- 1Medical Intensive Care Unit, Hôpital Saint-Louis, Assistance Publique des Hôpitaux de Paris, Paris, France
- 2INSERM UMR 976, University of Paris Cité, Paris, France
The vascular endothelium is crucial for the maintenance of vascular homeostasis. Moreover, in sepsis, endothelial cells can acquire new properties and actively participate in the host's response. If endothelial activation is mostly necessary and efficient in eliminating a pathogen, an exaggerated and maladaptive reaction leads to severe microcirculatory damage. The microcirculatory disorders in sepsis are well known to be associated with poor outcome. Better recognition of microcirculatory alteration is therefore essential to identify patients with the worse outcomes and to guide therapeutic interventions. In this review, we will discuss the main features of endothelial activation and dysfunction in sepsis, its assessment at the bedside, and the main advances in microcirculatory resuscitation.
Introduction
Sepsis is a life-threatening condition defined by multi-organ dysfunction secondary to a dysregulated host response to infection (1). Incidence of sepsis represents one of the leading causes of hospitalization in the Intensive Care Unit (ICU) and mortality remains high despite several improvements in early resuscitation (2).
The vascular endothelium consists of a single cell layer at the interface of the circulating blood and vessel wall. Composed of about 1013 cells representing 1.5 kg, the endothelium maintains microvascular homeostasis, regulating vascular tone, primary hemostasis, and cellular traffic. By its privileged localization, the vascular endothelium plays a crucial role in the response to infection. However, the exaggerated host response can cause structural and functional endothelial damage. Thus, most endothelial functions are disrupted in sepsis, leading to microthrombi, tissue edema, interstitial leakage, and dysregulated vascular tone.
Experimental and clinical studies have evidenced microcirculatory abnormalities in sepsis, which are strongly associated with organ dysfunction and mortality (3). The correction of systemic hemodynamic variables fails to restore microcirculatory perfusion and suggests a need for new axes and new therapeutics.
In this review we will discuss the key role of the endothelium in the microvascular response to sepsis.
Vascular Endothelium
Structure
The vascular endothelium, comprising a cell monolayer, covers the interior surface of blood vessels all along the vasculature. About 1013 endothelial cells (ECs), covering 1000 m2, provide a direct interface between the circulating blood cells and the vessel wall. ECs share common properties but are also heterogeneous according to specific organs and vascular beds, both concerning their structure and their function (4). This also implies distinct responses to pathological conditions with various structural and functional modifications.
At their surface, ECs are covered by a multicomponent layer, the glycocalyx, consisting of proteoglycans, glycoprotein, and glycosaminoglycans (5).The glycocalyx constitutes a first-line barrier which provides the regulation of cellular and molecular traffic. In addition, because of its negative electrical charge, the glycocalyx acts as an anticoagulant layer. The glycocalyx participates in vascular homeostasis as a vascular barrier, powerful antioxidant, and transducer of shear stress to the endothelium (6).
Regarding its weight and its numerous functions, the endothelium should be considered as a fully-fledged organ.
Resting Endothelium
The integrity of ECs is a chief regulator of vascular homeostasis. The vascular endothelium has a fundamental role in several physiologic processes such as vasomotor tone regulation, primary hemostasis, osmotic balance, and vascular barrier function. ECs also perform important immunologic functions. By sensing pathogen components present in blood, ECs can initiate the immune response. ECs are also conditional antigen-presenting cells (APCs) and, in some specific situations, allow the initiation of adaptive immunity (7). In normal conditions, ECs do not interact with circulation leukocytes, mainly because of the glycocalyx barrier and internalized membrane adhesion molecules.
Primary hemostasis is the process in which platelets adhere, activate, and aggregate to restore vascular integrity after an aggression. The endothelium synthesizes and expresses key hemostasis regulating factors, such as the von Willebrand factor (vWF) and tissue factor (TF). When the endothelium is damaged, the vWF is exposed to the circulation and can initiate platelet recruitment to the lesion. Then, binding to glycoproteins GPIa, GPVI, GPIb-IX-V, platelets activate and can enhance recruitment and activation of circulating platelets in order to form a clot.
At rest, the endothelium has anticoagulant and profibrinolytic properties. While the endothelium is one of the main producers of TF, it also negatively regulates the TF pathway, producing the TF pathway inhibitor (TFPI). TFPI limits thrombin generation, binding to activated factor X and inhibiting aFVII complex. In addition, ECs are responsible for thrombomodulin (TM) production and release. TM, combined with endothelial protein C receptor (EPCR), regulates activated protein C, which inhibits factor V, factor VIII, and Plasminogen activation inhibitor (PAI-1). Beside its role of co-factor, TM has its own anticoagulant properties. ECs also express and release tissue plasminogen activator (t-PA), the main initiator of fibrinolysis.
The endothelium is the main regulator of the vasomotor tone via its capacity to produce and release vasoactive substances in response to several environment signals. Nitric oxide (NO) is the most important vasodilator agent and is constitutively produced by ECs. NO is generated by the endothelial nitric oxide synthase (eNOS) and derived from L-arginine. NO is constitutively released by ECs and eNOS is induced by chemical (ADP, bradykinin) or physical (shear stress) factors to adapt blood flow to various conditions. Then, NO applies its vasodilatory function, diffusing in vascular smooth cells to activate cyclic GMP production by guanylate cyclase. Moreover, besides its vasoactive action, NO possesses many other biological properties (e.g., anti-agregant, endothelial cell growth) contributing to vascular homeostasis.
Endothelium In Sepsis
Because of their privileged contact with circulating blood, ECs are the first to interact with the microbial component and act as a “sentinel” for circulating micro-organisms. Moreover, the vascular endothelium is very sensitive to various stimulating factors that induce different phenotypes and initiate the immune response to infection.
ECs express Toll-like receptors (TLRs), surface receptors recognizing a pathogen antigen, such as Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs). Some TLRs are ubiquitous whereas others depend on organs or are expressed under specific circumstances. Moreover, TLR expression and signalization can be modulated by inflammatory cytokines (8). ECs predominantly express TLR4, which is the main receptor for lipopolysaccharide (LPS), a Gram-negative bacteria molecule. However, TLR2 expression might also be triggered by inflammatory conditions. This recognition initiates intracellular signalization, resulting in expression of proinflammatory transcription factors such as Nuclear factor of the k-chain in B cells (NF-kB) (9).
Endothelial Activation
During sepsis, activated ECs recruit immune cells (leukocytes) at the site of infection to eliminate the micro-organism and limit the spread of infection.
Proinflammatory Phenotype
NF-kB is a ubiquitous transcription factor involved in all major inflammatory reactions. It is implicated in cytokine production, molecule expression, cell survival, and differentiation (10). NF-kB activity can be induced in ECs by several stimuli, including inflammatory cytokines [Tumor necrosis factor (TNFα), Interleukin (IL-1)], and microbial components (LPS). Moreover, inhibiting NF-kB activity in mice stimulated by LPS led to decreased tissue neutrophilic infiltration and damage, suggesting its central role in sepsis (11).
During infection, ECs reprogram toward a proinflammatory and secretory phenotype. Numerous proteins produced by ECs are stocked in intracellular vesicles, the Weibel-Palade bodies. Under stimulation, ECs degranulate and release the content of these bodies (TF, P-selectin, vWF, angiopoietin-2) into the vasculature. ECs may then produce and release proinflammatory cytokines (IL-6) in the circulation, amplifying and spreading the inflammatory response in order to recruit immune cells at the infected site.
Pro-adhesive Phenotype
Activated endothelium and shedding of the glycocalyx in septic conditions lead to increased membrane expression of adhesion molecules that mediate leukocyte trafficking and recruitment to the area of infection. First, the selectins (E- and L-selectin) orchestrate the first phase of contact, rolling between circulating leukocytes and the endothelium. Then, prolonged and firm adhesion depends on immunoglobulin molecules, intercellular adhesion molecules (ICAM-1, ICAM-2), and the vascular adhesion molecule (VCAM). Finally, trans endothelial migration involves disruption of the tight junction and platelet endothelial cell adhesion molecule (PECAM). The final goal of this process is the diapedesis of leukocytes and extravasation into the tissues. The infiltration of immune cells in infected tissues is crucial to eliminate the pathogen. In experimental studies, LPS-exposed ECs expressed ICAM-1 and E-selectin mRNA, and blocking proinflammatory cytokines resulted in inhibition of mRNA transcription of adhesion molecules (12). Moreover, ECs release adhesion molecules in the circulation. E-selectin (13) and ICAM1 (14) plasma levels have been found to be increased in septic patients. The soluble circulating form of the adhesion molecules are increased in septic patients compared to patients admitted for a trauma (15), and the plasma levels of these adhesion molecules are closely related to sepsis severity (16).
Procoagulant Phenotype
In sepsis, ECs acquire procoagulant and antifibrinolytic properties which help to prevent dissemination of infection. Either expressed by activated immune cells or released from Webel-Palade bodies, TF level is increased in septic conditions. TF then initiates the coagulation cascade. In parallel, anticoagulant proteins [protein C, TFPI (17), TM] are downregulated by increased consumption and decreased production. Endothelial TM expression is decreased in sepsis and inactivated by leukocyte elastase release, limiting protein C activation. Skin biopsies of patients with purpura fulminans revealed a decreased expression of endothelial TM and ECPR in parallel with low blood levels of protein C, protein S and antithrombin (AT) (18). Moreover, decreased circulating activated protein C in neutropenic septic patients was associated with disease severity (19). In addition, the endothelium releases large amounts of PAI-1 when stimulated by IL-1 and TNF-α, resulting in an antifibrinolytic condition (20).
In addition, degradation of glycocalyx and EC apoptosis induce the release of large amounts of vWF. This permits the recruitment and aggregation of platelets at the infected site.
Endothelium activation is therefore an appropriate and necessary response of the host to an acute infection. Thus, this microcirculatory response (leukocyte recruitment, coagulation) is adaptive and often successful in localizing and eliminating infectious insults (21). However, in extreme cases of overwhelming infection, these processes may contribute to overall morbidity, organ failure, and death. The challenge is in the balance between the adaptative immune response and endothelial activation to control the infectious process and excessive and maladaptive response. Moreover, this is a dynamic process and an adaptative response at one timepoint can become deleterious at another (Figure 1).
