
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 13 October 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.923991
This article is part of the Research Topic Stevens Johnson Syndrome: Past, Present, and Future Directions View all 11 articles
Delayed drug T-cell immune-mediated hypersensitivity reactions have a large clinical heterogeneity varying from mild maculopapular exanthema (MPE) to severe cutaneous adverse reactions (SCARs) such as acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) and severe skin necrosis and blistering as seen in Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Given the knowledge gaps related to the immunopathogenesis of these conditions, the absence of validated diagnostic tools and the significant associated morbidity and mortality, patients with SCARs often have limited drug choices. We performed a comprehensive review aiming to evaluate in vivo diagnostic tools such as delayed intradermal skin and patch testing and ex vivo/in vitro research assays such as the lymphocyte transformation test (LTT) and the enzyme-linked ImmunoSpot (ELISpot) assay. We searched through PubMed using the terms “drug allergy,” “in vivo” and “ex vivo” for original papers in the last 10 years. A detailed meticulous approach adapted to the various clinical phenotypes is recommended for the diagnostic and management of delayed drug hypersensitivity reactions. This review highlights the current diagnostic tools for the delayed drug hypersensitivity phenotypes.
Delayed immune-mediated drug hypersensitivity reactions (DHR) are inflammatory reactions with a predominant manifestation in the skin that can be associated with systemic manifestations, and are hypothesized to be T-cell mediated. These reactions are not anticipated and not dependent on the dose administered (1).
Severe cutaneous adverse reactions (SCARs) are DHR that cause severe damage to the skin and/or internal organs and are associated with significant acute and long-term morbidity and increased mortality risk (2). Risk factors include cystic fibrosis, severe asthma, chronic lymphatic leukemia, human immunodeficiency virus or genetic susceptibility (3). For the purpose of this review, we will focus on mild maculopapular exanthema (MPE) as well as SCAR syndromes: acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS) and Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). Our main goal is to portray the diagnostic methods, including a description of the currently used clinical skin testing and novel investigational ex vivo methods for the delayed DHR.
We formulated a research question focusing on the available diagnostic tools aimed to improve the diagnosis and management of delayed T-cell mediated drug reactions. The objective of the comprehensive review was established using the PICO method, including population, interventions, comparators and outcomes. We searched PubMed for peer-reviewed original articles with the terms drug, antibiotic, antimicrobial, sulfonamide, non-steroidal anti-inflammatory, anti-epileptic or anti-convulsant; allergy, hypersensitivity or T-cell mediated; and in vivo as well as ex vivo diagnostic methods.
We used the key words: {[drug*(Title/Abstract)] OR [antibiotic*(Title/Abstract)] OR [antimicrobial*(Title/Abstract)] OR [sulfonamide*(Title/Abstract)] OR [non-steroidal anti-inflammator*(Title/Abstract)] OR [amoxicillin* (Title/Abstract)] OR [anti-epileptic*(Title/Abstract)] OR [anti-convulsant*(Title/Abstract)]} AND {[ex vivo (Title/Abstract)] OR [in vitro (Title/Abstract)] OR [skin testing*(Title/Abstract)] OR [patch testing*(Title/Abstract)] OR [enzyme-linked immunoSpot assay*(Title/Abstract)] OR [ELISpot(Title/Abstract)] OR [lymphocyte transformation test*(Title/Abstract)] OR [lymphocyte proliferation* (Title/Abstract)] OR [stimulation test*(Title/Abstract)] OR [IFN*(Title/Abstract)] OR [flow cytometry*(Title/Abstract)]} AND {[allergy*[Title/Abstract)] OR [hypersensitivity*(Title/Abstract)] OR [T-cell mediated*(Title/Abstract)]}.
Articles relevant to the topic of interest were examined following the inclusion criteria: (1) original human studies (pediatric and adult population), (2) academic articles published in peer-reviewed journals, (3) available in English or French language, and (4) published between January 1st 2012 and June 2nd 2022. The search provided 1,440 results (Figure 1). The first screening was based on the titles and abstracts followed by a second round of screening performed by reviewing the full-text articles for selected studies. For the purpose of this study, meta-analysis-based research articles were not considered in the original studies subcategory. Articles on immediate and vaccine hypersensitivity were excluded as these were considered beyond the scope of this review. To better illustrate the existing literature, original articles were further sub-categorized in studies containing information on in vivo tools, ex vivo tools and HLA-related research. The descriptive/epidemiological reports published that did not address any diagnostic tools were added to another subgroup (Figure 1).
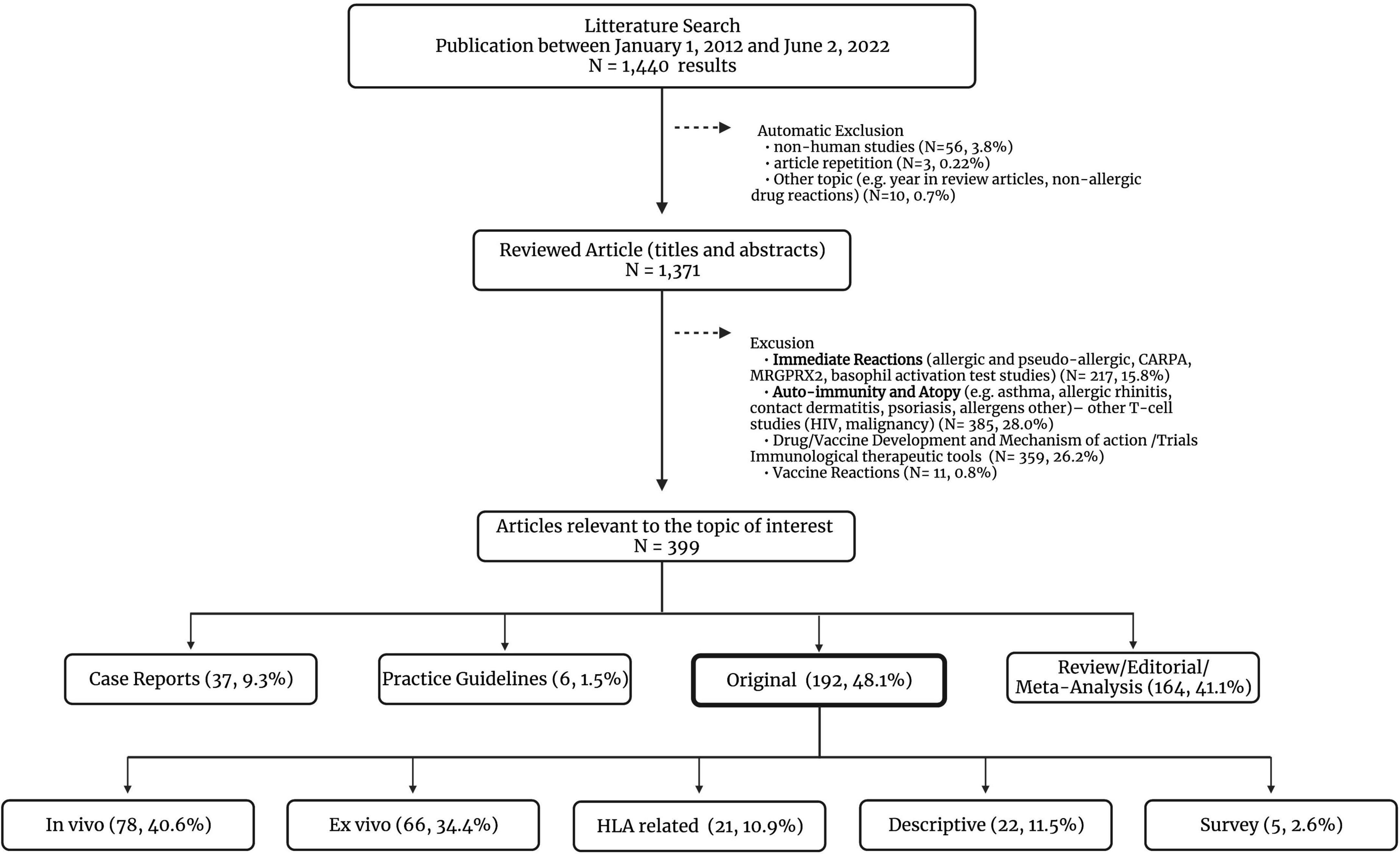
Figure 1. Comprehensive literature review–article selection. CARPA, complement activation-related pseudoallergy; HIV, human immunodeficiency virus; HLA, human leukocyte antigen; MRGPRX2, Mas-related G-protein coupled receptor member X2. The ex vivo original studies can also describe the use of in vivo diagnostic tools in the methods or study design.
Delayed hypersensitivity reactions can occur hours to days following exposure to a drug or drug metabolite. It is hypothesized that uncontrolled T-cell production triggers the different immune manifestations (Figure 2). Matured antigen-presenting cells such as dendritic cells and macrophages interact with antigen-specific T CD4+ helper cells as well as CD8+ cytotoxic T-cells leading to drug-specific cell-mediated immunity (4). While adaptive immunity plays an essential role, an implication of the innate immune response has been demonstrated in vitro for agents such as allopurinol (5).
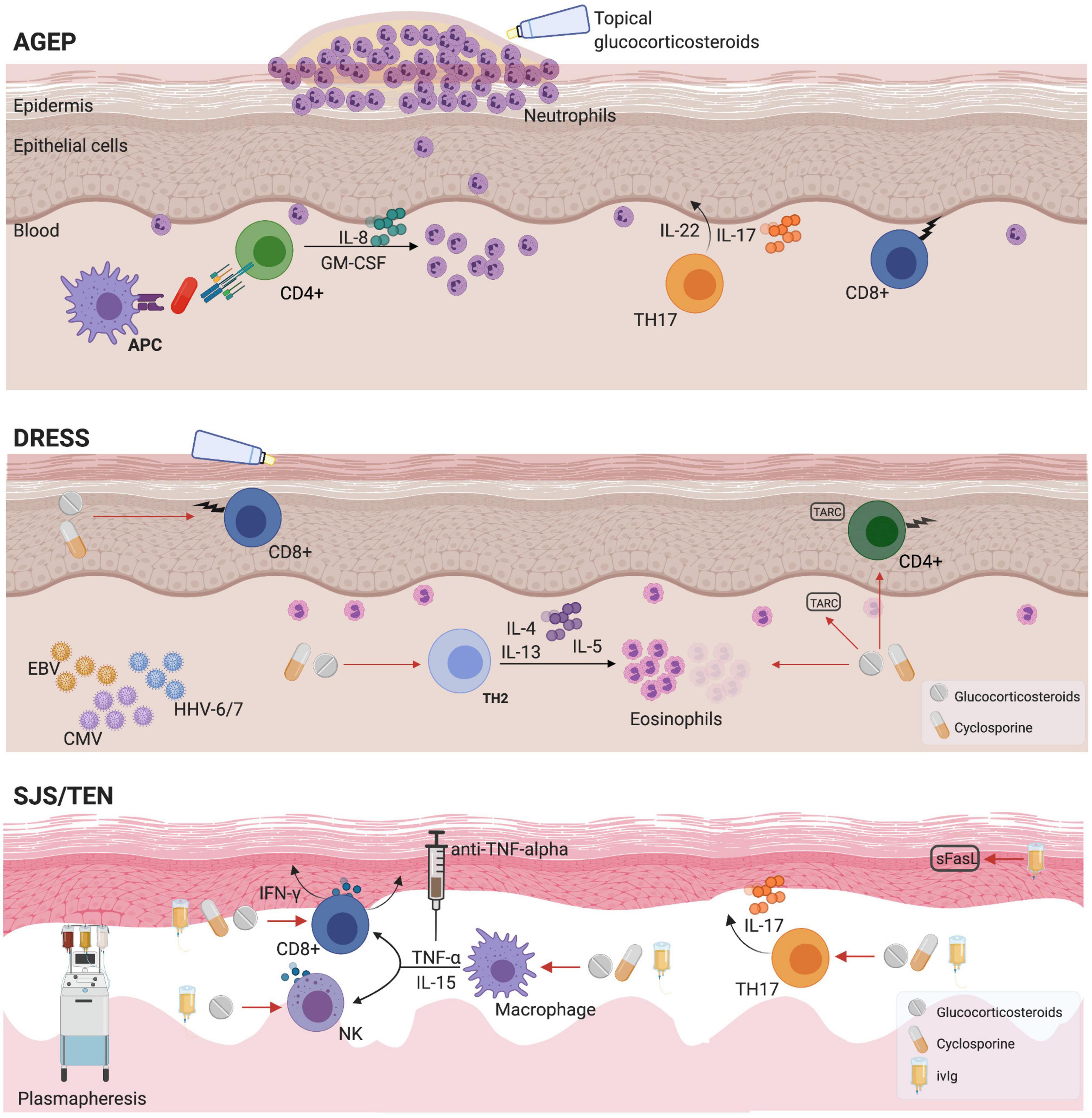
Figure 2. Mechanisms and pharmacological management for T-cell mediated reactions. AGEP, acute generalized exanthematous pustulosis; APC, antigen presenting cell; CMV, cytomegalovirus; DRESS, drug reaction with eosinophilia and systemic symptoms; EBV, Epstein-Barr virus; HHV, Human Herpesvirus; GM-CSF, granulocyte macrophage-colony stimulating factor; IFN-γ, Interferon gamma; IL, interleukin; sFasL, soluble Fas ligand; SJS, Stevens-Johnson syndrome; TARC, thymus and activation-regulated chemokine; TEN, toxic epidermal necrolysis; TNF, tumor necrosis factor.
