux J, Krivine A. Pregnancy outcomes in women with HIV type-1 receiving a lopinavir/ritonavir-containing regimen. Antivir Ther. (2009) 14:423–32. doi: 10.1177/135965350901400302
23. Bailey H, Townsend C, Semenenko I, Malyuta R, Cortina-Borja M, Thorne C. Impact of expanded access to combination antiretroviral therapy in pregnancy: results from a cohort study in Ukraine. Bull World Health Organ. (2013) 91:491–500. doi: 10.2471/BLT.12.114405
24. Balogun K, Lenis M, Papp E, Loutfy M, Yudin M, MacGillivray J, et al. Elevated levels of estradiol in human immunodeficiency virus-infected pregnant women on protease inhibitor-based regimens. Clin Infect Dis. (2018) 66:420–7. doi: 10.1093/cid/cix761
25. Bengtson A, Phillips T, Le Roux S, Brittain K, Zerbe A, Madlala H, et al. Does HIV infection modify the relationship between pre-pregnancy body mass index and adverse birth outcomes? Paediatr Perinat Epidemiol. (2020) 34:713–23. doi: 10.1111/ppe.12688
26. Boer K, Nellen J, Patel D, Timmermans S, Tempelman C, Wibaut M, et al. The AmRo study: pregnancy outcome in HIV-1-infected women under effective highly active antiretroviral therapy and a policy of vaginal delivery. BJOG. (2007) 114:148–55. doi: 10.1111/j.1471-0528.2006.01183.x
27. Boyajian T, Shah P, Murphy K. Risk of preeclampsia in HIV-positive pregnant women receiving HAART: a matched cohort study. J Obstetr Gynaecol Can. (2012) 34:136–41. doi: 10.1016/S1701-2163(16)35156-8
28. Carceller A, Ferreira E, Alloul S, Lapointe N. Lack of effect on prematurity, birth weight, and infant growth from exposure to protease inhibitors in utero and after birth. Pharmacotherapy. (2009) 29:1289–96. doi: 10.1592/phco.29.11.1289
29. Chagomerana M, Miller W, Pence B, Hosseinipour M, Hoffman I, Flick R, et al. PMTCT option b+ does not increase preterm birth risk and may prevent extreme prematurity: a retrospective cohort study in Malawi. J Acquir Immune Defic Syndr. (2017) 74:367–74. doi: 10.1097/QAI.0000000000001253
30. Chen J, Ribaudo H, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in botswana. J Infect Dis. (2012) 206:1695–705. doi: 10.1093/infdis/jis553
31. Chibwesha C, Zanolini A, Smid M, Vwalika B, Phiri Kasaro M, Mwanahamuntu M, et al. Predictors and outcomes of low birth weight in Lusaka, Zambia. Int J Gynecol Obstetr. (2016) 134:309–14. doi: 10.1016/j.ijgo.2016.03.021
32. Cooper E, Charurat M, Mofenson L, Hanson I, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1–infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. (2002) 29:484–94. doi: 10.1097/00042560-200204150-00009
33. Cotter A, Garcia A, Duthely M, Luke B, O’Sullivan M. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. (2006) 193:1195–201. doi: 10.1086/503045
34. Dadabhai S, Gadama L, Chamanga R, Kawalazira R, Katumbi C, Makanani B, et al. Pregnancy outcomes in the era of universal antiretroviral treatment in Sub-Saharan Africa (POISE Study). J Acquir Immune Defic Syndr. (2019) 80:7–14. doi: 10.1097/QAI.0000000000001875
35. de Souza R, Gómez-Marín O, Scott G, Guasti S, O’Sullivan M, Oliveira R, et al. Effect of prenatal zidovudine on disease progression in perinatally HIV-1–infected infants. J Acquir Immune Defic Syndr. (2000) 24:154–61. doi: 10.1097/00042560-200006010-00010
36. Djeha A, Girard S, Trottier H, Kakkar F, Soudeyns H, Boucher M, et al. No association between early antiretroviral therapy during pregnancy and plasma levels of angiogenic factors: a cohort study. BMC Pregnancy Childbirth. (2019) 19:482. doi: 10.1186/s12884-019-2600-4
