
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 22 August 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.907126
This article is part of the Research Topic Clinical Application of Artificial Intelligence in Emergency and Critical Care Medicine, Volume III View all 13 articles
Background: This retrospective study was designed to explore the types of postoperative pain trajectories and their associated factors after spine surgery.
Materials and methods: This study was conducted in a single medical center, and patients undergoing spine surgery with intravenous patient-controlled analgesia (IVPCA) for postoperative pain control between 2016 and 2018 were included in the analysis. Maximal pain scores were recorded daily in the first postoperative week, and group-based trajectory analysis was used to classify the variations in pain intensity over time and investigate predictors of rebound pain after the end of IVPCA. The relationships between the postoperative pain trajectories and the amount of morphine consumption or length of hospital stay (LOS) after surgery were also evaluated.
Results: A total of 3761 pain scores among 547 patients were included in the analyses and two major patterns of postoperative pain trajectories were identified: Group 1 with mild pain trajectory (87.39%) and Group 2 with rebound pain trajectory (12.61%). The identified risk factors of the rebound pain trajectory were age less than 65 years (odds ratio [OR]: 1.89; 95% CI: 1.12–3.20), female sex (OR: 2.28; 95% CI: 1.24–4.19), and moderate to severe pain noted immediately after surgery (OR: 3.44; 95% CI: 1.65–7.15). Group 2 also tended to have more morphine consumption (p < 0.001) and a longer length of hospital stay (p < 0.001) than Group 1.
Conclusion: The group-based trajectory analysis of postoperative pain provides insight into the patterns of pain resolution and helps to identify unusual courses. More aggressive pain management should be considered in patients with a higher risk for rebound pain after the end of IVPCA for spine surgery.
The indications for spine surgeries vary from herniated disks, spondylolisthesis, fractures, and tumors to scoliosis correction surgeries, and most of these patients need decompression and spine fusion surgeries. As the knowledge of spinal biomechanics, imaging diagnostics, and medical technology is improving over time, the complexity and diversity of spine surgery are increasing as well (1). Although these complex surgeries may benefit those suffering from spinal disease (2, 3), intense pain following the procedures, especially in the immediate and early postoperative period (4–6), often results in clinical problems such as delayed recovery induced by a reduction in patient mobility (7–9). As a result, effective postoperative pain control is of paramount importance and has been connected with better surgical outcomes (10, 11), reduced length of hospital stays (LOSs) (10, 11), lower incidence of chronic postsurgical pain (12), and decreased opioid dependence (7, 13). However, how to well control acute pain after spine surgery remains a major challenge for clinicians (1, 6, 7, 14, 15).
Intravenous patient-controlled analgesia (IVPCA) is a common and effective method to relieve acute pain after spine surgery (16–18) and it optimizes the delivery of analgesics and minimizes the interindividual variability in pharmacokinetics and pharmacodynamics (19). While some studies emphasized the importance of multimodal analgesia in spine surgery (1, 6, 14), IVPCA remains the gold standard for postoperative pain control for spine surgery worldwide (15–18). In addition, IVPCA provides better analgesia after spine surgery than conventional as-needed analgesic regimens do and improves patient satisfaction in the early postoperative days as well (20). However, moderate to severe rebound pain after the discontinuation of IVPCA was noted in other types of surgeries (21), and it is not clear whether this phenomenon also exists in patients receiving IVPCA for pain control after spine surgery. Accordingly, we hypothesized that some patients undergoing spine surgery were at risk of having rebound pain after the end of IVPCA and that there were risk factors associated with the development of rebound pain and designed this retrospective study to investigate these issues. The group-based trajectory analysis was used to classify the variations in postoperative pain scores over time and identify patients with rebound pain after discontinuing IVPCA. The risk factors of rebound pain were also explored, and the influence of rebound pain trajectory on the total amount of IVPCA consumption and LOS after surgery were evaluated as well.
This study was approved by the Institutional Review Board of Taipei Veterans General Hospital, Taipei, Taiwan (IRB-TPEVGH no. 2020-01-003AC). Written informed consent was waived and all the included patients were de-identified before analysis. We carefully reviewed the electronic medical records of patients receiving spine surgery and postoperative IVPCA for postoperative pain control in our hospital from January 2016 to December 2018 and collected all records. Those with severe postoperative complications, less than three pain assessments in the first postoperative week, IVPCA use of fewer than 48 h, age < 20 years old, staged surgery, re-operation, or missing key data, such as operation records, were excluded from the analysis.
In this study, all patients were administered general anesthesia with fentanyl (2–3 μg/kg) followed by propofol (1–2 mg/kg) and cisatracurium (0.2 mg/kg) or rocuronium (0.8 mg/kg) for induction. After endotracheal intubation, general anesthesia was maintained using desflurane or sevoflurane with the aforementioned neuromuscular blocking agents. Toward the end of the surgery, the inhalation agent was tapered off and the residual neuromuscular block was reversed with neostigmine and atropine. All patients were transferred to our post-anesthesia care unit where an infusion pump for IVPCA was connected to the patients with a loading dose of morphine of 2–4 mg and a bolus dose of 1 mg. No adjunct analgesics, such as acetaminophen and non-steroidal anti-inflammatory drugs, were administered with IVPCA. After the discontinuation of IVPCA on the fourth postoperative day (POD 4), pain management was shifted to oral medications, including Ultracet (acetaminophen 325 mg + tramadol 37.5 mg) every 6 h and 25 mg diclofenac every 8 h as needed.