There is a limited number of cohort studies that focus on providing a better understanding of the incidence, clinical description and mortality of DHR. The majority of the data is extrapolated from small older studies. Some reports suggest that drug-induced SCARs are less prevalent in the pediatric population compared to an adult population (6–9). A description of the main delayed drug related T-cell mediated hypersensitivity reactions is portrayed in Table 1 with an illustration of the immunopathogenesis and treatment options in Figure 2.
The MPE, morbilliform drug eruption or benign exanthem is the most common benign skin reaction associated with drugs. This condition is characterized by a maculopapular erythematous eruption that can become widespread and confluent and can be associated with pruritus and/or mild eosinophilia (10). The onset of the reaction typically occurs in the first 7–10 days of treatment for patients not previously exposed to the medication. However, in previously sensitized individual, re-exposure can lead to a skin eruption as rapid as 6–72 h after treatment initiation. In the pediatric population, viral exanthemas are an important differential diagnosis (11).
Early studies suggest a prevalence of 2% for cutaneous drug eruptions in general (12), with up to 90% representing a mild phenotype. However, there is limited recent reliable data describing this non-severe type phenotypes. Another aspect is the non-immune mediated nature of some MPE that may result in overestimating the prevalence of this condition (13).
All drug categories could, in theory, induce a skin eruption and there is a fine line between a recognized side effect and a mild skin hypersensitivity reaction. However, few studies that focus on a limited number of drugs have demonstrated how drugs induce T-cell mediated reaction mainly looking at antibiotics (penicillins, cephalosporins, sulfonamides) and anticonvulsants.
Treating through in MPE is part of the accepted management options especially when the treatment alternatives could jeopardize the quality of the treatment or the treatment outcome (14, 15). The skin manifestation can be controlled with oral second-generation antihistamines as well as topical corticosteroids (15). A multidisciplinary approach is suggested for all delayed hypersensitivity conditions from MPE to TEN. Specialists implicated in the management vary depending on the organ involvement with allergy immunology, dermatology and infectious disease usually at the center of the management team (16).
The AGEP is a non-follicular, sterile, pustular rash over widespread erythema, with a preference for the flexural folds. This condition can be accompanied by systemic symptoms such as fever and/or biological abnormalities (10). A validation score from the EuroSCAR group criteria can be used to confirm the clinical diagnostic for AGEP cases (17). Part of the differential diagnosis of pustules localized on an erythematous skin is generalized pustular psoriasis (GPP), a rare subtype of psoriasis (18). During the initial clinical presentation, AGEP and GPP can be difficult to distinguish. The clinical evolution, with a shorter disease course for AGEP, as well as the biopsy with psoriasiform changes of the epidermis seen with GPP and absent in AGEP, allows the clinician to clarify the diagnosis (19, 20).
A landmark study for AGEP comes from the 2001 EuroSCAR group that reports an incidence of 1–5 cases per million persons per year (17). The mortality rate was reported to be 2–4% (21, 22) while understanding that this condition has a favorable prognosis following culprit drug withdrawal (23).
Multiple agents have been associated with AGEP (17) with antibiotics and antimycotics commonly described (21, 22). The short latency period for AGEP and certain specific clinical characteristics are considered agent specific (24). Case reports have described an association with infections (viral, bacterial or parasitic), spider insect bites and contrast agents (24).
The main goal is to offer supportive care and to control the skin inflammation and pruritus. Similar to MPE, topical medium potency corticosteroids and second-generation antihistamines are commonly prescribed (25). In a retrospective review of electronic medical records from Singapore of 43 AGEP cases, where 9 (21%) patients were treated with systemic corticosteroids, the use of systemic corticosteroids compared with topical corticosteroids was associated with a reduction in the hospital stay (26). During the acute reaction, a skin biopsy can aid with the identification of the underlying phenotype. While this is not routinely performed for the mild drug eruption or for some of the classic manifestations, the histopathologic findings can support the diagnostic of a drug related reaction particularly in atypical cases or when GPP is suspected (Table 1).
Drug reaction with eosinophilia and systemic symptoms or drug-induced hypersensitivity syndrome (DIHS) is a polymorphic erythematous urticaria-like or violaceous skin eruption that can progress to exfoliative dermatitis, facial and extremity edema. Patients can present with lymphadenopathy as well as fever, biological abnormalities and internal organ involvement. It is suggested that reactivation of viruses from the Herpesviridae family such as human herpesvirus (HHV)-6, HHV-7, Epstein-Barr virus (EBV), cytomegalovirus (CMV) play a major role in the pathogenesis (27, 28). This condition is characterized by a delayed onset, the time from the drug exposure varying from 2 to 6 weeks (29). Recent reports have described a shorter latency period (less than 15 days) for antibiotics and contrast agents (30). The RegiSCAR is calculated using clinical and laboratory data to estimate the probability of this condition (definite, probable, possible, or no case) (31).
There are no large cohort studies or registries for DRESS. Using electronic health records, a recent report calculated the incidence at 2/100,000 (32) and a Spanish pharmacovigilance program described an incidence of 4/10,000 patients (33). The incidence of DRESS is drug and population dependent.
The primary culprit drugs are antibiotics and anticonvulsants (32) as well as allopurinol (34). Recently, other agents such as contrast product have been described (35).
Drug withdrawal is an essential part of acute management with patients often being restricted in terms of future drug options. The culprit agents and all possible cross-reactive drugs are avoided. As multiple organ involvement is frequent, systemic corticosteroids are usually initiated besides the usual supportive care (36–38). For refractory cases of DRESS with persistent elevated liver function, viral infections should be rule out as possible mimickers include infectious mononucleosis (EBV), CMV, and HIV (39). Case reports and small cases series demonstrate a role for cyclosporine as a second line agent (40, 41). There might be a role for other immunosuppressive agents but no randomized trials have showed a benefit and they are not part of routine management.
Stevens-Johnson syndrome and toxic epidermal necrolysis are characterized by skin necrosis, skin detachment (positive Nikolsky sign) and blistering of the mucous membranes accompanied by serious systemic manifestations. The mortality for this condition can reach 30–50% (42). The distinction between SJS and TEN is determined by affected body surface area (BSA): 1–10% for SJS, 10–30% for SJS/TEN overlap and >30% for TEN (10). The time interval from drug exposure to the development of symptoms can vary from 4 to 28 days and in a third of cases no causal agent is identified (29). In the pediatric population, Mycoplasma pneumoniae infection has been associated with SJS (43). A clinical score (SCORTEN) can be calculated to indicate prognostic value (44). The ALDEN score is an algorithm that helps identify the most likely culprit drug based on criteria such as type of drug, timing and possible alternative causes (45, 46). An ALDEN score of 4 or more is usually required for the SJS/TEN phenotype.
The incidence of SJS/TEN is estimated at 2–7 cases per million people per year using a German population based-registry with an increase prevalence of SJS cases compared to TEN (47, 48). Recently, data from the FDA adverse event reporting system (FAERS) indicated a rate of 0.15% with 30,202 reactions among the 20,406,852 adverse drug events reported in the database (49). In lower- and middle-income countries where TB and HIV are more prevalent, the rates of SJS/TEN are up to 10-fold higher (10).
The agents most commonly implicated are allopurinol, anticonvulsants and antibiotics (50). However, in about one third of cases, a drug cannot clearly be associated with the development of the SJS/TEN (46).
Following drug withdrawal and avoidance of cross-reactive medications, for SJS/TEN, given the multiorgan involvement, various specialties must be involved in the acute setting such as ophthalmology, head and neck, gastroenterology, gynecology, etc. Patients are usually transferred to burn units in order to be able to receive the adequate wound care, nutritional and fluid support (51, 52). The role of adjunctive therapies is unclear at this time with the use of systemic corticosteroids being controversial (47). While reports on mortality show contradictory results, a meta-analysis regrouping 1,209 patients indicated a benefit with corticosteroid treatment (decreased mortality) compared to supportive treatment alone (53). Intravenous immunoglobulins (IVIG), while part of the management in various centers, have an unclear clinical benefit (54). The combination of systemic corticosteroids and IVIG seems to be associated with the lowest mortality rates compared to each treatment alone (55). Cyclosporine has also been used with promising results in terms on mortality reduction (56, 57). Considering the high mortality rate for this condition, novel therapies are required. Recent studies have shown a possible benefit in the acute phase of the disease following the use of TNF-alpha inhibitors such as etanercept. These agents improved skin healing and decreased mortality as estimated by predictive scores (58).
The generalized bullous fixed drug eruption (GBFDE) is considered a rare type of fixed drug eruption that is multifocal and widespread, characterized by sharply defined bullae at the same site following recurrent administration of offending drug (59). The skin surface under the large flaccid bullae is often widespread red or brown (59). Systemic symptoms such as fever and arthralgias have also been described. The main differential diagnosis for this condition is SJS/TEN but GBFDE has a milder course with rapid skin healing in absence of scarring following drug discontinuation (60, 61).
While fixed drug eruption (FDE) has been commonly described with an incidence of 14–22% (61), the incidence of GBFDE is unknown at this time.
Fixed drug eruption has been associated with numerous drugs from antibiotics to analgesics and NSAIDS as well as sedatives (61). In a cohort of 48 GBFDE cases, the mean time to disease after drug administration was 2.9 days and the suspected drugs varied from antibiotics to analgesics and NSAIDS (59).
As for all the previously described conditions, the main treatment is culprit drug removal followed by symptomatic management to decrease pain or related pruritus (61). A biopsy excluding alternative cause (e.g., SJS/TEN, TEN-like lupus and immunobullous disease such as bullous pemphigoid, linear IgA disease) is required. The biological marker granulysin has been shown to help differentiate SJS/TEN from other conditions (62). While the aim of this review is to present diagnostic tools, the GBFDE has been presented as part of the differential diagnostic for SJS/TEN and will not discussed in detail in the subsequent sections.
A detailed clinical history is crucial to diagnose drug-related reactions. For beta-lactam allergy, it has been demonstrated that beta-lactam allergy interviews, in absence of skin testing, can assist in ruling out an allergy and reduce the use of non-beta-lactam antibiotics such as fluoroquinolones, considered high-Clostridioides difficile infection-risk antibiotics (63, 64). However, this has been infrequently deployed in moderate to severe presumed T-cell mediated reactions.
Following a detailed history, assessing the temporal association between symptoms and drug exposure with the help of a drug timeline is crucial. Any drug started more than 6–8 weeks before the reaction is less likely to be causal (65). The drug half-life must also be considered. SJS/TEN reactions associated with drugs that have a long half-life (more than 20 h) have been associated with an increase in mortality (26%) compared to drugs with shorter half-life (5% mortality) (66). This suggests that the time of drug discontinuation is also important. Using validated causality scores such as the Naranjo score can help guide clinicians in identifying the culprit agents. All agents administered must be considered causal with recent reports showing that T-cell mediated reactions can rarely occur after the administration of agents such as proton pump inhibitors (67) or anti-histamine receptors such as ranitidine (68). Among the agents commonly used in the hospital setting, contrast agents are often reported to be culprit (69, 70). The nursing and the pharmacy team can provide valuable assistance with identifying the agents for the drug timeline. Further, the pharmacy team can assist with pharmacovigilance researches by exploring existing databases (71).
Previous prospective studies and both international and local allergy society guidelines support the use of skin prick and intradermal testing (IDT) for drug allergy assessment (72–80). The concentration administered is designed to cause the least amount of irritation as per published guidelines (72, 74, 81–83), although validated concentrations for T-cell mediated reactions are less well described. Further, the true concentrations required to induce a positive T-cell response are unknown with recent studies showing that the use drugs such as vancomycin at the highest non-irritating concentrations are not enough to evoke a T-cell mediated reaction at the injection site (84). All the agents used are usually approved by local health regulations and have been safely administered via the intradermal route (77, 85–89). However, the sensitivity and specificity of skin tests are not validated for non-immediate reactions and, apart from penicillin, there are no current standardized extracts for skin tests.
Intradermal testing implies that a small quantity (0.02–0.05 mL) of a drug at a non-irritant concentration is gently injected under the skin. The testing is usually performed on the volar surface of the forearm and it is recommended to keep sufficient space (approximately 2–2.5 cm) between each injected agent. The preferred area is 5 cm from the wrist and 3 cm from the antecubital fossa. An immediate reading is performed after 15–20 min and a delayed reading after 24–48 h. A positive reaction translates as erythema and a local reaction when compared with the injection of a negative control, usually saline. A histamine prick test is used as a positive control for immediate reactions and several medications such as antihistamines have been identified as being able to suppress this local reaction. In this context, all drug known to affect the skin testing should be stopped depending on the described duration of suppression. There is no positive control for delayed reactions.