37. Duryea E, Nicholson F, Cooper S, Roberts S, Rogers V, McIntire D, et al. Use of protease inhibitors in pregnancy is not associated with preterm birth or small for gestational age infants. Am J Obstet Gynecol. (2015) 212:S41. doi: 10.1016/j.ajog.2014.10.106
38. European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr. (2003) 32:380–7. doi: 10.1097/00126334-200304010-00006
39. Gagnon L, MacGillivray J, Urquia M, Caprara D, Murphy K, Yudin M. Antiretroviral therapy during pregnancy and risk of preterm birth. Eur J Obstet Gynecol Reprod Biol. (2016) 201:51–5. doi: 10.1016/j.ejogrb.2016.03.028
40. García-Otero L, López M, Guitart-Mampel M, Morén C, Goncé A, Esteve C, et al. Cardiac and mitochondrial function in HIV-uninfected fetuses exposed to antiretroviral treatment. PLoS One. (2019) 14:e0213279. doi: 10.1371/journal.pone.0213279
41. Gibango N, Mda S, Ntuli T. Factors associated with delivering premature and/or low birth weight infants among pregnant HIV-positive women on antiretroviral treatment at Dr George Mukhari hospital, South Africa. S Afr J Infect Dis. (2018) 33:42–5. doi: 10.4102/sajid.v33i2.18
42. Goetghebuer T, Smolen K, Adler C, Das J, Mcbride T, Smits G, et al. Initiation of antiretroviral therapy before pregnancy reduces the risk of infection-related hospitalization in human immunodeficiency virus-exposed uninfected infants born in a high-income country. Clin Infect Dis. (2019) 68:1193–203. doi: 10.1093/cid/ciy673
43. González R, Rupérez M, Sevene E, Vala A, Maculuve S, Bulo H, et al. Effects of HIV infection on maternal and neonatal health in southern Mozambique: a prospective cohort study after a decade of antiretroviral drugs roll out. PLoS One. (2017) 12:e0178134. doi: 10.1371/journal.pone.0178134
44. Habib N, Daltveit A, Bergsjø P, Shao J, Oneko O, Lie R. Maternal HIV status and pregnancy outcomes in northeastern Tanzania: a registry-based study. BJOG. (2008) 115:616–24. doi: 10.1111/j.1471-0528.2008.01672.x
45. Haeri S, Shauer M, Dale M, Leslie J, Baker A, Saddlemire S, et al. Obstetric and newborn infant outcomes in human immunodeficiency virus-infected women who receive highly active antiretroviral therapy. Am J Obstet Gynecol. (2009) 201:.e1–5. doi: 10.1016/j.ajog.2009.06.017
46. Hernández S, Catalán-García M, Morén C, García-Otero L, López M, Guitart-Mampel M, et al. Placental mitochondrial toxicity, oxidative stress, apoptosis, and adverse perinatal outcomes in HIV pregnancies under antiretroviral treatment containing zidovudine. J Acquir Immune Defic Syndr. (2017) 75:e113–9. doi: 10.1097/QAI.0000000000001334
47. Hofer C, Keiser O, Zwahlen M, Lustosa C, Frota A, de Oliveira R, et al. In utero exposure to antiretroviral drugs: effect on birth weight and growth among HIV-exposed uninfected children in Brazil. Pediatr Infect Dis J. (2016) 35:71–7. doi: 10.1097/INF.0000000000000926
48. Hu F, Liang J, Lu J, Hu Y, Hu Y, Yu J, et al. Effects of antiretroviral therapy and HIV exposure in utero on adverse pregnancy and infant outcomes: a prospective cohort study in Guangzhou, China. Biomed Environ Sci. (2019) 32:719–29.
49. Joseph O, Biodun O, Michael E. Pregnancy outcome among HIV positive women receiving antenatal HAART versus untreated maternal HIV infection. J Coll Phys Surg Pak. (2011) 21:356–9.
