- 1Department of Critical Care Medicine, Chongqing Emergency Medical Center, Chongqing, China
- 2Department of Osteology, Army Medical Center of PLA, Third Military Medical University (Army Medical University), Chongqing, China
- 3Department of Emergency, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China
- 4Department of Military Cognitive Psychology, School of Psychology, Third Military Medical University (Army Medical University), Chongqing, China
Introduction: Resistin is a small secretory adipokine which is implicated to obesity and associated diseases. Recently, plenty of research papers have been conducted to explore the association between peripheral resistin and the severity of acute pancreatitis (AP). However, the results were controversial. In this study, we aimed to confirm the effect of peripheral resistin and the development of acute pancreatitis.
Methods: A comprehensive online search was performed using the PubMed, Embase, Web of Science, CNKI, and Wanfang databases up through January 20, 2022. The retrieved records and their references were screened to identify additional studies. Data were extracted to calculate the pooled Hedges' g and its 95% CI, which were selected to assess peripheral resistin levels and the severity of acute pancreatitis. Subgroup analyses, sensitivity analyses, meta-regression, and publication bias tests were also undertaken based on obtained information.
Results: A total of eleven studies with 892 acute pancreatitis patients were enrolled in the study. Peripheral resistin levels were significantly increased in severe acute pancreatitis compared with mild acute pancreatitis (Hedges' g = 2.092, 95% CI: 0.994–3.190, P < 0.001). Subgroup analyses based on sample types and ethnicity also showed similar results. A single study did not affect our results, which was verified by sensitivity analysis. Meta-regression analyses revealed that age, gender of the included subjects, sample size, and publication year did not moderate effects on the present results.
Conclusion: In our study, peripheral resistin levels were significantly elevated in patients with severe AP compared with patients with mild AP. Abnormal resistin levels may provide us some new insights in predicting the severity of AP.
Introduction
Acute pancreatitis (AP) is one of the most common clinical syndrome initiated by pancreatic injury from a variety of pathologic mechanisms (1). Pancreatic injury is mild and self-limiting in approximately 80% of patients with AP that would find their health after brief hospitalization. Unfortunately, the other 20% of patients with AP would develop lethal severe acute pancreatitis (SAP) that requires intensive care (2, 3). Despite improvements in treatment measurements and supportive care, SAP remains one of the important causes of death during the past decade (4). Therefore, identification of patients with SAP from patients with AP is urgently needed to help determine therapeutic interventions as early as possible.
Obesity is a chronic, systemic low-grade inflammatory state characterized by high levels of peripheral proinflammatory cytokines. Currently, about 40% of adults are overweight, and obesity has now become an important health problem (5, 6). A number of recent studies have suggested that obesity is associated with the risk of severe acute pancreatitis (7, 8). It is common knowledge that abnormal release of destructive digestive enzymes from pancreatic acinar cells and systemic inflammatory response play critical roles in the development of AP (9). Pancreatic microcirculation is poorer in obese patients that are more prone to infection and SAP (10). The main problem in managing acute pancreatitis is the lack of availability of convenient indicators or scoring systems for predicting severity and necrosis in the first hours of the disease (11). Lots of animal studies have shown that obesity-related cytokines, such as leptin and adiponectin, contributed to the development of AP (12, 13). Multiple clinical trials have found these abnormal cytokine profiles in patients with AP (14, 15). It seems that adipokines are associated with acute pancreatitis.
Resistin, coded by the RETN gene, belongs to the family of resistin-like molecules. It is mainly produced by adipose tissue, inflammatory cells, and functions as a pro-inflammatory adipokine (16, 17). There are many studies reported resistin plays a major role in a series of inflammation-related diseases, such as atherosclerosis, sepsis, and cancer (18–20). Animal studies have revealed that resistin could enhance intracellular Ca2+ levels, NADPH oxidase activity, intracellular reactive oxygen species (ROS) production, NF-κB activity, and IL-6 expression in cerulein-stimulated AR42J cells, which may contribute to the development of AP (21). Peripheral resistin was initially thought to be a promising biomarker, which could predict severe acute pancreatitis in the early phase (22). So far, many clinical studies have tried to determine whether the levels of peripheral resistin are related to the severity of AP. However, the results were inconsistent. Thus, we conducted a meta-analysis to further investigate the effect of peripheral resistin on the severity of AP based on larger sample size.
Materials and Methods
Search Strategy
A systematic literature search was performed for the relevant literature in five databases (PubMed, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), and Wanfang databases) up through January 20, 2022 according to the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. The retrieval key search terms were included the following: “acute pancreatitis,” “pancreatic necrosis,” or “peripancreatic necrosis”; and “resistin.” To obtain all eligible studies, all retrieved articles and their references were checked.
Inclusion and Exclusion Criteria
The records retrieved were screened according to the inclusion and exclusion criteria. In our meta-analysis, the inclusion criteria for the studies were as follows: (1) clinical studies evaluating the levels of resistin in severe and mild acute pancreatitis. (2) resistin detected in plasma or serum. (3) a mean and standard deviation, or sample size and P-value were presented in records. The exclusion criteria for the studies were as follows: (1) studies with insufficient information such as concentration or P-value. (2) animal study, review, comment or meeting abstract. (3) in vitro studies. When the retrieved studies contained overlapping data, only the data of study with the largest sample size was extracted. In addition, some data based on graphical figures or other formats (median, interquartile range) were not estimated in our study for the unwanted error.
Data Extraction
A standardized Excel spreadsheet was prepared for data extraction. Data from the included studies were extracted and summarized independently by two independent authors according to the inclusion and exclusion criteria. The information from each study was extracted as follows: author, publication year, country, diagnostic criteria of acute pancreatitis, source of acute pancreatitis patients, detection method for resistin, sample type, age and gender of samples, sample size, the levels of resistin (mean, standard deviation, or P-value). When discrepancies occurred during in the process of data extraction, a third person was consulted to resolve these issues. Finally, data were rechecked by an independent author for accuracy.
