
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 30 May 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.920688
This article is part of the Research TopicEye in Systemic DiseasesView all 21 articles
 Alessandro Meduri1
Alessandro Meduri1 Giovanni William Oliverio1*
Giovanni William Oliverio1* Antonio Valastro1
Antonio Valastro1 Claudia Azzaro1
Claudia Azzaro1 Umberto Camellin1
Umberto Camellin1 Francesco Franchina1
Francesco Franchina1 Leandro Inferrera2
Leandro Inferrera2 Anna Roszkowska1,3
Anna Roszkowska1,3 Pasquale Aragona1
Pasquale Aragona1Purpose: To evaluate the prevalence, clinical ocular presentation and corneal healing in moderate and severe neurotrophic keratopathy (NK) caused by systemic diseases and treated with rh-NGF.
Setting: Department of Biomedical and Dental Sciences and Morphofunctional Imaging, Ophthalmology Clinic, University of Messina, Italy.
Design: Retrospective observational study of case series.
Materials and Methods: In this retrospective observational study 11 patients (five female and six males) aged from 24 to 88 years (55.4 ± 21.3 years) with moderate and severe NK caused by systemic diseases were enrolled. The VAS questionnaire was dispensed. The ocular examination comprised slit lamp evaluation, ocular surface assessment with Keratograph 5M (Oculus, Germany), corneal sensitivity with Cochet-Bonnet esthesiometer (Lunneaux, France) and corneal thickness measurement with AC-OCT (DRI, Triton, Topcon, Japan). The underlying systemic causes of NK were determined.
Results: The main cause of NK was post-neuroma surgery (36%), followed by diabetes (18%). The remaining causes were rheumatoid arthritis (9%), post-traumatic (9%), post-surgery (9%), atopia (9%), Graves' disease (9%). Seven eyes presented severe grade of NK with corneal ulcer and in four a moderate grade was registered. The rh-NGF (Cenegermin) was administered with a standard protocol one drop six times daily for 8 weeks. The complete healing of all corneal defects was registered at the end of the treatment.
Conclusions: The post-neuroma surgery was the most common cause of NK and severe grade was clinically more represented. The rh-NGF proved effective to promote corneal recovery with all defects healed after the treatment.
Neurotrophic keratopathy (NK) is a degenerative corneal disease that affects the health and integrity of the ocular surface, resulting from impairment of corneal nerves that causes alterations in their sensory and trophic function (1). When the corneal epithelium is damaged, a coordinated and collaborative system of communication between epithelial and neuronal cells is required to promote the resynthesis of the damaged matrix, cell migration, and the restoration of architectural integrity (2, 3). As a result of permanent impairment of epithelial repair, the exposed stroma becomes subject to enzymatic deterioration, melting, and in severe forms perforation, which are all characteristics of NK (2). NK is defined as a rare disease with a prevalence estimated between 1.6 and 4.2/10,000 individuals. However, the recent studies demonstrated that this condition is commonly underestimated (4).
The etiology of corneal nerves alteration in NK could be linked to numerous ocular or systemic conditions (4, 5).
The main local causes reported are herpetic infections, chemical injuries, while the corneal surgery could directly damage the corneal nerves (5).
Although the etiology of neurotrophic keratitis is commonly related to primary ocular diseases, there are several systemic or genetic diseases, and central nervous disorders that may underlie this corneal affection (4–6).
Recent knowledge in pathogenesis of NK and the introduction of topical recombinant human Nerve Growth factor (rh-NGF) has significantly changed the natural history of the disease (1, 2).
The purpose of this study is to analyze the prevalence of moderate and severe NK resulting from systemic diseases in affected patients treated in our center with rh-NGF, aiming to identify the most frequent cause and the grade of corneal involvement. The additional aim is to assess the corneal healing process during the treatment.
In this retrospective observational cohort study, 21 eyes of 21 patients with moderate and severe NK treated with rh-NGF between January 2017 and March 2020 at Excellence Regional Center for Ocular Surface diseases of the Ophthalmology Clinic of the University Hospital of Messina were enrolled. The study was conducted with respect of tenets of Declaration of Helsinki and obtained approval of the Ethical Committee of the University Hospital of Messina. For the study purposes only patients with underlying systemic diseases that caused NK were included with the aim to determine the percentage of presentation of moderate and severe forms accordingly to the underlying pathology. Therefore, 10 patients were excluded from the study as they were affected by NK which had an ocular pathology as the primary cause. The enrolled sample comprised 11 patients with systemic diseases. Five patients were female and 6 were male and their age ranged from 24 to 88 years (mean 55.4 ± 21.3 years).
