- 1Department of Ophthalmology, “Iuliu Haţieganu” Medicine and Pharmacy University, Cluj-Napoca, Romania
- 2Oculens Clinic, Cluj-Napoca, Romania
- 3Department of Physiopathology, “Iuliu Haţieganu” Medicine and Pharmacy University, Cluj-Napoca, Romania
- 4Emergency County Hospital, Târgu Mureş, Romania
- 5Department of Ophthalmology, “George Emil Palade” University of Medicine, Pharmacy, Sciences and Technology, Târgu Mureş, Romania
- 6Department of Medical Informatics and Biostatistics, “Iuliu Haţieganu” Medicine and Pharmacy University, Cluj-Napoca, Romania
Aim: The purpose of the study was to assess the efficacy of topographical and tomographical indices given by the Pentacam (pachymetric, tomopetric, and aberometric) in clinical and subclinical keratoconus (KCN) diagnosis.
Material and Methods: In this observational analytic retrospective study, patients with abnormal findings in topography and tomography maps but with no signs on clinical examination (subclinical KCN group, sKCN), patients with clinical keratoconus (KCN group), and healthy subjects (Control group) were evaluated.
Results: The KCN group proved significantly different (p < 0.001) values of the investigated parameters than the Control group. Eleven out of 28 investigated parameters proved significantly different in the sKCN group compared to controls (p < 0.001). Two topographic measurements, namely I-S (cut-off = 1.435, a large value indicates the presence of KCN) and CCT (cut-off = 537, a small value indicates the presence of KCN), showed AUCs equal to 1 [0.999 to 1]. Six other Pentacam measurements, including Back maximum keratometry (Back Kmax) proved to be excellent parameters for case-finding and screening. In distinguishing sKCN from normal eyes, Pentacam index of vertical asymmetry (IVA), inferior-superior difference (I-S) value, thinnest point (TP), Belin Ambrosio Enhanced Ectasia Display (BAD_D) and root mean square total (RMS total) performed best.
Conclusions: In distinguishing sKCN from normal eyes, Back Kmax, IVA, I-S, and RMS total values demonstrated higher accuracy and utility. Six indices, namely ISV, IVA, KISA, PRC, RMS-HOA, and Back Kmax demonstrate excellent utility in case-finding and screening for clinical KCN.
Introduction
Keratoconus (KCN) is a progressive corneal ectatic disease that may appear at any age, it is frequently seen in teenagers and dramatically progresses without treatment (1). It is characterized by progressive central thinning of the cornea, irregular astigmatism, and decreased visual acuity. The prevalence of KCN show large variations from population to population, as 0.15% in the United States (2), 4% in the Iranian rural population (3), 37.4 cases (95% CI–confidence interval [36.8 to 37.9]) per 100,000 people in South Korea (4), 192.1 per 100,000 (95% CI [188.3 to 195.9]) in Norway (5). The KCN is more frequently observed in males (OR = 2.30, P-value <0.05) (2) and has a family aggregation (3, 6). Index surface variance and index of vertical asymmetry confirm a high association between phenotypes and genetic factors, supporting further investigation of the genetic mechanisms in keratoconus (6). Changes in oxidative stress markers were detected in KCN, indicating that oxidative stress may play a role in the development and disease progression (7). Non-enzymatic antioxidants and decreased expression of genes encoding antioxidative enzymes (aldehyde dehydrogenase, superoxide dismutase, peroxiredoxins, thioredoxin reductase) have been reported as decreased in patients with KCN (8, 9). Eye rubbing, atopy, allergy, asthma, Down syndrome, contact lens wear, myopia and eczema are other risk KCN factors reported in the scientific literature (3, 10, 11). Proteomic studies of tears from keratoconic patients have demonstrated the presence of elevated inflammatory markers, including tumor necrosis factor alfa, interleukin-6, interleukin-17, implicating inflammation in the pathogenesis of KCN (12).
