- 1Department of Radiology, Suining Central Hospital, Suining, China
- 2Department of Radiology, The First Hospital of Suining, Suining, China
- 3Department of Radiology, The Third Hospital of Mianyang and Sichuan Mental Health Center, Mianyang, China
- 4Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 5Department of Gastroenterology, The First Hospital of Suining, Suining, China
- 6Sichuan Key Laboratory of Medical Imaging, Department of Radiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 7Department of Radiology and Imaging, Institute of Rehabilitation and Development of Brain Function, The Second Clinical Medical College of North Sichuan Medical College, Nanchong Central Hospital, Nanchong, China
Radiomics involves high-throughput extraction and analysis of quantitative information from medical images. Since it was proposed in 2012, there are some publications on the application of radiomics for (1) predicting recurrent acute pancreatitis (RAP), clinical severity of acute pancreatitis (AP), and extrapancreatic necrosis in AP; (2) differentiating mass-forming chronic pancreatitis (MFCP) from pancreatic ductal adenocarcinoma (PDAC), focal autoimmune pancreatitis (AIP) from PDAC, and functional abdominal pain (functional gastrointestinal diseases) from RAP and chronic pancreatitis (CP); and (3) identifying CP and normal pancreas, and CP risk factors and complications. In this review, we aim to systematically summarize the applications and progress of radiomics in pancreatitis and it associated situations, so as to provide reference for related research.
Introduction
Radiomics and Its Process
Inspired by the knowledge systems and research fields of such as genomics, proteomics, radiogenomics, etc., Lambin et al. first proposed the concept of radiomics in 2012 (1–6). Radiomics refers to high-throughput extraction and analysis of a large number of advanced quantitative imaging features from medical images obtained by computed tomography (CT), magnetic resonance imaging (MRI) or positron emission tomography (PET) (2). The workflow of radiomics mainly includes the following steps (1–6). (1) Image acquisition is the first step of radiomics. The images may come from CT, MRI, PET, as well as X-ray radiography and ultrasonography (US), etc. (7–10). Because the distribution of images features may be affected by many factors such as equipment vendors, scanning protocols, imaging parameters, reconstruction algorithms, etc., it is of great importance to establish standards and consensus imaging protocols. (2) Image segmentation uses dedicated software to draw two dimensions (2-D) or three dimensions (3-D) of regions of interest (ROIs) of lesions or organs by means of manual, semi-automatic, or automatic segmentations.
(3) Image preprocessing is to homogenize the data before extracting radiomics features which mainly includes two methods: image resampling and gray-level discretization (4). Features extraction uses dedicated software or software packages to extract morphological features, first-order statistical features, second-order statistical features, and high-order statistical features from 2-D or 3-D ROIs after segmentation. Morphological features (n = 16) are used to describe the 3-D shape and size of a ROI including asphericity, compactness, maximum diameter, sphericity, surface area, surface to volume ratio, volume, etc. The first-order statistical feature (n = 18) represents the histogram of voxel intensity values contained within a ROI to include mean, median, maximum, minimum, standard deviation, percentile, skewness, kurtosis, uniformity, energy, entropy, etc. Second order statistical features are used to describe the spatial distribution of voxel intensities within a ROI to include gray-level co-occurrence matrix (GLCM), gray-level run-length matrix (GLRLM), gray-level size-zone matrix (GLSZM), gray-level distance-zone matrix (GLDZM), neighborhood gray tone difference matrix (NGTDM), and neighboring gray level dependence (NGLDM). After applying filters or mathematical transformations to the images, the higher-order statistics features can be obtained (5). Feature selection is the process of removing redundant features and selecting the most relevant features according to specific research tasks. Common methods are univariate analysis, logistic regression analysis, least absolute shrinkage and selection operator (LASSO), minimum redundancy maximum relevance (MRMR), etc. (6). Modelization and validation is after a classification or prediction model is established, it needs to be tested internally and externally to evaluate the robustness and repeatability of the model.
In recent years, due to the progress and rapid developments of various hardware and software technologies, radiomics has gradually developed into a relatively mature discipline or medical image analysis method (1). There are more and more publications on the application of radiomics for the diagnosis, differential diagnosis, treatment options, and prognosis evaluation of many human diseases (11–16). Among them, Hong et al. (13) extracted 10 radiomics features from the contrast-enhanced CT (CECT) images of 241 patients with a bone island or osteoblastic metastasis to establish a random forest (RF) prediction model. The results showed that the RF model based on CT was helpful to differentiate bone islands from osteoblastic metastases, and its diagnostic performance was higher than that of inexperienced radiologists but equivalent to that of experienced radiologists. In another study, Tian et al. (16) reported the diagnostic value of preoperative evaluation of microvascular invasion of solitary small hepatocellular carcinoma (HCC) based on nomogram of gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB-DTPA) enhanced MRI. The results indicated that the clinical-radiological-radiomics model achieved the highest diagnostic performance with area under the receiver operating characteristic curves (AUCs) of 0.934, 0.889 and 0.875 for the training, internal and external validation sets, respectively.
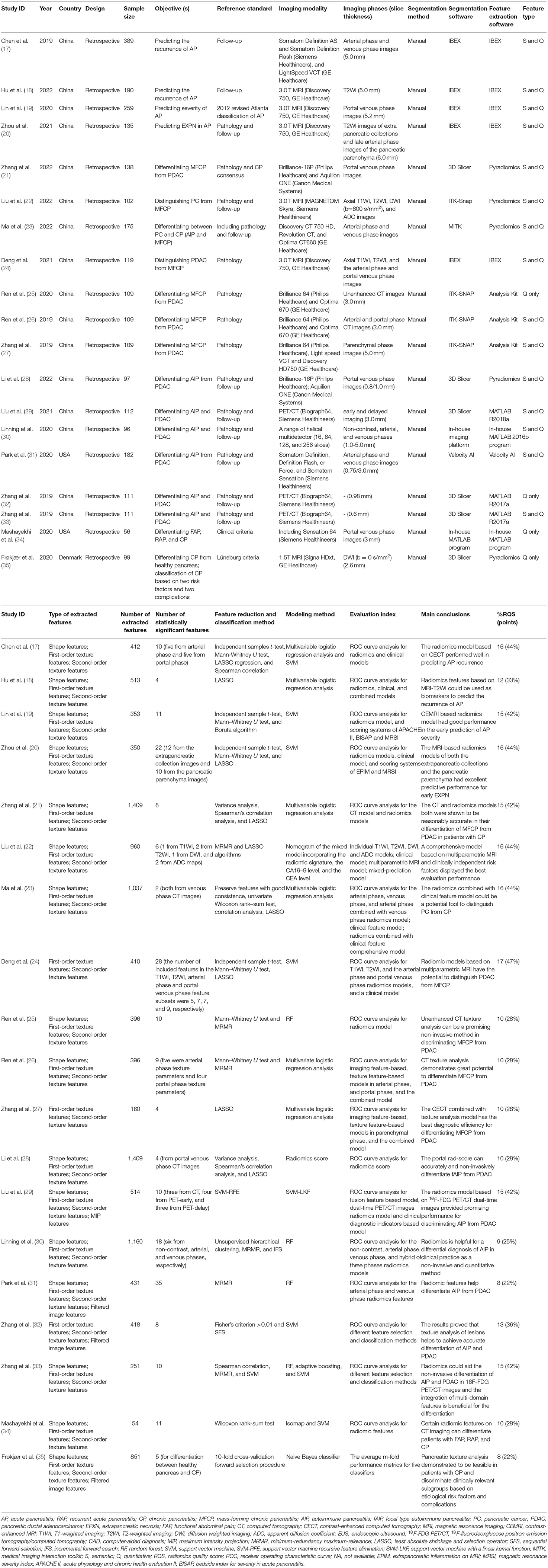
In this review, we aim to systematically summarize the applications and progress of radiomics in pancreatitis and associated situations (Table 1) so as to provide reference for related research.