All patients underwent ocular examination that included slit lamp evaluation, ocular surface assessment with Keratograph 5M (Oculus, Germany), corneal sensitivity with Cochet-Bonnet esthesiometer (Lunneaux, France) and corneal thickness measurement with AS-OCT (DRI, Triton, Topcon). Dua classification for NK severity determination was used (1) and moderate form defined as persistent epithelial defects (PED) was differentiated from severe one (ulcer) in relation to the corneal involvement. The corneal surface was analyzed with Keratograph 5M using fluorescein staining.
Corneal sensitivity was measured in the center of the cornea three times with the Cochet-Bonnet esthesiometer and reported in filament length (cm). The average of the three measurements was used. In cases of severe NK, the thinnest point in the ulcer bed was measured using Swept source AS-OCT (DRI Triton, Topcon, Japan). The ocular discomfort was assessed using the Visual Analog Scale (VAS). All patients received Cenegermin drops (20 μg/ml) (Oxervate ®, Dompè, farmaceutici Spa, Milan, Italy) accordingly to the standardized protocol with one drop for six times daily for 8 weeks.
Corneal healing was defined as <0.5 mm fluorescein staining in the lesion area, according to REPARO 2 (7).
In a total of 21 patients, 11 (52.4%) presented NK related to systemic diseases, and 10 (47.6%) to ocular affections.
Furthermore, the group with underlying systemic causes was evaluated. The main cause of NK was post-neuroma surgery (36%), followed by diabetes (18%), previous surgery (9%), complications of Graves' disease (9%), previous trauma (9%), ocular complications of rheumatoid arthritis (9%) and severe atopic dermatitis (9%).
Severe NK (ulcer) was observed in seven patients (mean age 62.3 ± 21.7 years) and moderate NK (PED) in four patients (mean age 43.3 ± 16.1 years). The causes and severity distribution of NK are shown in Figure 1. Moderate NK was related to post-neuroma surgery (75%) and Graves' disease (25%), whereas severe NK to diabetes (30%), and other causes (Figure 1). Total VAS score was 20.27 ± 4.11 mm before the treatment, 20.98 ± 3.63 mm after 4 weeks and 10.92 ± 7.19 mm after 8 weeks (Table 1). Corneal sensitivity improved in all eyes. The changes of sensitivity and VAS score from baseline to 8 weeks after rh-NGF administration are shown in (Tables 1, 2).
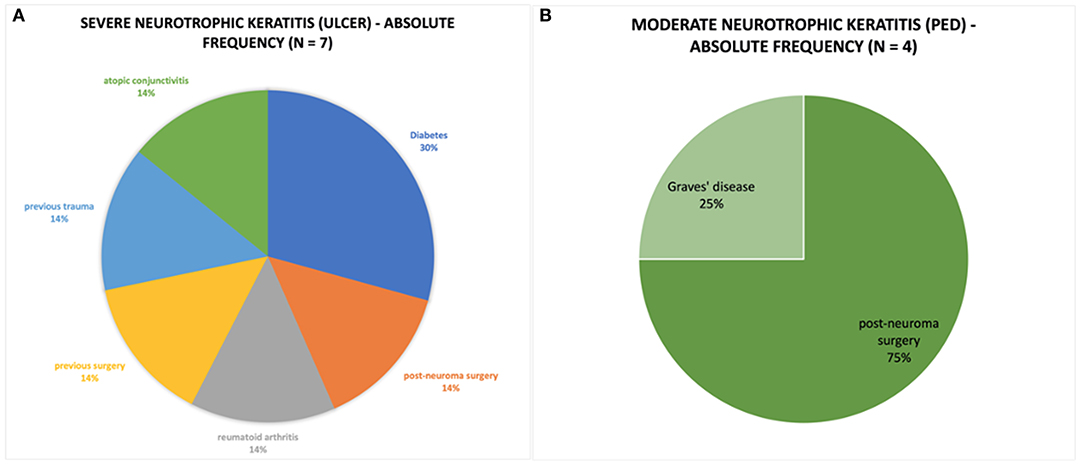
Figure 1. (A) Pie chart represent the absolute frequency of cases of severe neurotrophic keratitis caused by systemic pathology. (B) Pie chart representing the absolute frequency of cases of moderate neurotrophic keratitis caused by systemic pathology.
As to the corneal surface recovery after 4 weeks of the treatment a complete healing was registered in 100% of ulcers and 50% of PED. After 8 weeks a complete corneal recovery was observed in all patients.
Corneal thinnest location pachymetry in patients with severe NK was 285.25 ± 71.83 μm before treatment, 385.5 ± 16.33 μm at 4 weeks, and 448.25 ± 37.71 μm at 8 weeks (Table 2).
In our series there was a higher prevalence of NK resulted from several systemic diseases (52.4%); whereas the ocular causes of NK were related mainly to herpetic infections (47.6%).