Statistical Analysis
Pre-extracted means and standard deviations or sample size and P-values were employed to calculate Hedges' g statistic. Hedges' g and its 95% CI were selected to evaluate the significant difference of peripheral resistin levels in severe and mild acute pancreatitis. Statistical heterogeneity test across studies was carried out using the I2 value and a chi-square-based Q-test. When obvious heterogeneity existed, a random-effect model was selected to calculate the corresponding effect size; otherwise, a fixed-effect model was selected. To verify the effect of a single study on the comprehensive results directly, we conducted sensitivity analyses for statistically significant ES estimates by removing each study from the analysis in turn. A funnel plot was drawn to test the publication bias. When the P-value < 0.05, it was considered statistically significantly different in the present study.
To explore the source of heterogeneity, subgroup analyses for sample type and ethnicity based on available information. A Galbraith plot was drawn to identify potential studies affecting heterogeneity across studies. Unrestricted maximum-likelihood random-effects meta-regressions of effect sizes were performed to verify whether some theoretical covariates (mean age, gender (male proportion) of samples, sample size, and publication year) serve as confounders that affected our results. We conducted all statistical analyses using Comprehensive Meta-Analysis version 3 software (version 3; Biostat Inc) and STATA15 software (StataCorp, College Station, TX, USA).
Results
Literature Search Results
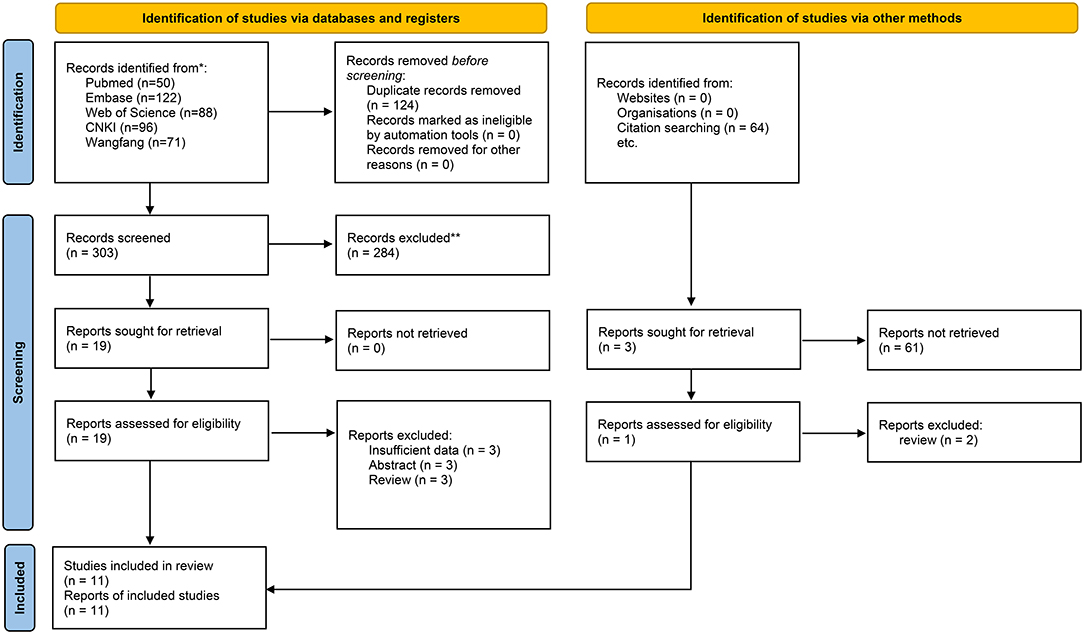
There were 428 records identified by electronic search with the retrieval key search terms. A total of 50 records were retrieved from PubMed, 122 records from Embase, 88 records from Web of Science, 96 records from CNKI, 71 records from Wan-fang database, and one additional record identified through other sources. After removing 124 duplications and 284 unrelated records based