For penicillin non-severe allergic reactions, performing testing with the major allergenic determinant (penicilloyl polylysine), a minor determinant mixture (penicillin G, penicilloate, penilloate), and amoxicillin translated to a negative predictive value of 97.9% (90) for immediate reactions. There is currently a clear recommendation for skin testing followed by challenge for pregnant women with a history of penicillin allergy considering the importance of a beta-lactam treatment for Group B Streptococcus (91–93). In a cohort of children with low risk beta-lactam delayed-type reactions, delayed IDT was considered a useful tool (94).
There is a clear role for delayed IDT reading in delayed reactions to penicillin with evidence showing that delayed reading would have identified an additional 25% of patients in a prospective cohort of 37 patients (95). Furthermore, there is increasing evidence that IDT is safe even for the severe delayed phenotypes (96, 97). Cases of disease reactivation with mild isolated skin symptoms following skin testing have been described, especially when the testing was performed in the first 4–6 weeks following the acute reaction (98). The sensitivity of delayed IDT for antimicrobials ranges from 40% (96, 99) to 56% (98) for the severe phenotypes, excluding SJS/TEN (Table 2). However, the specificity and the false positive rate are not known.
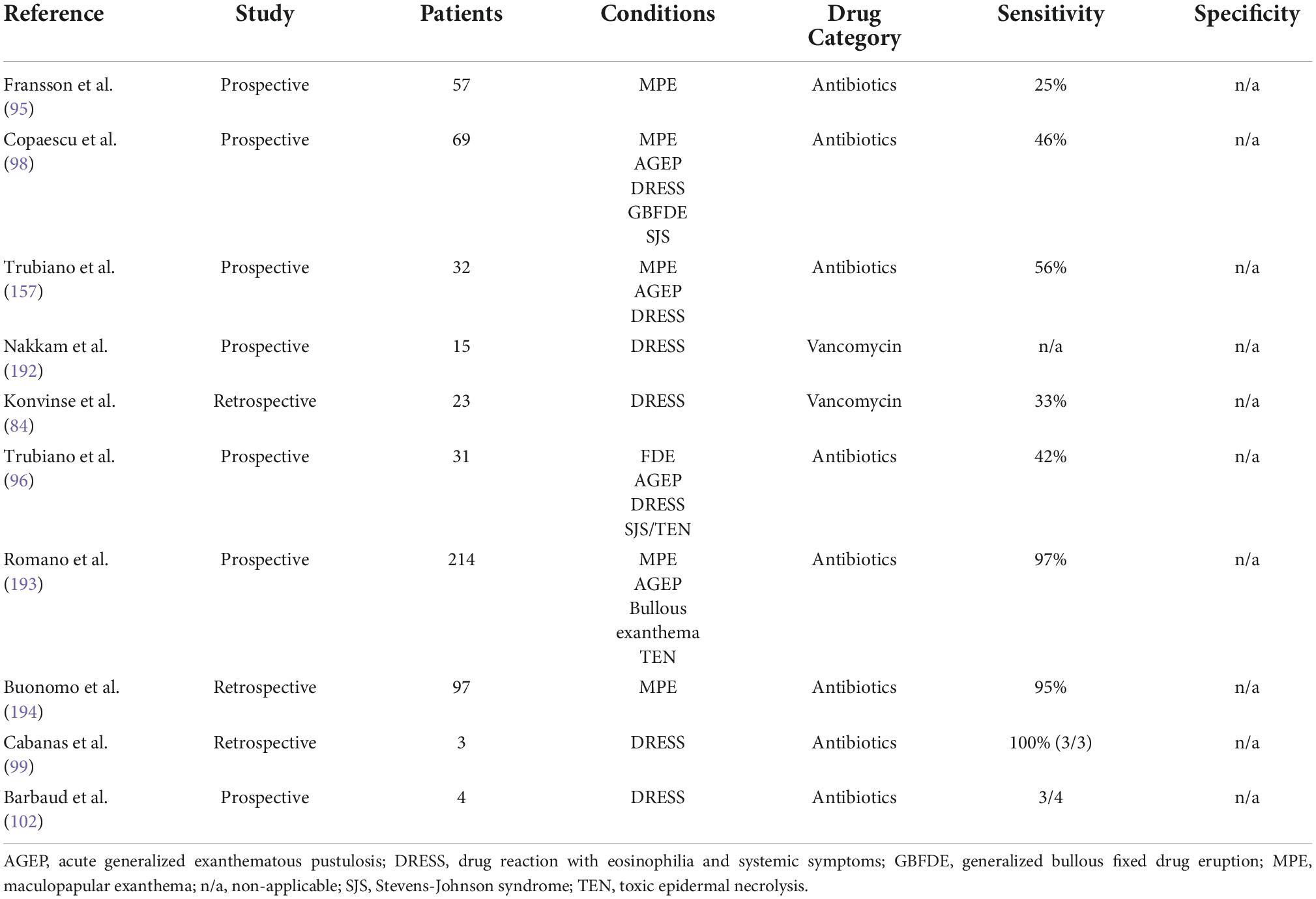
Table 2. Recent reported sensitivity and specificity for delayed intradermal testing in drug allergy.
In patients considered sensitized or allergic, antigen specific T-cells can be found on the surface of the skin. By applying non-irritant drug allergen concentrations under occlusion on the intact skin, patch testing (PT) aims to reproduce in the small limited area of the test the original delayed reaction. The PT is usually applied on the back or lateral upper arm area. There is no positive control that has been used with PT but the testing uses a negative control such as petroleum gel. Patch testing is usually left in place for a duration of 48 h with some studies showing benefit of performing a 7-day reading especially for certain preservatives (100). This is a time-consuming process as patients are asked to avoid showers and an increase in heat/humidity.
Non-irritant concentrations of various drugs for use in patch testing have been established (101, 102). However, there are currently no international guidelines for PT preparation as to ensure the quality of the products with large differences in active ingredient concentrations when using commercially available pure drugs compared with commercialized forms (103). Some alternatives for the classic PT method have been provided such as the scratch-patch involving the scarification or stripping of the epidermis with specialized tapes prior applying the PT (104). While this method proved to be non-irritant compared to the PT, carefully consideration is required especially for the severe phenotypes such as SJS/TEN. Indeed, cases of disease reactivation following PT have been reported in the literature, particularly in the immunosuppressed population (105).
The current published clinical studies underline a low sensitivity of this tool while the specificity is elevated, favoring a role of this tool for the more severe immune-mediated hypersensitivity reactions (102, 106, 107) (Table 3). Another advantage of this tool, compared to the IDT, is the possibility to use non-sterile and oral drug formulations. It is also interesting to note that the positivity of this tool seems to depend on the assessed drug as well as the reported reaction (108). In the clinical setting, considering this low reported sensitivity, lack of a validated positive control and less than 100% negative predictive value, removal of the allergy label should not be performed following a negative PT. In the pediatric population, while the literature is very limited, the sensitivity seems to be lower compared to the adult population (94). A positive PT should help confirm an immunologic mechanism with studies showing an increased reproducibility with positive PT not been affected by the time interval between testing, sex or age (111, 112). However, this is still dependent on the drug and the use of patch testing, IDT and ex vivo/in vitro testing and genetic testing are likely to be complementary (109, 110).
Several protocols have been suggested for challenge testing in non-severe delayed reactions: (1) single step direct challenge (113–115), (2) 2-step graded challenge (116), (3) single or multiple step challenge following negative delayed intradermal skin testing/patch testing (117–120), (4) direct multiple days challenge or (5) multiple days challenge following negative skin testing (117, 121). In absence of an immediate objective reaction, the “immediate” protocols have often led to the removal of the allergy label even in the context on a reported delayed reaction.
The benefits of penicillin allergy assessment based on clinical history (in person or telemedicine visit) (122, 123), skin testing (124) and challenge have been demonstrated in various studies in recent years (122, 125). Furthermore, for the non-severe delayed reactions such as MPE, algorithms based on direct challenge (with no prior skin tests) are considered a safe and cost-effective option (64, 126–129). However, currently, there are no clear guidelines on the optimal assessment tools for these low risk penicillin allergies with a need to compare skin testing followed by oral challenge, if negative, to direct oral challenge. Pharmacist led protocols have been instrumental in providing safe and rapid in hospital delabeling (130–132). This literature has evolved from pediatric penicillin and aminopenicillin allergic cohorts, where direct challenge without skin testing is considered part of standard of care (133–135). In these non-severe cases, the presence of an underlying immune mechanism is unclear and the majority of the skin isolated drug eruptions could be related to a non-allergic condition such as a viral illness or a drug-viral interaction (13). For the pediatric population, there is a need to develop clinical decision scores that can be used outside the allergy clinic assessment as to allow improvement of antibiotic stewardship.
While the literature provides interesting evidence for the non-severe reactions, strict drug avoidance is still part of the recommendations for the severe phenotypes associated with an increased mortality (16, 65). In these cases, the use of structurally non-related drugs in recommended. In particular scenarios such as reported in a South African study with anti-tuberculosis drugs, drug re-challenge with empirically initiated intravenous corticosteroids following the first clinical signs has been associated with a majority of mild to moderate reactions (136, 137). There is also evidence that ex vivo assays such as the enzyme-linked immunoSpot (ELISpot) could help risk stratify patients providing diagnostic accuracy compared to the current gold standard, the drug ingestion challenge (138). Large, multi-center international studies are required to further characterize drug re-challenge as a tool to provide optimal drug treatment following in vivo and ex vivo testing.
The lymphocyte transformation test (LTT) has been widely used for past 30 years and is considered the forefather of ex vivo testing in drug allergy (139). It is reported that patient isolated memory T-cells can be stimulated with causal agents leading to a drug-specific T-cell proliferation. Because of this mechanism, the LTT is also addressed as a lymphocyte proliferation test of a lymphocyte stimulation or activation test (140). This cell proliferation is defined according to a stimulation index (SI) or the proportion between the drug stimulated lymphocytes and the background lymphocyte proliferation. This ratio aims to take into consideration the biological variation. For the classic LTT, it is calculated based on a radioactive uptake marker directly proportional to the degree of T-cell proliferation in response to a drug antigen (140). In recent years, variations of the LTT platform have been proposed in the literature.
The reported sensitivity of LTT in delayed hypersensitivity reactions ranges from 27% (141) to 74% (142) and specificity was quoted as 85–100% (141–144) (Table 4). When this tool was studied for a specific phenotype, its accuracy greatly improved. For example, in a cohort of 41 DRESS patients, the reported sensitivity was 73% and the specificity was 82%, using samples from a recovery phase and not an acute phase (145). Further, the sensitivity can vary depending on the drug studied and expression of either granulysin, granzyme B or IFN-γ. In a cohort of 63 patients with SCAR associated to the use of anti-epileptics, the sensitivity increased when using granulysin-based lymphocyte activation tests stimulated with carbamazepine (73.9%). Other experimental techniques to increase the sensitivity of this tool have been described such CTLA-4 blocking of lymphocytes, demonstrating the importance of T-cell regulatory pathways (146, 147).
The value of this test was exemplified in various cohort studies and case reports where this tool provided clinical assistance in determining the optimal drug options in both a pediatric (148, 149) and an adult population (141, 150). However, some of these cases can be subject to misclassification bias as the initial reported phenotype was not always consistent with a hypersensitivity reaction (148).
The T-cell ELISpot assays measuring IFN-γ cytokine response to different agents has been used to assist drug hypersensitivity causality investigations in patients with drug allergy (143, 151–154). Compared to LTT, in an adult cohort of 23 SCAR patients, the ELISpot IFN-γ helped identify more drug-specific IFN-γ releasing cells (155). Similar to LTT, this laboratory technique requires viable well-preserved patient T lymphocytes and involves the use of complex manipulations for which an operator-dependent variability could influence the assay results.
In general, standardized concentrations for ex vivo diagnostics can be based on confirmatory data from performed cytotoxicity assays (97, 156). However, various studies using non-studied concentrations have been published. Given that antibiotics are a major culprit for SCAR, these agents have been commonly used for the ELISpot assays (98, 157, 158). Other commonly reviewed agents are anticonvulsants, antituberculosis drugs and allopurinol (141, 155, 158, 159).
Depending on the used definition and the studied drugs, the sensitivity of this assay varied from 35% (158) to 86% (84, 160) with a reported specificity of 100% (Table 5). As very few cohort studies from specialized centers are available, there is a need to further explore this promising ex vivo method. In the pediatric population, an interesting study regrouping a cohort of 9 SCAR and 7 MPE compared LTT with ELISpot in both an acute and post-recovery phase. The authors showed the ELISpot assay using IFN-γ and IL-4 as cytokine outputs, produced a higher drug-specific response contributing to the diagnosis of the culprit drugs (159). However, the sample size is relatively small and hence results are non-conclusive at this point.
The increase in serum level of the IFN-γ cytokine in conditions such as MPE and SJS/TEN has been previously documented (161). But other cytokines have been identified such as IL-8, IL-17, and IL-22 in AGEP (162–164), IL-4, IL-5, IL-13, and TARC In DRESS (165, 166) and IL-15 in SJS/TEN (167, 168). This provides relevance for possible outputs to explore in functional assays as to increase the sensitivity of these tools.