Systemic diseases such as diabetes, multiple sclerosis, central nervous system diseases, genetic syndromes and autoimmune diseases could be associated with NK (1, 4–12).
A recent epidemiologic study on 335 patients showed that central nervous systems diseases followed by diabetes are the main causes of NK due to the systemic conditions (13).
In our study, the main systemic causes of NK were post neuroma surgery (36%) and diabetes (18%) and such finding confirms these recently published data.
As to the central nervous system diseases, intracerebral tumors play a primary role, and they could be represented by both head and neck cancers with intracranial spreading and trigeminal involvement (14–18).
The effects of the different therapies of cerebral tumors such as surgery (19–22) radiotherapy, (23) and systemic chemotherapy (24, 25) may also alter the nerve fibers or induce limbal stem cell deficiency (26, 27) resulting clinically in NK.
Association between diabetes and NK was identified already in 1977, as a consequence of the neuropathy of ophthalmic branch of trigeminal nerve due to microvascular damages of myelinated fibers (6, 28, 29).
The severity and progression of NK in diabetic patients are related to peripheral neuropathy, so the corneal nerve plexus is considered as an important marker of this latter's evolution and management effectiveness (6, 30). In particular NFL is considered a good parameter to evaluate the diabetic sensorimotor polyneuropathy (8).
Additionally, diabetes has further negative effects on the ocular surface inducing tear film instability and ocular surface microbiome alterations, that increase corneal erosion and infection susceptibility (31–33).
As to the other systemic diseases that may induce NK, the autoimmune diseases such as rheumatoid arthritis, Grave's disease are reported and were observed in 9% of patients in our group (34, 35).
Other systemic causes of NK are rare and are represented by amyloidosis, leprosis, CIPD, disseminated lymphangiomatosis, T-cell lymphoma, syringomyelia, vitamin B12 deficiency, HIV, and ischemic conditions like Wallenberg syndrome or cocaine snorting (36–50).
Furthermore, in pediatric patients with NK different genetic syndromes were described.
They comprehend above all HSN, congenital insensitivity to pain with anhidrosis, Gómez-López-Hernández syndrome, Goldenhar syndrome, congenital trigeminal nerve aplasia, and other more infrequent conditions like APS, familial dysautonomia, and Crisponi/CISS1 syndrome (34, 50–67).
In our study the severity of neurotrophic keratopathy interestingly appeared to be related to the patients' age. The patients with PED were younger with respect to the patients with ulcers. In a previous study, Roszkowska et al. (6, 8) demonstrated that the age is the most important element that influence corneal sub-basal nerve plexus (SBNP) nerves length, tortuosity and density. This could explain the finding that more severe corneal defects were registered in older patients who already have lower SBNP parameters. We therefore speculate that at the basis of the severity of NK there is a component of cellular tropism linked to the age and general condition of the patient.
The diagnosis of NK is based firstly on medical and surgical history of the patient to investigate all those conditions that may underlie the pathology (9, 68). Indeed, it is mandatory to consider both ocular and systemic treatments which the patient is undergoing, focusing on those that could damage corneal sensory innervation. Since NK is characterized by damage to the trigeminal sensory innervation, patients commonly do not experience any symptoms, making the diagnosis of NK particularly challenging (67). It is for the same reason that patients often see the specialist only in late phases of the disease when the pathology is already at an advanced stage (69, 70). It is important to perform a complete neurological assessment to reveal any cranial nerve damage, because a trigeminal nerve impairment may be associated with other cranial nerves injuries (1). NK's treatment consists of medical therapies, non-surgical and surgical intervention, depending on disease's stage (1, 10, 71–77).
Cenegermin is a recombinant human nerve growth factor, and it is the first EMA and FDA approved medication for moderate and severe NK in adults. It acts by promoting the growth of corneal nerves, differentiation, proliferation, and migration of corneal epithelial cells and maintaining corneal epithelial limbal stem cells (72–74).
Cenegermin resulted effective in different clinical studies on NK, but only few reports discussed its efficacy in disease related to systemic causes (72, 75–77). In this study, Cenegermin was effective in promoting corneal healing in both moderate and severe NK related to systemic diseases, with improvement of corneal sensitivity and complete recovery of the surface defects. Interestingly the severe forms healed faster with respect to PED. We reported the same results in our recent study when we analyzed the efficacy of Cenegermin in all patients with NK independently of underlying cause. About this we hypothesized that in ulcers the rh-NGF promotes better stromal healing with restore of the corneal thickness that induces the faster epithelial resurfacing as compared to PED (78–81).
This interesting finding needs further investigation to be confirmed on higher number of treated patients.