on review of titles or abstracts, twenty records were further screened by full-text reading. Three records without sufficient data were excluded. Three records were meeting abstracts, and three records were reviews. Therefore, eleven records (11 studies) with 892 acute pancreatitis patients were included in the present meta-analysis to evaluate the association between the levels of resistin and the severity of AP (11, 22–31). The flow diagram is presented in Figure 1.
Study Characteristics
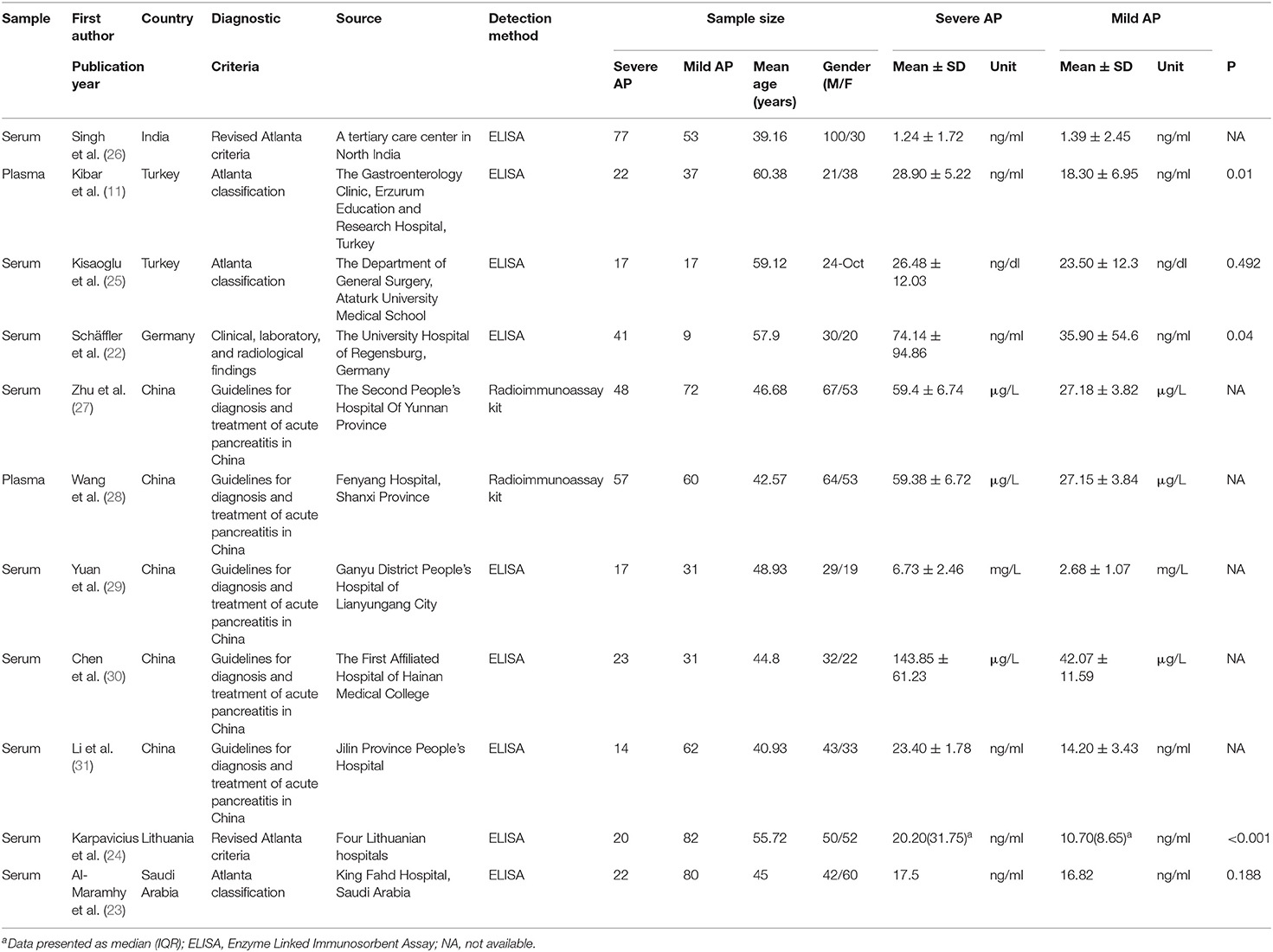
Of the eleven included studies, five studies were carried out in China, two studies in Turkey, one study in India, one study in Germany, one study in Lithuania, and one in Saudi Arabia. All of the included studies were published after 2010. We noted that serum resistin concentration was detected in nine studies and plasma resistin concentration was tested in two. The levels of peripheral resistin were determined using ELISA or radioimmunoassay kit. The minimum and maximum mean ages of the samples of the included studies were 39.16 and 60.38, respectively. The levels of peripheral resistin were detected in patients with acute pancreatitis within 24 h of admission in nine studies (11, 22–25, 27–30). The characteristics of the included studies are presented in Table 1.
Quantitative Data Synthesis
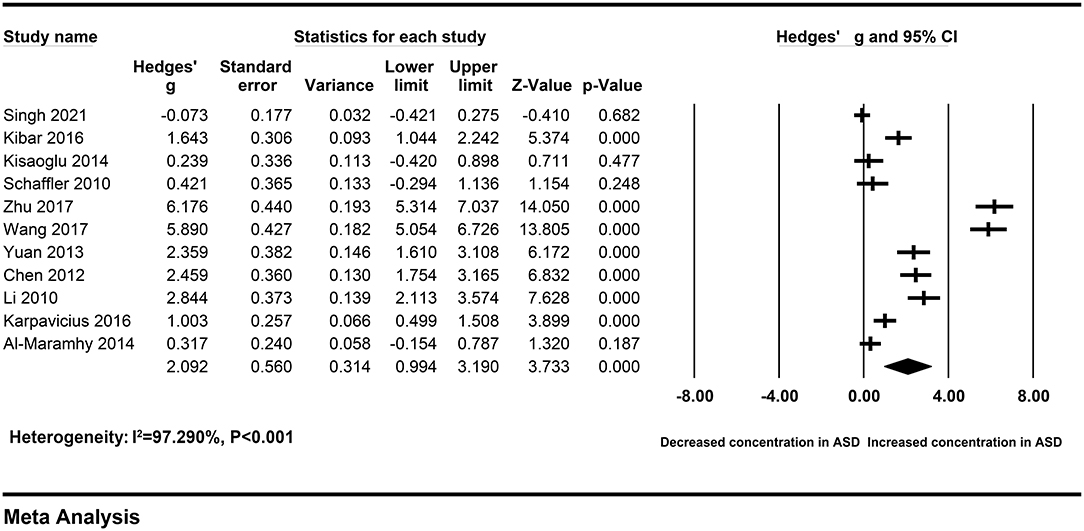
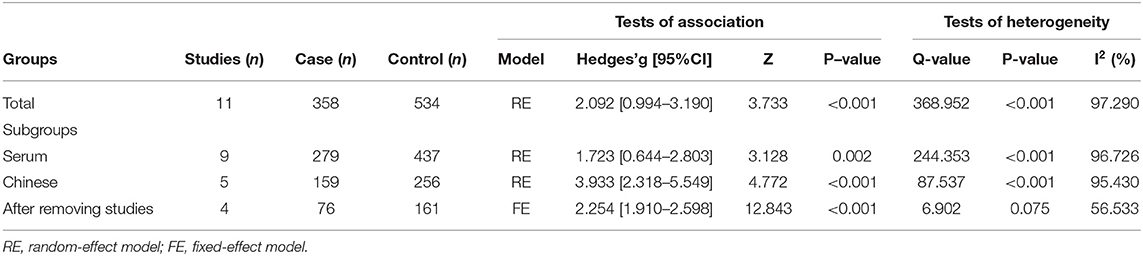
The pooled Hedges' g and its corresponding 95% CI were calculated based on the extracted data of eleven studies compassing 358 patients with severe AP and 534 patients with mild AP. Obvious heterogeneity across studies were found in main analysis (I2 = 97.290%, P < 0.001). Therefore, a random-effect model was used to calculate the pooled ES and its corresponding 95% CI. The results of the overall comparison revealed that, peripheral resistin levels were statistically elevated in patients with severe AP compared with patients with mild AP (Hedges' g = 2.092, 95% CI: 0.994–3.190, P < 0.001) (Figure 2) (Table 2).