Clinical Applications
Predicting Recurrent Acute Pancreatitis
Acute pancreatitis (AP) is a common disease in clinical practice and meta-analysis showed that the annual incidence rate of AP in the world is about 33.74/100 000, along with an annual mortality rate of about 1.16/100 000 (36). With the increase in population aging, biliary calculus, hyperlipidemia, obesity, and many other AP risk factors, the incidence of AP is also gradually increasing (37–39). Recurrent acute pancreatitis (RAP) is a special type of pancreatitis, and it is different from AP and chronic pancreatitis (CP). The definition of RAP is that patients should experience at least two separate episodes of AP at least 3 months apart, and there are no abnormities in pancreatic tissue structure or function in remission (40). It is reported that the recurrence rate of AP is about 10–30% (17). About 10% of patients with first-episode of AP and 36% of patients with RAP may progress to CP, and the risk is higher among men, smokers, and alcoholics (41). Another study also reported that CP may increase the risk of pancreatic cancer (PC) in patients (42). After 5 and 9 years of the diagnosis of CP, the risk of PC in CP patients increased by eight times and three times, respectively. Therefore, early prediction of RAP and appropriate management measures can not only decrease the recurrence of AP, but it also prevents or delays its progression to CP and even PC.
Chen et al. (17) included 389 first-episode AP patients. On the CT images of arterial and venous phases, 412 radiomics features were extracted from the ROIs of the whole pancreatic parenchyma, and 10 features were finally selected to establish the prediction model. In the training cohort (n = 271, including 145 patients with AP and 126 patients with RAP), the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy and AUC of the radiomics model in predicting patients with RAP were 86.7%, 87.6%, 89.7%, 84.1%, 87.1%, and 0.941%, respectively. In the validation cohort (n = 118, including 63 patients with AP and 55 patients with RAP), the same diagnostic indexes of the radiomics model in predicting patients with RAP were 83.8%, 97.7%, 98.4%, 78.2%, 89.0%, and 0.929%, respectively. The results in the training and validation cohorts were all significantly higher than those of the clinical model (all P-values < 0.05).
Quantitative investigation on predicting RAP is still in a paucity at present. Previous studies mostly focused on the risk factors of RAP after the first attack of AP such as demography (like gender, age, etc.), and clinical characteristics (like etiology, local complications, etc.) (43–45). Chen et al. (17) first showed that the radiomics model based on CECT exhibits promising value in the early prediction of RAP. In another similar study, Hu et al. (18) constructed a multivariate logistic regression radiomics model, radiomics, and clinical characteristics combined model based on MRI-T2WI, and their results were consistent with those of Chen et al. (17).
Predicting Clinical Severity of AP
Based on the 2012 revised Atlanta classification and definition (2012-RACD) by international consensus, AP can be divided into three categories stratified by its clinical severity: mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP) (46). MAP is characterized by no organ failure and local or systemic complications. It can return to normal within 1–2 weeks. Usually, there is no need for an imaging examination of the pancreas, and the mortality rate is very low. MSAP is characterized by transient organ failure (<48 h), or accompanied by local or systemic complications, while no persistent organ failure (more than 48 h) exists. MSAP can be cured without intervention or may require long-term specialist care. The mortality rate of MSAP is much lower than that of SAP. SAP is characterized by persistent single or multiple organ failure (more than 48 h). Patients with persistent organ failure usually have one or more local complications. In the first few days after AP onset, patients with persistent organ failure have an increased risk of death, and the mortality reported in the literature is as high as 36–50% (46), and the mortality rate of patients with persistent organ failure complicated with infectious necrosis is very high (46). Therefore, early prediction of the clinical severity of AP is of utmost importance, which is not only good for the early diagnosis and treatment of MSAP and SAP patients, and also in favor of the early diversion or referral of MSAP and SAP patients.
Currently, methods of early predicting the clinical severity of AP mainly depend on clinical characteristics [such as scoring systems of acute physiology and chronic health evaluation II (APACHE II, ≥eight points), bedside index for severity in acute pancreatitis (BISAP, ≥three points), Ranson (≥three points) and modified Marshall score (≥two points)], laboratory tests [such as C-reactive protein concentration (≥150 mg/l), serum procalcitonin (>0.5 ng/ml), interleukin-6 (>50 pg/l) and neutrophil/lymphocyte ratio (>10)] as well as findings on imaging examinations [such as computed tomography severity index (CTSI, ≥four points), modified computed tomography severity index (mCTSI, ≥four points), and extrapancreatic inflammation on computed tomography (EPIC, ≥four points)] (47–50).
Lin et al. (19) first reported a contrast-enhanced MRI (CEMRI) based radiomics model to predict the clinical severity of AP (MAP vs. MSAP and SAP). In their study, they included 259 AP patients into the training (n = 180, with 99 MAP and 81 MSAP and SAP patients) and validation cohorts (n = 79, with 43 MAP and 36 MSAP and SAP patients). From the portal vein phase images, Lin et al. (19) extracted 353 radiomics features from the ROIs that contained the whole pancreatic parenchyma, and finally they selected 11 features to establish the support vector machine (SVM) model. In the training cohort, the sensitivity, specificity, PPV, NPV, accuracy, and AUC of the radiomics model to distinguish MAP from MSAP or SAP patients were 77.8%, 91.9%, 88.7%, 83.5%, 85.6%, and 0.917%, respectively. In the validation cohort, the corresponding diagnostic indexes of the radiomics model in distinguishing MAP from MSAP or SAP patients were 75.0%, 86.0%, 81.8%, 80.4%, 81.0%, and 0.848%, respectively. The both AUCs were significantly higher than that of APACHE II, BISAP, and MRSI scoring systems (all P-values were < 0.05). This study showed that when compared with some existing clinical and radiological scoring systems, the portal phase MRI radiomics model may be more accurate in early predicting the clinical severity of AP.