50. Jumare J, Datong P, Osawe S, Okolo F, Mohammed S, Inyang B, et al. Compromised growth among HIV-exposed uninfected compared with unexposed children in Nigeria. Pediatr Infect Dis J. (2019) 38:280–6. doi: 10.1097/INF.0000000000002238
51. Kakkar F, Boucoiran I, Lamarre V, Ducruet T, Amre D, Soudeyns H, et al. Risk factors for pre-term birth in a Canadian cohort of HIV-positive women: role of ritonavir boosting? J Int AIDS Soc. (2015) 18:19933. doi: 10.7448/IAS.18.1.19933
52. Kowalska A, Niemiec T, El Midaoui A, Burkacka E. Effect of antiretroviral therapy on pregnancy outcome in HIV-1 positive women. Med Wieku Rozwoj. (2003) 7:459–68.
53. Li H, Liu J, Tan D, Huang G, Zheng J, Xiao J, et al. Maternal HIV infection and risk of adverse pregnancy outcomes in hunan province, China: a prospective cohort study. Medicine (United States). (2020) 99:e19213. doi: 10.1097/MD.0000000000019213
54. Liff I, Zash R, Mingochi D, Gaonakala F, Diseko M, Mayondi G, et al. Mid-trimester cervical length not associated with HIV status among pregnant women in Botswana. PLoS One. (2020) 15:e0229500. doi: 10.1371/journal.pone.0229500
55. Lopez M, Figueras F, Hernandez S, Lonca M, Garcia R, Palacio M, et al. Association of HIV infection with spontaneous and iatrogenic preterm delivery: effect of HAART. AIDS. (2012) 26:37–43. doi: 10.1097/QAD.0b013e32834db300
56. Malaba T, Phillips T, Le Roux S, Brittain K, Zerbe A, Petro G, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol. (2017) 46:1678–89. doi: 10.1093/ije/dyx136
57. Malaba T, Newell M, Madlala H, Perez A, Gray C, Myer L. Methods of gestational age assessment influence the observed association between antiretroviral therapy exposure, preterm delivery, and small-for-gestational age infants: a prospective study in Cape Town, South Africa. Ann Epidemiol. (2018) 28:893–900. doi: 10.1016/j.annepidem.2018.08.011
58. Mandelbrot L, Le Chenadec J, Berrebi A, Bongain A, Bénifla J, Delfraissy J, et al. Perinatal HIV-1 transmission: interaction between zidovudine prophylaxis and mode of delivery in the French perinatal cohort. J Am Med Assoc. (1998) 280:55–60. doi: 10.1001/jama.280.1.55
59. Marazzi M, Palombi L, Nielsen-Saines K, Haswell J, Zimba I, Magid N, et al. Extended antenatal use of triple antiretroviral therapy for prevention of mother-to-child transmission of HIV-1 correlates with favorable pregnancy outcomes. AIDS. (2011) 25:1611–8. doi: 10.1097/QAD.0b013e3283493ed0
60. Martí C, Peña J, Bates I, Madero R, de José I, Pallardo L, et al. Obstetric and perinatal complications in HIV-infected women. Analysis of a cohort of 167 pregnancies between 1997 and 2003. Acta Obstet Gynecol Scand. (2007) 86:409–15. doi: 10.1080/00016340601148531
61. Matheson P, Abrams E, Thomas P, Hernan M, Thea D, Lambert G, et al. Efficacy of antenatal zidovudine in reducing perinatal transmission of human immunodeficiency virus type 1. J Infect Dis. (1995) 172:353–8. doi: 10.1093/infdis/172.2.353
62. Mehta U, van Schalkwyk C, Naidoo P, Ramkissoon A, Mhlongo O, Maharaj N, et al. Birth outcomes following antiretroviral exposure during pregnancy: initial results from a pregnancy exposure registry in South Africa. S Afr J HIV Med. (2019) 20:971. doi: 10.4102/sajhivmed.v20i1.971