Heterogeneity Analysis
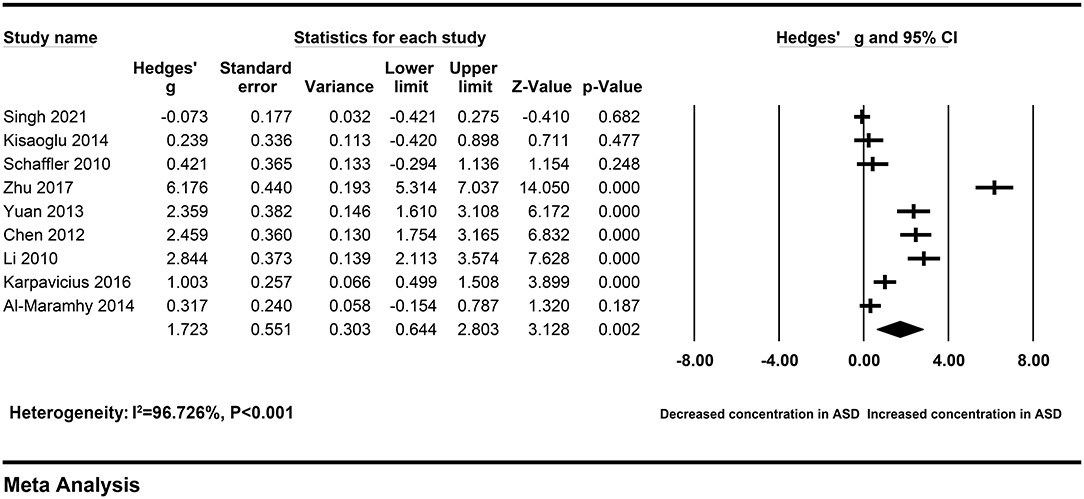
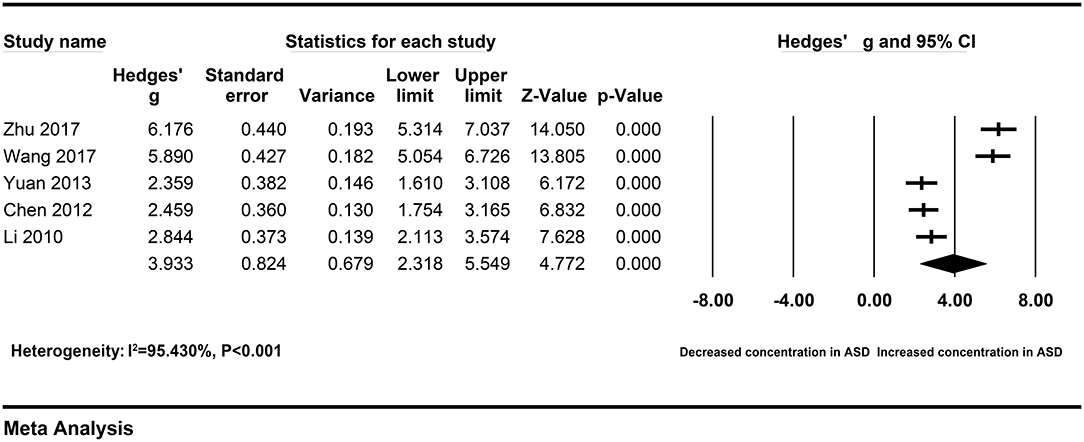
To explore the sources of heterogeneity across studies, subgroup analyses were according to different sample types and ethnicity. The correlation between elevated peripheral resistin levels and severity of AP did not change obviously in the subgroup with the serum samples (Hedges' g = 1.723, 95% CI: 0.644–2.803, P = 0.002) and Chinese population (Hedges' g = 3.933, 95% CI: 2.318–5.549, P < 0.001) (Figures 3, 4). However, heterogeneity across studies did not decrease significantly in serum (I2 = 96.726%, P < 0.001) or Chinese (I2 = 95.430%, P < 0.001) subgroups. It suggested that sample types and ethnicity did not address the source of heterogeneity across studies.
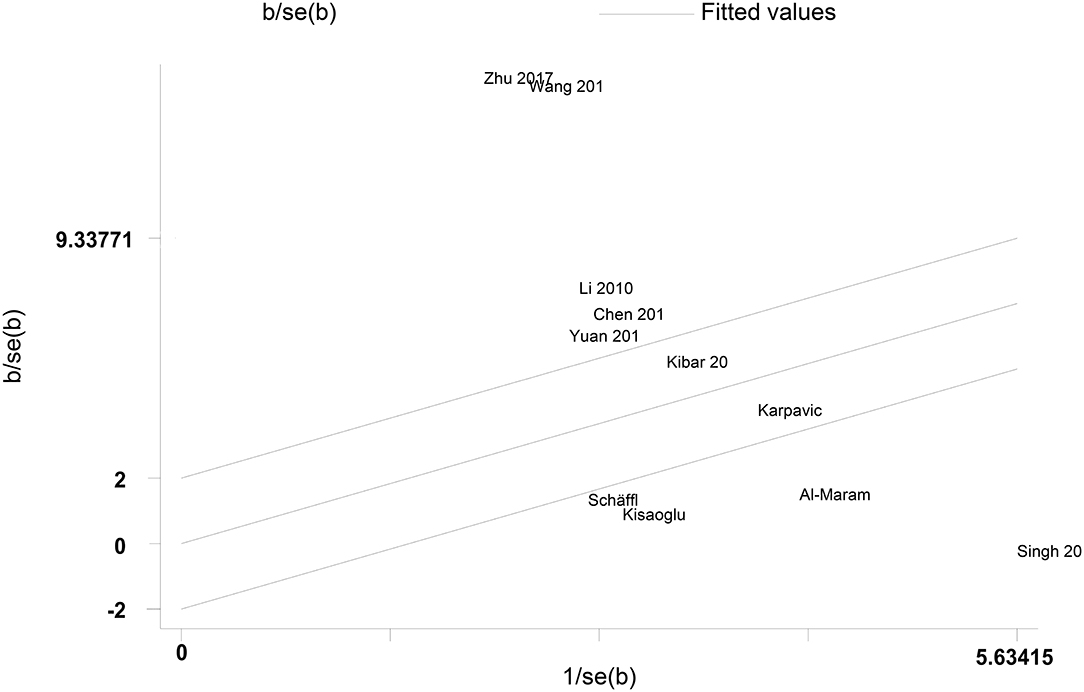
A Galbraith plot was drawn according to the extracted data of included studies to identify the studies deviated from baseline, which may affect heterogeneity across studies. As shown in Figure 5, seven studies (22–28) were labeled for displacing from the center of the zero line in the Galbraith plot. After removing these studies, heterogeneity across studies decreased obviously (I2 = 56.533%, P = 0.075). Moreover, peripheral resistin levels were still significantly higher in patients with severe AP than patients with mild AP (Hedges' g = 2.254, 95% CI: 1.910–2.598, P < 0.001) (Figure 6) (Table 2).