After surgery, patient-reported pain scores on a numeric rating scale (NRS) from 0 to 10 for no pain to the worst pain were recorded by the nurses in charge at least one time per day. Postoperative maximal daily pain scores were collected in series and used in the trajectory analyses. Patient attributes, such as age, sex, body mass index (BMI), and comorbidities, surgical features, such as surgical time and blood loss, PCA pump settings, and LOS after surgery were collected. The primary endpoint was the patterns of postoperative pain trajectories, and the secondary endpoints were the total amount of PCA consumption and LOS after surgery.
Group-based trajectory analysis was employed to categorize the variations in postoperative pain over time and the technical details refer to Jones et al. (22). The numbers and features of postoperative trajectories were decided by comparing the Bayesian information criteria of different models and examining the generated trajectories and estimated parameters (23, 24). Two main patterns of pain trajectories were identified, and we compared patient characteristics between the two groups with the Student’s t-test, the Mann–Whitney U–tests, or the chi-squared tests as appropriate. The relationships between the types of postoperative pain trajectories and collected variables were evaluated and presented as odds ratios (OR) with 95% confidence intervals (CI) as well. Backward model selection with an exit criterion of significance level greater than 0.05 was performed to determine the final model for the prediction of postoperative pain trajectories. In addition, a simplified risk scoring system was developed to predict a rebound pain trajectory after the discontinuation of IVPCA for spine surgery. The area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the predictive power of the final model and the simplified risk scoring system. Besides, linear backward regression analysis with an exit significance level of 0.05 was used to select independent predictors of total morphine consumption and log-transformed LOS after surgery. The adjusted association between the types of pain trajectories and total morphine consumption or LOS was also evaluated after the final predictive models were determined. A p-value less than 0.05 was considered statistically significant in this study. All the analyses were conducted using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, United States).
There were 547 patients with a pain score of 3,761 included in the analysis, and the average maximal pain scores on the first five PODs ranged between 2.98 and 3.33 (Figure 1, blue line). The mean morphine consumption was 52.6 mg and the median LOS was 7 days. The two postoperative pain trajectory groups were identified after the analysis: Group 1 with a mild pain trajectory (87.4%) and Group 2 (12.6%) with a rebound pain trajectory after the end of IVPCA (Figure 1, black line and red line, respectively). Table 1 shows the comparisons of patient attributes and no significant differences in the surgical features were found between the two groups. However, significant differences in the distributions of age, sex, and body weight were noted between those with rebound pain and their counterparts without it. Moreover, patients in Group 2 also tended to have more morphine consumption and longer LOS after surgery (both p < 0.001).
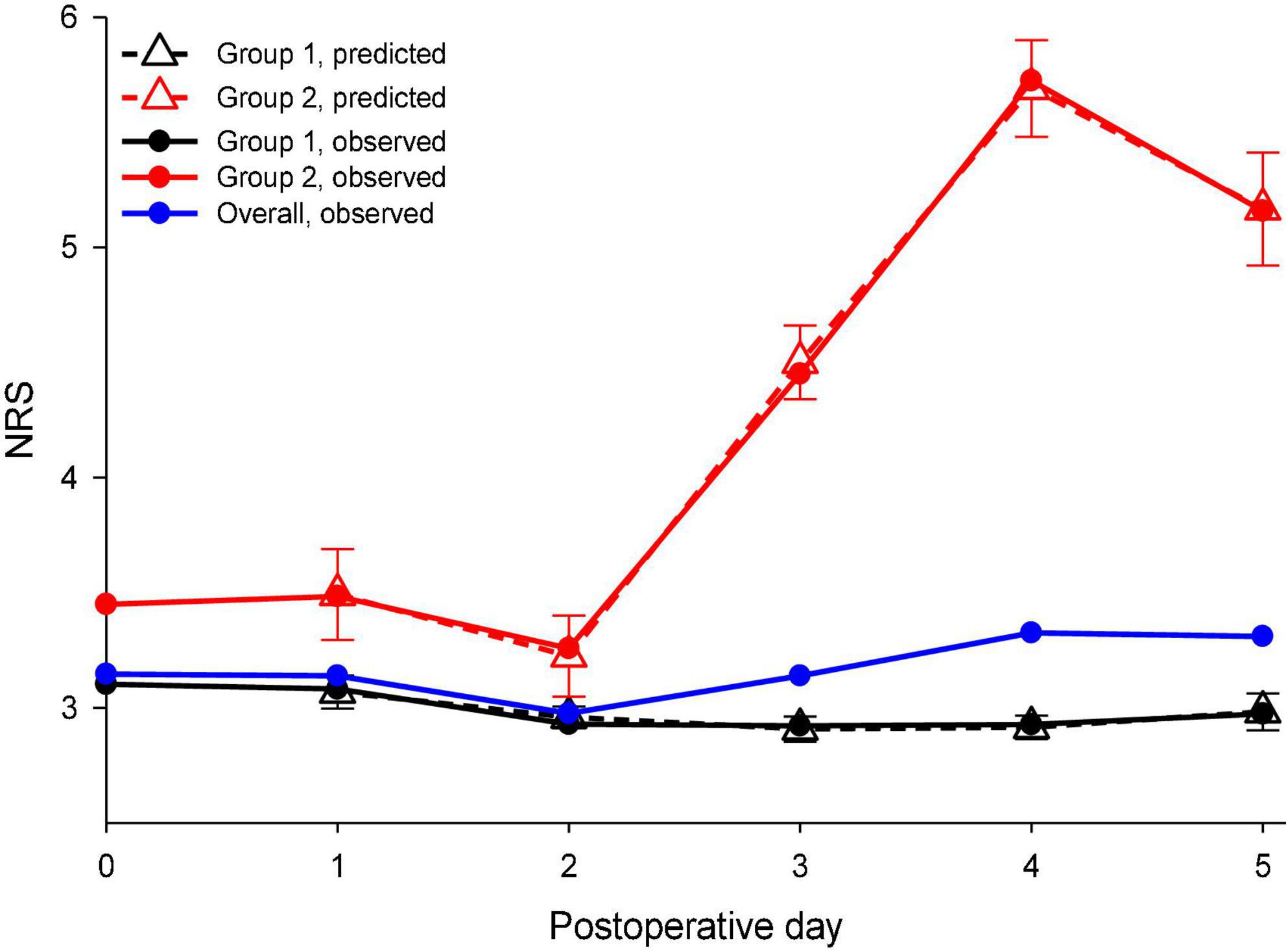
Figure 1. Observed and predicted maximal daily pain scores during the first postoperative week were stratified by distinct pain trajectories after spine surgery. NRS, a numeric rating scale for pain intensity. Solid blue line: observed overall pain scores during the first postoperative week; solid black line: observed pain scores of the mild pain trajectory group; solid red line: observed pain scores of the rebound pain trajectory group; dashed black line: predicted pain scores with their 95% confidence interval (CI) for the mild pain trajectory group; and dashed red line: predicted pain scores with their 95% confidence interval for the rebound pain trajectory group.