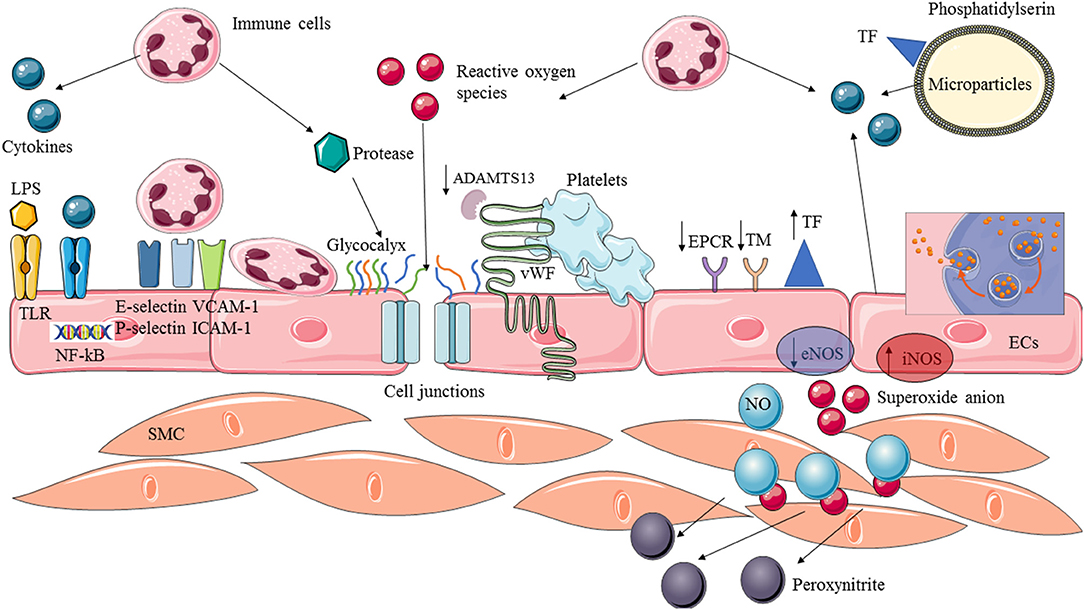
Figure 1. Endothelial activation and dysfunction in sepsis. Endothelial cells are provided with membrane receptors (ex:TLRs) and can be activated by various stimuli factors such as microbial components (LPS) or pro-inflammatory cytokines. The recognition of circulating stimuli leads to the activation of NF-kB transcription factor allowing expression of adhesion molecules and switch to a pro-inflammatory and pro-coagulant phenotype. Circulating leukocytes are recruited, adhere to the endothelium and extravasate into the tissue. Pro-coagulant protein are overexpressed whereas anticoagulant and profibrinolytic proteins are downregulated. Cytokines, ROS and protease released by activated immune cells can cause structural damage, leading to an increased permeability and exposition of endothelial-derived proteins. Vasoreactivity is impaired by (1) decreased production of NO by the eNOS, and (2) complexed available NO with superoxide anion to produce peroxynitrite. Moreover, ECs play a crucial role in spreading the inflammatory reaction which, in turn, produces cytokines and free radicals, and the release of microparticles. ADAMTS13, A disintegrin and metalloprotease with thrombospondin type I repeats-13; ECs, Endothelial cells; SMC, Smooth muscular cells; TF, Tissue factor; TM, Thrombomodulin; EPCR, Endothelial protein C receptor; vWF, Von Willebrand factor; LPS, Lipopolysaccharide; TLR, Toll like receptor; eNOS, Endothelial nitric oxide synthase; iNOS, Inducible nitric oxide synthase; NO, Nitric oxide; ICAM, Intercellular adhesion molecules; VCAM, Vascular cells adhesion molecules; NF-kB, Nuclear factor of k-chain of B.
Microvascular Endothelial Dysfunction
Endothelial dysfunction is defined by the loss of or exaggerated endothelial function.
The uncontrolled amplification of the host's proinflammatory response can lead to septic shock and the failure of distant, non-infected organs.
Structural Damage/Increased Permeability
In animal models of sepsis, ECs experienced morphological changes, including nuclear and cytoplasm lesion, rupture of cells, and even apoptosis. These kinds of damage have been confirmed in septic patients who demonstrate an increased number of circulating ECs and apoptotic bodies (22).
During sepsis, the damaged glycocalyx exposes a denuded endothelium. In an endotoxemic mouse model, the authors revealed pulmonary microvascular glycocalyx degradation related to TNF-alpha and heparinase. Interestingly, in this experimental study, inhibiting heparinase maintained glycocalyx integrity and reduced neutrophil adhesion and tissue damage (23). There are several consequences for the microcirculation following glycocalyx degradation, including decreased capillary density and increased permeability of macromolecules, although there are no macrohemodynamic changes (24).
In septic patients, glycocalyx impairment can be evidenced by the presence of glycocalyx shedding components in the circulation. The levels of circulating serum hyaluronan and syndecan are associated with the prognosis of septic patients (25). Moreover, new techniques can evaluate glycocalyx thickness in sublingual microcirculation. The decreased microvascular glycocalyx thickness in sepsis is correlated to microcirculation perfusion (26) and is an early predictor of mortality (27).
In addition, several pathways, like the Angiopoietin/Tie-2 (Ang/Tie2) axis, are altered during sepsis. Ang/Tie2 signaling is critical for maintaining vascular barrier integrity. During sepsis, the Ang/Tie2 system is disrupted, leading to increased microvascular permeability which contributes to organ failure and death (28). Moreover, the neutrophilic accumulation may enhance tissue and cell damage by generating inflammatory cytokines, reactive oxygen, and proteases (29).
All together, these structural damages imply disrupted barrier function and increased permeability, leading to interstitial leakage and edema. Moreover, the denuded endothelium and apoptotic ECs display a proinflammatory and procoagulant phenotype, enhancing the systemic response to infection. Furthermore, leukocyte adherence to the endothelium may participate in microvascular blood flow alterations (30).
Impaired Vasoreactivity
Microvascular endothelium is the chief regulator of vascular tone, mainly through the production of NO, a vasoactive soluble gas. During sepsis, NO production is dysregulated, with various changes depending on the course of the disease, leading to an impaired endothelium-dependent vasorelaxation and ultimately an alteration of microvascular blood flow.
First, there is a decreased production of NO by endothelial NO synthase, precipitating so-called “nitrosopenia”. The causes are multifactorial, with the downregulated expression of eNOS mRNA (31), a modified membrane receptor, and impaired signal transduction (32). Moreover, septic ECs are depleted of tetrahydrobiopterin, the limiting substrate for eNOS, uncoupling the enzyme and resulting in production of superoxide anion instead of NO.
The inducible NO synthase (iNOS) is also increased during sepsis (33). iNOS produces large amounts of NO, about 1,000-fold more than eNOS, and is responsible for intense and diffuse microvascular vasodilatation. This effect can cause an impaired response to norepinephrine. Moreover, the overproduced NO can complex with oxygen reactive species (superoxide anion) to generate peroxynitrite, a highly cytotoxic oxidant product. The rapid formation of peroxynitrite in an oxidative environment leads to reduced NO availability.
Overall, sepsis is characterized by a decrease of NO bioavailability and impaired vasoreactivity. Moreover, besides its effects on vascular tone, the lack of NO also results in dysregulated platelet adhesion and endothelium integrity. However, the pathophysiology of NO in sepsis is not fully understood as showed by the controversial therapeutic interventions targeting NO.
Oxidative Stress
Large amounts of reactive species are produced during sepsis, causing structural and cellular damage (41). There is an imbalance in the production of reactive species and a decrease in antioxidant agents. Firstly, oxidative species are produced in large amounts by inflammatory cells (42) and play a role in cell adhesion and inflammatory reaction (43). Besides being a target, ECs also produce and release reactive species (superoxide anion, hydrogen peroxide, radical hydroxyl) in sepsis through the NADPH pathway (44).
Inside cells, the accumulation of reactive species in the form of hydrogen peroxide and peroxynitrite causes protein and DNA damage, contributing to endothelial dysfunction (45). Moreover, besides complexing the NO to generate highly unstable peroxynitrite, oxidant agents induce uncoupling of eNOS (46), thus creating a vicious circle and amplifying oxidative stress (47). Overall, this results in decreased NO bioavailability. On the other hand, reactive oxygen and nitrogen species can enhance NF-kB activation and thus endothelial activation (48).
Microthrombi
Dysfunctional endothelium activates primary hemostasis and coagulation in a supra-physiologic way by decreased anticoagulant signaling. Generalized activation of coagulation during sepsis may enhance widespread microvascular injury (49, 50).
Autopsy studies of patients who died from Streptococcus suis infection found multiple microthrombi in the capillaries of the lung, kidney, and intestine (51). Several post-mortem studies have confirmed disseminated microthrombosis in the kidney, liver, brain, and gut microcirculation of septic patients (52).
Therefore, a new pathogenic entity in sepsis was defined as “endotheliopathy-associated vascular microthrombotic disease” involving microthrombosis mainly through unusually large vWF multimers (53). Indeed, sepsis may lead to acquired a disintegrin and metalloprotease with thrombospondin type I repeats-13 (ADAMTS13) deficiency since inflammatory mediators can inactivate ADAMTS13. ADAMTS13 is a metalloproteinase that cleaves the multimers of vWF in order to limit platelet activation in physiological conditions. Sepsis results in overexpressed vWF from ECs and a decreased availability of ADAMTS13. Then, large multimers of vWF recruit circulating platelets, thereby contributing to platelet-endothelium interactions (54, 55).
In parallel to leukocyte adherence to the endothelium (30), the presence of disseminated microthrombosis may participate in alterations to the microvascular blood flow (56). Platelet-to-endothelium adhesion is associated with stopped-flow capillaries and the inhibiting adhesion process may lead to an improved microvascular blood flow (57).
Microparticles
Microparticles (MPs) are small vesicles derived from activated or apoptotic cells which are released into the circulation with the detachment of cell membrane. MPs are embedded with various surface antigens. In physiological conditions, microparticles are essential to intercellular traffic and act as messengers. In response to an inflammatory environment, ECs release microparticles that can activate coagulation, amplifying the inflammatory and procoagulant response and dispersing it away from the initial site of infection. In sepsis, MPs express proinflammatory and procoagulant mediators. Endothelium-derived MPs expose phosphatidylserine at their surface, providing a privileged site to initiate coagulation cascade (58). Moreover, MPs express high levels of TF, which contribute to coagulation activation (59).
Delabranche and co-authors have suggested that circulating levels of endothelium-derived MPs in the plasma of septic patients may be a good predicting factor of outcome (60).
Microcirculation In Sepsis
The microcirculation, composed of a series of <100 micron-diameter arterioles, venules and capillaries, is the terminus of the vascular tree (61). Arterioles mostly guarantee vascular tone because of their muscular surface, whereas capillaries provide oxygen delivery, nutriments, and solute exchange depending on the tissue need (61).
Sepsis is associated with profound changes in microcirculation (62).