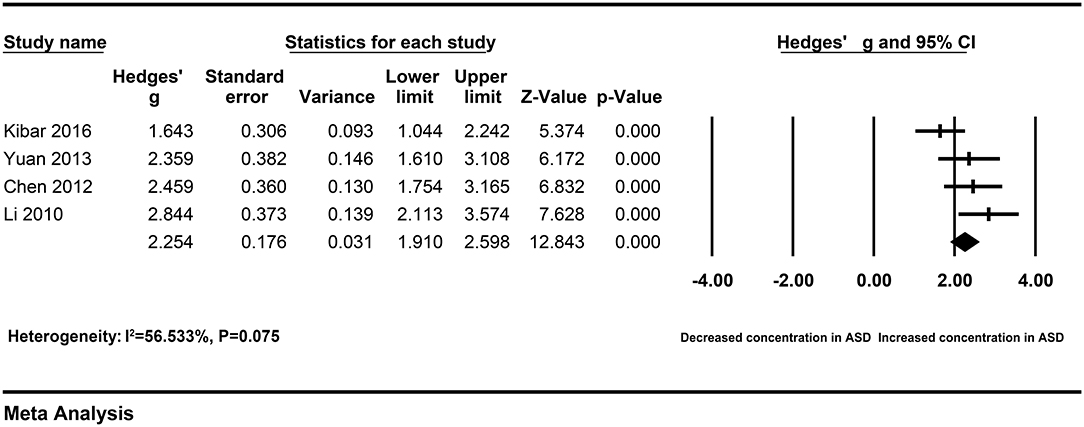
Figure 6. Forest plot for the fixed-effect meta-analysis after removing the studies outside the boundaries of the Galbraith plot.
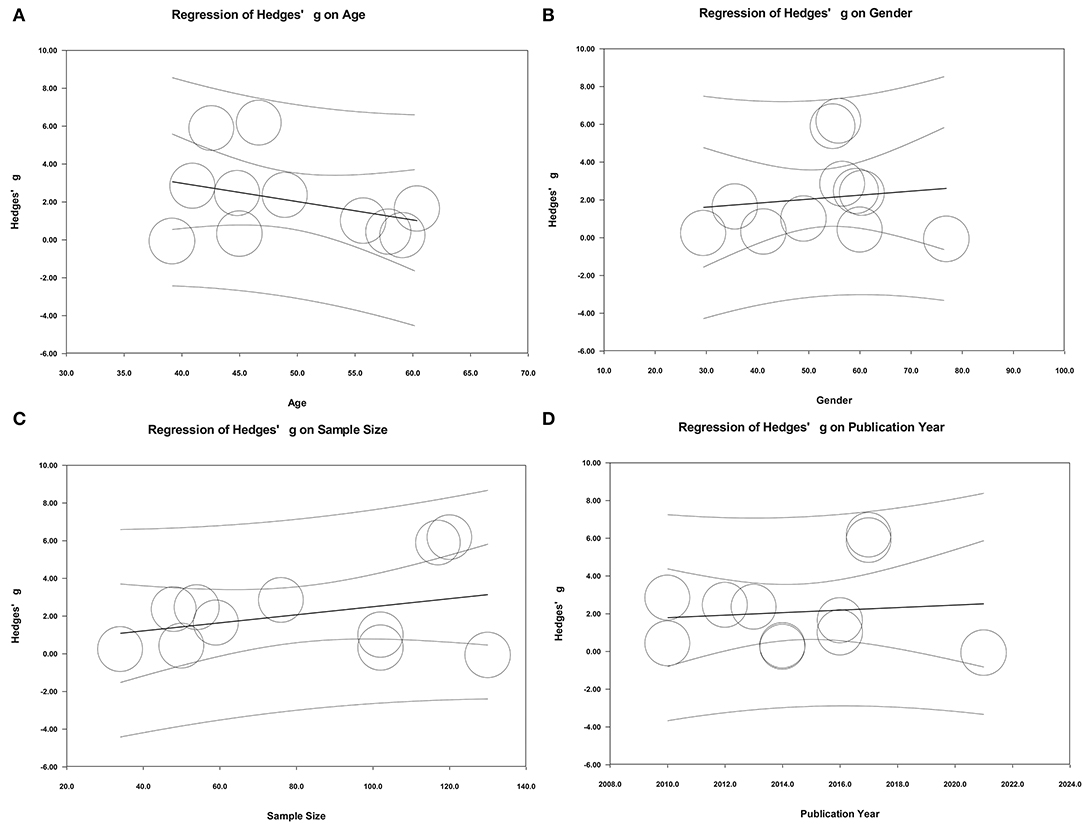
In addition to the above relevant categorical variables (sample type and ethnicity) and potential studies affecting heterogeneity, the effects of some potential continuous variables (age, gender, and sample size) on heterogeneity were also evaluated by performing meta-regression analyses. The results of meta-regression suggested that age, gender of samples, sample size, and publication year did not play important roles in moderating effects on the present results (Figure 7).