Predicting Extrapancreatic Necrosis in AP
Based on the 2012-RACD (46), AP can be divided into two categories according to its morphological manifestations on imaging examination: (1) interstitial edematous pancreatitis (IEP; about 85%); and (2) necrotizing pancreatitis (NP; about 15%). Based on the distribution and location of necrosis, NP can be further subdivided into three subtypes (46): (1) combined pancreatic and peripancreatic necrosis (about 75.0%); (2) peripancreatic necrosis only (about 20.0%); and (3) pancreatic necrosis only (about 5.0%). The literature indicates that compared with NP, the mortality rate of IEP is about 3.0% while the mortality rate of NP is about 17%; and if combined with infection, the mortality rate of NP can rise to about 30% (46, 51). Consequently, it is of great clinical significance to distinguish IEP from NP for predicting the prognosis of AP patients. In the international structured reporting template of AP based on CECT published in 2020, experts also highlighted the importance of radiologists to clarify the morphologic subtypes of AP, the degree and anatomic area involvement of NP, the type and location of peripancreatic collections, and some other key points in the CT reports (51).
Zhou et al. (20) used an MRI based radiomics model to predict early extrapancreatic necrosis (EXPN) in patients with AP. They enrolled 135 AP patients who were divided into the training (n = 94, with 47 EXPN and 47 APFC patients) and validation cohorts (n = 41, with 20 EXPN and 21 APFC patients). On the T2WI and late arterial phase images, Zhou et al. (20) extracted 350 image radiomics features from ROI of the peripancreatic collections (T2WI) and entire pancreatic parenchyma (late arterial phase). After dimension reduction and feature selection, 22 features (12 from the T2WI and 10 from the late arterial phase images) were selected for establishing SVM model. In the training cohort, the sensitivity, specificity, PPV, NPV, accuracy, and AUC of the T2WI peripancreatic collections and late arterial phase pancreatic parenchyma radiomics models for predicting EXPN were 97.9% and 87.2%, 85.1% and 87.2%, 86.8% and 87.2%, 97.6% and 87.2%, 91.5% and 87.2%, 0.969% and 0.931%, respectively. In the validation cohort, the corresponding diagnostic parameters of the T2WI peripancreatic collections and late arterial phase pancreatic parenchyma radiomics models for predicting EXPN were 95.0% and 75.0%, 90.5% and 90.5%, 90.5% and 88.2%, 95.0% and 79.2%, 92.7% and 82.9%, 0.976 and 0.921%, respectively. Both of the AUCs were significantly higher than those of clinical model, EPIM and MRSI scoring systems (all P-values < 0.05). This investigation showed that when compared with some existing clinical model and radiological scoring systems, the MRI radiomics model based on T2WI peripancreatic collections and late arterial phase pancreatic parenchyma may be able to accurately predict EXPN in AP patients at an early stage.
Differentiating Mass-Forming Chronic Pancreatitis From Pancreatic Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PDAC) is a malignant tumor that originating from pancreatic ductal epithelial cells, accounting for about 80–90% of all the pancreatic cancer (PC) patients with about 60–70% of the PDACs occur in the pancreatic heads (52, 53). The prognosis of PDAC is very poor (<10%) and surgery has always been considered the first choice for the treatment of PDAC (52, 53). The mass-forming chronic pancreatitis (MFCP) is a special type of CP. Documents reported that MFCP accounts for about 27–50% of CP, and the vast majority of MFCP is located in the pancreatic heads (about 71%) (54–56). MFCP and PDAC share significant overlaps in the clinical manifestations (such as upper abdominal pain, nausea, weight loss, jaundice, diabetes, etc.), risk factors (such as alcohol, smoking, etc.), laboratory tests (such as elevated carbohydrate antigen 199 (CA199) and carcinoembryonic antigen (CEA) levels), and imaging findings (such as delayed enhancement) (57, 58). CT and endoscopic ultrasonography guided fine needle aspiration biopsy (EUS-FNA) can be used to improve the differential diagnosis accuracy of MFCP and PDAC, but both modalities are invasive examinations, which not only have sampling error, and also carry the risks of needle tract tumor seeding, bleeding, pancreatic juice leakage, etc. (59, 60). As a result, it is very difficult to accurately distinguish MFCP from PDAC prior to operation, yet it has very important clinical significance. Because accurate preoperative diagnosis of early PADC can prevent it from being resectable to unresectable, and accurate diagnosis of MFCP can avoid unnecessary surgery.
With the rapid development of medical imaging technologies, radiomics has begun to be used in the differential diagnosis of MFCP and PDAC (21–27). For example, Deng et al. (24) studied 96 patients with PDAC and 23 patients with MFCP. They extracted four sets of radiomics features from T1WI, T2WI, as well as arterial and portal phase images of MRI to establish SVM models. When compared with the clinical model based on clinical characteristics and the evaluation results of two radiologists, the results demonstrated that in the primary cohort (n = 64, with 51 PDAC and 13 MFCP patients), the sensitivity, specificity and AUC of T1WI, T2WI, arterial phase and portal phase radiomics models, and the clinical model were 0.961, 0.769, and 0.893; 0.941, 0.769, and 0.911; 0.961, 0.923, and 0.958; 0.980, 1.000, and 0.997; 0.529, 0.692, and 0.516, respectively. In the testing cohort (n = 55, with 45 PDAC and 10 MFCP patients), the corresponding diagnostic data were 1.000, 0.733, and 0.882; 0.844, 0.900, and 0.902; 0.956, 0.900, and 0.920; 0.978, 0.900, and 0.962; 0.422, 0.900, and 0.649, respectively. There were no significant differences in the diagnostic performances between the four radiomics models (all P-values > 0.05), but they were all better than that of the clinical model and the radiologists' evaluation (all P-values < 0.05). This study demonstrated that radiomics may be used to improve the differential diagnosis accuracy of MFCP and PDAC.