Corneal cross-linking riboflavin-ultraviolet A (UVA) introduced by Wollensak et al. (13) stops the illness's progression, mainly in the early or moderate stages. In the early stages, the visual acuity may be normal and slit-lamp examination cannot give us features of KCN, so corneal topography and tomography re the gold standard examination for diagnosing corneal ectasia. Identifying subclinical keratoconus (sKCN), described by Amsler in 1946 (14), is important in order to assess the candidates for refractive surgery (15). Corneal topography is a non-contact imaging tool that gives information about the anterior surface of the cornea (16). Corneal tomography evaluates anterior and posterior corneal surfaces and is mandatory for preoperatory evaluation before refractive surgery (16, 17). The first signal of an ectatic corneal disease is the posterior elevation (16–20). Most corneal topographical systems are built on Placido disc that analyses rings reflected on the corneal surface, and cannot evaluate the posterior corneal surface. Scheimpflug imaging is the technique used for corneal tomography (17). The Oculus Pentacam® (Oculus Optikgerate GmbH, Wetzlar, Germany) implements the Scheimpflug technology to create topographic reports. The cross-sectional images generated by a rotating Scheimpflug camera are used to locate the anterior and posterior corneal plane (17). The Global Consensus on Keratoconus and Ectatic Disease established in 2015 the following criteria for diagnosing keratoconus: abnormal posterior elevation, abnormal corneal thickness, and corneal thinning (21). The corneal indices offered by Pentacam (I-S index - inferior-superior index, KISA% index - keratoconus percentage index, central K value, AST - astigmatism index, SRAX - steepest radial axes, and BAD_D-Belin/Ambrosio enhanced ectasia display) are used to diagnose the KCN and to establish the disease severity. The KCN staging is concordant with the ABCD grading system proposed in 2016, which is linked with the topographical findings: anterior and posterior curvature within a 3-mm zone centered around the thinnest point of the cornea, thinnest pachymetry, and distance best-corrected visual acuity (22). Rabinowitz (23) reported KISA% index as the best metric for diagnosis (99.6% rate of correct diagnosis). Hashemi et al. (24) showed that index vertical asymmetry (IVA) is the best diagnostic index for KCN, and recommended a combination of indices to obtain accurate results. Bühren et al. (25) reported in a sample fi 17 eyes an AUC (area under the ROC curve) of 0.980, cut-off = −0.2 μm of corneal wavefront (vertical coma) as a diagnosis metric for sKCN. No consent regarding the best cut-off values for diagnosis of KCN was reached (26–32), and multi-parameter evaluation is recommended (33). Furthermore, the cut-off values for diagnosis of sKCN remain to be established. The sKCN term is used to define a very early preclinical stage of the illness in eyes with slight topographic features like clinical KCN, but without the classical keratometry or slit lamp signs (1, 18, 34–36). Previous studies reported some reliable indices from Pentacam to distinguish sKCN from normal eyes (20, 37–40), respectively KCN from normal eyes (14, 37, 38, 41–43).
Several studies revealed the long-term efficacy of standard collagen cross-linking (CXL) (irradiance of 3 mW/cm2 for 30 min) in stopping the progression of KCN (44, 45). The accelerated CXL procedure was introduced to reduce treatment time (46–48). Mazzotta et al. (49) showed the effectiveness of 15 mW/cm2 pulsed-light epithelium-off accelerated CXL in stabilizing KCN progression. A non-statistically significant intraoperative corneal reduction was also demonstrated in patients undergoing pulsed-light epithelium-off accelerated CXL by using dextran free 0.1% riboflavin solution with hydroxyl-propyl methylcellulose (50). The efficiency of corneal epithelial-disruption CXL in medium-term stabilization of KCN and better comfort for the patient was also reported (51). Improvement of the refraction by corneal reshaping treatments, with a decrease of vertical asymmetry and high order aberrations, is important in treating patients with KCN (52). Selective transepithelial topography guided photorefractive keratectomy combined with accelerated CXL treatment (STARE X protocol) in improving the visual acuity, manifest refraction and high-order aberrations have been reported (53). The placement of intracorneal polymethyl methacrylate segments or rings in the mid-stroma of the peripheral cornea inducing an arc-shortening effect of the corneal geometry that flattens the central area of the corneal tissue is another treatment option that proved efficient (54). Keratoplasty is usually recommended in advanced KCN cases (55).
The scientific literature search has identified no study reporting the Pentacam diagnosis of KCN or sKCN in the Romanian population. Our study aimed to assess the efficacy of topographical and tomographical indices given by the Pentacam (pachymetric, topometric, and aberometric) in clinical and subclinical keratoconus diagnosis and to establish the cut-off values able to discriminate KCN and subclinical KCN from normal corneas.
Materials and Methods
A retrospective, observational single-center study was performed at the Oculens Clinic in Cluj-Napoca, Romania. According to the Declaration of Helsinki, our study met the bioethical standards and was approved by the Ethical Committee of the Clinic (1/2022).
Participants
Patients older than 18 years, regardless of gender and with a corneal tomography treated at our clinic between 15 January 2019 and 15 January 2020, were eligible for the study. Patients diagnosed with KCN confirmed by slit-lamp examination (conical protrusion, stromal thinning, Fleisher ring, and Vogt's striae), keratometry and corneal topography, and tomography were included in the KCN group. In our study, KCN was considered as any eye that had an asymmetric bow tie pattern or an abnormal localized steepening, merged with at least one of the following signs: steep keratometry >47 diopter (D), maximum keratometry (Kmax)> 48.7D, oblique cylinder >1.5 D, corneal thickness under 500 μm, abnormal topographical patterns (asymmetric bow-tie), inferior-superior (I-S) difference value>1.9D at 6 mm (3 mm radii), distorted keratometry mires, distortion of the ophthalmoscopic red reflex or clinical KCN in the fellow eye (56, 57). The KCN eyes met the criteria of the Collaborative Longitudinal Evaluation of Keratoconus study (58). The sKCN was considered if the following features were present: no identifiable slit lamp exam, keratometry or ophthalmoscopy signs; asymmetric bow-tie pattern at topography, K max between 47.2–48.7D, I-S values between 1.4D and 1.9D at 6 mm (3 mm radii), abnormal anterior and posterior elevation values, and no history of ocular trauma, ocular surgery or contact lens wear (56, 59).
The control group comprises subjects selected from the candidates for refractive corneal surgery with myopia (< -8.5D) and/or myopic astigmatism (< -3.5D) with stable ocular refraction (unchanged keratometry, spherical equivalent, cylinder) for almost 1 year, and a normal corneal tomography and healthy eyes. No patients with suspect signs of KCN were included among refractive surgery candidates.