It can be concluded that this study emphasizes the crucial relation between NK and systemic diseases and particularly neurological diseases and diabetes emerged as main conditions. Given the high prevalence of these systemic diseases and their socio-economic impact, the prevention and a proper early management of NK is of high importance. We believe that accurate preventing, managing and monitoring of these systemic diseases, can help to reduce the risk of presenting of moderate and severe forms of NK.
Additionally, we demonstrated on our sample that despite the associated systemic pathology, the use of rh-NGF was equally effective in all studied subjects. However, further studies with a larger number of participants are necessary to understand better the relationships between NK presentation and systemic diseases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethical Committee of the University Hospital of Messina. The patients/participants provided their written informed consent to participate in this study.
GO: conceptualization, writing, review, editing, and data analysis. AM: conceptualization, data collection, and supervisor. AV: data collection and analysis. LI: data collection and original draft preparation. CA, FF, and UC: conceptualization, writing, review, and editing. PA: conceptualization, writing, review and editing, original draft preparation, data analysis, and supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dua HS, Said DG, Messmer EM, Rolando M, Benitez-Del-Castillo JM, Hossain PN, et al. Neurotrophic keratopathy. Prog Retin Eye Res. (2018) 66:107–31. doi: 10.1016/j.preteyeres.2018.04.003
2. Fini ME, Cook JR, Mohan R. Proteolytic mechanisms in corneal ulceration and repair. Arch Dermatol Res. (1998) 290:S12–23. doi: 10.1007/PL00007449
3. Tan MH, Bryars J, Moore J. Use of nerve growth factor to treat congenital neurotrophic corneal ulceration. Cornea. (2006) 25:352–5. doi: 10.1097/01.ico.0000176609.42838.df
4. Feroze K, Patel B. StatPearls. (2019). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK431106/ (accessed November 2, 2021).
5. Bonini S, Rama P, Olzi D, Lambiase A. Neurotrophic keratitis. Eye. (2003) 17:989–95. doi: 10.1038/sj.eye.6700616
6. Roszkowska AM, Licitra C, Tumminello G, Postorino EI, Colonna MR, Aragona P. Corneal nerves in diabetes-the role of the in vivo corneal confocal microscopy of the subbasal nerve plexus in the assessment of peripheral small fiber neuropathy. Surv Ophthalmol. (2021) 66:493–513. doi: 10.1016/j.survophthal.2020.09.003
7. Bonini S, Lambiase A, Rama P, Sinigaglia F, Allegretti M, et al. Phase II randomized, double-masked, vehicle-controlled trial of recombinant human nerve growth factor for neurotrophic keratitis. Ophthalmology. (2018) 125:1332–43. doi: 10.1016/j.ophtha.2018.02.022
8. Roszkowska AM, Wylegała A, Gargano R, Spinella R, Inferrera L, Orzechowska-Wylegała B, et al. Impact of corneal parameters, refractive error and age on density and morphology of the subbasal nerve plexus fibers in healthy adults. Sci Rep. (2021) 11:6076. doi: 10.1038/s41598-021-85597-5
9. Sacchetti M, Lambiase A. Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol. (2014) 8:571–9. doi: 10.2147/OPTH.S45921
10. Groos EB Jr. Neurotrophic keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea: Clinical Diagnosis and Management. Mosby: St Louis (1997). p. 1340
11. Puca A, Meglio M, Vari R, Tamburrini G, Tancredi A. Evaluation of fifth nerve dysfunction in 136 patients with middle and posterior cranial fossae. Eur Neurol. (1995) 35:33–7. doi: 10.1159/000117086
12. Al-Aqaba MA, Dhillon VK, Mohammed I, Said DG, Dua HS. Corneal nerves in health and disease. Prog Retin Eye Res. (2019) 73:100762. doi: 10.1016/j.preteyeres.2019.05.003
13. Saad S, Abdelmassih Y, Saad R, Guindolet D, Khoury SE, Doan S, et al. Neurotrophic keratitis: frequency, etiologies, clinical management and outcomes. Ocul Surf. (2020) 18:231–6. doi: 10.1016/j.jtos.2019.11.008
14. Ableman TB, Newman SA. Perineural spread of head and neck cancer: ophthalmic considerations. J Neurol Surg B Skull Base. (2016) 77:131–9. doi: 10.1055/s-0036-1582239
15. Sánchez Orgaz M, Gonzalez Pessolani T, Pozo Kreilinger JJ, Zamora P, Martí Álvarez C, Boto-de-Los-Bueis A. Orbital and conjunctival metastasis from lobular breast carcinoma. Orbit. (2017) 36:197–200. doi: 10.1080/01676830.2017.1310255
16. Solari HP, Ventura MP, Cheema DP, Odashiro AN, Burnier MN Jr. Orbital metastasis from breast carcinoma presenting as neurotrophic keratitis. Can J Ophthalmol. (2006) 41:93–6. doi: 10.1016/S0008-4182(06)80075-X
17. Bonzano C, Bonzano E, Cutolo CA, Scotto R, Traverso CE. A case of neurotrophic keratopathy concomitant to brain metastasis. Cureus. (2018) 10:e2309. doi: 10.7759/cureus.2309
18. Ibáñez Flores N, Sanz Moreno S. Queratopatía neurotrófica bilateral secundaria a metastasis en tronco del encéfalo [Bilateral neurotrophic keratitis secondary to encephalic trunk metastasis]. Arch Soc Esp Oftalmol. (2002) 77:681–4. doi: 10.4321/S0365-66912002001200008
19. Newman SA. Prospective study of cavernous sinus surgery for meningiomas and resultant common ophthalmic complications (An American Ophthalmological Society Thesis) Trans. Am Ophthalmol Soc. (2007) 105:392–447.