63. Moodley T, Moodley D, Sebitloane M, Maharaj N, Sartorius B. Improved pregnancy outcomes with increasing antiretroviral coverage in South Africa. BMC Pregnancy Childbirth. (2016) 16:35. doi: 10.1186/s12884-016-0821-3
64. Moseholm E, Helleberg M, Sandholdt H, Katzenstein T, Storgaard M, Pedersen G, et al. Children exposed or unexposed to human immunodeficiency virus: weight, height, and body mass index during the first 5 years of life-a danish nationwide cohort. Clin Infect Dis. (2020) 70:2168–77. doi: 10.1093/cid/ciz605
65. Olagbuji B, Ezeanochie M, Ande A, Oboro V. Obstetric and perinatal outcome in HIV positive women receiving HAART in urban Nigeria. Arch Gynecol Obstet. (2010) 281:991–4. doi: 10.1007/s00404-009-1186-x
66. Orloff S, Bulterys M, Vink P, Nesheim S, Abrams E, Schoenbaum E, et al. Maternal characteristics associated with antenatal, intrapartum, and neonatal zidovudine use in four U.S. cities, 1994-1998. J Acquir Immune Defic Syndr. (2001) 28:65–72. doi: 10.1097/00042560-200109010-00010
67. Phiri K, Williams P, Dugan K, Fischer M, Cooper W, Seage G, et al. Antiretroviral therapy use during pregnancy and the risk of small for gestational age birth in a medicaid population. Pediatr Infect Dis J. (2015) 34:e169–75. doi: 10.1097/INF.0000000000000712
68. Ramokolo V, Goga A, Lombard C, Doherty T, Jackson D, Engebretsen I. In utero ART exposure and birth and early growth outcomes among HIV-exposed uninfected infants attending immunization services: results from national PMTCT surveillance, South Africa. Open Forum Infect Dis. (2017) 4:ofx187. doi: 10.1093/ofid/ofx187
69. Rempis E, Schnack A, Decker S, Braun V, Rubaihayo J, Tumwesigye N, et al. Option B+ for prevention of vertical HIV transmission has no influence on adverse birth outcomes in a cross-sectional cohort in Western Uganda. BMC Pregnancy Childbirth. (2017) 17:82. doi: 10.1186/s12884-017-1263-2
70. Rudin C, Spaenhauer A, Keiser O, Rickenbach M, Kind C, Aebi-Popp K, et al. Antiretroviral therapy during pregnancy and premature birth: analysis of swiss data. HIV Med. (2011) 12:228–35. doi: 10.1111/j.1468-1293.2010.00876.x
71. Santosa W, Staines-Urias E, Tshivuila-Matala C, Norris S, Hemelaar J. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS. (2019) 33:1623–33. doi: 10.1097/QAD.0000000000002222
72. Saums M, King C, Adams J, Sheth A, Badell M, Young M, et al. Combination antiretroviral therapy and hypertensive disorders of pregnancy. Obstet Gynecol. (2019) 134:1205–14. doi: 10.1097/AOG.0000000000003584
73. Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler M. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: pediatric spectrum of HIV disease, 1989-2004. Pediatrics. (2007) 119:e900–6. doi: 10.1542/peds.2006-1123
74. Sebitloane H, Moodley J. Maternal and obstetric complications among HIV-infected women treated with highly active antiretroviral treatment at a regional Hospital in Durban, South Africa. Niger J Clin Pract. (2017) 20:1360–7. doi: 10.4103/njcp.njcp_328_16
75. Short C, Douglas M, Smith J, Taylor G. Preterm delivery risk in women initiating antiretroviral therapy to prevent HIV mother-to-child transmission. HIV Med. (2014) 15:233–8. doi: 10.1111/hiv.12083
76. Silverman M. Low birth weight associated with HAART in pregnancy in Zambia. Proceedings of the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) H-1662. Boston, MA: (2010).