There have been an increasing number of HLA associations described with many drugs and SCAR (Table 6). Some examples include HLA-B*57:01 screening prior prescription of the anti-retroviral drug abacavir (169–171) and HLA-B*15:02 screening before carbamazepine prescription in many South-East Asian countries where this allele is prevalent (172, 173). A study from Thailand reported that 21.2% of SCAR could have been prevented by screening for HLA-B alleles prior to drug exposure (158). Recently, studies have reported that DNA methylation, identified using genome-scale methylation analysis, might play a role in allopurinol SJS/TEN (174). The presence of HLA-B*58:01 is considered a predisposing factor for developing allopurinol/oxypurinol induced SCAR in Southeast Asian populations but not in European and African ancestry populations (175).
Vancomycin induced DRESS was associated with the expression of HLA-A*32:01 (84) and evidence shows that vancomycin directly interacts with naïve T-cells expressing HLA-A*32:01 (176). In the Thai population, HLA-B*15:02, HLA-C*06:02, HLA-C*08:01, and HLA-B*13:01 were associated with co-trimoxazole hypersensitivity reactions and mostly SJS/TEN (177, 178). Dapsone and its reactive metabolite, nitroso dapsone, induced hypersensitivities such as DRESS in individuals with HLA-B*13:01 (179, 180). Carbamazepine triggered SCAR, was linked to HLA-A*31:01 in Caucasian and Japanese populations (181). Following genome-wide association studies, HLA-B*57:01 and HLA-B*57:03 were reported in patients with drug-induced liver injury caused by flucloxacillin (182, 183) and (HLA)-DRB1*01:01 has been associated with nevirapine-induced hepatic hypersensitivity reactions (184). Anti-osteoporotic agents induced SJS were suggested to be associated with HLA-A*33:03 (185).
However, genetic screening is not currently integrated in routine practice and a comprehensive description of the current identified genetic markers is beyond the scope of this review. The biggest concern with HLA screening for many drugs is the fact that HLA risk is necessary but not sufficient for the development of the hypersensitivity in question. In many cases this means that an extremely high number of patients would need to be tested in order to prevent one case of hypersensitivity and hence this is not a cost-effective confirmatory test. However, there could be scenarios where HLA testing could be used beyond screening and could have a diagnosis role such as the HLA-A*32:01 testing for vancomycin DRESS in the setting of multiple implicated drugs.
Drug allergy labels have important impact on patient care by limiting not only the use of appropriate medications but also by increasing costs and quality of patient care (10, 124, 186). A multidisciplinary patient-centered risk/benefit-based assessment must be part of the management plan (Figure 3). What is the optimal management for the patient’s acute condition? What is the reported reaction or described phenotype and what was the most likely causal drug? If the culprit drug is stopped, are there any other drug alternatives available for the patient? Another important inquiry often unexplored is regarding the patient’s willingness to take the medication or alternative drugs again. Unfortunately, the clinical investigations can sometimes be limited by the patient’s refusal of in vivo investigations. In this scenario, ex vivo tools are appealing as the safety of the procedure can be guaranteed (Figure 3). However, as discussed, these tools are not available in the majority of health facilities. Another limit of these tools is their lack of validity. It is possible that the low sensitivity of these diagnostic tools is due to the fact that current assays rely on drug or drug metabolites that are not effectively recognized by the immune system (187). Also, considering that none of these diagnostic tools have a 100% negative predictive value, their use should aim to complement each other as to improve the sensitivity and the specificity of the diagnosis.
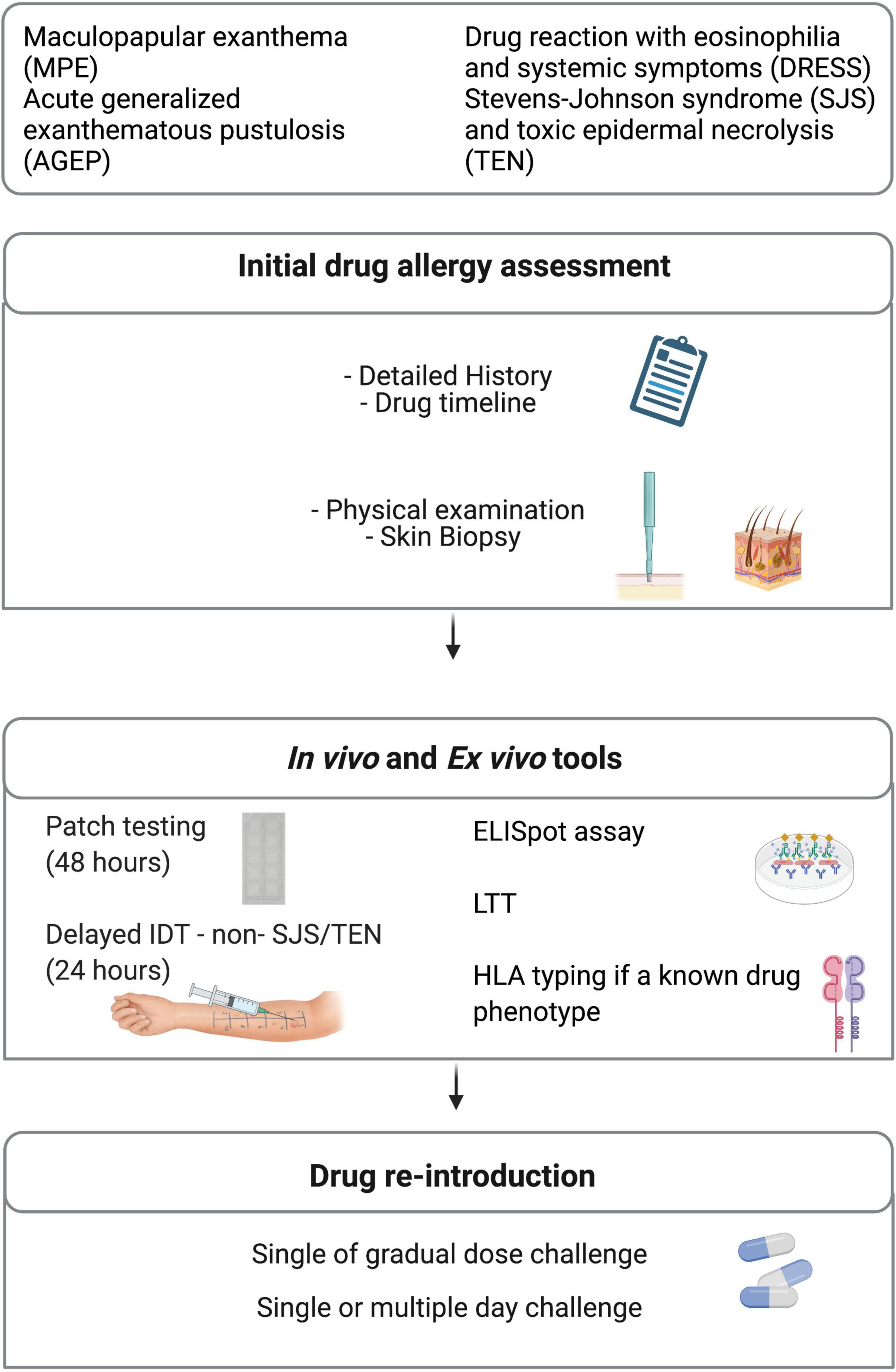
Figure 3. Diagnostic management. ELISpot, enzyme-linked immunoSpot; HLA, human leukocyte antigen; IDT, intradermal testing; LTT, Lymphocyte transformation test.
There is a current need to provide internationally accepted management algorithms for in vivo and ex vivo diagnostic tools and/or challenge while understanding the possibility that these algorithms might not apply to all phenotypes. The currently available tools must be prospectively used as to allow safe drug re-introduction.
Despite the increased mortality associated with SCAR, diagnostic tools remain limited and unstandardized. Ongoing research is required to better understand the epidemiology, the diagnostic approach and management strategies for these delayed drug reactions. Furthermore, large scale studies validating clinical diagnostic tools used for DHR are required.
AC performed the literature review and wrote the manuscript text with supervision from MB-S and JT. All authors reviewed the manuscript, made a substantial, direct, and intellectual contribution to the work, and approved the manuscript for publication.
AC receives support from The Montreal General Hospital Foundation and The Research Institute of the McGill University Health Centre (RI-MUHC) and was awarded University of Melbourne Research Scholarship, The Anna Maria Solinas Laroche Career Award in Immunology, and the Anita Garbarino Girard, Anna Maria Solinas, Dr. Phil Gold Award of Distinction. JT was supported by the Austin Medical Research Foundation and by a National Health and Medical Research Council postgraduate scholarship (GNT 1139902).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AGEP, acute generalized exanthematous pustulosis; DRESS, drug reaction with eosinophilia and systemic symptoms; ELISpot, enzyme-linked ImmunoSpot; MPE, maculopapular exanthema; SCAR, severe cutaneous adverse reaction; SJS, Stevens-Johnson syndrome; TEN, toxic epidermal necrolysis.
1. Pichler WJ. Immune pathomechanism and classification of drug hypersensitivity. Allergy. (2019) 74:1457–71. doi: 10.1111/all.13765
2. Trubiano JA, Aung AK, Nguyen M, Fehily SR, Graudins L, Cleland H, et al. A comparative analysis between antibiotic– and nonantibiotic-associated delayed cutaneous adverse drug reactions. J Allergy Clin Immunol Pract. (2016) 4:1187–93. doi: 10.1016/j.jaip.2016.04.026
3. Greenberger PA. Drug allergy. Allergy Asthma Proc. (2019) 40:474–9. doi: 10.2500/aap.2019.40.4275
4. Iulini M, Maddalon A, Galbiati V, Marinovich M, Corsini E. In vitro identification of drugs inducing systemic hypersensitivity reactions known in vivo to be associated with specific HLA genotypes. Toxicol In Vitro. (2020) 68:104953. doi: 10.1016/j.tiv.2020.104953
5. Nakajima A, Oda S, Yokoi T. Allopurinol induces innate immune responses through mitogen-activated protein kinase signaling pathways in HL-60 cells. J Appl Toxicol. (2016) 36:1120–8. doi: 10.1002/jat.3272
6. Jares EJ, Sanchez-Borges M, Cardona-Villa R, Ensina LF, Arias-Cruz A, Gomez M, et al. Multinational experience with hypersensitivity drug reactions in Latin America. Ann Allergy Asthma Immunol. (2014) 113:282–9. doi: 10.1016/j.anai.2014.06.019
7. Dibek Misirlioglu E, Guvenir H, Bahceci S, Haktanir Abul M, Can D, Usta Guc BE, et al. Severe cutaneous adverse drug reactions in pediatric patients: a multicenter study. J Allergy Clin Immunol Pract. (2017) 5:757–63. doi: 10.1016/j.jaip.2017.02.013
8. Cekic S, Canitez Y, Sapan N. Evaluation of the patients diagnosed with Stevens Johnson syndrome and toxic epidermal necrolysis: a single center experience. Turk Pediatri Ars. (2016) 51:152–8. doi: 10.5152/TurkPediatriArs.2016.3836
9. Kim GY, Anderson KR, Davis DMR, Hand JL, Tollefson MM. Drug reaction with eosinophilia and systemic symptoms (DRESS) in the pediatric population: a systematic review of the literature. J Am Acad Dermatol. (2020) 83:1323–30. doi: 10.1016/j.jaad.2020.03.081
10. Peter JG, Lehloenya R, Dlamini S, Risma K, White KD, Konvinse KC, et al. Severe delayed cutaneous and systemic reactions to drugs: a global perspective on the science and art of current practice. J Allergy Clin Immunol Pract. (2017) 5:547–63. doi: 10.1016/j.jaip.2017.01.025
11. Tsabouri S, Atanaskovic-Markovic M. Skin eruptions in children: drug hypersensitivity vs viral exanthema. Pediatr Allergy Immunol. (2021) 32:824–34. doi: 10.1111/pai.13485
12. Bigby M, Jick S, Jick H, Arndt K. Drug-induced cutaneous reactions. A report from the Boston collaborative drug surveillance program on 15,438 consecutive inpatients, 1975 to 1982. JAMA. (1986) 256:3358–63. doi: 10.1001/jama.256.24.3358
13. Ben-Said B, Arnaud-Butel S, Rozieres A, Rodet K, Berard F, Nicolas JF, et al. Allergic delayed drug hypersensitivity is more frequently diagnosed in drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome than in exanthema induced by beta-lactam antibiotics. J Dermatol Sci. (2015) 80:71–4. doi: 10.1016/j.jdermsci.2015.07.014
14. Trubiano JA, Soria A, Torres MJ, Trautmann A. Treating through drug-associated exanthems in drug allergy management: current evidence and clinical aspects. J Allergy Clin Immunol Pract. (2021) 9:2984–93. doi: 10.1016/j.jaip.2021.04.008
15. Trautmann A, Benoit S, Goebeler M, Stoevesandt J. “Treating through” decision and follow-up in antibiotic therapy-associated exanthemas. J Allergy Clin Immunol Pract. (2017) 5:1650–6. doi: 10.1016/j.jaip.2017.03.032
16. Phillips EJ, Bigliardi P, Bircher AJ, Broyles A, Chang YS, Chung WH, et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol. (2019) 143:66–73. doi: 10.1016/j.jaci.2018.10.030
17. Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. (2001) 28:113–9. doi: 10.1034/j.1600-0560.2001.028003113.x
18. Lofvendahl S, Norlin JM, Schmitt-Egenolf M. Prevalence and incidence of generalized pustular psoriasis in Sweden: a population-based register study. Br J Dermatol. (2022) 186:970–6. doi: 10.1111/bjd.20966
19. Isom J, Braswell DS, Siroy A, Auerbach J, Motaparthi K. Clinical and histopathologic features differentiating acute generalized exanthematous pustulosis and pustular psoriasis: a retrospective series. J Am Acad Dermatol. (2020) 83:265–7. doi: 10.1016/j.jaad.2020.03.015
20. Kardaun SH, Kuiper H, Fidler V, Jonkman MF. The histopathological spectrum of acute generalized exanthematous pustulosis (AGEP) and its differentiation from generalized pustular psoriasis. J Cutan Pathol. (2010) 37:1220–9. doi: 10.1111/j.1600-0560.2010.01612.x
21. Saissi EH, Beau-Salinas F, Jonville-Bera AP, Lorette G, Autret-Leca E. Centres Régionaux de Pharmacovigilance. [Drugs associated with acute generalized exanthematic pustulosis]. Ann Dermatol Venereol. (2003) 130:612–8.