20. Lam BC, Saboo US, Kheirkhah A. Acute neurotrophic keratitis with trigeminal trophic syndrome after craniotomy. J AAPOS. (2020) 24:376–9 doi: 10.1016/j.jaapos.2020.07.011
21. Galvis V, Nino V, Tello A, Grice JM, Gómez MA. Topical insulin in neurotrophic keratopathy after resection of acoustic neuroma Arch Soc Esp Oftalmol. (2019) 94:100–4. doi: 10.1016/j.oftale.2018.06.012
22. Kloek CE, Jeng-Miller KW, Jacobs DS, Dunn IF. Prosthetic replacement of the ocular surface ecosystem treatment of ocular surface disease after skull base tumor resection. World Neurosurg. (2018) 110:e124–8. doi: 10.1016/j.wneu.2017.10.111
23. Ting DSJ, Rana-Rahman R, Ng JY, Wilkinson DJP, Ah-Kine D, Patel T. Clinical spectrum and outcomes of ocular and periocular complications following external-beam radiotherapy for inoperable malignant maxillary sinus tumors. Ocul Oncol Pathol. (2021) 7:36–43. doi: 10.1159/000511011
24. Gozzi F, Tiseo M, Facchinetti F, Gandolfi S, Rubino P. Bilateral severe corneal ulcer in a patient with lung adenocarcinoma treated with gefitinib. Case Rep Ophthalmol. (2021) 12:288–92. doi: 10.1159/000514696
25. Sekhon A, Wang JYF, Tan JCH, Holland SP, Yeung SN. Limbal stem cell deficiency secondary to systemic paclitaxel (Taxol) for breast cancer: a case report. BMC Ophthalmol. (2020) 20:e400. doi: 10.1186/s12886-020-01672-x
26. Ellies P, Anderson DF, Topuhami A, Tseng SC. Limbal stem cell deficiency arising from systemic chemotherapy. Br J Ophthalmol. (2001) 85:373–4. doi: 10.1136/bjo.85.3.371c
27. Ding X, Bishop RJ, Herzlich AA, Patel M, Chan CC. Limbal stem cell deficiency arising from systemic chemotherapy with hydroxycarbamide. Cornea. (2009) 28:221–3. doi: 10.1097/ICO.0b013e318183a3bd
28. Hyndiuk RA, Kazarian EL, Schultz RO, Seideman S. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. (1977) 95:2193–6. doi: 10.1001/archopht.1977.04450120099012
29. Kara-corlu MA, Cakiner T, Saylan T. Neurotrophic corneal ulcers in diabetes mellitus. Arch Ophthalmol. (1977) 95:2193–96.