Differentiating Focal Autoimmune Pancreatitis From PDAC
Autoimmune pancreatitis (AIP) is a special type of CP. Yoshida et al. first proposed the concept of AIP in 1995; and the annual incidence rate of AIP is about 3.1/100 000, accounting for about 1.9%-6.6% of CP (61, 62). Pathologically, AIP is classified into two subtypes: (1) Type I, lymphoplasmacytic sclerosing pancreatitis (LPSP); and (2) Type II, idiopathic duct-centric chronic pancreatitis (IDCP) (61, 63). At present, Type I AIP has been considered as the pancreatic manifestation of a systemic disease named IgG4-related disease (IgG4-RD) and there are now dedicated criteria for IgG4-RD and some specific organs (like pancreas, biliary tract, kidney, ophthalmic tissues, and chest) (64–67). On imaging, AIP can be manifested as diffuse AIP and focal AIP, and about 40% of Type I AIP and 85% of Type II AIP are localized (64, 65). Focal AIP overlaps obviously with PDAC in clinical manifestations (such as obstructive jaundice, epigastric pain or discomfort, weight loss, etc.) and imaging findings (focal mass in the pancreas), and an accurate differential diagnosis is very challenging. However, the treatment methods after the establishment of diagnosis are very different because AIP responds well to glucocorticoid drugs while PADC mainly needs comprehensive treatment methods such as surgery, chemotherapy and radiotherapy. Therefore, the accurate differential diagnosis of focal AIP and PDAC before the treatments has very important clinical value. Once focal AIP is misdiagnosed as PDAC, it will lead to unnecessary surgery, and once PDAC is misdiagnosed as focal AIP, it may delay the effective treatments of PDAC.
Radiomics may play a positive role in the differential diagnosis of focal AIP and PDAC (28–33). Among the studies, Linning et al. (30) studied 45 patients with focal AIP and 51 patients with PDAC to evaluate the value of radiomics model based on multi-phase CECT for the differential diagnosis of focal AIP from PDAC. The results showed that the sensitivity, specificity, PPV, NPV and accuracy of unenhanced, arterial phase, portal phase, and hybrid radiomics models were 71.11%, 86.27%, 77.19%, 82.05%, and 79.17%; 82.22%, 90.20%, 85.19%, 88.10%, and 86.46%; 93.33%, 96.08%, 92.00%, 89.13%, and 90.63%; 93.33%, 96.08%, 94.23%, 95.45%, and 94.80%, respectively. The AUCs were 0.827, 0.890, 0.953, and 0.977, respectively. The diagnostic performances were higher than those of the two radiologists (P < 0.05). In another study, Li et al. (28) used propensity score matching (PSM) in 45 patients with focal AIP and 51 patients with PDAC who were matched in gender, age, body mass index (BMI), and CT characteristics. They evaluated the diagnostic performance of radiomics model based on portal phase CECT images in the differential diagnosis of focal AIP and PDAC. Their results were consistent with the research of Linning et al. (30) The above two studies have shown that radiomics may play a positive role in the differential diagnosis of focal AIP and PDAC.
Differentiating Functional Abdominal Pain, RAP, and CP
Abdominal pain is a common clinical symptom and one of the most important reasons for patients to see a doctor. Its etiologies may come from abdominal solid organs, gastrointestinal tract, biliary system, urinary system, reproductive system, chest diseases, or systemic diseases. Because abdominal pain is a non-specific clinical symptom, early identifying the causes of abdominal pain helps the clinicians and patients to choose the appropriate treatment methods. In a study, Mashayekhi et al. (34) studied 19 patients with functional abdominal pain (functional gastrointestinal diseases, FGD), 20 patients with RAP, and 17 patients with CP and explored the value of a SVM classifier based on venous phase images of CECT in distinguishing FGD, RAP, and CP. The results showed that the overall predictive accuracy of the SVM classifier was 82.1%. In the one-to-one comparison of the three groups, the sensitivity, specificity, and AUC of the FGD group were 79%, 100%, and 0.91%, respectively; the same diagnostic parameters of the RAP group were 95%, 78%, and 0.88%, respectively; while the sensitivity, specificity and AUC of the CP group were 71%, 95%, and 0.90%, respectively. The results suggested that some radiomics features may be an effective method for radiologists and gastroenterologists to distinguish FGD, RAP, and CP.
Identifying CP and Normal Pancreas, CP Risk Factors, and Complications
Frøkjær et al. (35) studied 77 CP patients and 22 healthy controls, extracted 851 MRI texture features from diffusion-weighted imaging (DWI) images, and finally constructed five classifier models to address the potential use of MRI texture analysis of the pancreas in CP patients. The five radiomics classifiers were: (1) CP vs. healthy controls (with five selected radiomics features), (2) alcoholic vs. non-alcoholic etiology of CP (with nine selected radiomics features), (3) use of tobacco vs. no use of tobacco (with 10 selected radiomics features), (4) diabetes vs. no diabetes (with four selected radiomics features), and (5) pancreatic exocrine insufficiency vs. normal exocrine function (with three selected radiomics features). The results showed that the sensitivity, specificity, PPV, and accuracy of the above five radiomics classifiers were 0.71–0.97, 0.84–1.00, 0.71–1.00, and 0.82–0.98, respectively. These results implied that radiomics may be a potentially promising tool used to depict early-stage CP and monitor disease progression.
Limitations and Solutions
Since it was proposed in 2012, due to the progress and rapid developments of various hardware and software technologies, radiomics has gradually developed into a relatively mature research field and knowledge system (1–6, 68). The authors performed a literature search in the PubMed database with the strategy of “(Radiomics [Title/Abstract]) OR (Radiomic [Title/Abstract]).” There were no restrictions on the publication time, language or research object. As of April 17, 2022, a total of 5,580 relevant publications were retrieved. This search result has proved the degree of attention paid by researchers and related fields to radiomics in the past 10 years. However, the vast majority of radiomics models reported in the current literature are still in the stage of developing research, and their clinical applications have not really been implemented. The authors believe that this phenomenon is mainly caused by the limitations of radiomics. The current radiomics research and clinical applications still have the following limitations and difficulties (69): (1) the standardization of medical imaging data is insufficient; (2) the generalization ability of the models is not good enough; (3) poor biological interpretability; and (4) the clinical utility of the models needing to be improved.
Standardization of Medical Image Data
Standardized, homogeneous, and high-quality training data is an important cornerstone of radiomics research and clinical applications. Radiomics may refer to the FAIR guiding principles for scientific data management and stewardship that were proposed by the international community named Force 11 (The Future of Research Communications and e-Scholarship 2011) in 2016 (70). This international community emphasizes that scientific data management and stewardship should follow the principles of Findable (F), Accessible (a), Interoperable (I), and Reusable (R).
Generalization of the Models
The performance of a radiomics model in similar and different distribution of datasets (such as various times, treatment plans, geographical locations, etc.) is called the generalization of a radiomics model. That is to say the reproducibility and transferability of a radiomics model (71) which is an important premise for the clinical applications of radiomics. It is also an important problem that needs to be solved urgently in radiomics (72, 73). In addition to increasing the data sample size and data diversity, full-automatic and semi-automatic image segmentation methods need to be advocated, and reasonable features selection and dimensionality reduction methods also need to be adopted (69, 74). Federated machine learning is also expected to provide effective solutions to the above difficulties (75, 76).