Patients with previously intracorneal ring placement, corneal collagen cross-linking, corneal pachymetry <400 μ, concomitant corneal disease, central corneal scar, dry eye syndrome, and ocular eye surgery or trauma were excluded.
Eyes Evaluation
All eyes had an ocular examination consisting of distance best-corrected visual acuity examination (DCVA), ocular refraction, slit lamp exam, fundus, and intraocular pressure investigation. All patients were requested to discontinue contact lens use for almost 1 month before the corneal topography/tomography. The corneal topography and tomography were performed with the Pentacam® (HR Premium; Oculus Optikgerate GmbH, Wetzlar, Germany) under scotopic conditions, without pupillary dilation, by the same experienced person. All patients had an appropriate position during the examination and were asked to blink a few times before the examination, open both eyes, and gaze at the fixation target. The automatic-release mode started the scan after we obtained a perfect lining up. Twenty-five Scheimpflug images were taken on an optical zone of 9 mm on the cornea on each evaluated eye. For anterior and posterior corneal elevation, a best-fit sphere (BFS) was used as reference surface measurement. The anterior elevation map represents the radial difference between the sphere and the anterior corneal surface. The posterior elevation map is defined as the radial difference between the sphere and the posterior corneal surface. All the elevation maps were displayed with 2.5 μm color-coded scales.
Curved-based parameters, elevation-based data, pachymetric, aberometric, and integrated indices were extracted from the Pentacam software for evaluated eyes, collected, and stored in an Excel database (Table 1).
We analyzed the Belin/Ambrósio Enhanced Ectasia Display (BAD_D) to establish deviations from normal front and back elevation limits, which represented a meaningful sign of early KCN and was included as an important sign integrated index. Aberometric indices involved the [root mean square total (RMS total)] and the root mean square high order aberration (RMS –HOA).
We used the ABCD staging (22) criteria included in Pentacam to establish the severity scale of KCN (subclinical - sKCN, clinical mild, moderate, advanced).
Statistical Analysis
The eye was the statistical unit in our study. Participants' sex was reported as number (%) by group and the differences were tested with the Chi-squared test. We reported the measurements as mean±standard deviation or median (Q1 to Q3), where Q is the quartile's value according to the distribution (Kolmogorov-Smirnov test at a significance level of 5%) of data by each group (KCN, sKCN, and control). Comparisons between the three groups were made with the ANOVA test for normally distributed data and Kruskal-Wallis whenever data proved a deviation from the normal distribution. Posthoc analysis was applied when ANOVA or Kruskal-Wallis test showed statistically significant results (adjusted significance level of 1.7%).
The analysis of significant differences between groups was further investigated using ROC (receiver operating characteristic). The cut-off value that discriminates between the two groups was established with Youden's index (max(Se+Sp-1), where Se, sensitivity, Sp, specificity). The performances of individual topographical parameters were assessed whenever the AUC (area under the curve) lower bound of the confidence interval showed at least a good accuracy (0.8≤AUC <0.9) (60). The following statistical metrics were used to evaluate performances of the eligible individual topographical parameters: sensitivity (Se, the highest, the better), specificity (Sp, the highest, the better), positive predictive value (PPV, the highest, the better), negative predictive value (NPV, the highest, the better), positive likelihood ratio (PLR>10 indicates convincing diagnostic evidence; 5 < PLR < 10 indicates strong diagnostic evidence), negative likelihood ratio (NLR < 0.10 indicates convincing diagnostic evidence; 0.2 < NLR < 0.1 indicates strong diagnostic evidence), +CUI (positive clinical utility index; +CUI>0.81 indicates an excellent utility in case-findings, 0.64 ≤ +CUI ≤ 0.81 indicates a good utility in case-findings) and -CUI (negative clinical utility index; -CUI>0.
Results
One hundred and fifty-nine patients aged from 18 to 63 years were evaluated. The number of subjects and the main characteristics are presented in Figure 1. The subjects in the sKCN groups were younger than those in the KCN and Control group, but the difference was not statistically significant (ANOVA test: P-value = 0.0632). Significantly more men were in the sKCN and KCN groups than in the Control group (Chi-squared test: P-value = 0.0008).

Figure 1. Flowchart with the main characteristics of the evaluated patients. Age is reported as mean ±standard deviation; men are reported as no. and associated percentage.
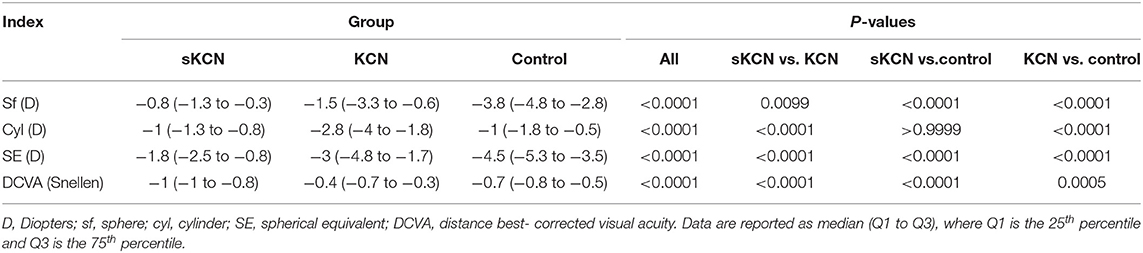
Refractive parameters (sphere, cylinder, spherical equivalent) and distance best-corrected visual acuity of each group are presented in Table 2.