30. Mansoor H, Tan HC, Lin MT, Mehta JS, Liu YC. Diabetic corneal neuropathy. J Clin Med. (2020) 9:3956. doi: 10.3390/jcm9123956
31. Markoulli M, Flanagan J, Tummanapalli SS, Wu J, Willcox M. The impact of diabetes on corneal nerve morphology and ocular surface integrity. Ocul Surf. (2018) 16:45–57. doi: 10.1016/j.jtos.2017.10.006
32. Margolis TP. Neurotrophic keratopathy: ophthalmology's diabetic foot problem. Eye Contact Lens. (2021) 47:136–9. doi: 10.1097/ICL.0000000000000774
33. Patel SN, Shetlar DJ, Pflugfelder SC. Bilateral candida parapsilosis infiltration of nonhealing indolent epithelial defects in a diabetic patient with neurotrophic keratopathy. Can J Ophthalmol. (2018) 53:e224–6. doi: 10.1016/j.jcjo.2018.01.025
34. Wu PY, Chang HW, Chen WL. Neurotrophic keratitis in autoimmune polyglandular syndrome type 1: a case report. BMC Ophthalmol. (2021) 21:e17. doi: 10.1186/s12886-020-01770-w
35. Roszkowska AM, Oliverio GW, Aragona E, Inferrera L, Severo AA, Alessandrello F, et al. Ophthalmologic manifestations of primary sjögren's syndrome. Genes. (2021) 12:365. doi: 10.3390/genes12030365
36. Nguyen VT, Hwang TN, Shamie N, Chuck RS, McCulley TJ. Amyloidosis-associated neurotrophic keratopathy precipitated by overcorrected blepharoptosis. Cornea. (2009) 28:575–6. doi: 10.1097/ICO.0b013e318191bdae
37. Reynolds MM, Veverka KK, Gertz MA, Dispenzieri A, Zeldenrust SR, Leung N, et al. Ocular manifestations of familial transthyretin amyloidosis. Am J Ophthalmol. (2017) 183:156.f f doi: 10.1016/j.ajo.2017.09.001
38. de Carvalho Mendes Castenheira AM, Pujol Vives P, Asaad Ammaar M. Neurotrophic keratopathy in a patient with familial amyloidosis. Arch Soc Esp Oftalmol. (2017) 92:447–50. doi: 10.1016/j.oftale.2017.07.004
39. Iraha S, Kondo S, Yamaguchi T, Inoue T. Bilateral corneal perforation caused by neurotrophic keratopathy associated with leprosy: a case report. BMC Ophthalmol. (2022) 22:e42. doi: 10.1186/s12886-022-02265-6
40. Grzybowski A, Nita M, Virmond M. Ocular leprosy. Clin Dermatol. (2015) 33:79–89. doi: 10.1016/j.clindermatol.2014.07.003
41. Bansal S, Myneni AA, Mu L, Myers BH, Patel SP. Corneal sensitivity in chronic inflammatory demyelinating polyneuropathy. Cornea. (2014) 33:703–6. doi: 10.1097/ICO.0000000000000145
42. Knickelbein JE, Stefko ST, Charukamnoetkanok P. Neurotrophic keratitis in a patient with disseminated lymphangiomatosis. Eye Brain. (2009) 1:1–4. doi: 10.2147/EB.S6957
43. Lin TC, Lin PY, Wang LC, Chen SJ, Chang YM, Lee SM. Intraocular involvement of T-cell lymphoma presenting as inflammatory glaucoma, neurotrophic keratopathy, and choroidal detachment. J Chin Med Assoc. (2014) 77:385oc. doi: 10.1016/j.jcma.2014.04.002
44. Amalric P, Bessou P, Vergnes H. Syringomyelia and bilateral corneal perforation caused by neurotrophic keratitis. Rev Otoneuroophtalmol. (1964) 36:62–4.
45. Nassiri N, Assarzadegan F, Shahriari M, Norouzi H, Kavousnezhad S, Nassiri N, et al. Vitamin B12 deficiency as a cause of neurotrophic keratopathy. Open Ophthalmol J. (2018) 12:7–11. doi: 10.2174/1874364101712010007
46. Paz Moreno-Arrones J, Benítez-Herreros J, Drake-Rodríguez P, Romero-García Tenorio A. Neurotrophic corneal ulcer in an HIV patient. Arch Soc Esp Oftalmol. (2011) 86:27–30. doi: 10.1016/S2173-5794(11)70006-8
47. Mandarà E, Brocca D, Pellegrini F, Interlandi E. Topical nerve growth factor for the treatment of neurotrophic keratopathy caused by wallenberg syndrome. Cornea. (2021) 41:647–8. doi: 10.1097/ICO.0000000000002928
48. Hipps WM, Wilhelmus KR. Persistent visual loss from neurotrophic corneal ulceration after dorsolateral medullary infarction (Wallenberg syndrome). J Neuroophthalmol. (2004) 24:345–6. doi: 10.1097/00041327-200412000-00015
49. Pellegrini F, Interlandi E, Cuna A, Mandarn E, Lee AG. Corneal involvement in wallenberg syndrome: case report and literature review. Neuroophthalmology. (2019) 44:54olo doi: 10.1080/01658107.2019.1602147
50. Mantelli F, Lambiase A, Sacchetti M, Orlandi V, Rosa A, Casella P, et al. Cocaine snorting may induce ocular surface damage through corneal sensitivity impairment. Graefes Arch Clin Exp Ophthalmol. (2015) 253:765–72. doi: 10.1007/s00417-015-2938-x
51. Donaghy M, Hakin RN, Bamford JM, Garner A, Kirkby GR, Noble BA, et al. Hereditary sensory neuropathy with neurotrophic keratitis. Description of an autosomal recessive disorder with a selective reduction of small myelinated nerve fibres and a discussion of the classification of the hereditary sensory neuropathies. Brain. (1987) 110:563–83. doi: 10.1093/brain/110.3.563
52. Bhaskar PA. Hereditary sensory neuropathy (HSN) type I with neurotrophic keratitis. J Assoc Physicians India. (1986) 34:379–81.