Microcirculatory Disorders
Microcirculatory alterations in sepsis mainly consist of a decreased capillary density and an increased heterogeneity of blood flow. As compared to hemorrhagic shock, endotoxemic shock leads to considerably greater impaired gut microvascular perfusion (63). During sepsis, microcirculation suffers from quantitative and qualitative alterations.
Firstly, sepsis is associated with a decrease in functional capillary density and a reduction in the proportion of perfused small vessels, whereas stopped and intermittently perfused capillaries are increased (64, 65). Altered capillary density has been observed in gut (64), brain (65), skin, and tongue (66) microcirculation in various septic animal models.
Sepsis-associated microcirculatory dysfunction is responsible for suboptimal capillary perfusion, resulting in impaired oxygen extraction by tissues, a finding which is not explained by impaired delivery of oxygen by systemic circulation (67). It has been shown that muscular microcirculation in a cecal ligation and puncture (CLP) rat model heterogeneously delivers oxygen to capillaries (68). Heterogeneous perfusion is defined by the presence of intermittently or non-perfused capillaries close to well perfused capillaries, and this process is dynamic over time. Minimal under physiological conditions, the heterogeneity of perfusion is highly increased in sepsis. Thus, heterogeneous perfusion exists between organs, with redistribution of blood flow to vital organs at the expense of other tissues, but it is also present within the same organ. Experimental studies evidenced microvascular shunting in sepsis, especially in the gut, creating local zones of hypoxia (69, 70). One of the main features of microvascular shunting can be attributed to the heterogeneity of perfusion (71).
The gut and splanchnic circulation represents an elective measurement site because of its early impairment in sepsis. Experimental studies provided evidence that the disturbance in oxygen extraction was related to the heterogeneity of microcirculatory perfusion. Heterogeneous perfusion is therefore considerably more deleterious than uniformly perfused capillaries regarding tissue oxygen extraction (72).
This heterogeneous perfusion also implies different expression of key proteins, enzymes, and molecules, which are increased during sepsis. For example, in endotoxemia rats, iNOS is differently expressed along the gut tractus, suggesting that the vasomotor tone within the gut is different (73). This observation has been confirmed in a sheep sepsis model in which animals were treated with iNOS inhibitors resulting in various microvascular blood flow responses in the gut, pancreas, and spleen (74).
Together, the decreased capillary density and heterogeneous alterations in microcirculation contribute to an increased diffusion distance for oxygen and an impaired extraction capacity, thus creating hypoxic zones. Heterogeneous expression of hypoxic genes within myocardial microcirculation has been demonstrated in a murine model of sepsis. Indeed, during entoxemia, Hypoxia inducible factor (HIF)-1a and Glucose transporter (GLUT)1 expression co-existed with ICAM-1 expression in malfunctioning capillaries, suggesting that microcirculatory alterations were associated with hypoxic zones (75).
Association Between Microvascular Dysfunction And Outcome
Hemodynamic coherence is the hypothesis that normalization of systemic variables will be accompanied by improvement of microcirculatory perfusion in resuscitated patients (76). In experimental studies, microcirculatory disorders poorly correlate with systemic variables. Several authors have highlighted the loss of hemodynamic coherence in critically ill patients, especially in sepsis. The resulting consequences are: (1) conventional systemic parameters fail to reflect the state of microcirculation; (2) therapeutic strategies which aim to correct macrohemodynamic parameters are probably ineffective in improving microcirculation.
Knowing that microcirculatory disorders are central to the pathophysiology of sepsis, it is essential to assess their clinical relevance in the course of the disease and in patient outcome. Although macrohemodynamic parameters can be improved with conventional resuscitation interventions, the persistence of microcirculation abnormalities may explain unfavorable outcomes. Several experimental studies have found a correlation between survival and microcirculatory perfusion (77). Moreover, Zhang et al. provided strong evidence that micro- and macrocirculation are dissociated in endotoxemic shock, and that microcirculatory disorders precede the alterations in macrocirculation (78). Sakr and colleagues (3) demonstrated that, with equal baseline alterations, sublingual capillary density increased over the time course of septic shock in survivors, whereas this did not occur in non-survival patients. These microvascular alterations could predict ICU mortality contrary to global hemodynamic parameters. In a pilot observational study, the authors assessed sublingual microvascular perfusion by Sidestream Dark Field (SDF) in the early course of sepsis, with repeated measures during the early goal-directed therapy. They found that an improvement in microcirculatory perfusion during resuscitation was associated with less organ failure (79). Finally, De Backer et al. (80) confirmed the independence of micro- and macrovascular parameters and the strong association of sublingual microcirculatory parameters with outcome.
Although it is difficult to assess a causal relationship in the absence of randomized controlled trials, the development of more accurate techniques allows better identification of prognostic factors. Concordant data found several microcirculatory variables to be independent prognostic factors in sepsis and septic shock. Thus, the loss of hemodynamic coherence, and the association of microcirculatory disorders with outcome, support the extension of microcirculatory assessments and the use of microvascular variables to guide resuscitation.
Microcirculatory Dysfunction Assessment
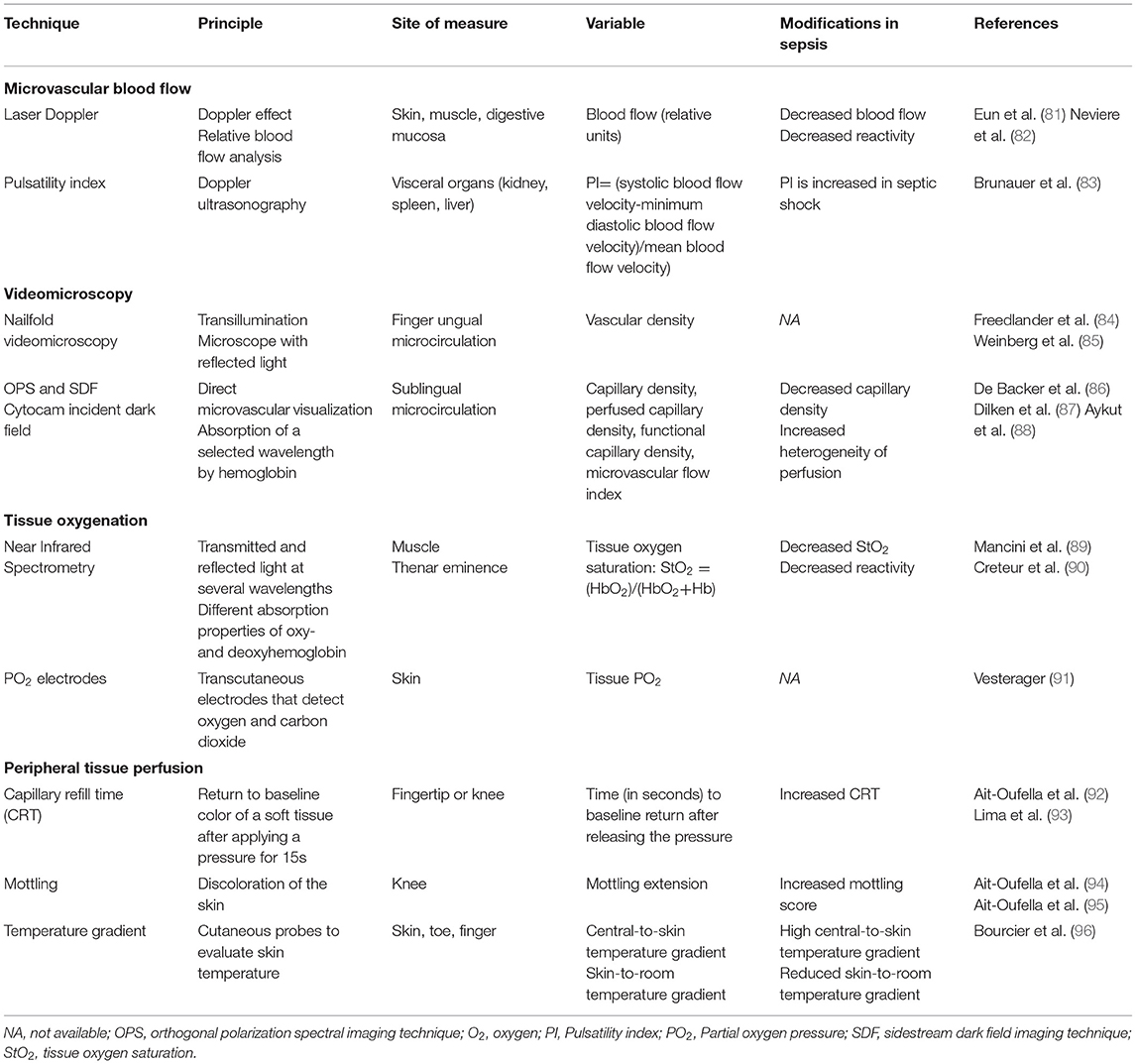
Microcirculatory dysfunction plays a crucial role in the pathophysiology of sepsis. Early recognition of critical illness severity, leading to an early appropriate resuscitation, is essential to improve outcome (1). For a long time, management of critically ill patients has focused on restoring systemic hemodynamic parameters. Indeed, patient monitoring has comprised assessment of macrohemodynamic parameters such as arterial pressure, and both urine and cardiac output. However, in the past few years, it has been shown that classic hemodynamic parameters fail to identify microcirculatory disorders. Beyond classic systemic monitoring, we need specific tools to assess microcirculation dysfunction (Table 1).
Microcirculatory Perfusion
Evidence for microcirculatory dysfunction was first found in experimental studies using intravital microscopy, allowing direct visualization of microcirculation with large microscopes on fixed tissues. Bedside assessment of microcirculation in critically ill patients is challenging, as intravital microscopy cannot be used in humans. The main concerns are the site of measurement, the timing (80), and the availability of validated tools. In the past decade, several techniques have been examined in the study of microcirculation.
Microvascular Blood Flow
The Laser Doppler flowmetry technique applies the Doppler effect, in which laser light shifts its frequency after reflecting from red blood cells. Laser Doppler provides a measure of relative microcirculatory blood flow defined by the average of the velocities in vessels in the area of interest. Thus, this technique does not give an absolute value of blood flow but a quantitative index of flux (81).
Laser Doppler flowmetry can be performed in skin, muscle, and mucosal microcirculation. Interestingly, gut mucosal perfusion is accessible to Laser Doppler using specific probes, and is of particular interest in critically ill patients to assess visceral organ perfusion (97).
This technique allows continuous microcirculatory blood flow recording and measurement of microvascular blood flow reactivity to various interventions (vascular occlusion test, pharmacologic products) (98). The method is advantageous in critically ill patients as it can be applied to the skin, an easily accessible site. It has been observed that basal blood flow is decreased in critically ill patients as compared to healthy volunteers. Moreover, vascular reactivity is impaired during sepsis (82).