Topographic and tomographic parameters of all groups from Pentacam are shown in Tables 3, 4. The KCN group proved significantly different values (p <0.001) of the investigated parameters compared to the control group, except corneal volume (VOL) and I-T significant values, compared to sKCN (see Tables 3, 4). Eleven out of 28 investigated parameters proved significantly different in the sKCN group compared with the Control group (Tables 3, 4).
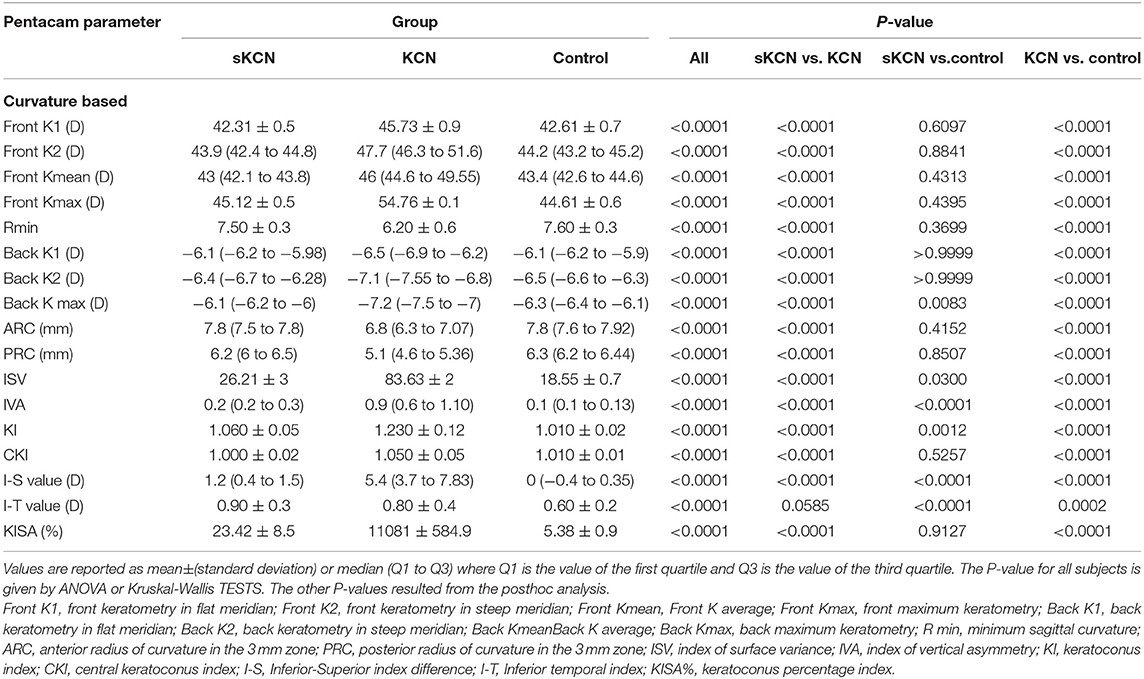
Table 3. Pentacam curvature-based parameters by groups: descriptive statistics and comparisons between groups.

Table 4. Pachymetric parameters, elevation based data, integrated indices and aberometric values by groups: descriptive statistics and comparisons between groups.
Excepting KI, I-T, BFS-front and BFS-back, showed AUCs higher than 0.8 regarding discrimination of the KCN by controls (Table 5). Discrimination between subjects suspected by KCN and controls is limited to eighteen topographic measurements, but only I-S, RMS Total, and RMS-HOA showed AUCs excellent accuracy classification (AUC>0.9, Tables 5, 6). Excellent accuracy classification is observed for most investigated topographic parameters when subjects with KCN are compared to those suspected by KCN (Tables 5, 6).
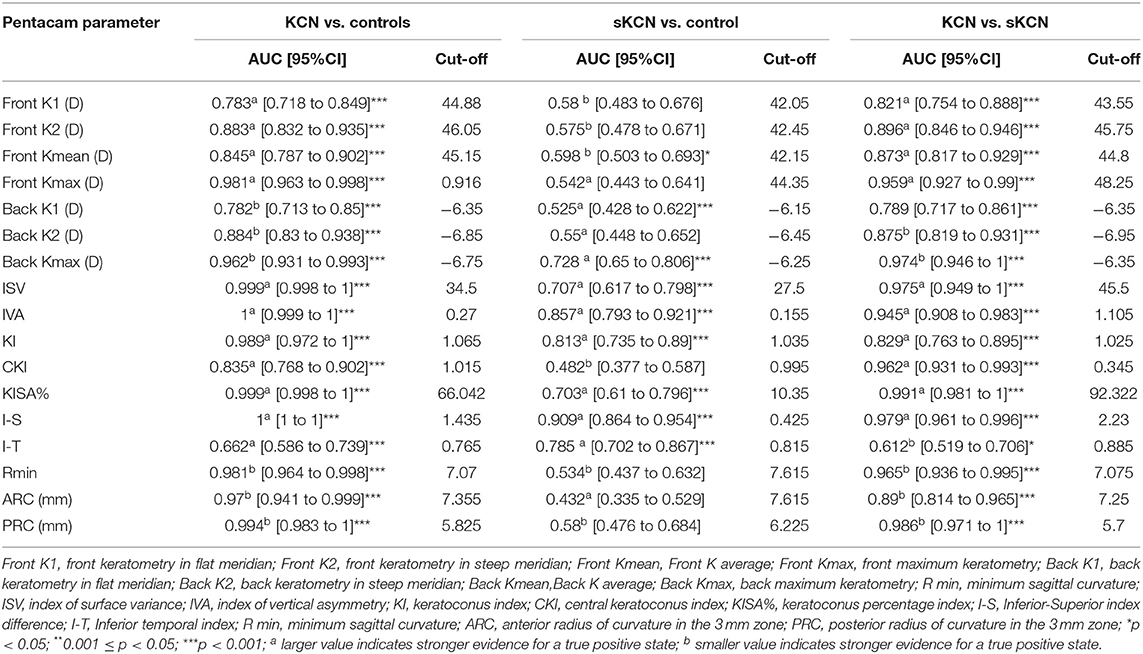
Table 5. Curvature based Pentacam parameters AUCs and associated cut-off: KCN vs. controls, sKCN vs. controls, KCN vs. sKCN.