Biological Interpretability
Radiomics researchers hope to explore the relationships between certain features and some diseases or clinical endpoints (such as the diagnosis and differential diagnosis of diseases, options of treatment schemes, predictions of treatment effects, pathological classification and grading, gene and protein phenotypes, etc.) by quantitatively extracting and analyzing image information (features) that cannot be recognized by human naked eye. This will provide more help for clinicians and patients for disease diagnosis and treatments. However, the biological interpretability of radiomics is still lacking, and the potential biological significance of each features is still unclear, which seriously hinders its clinical applications (77–79). Therefore, how can we improve the biological interpretability of radiomics is an important problem to be faced in this field.
Clinical Utility
Radiomics models or systems with characteristics of easy to operate, short learning curve, good user experience, fast running speed, and broad use scenarios are often more in line with clinicians' work habits (80). Applications developed for mobile phones and internet users may become an effective carrier for the clinical applications of radiomics models or systems in the future.
Conclusions and Future Perspectives
Since it was proposed in 2012, radiomics has begun to demonstrate a promising potential both in scientific research and in clinical applications, such as predicting RAP, clinical severity of AP and EXPN of AP, and differentiating MFCP and focal AIP from PDAC (Table 1). However, most of the published studies hold the limitations of a single-center, retrospective, limited sample size, and low radiomics quality score (RQS) (4). In looking forward to the future, researchers may successively report some multicenter, prospective, large sample size, and high RQS studies. In addition to these, predicting AP clinical outcomes of organ failure, infection, death, hospitalization, admission to intensive care unit (ICU) and invasive intervention; quantifying pancreatic exocrine or (and) endocrine insufficiency; predicting the possibility of AP progress to CP or CP progress to PC; and effectively combining deep learning or some other technologies with radiomics may become the potential directions (81–87).
Author Contributions
GaowuY, GaoweY, HLi, HLia, and CP designed the study and the drafting of the paper. GaowuY, GuY, YL, and YML revised the paper critically for intellectual content. All authors participated in the literature search and data collection. All authors approved the final version of the paper to be published.
Funding
This project was supported by grants from the Sichuan Provincial Commission of Health (Grant Nos. 18PJ138, 19PJ283, 19PJ284, and 20PJ284), Sichuan Provincial Department of Science and Technology (Grant No. 2019YFQ0028), and Science and Technology Association of Suining City (Grant Nos. 6 and 10).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank co-authors AB and MM for their help in proofreading the article.
References
1. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. (2012) 48:441–6. doi: 10.1016/j.ejca.2011.11.036
2. Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, et al. Radiomics: the process and the challenges. Magn Reson Imaging. (2012) 30:1234–48. doi: 10.1016/j.mri.2012.06.010
3. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures. They are data. Radiology. (2016) 278:563–77. doi: 10.1148/radiol.2015151169
4. Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. (2017) 14:749–62. doi: 10.1038/nrclinonc.2017.141
5. Bartoli M, Barat M, Dohan A, Gaujoux S, Coriat R, Hoeffel C, et al. CT and MRI of pancreatic tumors: an update in the era of radiomics. Jpn J Radiol. (2020) 38:1111–24. doi: 10.1007/s11604-020-01057-6
6. Shur JD, Doran SJ, Kumar S, Ap Dafydd D, Downey K, O'Connor JPB, et al. Radiomics in oncology: a practical guide. Radiographics. (2021) 41:1717–32. doi: 10.1148/rg.2021210037
7. Siviengphanom S, Gandomkar Z, Lewis SJ, Brennan PC. Mammography-based radiomics in breast cancer: a scoping review of current knowledge and future needs. Acad Radiol. 2021:S1076–6332(21)00468-2. doi: 10.1016/j.acra.2021.09.025
8. Hu Z, Yang Z, Lafata KJ, Yin FF, Wang C. A radiomics-boosted deep-learning model for COVID-19 and non-COVID-19 pneumonia classification using chest x-ray images. Med Phys. (2022) 49:3213–22. doi: 10.1002/mp.15582
9. Li MD, Cheng MQ, Chen LD, Hu HT, Zhang JC, Ruan SM, et al. Reproducibility of radiomics features from ultrasound images: influence of image acquisition and processing. Eur Radiol. (2022). doi: 10.1007/s00330-022-08662-1. [Epub ahead of print].
10. Li C, Qiao G, Li J, Qi L, Wei X, Zhang T, et al. An Ultrasonic-based radiomics nomogram for distinguishing between benign and malignant solid renal masses. Front Oncol. (2022) 12:847805. doi: 10.3389/fonc.2022.847805
11. Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. Development and validation of a radiomics nomogram for preoperative prediction of lymph node metastasis in colorectal Cancer. J Clin Oncol. (2016) 34:2157–64. doi: 10.1200/JCO.2015.65.9128
12. Rigiroli F, Hoye J, Lerebours R, Lafata KJ, Li C, Meyer M, et al. CT Radiomic features of superior mesenteric artery involvement in pancreatic ductal adenocarcinoma: a pilot study. Radiology. (2021) 301:610–22. doi: 10.1148/radiol.2021210699
13. Hong JH, Jung JY, Jo A, Nam Y, Pak S, Lee SY, et al. Development and validation of a radiomics model for differentiating bone islands and osteoblastic bone metastases at abdominal CT. Radiology. (2021) 299:626–32. doi: 10.1148/radiol.2021203783
14. Guo W, She D, Xing Z, Lin X, Wang F, Song Y, et al. Multiparametric MRI-based radiomics model for predicting H3 K27M mutant status in diffuse midline glioma: a comparative study across different sequences and machine learning techniques. Front Oncol. (2022) 12:796583. doi: 10.3389/fonc.2022.796583
15. Jimenez JE, Abdelhafez A, Mittendorf EA, Elshafeey N, Yung JP, Litton JK, et al. A model combining pretreatment MRI radiomic features and tumor-infiltrating lymphocytes to predict response to neoadjuvant systemic therapy in triple-negative breast cancer. Eur J Radiol. (2022) 149:110220. doi: 10.1016/j.ejrad.2022.110220
16. Tian Y, Hua H, Peng Q, Zhang Z, Wang X, Han J, et al. Preoperative evaluation of Gd-EOB-DTPA-enhanced MRI radiomics-based nomogram in small solitary hepatocellular carcinoma (≤ 3.0 cm) with microvascular invasion: a two-center study. J Magn Reson Imaging. (2022). doi: 10.1002/jmri.28157. [Epub ahead of print].