Sensitivity Analysis
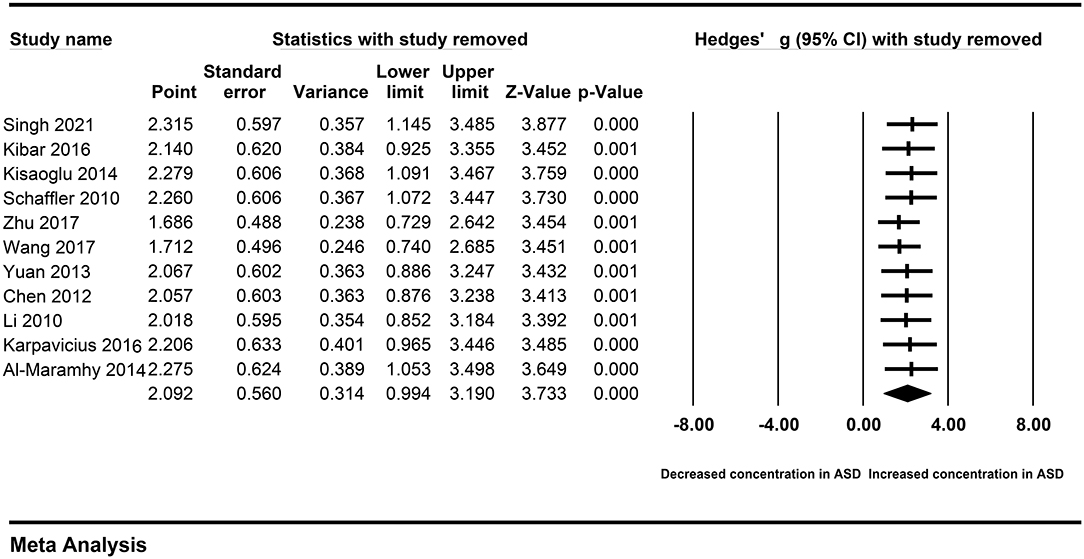
A single study may affect the overall result, and cause obvious heterogeneity across studies. Therefore, we performed the sensitivity analysis by removing each study sequentially. The association between peripheral resistin and the severity of AP was also observed after removing a single study sequentially (Figure 8). Similarly, we did not found heterogeneity across studies decreased significantly. Therefore, a single study did not affect our results.
Publication Bias
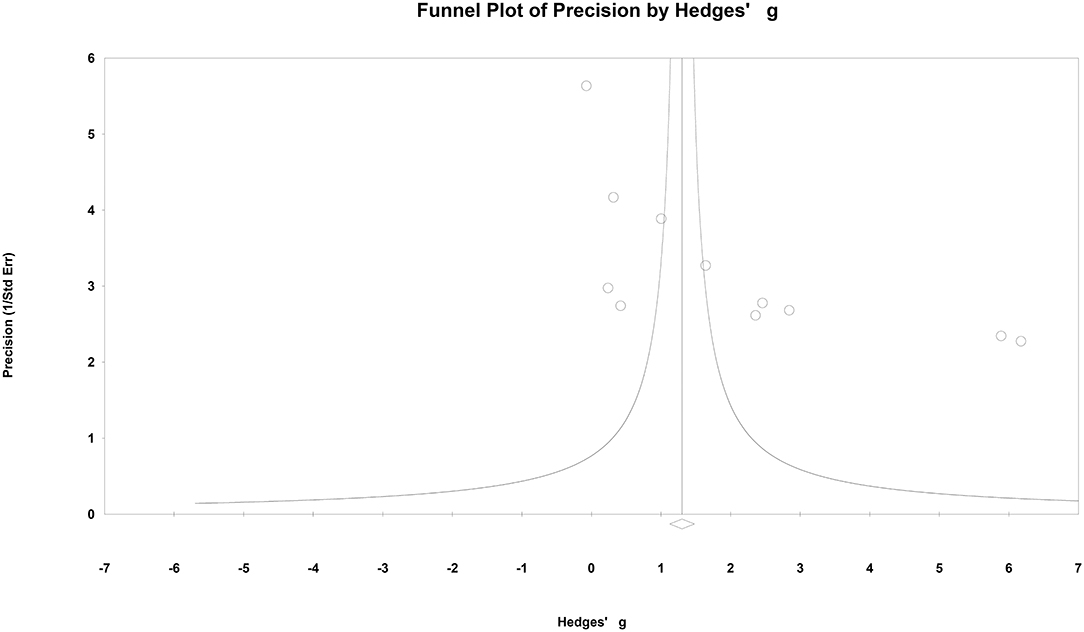
A funnel plot was presented in Figure 9. No obvious publication bias was found in this meta-analysis.
Discussion
So far as we know, this is the first meta-analysis to investigate the relation of peripheral resistin levels to severity of acute pancreatitis. The main finding of our meta-analysis was that there was a significant association between peripheral levels of resitin and severity of acute pancreatitis. There was no single study affecting the overall outcomes, which was verified by performing a sensitivity analysis. The funnel plot showed that no obvious publication bias in our study. Resistin has been implicated to play important roles in the development of insulin resistance and affecting pancreatic function (20). Despite the disagreements in the effects of peripheral resistin on severity of AP, our present study combining the published data suggested that peripheral levels of resistin were significantly higher in patients with severe AP compared with patients with mild AP, which may provide us some clues about the marker for the accurate early prediction of the severity of acute pancreatitis. We noticed that the levels of peripheral resistin were detected in patients with acute pancreatitis within 24 h of admission in most of the included studies. Early screening the levels of resistin in peripheral blood of patients with acute pancreatitis after admission may help clinicians better master the patient's condition and assess the risk of severe acute pancreatitis in patients. In addition, further developing effective interventions after early prediction of the severity of acute pancreatitis may enhance the prognosis of acute pancreatitis.
It has not escaped our notice that there was obvious heterogeneity across studies in both overall analysis and subgroup analysis. Although subgroup analyses with limited information did not define the source of heterogeneity in our study, peripheral resistin levels remained significantly higher in both serum and Chinese subgroups. A Galbraith plot was drawn to identify the source of heterogeneity in the included studies. Seven studies were removing from the overall analysis and heterogeneity across studies was statistically decreased. These studies may cause obvious heterogeneity. In addition, meta-regression analyses to adjust for potential moderators were performed to explore the source of heterogeneity. The results of meta-regression indicated that age and gender of samples, sample size and publication year did not play an important role in moderating the outcomes of the meta-analysis. These factors may be not the potential confounding factor. More information was needed to identify the potential source of heterogeneity.