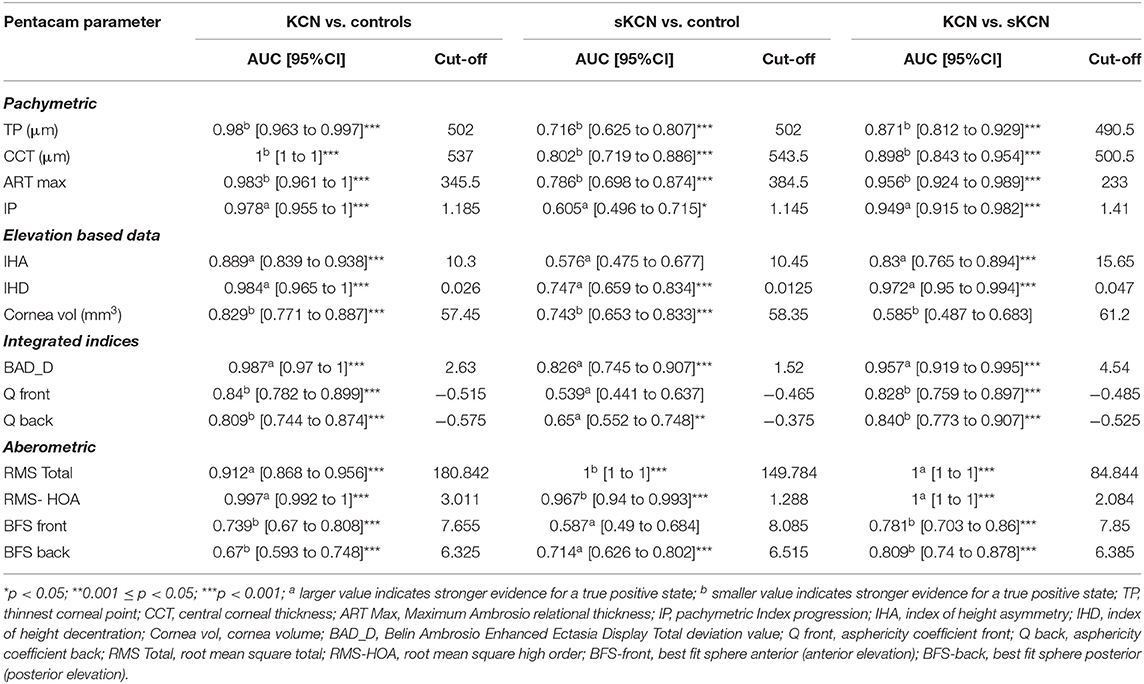
Table 6. Summary of the pachymetric, elevation, integrated, and aberometric Pentacam parameters: AUCs and associated cut-off for KCN vs. controls, sKCN vs. controls, KCN vs. sKCN.