53. Bowe BE, Levartovsky S, Eiferman RA. Neurotrophic corneal ulcers in congenital sensory neuropathy. Am J Ophthalmol. (1989) 107:303–4. doi: 10.1016/0002-9394(89)90324-3
54. Sethi A, Ramasubramanian S, Swaminathan M. The painless eye: neurotrophic keratitis in a child suffering from hereditary sensory autonomic neuropathy type IV. Indian J Ophthalmol. (2020) 68:2270–2. doi: 10.4103/ijo.IJO_2101_19
55. Jarade EF, El-Sheikh HF, Tabbara KF. Indolent corneal ulcers in a patient with congenital insensitivity to pain with anhidrosis: a case report and literature review. Eur J Ophthalmol. (2002) 12:60–5. doi: 10.1177/112067210201200112
56. John D, Thomas M, Jacob P. Neurotrophic keratitis and congenital insensitivity to pain with anhidrosis–a case report with 10-year follow-up. Cornea. (2011) 30:100–2. doi: 10.1097/ICO.0b013e3181e458e4
57. Yagev R, Levy J, Shorer Z, Lifshitz T. Congenital insensitivity to pain with anhidrosis: ocular and systemic manifestations. Am J Ophthalmol. (1999) 127:322–6. doi: 10.1016/S0002-9394(98)00370-5
58. Indo Y. NTRK1 congenital insensitivity to pain with anhidrosis. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Gripp KW, et al. editors. GeneReviews®. Seattle, WA: University of Washington (1993–2022).
59. Chao J, Rao R, Gupta C. Gómez-López-Hernández syndrome: a case report on pediatric neurotrophic corneal ulcers and review of the literature. J AAPOS. (2021) 25:373–5. doi: 10.1016/j.jaapos.2021.08.299
60. Pastor-Idoate S, Carreño E, Tesón M, Herreras JM. Gómez-López-Hernández syndrome: another consideration in corneal neurotrophic ulcers. Eur J Ophthalmol. (2012) 22:826–9. doi: 10.5301/ejo.5000138
61. Rollon-Mayordomo A, Mataix-Albert B, Espejo-Arjona F, Herce-Lopez J., Lledo- Villar L, Caparros-Escudero C, et al. Neurotrophic keratitis in a pediatric patient with goldenhar syndrome and trigeminal aplasia successfully treated by corneal neurotization. Ophthalmic Plast Reconstr Surg. (2022) 38:e49–51. doi: 10.1097/IOP.0000000000002086
62. Olavarri González G, García-Valcarcel González B, Baeza Autillo A, Balado Vazquez P. Neurotrophic keratopathy secondary to trigeminal nerve aplasia in patient with goldenhar syndrome. Arch Soc Esp Oftalmol. (2016) 91:191–4. doi: 10.1016/j.oftale.2016.03.002
63. Kamal SM, Riccobono K, Kwok A, Edmond JC, Pflugfelder SC. Unilateral pediatric neurotrophic keratitis due to congenital left trigeminal nerve aplasia with PROSE (prosthetic replacement of the ocular surface ecosystem) treatment. Am J Ophthalmol Case Rep. (2020) 20:100854. doi: 10.1016/j.ajoc.2020.100854
64. Ohana M, Lipsker D, Chaigne D, Speeg-Schatz C, Sauer A. Unilateral ulceration of the cornea secondary to congenital trigeminal nerve agenesis. Eur J Ophthalmol. (2015) 25:e35–7. doi: 10.5301/ejo.5000552
65. Scanzera AC, Shorter E. Case series: management of neurotrophic keratitis from familial dysautonomia. Optom Vis Sci. (2018) 95:678–81. doi: 10.1097/OPX.0000000000001255
66. Agresta A, Fasciani R, Padua L, Petroni S, La Torraca I, Dickmann A, et al. Corneal alterations in Crisponi/CISS1 syndrome: a slit-lamp biomicroscopy and in vivo confocal microscopy corneal report. Ophthalmic Genet. (2017) 38:83–7. doi: 10.3109/13816810.2015.1137326
67. Jabbour S, Harissi-Dagher M. Recessive mutation in a nuclear-encoded mitochondrial tRNA synthetase associated with infantile cataract, congenital neurotrophic keratitis, and orbital myopathy. Cornea. (2016) 35:894–6. doi: 10.1097/ICO.0000000000000847
68. Inferrera L, Aragona E, Wylegała A, Valastro A, Latino G, Postorino EI, et al. The role of Hi-Tech devices in assessment of corneal healing in patients with neurotrophic keratopathy. J Clin Med. (2022) 11:1602. doi: 10.3390/jcm11061602
69. Feroze KB, Patel BC. Neurotrophic keratitis. In: Hauber S, editor. StatPearls. Treasure Island, FL: StatPearls Publishing (2022).