What is the role of the elevated resistin levels on the severity of AP? Oxidative stress and inflammatory response are regarded as major causative factors in acute pancreatitis. Peripheral resistin was reported enhancing environmental factors-induced IL-6 and ROS expression in pancreatic acinar cells, which resulted in endothelial cells injury and the release of endothelin-1 (21, 32). Endothelin-1 plays an important role in the pathogenesis of pancreatitis by a decrease in pancreatic perfusion and contribute to oxidative stress and inflammatory response (33). Thus, there seems to be a causal link between peripheral resistin and the severity of AP. The exact mechanism of peripheral resistin affecting the severity of AP should be further verified by well-designed animal studies.
Some inherent limitations should be pointed out in our meta-analysis. First, the sample size was moderate, especially in some subgroups, and lots of information was unavailable. In terms of included studies, substantial variability in sampling methods, sample sizes, detecting methods, and diagnostic criteria should be further assessed despite of some factors detected in the study. We should realize that obvious heterogeneity still exists. The source of heterogeneity may be further explored with more detailed information. Second, the numbers of studies that analyzed plasma resistin levels from patients with AP were small. Whether the association between resistin levels and acute pancreatitis was applicable in plasma should be further verified with more well-designed studies. Third, despite of elevated resistin levels in patients with severe AP, whether an increase in resistin levels is a cause for onset and exacerbation of acute pancreatitis or an epiphenomenon remains unclear. We should recognize the clinical significance of elevated peripheral resistin levels dialectically. More basic studies were needed to confirm the causal relationship of resistin and acute pancreatitis. Four, more longitudinal studies on resistin levels and acute pancreatitis should be performed to explore the development of acute pancreatitis, instead of cross-sectional studies.
Conclusions
Our meta-analysis suggested that increased resistin levels in peripheral blood may be a hallmark of severe acute pancreatitis, which may provide us some new insights about the early diagnosis. Well-designed studies are needed to verify the mechanism of peripheral resistin on the severity of AP.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
JY, ML, and JG were the main researchers in this study and took part in the study conceptualization, literature review, data extraction, analysis, and writing of the manuscript. SW and YG revised the manuscript editing. JG, YT, and XC planned the study and revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AP, acute pancreatitis; SAP, severe acute pancreatitis; CNKI, China National Knowledge Infrastructure; ES, effect size; CI, confidence interval.
References
1. Lee PJ, Papachristou GI. New insights into acute pancreatitis. Nat Rev Gastroenterol Hepatol. (2019) 16:479–96. doi: 10.1038/s41575-019-0158-2
2. Tang Y, Sun M, Liu Z. Phytochemicals with protective effects against acute pancreatitis: a review of recent literature. Pharm Biol. (2022) 60:479–90. doi: 10.1080/13880209.2022.2039723
3. Iannuzzi JP, King JA, Leong JH, Quan J, Windsor JW, Tanyingoh D, et al. Global incidence of acute pancreatitis is increasing over time: a systematic review and meta-analysis. Gastroenterology. (2022) 162:122–34. doi: 10.1053/j.gastro.2021.09.043
4. Ladd AM, Conwell D, Burroughs TE, Satish M. Prior exposure to nonsteroidal anti-inflammatory drugs reduces the rate of organ failure and in-hospital mortality in acute pancreatitis. Am J Med. (2021). doi: 10.1016/j.amjmed.2021.10.020
5. Ince AT, Seven G, Koçhan K, Kiremitçi S, Yildiz K, Sentürk H. The course of acute pancreatitis in patients with different bmi groups. Pancreatology. (2022) 22:348–355. doi: 10.1016/j.pan.2022.03.009
6. Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. (2005) 5:70–5. doi: 10.1007/s11892-005-0071-7
7. Yang X, He J, Ma S, Wang T, Zhu Q, Cao F, et al. The role of comorbid hypertriglyceridemia and abdominal obesity in the severity of acute pancreatitis: a retrospective study. Lipids Health Dis. (2021) 20:171. doi: 10.1186/s12944-021-01597-4
8. Kuan LL, Dennison AR, Garcea G. Association of visceral adipose tissue on the incidence and severity of acute pancreatitis: a systematic review. Pancreatology. (2020) 20:1056–61. doi: 10.1016/j.pan.2020.05.027
9. Nawaz H, Koutroumpakis E, Easler J, Slivka A, Whitcomb DC, Singh VP, et al. Elevated serum triglycerides are independently associated with persistent organ failure in acute pancreatitis. Am J Gastroenterol. (2015) 110:1497–503. doi: 10.1038/ajg.2015.261
10. Navina S, Acharya C, DeLany JP, Orlichenko LS, Baty CJ, Shiva SS, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. (2011) 3:107ra10. doi: 10.1126/scitranslmed.3002573
11. Kibar YI, Albayrak F, Arabul M, Dursun H, Albayrak Y, Ozturk Y. Resistin: new serum marker for predicting severity of acute pancreatitis. J IntMed Res. (2016) 44:328–37. doi: 10.1177/0300060515605428
12. Ye C, Wang R, Wang M, Huang Z, Tang C. Leptin alleviates intestinal mucosal barrier injury and inflammation in obese mice with acute pancreatitis. Int J Obes (Lond). (2018) 42:1471–9. doi: 10.1038/s41366-018-0125-y