The main limitation of this technique is the relatively large sample volume of analysis (0.5–1 mm3) in which arterioles, capillaries, and venules of diverse size and perfusion are analyzed within the same timeframe. In sepsis, where perfusion is particularly heterogeneous, even in the same area, this method may miss some perfusion abnormalities.
Pulsatility Index
The pulsatility index (PI) is used to estimate the organ vascular tone rather than to directly assess blood flow. The PI is assessed using a Doppler sonography technique which can explore visceral organs [kidney (99), spleen, liver (100)]. Using color Doppler, an artery of interest is identified and pulse wave Doppler is performed. The resulting blood flow signal is recorded, and PI is calculated as the ratio of systolic blood flow velocity-minimum diastolic blood flow velocity/mean blood flow velocity.
Brunauer and colleagues (83) reported alteration of the PI of the liver, spleen, intestine, and kidney in septic shock patients, which were correlated to peripheral perfusion alterations.
Tissue Oxygenation
Near infrared spectroscopy (NIRS) is a procedure which measures tissue oxygenation. It can reach vessels at 0 to 25 mm from the applied area. Limited to vessels of <1 mm diameter, NIRS focuses on microcirculation oxygenation (89).
In critically ill patients, the thenar eminence is the preferential site of measure, limiting confounding factors such as edema. Using transmitted and reflected light at different wavelengths, tissue oxygenation is estimated by the different absorption properties of oxy- and deoxyhemoglobin. Septic patients had a reduced tissular oxygen saturation (StO2) whose value correlated with organ failure and which was associated with poor outcome (101). This technique does not assess microvascular blood flow directly; however, it can be used to evaluate vascular reactivity. Septic patients evidenced reduced vascular reactivity, defined by a decreased slope in StO2 changes after occlusion challenge (90).
Microvascular partial oxygen pressure (PO2) electrodes can be used for direct tissue oxygenation assessment. This provides a reliable tissue PO2 in conditions of homogenous microcirculation (91). However, in the context of heterogeneous microcirculation, its accuracy decreases, limiting its use in critically ill patients.
Videomicroscopy
Nailfold videomicroscopy is historically the first procedure used in the clinical setting. It relies on the principle that organs can become translucent using reflecting light (84). The nailfold area provides an easily accessible, non-invasive site for direct visualization of the microcirculation under an ordinary microscope. Ungual microvascular blood flow undergoes severe impairment under various conditions (85). However, this technique has not been studied specifically in septic patients and is limited by its sensitivity to room temperature and local vasoconstriction.
Orthogonal polarization spectral imaging (OPS) and SDF are both techniques that rely on the principle of transillumination, which permits direct visualization of the microcirculation (86). The OPS light source emits polarized light which is reflected as: (1) (still) polarized light by the superficial layers, and (2) scattered depolarized light by the deeper layers of tissue which is absorbed by red blood cells. SDF uses green light and, like OPS, the superficial layer reflected light is not analyzed by the apparatus whereas reflected light from deep tissue reaches the center of the device. Red blood cells are seen as black bodies because of absorption of the light as a selected wavelength.
These two techniques allow direct visualization of the microcirculation with high contrast images provided by the reflected light from deeper layers. However, the visualization of microcirculation can be biased by the presence of red blood cells from the vessels.
Visualization has been mainly tested and validated in sublingual microcirculation because of the thin epithelial layer providing better images (87). The analyzed variables comprise vascular density, heterogeneity of perfusion, and capillary density (102).
In septic patients, De Backer et al. evidenced a decrease in the proportion of perfused small vessels (mainly capillaries) in addition to an increase in non or intermittently perfused capillaries (30).
These alterations are depicted even in the very early stages of sepsis. In addition, there are more severe sublingual microcirculatory alterations in non-survivor patients when compared to survivors (79).
One could question whether there is a correlation between sublingual microcirculation and vital organ perfusion. In pigs, sublingual and gut perfusion were similar during sepsis (103). Boerma and co-workers showed that sublingual microcirculation alterations are related to intestinal perfusion in septic patients (104). However, sublingual assessment presents some limitations (secretion, movement artifact), and cannot be used in patients with non-invasive ventilation.
These techniques use semi-quantitative analysis to perform microcirculatory evaluations and can only be used reliably by trained investigators (105). More recently, a third generation of handheld videomicroscope has been developed based on incident dark field imaging (IDF). Illuminating the vessel on all sides, IDF provides a three-dimensional effect and allows better optical resolution compared to SDF imaging (88). The last generation, Cytocam-IDF videomicroscope is a fully digitalized device computer-controlled, with a lower weight, high resolution sensor and a shortened pulse time. Therefore, IDF overcomes many of handheld microscopy previous limitations (106). Compared to SDF, Cytocam IDF provides superior image quality and better microcirculatory analysis (107) and in a preterm neonate's population, IDF imaging allowed visualization of 20 % more vessels than SDF (108).
The development of a bedside assessment of microcirculatory blood flow and oxygenation should provide a better and earlier recognition of microcirculatory dysfunction and guide resuscitation.
However, despite recent improvements, the application of these techniques is mainly confined to the field of research and rarely available in routine practice.
Peripheral Tissue Perfusion Assessment
In critically ill patients, systemic hemodynamic parameters and biomarkers are not always an accurate reflection of microcirculatory disorders. The primary marker at the bedside is arterial lactate, which recognizes circulatory failure and guides resuscitation. However, hyperlactatemia is not specific to hypoperfusion and, in many cases, persistent hyperlactatemia is not related to circulatory dysfunction and can thus lead to over-resuscitation (92).
During septic shock, sympathetic activation redistributes blood flow toward the “noble organ” at the expense of less important tissue, such as the skin, which is deprived of autoregulation. Evaluation of perfusion of those tissues with non-invasive parameters can therefore provide a good estimation of microcirculatory disorders. Skin provides an easily accessible site at the bedside of critically ill patients.
Capillary Refill Time
The capillary refill time (CRT) is assessed by applying a pressure of 3 to 7 Newtons on the knee or on the fingertip for at least 2 s (93). There is agreement in the literature that “good” pressure is that needed to produce a “thin white distal crescent” under the physician's nail. After releasing the pressure, the time in seconds necessary to return the skin color to baseline defines CRT. It provides an easy-to-use clinical tool to assess skin perfusion and microcirculatory dysfunction. In trained physicians using standardized pressure and chronometer time recording, CRT has a good reproducibility (109).
This quantitative tool can reliably provide information on critical illness severity. Lima et al. demonstrated that a prolonged fingertip CRT longer than 4.5 s is associated with high arterial lactate level and organ failure in critically ill patients (110). In septic patients, Brunauer and colleagues showed that CRT is related to the PI (83). Moreover, the persistence of prolonged CRT (>2.4 s at the fingertip and >4.9 s at the knee) after resuscitation of septic shock patients is a reliable predictive factor of 14-day mortality (109).
Recently, it has been suggested that CRT could guide resuscitation therapeutics. The ANDROMEDA-SHOCK study (111) compared a fluid resuscitation initiation and cessation guided by arterial lactate or by CRT. The authors found no significant difference in primary outcome between the groups. However, this study suggests that a resuscitation strategy based on CRT can decrease fluid and vasopressor administration. Moreover, the latest guidelines from the Surviving Sepsis Campaign recommends taking into account CRT measurement in the management of septic shock patients (96).
Skin Temperature
The aspect of the skin in circulatory failure circumstances has historically been described as “pale and cold” (94). Using probes, skin temperature is easily accessible in critically ill patients. Septic shock patients experience increased central-to-skin and decreased skin-to-room temperature gradients. This provides quantitative information which is related to ICU mortality (95).
Mottling
Mottling is described as a purple discoloration of the skin, primarily localized in the knee area. Although the pathophysiology of mottling is not completely understood, it is related to alterations of skin perfusion (112). Ait-Oufella et al. provided a semi-quantitative evaluation score of mottling which depended on skin extension of mottling around the knee area. This score can reliably predict sepsis severity and mortality (113).
Mottling provides an excellent risk stratification tool. This is a non-invasive and easily used bedside tool, with excellent reproducibility even in non-trained clinicians. In septic shock patients, the mottling score is correlated to organ failure and is a strong predictor of 14-day mortality (114). De Moura et al. confirmed the excellent positive predictive value of a mottling score of 4 or more to predict 28-day mortality (115).
A multimodal approach taking into account these markers should be recommended for personalized management of septic patients. Lavelligrand et al. showed in a retrospective observational study that daily clinical evaluation, including the mottling score, oliguria, lactatemia, and neurological exam, may allow physicians to tolerate a mild arterial hypotension in septic patients (116).
Peripheral perfusion assessment suffers from several limitations. First, CRT and mottling are difficult to assess in patients with dark skin. Second, due to the high heterogeneity of microcirculation in organs during sepsis, these markers may not accurately reflect visceral organ perfusion.
Therapeutic Axes
Sepsis and septic shock represent one of the leading causes of critically ill mortality despite improvement in management (early antibiotic therapy, fluid resuscitation, vasopressors). Alterations in microcirculatory perfusion play a key role in the pathophysiology of sepsis and are associated with organ failure. Resuscitating the microcirculation should be considered a major therapeutic goal in septic patients (117). Considering the loss of hemodynamic coherence, one of the foremost questions is whether the most common therapeutics used to resuscitate macrohemodynamic parameters impact the microcirculation.
Fluid Resuscitation
Fluid resuscitation is the administration of fluids (crystalloids, colloids) in hypotensive patients to restore organ perfusion. However, there is some debate about the microcirculatory consequences. In an experimental study, fluid resuscitation worsened shock severity and impaired the microcirculation (118). Conversely, Santos et al. found an improvement of capillary density and blood flow following fluid resuscitation in a rodent model (77). In humans, Ospina-Tascon and team (119) found that fluid administration might improve sublingual microvascular perfusion for patients in the early sepsis phase. However, this effect was not confirmed in later phases of sepsis. Interestingly, the authors demonstrated a dissociation between micro- and macrohemodynamic response to fluid resuscitation in some patients with no microvascular response despite improvement of cardiac output. Moreover, the type of fluid used, whether colloids or crystalloids, had no impact on sublingual microcirculation in this study (120). Recently, in a pilot study of 35 septic shock patients, Hariri et al. (121) found an improvement in skin microvascular endothelial function in patients receiving albumin compared to crystalloids.