17. Chen Y, Chen TW, Wu CQ, Lin Q, Hu R, Xie CL, et al. Radiomics model of contrast-enhanced computed tomography for predicting the recurrence of acute pancreatitis. Eur Radiol. (2019) 29:4408–17. doi: 10.1007/s00330-018-5824-1
18. Hu Y, Liu N, Tang L, Liu Q, Pan K, Lei L, et al. Three-dimensional radiomics features of magnetic resonance T2-weighted imaging combined with clinical characteristics to predict the recurrence of acute pancreatitis. Front Med. (2022) 9:777368. doi: 10.3389/fmed.2022.777368
19. Lin Q, Ji YF, Chen Y, Sun H, Yang DD, Chen AL, et al. Radiomics model of contrast-enhanced MRI for early prediction of acute pancreatitis severity. J Magn Reson Imaging. (2020) 51:397–406. doi: 10.1002/jmri.26798
20. Zhou T, Xie CL, Chen Y, Deng Y, Wu JL, Liang R, et al. Magnetic resonance imaging-based radiomics models to predict early extra pancreatic necrosis in acute pancreatitis. Pancreas. (2021) 50:1368–75. doi: 10.1097/MPA.0000000000001935
21. Zhang H, Meng Y, Li Q, Yu J, Liu F, Fang X, et al. Two nomograms for differentiating mass-forming chronic pancreatitis from pancreatic ductal adenocarcinoma in patients with chronic pancreatitis. Eur Radiol. (2022). doi: 10.1007/s00330-022-08698-3. [Epub ahead of print].
22. Liu J, Hu L, Zhou B, Wu C, Cheng Y. Development and validation of a novel model incorporating MRI-based radiomics signature with clinical biomarkers for distinguishing pancreatic carcinoma from mass-forming chronic pancreatitis. Transl Oncol. (2022) 18:101357. doi: 10.1016/j.tranon.2022.101357
23. Ma X, Wang YR, Zhuo LY, Yin XP, Ren JL, Li CY, et al. Retrospective analysis of the value of enhanced CT radiomics analysis in the differential diagnosis between pancreatic cancer and chronic pancreatitis. Int J Gen Med. (2022) 15:233–41. doi: 10.2147/IJGM.S337455
24. Deng Y, Ming B, Zhou T, Wu JL, Chen Y, Liu P, et al. Radiomics model based on MR images to discriminate pancreatic ductal adenocarcinoma and mass-forming chronic pancreatitis lesions. Front Oncol. (2021) 11:620981. doi: 10.3389/fonc.2021.620981
25. Ren S, Zhao R, Zhang J, Guo K, Gu X, Duan S, et al. Diagnostic accuracy of unenhanced CT texture analysis to differentiate mass-forming pancreatitis from pancreatic ductal adenocarcinoma. Abdom Radiol. (2020) 45:1524–33. doi: 10.1007/s00261-020-02506-6
26. Ren S, Zhang J, Chen J, Cui W, Zhao R, Qiu W, et al. Evaluation of texture analysis for the differential diagnosis of mass-forming pancreatitis from pancreatic ductal adenocarcinoma on contrast-enhanced CT images. Front Oncol. (2019) 9:1171. doi: 10.3389/fonc.2019.01171
27. Zhang JJ, Li QZ, Wang JH, Chen X, Ren S, Ye DD, et al. [Contrast-enhanced CT and texture analysis of mass-forming pancreatitis and cancer in the pancreatic head]. Zhonghua Yi Xue Za Zhi. (2019) 99:2575–80. doi: 10.3760/cma.j.issn.0376-2491.2019.33.004
28. Li J, Liu F, Fang X, Cao K, Meng Y, Zhang H, et al. CT Radiomics features in differentiation of focal-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma: a propensity score analysis. Acad Radiol. (2022) 29:358–66. doi: 10.1016/j.acra.2021.04.014
29. Liu Z, Li M, Zuo C, Yang Z, Yang X, Ren S, et al. Radiomics model of dual-time 2-[18F]FDG PET/CT imaging to distinguish between pancreatic ductal adenocarcinoma and autoimmune pancreatitis. Eur Radiol. (2021) 31:6983–91. doi: 10.1007/s00330-021-07778-0
30. Linning E, Xu Y, Wu Z, Li L, Zhang N, Yang H, et al. Differentiation of focal-type autoimmune pancreatitis from pancreatic ductal adenocarcinoma using radiomics based on multiphasic computed tomography. J Comput Assist Tomogr. (2020) 44:511–8. doi: 10.1097/RCT.0000000000001049
31. Park S, Chu LC, Hruban RH, Vogelstein B, Kinzler KW, Yuille AL, et al. Differentiating autoimmune pancreatitis from pancreatic ductal adenocarcinoma with CT radiomics features. Diagn Interv Imaging. (2020) 101:555–64. doi: 10.1016/j.diii.2020.03.002
32. Zhang Y, Cheng C, Liu Z, Wang L, Pan G, Sun G, et al. Radiomics analysis for the differentiation of autoimmune pancreatitis and pancreatic ductal adenocarcinoma in 18 F-FDG PET/CT. Med Phys. (2019)46:4520–30. doi: 10.1002/mp.13733
33. Zhang Y, Cheng C, Liu Z, Pan G, Sun G, Yang X, et al. [Differentiation of autoimmune pancreatitis and pancreatic ductal adenocarcinoma based on multi-modality texture features in 18F-FDG PET/CT]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. (2019) 36:755–62. doi: 10.7507/1001-5515.201807012
34. Mashayekhi R, Parekh VS, Faghih M, Singh VK, Jacobs MA, Zaheer A. Radiomic features of the pancreas on CT imaging accurately differentiate functional abdominal pain, recurrent acute pancreatitis, and chronic pancreatitis. Eur J Radiol. (2020) 123:108778. doi: 10.1016/j.ejrad.2019.108778
35. Frøkjær JB, Lisitskaya MV, Jørgensen AS, Østergaard LR, Hansen TM, Drewes AM, et al. Pancreatic magnetic resonance imaging texture analysis in chronic pancreatitis: a feasibility and validation study. Abdom Radiol. (2020) 45:1497–506. doi: 10.1007/s00261-020-02512-8
36. Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. (2016) 1:45–55. doi: 10.1016/S2468-1253(16)30004-8
37. Li CL, Jiang M, Pan CQ, Li J, Xu LG. The global, regional, and national burden of acute pancreatitis in 204 countries and territories, 1990–2019. BMC Gastroenterol. (2021) 21:332. doi: 10.1186/s12876-021-01906-2
38. Roberts SE, Morrison-Rees S, John A, Williams JG, Brown TH, Samuel DG. The incidence and aetiology of acute pancreatitis across Europe. Pancreatology. (2017) 17:155–65. doi: 10.1016/j.pan.2017.01.005