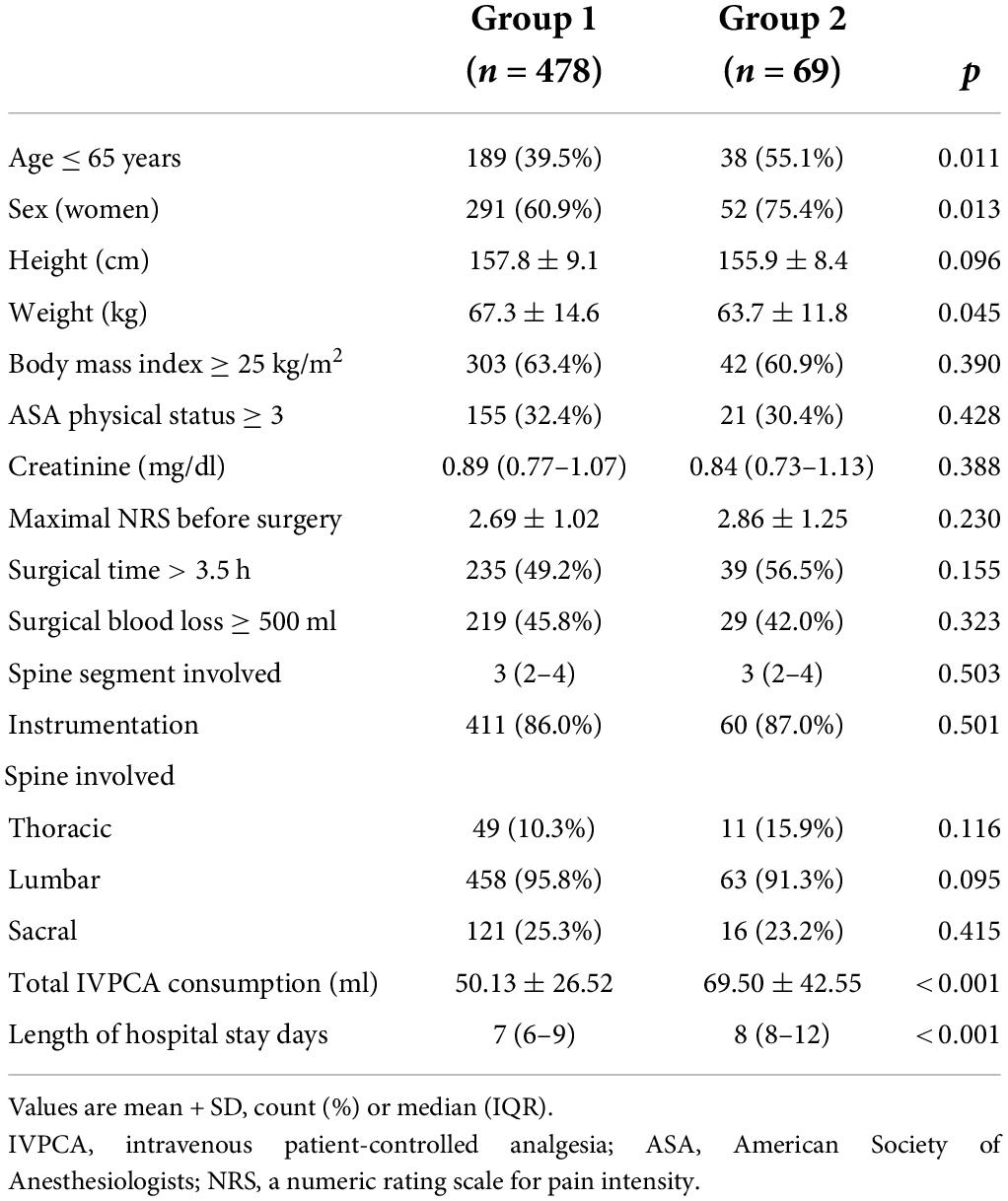
Table 1. Comparisons of patient characteristics between the two postoperative pain trajectory groups after spine surgery.