22. Sidoroff A, Dunant A, Viboud C, Halevy S, Bavinck JN, Naldi L, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. (2007) 157:989–96. doi: 10.1111/j.1365-2133.2007.08156.x
23. Mebazaa A, Kort R, Zaiem A, Elleuch D, Moula H, Cheikhrouhou R, et al. [Acute generalized exanthematous pustulosis. Study of 22 cases]. Tunis Med. (2010) 88:910–5.
24. Creadore A, Desai S, Alloo A, Dewan AK, Bakhtiar M, Cruz-Diaz C, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized exanthematous pustulosis in the US. JAMA Dermatol. (2022) 158:176–83. doi: 10.1001/jamadermatol.2021.5390
25. Ingen-Housz-Oro S, Hotz C, Valeyrie-Allanore L, Sbidian E, Hemery F, Chosidow O, et al. Acute generalized exanthematous pustulosis: a retrospective audit of practice between 1994 and 2011 at a single centre. Br J Dermatol. (2015) 172:1455–7. doi: 10.1111/bjd.13540
26. Oh DAQ, Yeo YW, Choo KJL, Pang SM, Oh CC, Lee HY. Acute generalized exanthematous pustulosis: epidemiology, clinical course, and treatment outcomes of patients treated in an Asian academic medical center. JAAD Int. (2021) 3:1–6. doi: 10.1016/j.jdin.2020.12.004
27. Hashizume H, Fujiyama T, Kanebayashi J, Kito Y, Hata M, Yagi H. Skin recruitment of monomyeloid precursors involves human herpesvirus-6 reactivation in drug allergy. Allergy. (2013) 68:681–9. doi: 10.1111/all.12138
28. Neuman MG, McKinney KK, Nanau RM, Kong V, Malkiewicz I, Mazulli T, et al. Drug-induced severe adverse reaction enhanced by human herpes virus-6 reactivation. Transl Res. (2013) 161:430–40. doi: 10.1016/j.trsl.2012.12.012
29. Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. (2017) 390:1996–2011. doi: 10.1016/S0140-6736(16)30378-6
30. Soria A, Bernier C, Veyrac G, Barbaud A, Puymirat E, Milpied B. Drug reaction with eosinophilia and systemic symptoms may occur within 2 weeks of drug exposure: a retrospective study. J Am Acad Dermatol. (2020) 82:606–11. doi: 10.1016/j.jaad.2019.09.036
31. Kardaun SH, Sidoroff A, Valeyrie-Allanore L, Halevy S, Davidovici BB, Mockenhaupt M, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. (2007) 156:609–11. doi: 10.1111/j.1365-2133.2006.07704.x
32. Wolfson AR, Zhou L, Li Y, Phadke NA, Chow OA, Blumenthal KG. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome identified in the electronic health record allergy module. J Allergy Clin Immunol Pract. (2019) 7:633–40. doi: 10.1016/j.jaip.2018.08.013
33. Ramirez E, Medrano-Casique N, Tong HY, Bellon T, Cabanas R, Fiandor A, et al. Eosinophilic drug reactions detected by a prospective pharmacovigilance programme in a tertiary hospital. Br J Clin Pharmacol. (2017) 83:400–15. doi: 10.1111/bcp.13096
34. Bluestein S, Yu R, Stone CA, Phillips E. A review of drug reaction with eosinophilia and systemic symptoms in the FDA adverse event reporting system (FAERS). J Allergy Clin Immunol. (2021) 147:AB12. doi: 10.1016/j.jaci.2020.12.085
35. Soria A, Amsler E, Bernier C, Milpied B, Tetart F, Morice C, et al. DRESS and AGEP reactions to iodinated contrast media: a french case series. J Allergy Clin Immunol Pract. (2021) 9:3041–50. doi: 10.1016/j.jaip.2021.02.060
36. Shiohara T, Kano Y. Drug reaction with eosinophilia and systemic symptoms (DRESS): incidence, pathogenesis and management. Expert Opin Drug Saf. (2017) 16:139–47. doi: 10.1080/14740338.2017.1270940
37. Roujeau JC, Haddad C, Paulmann M, Mockenhaupt M. Management of nonimmediate hypersensitivity reactions to drugs. Immunol Allergy Clin North Am. (2014) 34:473–87; vii. doi: 10.1016/j.iac.2014.04.012
38. Sandhu S, Neema S, Vashisht D, Venugopal R, Sengupta P, Radhakrishnan S. Drug reaction with eosinophilia and systemic symptoms: a single center descriptive observational study. Dermatol Ther. (2021) 34:e14670. doi: 10.1111/dth.14670
39. Anci E, Braun C, Marinosci A, Rodieux F, Midun E, Torres MJ, et al. Viral infections and cutaneous drug-related eruptions. Front Pharmacol. (2020) 11:586407. doi: 10.3389/fphar.2020.586407
40. Kuschel SL, Reedy MS. Cyclosporine treatment of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a case report and brief review of the literature. Pract Dermatol. (2018) 2018:41–3.
41. Su HJ, Chen CB, Yeh TY, Chung WH. Successful treatment of corticosteroid-dependent drug reaction with eosinophilia and systemic symptoms with cyclosporine. Ann Allergy Asthma Immunol. (2021) 127:674–81. doi: 10.1016/j.anai.2021.08.012
42. Rive CM, Bourke J, Phillips EJ. Testing for drug hypersensitivity syndromes. Clin Biochem Rev. (2013) 34:15–38.
43. Olson D, Watkins LK, Demirjian A, Lin X, Robinson CC, Pretty K, et al. Outbreak of mycoplasma pneumoniae-associated stevens-johnson syndrome. Pediatrics. (2015) 136:e386–94. doi: 10.1542/peds.2015-0278
44. Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz J, Wolkenstein P. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. (2000) 115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x
45. Shear NH, Dodiuk-Gad RP. Advances in Diagnosis and Management of Cutaneous Adverse Drug Reactions. Singapore: ADIS (2019). p. 307 doi: 10.1007/978-981-13-1489-6
46. Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Ther. (2010) 88:60–8. doi: 10.1038/clpt.2009.252
47. Sekula P, Dunant A, Mockenhaupt M, Naldi L, Bouwes Bavinck JN, Halevy S, et al. Comprehensive survival analysis of a cohort of patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. J Invest Dermatol. (2013) 133:1197–204. doi: 10.1038/jid.2012.510
48. Rzany B, Mockenhaupt M, Baur S, Schroder W, Stocker U, Mueller J, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990-1992): structure and results of a population-based registry. J Clin Epidemiol. (1996) 49:769–73. doi: 10.1016/0895-4356(96)00035-2
49. Krantz MS, Yoon B, Stone CA, Yu R, Phillips E. Stevens-Johnson syndrome and toxic epidermal necrolysis in the FDA adverse event reporting system (FAERS) from 1995-2020. In: Proceeding of the 2022 AAAAI Annual Meeting. Phoenix (2022). doi: 10.1016/j.jaci.2021.12.233
50. Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S, Bouwes Bavinck JN, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The EuroSCAR-study. J Invest Dermatol. (2008) 128:35–44. doi: 10.1038/sj.jid.5701033
51. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol. (2013) 69:e1–16. doi: 10.1016/j.jaad.2013.05.003
52. Seminario-Vidal L, Kroshinsky D, Malachowski SJ, Sun J, Markova A, Beachkofsky TM, et al. Society of dermatology hospitalists supportive care guidelines for the management of Stevens-Johnson syndrome/toxic epidermal necrolysis in adults. J Am Acad Dermatol. (2020) 82:1553–67. doi: 10.1016/j.jaad.2020.02.066
53. Zimmermann S, Sekula P, Venhoff M, Motschall E, Knaus J, Schumacher M, et al. Systemic immunomodulating therapies for Stevens-Johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. JAMA Dermatol. (2017) 153:514–22. doi: 10.1001/jamadermatol.2016.5668
54. Huang YC, Li YC, Chen TJ. The efficacy of intravenous immunoglobulin for the treatment of toxic epidermal necrolysis: a systematic review and meta-analysis. Br J Dermatol. (2012) 167:424–32. doi: 10.1111/j.1365-2133.2012.10965.x
55. Micheletti RG, Chiesa-Fuxench Z, Noe MH, Stephen S, Aleshin M, Agarwal A, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective Study of 377 adult patients from the united States. J Invest Dermatol. (2018) 138:2315–21. doi: 10.1016/j.jid.2018.04.027
56. Gonzalez-Herrada C, Rodriguez-Martin S, Cachafeiro L, Lerma V, Gonzalez O, Lorente JA, et al. Cyclosporine use in epidermal necrolysis is associated with an important mortality reduction: evidence from three different approaches. J Invest Dermatol. (2017) 137:2092–100. doi: 10.1016/j.jid.2017.05.022
57. Ng QX, De Deyn M, Venkatanarayanan N, Ho CYX, Yeo WS. A meta-analysis of cyclosporine treatment for Stevens-Johnson syndrome/toxic epidermal necrolysis. J Inflamm Res. (2018) 11:135–42. doi: 10.2147/JIR.S160964
58. Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF-alpha antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. (2018) 128:985–96. doi: 10.1172/JCI93349
59. Perron E, Viarnaud A, Marciano L, Karkouche R, Wechsler J, De Prost N, et al. Clinical and histological features of fixed drug eruption: a single-centre series of 73 cases with comparison between bullous and non-bullous forms. Eur J Dermatol. (2021) 31:372–80. doi: 10.1684/ejd.2021.4051
60. Lipowicz S, Sekula P, Ingen-Housz-Oro S, Liss Y, Sassolas B, Dunant A, et al. Prognosis of generalized bullous fixed drug eruption: comparison with Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. (2013) 168:726–32. doi: 10.1111/bjd.12133
61. Patel S, John AM, Handler MZ, Schwartz RA. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. (2020) 21:393–9. doi: 10.1007/s40257-020-00505-3
62. Chung WH, Pan RY, Chu MT, Chin SW, Huang YL, Wang WC, et al. Oxypurinol-specific T cells possess preferential TCR clonotypes and express granulysin in allopurinol-induced severe cutaneous adverse reactions. J Invest Dermatol. (2015) 135:2237–48. doi: 10.1038/jid.2015.165
63. Covington EW, Baldwin BJ, Warren E. Pharmacy-led beta-lactam allergy interview (BLAI) reduces duration of fluoroquinolones within a community hospital. Ann Pharmacother. (2019) 53:588–95. doi: 10.1177/1060028019826223
64. Turner NA, Wrenn R, Sarubbi C, Kleris R, Lugar PL, Radojicic C, et al. Evaluation of a pharmacist-led penicillin allergy assessment program and allergy delabeling in a tertiary care hospital. JAMA Netw Open. (2021) 4:e219820. doi: 10.1001/jamanetworkopen.2021.9820
65. Lehloenya RJ, Peter JG, Copaescu A, Trubiano JA, Phillips EJ. Delabeling delayed drug hypersensitivity: how far can you safely go? J Allergy Clin Immunol Pract. (2020) 8:2878–95.e6. doi: 10.1016/j.jaip.2020.07.005
66. Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. (2000) 136:323–7. doi: 10.1001/archderm.136.3.323
67. Lin CY, Wang CW, Hui CR, Chang YC, Yang CH, Cheng CY, et al. Delayed-type hypersensitivity reactions induced by proton pump inhibitors: a clinical and in vitro T-cell reactivity study. Allergy. (2018) 73:221–9. doi: 10.1111/all.13235
68. Bouvette G, Copaescu A, Masse MS. A case of ranitidine induced exanthema. Allergy Asthma Clin Immunol. (2019) 15:1–31.