39. Bai X, Jin M, Zhang H, Lu B, Yang H, Qian J. Evaluation of Chinese updated guideline for acute pancreatitis on management of moderately severe and severe acute pancreatitis. Pancreatology. (2020) 20:1582–6. doi: 10.1016/j.pan.2020.09.013
40. Guda NM, Muddana V, Whitcomb DC, Levy P, Garg P, Cote G, et al. Recurrent acute pancreatitis: international state-of-the-science conference with recommendations. Pancreas. (2018) 47:653–66. doi: 10.1097/MPA.0000000000001053
41. Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. (2015) 149:1490–500.e1. doi: 10.1053/j.gastro.2015.07.066
42. Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta-analysis. Am J Gastroenterol. (2017) 112:1366–72. doi: 10.1038/ajg.2017.218
43. Magnusdottir BA, Baldursdottir MB, Kalaitzakis E, Björnsson ES. Risk factors for chronic and recurrent pancreatitis after first attack of acute pancreatitis. Scand J Gastroenterol. (2019) 54:87–94. doi: 10.1080/00365521.2018.1550670
44. Yu B, Li J, Li N, Zhu Y, Chen Y, He W, et al. Progression to recurrent acute pancreatitis after a first attack of acute pancreatitis in adults. Pancreatology. (2020) 20: 1340–6. doi: 10.1016/j.pan.2020.09.006
45. Sun Y, Jin J, Zhu A, Hu H, Lu Y, Zeng Y, et al. Risk factors for recurrent pancreatitis after first episode of acute pancreatitis. Int J Gen Med. (2022) 15:1319–28. doi: 10.2147/IJGM.S344863
46. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis-2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
47. Kuo DC, Rider AC, Estrada P, Kim D, Pillow MT. Acute pancreatitis: what's the score? J Emerg Med. (2015) 48:762–70. doi: 10.1016/j.jemermed.2015.02.018
48. Van den Berg FF, de Bruijn AC, van Santvoort HC, Issa Y, Boermeester MA. Early laboratory biomarkers for severity in acute pancreatitis; a systematic review and meta-analysis. Pancreatology. (2020) 20:1302–11. doi: 10.1016/j.pan.2020.09.007
49. Yan G, Li H, Bhetuwal A, McClure MA, Li Y, Yang G, et al. Pleural effusion volume in patients with acute pancreatitis: a retrospective study from three acute pancreatitis centers. Ann Med. (2021) 53:2003–18. doi: 10.1080/07853890.2021.1998594
50. Zhou T, Chen Y, Wu JL, Deng Y, Zhang J, Sun H, et al. Extrapancreatic inflammation on magnetic resonance imaging for the early prediction of acute pancreatitis severity. Pancreas. (2020) 49:46–52. doi: 10.1097/MPA.0000000000001425
51. Khurana A, Nelson LW, Myers CB, Akisik F, Jeffrey BR, Miller FH, et al. Reporting of acute pancreatitis by radiologists-time for a systematic change with structured reporting template. Abdom Radiol. (2020) 45:1277–89. doi: 10.1007/s00261-020-02468-9
52. Zaky AM, Wolfgang CL, Weiss MJ, Javed AA, Fishman EK, Zaheer A. Tumor-vessel relationships in pancreatic ductal adenocarcinoma at multi detector CT: different classification systems and their influence on treatment planning. Radiographics. (2017) 37:93–112. doi: 10.1148/rg.2017160054
53. Schawkat K, Manning MA, Glickman JN, Mortele KJ. Pancreatic ductal adenocarcinoma and its variants: pearls and perils. Radiographics. (2020) 40:1219–39. doi: 10.1148/rg.2020190184
54. Schima W, Böhm G, Rösch CS, Klaus A, Függer R, Kopf H. Mass-forming pancreatitis versus pancreatic ductal adenocarcinoma: CT and MR imaging for differentiation. Cancer Imaging. (2020) 20:52. doi: 10.1186/s40644-020-00324-z
55. Kothari K, Lopes Vendrami C, Kelahan LC, Shin JS, Mittal P, Miller FH. Inflammatory mimickers of pancreatic adenocarcinoma. Abdom Radiol. (2020) 45:1387–96. doi: 10.1007/s00261-019-02233-7
56. Wolske KM, Ponnatapura J, Kolokythas O, Burke LMB, Tappouni R, Lalwani N. Chronic pancreatitis or pancreatic tumor? A problem-solving approach. Radiographics. (2019) 39:1965–82. doi: 10.1148/rg.2019190011
57. Elsherif SB, Virarkar M, Javadi S, Ibarra-Rovira JJ, Tamm EP, Bhosale PR. Pancreatitis and PDAC: association and differentiation. Abdom Radiol. (2020) 45:1324–37. doi: 10.1007/s00261-019-02292-w
58. Jia H, Li J, Huang W, Lin G. Multimodel magnetic resonance imaging of mass-forming autoimmune pancreatitis: differential diagnosis with pancreatic ductal adenocarcinoma. BMC Med Imaging. (2021) 21:149. doi: 10.1186/s12880-021-00679-0
59. Tanaka H, Matsusaki S. The Utility of endoscopic-ultrasonography-guided tissue acquisition for solid pancreatic lesions. Diagnostics. (2022) 12:753. doi: 10.3390/diagnostics12030753
60. DelMaschio A, Vanzulli A, Sironi S, Castrucci M, Mellone R, Staudacher C, et al. Pancreatic cancer versus chronic pancreatitis: diagnosis with CA 19-9 assessment, US, CT, and CT-guided fine-needle biopsy. Radiology. (1991) 178:95–9. doi: 10.1148/radiology.178.1.1984331
61. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. (2011) 40:352–8. doi: 10.1097/MPA.0b013e3182142fd2
62. Masamune A, Kikuta K, Hamada S, Tsuji I, Takeyama Y, Shimosegawa T, et al. Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2016. J Gastroenterol. (2020) 55:462–70. doi: 10.1007/s00535-019-01658-7
63. Okazaki K, Kawa S, Kamisawa T, Ikeura T, Itoi T, Ito T, et al. Amendment of the Japanese consensus guidelines for autoimmune pancreatitis, 2020. J Gastroenterol. (2022) 57:225–45. doi: 10.1007/s00535-022-01857-9
64. Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. (2011) 31:1379–402. doi: 10.1148/rg.315105735
65. Martínez-de-Alegría A, Baleato-González S, García-Figueiras R, Bermúdez-Naveira A, Abdulkader-Nallib I, Díaz-Peromingo JA, et al. IgG4-related disease from head to toe. Radiographics. (2015) 35:2007–25. doi: 10.1148/rg.357150066