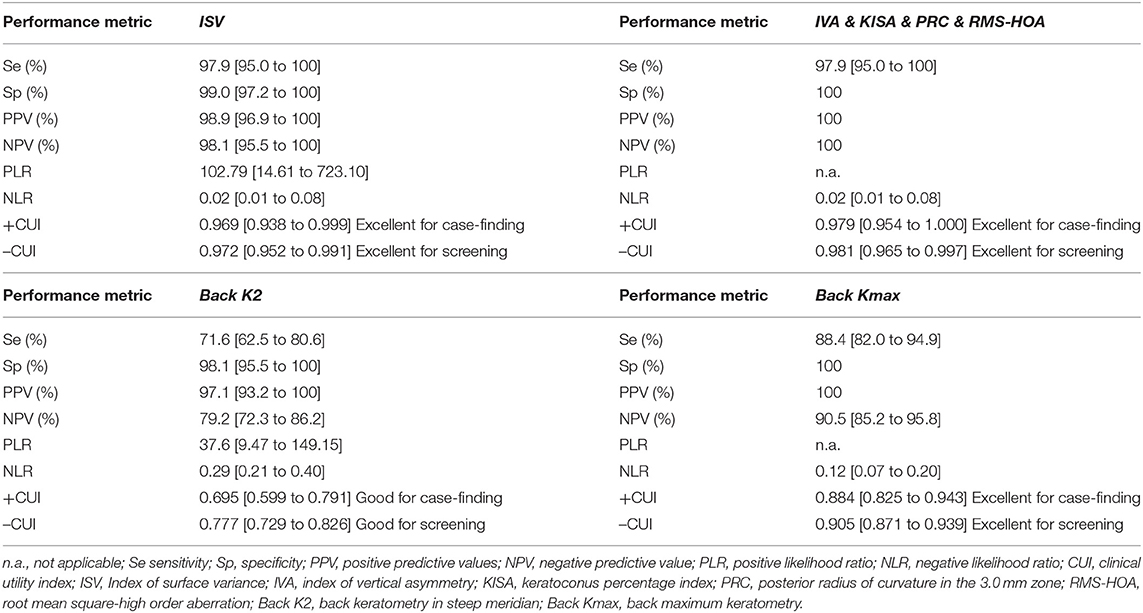
According to AUC, refractive parameters and visual acuity show limited diagnosis performances (Table 7). Two topographic measurements, namely I-S (cut-off = 1.435, the large value indicates the presence of KCN) and CCT (cut-off = 537, the small value indicates the presence of KCN), showed AUCs equal to 1 [0.999 to 1] in the differentiation of KCN by controls. All showed the performance metrics of 100% for Se, Sp, PPV, NPP, +CUI and -CUI. Other seven Pentacam measurements, including back Kmax proved excellent for case-finding and screening (Table 8).
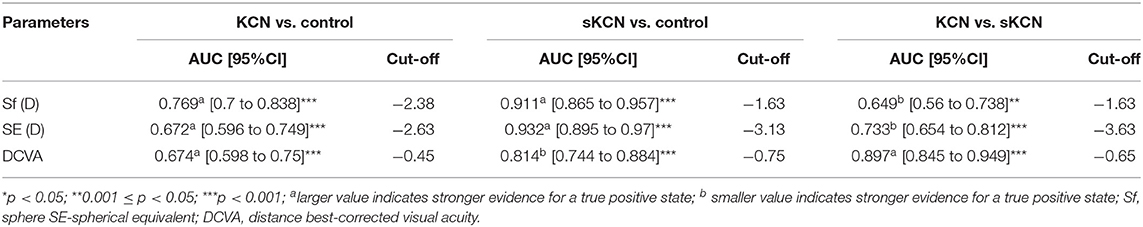
Table 7. Refractive parameters and visual acuity AUCs and associated cut-off: KCN vs. control, sKCN vs. control, KCN vs. sKCN.
Six measurements, including back Kmax proved excellent capacities for case-finding and good or excellent capacity for screening in the differentiation of sKNC by KCN, but KISA% showed higher sensitivity, specificity, and clinical specificity utility for case finding and screening (Table 9).
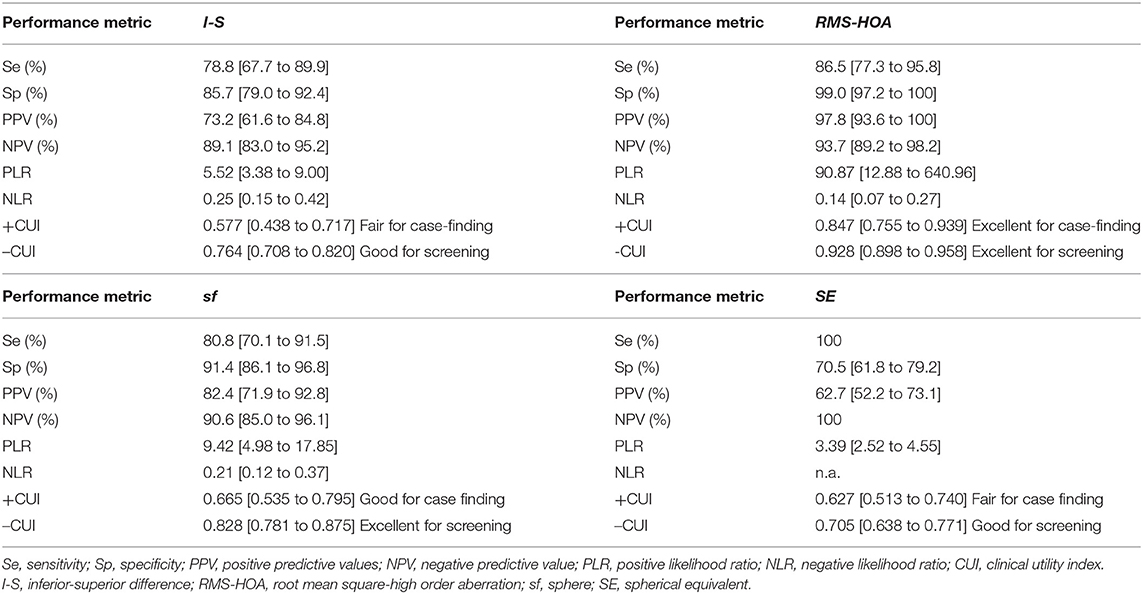
Only two measurements showed performance in identifying patients with sKCN compared to the controls, but RMS-HOA showed the best performances that support strong diagnostic evidence (Table 10).