69. Berti A, Della-Torre E, Yacoub M, Tombetti E, Canti V, Sabbadini MG, et al. Patients with breakthrough reactions to iodinated contrast media have low incidence of positive skin tests. Eur Ann Allergy Clin Immunol. (2016) 48:137–44.
70. Trautmann A, Brockow K, Behle V, Stoevesandt J. Radiocontrast media hypersensitivity: skin testing differentiates allergy from nonallergic reactions and identifies a safe alternative as proven by intravenous provocation. J Allergy Clin Immunol Pract. (2019) 7:2218–24. doi: 10.1016/j.jaip.2019.04.005
71. Shrestha S, Danekhu K, Kc B, Palaian S, Ibrahim MIM. Bibliometric analysis of adverse drug reactions and pharmacovigilance research activities in Nepal. Ther Adv Drug Saf. (2020) 11:2042098620922480. doi: 10.1177/2042098620922480
72. Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM, Greenberger PA, et al. International consensus on drug allergy. Allergy. (2014) 69:420–37. doi: 10.1111/all.12350
73. Bergmann KC, Demoly P, Worm M, Fokkens WJ, Carrillo T, Tabar AI, et al. Efficacy and safety of sublingual tablets of house dust mite allergen extracts in adults with allergic rhinitis. J Allergy Clin Immunol. (2014) 133:1608–14e6. doi: 10.1016/j.jaci.2013.11.012
74. Joint Task Force on Practice Parameters, American Academy of Allergy Asthma Immunology, American College of Allergy Asthma Immunology, Joint Council of Allergy Asthma Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. (2010) 105:259–73. doi: 10.1016/j.anai.2010.08.002
75. Blanca M, Romano A, Torres MJ, Fernandez J, Mayorga C, Rodriguez J, et al. Update on the evaluation of hypersensitivity reactions to betalactams. Allergy. (2009) 64:183–93. doi: 10.1111/j.1398-9995.2008.01924.x
76. Australasian Society of Clinical Immunology and Allergy. Skin Prick Testing for the Diagnosis of Allergic Disease [Manual]. Brookvale, NSW: ASCIA (2013).
77. Fernandez J, Torres MJ, Campos J, Arribas-Poves F, Blanca M, Group DA-D. Prospective, multicenter clinical trial to validate new products for skin tests in the diagnosis of allergy to penicillin. J Investig Allergol Clin Immunol. (2013) 23:398–408.
78. Romano A, Viola M, Bousquet PJ, Gaeta F, Valluzzi R, Caruso C, et al. A comparison of the performance of two penicillin reagent kits in the diagnosis of beta-lactam hypersensitivity. Allergy. (2007) 62:53–8. doi: 10.1111/j.1398-9995.2006.01272.x
79. Mirakian R, Leech SC, Krishna MT, Richter AG, Huber PA, Farooque S, et al. Management of allergy to penicillins and other beta-lactams. Clin Exp Allergy. (2015) 45:300–27. doi: 10.1111/cea.12468
80. Broyles AD, Banerji A, Barmettler S, Biggs CM, Blumenthal K, Brennan PJ, et al. Practical guidance for the evaluation and management of drug hypersensitivity: specific drugs. J Allergy Clin Immunol Pract. (2020) 8:S16–116. doi: 10.1016/j.jaip.2020.08.002
81. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs – an ENDA/EAACI drug allergy interest group position paper. Allergy. (2013) 68:702–12. doi: 10.1111/all.12142
82. Empedrad R, Darter AL, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol. (2003) 112:629–30. doi: 10.1016/S0091-6749(03)01783-4
83. Testi S, Severino M, Iorno ML, Capretti S, Ermini G, Macchia D, et al. Nonirritating concentration for skin testing with cephalosporins. J Investig Allergol Clin Immunol. (2010) 20:171–2.
84. Konvinse KC, Trubiano JA, Pavlos R, James I, Shaffer CM, Bejan CA, et al. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol. (2019) 144:183–92. doi: 10.1016/j.jaci.2019.01.045
85. Yoon SY, Park SY, Kim S, Lee T, Lee YS, Kwon HS, et al. Validation of the cephalosporin intradermal skin test for predicting immediate hypersensitivity: a prospective study with drug challenge. Allergy. (2013) 68:938–44. doi: 10.1111/all.12182
86. Romano A, Torres MJ, Namour F, Mayorga C, Artesani MC, Venuti A, et al. Immediate hypersensitivity to cephalosporins. Allergy. (2002) 57:52–7. doi: 10.1034/j.1398-9995.57.s72.18.x
87. Seitz CS, Brocker EB, Trautmann A. Diagnostic testing in suspected fluoroquinolone hypersensitivity. Clin Exp Allergy. (2009) 39:1738–45. doi: 10.1111/j.1365-2222.2009.03338.x
88. Romano A, Gaeta F, Valluzzi RL, Alonzi C, Maggioletti M, Zaffiro A, et al. Absence of cross-reactivity to carbapenems in patients with delayed hypersensitivity to penicillins. Allergy. (2013) 68:1618–21. doi: 10.1111/all.12299
89. Broz P, Harr T, Hecking C, Grize L, Scherer K, Jaeger KA, et al. Nonirritant intradermal skin test concentrations of ciprofloxacin, clarithromycin, and rifampicin. Allergy. (2012) 67:647–52. doi: 10.1111/j.1398-9995.2012.02807.x
90. Solensky R, Jacobs J, Lester M, Lieberman P, McCafferty F, Nilsson T, et al. Penicillin allergy evaluation: a prospective, multicenter, open-label evaluation of a comprehensive penicillin skin test kit. J Allergy Clin Immunol Pract. (2019) 7:1876–85.e3. doi: 10.1016/j.jaip.2019.02.040
91. Dhudasia MB, Flannery DD, Pfeifer MR, Puopolo KM. Updated guidance: prevention and management of perinatal group B streptococcus infection. Neoreviews. (2021) 22:e177–88. doi: 10.1542/neo.22-3-e177
92. Kuder MM, Lennox MG, Li M, Lang DM, Pien L. Skin testing and oral amoxicillin challenge in the outpatient allergy and clinical immunology clinic in pregnant women with penicillin allergy. Ann Allergy Asthma Immunol. (2020) 125:646–51. doi: 10.1016/j.anai.2020.08.012
93. Patel V, Gleeson PK, Delaney K, Ralston SJ, Feldman S, Fadugba O. Safety and outcomes of penicillin allergy evaluation in pregnant women. Ann Allergy Asthma Immunol. (2022) 128:568–74. doi: 10.1016/j.anai.2022.01.032
94. Atanaskovic-Markovic M, Gaeta F, Medjo B, Gavrovic-Jankulovic M, Cirkovic Velickovic T, Tmusic V, et al. Non-immediate hypersensitivity reactions to beta-lactam antibiotics in children – our 10-year experience in allergy work-up. Pediatr Allergy Immunol. (2016) 27:533–8. doi: 10.1111/pai.12565
95. Fransson S, Mosbech HF, Elberling J, Kappel M, Garvey LH. Intradermal Testing Identifies 1 in 4 Patients with Nonimmediate Penicillin Allergy. Int Arch Allergy Immunol. (2021) 182:827–34. doi: 10.1159/000515080
96. Trubiano JA, Douglas AP, Goh M, Slavin MA, Phillips EJ. The safety of antibiotic skin testing in severe T-cell-mediated hypersensitivity of immunocompetent and immunocompromised hosts. J Allergy Clin Immunol Pract. (2019) 7:1341–43.e1. doi: 10.1016/j.jaip.2018.09.014
97. Trubiano JA, Strautins K, Redwood AJ, Pavlos R, Konvinse KC, Aung AK, et al. The combined utility of ex vivo IFN-gamma release enzyme-linked immunospot assay and in vivo skin testing in patients with antibiotic-Associated severe cutaneous adverse reactions. J Allergy Clin Immunol Pract. (2018) 6:1287–96.e1. doi: 10.1016/j.jaip.2017.09.004
98. Copaescu A, Mouhtouris E, Vogrin S, James F, Chua KYL, Holmes NE, et al. The role of in vivo and Ex vivo diagnostic tools in severe delayed immune-mediated adverse antibiotic drug reactions. J Allergy Clin Immunol Pract. (2021) 9:2010-15.e4. doi: 10.1016/j.jaip.2020.12.052
99. Cabanas R, Calderon O, Ramirez E, Fiandor A, Prior N, Caballero T, et al. Piperacillin-induced DRESS: distinguishing features observed in a clinical and allergy study of 8 patients. J Investig Allergol Clin Immunol. (2014) 24:425–30.
100. Chaudhry HM, Drage LA, El-Azhary RA, Hall MR, Killian JM, Prakash AV, et al. Delayed patch-test reading after 5 days: an update from the mayo clinic contact dermatitis group. Dermatitis. (2017) 28:253–60. doi: 10.1097/DER.0000000000000297
101. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy. (2002) 57:45–51. doi: 10.1046/j.0105-4538.2001.00001.x-i8
102. Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. (2013) 168:555–62. doi: 10.1111/bjd.12125
103. Agui Callejas A, Cuervas-Mons Vendrell M, Bernaola Abraira M, González Andrés D, Arrieta Loitegui M, Ranz Ortega P, et al. 3PC-013 Pharmaceutical compounding in paediatric patch testing: are we sure about the actual active ingredient concentration? Eur J Hosp Pharm. (2022) 29:A1–187. doi: 10.1136/ejhpharm-2022-eahp.38
104. Hug K, Yawalkar N, Helbling A, Pichler WJ. Scratch-patch and patch testing in drug allergy–an assessment of specificity. J Investig Allergol Clin Immunol. (2003) 13:12–9.