Inotropes/Vasopressors
Vasopressive and inotropic agents are used in sepsis to maintain organ perfusion pressure. However, their effects on microcirculation may vary among patients and between different organs (122). Experimental studies have suggested that dobutamine might improve hepatic (123) and mesenteric (124) but not renal perfusion (125). Using the OPS imaging technique, De Backer et al. additionally showed that administration of dobutamine partially recruited the microcirculation independently of systemic hemodynamic parameters in the early phase of sepsis. However, this effect was not maintained in the later phases of the disease (126). In a randomized controlled trial, dobutamine failed to improve sublingual, peripheral, and splanchnic perfusion in septic shock patients, when compared to placebo (127). Conversely, Morelli and team (128) found that, due to its vasodilatory effects, levosimendan significantly improved microcirculatory blood flow and increased perfused capillary density in septic shock patients, as compared to dobutamine.
Similarly, data focusing on the microvascular effects of vasopressor agents are controversial. While norepinephrine improved both hepatic (129) and renal blood flow (130) in endotoxemic shock, this observation was not confirmed in another study focusing on the liver (131) and gut (132) microcirculations. In septic shock patients, norepinephrine failed to improve sublingual microvascular blood flow despite restoring macrohemodynamic parameters (mean arterial pressure) (133). Indeed, increasing blood pressure in septic patients did not affect various microcirculatory and perfusion parameters (134). Due to the complex pathophysiology of sepsis, which implies an imbalance between vasodilatation and vasoconstriction, it is reasonable to expect that the microvascular response might be highly unpredictable.
Microhemodynamic parameter assessment is required to evaluate the effects of the main drugs used during sepsis, as well as to select the patients for randomized trials in a tailored strategy, taking into account both macro- and microhemodynamic parameters.
Many strategies targeting endothelial and microvascular dysfunction have been proposed. Therapeutic drugs focus on endothelial vasoreactivity, inflammation, oxidative stress, and coagulation/anticoagulation balance. Table 2 (34–40) presents the major recent trials focusing on microcirculation during sepsis. Overall, while some studies showed an improvement in microcirculatory parameters, very few interventions succeeded in reducing mortality in critically ill septic patients. As endothelial activation is part of the host's required response during sepsis, its inhibition may be deleterious. Conversely, targeting one pathway may not be sufficient to improve outcome, and combination therapy should be discussed for future trials.
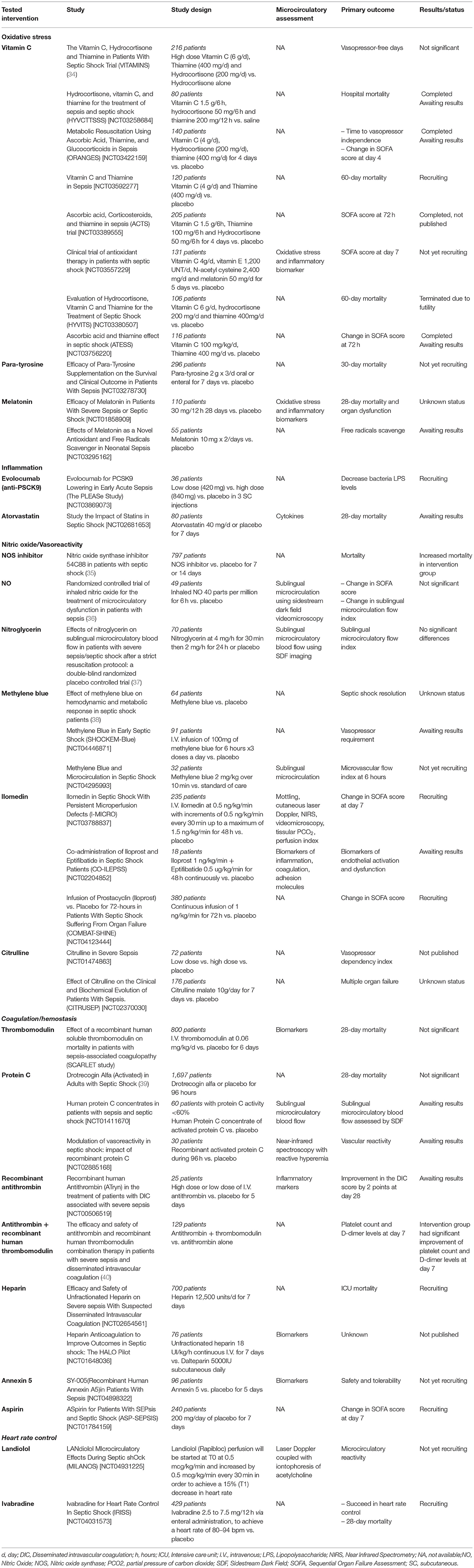
Table 2. Human randomized controlled trials targeting endothelium and microcirculation in sepsis and septic shock.
Given the encouraging results in recent studies and incoming trials, new therapeutic targets deserve attention.
In the past few years, many authors have evidenced a decrease of antioxidant defense in septic patients (135). Vitamin C, or ascorbic acid, is a well-known antioxidant molecule, easily accessible and safe to use at the bedside (136). Several trials have studied the combination of vitamin C, thiamine, and hydrocortisone in septic shock patients. A meta-analysis of nine randomized controlled trials confirmed that this combination therapy could improve Sequential Organ Failure Assessment (SOFA) score and vasopressor-free days. However its benefit for survival is still under debate (137). Recently, Lavillegrand et al. found that supplementation of vitamin C in septic shock patients with persistent peripheral tissue impairment improved skin microvascular reactivity and peripheral perfusion in patients with or without vitamin C deficiency (138).
Other studies have focused on the adrenergic system. Indeed, the deleterious effects of sympathetic overstimulation in septic shock led several authors to study beta blockers. Morelli and co-workers (139) studied esmolol in order to reduce heart rate in septic shock patients. The authors found that the use of esmolol was safe, and improved outcomes of septic shock patients, and that it also improved microvascular blood flow (140). These results led to other trials that aimed to reduce heart rate and adrenergic stress in septic patients (Table 2).
Conclusion
In the past two decades, the endothelium has been the focus of particular interest, especially in sepsis. As the major regulator of vascular homeostasis, the endothelium is one of the leading actors in response to aggression. In sepsis, the exaggerated and systemic endothelial activation leads to microcirculatory alterations which thus participate in organ failure and death. Recent advances in the assessment of the microcirculation promote a better understanding of microcirculatory impairments in sepsis. However, their use in clinical practice is limited by their availability and difficulty of use in critically ill patients with multiple confusing factors. New technical devices and clinical tools can be useful at the bedside to recognize microcirculatory impairments.
Despite improvements in patient care, sepsis and septic shock lead to high morbidity and mortality in critical care. Testing the prognostic value of microcirculatory disorders in sepsis, several trials have studied new molecules targeting endothelial functions and dysfunctions. However, despite some promising leads, the foremost studies have shown unfavorable results for outcomes of mortality or organ failure. We believe that microcirculatory resuscitation should be one of the goals in the management of septic patients. It is therefore crucial to identify microvascular endothelial dysfunction more effectively to better select patients for future trials.
Author Contributions
LR and LZ both performed the review of the literature and wrote the study. All authors contributed to the article and approved the submitted version.
Conflict of Interest
LZ has received a research grant from Jazz Pharmaceuticals, not related to this study.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Ang/Tie2, Angiopoietin-1/Tie2 receptor; APC, Antigen-presenting cell; AT, Antithrombin; CLP, Cecal ligation and puncture; CRT, Capillary refill time; DAMPs, Damage-Associated Molecular Patterns; ECs, Endothelial cells; EPCR, Endothelial protein C receptor; GLUT 1, Glucose transporter; HIF, Hypoxia inducible factor; ICU, Intensive Care Unit; IDF, Incident dark field imaging; IL-1, Interleukin; iNOS, Inducible NO synthase; LPS, Lipopolysaccharide; MPs, Microparticles; NF-kB, Nuclear factor of the k-chain in B-cells; NIRS, Near infrared spectroscopy; NO, Nitric oxide; eNOS, Endothelial nitric oxide synthase; OPS, Orthogonal polarization spectral imaging; O2, Oxygen; PAI-1, Plasminogen activation inhibitor 1; PAMPs, Pathogen-Associated Molecular Patterns; PECAM, Platelet endothelial cell adhesion molecule; PI, Pulsatility index; PO2, Partial oxygen pressure; SDF, Sidestream dark field; SOFA, Sequential Organ Failure Assessment; StO2, Tissular oxygen saturation; t-PA, Tissue plasminogen activator; TF, Tissue factor; TFPI, Tissue factor pathway inhibitor; TLR, Toll-like receptor; TM, Thrombomodulin; TNF-α, Tumor necrosis factor; VCAM, Vascular adhesion molecule; vWF, Von Willebrand factor.