70. Dhillon VK, Elalfy MS, Al-Aqaba M, Gupta A, Basu S, Dua HS. Corneal hypoesthesia with normal sub-basal nerve density following surgery for trigeminal neuralgia. Acta Ophthalmol. (2016) 94:e6–10. doi: 10.1111/aos.12697
71. Oliverio GW, Spinella R, Postorino EI, Inferrera L, Aragona E, Aragona P, et al. Safety and tolerability of an eye drop based on 0.6% povidone-iodine nanoemulsion in dry eye patients. J Ocul Pharmacol Ther. (2021) 37:90–6. doi: 10.1089/jop.2020.0085
72. Blanco-Mezquita T, Martinez-Garcia C, Proença R, Zieske JD, Bonini S, Lambiase A, et al. Nerve growth factor promotes corneal epithelial migration by enhancing expression of matrix metalloprotease-9. Investig Ophthalmol Vis Sci. (2013) 54:3880–90. doi: 10.1167/iovs.12-10816
73. Joo MJ, Yuhan KR, Hyon JY, Lai H, Hose S, Sinha D, et al. The effect of nerve growth factor on corneal sensitivity after laser in situ keratomileusis. Arch Ophthalmol. (2004) 122:1338–41. doi: 10.1001/archopht.122.9.1338
74. Lambiase A, Bonini S, Aloe L, Rama P, Bonini S. Antiinflammatory and healing properties of nerve growth factor in immune corneal ulcers with stromal melting. Arch Ophthalmol. (2000) 118:1446–49. doi: 10.1001/archopht.118.10.1446
75. Roszkowska AM, Inferrera L, Aragona E, Gargano R, Postorino EI, Aragona P. Clinical and instrumental assessment of the corneal healing in moderate and severe neurotrophic keratopathy treated with rh-NGF (Cenegermin). Eur J Ophthalmol. (2022) 27:11206721221097584. doi: 10.1177/11206721221097584
76. Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med. (1998) 338:1174cal doi: 10.1056/NEJM199804233381702
77. Pflugfelder SC, Massaro-Giordano M, Perez VL, Hamrah P, Deng SX, Espandar L, et al. Topical recombinant human nerve growth factor (cenegermin) for neurotrophic keratopathy: a multicenter randomized vehicle-controlled pivotal trial. Ophthalmology. (2020) 127:14phic doi: 10.1016/j.ophtha.2019.08.020
78. Scorolli L, Meduri A, Morara M, Scalinci SZ, Greco P, Meduri RA, et al. Effect of cysteine in transgenic mice on healing of corneal epithelium after excimer laser photoablation. Ophthalmologica. (2008) 222:380–5. doi: 10.1159/000151691
79. Scalinci SZ, Scorolli L, Meduri A, Grenga PL, Corradetti G, Metrangolo C. Effect of basic fibroblast growth factor and cytochrome c peroxidase combination in transgenic mice corneal epithelial healing process after excimer laser photoablation. Clin Ophthalmol. (2011) 5:215–21. doi: 10.2147/OPTH.S16866
80. Meduri A, Scorolli L, Scalinci SZ, Grenga PL, Lupo S, Rechichi M, et al. Effect of the combination of basic fibroblast growth factor and cysteine on corneal epithelial healing after photorefractive keratectomy in patients affected by myopia. Indian J Ophthalmol. (2014) 62:424–8. doi: 10.4103/0301-4738.119420
Keywords: neurotrophic keratopathy, rh-NGF, neurotrophic keratitis, Cenegermin, nerve growing factor
Citation: Meduri A, Oliverio GW, Valastro A, Azzaro C, Camellin U, Franchina F, Inferrera L, Roszkowska A and Aragona P (2022) Neurotrophic Keratopathy in Systemic Diseases: A Case Series on Patients Treated With rh-NGF. Front. Med. 9:920688. doi: 10.3389/fmed.2022.920688
Received: 14 April 2022; Accepted: 11 May 2022;
Published: 30 May 2022.
Edited by:
Paolo Fogagnolo, University of Milan, ItalyReviewed by:
Alessandro Arrigo, San Raffaele Hospital (IRCCS), ItalyCopyright © 2022 Meduri, Oliverio, Valastro, Azzaro, Camellin, Franchina, Inferrera, Roszkowska and Aragona. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni William Oliverio, Zy53ODlAbWUuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.