77. Simonds R, Steketee R, Nesheim S, Matheson P, Palumbo P, Alger L, et al. Impact of zidovudine use on risk and risk factors for perinatal transmission of HIV. AIDS. (1998) 12:301–8. doi: 10.1097/00002030-199803000-00008
78. Snijdewind I, Smit C, Godfried M, Bakker R, Nellen J, Jaddoe VV, et al. Preconception use of cART by HIV-positive pregnant women increases the risk of infants being born small for gestational age. PLoS One. (2018) 13:e0191389. doi: 10.1371/journal.pone.0191389
79. Tiam A, Kassaye S, Machekano R, Tukei V, Gill M, Mokone M, et al. Comparison of 6-week PMTCT outcomes for HIV-exposed and HIV-unexposed infants in the era of lifelong ART: results from an observational prospective cohort study. PLoS One. (2019) 14:e0226339. doi: 10.1371/journal.pone.0226339
80. Townsend C, Schulte J, Thorne C, Dominguez K, Tookey P, Cortina-Borja M, et al. Antiretroviral therapy and preterm delivery-a pooled analysis of data from the United States and Europe. BJOG. (2010) 117:1399–410. doi: 10.1111/j.1471-0528.2010.02689.x
81. Tuomala R, Shapiro D, Mofenson L, Bryson Y, Culnane M, Hughes M, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. (2002) 346:1863–70. doi: 10.1056/NEJMoa991159
82. van der Merwe K, Hoffman R, Black V, Chersich M, Coovadia A, Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. (2011) 14:42. doi: 10.1186/1758-2652-14-42
83. von Linstow M, Rosenfeldt V, Lebech A, Storgaard M, Hornstrup T, Katzenstein T, et al. Prevention of mother-to-child transmission of HIV in Denmark, 1994-2008. HIV Med. (2010) 11:448–56. doi: 10.1111/j.1468-1293.2009.00811.x
84. Watts D, Williams P, Kacanek D, Griner R, Rich K, Hazra R, et al. Combination antiretroviral use and preterm birth. J Infect Dis. (2013) 207:612–21. doi: 10.1093/infdis/jis728
85. Wedderburn C, Yeung S, Rehman A, Stadler J, Nhapi R, Barnett W, et al. Neurodevelopment of HIV-exposed uninfected children in South Africa: outcomes from an observational birth cohort study. Lancet Child Adolesc Health. (2019) 3:803–13. doi: 10.1016/S2352-4642(19)30250-0
86. Wilkinson A, Pedersen S, Urassa M, Michael D, Todd J, Kinung’hi S, et al. Associations between gestational anthropometry, maternal HIV, and fetal and early infancy growth in a prospective rural/semi-rural Tanzanian cohort, 2012-13. BMC Pregnancy Childbirth. (2015) 15:277. doi: 10.1186/s12884-015-0718-6
87. Yu L, Li W, Chen R, Tang Z, Pang J, Gui X, et al. Pregnancy outcomes and risk factors for low birth weight and preterm delivery among HIV-infected pregnant women in Guangxi, China. Chin Med J (Engl). (2012) 125:403–9.
88. Zash R, Jacobson D, Diseko M, Mayondi G, Mmalane M, Essex M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health. (2018) 6:e804–10. doi: 10.1016/S2214-109X(18)30218-3
89. Ziske J, Kunz A, Sewangi J, Lau I, Dugange F, Hauser A, et al. Hematological changes in women and infants exposed to an AZT-containing regimen for prevention of mother-to-child-transmission of HIV in Tanzania. PLoS One. (2013) 8:e55633. doi: 10.1371/journal.pone.0055633
90. Shinar S, Agrawal S, Ryu M, Walmsley S, Serghides L, Yudin M, et al. Perinatal outcomes in women living with HIV-1 and receiving antiretroviral therapy-a systematic review and meta-analysis. Acta Obstet Gynecol Scand. (2022) 101:168–82. doi: 10.1111/aogs.14282
91. Kintu K, Malaba T, Nakibuka J, Papamichael C, Colbers A, Byrne K, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV. (2020) 7:e332–9. doi: 10.1016/S2352-3018(20)30050-3