105. Shebe K, Ngwanya MR, Gantsho N, Lehloenya RJ. Severe recurrence of drug rash with eosinophilia and systemic symptoms syndrome secondary to rifampicin patch testing in a human immunodeficiency virus-infected man. Contact Dermatitis. (2014) 70:125–7. doi: 10.1111/cod.12155
106. Hassoun-Kheir N, Bergman R, Weltfriend S. The use of patch tests in the diagnosis of delayed hypersensitivity drug eruptions. Int J Dermatol. (2016) 55:1219–24. doi: 10.1111/ijd.13306
107. Gilissen L, Huygens S, Goossens A, Breynaert C, Schrijvers R. Utility of patch testing for the diagnosis of delayed-type drug hypersensitivity reactions to clindamycin. Contact Dermatitis. (2020) 83:237–9. doi: 10.1111/cod.13575
108. Woodruff CM, Botto N. The Role of patch testing in evaluating delayed hypersensitivity reactions to medications. Clin Rev Allergy Immunol. (2022) 62:548–61. doi: 10.1007/s12016-022-08924-2
109. Kaplan Y, Goldberg I, Sprecher E, Slodownik D. Patch testing versus interferon-gamma release assay in evaluation of drug eruptions. Fundam Clin Pharmacol. (2022) 36:414–20. doi: 10.1111/fcp.12733
110. de Groot AC. Patch testing in drug reaction with eosinophilia and systemic symptoms (DRESS): a literature review. Contact Dermatitis. (2022) 86:443–79. doi: 10.1111/cod.14090
111. Pinho A, Marta A, Coutinho I, Goncalo M. Long-term reproducibility of positive patch test reactions in patients with non-immediate cutaneous adverse drug reactions to antibiotics. Contact Dermatitis. (2017) 76:204–9. doi: 10.1111/cod.12720
112. Horita K, Tanoue C, Yasoshima M, Ohtani T, Matsunaga K. Study of the usefulness of patch testing and use test to predict the safety of commercial topical drugs. J Dermatol. (2014) 41:505–13. doi: 10.1111/1346-8138.12505
113. Vezir E, Dibek Misirlioglu E, Civelek E, Capanoglu M, Guvenir H, Ginis T, et al. Direct oral provocation tests in non-immediate mild cutaneous reactions related to beta-lactam antibiotics. Pediatr Allergy Immunol. (2016) 27:50–4. doi: 10.1111/pai.12493
114. Kuruvilla M, Shih J, Patel K, Scanlon N. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. (2019) 40:57–61. doi: 10.2500/aap.2019.40.4184
115. Li J, Cvetanovski V, Fernando S. Single-step direct drug provocation testing is safe for delabelling selected non-low-risk penicillin allergy labels. Ann Allergy Asthma Immunol. (2021) 127:232–5. doi: 10.1016/j.anai.2021.04.008
116. Iammatteo M, Alvarez Arango S, Ferastraoaru D, Akbar N, Lee AY, Cohen HW, et al. Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. J Allergy Clin Immunol Pract. (2019) 7:236–43. doi: 10.1016/j.jaip.2018.05.008
117. Hjortlund J, Mortz CG, Skov PS, Bindslev-Jensen C. Diagnosis of penicillin allergy revisited: the value of case history, skin testing, specific IgE and prolonged challenge. Allergy. (2013) 68:1057–64. doi: 10.1111/all.12195
118. Rimawi RH, Cook PP, Gooch M, Kabchi B, Ashraf MS, Rimawi BH, et al. The impact of penicillin skin testing on clinical practice and antimicrobial stewardship. J Hosp Med. (2013) 8:341–5. doi: 10.1002/jhm.2036
119. Marwood J, Aguirrebarrena G, Kerr S, Welch SA, Rimmer J. De-labelling self-reported penicillin allergy within the emergency department through the use of skin tests and oral drug provocation testing. Emerg Med Australas. (2017) 29:509–15. doi: 10.1111/1742-6723.12774
120. Cook DJ, Barbara DW, Singh KE, Dearani JA. Penicillin skin testing in cardiac surgery. J Thorac Cardiovasc Surg. (2014) 147:1931–5. doi: 10.1016/j.jtcvs.2014.01.019
121. Confino-Cohen R, Rosman Y, Meir-Shafrir K, Stauber T, Lachover-Roth I, Hershko A, et al. Oral challenge without skin testing safely excludes clinically significant delayed-onset penicillin hypersensitivity. J Allergy Clin Immunol Pract. (2017) 5:669–75. doi: 10.1016/j.jaip.2017.02.023
122. Staicu ML, Holly AM, Conn KM, Ramsey A. The use of telemedicine for penicillin allergy skin testing. J Allergy Clin Immunol Pract. (2018) 6:2033–40. doi: 10.1016/j.jaip.2018.04.038
123. Livirya S, Pithie A, Chua I, Hamilton N, Doogue M, Isenman H. Oral amoxicillin challenge for low-risk penicillin allergic patients. Intern Med J. (2022) 52:295–300. doi: 10.1111/imj.14978
124. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. (2016) 117:67–71. doi: 10.1016/j.anai.2016.04.021
125. Ramsey A, Staicu ML. Use of a penicillin allergy screening algorithm and penicillin skin testing for transitioning hospitalized patients to first-line antibiotic therapy. J Allergy Clin Immunol Pract. (2018) 6:1349–55. doi: 10.1016/j.jaip.2017.11.012
126. Banks TA, Tucker M, Macy E. Evaluating penicillin allergies without skin testing. Curr Allergy Asthma Rep. (2019) 19:27. doi: 10.1007/s11882-019-0854-6
127. Chua KYL, Vogrin S, Bury S, Douglas A, Holmes NE, Tan N, et al. The penicillin allergy delabeling program: a multicenter whole-of-hospital health services intervention and comparative effectiveness study. Clin Infect Dis. (2021) 73:487–96. doi: 10.1093/cid/ciaa653
128. Trubiano JA, Vogrin S, Chua KYL, Bourke J, Yun J, Douglas A, et al. Development and validation of a penicillin allergy clinical decision rule. JAMA Intern Med. (2020) 180:745–52. doi: 10.1001/jamainternmed.2020.0403
129. Iammatteo M, Lezmi G, Confino-Cohen R, Tucker M, Ben-Shoshan M, Caubet JC. Direct challenges for the evaluation of beta-lactam allergy: evidence and conditions for not performing skin testing. J Allergy Clin Immunol Pract. (2021) 9:2947–56. doi: 10.1016/j.jaip.2021.04.073
130. Gugkaeva Z, Crago JS, Yasnogorodsky M. Next step in antibiotic stewardship: pharmacist-provided penicillin allergy testing. J Clin Pharm Ther. (2017) 42:509–12. doi: 10.1111/jcpt.12530
131. Jones BM, Bland CM. Penicillin skin testing as an antimicrobial stewardship initiative. Am J Health Syst Pharm. (2017) 74:232–7. doi: 10.2146/ajhp160233
132. Torney NP, Tiberg MD. Description of a pharmacist-managed/administered penicillin allergy skin testing service at a community hospital. Am J Health Syst Pharm. (2021) 78:1066–73. doi: 10.1093/ajhp/zxab068
133. Prieto A, Munoz C, Bogas G, Fernandez-Santamaria R, Palomares F, Mayorga C, et al. Single-dose prolonged drug provocation test, without previous skin testing, is safe for diagnosing children with mild non-immediate reactions to beta-lactams. Allergy. (2021) 76:2544–54. doi: 10.1111/all.14800
134. Mustafa SS, Conn K, Ramsey A. Comparing direct challenge to penicillin skin testing for the outpatient evaluation of penicillin allergy: a randomized controlled trial. J Allergy Clin Immunol Pract. (2019) 7:2163–70. doi: 10.1016/j.jaip.2019.05.037
135. Arikoglu T, Kuyucu S, Caubet JC. New diagnostic perspectives in the management of pediatric beta-lactam allergy. Pediatr Allergy Immunol. (2022) 33:e13745. doi: 10.1111/pai.13745
136. Lehloenya RJ, Isaacs T, Nyika T, Dhana A, Knight L, Veenstra S, et al. Early high-dose intravenous corticosteroids rapidly arrest Stevens Johnson syndrome and drug reaction with eosinophilia and systemic symptoms recurrence on drug re-exposure. J Allergy Clin Immunol Pract. (2021) 9:582–4.e1. doi: 10.1016/j.jaip.2020.08.012
137. Lehloenya RJ, Todd G, Badri M, Dheda K. Outcomes of reintroducing anti-tuberculosis drugs following cutaneous adverse drug reactions. Int J Tuberc Lung Dis. (2011) 15:1649–57. doi: 10.5588/ijtld.10.0698
138. Porter M, Choshi P, Pedretti S, Chimbetete T, Smith R, Meintjes G, et al. IFN-gamma ELISpot in severe cutaneous adverse reactions to first-line anti-tuberculosis drugs in an HIV endemic setting. J Invest Dermatol. (2022). [Epub ahead of print]. doi: 10.1016/j.jid.2022.05.1059
139. Srinoulprasert Y. Lymphocyte transformation test and cytokine detection assays: determination of read out parameters for delayed-type drug hypersensitivity reactions. J Immunol Methods. (2021) 496:113098. doi: 10.1016/j.jim.2021.113098
140. Sachs B, Fatangare A, Sickmann A, Glassner A. Lymphocyte transformation test: history and current approaches. J Immunol Methods. (2021) 493:113036. doi: 10.1016/j.jim.2021.113036
141. Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome – alternatives for lymphocyte transformation test. Clin Exp Allergy. (2013) 43:1027–37. doi: 10.1111/cea.12145
142. Nyfeler B, Pichler WJ. The lymphocyte transformation test for the diagnosis of drug allergy: sensitivity and specificity. Clin Exp Allergy. (1997) 27:175–81. doi: 10.1046/j.1365-2222.1997.d01-495.x
143. Rozieres A, Hennino A, Rodet K, Gutowski MC, Gunera-Saad N, Berard F, et al. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. (2009) 64:534–42. doi: 10.1111/j.1398-9995.2008.01674.x
144. Porebski G, Czarnobilska E, Bosak M. Cytotoxicbased assays in delayed drug hypersensitivity reactions induced by antiepileptic drugs. Pol Arch Med Wewn. (2015) 125:823–34. doi: 10.20452/pamw.3160
145. Cabanas R, Calderon O, Ramirez E, Fiandor A, Caballero T, Heredia R, et al. Sensitivity and specificity of the lymphocyte transformation test in drug reaction with eosinophilia and systemic symptoms causality assessment. Clin Exp Allergy. (2018) 48:325–33. doi: 10.1111/cea.13076
146. Sugita K, Kabashima K, Sawada Y, Haruyama S, Yoshioka M, Mori T, et al. Blocking of CTLA-4 on lymphocytes improves the sensitivity of lymphocyte transformation tests in a patient with nickel allergy. Eur J Dermatol. (2012) 22:268–9. doi: 10.1684/ejd.2012.1641
147. Hammond S, Thomson P, Meng X, Naisbitt D. In-vitro approaches to predict and study T-cell mediated hypersensitivity to drugs. Front Immunol. (2021) 12:630530. doi: 10.3389/fimmu.2021.630530
148. Wada S, Kumagai H, Yokoyama K, Ito T, Miyauchi A, Sakamoto S, et al. Mesalazine allergy in a boy with ulcerative colitis: clinical usefulness of mucosal biopsy criteria. Clin J Gastroenterol. (2016) 9:302–5. doi: 10.1007/s12328-016-0675-2
149. Dias de Castro E, Leblanc A, Sarmento A, Cernadas JR. An unusual case of delayed-type hypersensitivity to ceftriaxone and meropenem. Eur Ann Allergy Clin Immunol. (2015) 47:225–7.
150. Sun Q, Sha W, Gui XW, Xiao YJ, Zeng WH, Sun WW, et al. Drug-induced lymphocyte stimulation test in the prediction of drug-induced hypersensitivity to antituberculosis drugs. Diagn Microbiol Infect Dis. (2015) 82:172–6. doi: 10.1016/j.diagmicrobio.2015.03.008
151. Khalil G, El-Sabban M, Al-Ghadban S, Azzi S, Shamra S, Khalife S, et al. Cytokine expression profile of sensitized human T lymphocytes following in vitro stimulation with amoxicillin. Eur Cytokine Netw. (2008) 19:131–41.
152. Bensaid B, Rozieres A, Nosbaum A, Nicolas JF, Berard F. Amikacin-induced drug reaction with eosinophilia and systemic symptoms syndrome: delayed skin test and ELISPOT assay results allow the identification of the culprit drug. J Allergy Clin Immunol. (2012) 130:1413–4. doi: 10.1016/j.jaci.2012.05.042
153. Copaescu A, Rose M, Mouhtouris E, Chua KY, Holmes NE, Phillips EJ, et al. Delayed hypersensitivity associated with amoxicillin-clavulanate. Allergy. (2020) 75:2700–2. doi: 10.1111/all.14359
154. Copaescu A, Gibson A, Li Y, Trubiano J, Phillips E. An updated review of the diagnostic methods in delayed drug hypersensitivity. Front Pharmacol. (2020) 11:573573. doi: 10.3389/fphar.2020.573573
155. Suthumchai N, Srinoulprasert Y, Thantiworasit P, Rerknimitr P, Tuchinda P, Chularojanamontri L, et al. The measurement of drug-induced interferon gamma-releasing cells and lymphocyte proliferation in severe cutaneous adverse reactions. J Eur Acad Dermatol Venereol. (2018) 32:992–8. doi: 10.1111/jdv.14890
156. Copaescu A, Choshi P, Pedretti S, Mouhtouris E, Peter J, Trubiano JA. Dose dependent antimicrobial cellular cytotoxicity-implications for ex vivo diagnostics. Front Pharmacol. (2021) 12:640012. doi: 10.3389/fphar.2021.758192
157. Trubiano JA, Chua KYL, Holmes NE, Douglas AP, Mouhtouris E, Goh M, et al. Safety of cephalosporins in penicillin class severe delayed hypersensitivity reactions. J Allergy Clin Immunol Pract. (2020) 8:1142–46.e4. doi: 10.1016/j.jaip.2019.10.005
158. Klaewsongkram J, Sukasem C, Thantiworasit P, Suthumchai N, Rerknimitr P, Tuchinda P, et al. Analysis of HLA-B allelic variation and IFN-gamma ELISpot responses in patients with severe cutaneous adverse reactions associated with drugs. J Allergy Clin Immunol Pract. (2019) 7:219–27.e4. doi: 10.1016/j.jaip.2018.05.004
159. Haw WY, Polak ME, McGuire C, Erlewyn-Lajeunesse M, Ardern-Jones MR. In vitro rapid diagnostic tests for severe drug hypersensitivity reactions in children. Ann Allergy Asthma Immunol. (2016) 117:61–6. doi: 10.1016/j.anai.2016.04.017
160. El-Ghaiesh S, Monshi MM, Whitaker P, Jenkins R, Meng X, Farrell J, et al. Characterization of the antigen specificity of T-cell clones from piperacillin-hypersensitive patients with cystic fibrosis. J Pharmacol Exp Ther. (2012) 341:597–610. doi: 10.1124/jpet.111.190900
161. Wang F, Cai R, He D, Zhao Y, Ye Y, Zhang X. Serum IFN-gamma-inducible chemokines CXCL9 and CXCL10 are elevated in non-immediate drug hypersensitivity reactions. Asian Pac J Allergy Immunol. (2016) 34:236–41.