After the group-based trajectory analysis, we identified three factors associated with the rebound pain trajectory, such as age ≤ 65 years (adjusted OR: 1.89, 95%, CI: 1.12–3.20), female sex (OR: 2.28, 95% CI: 1.24–4.19), and moderate to severe pain (NRS ≥ 4) on POD 0 (OR: 3.44, 95% CI: 1.65–7.15; Table 2). Surgical features and other patient characteristics were not related to the rebound pain trajectory. Moreover, a simplified risk scoring system for predicting rebound pain trajectory after the discontinuation of IVPCA could be developed as the following formula:
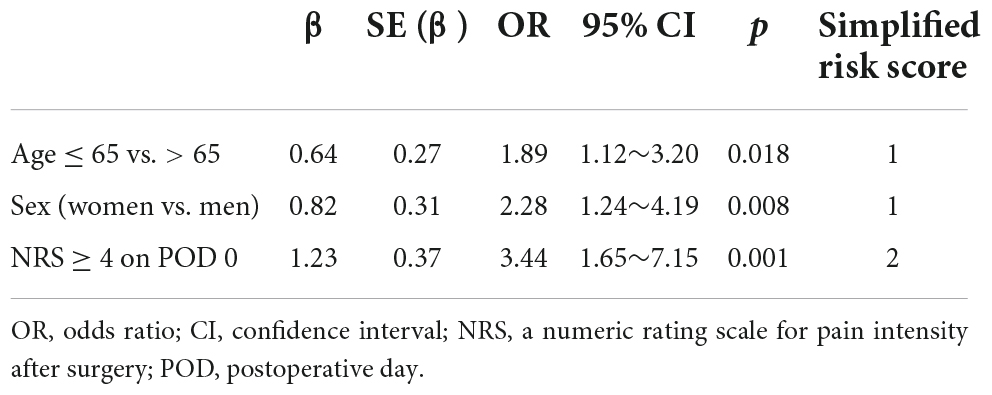
Table 2. Risk factors of rebound pain trajectory after the discontinuation of IVPCA following spine surgery.
Figure 2 illustrates the estimated probabilities of rebound pain trajectory at distinct risk scores. The risk of developing rebound pain after the end of IVPCA ranged from 5.6 to 42.4% for patients with no to all three risk factors. Figure 3 depicts the ROC curves of the original model and a simplified scoring system. The predictive power of the two models assessed by areas under ROC curves was similar (0.64).
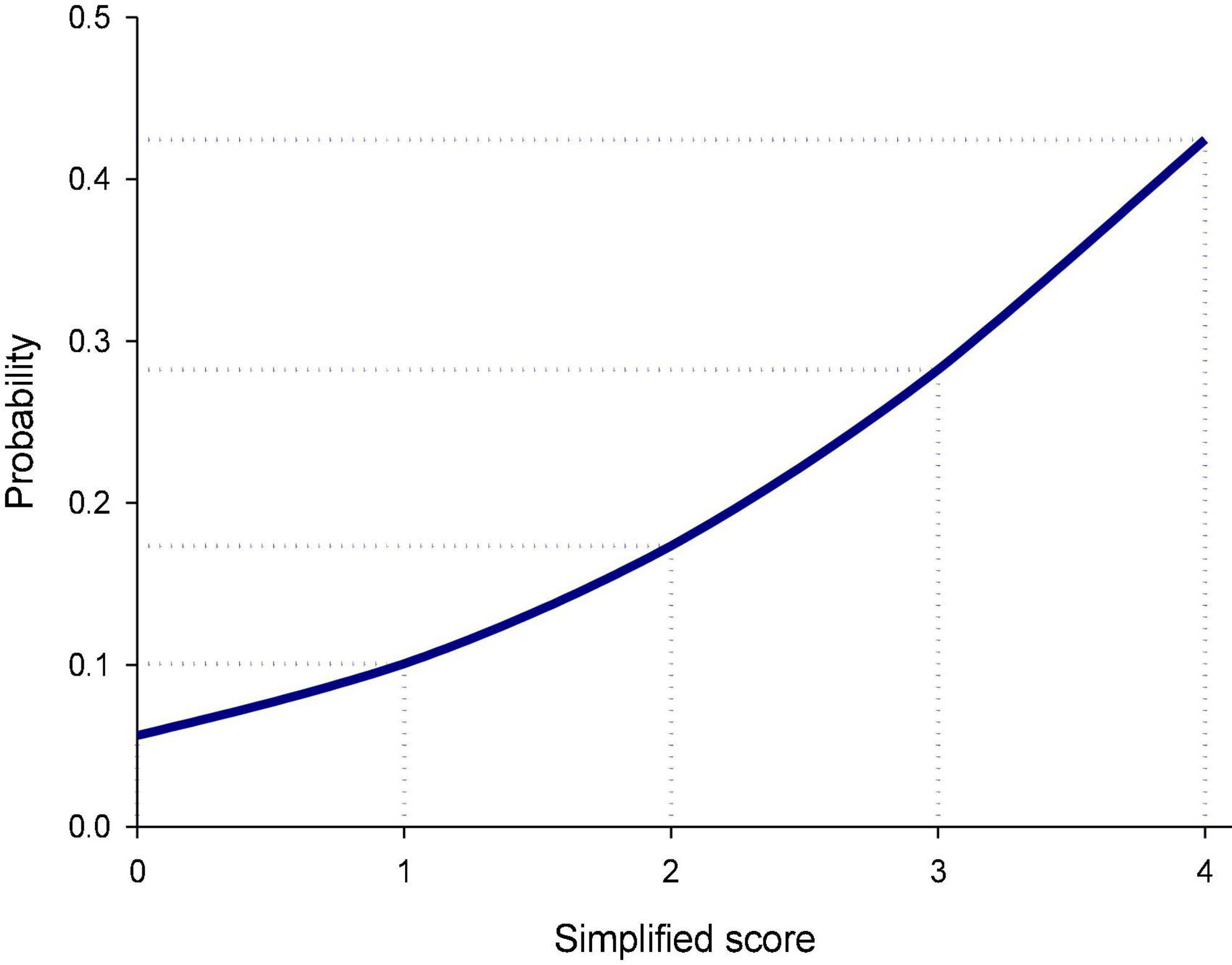
Figure 2. Predicted probability of the rebound pain trajectory for the simplified risk scoring systems after the discontinuation of intravenous patient-controlled analgesia (IVPCA) for spine surgery. The probability of developing rebound pain after the end of IVPCA for spine surgery increased gradually from 5.6% for the simplified score of 0–42.4% for the score of 4.