References
1. S Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. (2016) 315:801–10. doi: 10.1001/jama.2016.0287
2. Dupuis C, Bouadma L, Ruckly S, Perozziello A, Van-Gysel D, Mageau A, et al. Sepsis and septic shock in France: incidences, outcomes and costs of care. Ann Intensive Care. (2020) 10:145. doi: 10.1186/s13613-020-00760-x
3. Sakr Y, Dubois M-J, De Backer D, Creteur J, Vincent J-L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. (2004) 32:1825–31. doi: 10.1097/01.CCM.0000138558.16257.3F
4. Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. (2007) 100:158–73. doi: 10.1161/01.RES.0000255691.76142.4a
5. Reitsma S, Slaaf DW, Vink H, van Zandvoort MAMJ. oude Egbrink MGA. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. (2007) 454:345–59. doi: 10.1007/s00424-007-0212-8
6. Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. (2007) 9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959
7. Pons S, Arnaud M, Loiselle M, Arrii E, Azoulay E, Zafrani L. Immune Consequences of Endothelial Cells' Activation and Dysfunction During Sepsis. Crit Care Clin. (2020) 36:401–13. doi: 10.1016/j.ccc.2019.12.001
8. Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. (2001) 166:2018–24. doi: 10.4049/jimmunol.166.3.2018
9. Faure E, Equils O, Sieling PA, Thomas L, Zhang FX, Kirschning CJ, et al. Bacterial lipopolysaccharide activates NF-kappaB through toll-like receptor 4 (TLR-4) in cultured human dermal endothelial cells. Differential expression of TLR-4 and TLR-2 in endothelial cells. J Biol Chem. (2000) 275:11058–63. doi: 10.1074/jbc.275.15.11058
10. Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med. (2016) 8:227–41. doi: 10.1002/wsbm.1331
11. Ye X, Ding J, Zhou X, Chen G, Liu SF. Divergent roles of endothelial NF-kappaB in multiple organ injury and bacterial clearance in mouse models of sepsis. J Exp Med. (2008) 205:1303–15. doi: 10.1084/jem.20071393
12. Nooteboom A, van der Linden CJ, Hendriks T. Modulation of adhesion molecule expression on endothelial cells after induction by lipopolysaccharide-stimulated whole blood. Scand J Immunol. (2004) 59:440–8. doi: 10.1111/j.0300-9475.2004.01413.x
13. Kuhns DB, Alvord WG, Gallin JI. Increased circulating cytokines, cytokine antagonists, and E-selectin after intravenous administration of endotoxin in humans. J Infect Dis. (1995) 171:145–52. doi: 10.1093/infdis/171.1.145
14. Kayal S, Jaïs JP, Aguini N, Chaudière J, Labrousse J. Elevated circulating E-selectin, intercellular adhesion molecule 1, and von Willebrand factor in patients with severe infection. Am J Respir Crit Care Med. (1998) 157:776–84. doi: 10.1164/ajrccm.157.3.9705034
15. Boldt J, Muller M, Kuhn D, Linke LC, Hempelmann G. Circulating adhesion molecules in the critically ill: a comparison between trauma and sepsis patients. Intensive Care Med. (1996) 22:122–8. doi: 10.1007/BF01720718
16. Sessler CN, Windsor AC, Schwartz M, Watson L, Fisher BJ, Sugerman HJ, et al. Circulating ICAM-1 is increased in septic shock. Am J Respir Crit Care Med. (1995) 151:1420–7. doi: 10.1164/ajrccm.151.5.7735595
17. Tang H, Ivanciu L, Popescu N, Peer G, Hack E, Lupu C, et al. Sepsis-induced coagulation in the baboon lung is associated with decreased tissue factor pathway inhibitor. Am J Pathol. (2007) 171:1066–77. doi: 10.2353/ajpath.2007.070104
18. Faust SN, Levin M, Harrison OB, Goldin RD, Lockhart MS, Kondaveeti S, et al. Dysfunction of endothelial protein C activation in severe meningococcal sepsis. N Engl J Med. (2001) 345:408–16. doi: 10.1056/NEJM200108093450603
19. Mesters RM, Helterbrand J, Utterback BG, Yan B, Chao YB, Fernandez JA, et al. Prognostic value of protein C concentrations in neutropenic patients at high risk of severe septic complications. Crit Care Med. (2000) 28:2209–16. doi: 10.1097/00003246-200007000-00005
20. Levin EG, Marotti KR, Santell L. Protein kinase C and the stimulation of tissue plasminogen activator release from human endothelial cells. Dependence on the elevation of messenger. RNA J Biol Chem. (1989) 264:16030–6. doi: 10.1016/S0021-9258(18)71583-4
21. Echtenacher B, Weigl K, Lehn N, Männel DN. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect Immun. (2001) 69:3550–5. doi: 10.1128/IAI.69.6.3550-3555.2001
22. Schlichting DE, Waxman AB, O'Brien LA, Wang T, Naum CC, et al. Circulating endothelial and endothelial progenitor cells in patients with severe sepsis. Microvasc Res. (2011) 81:216–21. doi: 10.1016/j.mvr.2010.11.011
23. Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, Barthel L, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. (2012) 18:1217–23. doi: 10.1038/nm.2843
24. Cabrales P, Vázquez BYS, Tsai AG, Intaglietta M. Microvascular and capillary perfusion following glycocalyx degradation. J Appl Physiol (1985). (2007) 102:2251–2259. doi: 10.1152/japplphysiol.01155.2006
25. Anand D, Ray S, Srivastava LM, Bhargava S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin Biochem. (2016) 49:768–76. doi: 10.1016/j.clinbiochem.2016.02.014
26. Lee DH, Dane MJC, van den Berg BM, Boels MGS, van Teeffelen JW, de Mutsert R, et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS ONE. (2014) 9:e96477. doi: 10.1371/journal.pone.0096477
27. Beurskens DM, Bol ME, Delhaas T, van de Poll MC, Reutelingsperger CP, Nicolaes GA, et al. Decreased endothelial glycocalyx thickness is an early predictor of mortality in sepsis. Anaesth Intensive Care. (2020) 48:221–8. doi: 10.1177/0310057X20916471
28. Leligdowicz A, Richard-Greenblatt M, Wright J, Crowley VM, Kain KC. Endothelial Activation: The Ang/Tie Axis in Sepsis. Front Immunol. (2018) 9:838. doi: 10.3389/fimmu.2018.00838
29. Rossaint J, Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun. (2013) 5:348–57. doi: 10.1159/000345943
30. De Backer D, Creteur J, Preiser J-C, Dubois M-J, Vincent J-L. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. (2002) 166:98–104. doi: 10.1164/rccm.200109-016OC
31. Lu JL, Schmiege LM, Kuo L, Liao JC. Downregulation of endothelial constitutive nitric oxide synthase expression by lipopolysaccharide. Biochem Biophys Res Commun. (1996) 225:1–5. doi: 10.1006/bbrc.1996.1121
32. Zhou M, Wang P, Chaudry IH. Endothelial nitric oxide synthase is downregulated during hyperdynamic sepsis. Biochim Biophys Acta. (1997) 1335:182–90. doi: 10.1016/S0304-4165(96)00139-0
33. Preiser JC, Zhang H, Vray B, Hrabak A, Vincent JL. Time course of inducible nitric oxide synthase activity following endotoxin administration in dogs. Nitric Oxide. (2001) 5:208–11. doi: 10.1006/niox.2001.0342
34. Fujii T, Luethi N, Young PJ, Frei DR, Eastwood GM, French CJ, et al. Effect of Vitamin C, Hydrocortisone, and Thiamine vs. Hydrocortisone Alone on Time Alive and Free of Vasopressor Support Among Patients With Septic Shock: The VITAMINS Randomized Clinical Trial. JAMA. (2020) 323:423–31. doi: 10.1001/jama.2019.22176
35. López A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546C88: effect on survival in patients with septic shock. Crit Care Med. (2004) 32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6
36. Trzeciak S, Glaspey LJ, Dellinger RP, Durflinger P, Anderson K, Dezfulian C, et al. Randomized controlled trial of inhaled nitric oxide for the treatment of microcirculatory dysfunction in patients with sepsis*. Crit Care Med. (2014) 42:2482–92. doi: 10.1097/CCM.0000000000000549
37. Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, et al. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. (2010) 38:93–100. doi: 10.1097/CCM.0b013e3181b02fc1
38. Luis-Silva F, Menegueti MG, Sepeda CDR, Petroski-Moraes BC, Sato L, Peres LM, et al. Effect of methylene blue on hemodynamic and metabolic response in septic shock patients. Medicine (Baltimore). (2022) 101:e28599. doi: 10.1097/MD.0000000000028599
39. Ranieri VM, Thompson BT, Barie PS, Dhainaut J-F, Douglas IS, Finfer S, et al. Drotrecogin alfa (activated) in adults with septic shock. N Engl J Med. (2012) 366:2055–64. doi: 10.1056/NEJMoa1202290
40. Yasuda N, Goto K, Ohchi Y, Abe T, Koga H, Kitano T. The efficacy and safety of antithrombin and recombinant human thrombomodulin combination therapy in patients with severe sepsis and disseminated intravascular coagulation. J Crit Care. (2016) 36:29–34. doi: 10.1016/j.jcrc.2016.06.008
41. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. (2015) 4:180–3. doi: 10.1016/j.redox.2015.01.002
42. Victor VM, Rocha M. De la Fuente M. Immune cells: free radicals and antioxidants in sepsis. Int Immunopharmacol. (2004) 4:327–47. doi: 10.1016/j.intimp.2004.01.020
43. Cerwinka WH, Cooper D, Krieglstein CF, Ross CR, McCord JM, Granger DN. Superoxide mediates endotoxin-induced platelet-endothelial cell adhesion in intestinal venules. Am J Physiol Heart Circ Physiol. (2003) 284:H535–541. doi: 10.1152/ajpheart.00311.2002
44. Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. Am J Respir Cell Mol Biol. (2001) 24:762–8. doi: 10.1165/ajrcmb.24.6.4228
45. Chung HY, Yokozawa T, Kim MS, Lee KH, Kim KW, Yang R, et al. The mechanism of nitric oxide and/or superoxide cytotoxicity in endothelial cells. Exp Toxicol Pathol. (2000) 52:227–33. doi: 10.1016/S0940-2993(00)80034-2
46. Vásquez-Vivar J, Kalyanaraman B, Martásek P. The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic Res. (2003) 37:121–7. doi: 10.1080/1071576021000040655
47. Szabó C, Módis K. Pathophysiological roles of peroxynitrite in circulatory shock. Shock. (2010) 34:4–14. doi: 10.1097/SHK.0b013e3181e7e9ba
48. Joffre J, Hellman J. Oxidative Stress and Endothelial Dysfunction in Sepsis and Acute Inflammation. Antioxid Redox Signal. (2021) 35:1291–307. doi: 10.1089/ars.2021.0027