13. Dikmen K, Bostanci H, Gobut H, Yavuz A, Alper M, Kerem M. Recombinant adiponectin inhibits inflammation processes via Nf-Kb pathway in acute pancreatitis. Bratisl Lek Listy. (2018) 119:619–24. doi: 10.4149/BLL_2018_110
14. Yu P, Wang S, Qiu Z, Bai B, Zhao Z, Hao Y, et al. Efficacy of resistin and leptin in predicting persistent organ failure in patients with acute pancreatitis. Pancreatology. (2016) 16:952–7. doi: 10.1016/j.pan.2016.09.002
15. Sharma A, Muddana V, Lamb J, Greer J, Papachristou GI, Whitcomb DC. Low serum adiponectin levels are associated with systemic organ failure in acute pancreatitis. Pancreas. (2009) 38:907–12. doi: 10.1097/MPA.0b013e3181b65bbe
16. Shuldiner AR, Yang R, Gong DW. Resistin, obesity, and insulin resistance–the emerging role of the adipocyte as an endocrine organ. N Engl J Med. (2001) 345:1345–6. doi: 10.1056/NEJM200111013451814
17. Taouis M, Benomar Y. Is resistin the master link between inflammation and inflammation-related chronic diseases? Mol Cell Endocrinol. (2021) 533:111341. doi: 10.1016/j.mce.2021.111341
18. Ebihara T, Matsumoto H, Matsubara T, Matsuura H, Hirose T, Shimizu K, et al. Adipocytokine profile reveals resistin forming a prognostic-related cytokine network in the acute phase of sepsis. Shock. (2021) 56:718–26. doi: 10.1097/SHK.0000000000001756
19. Kollari E, Zografou I, Sampanis C, Athyros VG, Didangelos T, Mantzoros CS, et al. Serum adipokine levels in patients with type 1 diabetes are associated with degree of obesity but only resistin is independently associated with atherosclerosis markers. Hormones (Athens). (2022) 21:91–101. doi: 10.1007/s42000-021-00328-9
20. Deb A, Deshmukh B, Ramteke P, Bhati FK, Bhat MK. Resistin: a journey from metabolism to cancer. Transl Oncol. (2021) 14:101178. doi: 10.1016/j.tranon.2021.101178
21. Kwak MS, Lim JW, Kim H. Astaxanthin inhibits interleukin-6 expression in cerulein/resistin-stimulated pancreatic acinar cells. Mediators Inflamm. (2021) 2021:5587297. doi: 10.1155/2021/5587297
22. Schäffler A, Hamer O, Dickopf J, Goetz A, Landfried K, Voelk M, et al. Admission resistin levels predict peripancreatic necrosis and clinical severity in acute pancreatitis. Am J Gastroenterol. (2010) 105:2474–84. doi: 10.1038/ajg.2010.278
23. Al-Maramhy H, Abdelrahman AI, Sawalhi S. Resistin is not an appropriate biochemical marker to predict severity of acute pancreatitis: a case-controlled study. World J Gastroenterol. (2014) 20:15351–7. doi: 10.3748/wjg.v20.i41.15351
24. Karpavicius A, Dambrauskas Z, Gradauskas A, Samuilis A, Zviniene K, Kupcinskas J, et al. The Clinical value of adipokines in predicting the severity and outcome of acute pancreatitis. BMC Gastroenterol. (2016) 16:99. doi: 10.1186/s12876-016-0514-4
25. Kisaoglu A, Aydinli B, Ozturk G, Atamanalp SS, Ozogul B, Yildirgan MI, et al. Severity markers in patients with acute pancreatitis. Cent Eur J Med. (2014) 9:556–64. doi: 10.2478/s11536-014-0501-5
26. Singh AK, Dawra S, Rana S, Gupta P, Samanta J, Sinha SK, et al. Can serum resistin predict severity of acute pancreatitis? Biomarkers. (2021) 26:31–7. doi: 10.1080/1354750X.2020.1841295
27. Zhu QY, Wang MC, Tao QY, Zhou LH Li LL, Xue YS. Analysis of value of dynamic changes of monocyte chemotactic protein-1 and interleukin 8 in assessment of severity of acute pancreatitis. Chinese J General Surgery. (2017) 26:1365–70. doi: 10.3978/j.issn.1005-6947.2017.10.024
28. Wang SQ, Wang XL, Wang L, Lv FB, Jin WL, HQ X. Concentration changes of neutrophil gelatinase-associated lipocalin in peripheral blood plasma of acute pancreatitis patients and their significance. Chinese J General Surgery. (2017) 26:1217–22. doi: 10.3978/j.issn.1005-6947.2017.09.022
29. Yuan SL, Ding MS, Huang JY, Zhang GX. Prognostic values of serum resistin in patients with acute pancreatitis. Chi J Experimental Surgery. (2013) 30:2209–10. doi: 10.3760/cma.j.issn.1001-9030.2013.10.070
30. Chen J, Zeng SP, Tang J, Tan Y. Effect of somatostatin on serum resistin in patients with acute pancre-atitis. Modern Prevent Med. (2012) 39:786–7+9.
31. Li QX, Yang WY, Yang Z, Sun P, Rao M. Evaluation of the adipocytokine in the prediction of severity in acute pancreatitis. Chin J Lab Med Diagnosis. (2010) 14:1799–801. doi: 10.3969/j.issn.1007-4287.2010.11.043
32. Sciskalska M, Marek G, Grzebieniak Z, Milnerowicz H. Resistin as a prooxidant factor and predictor of endothelium damage in patients with mild acute pancreatitis exposed to tobacco smoke xenobiotics. Mediators Inflamm. (2017) 2017:3039765. doi: 10.1155/2017/3039765
Keywords: acute pancreatitis, resistin, serum, plasma, association
Citation: Yang J, Liu M, Wang S, Gan Y, Chen X, Tao Y and Gao J (2022) Alteration of Peripheral Resistin and the Severity of Acute Pancreatitis: A Meta-Analysis. Front. Med. 9:915152. doi: 10.3389/fmed.2022.915152
Received: 07 April 2022; Accepted: 16 May 2022;
Published: 13 June 2022.
Edited by:
Phunchai Charatcharoenwitthaya, Mahidol University, ThailandReviewed by:
Surasak Saokaew, University of Phayao, ThailandPochamana Phisalprapa, Mahidol University, Thailand
Copyright © 2022 Yang, Liu, Wang, Gan, Chen, Tao and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junwei Gao, bnV0ZGdqdyYjeDAwMDQwOzE2My5jb20=; Yang Tao, dGFveWFuZzQ4MzMmI3gwMDA0MDsxMjYuY29t; Xiangyu Chen, eGlhbmd5dWMmI3gwMDA0MDtob3RtYWlsLmNvbQ==
 Jianhua Yang
Jianhua Yang Mengyao Liu2
Mengyao Liu2 Junwei Gao
Junwei Gao