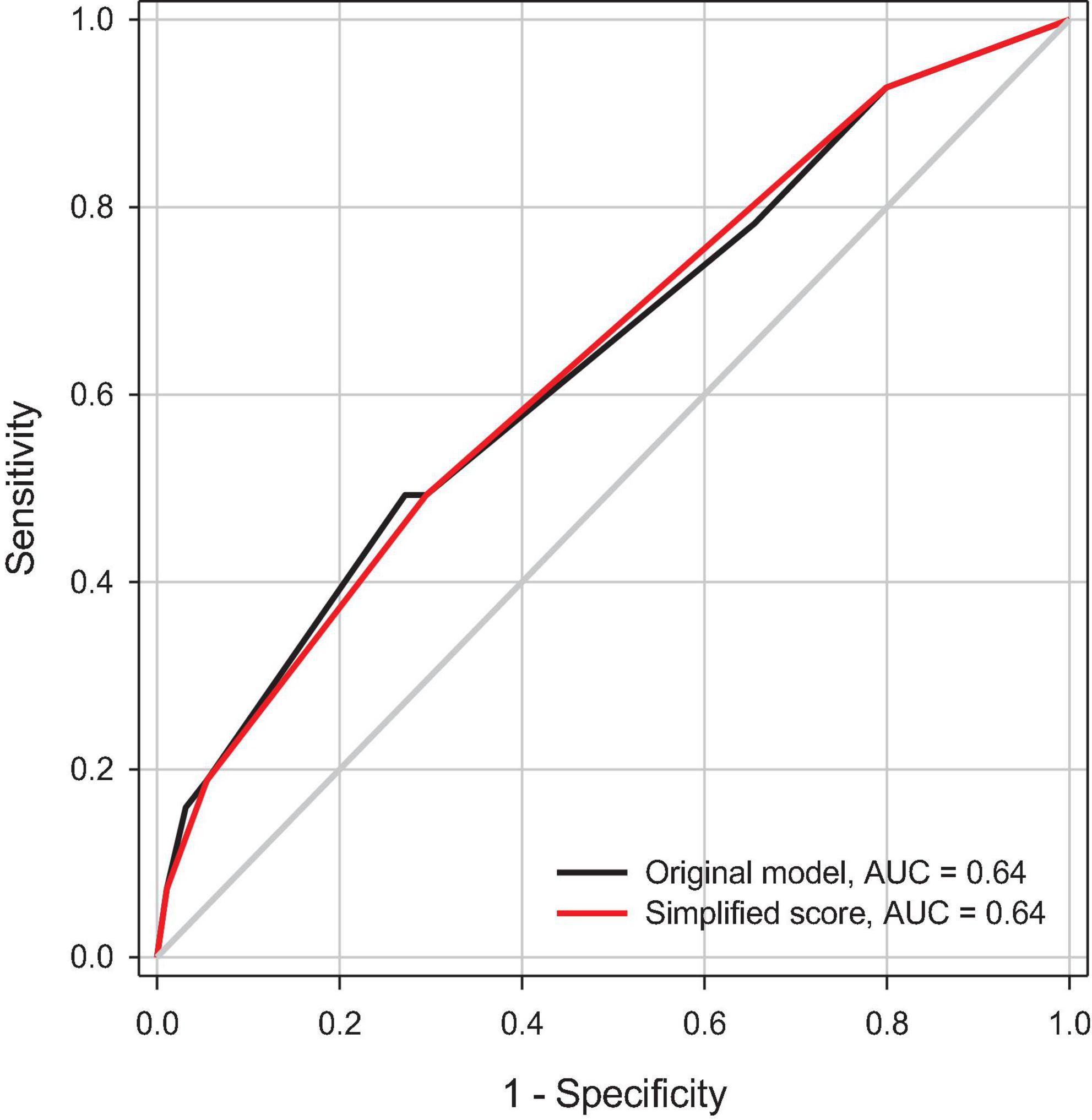
Figure 3. A receiver operating characteristic (ROC) curve analysis of predictive power for the selected model and the simplified risk scoring system for the rebound pain trajectory. AUC, area under ROC curve.
After the backward model selection processes, five independent predictors of increased morphine consumption were identified, such as age ≤ 65, male sex (both p < 0.001), greater preoperative pain (p = 0.001), more spine segment involved (p = 0.009), and rebound pain trajectory (p < 0.001; Table 3). On average, those with the rebound pain trajectory consumed 17.9 mg more morphine during their IVPCA course after controlling for the effects of other predictors in the final model.