49. Regoeczi E, Brain MC. Organ distribution of fibrin in disseminated intravascular coagulation. Br J Haematol. (1969) 17:73–81. doi: 10.1111/j.1365-2141.1969.tb05665.x
50. Levi M, van der Poll T, Schultz M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin Immunopathol. (2012) 34:167–79. doi: 10.1007/s00281-011-0283-7
51. Yang Q-P, Liu W-P, Guo L-X, Jiang Y, Li G-D, Bai Y-Q, et al. Autopsy report of four cases who died from Streptococcus suis infection, with a review of the literature. Eur J Clin Microbiol Infect Dis. (2009) 28:447–53. doi: 10.1007/s10096-008-0646-8
52. Dixon B. The role of microvascular thrombosis in sepsis. Anaesth Intensive Care. (2004) 32:619–29. doi: 10.1177/0310057X0403200502
53. Chang JC. Disseminated intravascular coagulation: new identity as endotheliopathy-associated vascular microthrombotic disease based on in vivo hemostasis and endothelial molecular pathogenesis. Thromb J. (2020) 18:25. doi: 10.1186/s12959-020-00231-0
54. de Stoppelaar SF. van 't Veer C, van der Poll T. The role of platelets in sepsis. Thromb Haemost. (2014) 112:666–77. doi: 10.1160/TH14-02-0126
55. Nguyen TC, Gushiken F, Correa JI, Dong J-F, Dasgupta SK, Thiagarajan P, et al. recombinant fragment of von Willebrand factor reduces fibrin-rich microthrombi formation in mice with endotoxemia. Thromb Res. (2015) 135:1025–30. doi: 10.1016/j.thromres.2015.02.033
56. De Backer D, Donadello K, Favory R. Link between coagulation abnormalities and microcirculatory dysfunction in critically ill patients. Curr Opin Anaesthesiol. (2009) 22:150–4. doi: 10.1097/ACO.0b013e328328d1a1
57. Secor D, Li F, Ellis CG, Sharpe MD, Gross PL, Wilson JX, et al. Impaired microvascular perfusion in sepsis requires activated coagulation and P-selectin-mediated platelet adhesion in capillaries. Intensive Care Med. (2010) 36:1928–34. doi: 10.1007/s00134-010-1969-3
58. Nieuwland R, Berckmans RJ, McGregor S, Böing AN, Romijn FP, Westendorp RG, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. (2000) 95:930–5. doi: 10.1182/blood.V95.3.930.003k46_930_935
59. Wang J-G, Manly D, Kirchhofer D, Pawlinski R, Mackman N. Levels of microparticle tissue factor activity correlate with coagulation activation in endotoxemic mice. J Thromb Haemost. (2009) 7:1092–8. doi: 10.1111/j.1538-7836.2009.03448.x
60. Delabranche X, Boisramé-Helms J, Asfar P, Berger A, Mootien Y, Lavigne T, et al. Microparticles are new biomarkers of septic shock-induced disseminated intravascular coagulopathy. Intensive Care Med. (2013) 39:1695–703. doi: 10.1007/s00134-013-2993-x
61. Guven G, Hilty MP, Ince C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. (2020) 49:143–50. doi: 10.1159/000503775
62. Ince C. The microcirculation is the motor of sepsis. Crit Care. (2005) 9 Suppl 4:S13–9. doi: 10.1186/cc3753
63. Nakajima Y, Baudry N, Duranteau J, Vicaut E. Microcirculation in intestinal villi: a comparison between hemorrhagic and endotoxin shock. Am J Respir Crit Care Med. (2001) 164:1526–30. doi: 10.1164/ajrccm.164.8.2009065
64. Farquhar I, Martin CM, Lam C, Potter R, Ellis CG, Sibbald WJ. Decreased capillary density in vivo in bowel mucosa of rats with normotensive sepsis. J Surg Res. (1996) 61:190–6. doi: 10.1006/jsre.1996.0103
65. Taccone FS, Su F, De Deyne C, Abdellhai A, Pierrakos C, He X, et al. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med. (2014) 42:e114–122. doi: 10.1097/CCM.0b013e3182a641b8
66. Wester T, Häggblad E, Awan ZA, Barratt-Due A, Kvernebo M, Halvorsen PS, et al. Assessments of skin and tongue microcirculation reveals major changes in porcine sepsis. Clin Physiol Funct Imaging. (2011) 31:151–8. doi: 10.1111/j.1475-097X.2010.00994.x
67. Hayes MA, Timmins AC, Yau EH, Palazzo M, Hinds CJ, Watson D. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. (1994) 330:1717–22. doi: 10.1056/NEJM199406163302404
68. Ellis CG, Bateman RM, Sharpe MD, Sibbald WJ, Gill R. Effect of a maldistribution of microvascular blood flow on capillary O(2) extraction in sepsis. Am J Physiol Heart Circ Physiol. (2002) 282:H156–164. doi: 10.1152/ajpheart.2002.282.1.H156
69. Humer MF, Phang PT, Friesen BP, Allard MF, Goddard CM, Walley KR. Heterogeneity of gut capillary transit times and impaired gut oxygen extraction in endotoxemic pigs. J Appl Physiol (1985). (1996) 81:895–904. doi: 10.1152/jappl.1996.81.2.895
70. Ince C, Sinaasappel M. Microcirculatory oxygenation and shunting in sepsis and shock. Crit Care Med. (1999) 27:1369–77. doi: 10.1097/00003246-199907000-00031
71. Østergaard L, Granfeldt A, Secher N, Tietze A, Iversen NK, Jensen MS, et al. Microcirculatory dysfunction and tissue oxygenation in critical illness. Acta Anaesthesiol Scand. (2015) 59:1246–59. doi: 10.1111/aas.12581
72. Walley KR. Heterogeneity of oxygen delivery impairs oxygen extraction by peripheral tissues: theory. J Appl Physiol. (1996) 81:885–94. doi: 10.1152/jappl.1996.81.2.885
73. Morin MJ, Unno N, Hodin RA, Fink MP. Differential expression of inducible nitric oxide synthase messenger RNA along the longitudinal and crypt-villus axes of the intestine in endotoxemic rats. Crit Care Med. (1998) 26:1258–64. doi: 10.1097/00003246-199807000-00031
74. Lange M, Hamahata A, Traber DL, Nakano Y, Traber LD, Enkhbaatar P. Heterogeneity of the effects of combined nitric oxide synthase inhibition on organ perfusion in ovine sepsis. Burns. (2013) 39:1565–70. doi: 10.1016/j.burns.2013.04.019
75. Bateman RM, Tokunaga C, Kareco T, Dorscheid DR, Walley KR. Myocardial hypoxia-inducible HIF-1alpha, VEGF, and GLUT1 gene expression is associated with microvascular and ICAM-1 heterogeneity during endotoxemia. Am J Physiol Heart Circ Physiol. (2007) 293:H448–456. doi: 10.1152/ajpheart.00035.2007
76. Ince C. Hemodynamic coherence and the rationale for monitoring the microcirculation. Crit Care. (2015) 19 Suppl 3:S8–S8. doi: 10.1186/cc14726
77. Santos AOMT, Furtado ES, Villela NR, Bouskela E. Microcirculatory effects of fluid therapy and dopamine, associated or not to fluid therapy, in endotoxemic hamsters. Clin Hemorheol Microcirc. (2011) 47:1–13. doi: 10.3233/CH-2010-1358
78. Zhang H, Li L, Wu J, Qu H-P, Tang Y-Q, Chen D-C. Time of dissociation between microcirculation, macrocirculation, and lactate levels in a rabbit model of early endotoxemic shock. Chin Med J (Engl). (2020) 133:2153–60. doi: 10.1097/CM9.0000000000000887
79. Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, et al. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. (2007) 49:88–98, 98.e1–2. doi: 10.1016/j.annemergmed.2006.08.021
80. De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, et al. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. (2013) 41:791–9. doi: 10.1097/CCM.0b013e3182742e8b
81. Eun HC. Evaluation of skin blood flow by laser Doppler flowmetry. Clin Dermatol. (1995) 13:337–47. doi: 10.1016/0738-081X(95)00080-Y
82. Neviere R, Mathieu D, Chagnon JL, Lebleu N, Millien JP, Wattel F. Skeletal muscle microvascular blood flow and oxygen transport in patients with severe sepsis. Am J Respir Crit Care Med. (1996) 153:191–5. doi: 10.1164/ajrccm.153.1.8542115
83. Brunauer A, Koköfer A, Bataar O, Gradwohl-Matis I, Dankl D, Bakker J, et al. Changes in peripheral perfusion relate to visceral organ perfusion in early septic shock: A pilot study. J Crit Care. (2016) 35:105–9. doi: 10.1016/j.jcrc.2016.05.007
84. Freedlander SO, Lenhart CH. Clinical observations on the capillary circulation. Arch Intern Med. (1922) 29:12–32. doi: 10.1001/archinte.1922.00110010017002
85. Weinberg JR, Boyle P, Thomas K, Murphy K, Tooke JE, Guz A. Capillary blood cell velocity is reduced in fever without hypotension. Int J Microcirc Clin Exp. (1991) 10:13–9.
86. De Backer D. Dubois M-J. Assessment of the microcirculatory flow in patients in the intensive care unit. Curr Opin Crit Care. (2001) 7:200–3. doi: 10.1097/00075198-200106000-00010
87. Dilken O, Ergin B, Ince C. Assessment of sublingual microcirculation in critically ill patients: consensus and debate. Ann Transl Med. (2020) 8:793–793. doi: 10.21037/atm.2020.03.222
88. Sherman H, Klausner S, Cook WA. Incident dark-field illumination: a new method for microcirculatory study. Angiology. (1971) 22:295–303. doi: 10.1177/000331977102200507
89. Mancini DM, Bolinger L, Li H, Kendrick K, Chance B, Wilson JR. Validation of near-infrared spectroscopy in humans. J Appl Physiol. (1994) 77:2740–7. doi: 10.1152/jappl.1994.77.6.2740
90. Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent J-L. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. (2007) 33:1549–56. doi: 10.1007/s00134-007-0739-3
91. Vesterager P. Transcutaneous pO2 electrode. Scand J Clin Lab Invest Suppl. (1977) 146:27–30. doi: 10.3109/00365517709098929
92. Hernandez G, Luengo C, Bruhn A, Kattan E, Friedman G, Ospina-Tascon GA, et al. When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care. (2014) 4:30. doi: 10.1186/s13613-014-0030-z
93. Kawaguchi R, Nakada T, Oshima T, Shinozaki M, Nakaguchi T, Haneishi H, et al. Optimal pressing strength and time for capillary refilling time. Critical Care. (2019) 23:4. doi: 10.1186/s13054-018-2295-3
94. Ebert RV, Stead EA. Circulatory failure in acute infections. J Clin Invest. (1941) 20:671–9. doi: 10.1172/JCI101260
95. Bourcier S, Pichereau C, Boelle P-Y, Nemlaghi S, Dubée V, Lejour G, et al. Toe-to-room temperature gradient correlates with tissue perfusion and predicts outcome in selected critically ill patients with severe infections. Ann Intensive Care. (2016) 6:63. doi: 10.1186/s13613-016-0164-2
96. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. (2021) 49:e1063.