66. Umehara H, Okazaki K, Nakamura T, Satoh-Nakamura T, Nakajima A, Kawano M, et al. Current approach to the diagnosis of IgG4-related disease - combination of comprehensive diagnostic and organ-specific criteria. Mod Rheumatol. (2017) 27:381–91. doi: 10.1080/14397595.2017.1290911
67. Nour E, Hammami A, Missaoui N, Bdioui A, Dahmani W, Ameur BW, et al. Multi-organ involvement of immunoglobulin g4-related disease. Gastroenterol. Insights. (2021) 12:350–7. doi: 10.3390/gastroent12030033
68. Rogers W, Thulasi Seetha S, Refaee TAG, Lieverse RIY, Granzier RWY, Ibrahim A, et al. Radiomics: from qualitative to quantitative imaging. Br J Radiol. (2020) 93:20190948. doi: 10.1259/bjr.20190948
69. Avery E, Sanelli PC, Aboian M, Payabvash S. Radiomics: a primer on processing workflow and analysis. Semin Ultrasound CT MR. (2022) 43:142–6. doi: 10.1053/j.sult.2022.02.003
70. Vesteghem C, Brøndum RF, Sønderkær M, Sommer M, Schmitz A, Bødker JS, et al. Implementing the FAIR data principles in precision oncology: review of supporting initiatives. Brief Bioinform. (2020) 21:936–45. doi: 10.1093/bib/bbz044
71. Van Soest J, Meldolesi E, van Stiphout R, Gatta R, Damiani A, Valentini V, et al. Prospective validation of pathologic complete response models in rectal cancer: transferability and reproducibility. Med Phys. (2017) 44:4961–7. doi: 10.1002/mp.12423
72. Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat Rev Cancer. (2018) 18:500–10. doi: 10.1038/s41568-018-0016-5
73. Lee SH, Park H, Ko ES. Radiomics in breast imaging from techniques to clinical applications: a review. Korean J Radiol. (2020) 21:779–92. doi: 10.3348/kjr.2019.0855
74. Traverso A, Wee L, Dekker A, Gillies R. Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys. (2018) 102:1143–58. doi: 10.1016/j.ijrobp.2018.05.053
75. Kaissis GA, Makowski MR, Rückert D, Braren RF. Secure, privacy-preserving and federated machine learning in medical imaging. Nat Mach Intellig. (2020) 2:305–11. doi: 10.1038/s42256-020-0186-1
76. Rieke N, Hancox J, Li W, Milletarì F, Roth HR, Albarqouni S, et al. The future of digital health with federated learning. NPJ Digit Med. (2020) 3:119. doi: 10.1038/s41746-020-00323-1
77. Bi WL, Hosny A, Schabath MB, Giger ML, Birkbak NJ, Mehrtash A, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. (2019) 69:127–57. doi: 10.3322/caac.21552
78. Tomaszewski MR, Gillies RJ. The biological meaning of radiomic features. Radiology. (2021) 298:505–16. doi: 10.1148/radiol.2021202553
79. Wang F, Kaushal R, Khullar D. Should health care demand interpretable artificial intelligence or accept “black box” medicine? Ann Intern Med. (2020) 172:59–60. doi: 10.7326/M19-2548
80. Kann BH, Hosny A, Aerts HJWL. Artificial intelligence for clinical oncology. Cancer Cell. (2021) 39:916–27. doi: 10.1016/j.ccell.2021.04.002
81. Ghandili S, Shayesteh S, Fouladi DF, Blanco A, Chu LC. Emerging imaging techniques for acute pancreatitis. Abdom Radiol. (2020) 45:1299–307. doi: 10.1007/s00261-019-02192-z
82. Parakh A, Tirkes T. Advanced imaging techniques for chronic pancreatitis. Abdom Radiol. (2020) 45:1420–38. doi: 10.1007/s00261-019-02191-0
83. Gorris M, Hoogenboom SA, Wallace MB, van Hooft JE. Artificial intelligence for the management of pancreatic diseases. Dig Endosc. (2021) 33:231–41. doi: 10.1111/den.13875
84. Goyal H, Mann R, Gandhi Z, Perisetti A, Zhang Z, Sharma N, et al. Application of artificial intelligence in pancreaticobiliary diseases. Ther Adv Gastrointest Endosc. (2021) 14:2631774521993059. doi: 10.1177/2631774521993059
85. Tong T, Gu J, Xu D, Song L, Zhao Q, Cheng F, et al. Deep learning radiomics based on contrast-enhanced ultrasound images for assisted diagnosis of pancreatic ductal adenocarcinoma and chronic pancreatitis. BMC Med. (2022) 20:74. doi: 10.1186/s12916-022-02258-8
86. Ziegelmayer S, Kaissis G, Harder F, Jungmann F, Müller T, Makowski M, et al. Deep convolutional neural network-assisted feature extraction for diagnostic discrimination and feature visualization in pancreatic ductal adenocarcinoma (PDAC) versus autoimmune pancreatitis (AIP). J Clin Med. (2020) 9:4013. doi: 10.3390/jcm9124013
Keywords: radiomics, acute pancreatitis, chronic pancreatitis, autoimmune pancreatitis, pancreatic ductal adenocarcinoma, computed tomography, magnetic resonance imaging, positron emission tomography/computed tomography
Citation: Yan G, Yan G, Li H, Liang H, Peng C, Bhetuwal A, McClure MA, Li Y, Yang G, Li Y, Zhao L and Fan X (2022) Radiomics and Its Applications and Progress in Pancreatitis: A Current State of the Art Review. Front. Med. 9:922299. doi: 10.3389/fmed.2022.922299
Received: 17 April 2022; Accepted: 31 May 2022;
Published: 23 June 2022.
Edited by:
Alessandro Granito, University of Bologna Department of Medical and Surgical Sciences, ItalyReviewed by:
Zubair Khan, University of Texas Health Science Center at Houston, United StatesLinda Beenet, University of California, Los Angeles, United States
Copyright © 2022 Yan, Yan, Li, Liang, Peng, Bhetuwal, McClure, Li, Yang, Li, Zhao and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmei Li, bHltemhhbmc3MEBhbGl5dW4uY29t; Guoqing Yang, MTM4OTA4OTMwNTdAMTYzLmNvbQ==; Yong Li, bG55MjAwOGh5QDE2My5jb20=
†These authors have contributed equally to this work
 Gaowu Yan
Gaowu Yan Gaowen Yan2†
Gaowen Yan2† Anup Bhetuwal
Anup Bhetuwal