92. Lockman S, Brummel S, Ziemba L, Stranix-Chibanda L, McCarthy K, Coletti A, et al. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet. (2021) 397:1276–92. doi: 10.1016/S0140-6736(21)00314-7
93. Committee on Obstetric Practice American Institute of Ultrasound in Medicine Society for Maternal-Fetal Medicine. Committee opinion No 700: methods for estimating the due date. American college of obstetricians and gynecologists. Obstet Gynecol. (2017) 129:e150–4. doi: 10.1097/AOG.0000000000002046
94. Bailey H, Zash R, Rasi V, Thorne C. HIV treatment in pregnancy. Lancet HIV. (2018) 5:e457–67. doi: 10.1016/S2352-3018(18)30059-6
95. de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. (2011) 11:171–80. doi: 10.1016/S1473-3099(10)70288-7
96. Portwood C, Murray C, Sexton H, Kumarendran M, Brandon Z, Johnson B, et al. Adverse perinatal outcomes associated with HAART and monotherapy. AIDS. (2022) 36:1409–27. doi: 10.1097/QAD.0000000000003248
97. Koss C, Natureeba P, Plenty A, Luwedde F, Mwesigwa J, Ades V, et al. Risk factors for preterm birth among HIV-infected pregnant ugandan women randomized to lopinavir/ritonavir-or efavirenz-based antiretroviral therapy. J Acquir Immune Defic Syndr. (2014) 67:128–35. doi: 10.1097/QAI.0000000000000281
98. World Health Organization. Consolidated Guidelines On HIV Prevention, Testing, Treatment, Service Delivery And Monitoring: Recommendations For A Public Health Approach. Geneva: World Health Organization (2021).
99. Barros F, Papageorghiou A, Victora C, Noble J, Pang R, Iams J, et al. The distribution of clinical phenotypes of preterm birth syndrome implications for prevention. JAMA Pediatr. (2015) 169:220–9. doi: 10.1001/jamapediatrics.2014.3040
100. Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev. (2013) 254:78–101. doi: 10.1111/imr.12079
101. Pacheco Y, Jarrin I, Rosado I, Campins A, Berenguer J, Iribarren J, et al. Increased risk of non-AIDS-related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res. (2015) 117:69–74. doi: 10.1016/j.antiviral.2015.03.002
102. Fiore S, Ferrazzi E, Newell M, Trabattoni D. Protease inhibitor-associated increased risk of preterm delivery is an immunological complication of therapy [with Reply]. J Infect Dis. (2007) 195:914–7. doi: 10.1086/511983
103. Akoto C, Chan C, Tshivuila-Matala C, Ravi K, Zhang W, Vatish M, et al. Innate lymphoid cells are reduced in pregnant HIV positive women and are associated with preterm birth. Sci Rep. (2020) 10:13265. doi: 10.1038/s41598-020-69966-0
104. Ravi K, Chan C, Akoto C, Zhang W, Vatish M, Norris S, et al. Changes in the Vα7.2+ CD161++ MAIT cell compartment in early pregnancy are associated with preterm birth in HIV-positive women. Am J Reprod Immunol. (2020) 83:e13240. doi: 10.1111/aji.13240
Keywords: HIV, perinatal, pregnancy, antiretroviral, preterm (birth)
Citation: Portwood C, Sexton H, Kumarendran M, Brandon Z, Kirtley S and Hemelaar J (2023) Adverse perinatal outcomes associated with antiretroviral therapy in women living with HIV: A systematic review and meta-analysis. Front. Med. 9:924593. doi: 10.3389/fmed.2022.924593
Received: 20 April 2022; Accepted: 20 December 2022;
Published: 03 February 2023.
Edited by:
Sahera Dirajlal-Fargo, Case Western Reserve University, United StatesReviewed by:
Wei Li Koay, Children’s National Hospital, United StatesSilvia Visentin, University of Padua, Italy
Copyright © 2023 Portwood, Sexton, Kumarendran, Brandon, Kirtley and Hemelaar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joris Hemelaar,  am9yaXMuaGVtZWxhYXJAbnBldS5veC5hYy51aw==
am9yaXMuaGVtZWxhYXJAbnBldS5veC5hYy51aw==
 Clara Portwood1
Clara Portwood1 Joris Hemelaar
Joris Hemelaar