There were six factors associated with LOS after surgery, such as surgical time, lumbar spine involved, preoperative pain (all p < 0.001), American Society of Anesthesiologists (ASA) physical status (p = 0.005), spine segment involved (p = 0.014), and rebound pain trajectory (Table 4). On average, patients with the postoperative rebound pain trajectory tended to stay 17.2% longer in hospital (p = 0.001) than those with normal pain resolution after the adjustment for the other selected predictors in the model.
This is the first study to describe the phenomenon of rebound pain after the discontinuation of IVPCA for spine surgery. Although Nicholson et al. (25) used “rebound pain” to describe the increase in pain score between 8 and 24 h after surgery in a patient still “receiving PCA,” this is totally different from our findings. Approximately one-eighth of the target population experienced this unpleasant journey after the end of IVPCA. With the aid of group-based trajectory analysis, patients with abnormal pain resolution after spine surgery could be recognized and the associated factors could be identified. Regional anesthesia (RA), such as short-lasting spinal anesthesia and peripheral nerve blocks, is widely used in various surgery due to effective pain relief in the early postoperative phase. However, severe pain was noted in up to 40% of patients when the RA wears off, and this phenomenon is known as “rebound pain” (26). Recently, rebound pain was also observed in patients receiving epidural analgesia for video-assisted thoracoscopic surgery (21). All these aforementioned rebound pain phenomena were developed after the transition from an effective analgesic intervention to other routine pain management. These findings highlight the importance of analgesic transition and the necessity of early identification and intervention. Our study provides important clues for clinicians to early detect high-risk patients, and thus, preventive strategies could be initiated in advance to refine the quality of postoperative care and pain management following spine surgery (27).
Several risk factors of rebound pain were identified in this study and among them, younger age (28–31) and female sex (28–30, 32) were associated with analgesic consumption. These two non-modifiable factors were identified as risk factors in rebound pain development in other studies as well (21, 27). In addition, some previous studies revealed that younger age (28–31), female sex (28–30, 32), preoperative NRS (30, 33), and the number of spine segments involvement (4, 34) were associated with higher postoperative pain scores and more analgesic consumption. Although preoperative pain has been proposed as a risk factor for inferior postoperative pain control and more morphine consumption in a previous study (33), our study demonstrated that the postoperative pain on POD 0, rather than the preoperative pain, was an independent predictor of rebound pain trajectory after the end of IVPCA for spine surgery. The discrepancy might result from the difference in outcomes of interest and study population since we focused on the rebound pain trajectory after the end of IVPCA for spine surgery instead of general pain scores observed after surgery. Since the IVPCA remains the gold standard for postoperative pain control in complex spine surgery, the prediction of rebound pain in advance is of paramount importance. In spite of the efforts which have been made to evaluate the effects of surgical time and blood loss and the complexity of the surgery, such as procedure types and the number of spine segments involved, none of these factors were significantly associated with rebound pain trajectory. A more comprehensive classification of spine surgery might be considered in future studies.
In this study, we used group-based trajectory analysis to model the variations in pain intensity over time and identify distinct patterns of postoperative pain trajectories and their associated factors. Similar to clinical decision-making, this approach directly focuses on postoperative pain observations. Patient characteristics were not involved in the group classification processes but evaluated post hoc to avoid untoward interference in trajectory recognition. In addition, the group-based trajectory analysis has a great advantage in handling missing data, which is commonly observed in retrospective studies (35). Furthermore, a simplified risk scoring system was developed based on the estimated results of group-based trajectory analysis. The risk of developing rebound pain after the end of IVPCA could be easily assessed with the help of this system. Among the three risk factors, moderate to severe pain noted immediately after spine surgery despite IVPCA in use should be regarded as an early sign of possible rebound pain after the transition from IVPCA to other analgesic modalities. Once moderate to severe pain is noted after surgery, more aggressive multimodal pain management should be considered to reduce the risk of rebound pain after the end of IVPCA. This scoring system has great potential to be applied in clinical practice to prevent rebound pain after the discontinuation of IVPCA (36, 37) and improve pain control quality following spine surgery. For example, a 70-year-old male patient who is satisfied with IVPCA had no to mild pain on POD 0 after spine surgery, and the simplified risk score of rebound pain is 0; while a 60-year-old female patient who has moderate to severe pain on POD 0 under IVPCA management had the simplified risk score of rebound pain of 4. The probability of developing rebound pain (group 2) after the end of IVPCA in these two patients would be 5.6 and 42.4%, respectively. The clinicians should introduce more vigorous pain management, such as prolonged PCA duration or multiple model pain management control to prevent or manage the rebound pain afterward. However, its validity and clinical utility of this risk scoring system await further investigation.
There were some limitations to our study. First, the impacts of unobserved variables on the patterns of variations in postoperative pain scores over time could not be further evaluated and more covariates should be included in future studies for better prediction of the rebound pain trajectory. Second, the preoperative analgesic prescriptions were not further investigated due to data unavailability. Third, we only evaluated the effects of surgical time, blood loss, instrumentation, and spine segments involved on the risk of having a rebound pain trajectory but did not further assess the associations between different kinds of spine surgical procedures and the incidence of rebound pain since there is still no consensus on the classification of complex spine surgery.