Discussions
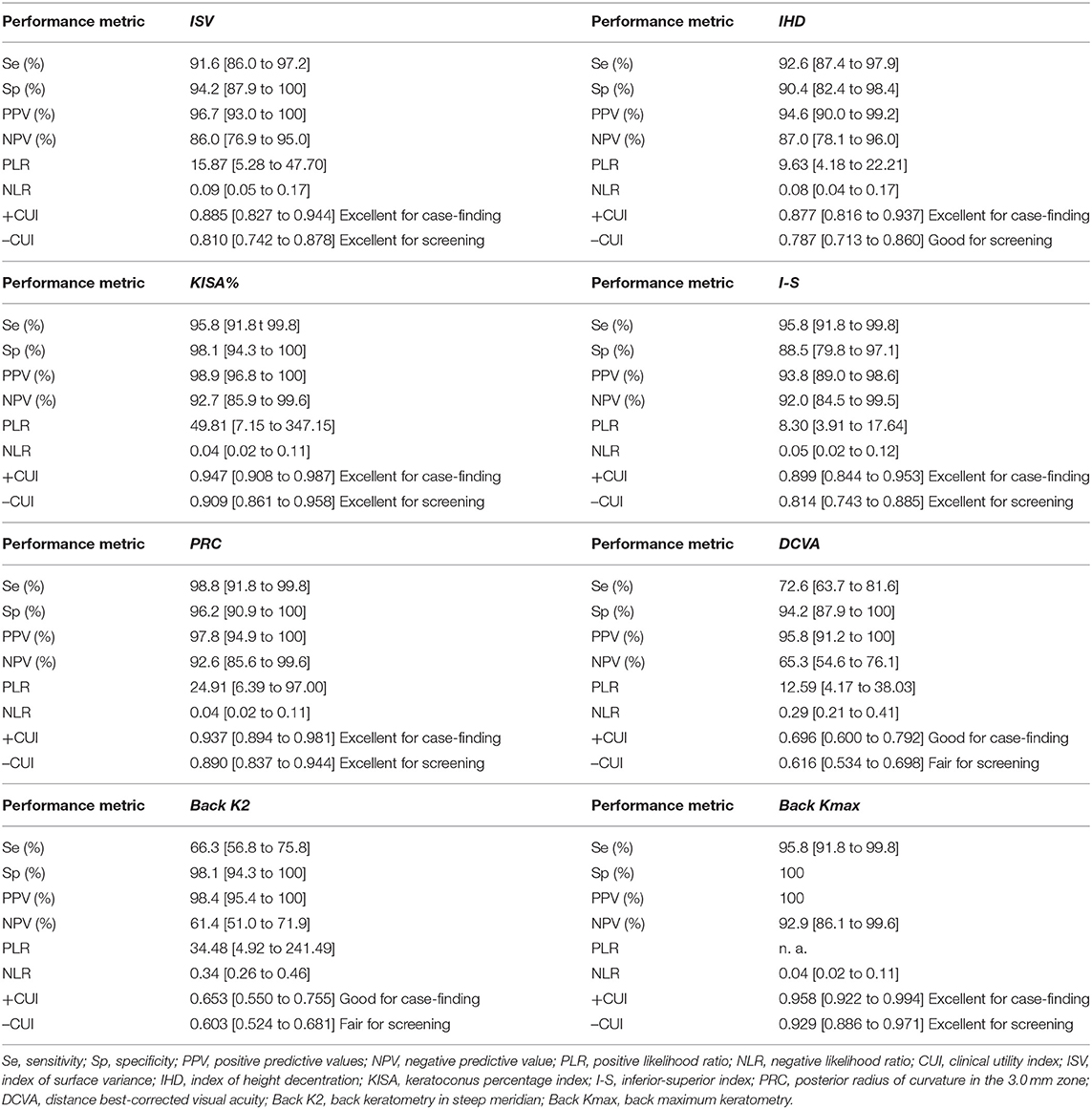
Our findings support the usefulness of five Pentacam parameters, ISV (AUC = 0.975), IHD (AUC = 0.972), KISA% (AUC = 0.991), I-S (AUC = 0.979), and PRC (AUC = 0.986), in the differentiation of sKCN from KCN.
De Sanctis (28) reported that posterior corneal elevation measured (100.74 ± 9.2 in KCN and 39.91 ± 5 in sKCN) with the Pentacam is a suitable index for differentiating sKCN from KCN but was less efficient in the diagnosis of sKCN. Nevertheless, a study suggested that an increase in posterior corneal elevation can be an early sign of sKCN (28). Solis-Vivanco et al. (61) reported high specificity (92%) and sensitivity (87%) of corneal topography than the clinical examination. Claude et al. (62) and Rabinowitz (56) support the need to combine different topographical indices to confirm the presence of KCN.
Our results demonstrated that the I-S (AUC = 0.909, cut-off = 0.425, Se = 78.8%, Sp = 85.7%; fair for case-findings and good for screening) and RMS-HOA (Se = 86.5%, Sp=99.0%, AUC=0.967, excellent for case-findings and screening) at the front surface of the cornea were the best variables for the diagnosis of sKCN (Tables 5, 6, 10). Similar results in diagnosing sKCN were reported for I-S by other researchers but with lower performances [AUC = 0.842, Se = 80.1%, Sp = 79.2% (43); AUC = 0.840 (38)]. Hashemi et al. (24) reported that ISV (>0.14) and IVA (>0.22) indices were the best parameters for detecting sKCN cases compared with the well-known indices such as Kmean or K max. Still, our results showed limited performances of these indices and different cut-off values (Table 3). Arbelaez et al. (63) and Heidari et al. (43), using corneal topography, tomography and biomechanical indices, showed that anterior and posterior curvature-based modifications could diagnose sKCN earlier than biomechanical analysis.