162. Schaerli P, Britschgi M, Keller M, Steiner UC, Steinmann LS, Moser B, et al. Characterization of human T cells that regulate neutrophilic skin inflammation. J Immunol. (2004) 173:2151–8. doi: 10.4049/jimmunol.173.3.2151
163. Padial MA, Alvarez-Ferreira J, Tapia B, Blanco R, Manas C, Blanca M, et al. Acute generalized exanthematous pustulosis associated with pseudoephedrine. Br J Dermatol. (2004) 150:139–42. doi: 10.1111/j.1365-2133.2004.05717.x
164. Kabashima R, Sugita K, Sawada Y, Hino R, Nakamura M, Tokura Y. Increased circulating Th17 frequencies and serum IL-22 levels in patients with acute generalized exanthematous pustulosis. J Eur Acad Dermatol Venereol. (2011) 25:485–8. doi: 10.1111/j.1468-3083.2010.03771.x
165. Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. (2006) 117:455–62. doi: 10.1016/j.jaci.2005.10.030
166. Ogawa K, Morito H, Hasegawa A, Daikoku N, Miyagawa F, Okazaki A, et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS). J Dermatol Sci. (2013) 69:38–43. doi: 10.1016/j.jdermsci.2012.10.002
167. Chung WH, Hung SI. Recent advances in the genetics and immunology of Stevens-Johnson syndrome and toxic epidermal necrosis. J Dermatol Sci. (2012) 66:190–6. doi: 10.1016/j.jdermsci.2012.04.002
168. Su SC, Mockenhaupt M, Wolkenstein P, Dunant A, Le Gouvello S, Chen CB, et al. Interleukin-15 is associated with severity and mortality in Stevens-Johnson syndrome/toxic epidermal necrolysis. J Invest Dermatol. (2017) 137:1065–73. doi: 10.1016/j.jid.2016.11.034
169. Norcross MA, Luo S, Lu L, Boyne MT, Gomarteli M, Rennels AD, et al. Abacavir induces loading of novel self-peptides into HLA-B*57: 01: an autoimmune model for HLA-associated drug hypersensitivity. AIDS. (2012) 26:F21–9. doi: 10.1097/QAD.0b013e328355fe8f
170. Thomson PJ, Illing PT, Farrell J, Alhaidari M, Bell CC, Berry N, et al. Modification of the cyclopropyl moiety of abacavir provides insight into the structure activity relationship between HLA-B*57:01 binding and T-cell activation. Allergy. (2020) 75:636–47. doi: 10.1111/all.14057
171. De Spiegelaere W, Philippe J, Vervisch K, Verhofstede C, Malatinkova E, Kiselinova M, et al. Comparison of methods for in-house screening of HLA-B*57:01 to prevent abacavir hypersensitivity in HIV-1 care. PLoS One. (2015) 10:e0123525. doi: 10.1371/journal.pone.0123525
172. Shen M, Lim JME, Chia C, Ren EC. CD39(+) regulatory T cells modulate the immune response to carbamazepine in HLA-B*15:02 carriers. Immunobiology. (2020) 225:151868. doi: 10.1016/j.imbio.2019.11.003
173. Zhou P, Zhang S, Wang Y, Yang C, Huang J. Structural modeling of HLA-B*1502/peptide/carbamazepine/T-cell receptor complex architecture: implication for the molecular mechanism of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis. J Biomol Struct Dyn. (2016) 34:1806–17. doi: 10.1080/07391102.2015.1092476
174. Cheng L, Sun B, Xiong Y, Hu L, Gao L, Li J, et al. WGCNA-based DNA methylation profiling analysis on allopurinol-induced severe cutaneous adverse reactions: a DNA methylation signature for predisposing drug hypersensitivity. J Pers Med. (2022) 12:1–13. doi: 10.3390/jpm12040525
175. Yun J, Mattsson J, Schnyder K, Fontana S, Largiader CR, Pichler WJ, et al. Allopurinol hypersensitivity is primarily mediated by dose-dependent oxypurinol-specific T cell response. Clin Exp Allergy. (2013) 43:1246–55. doi: 10.1111/cea.12184
176. Ogese MO, Lister A, Gardner J, Meng X, Alfirevic A, Pirmohamed M, et al. Deciphering adverse drug reactions: in vitro priming and characterization of vancomycin-specific T cells from healthy donors expressing HLA-A*32:01. Toxicol Sci. (2021) 183:139–53. doi: 10.1093/toxsci/kfab084
177. Kongpan T, Mahasirimongkol S, Konyoung P, Kanjanawart S, Chumworathayi P, Wichukchinda N, et al. Candidate HLA genes for prediction of co-trimoxazole-induced severe cutaneous reactions. Pharmacogenet Genomics. (2015) 25:402–11. doi: 10.1097/FPC.0000000000000153
178. Pratoomwun J, Thomson P, Jaruthamsophon K, Tiyasirichokchai R, Jinda P, Rerkpattanapipat T, et al. Characterization of T-Cell Responses to SMX and SMX-NO in Co-Trimoxazole Hypersensitivity Patients Expressing HLA-B*13:01. Front Immunol. (2021) 12:658593. doi: 10.3389/fimmu.2021.658593
179. Zhao Q, Alhilali K, Alzahrani A, Almutairi M, Amjad J, Liu H, et al. Dapsone- and nitroso dapsone-specific activation of T cells from hypersensitive patients expressing the risk allele HLA-B*13:01. Allergy. (2019) 74:1533–48. doi: 10.1111/all.13769
180. Chen WT, Wang CW, Lu CW, Chen CB, Lee HE, Hung SI, et al. The function of HLA-B*13:01 involved in the pathomechanism of dapsone-induced severe cutaneous adverse reactions. J Invest Dermatol. (2018) 138:1546–54. doi: 10.1016/j.jid.2018.02.004
181. Niihara H, Kakamu T, Fujita Y, Kaneko S, Morita E. HLA-A31 strongly associates with carbamazepine-induced adverse drug reactions but not with carbamazepine-induced lymphocyte proliferation in a Japanese population. J Dermatol. (2012) 39:594–601. doi: 10.1111/j.1346-8138.2011.01457.x
182. Nicoletti P, Aithal GP, Chamberlain TC, Coulthard S, Alshabeeb M, Grove JI, et al. Drug-induced liver injury due to flucloxacillin: relevance of multiple human leukocyte antigen alleles. Clin Pharmacol Ther. (2019) 106:245–53. doi: 10.1002/cpt.1375
183. Puig M, Ananthula S, Venna R, Kumar Polumuri S, Mattson E, Walker LM, et al. Alterations in the HLA-B*57:01 immunopeptidome by flucloxacillin and immunogenicity of drug-haptenated peptides. Front Immunol. (2020) 11:629399. doi: 10.3389/fimmu.2020.629399
184. Hirasawa M, Hagihara K, Abe K, Ando O, Hirayama N. Interaction of nevirapine with the peptide binding groove of HLA-DRB1*01:01 and its effect on the conformation of HLA-peptide complex. Int J Mol Sci. (2018) 19:1660. doi: 10.3390/ijms19061660
185. Chen CB, Chen YE, Chu MT, Wang CW, Hui RC, Lu CW, et al. The risk of anti-osteoporotic agent-induced severe cutaneous adverse drug reactions and their association with HLA. J Eur Acad Dermatol Venereol. (2021) 35:712–20. doi: 10.1111/jdv.16924
186. Steiner R, King M, Byrne D, Rose L. Inappropriate use of aztreonam. Am J Ther. (2021) 28:e14–8. doi: 10.1097/MJT.0000000000001058
187. Ariza A, Mayorga C, Fernandez TD, Barbero N, Martin-Serrano A, Perez-Sala D, et al. Hypersensitivity reactions to beta-lactams: relevance of hapten-protein conjugates. J Investig Allergol Clin Immunol. (2015) 25:12–25.
188. Muller P, Dubreil P, Mahé A, Lamaury I, Salzer B, Deloumeaux J, et al. Drug hypersensitivity syndrome in a west-Indian population. Eur J Dermatol. (2003) 13:478–81.
189. Chiou CC, Yang LC, Hung SI, Chang YC, Kuo TT, Ho HC, et al. Clinicopathological features and prognosis of drug rash with eosinophilia and systemic symptoms: a study of 30 cases in Taiwan. J Eur Acad Dermatol Venereol. (2008) 22:1044–9. doi: 10.1111/j.1468-3083.2008.02585.x
190. Hung SI, Chung WH, Liou LB, Chu CC, Lin M, Huang HP, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci USA. (2005) 102:4134–9. doi: 10.1073/pnas.0409500102
191. Zhang FR, Liu H, Irwanto A, Fu XA, Li Y, Yu GQ, et al. HLA-B*13:01 and the dapsone hypersensitivity syndrome. N Engl J Med. (2013) 369:1620–8.
192. Nakkam N, Gibson A, Mouhtouris E, Konvinse KC, Holmes NE, Chua KY, et al. Cross-reactivity between vancomycin, teicoplanin and telavancin in HLA-A*32:01 positive vancomycin DRESS patients sharing an HLA-Class II haplotype. J Allergy Clin Immunol. (2020) 147:403–5. doi: 10.1016/j.jaci.2020.04.056
193. Romano A, Gaeta F, Valluzzi RL, Maggioletti M, Caruso C, Quaratino D. Cross-reactivity and tolerability of aztreonam and cephalosporins in subjects with a T cell-mediated hypersensitivity to penicillins. J Allergy Clin Immunol. (2016) 138:179–86. doi: 10.1016/j.jaci.2016.01.025
194. Buonomo A, Nucera E, Pecora V, Rizzi A, Aruanno A, Pascolini L, et al. Cross-reactivity and tolerability of cephalosporins in patients with cell-mediated allergy to penicillins. J Investig Allergol Clin Immunol. (2014) 24:331–7.
195. Prasertvit P, Chareonyingwattana A, Wattanakrai P. Nevirapine patch testing in Thai human immunodeficiency virus infected patients with nevirapine drug hypersensitivity. Contact Dermatitis. (2017) 77:379–84. doi: 10.1111/cod.12849
196. Ye YM, Hur GY, Kim SH, Ban GY, Jee YK, Naisbitt DJ, et al. Drug-specific CD4(+) T-cell immune responses are responsible for antituberculosis drug-induced maculopapular exanthema and drug reaction with eosinophilia and systemic symptoms syndrome. Br J Dermatol. (2017) 176:378–86. doi: 10.1111/bjd.14839
197. Meller S, Gerber PA, Kislat A, Hevezi P, Göbel T, Wiesner U, et al. Allergic sensitization to pegylated interferon-alpha results in drug eruptions. Allergy. (2015) 70:775–83. doi: 10.1111/all.12618
198. Xiong H, Chen S, Luo X. Salazosulphapyridine-related Stevens-Johnson Syndrome Caused by Sulphapyridine and Confirmed by Enzyme-linked Immunospot Assay. J Crohns Colitis. (2018) 12:381–2. doi: 10.1093/ecco-jcc/jjx148
199. Trubiano JA, Redwood A, Strautins K, Pavlos R, Woolnough E, Chang CC, et al. Drug-specific upregulation of CD137 on CD8+ T cells aids in the diagnosis of multiple antibiotic toxic epidermal necrolysis. J Allergy Clin Immunol Pract. (2017) 5:823–6. doi: 10.1016/j.jaip.2016.09.043
200. Kato K, Kawase A, Azukizawa H, Hanafusa T, Nakagawa Y, Murota H, et al. Novel interferon-gamma enzyme-linked immunoSpot assay using activated cells for identifying hypersensitivity-inducing drug culprits. J Dermatol Sci. (2017) 86:222–9. doi: 10.1016/j.jdermsci.2017.03.007
201. Klaewsongkram J, Thantiworasit P, Suthumchai N, Rerknimitr P, Sukasem C, Tuchinda P, et al. In vitro test to confirm diagnosis of allopurinol-induced severe cutaneous adverse reactions. Br J Dermatol. (2016) 175:994–1002. doi: 10.1111/bjd.14701
202. Lucas A, Lucas M, Strhyn A, Keane NM, McKinnon E, Pavlos R, et al. Abacavir-reactive memory T cells are present in drug naive individuals. PLoS One. (2015) 10:e0117160. doi: 10.1371/journal.pone.0117160
203. Keane NM, Pavlos RK, McKinnon E, Lucas A, Rive C, Blyth CC, et al. HLA Class I restricted CD8+ and Class II restricted CD4+ T cells are implicated in the pathogenesis of nevirapine hypersensitivity. AIDS. (2014) 28:1891–901. doi: 10.1097/QAD.0000000000000345
204. Tanvarasethee B, Buranapraditkun S, Klaewsongkram J. The potential of using enzyme-linked immunospot to diagnose cephalosporin-induced maculopapular exanthems. Acta Derm Venereol. (2013) 93:66–9. doi: 10.2340/00015555-1386
205. Phatharacharukul P, Klaewsongkram J. A case of sulfasalazine-induced hypersensitivity syndrome confirmed by enzyme-linked immunospot assay. Allergy Asthma Immunol Res. (2013) 5:415–7. doi: 10.4168/aair.2013.5.6.415
206. Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. (2002) 359:727–32. doi: 10.1016/S0140-6736(02)07873-X
207. Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). (2012) 64:1431–46. doi: 10.1002/acr.21772
Keywords: drug allergy, in vivo, ex vivo, diagnostic tools, delayed hypersensitivity reaction, drug reaction with eosinophilia and systemic symptoms (DRESS), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), severe cutaneous adverse reactions (SCARs)
Citation: Copaescu AM, Ben-Shoshan M and Trubiano JA (2022) Tools to improve the diagnosis and management of T-cell mediated adverse drug reactions. Front. Med. 9:923991. doi: 10.3389/fmed.2022.923991
Received: 20 April 2022; Accepted: 26 August 2022;
Published: 13 October 2022.
Edited by:
Elizabeth Phillips, Vanderbilt University, United StatesReviewed by:
Claudia Zeidler, University Hospital Münster, GermanyCopyright © 2022 Copaescu, Ben-Shoshan and Trubiano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana Maria Copaescu, YW5hLmNvcGFlc2N1QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.