In conclusion, two major patterns of postoperative pain trajectories were recognized in patients receiving IVPCA for spine surgery using group-based trajectory analysis, and about one-eighth of them had a rebound pain trajectory. Three predictors of rebound pain trajectory were identified, namely, younger age, female sex, and moderate to severe pain on POD 0. A simplified risk scoring system was developed based on the analytical results but its clinical utility needs further investigation. Preventive strategies, such as early introduction of more aggressive multimodal analgesia, should be considered in high-risk patients to reduce the incidence of rebound pain since patients with rebound pain trajectory were inclined to have longer hospital stay after surgery and more opioid consumption. Group-based trajectory analysis provides valuable information to categorize variations in postoperative pain over time and detect unusual patterns of pain resolution for further optimization of perioperative pain management. More patient attributes and surgical features should be collected in future studies to further elucidate the underlying mechanism of rebound pain after the end of IVPCA for spine surgery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Taipei Veterans General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Y-SL contributed to data acquisition and manuscript drafting. W-KC contributed to data validation and draft preparation. S-PL contributed to study coordination and data acquisition. M-CC helped to review and revised the manuscript. K-YC contributed to conceptualization, statistical analysis, and manuscript revision. All authors have read and agreed to the published version of the manuscript.
This study was supported by grants from the Taipei Veterans General Hospital, Taipei, Taiwan (V111C-074), the Ministry of Science and Technology, Taipei, Taiwan (MOST 109-2511-H-075-003-MY2), and the Anesthesiology Research and Development Foundation, Taipei, Taiwan (ARDF11101).
We would like to thank Shih-Tien Wang (Department of Orthopedics, Taipei Veterans General Hospital) and Chun-Sung Sung (Department of Anesthesiology, Taipei Veterans General Hospital) for their kind assistance in data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Waelkens P, Alsabbagh E, Sauter A, Joshi GP, Beloeil H Prospect Working group** of the European Society of Regional Anaesthesia and Pain therapy (Esra). Pain management after complex spine surgery: a systematic review and procedure-specific postoperative pain management recommendations. Eur J Anaesthesiol. (2021) 38:985–94. doi: 10.1097/EJA.0000000000001448
2. Lamperti M, Tufegdzic B, Avitsian R. Management of complex spine surgery. Curr Opin Anaesthesiol. (2017) 30:551–6. doi: 10.1097/ACO.0000000000000494
3. Marquez-Lara A, Nandyala SV, Fineberg SJ, Singh K. Current trends in demographics, practice, and in-hospital outcomes in cervical spine surgery: a national database analysis between 2002 and 2011. Spine (Phila Pa 1976). (2014) 39:476–81. doi: 10.1097/BRS.0000000000000165
4. Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. (2013) 118:934–44. doi: 10.1097/ALN.0b013e31828866b3
5. Archer K, Bird ML, Haug C, Coronado R, Wegener S, Devin CJ, et al. Patients’ experience and expectations of lumbar spine surgery for degenerative conditions: a qualitative study. Spine J. (2015) 15:S99–100. doi: 10.1016/j.spinee.2015.07.046
6. Devin CJ, McGirt MJ. Best evidence in multimodal pain management in spine surgery and means of assessing postoperative pain and functional outcomes. J Clin Neurosci. (2015) 22:930–8. doi: 10.1016/j.jocn.2015.01.003
7. Debono B, Wainwright TW, Wang MY, Sigmundsson FG, Yang MMH, Smid-Nanninga H, et al. Consensus statement for perioperative care in lumbar spinal fusion: enhanced recovery after surgery (ERAS(R)) society recommendations. Spine J. (2021) 21:729–52. doi: 10.1016/j.spinee.2021.01.001
8. Street JT, Lenehan BJ, DiPaola CP, Boyd MD, Kwon BK, Paquette SJ, et al. Morbidity and mortality of major adult spinal surgery. A prospective cohort analysis of 942 consecutive patients. Spine J. (2012) 12:22–34. doi: 10.1016/j.spinee.2011.12.003
9. Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. (2016) 24:416–27. doi: 10.3171/2015.2.SPINE14973
10. Carli F, Kehlet H, Baldini G, Steel A, McRae K, Slinger P, et al. Evidence basis for regional anesthesia in multidisciplinary fast-track surgical care pathways. Reg Anesth Pain Med. (2011) 36:63–72. doi: 10.1097/AAP.0b013e31820307f7
11. Lenart MJ, Wong K, Gupta RK, Mercaldo ND, Schildcrout JS, Michaels D, et al. The impact of peripheral nerve techniques on hospital stay following major orthopedic surgery. Pain Med. (2012) 13:828–34. doi: 10.1111/j.1526-4637.2012.01363.x
12. Borghi B, D’Addabbo M, White PF, Gallerani P, Toccaceli L, Raffaeli W, et al. The use of prolonged peripheral neural blockade after lower extremity amputation: the effect on symptoms associated with phantom limb syndrome. Anesth Analg. (2010) 111:1308–15. doi: 10.1213/ANE.0b013e3181f4e848
13. Schoenfeld AJ, Nwosu K, Jiang W, Yau AL, Chaudhary MA, Scully RE, et al. Risk factors for prolonged opioid use following spine surgery, and the association with surgical intensity, among opioid-naive patients. J Bone Joint Surg Am. (2017) 99:1247–52. doi: 10.2106/JBJS.16.01075
14. Dunn LK, Durieux ME, Nemergut EC. Non-opioid analgesics: novel approaches to perioperative analgesia for major spine surgery. Best Pract Res Clin Anaesthesiol. (2016) 30:79–89. doi: 10.1016/j.bpa.2015.11.002
15. Dietz N, Sharma M, Adams S, Alhourani A, Ugiliweneza B, Wang D, et al. Enhanced recovery after surgery (ERAS) for spine surgery: a systematic review. World Neurosurg. (2019) 130:415–26. doi: 10.1016/j.wneu.2019.06.181
16. Reynolds RA, Legakis JE, Tweedie J, Chung Y, Ren EJ, Bevier PA, et al. Postoperative pain management after spinal fusion surgery: an analysis of the efficacy of continuous infusion of local anesthetics. Global Spine J. (2013) 3:7–14. doi: 10.1055/s-0033-1337119
17. Javed T, Ahad B, Singh P, Ahmad R. A prospective randomized study to compare tramadol and morphine for postoperative analgesia in spine surgeries using intravenous patient controlled analgesia. Int J Res Med Sci. (2017) 5:3350–3354. doi: 10.18203/2320-6012.ijrms20173140
18. Venkatraman R, Pushparani A, Balaji R, Nandhini P. Comparison of low dose intravenous fentanyl and morphine infusion for postoperative analgesia in spine fusion surgeries - a randomized control trial. Braz J Anesthesiol. (2021) 71:339–44. doi: 10.1016/j.bjane.2020.12.013
19. Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. (2001) 87:36–46. doi: 10.1093/bja/87.1.36