97. Duranteau J, Sitbon P, Teboul JL, Vicaut E, Anguel N, Richard C, et al. Effects of epinephrine, norepinephrine, or the combination of norepinephrine and dobutamine on gastric mucosa in septic shock. Crit Care Med. (1999) 27:893–900. doi: 10.1097/00003246-199905000-00021
98. Young JD, Cameron EM. Dynamics of skin blood flow in human sepsis. Intensive Care Med. (1995) 21:669–74. doi: 10.1007/BF01711546
99. Yura T, Yuasa S, Fukunaga M, Badr KF, Matsuo H. Role for Doppler ultrasound in the assessment of renal circulation: effects of dopamine and dobutamine on renal hemodynamics in humans. Nephron. (1995) 71:168–75. doi: 10.1159/000188707
100. Schneider AW, Kalk JF, Klein CP. Hepatic arterial pulsatility index in cirrhosis: correlation with portal pressure. J Hepatol. (1999) 30:876–81. doi: 10.1016/S0168-8278(99)80142-1
101. Macdonald SPJ, Kinnear FB, Arendts G, Ho KM, Fatovich DM. Near-infrared spectroscopy to predict organ failure and outcome in sepsis: the Assessing Risk in Sepsis using a Tissue Oxygen Saturation (ARISTOS) study. Eur J Emerg Med. (2019) 26:174–9. doi: 10.1097/MEJ.0000000000000535
102. De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. (2007) 11:R101–R101. doi: 10.1186/cc6118
103. Verdant CL, De Backer D, Bruhn A, Clausi CM, Su F, Wang Z, et al. Evaluation of sublingual and gut mucosal microcirculation in sepsis: a quantitative analysis. Crit Care Med. (2009) 37:2875–81. doi: 10.1097/CCM.0b013e3181b029c1
104. Boerma EC, van der Voort PHJ, Spronk PE, Ince C. Relationship between sublingual and intestinal microcirculatory perfusion in patients with abdominal sepsis. Crit Care Med. (2007) 35:1055–60. doi: 10.1097/01.CCM.0000259527.89927.F9
105. Ince C, Boerma EC, Cecconi M, De Backer D, Shapiro NI, Duranteau J, et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. (2018) 44:281–99. doi: 10.1007/s00134-018-5070-7
106. Aykut G, Veenstra G, Scorcella C, Ince C, Boerma C. Cytocam-IDF (incident dark field illumination) imaging for bedside monitoring of the microcirculation. Intensive Care Med Exp. (2015) 3:40. doi: 10.1186/s40635-015-0040-7
107. Gilbert-Kawai E, Coppel J, Bountziouka V, Ince C, Martin D. Caudwell Xtreme Everest and Xtreme Everest 2 Research Groups. A comparison of the quality of image acquisition between the incident dark field and sidestream dark field video-microscopes. BMC Med Imaging. (2016) 16:10. doi: 10.1186/s12880-015-0078-8
108. van Elteren HA, Ince C, Tibboel D, Reiss IKM, de Jonge RCJ. Cutaneous microcirculation in preterm neonates: comparison between sidestream dark field (SDF) and incident dark field (IDF) imaging. J Clin Monit Comput. (2015) 29:543–8. doi: 10.1007/s10877-015-9708-5
109. Ait-Oufella H, Bige N, Boelle PY, Pichereau C, Alves M, Bertinchamp R, et al. Capillary refill time exploration during septic shock. Intensive Care Med. (2014) 40:958–64. doi: 10.1007/s00134-014-3326-4
110. Lima A, Jansen TC, van Bommel J, Ince C. Bakker J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. (2009) 37:934–8. doi: 10.1097/CCM.0b013e31819869db
111. Hernández G, Ospina-Tascón GA, Damiani LP, Estenssoro E, Dubin A, Hurtado J, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs. serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. (2019) 321:654. doi: 10.1001/jama.2019.0071
112. Ait-Oufella H, Bourcier S, Alves M, Galbois A, Baudel J-L, Margetis D, et al. Alteration of skin perfusion in mottling area during septic shock. Ann Intensive Care. (2013) 3:31. doi: 10.1186/2110-5820-3-31
113. Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, et al. Mottling score predicts survival in septic shock. Intensive Care Med. (2011) 37:801–7. doi: 10.1007/s00134-011-2163-y
114. Dumas G, Lavillegrand J-R, Joffre J, Bigé N., de-Moura EB, Baudel J-L, et al. Mottling score is a strong predictor of 14-day mortality in septic patients whatever vasopressor doses and other tissue perfusion parameters. Crit Care. (2019) 23:211. doi: 10.1186/s13054-019-2496-4
115. de Moura EB, Amorim FF, da Cruz Santana AN, Kanhouche G, de Souza Godoy LG, de Jesus Almeida L, et al. Skin mottling score as a predictor of 28-day mortality in patients with septic shock. Intensive Care Med. (2016) 42:479–80. doi: 10.1007/s00134-015-4184-4
116. Lavillegrand J-R, Dumas G, Bigé N, Zafimahazo D, Guidet B, Maury E, et al. Should we treat mild hypotension in septic patients in the absence of peripheral tissue hypoperfusion? Intensive Care Med. (2018) 44:1593–4. doi: 10.1007/s00134-018-5315-5
117. Legrand M, De Backer D, Dépret F, Ait-Oufella H. Recruiting the microcirculation in septic shock. Ann Intensive Care. (2019) 9:102, s13613-019-0577–9. doi: 10.1186/s13613-019-0577-9
118. Byrne L, Obonyo NG, Diab SD, Dunster KR, Passmore MR, Boon A-C, et al. Unintended Consequences: Fluid Resuscitation Worsens Shock in an Ovine Model of Endotoxemia. Am J Respir Crit Care Med. (2018) 198:1043–54. doi: 10.1164/rccm.201801-0064OC
119. Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Büchele G, Simion D, et al. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. (2010) 36:949–55. doi: 10.1007/s00134-010-1843-3
120. Hippensteel JA, Uchimido R, Tyler PD, Burke RC, Han X, Zhang F, et al. Intravenous fluid resuscitation is associated with septic endothelial glycocalyx degradation. Crit Care. (2019) 23:259. doi: 10.1186/s13054-019-2534-2
121. Hariri G, Joffre J, Deryckere S, Bigé N, Dumas G, Baudel J-L, et al. Albumin infusion improves endothelial function in septic shock patients: a pilot study. Intensive Care Med. (2018) 44:669–71. doi: 10.1007/s00134-018-5075-2
122. Boerma EC, Ince C. The role of vasoactive agents in the resuscitation of microvascular perfusion and tissue oxygenation in critically ill patients. Intensive Care Med. (2010) 36:2004–18. doi: 10.1007/s00134-010-1970-x
123. Secchi A, Ortanderl JM, Schmidt W, Walther A, Gebhard MM, Martin E, et al. Effects of dobutamine and dopexamine on hepatic micro- and macrocirculation during experimental endotoxemia: an intravital microscopic study in the rat. Crit Care Med. (2001) 29:597–600. doi: 10.1097/00003246-200103000-00023
124. Secchi A, Wellmann R, Martin E, Schmidt H. Dobutamine maintains intestinal villus blood flow during normotensive endotoxemia: an intravital microscopic study in the rat. J Crit Care. (1997) 12:137–41. doi: 10.1016/S0883-9441(97)90043-5
125. De Backer D, Zhang H, Manikis P, Vincent JL. Regional effects of dobutamine in endotoxic shock. J Surg Res. (1996) 65:93–100. doi: 10.1006/jsre.1996.0349
126. De Backer D, Creteur J, Dubois M-J, Sakr Y, Koch M, Verdant C. Vincent J-L. The effects of dobutamine on microcirculatory alterations in patients with septic shock are independent of its systemic effects*. Crit Care Med. (2006) 34:403–8. doi: 10.1097/01.CCM.0000198107.61493.5A
127. Hernandez G, Bruhn A, Luengo C, Regueira T, Kattan E, Fuentealba A, et al. Effects of dobutamine on systemic, regional and microcirculatory perfusion parameters in septic shock: a randomized, placebo-controlled, double-blind, crossover study. Intensive Care Med. (2013) 39:1435–43. doi: 10.1007/s00134-013-2982-0
128. Morelli A, Donati A, Ertmer C, Rehberg S, Lange M, Orecchioni A, et al. Levosimendan for resuscitating the microcirculation in patients with septic shock: a randomized controlled study. Crit Care. (2010) 14:R232. doi: 10.1186/cc9387
129. Zhang H, Smail N, Cabral A, Rogiers P, Vincent JL. Effects of norepinephrine on regional blood flow and oxygen extraction capabilities during endotoxic shock. Am J Respir Crit Care Med. (1997) 155:1965–71. doi: 10.1164/ajrccm.155.6.9196103
130. Bellomo R, Kellum JA, Wisniewski SR, Pinsky MR. Effects of norepinephrine on the renal vasculature in normal and endotoxemic dogs. Am J Respir Crit Care Med. (1999) 159:1186–92. doi: 10.1164/ajrccm.159.4.9802055
131. Regueira T, Bänziger B, Djafarzadeh S, Brandt S, Gorrasi J, Takala J, et al. Norepinephrine to increase blood pressure in endotoxaemic pigs is associated with improved hepatic mitochondrial respiration. Crit Care. (2008) 12:R88. doi: 10.1186/cc6956
132. Krouzecky A, Matejovic M, Radej J, Rokyta R, Novak I. Perfusion pressure manipulation in porcine sepsis: effects on intestinal hemodynamics. Physiol Res. (2006) 55:527–33. doi: 10.33549/physiolres.930821
133. Dubin A, Pozo MO, Casabella CA, Pálizas F, Murias G, Moseinco MC, et al. Increasing arterial blood pressure with norepinephrine does not improve microcirculatory blood flow: a prospective study. Crit Care. (2009) 13:R92. doi: 10.1186/cc7922
134. LeDoux D, Astiz ME, Carpati CM, Rackow EC. Effects of perfusion pressure on tissue perfusion in septic shock. Crit Care Med. (2000) 28:2729–32. doi: 10.1097/00003246-200008000-00007
135. Borrelli E, Roux-Lombard P, Grau GE, Girardin E, Ricou B, Dayer J-M, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med. (1996) 24:392–7. doi: 10.1097/00003246-199603000-00006
136. Medical Respiratory Intensive Care Unit Nursing Fowler AA Syed AA Knowlson S Sculthorpe R Farthing D. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. (2014) 12:32. doi: 10.1186/1479-5876-12-32
137. Na W, Shen H, Li Y, Qu D. Hydrocortisone, ascorbic acid, and thiamine (HAT) for sepsis and septic shock: a meta-analysis with sequential trial analysis. J Intens Care. (2021) 9:75. doi: 10.1186/s40560-021-00589-x
138. Lavillegrand J-R, Raia L, Urbina T, Hariri G, Gabarre P, Bonny V, et al. Vitamin C improves microvascular reactivity and peripheral tissue perfusion in septic shock patients. Crit Care. (2022) 26:25. doi: 10.1186/s13054-022-03891-8
139. Morelli A, Ertmer C, Westphal M, Rehberg S, Kampmeier T, Ligges S, et al. Effect of Heart Rate Control With Esmolol on Hemodynamic and Clinical Outcomes in Patients With Septic Shock: A Randomized Clinical Trial. JAMA. (2013) 310:1683. doi: 10.1001/jama.2013.278477
Keywords: endothelial dysfunction, sepsis, microcirculation, organ failure, peripheral perfusion, endothelium
Citation: Raia L and Zafrani L (2022) Endothelial Activation and Microcirculatory Disorders in Sepsis. Front. Med. 9:907992. doi: 10.3389/fmed.2022.907992
Received: 30 March 2022; Accepted: 16 May 2022;
Published: 03 June 2022.
Edited by:
W. Conrad Liles, University of Washington, United StatesCopyright © 2022 Raia and Zafrani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lara Zafrani, bGFyYS56YWZyYW5pQGFwaHAuZnI=
 Lisa Raia
Lisa Raia Lara Zafrani
Lara Zafrani