20. McNicol ED, Ferguson MC, Hudcova J Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev. (2015) 6:CD003348.
21. Chang WK, Li YS, Wu HL, Tai YH, Lin SP, Chang KY. Group-based trajectory analysis of postoperative pain in epidural analgesia for video-assisted thoracoscopic surgery and risk factors of rebound pain. J Chin Med Assoc. (2022) 85:216–221. doi: 10.1097/JCMA.0000000000000647
22. Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. (2018) 27:2015–23. doi: 10.1177/0962280216673085
23. Chang WK, Tai YH, Lin SP, Wu HL, Tsou MY, Chang KY. An investigation of the relationships between postoperative pain trajectories and outcomes after surgery for colorectal cancer. J Chine Med Assoc. (2019) 82:865–71. doi: 10.1097/JCMA.0000000000000166
24. Teng WN, Wu HL, Tai YH, Lei HJ, Tsou MY, Chang KY. Group-based trajectory analysis of postoperative pain and outcomes after liver cancer surgery. J Chine Med Assoc. (2021) 84:95–100. doi: 10.1097/JCMA.0000000000000446
25. Nicholson T, Maltenfort M, Getz C, Lazarus M, Williams G, Namdari S. Multimodal pain management protocol versus patient controlled narcotic analgesia for postoperative pain control after shoulder arthroplasty. Arch Bone Jt Surg. (2018) 6:196–202.
26. Lavand’homme P. Rebound pain after regional anesthesia in the ambulatory patient. Curr Opin Anaesthesiol. (2018) 31:679–84. doi: 10.1097/ACO.0000000000000651
27. Barry GS, Bailey JG, Sardinha J, Brousseau P, Uppal V. Factors associated with rebound pain after peripheral nerve block for ambulatory surgery. Br J Anaesth. (2021) 126:862–71. doi: 10.1016/j.bja.2020.10.035
28. Mei W, Seeling M, Franck M, Radtke F, Brantner B, Wernecke KD, et al. Independent risk factors for postoperative pain in need of intervention early after awakening from general anaesthesia. Eur J Pain. (2010) 14:e1–7. doi: 10.1016/j.ejpain.2009.03.009
29. Murray AA, Retief FW. Acute postoperative pain in 1 231 patients at a developing country referral hospital: incidence and risk factors. South Afr J Anaesth Analg. (2015) 22:19–24. doi: 10.1080/22201181.2015.1115608
30. Gerbershagen HJ, Pogatzki-Zahn E, Aduckathil S, Peelen LM, Kappen TH, van Wijck AJ, et al. Procedure-specific risk factor analysis for the development of severe postoperative pain. Anesthesiology. (2014) 120:1237–45. doi: 10.1097/ALN.0000000000000108
31. Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. (2009) 111:657–77. doi: 10.1097/ALN.0b013e3181aae87a
32. Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. (2005) 103:156–60. doi: 10.1097/00000542-200507000-00023
33. Yang MMH, Hartley RL, Leung AA, Ronksley PE, Jetté N, Casha S, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. (2019) 9:e025091. doi: 10.1136/bmjopen-2018-025091
34. Chaterjee S, Ghosh S, Ray S, Rudra A. Pain management after spinal surgery. Indian J Pain. (2015) 29:14. doi: 10.4103/0970-5333.145916
35. Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. (2010) 6:109–38. doi: 10.1146/annurev.clinpsy.121208.131413
36. Marshall K, McLaughlin K. Pain management in thoracic surgery. Thorac Surg Clin. (2020) 30:339–46. doi: 10.1016/j.thorsurg.2020.03.001
Keywords: group-based trajectory analysis, spine surgery, patient-controlled analgesia (PCA), rebound pain, multimodal analgesia (MMA)
Citation: Li Y-S, Chang K-Y, Lin S-P, Chang M-C and Chang W-K (2022) Group-based trajectory analysis of acute pain after spine surgery and risk factors for rebound pain. Front. Med. 9:907126. doi: 10.3389/fmed.2022.907126
Received: 20 May 2022; Accepted: 29 July 2022;
Published: 22 August 2022.
Edited by:
Zhongheng Zhang, Sir Run Run Shaw Hospital, ChinaReviewed by:
Sangseok Lee, Inje University Sanggye Paik Hospital, South KoreaCopyright © 2022 Li, Chang, Lin, Chang and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Kuei Chang, d2tjaGFuZ0B2Z2h0cGUuZ292LnR3
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.