In our study, the BAD_D index had an AUC = 0.826 with a cut-off point equal to 1.52 in discriminating sKCN than normal eyes. This cut-off value is like the one reported by Hashemi et al. (24) (1.54), with higher accuracy than the ART Max parameter proposed by Ambrosio et al. (64) in detecting sKCN (cut-off value = 1.45). Correia et al. (65) also supported the idea that BAD_D is an important parameter in diagnosing sKCN and KCN, results also demonstrated in our study (Table 6).
Hashemi et al. (24) reported that BAD_D, IVA, ISV, and 5th order vertical coma aberration are the best criteria for sKCN. Shaag (66) suggested that aberration indices should be simultaneously used with vertical asymmetry.
Our findings revealed that the best indices for discriminating KCN from normal corneas were ISV (AUC = 0.999), ISA (AUC = 0.999, KISA% (AUC = 0.999), PRC (AUC = 0.999), BAD_D (AUC = 0.987), ART Max (AUC = 0.983) and RMS-HOA (AUC = 0.997) (Tables 5, 6). In our study, we took into consideration the ART Max index as a parameter for the diagnosis of KCN with a cut-off value of 345.5 and an AUC = 0.983 (Table 6), a result similar to Muftuoglu et al. (67) that showed high confidence of the ART Max index for the diagnosis of clinical KCN in contrast with sKCN. Muftuoglu et al. (67) introduced the D index as a combination of keratometric, pachymetric, pachymetric progression and back elevation parameters to differentiate KCN from the sKCN. The cut-off value for D index was 1.3, with a sensitivity of 60% and specificity of 90%, suggesting the possible false-negative results (67). In our study, RMS-HOA had a sensitivity of 97.9 and a specificity of 100 (Table 8). Similarly, Hashemi et al. (24) revealed that BAD_D, mean K, and 3rd order vertical coma was optimal for diagnosing clinical KCN. Moreover, Shetty et al. (42) showed that RMS was the best index to distinguish KCN from normal eyes (AUC = 0.983).
Sedgipour et al. (41) showed that KISA% (cut-off value for correct diagnosis of KCN = 100%.) was the single index with specificity and sensitivity >90% demonstrating positive and negative predictive values >95%. Li et al. (36) successfully used K value, I-S value, and KISA% indices to detect KCN.
In our study, corneal volume distribution proved significantly smaller in sKCN group than in controls (p < 0.0001, Table 4), supporting its potential in diagnosing sKCN. Similar results were also reported by Ambrosio et al. (29), showing that corneal-thickness spatial profile and corneal volume distribution may differentiate keratoconus corneas from the normal ones.
Our results showed that two topographic measurements, namely I-S (cut-off = 1.435) and CCT (cut-off = 537) were other important parameters in diagnosing KCN. Similarly, Sedhagat et al. (68) demonstrated that I-S value (AUC = 0.986) had the highest capacity among curvature parameters to distinguish KCN from normal eyes. Heidari et al. (43) showed that IHD had better accuracy in diagnosing KCN cases.
Our findings demonstrated the potential of Kmax of the back cornea (Se = 88.4%, Sp = 100%, Table 8) with excellent utility in case-finding and screening of the disease. Similarly, Bae et al. (69) demonstrated that curvature data are more accurate than pachymetric and elevation parameters for early diagnosing KCN. Other studies (70, 71) also reported the usefulness of the posterior corneal surface in the differentiation of the normal eyes from KCN, as the earliest indicator for ectasia. Correia et al. (65) demonstrated that front surface curvature parameters may be applied as objective parameters to diagnose KCN but can be normal in mild ectasia cases. This can give a later ectasia diagnosis than tomographic indices based on posterior elevation and pachymetry distribution (65).
Our study turned its special attention to sKCN cases, proposing effective indices for the diagnosis of this cases, which are applicable and can be evaluated. In our opinion, simultaneous evaluation of BAD_D, back Kmax, IVA, I-S, ART Max and RMS total values can help diagnose cases of sKCN. Even though, these findings do not reduce the importance of the classical indices such as front keratometry. The cut-off points suggested in our study have acceptable specificity and sensitivity. Further studies in this regard would probably conclude in more accurate cut-off values.
Our study has some limitations that need to be highlighted. First, we did not collect the cornea biomechanical parameters, so topographical and tomographical data association was not done. This association could be extremely useful in the early diagnosis of sKCN. Second, based on applied design and collected data, we could not evaluate the sKCN progression to clinical KCN. A prospective cohort study with follow-up evaluation of patients with sKCN will allow the assessment of the rate of progression and is currently conducted by our team. Third, the reported cut-off values must be evaluated on external samples to prove their reliability and validity.
Conclusions
In distinguishing sKCN from normal eyes, Back Kmax, IVA, I-S and RMS total values were the most accurate indices. All topographical and tomographical parameters had good specificity and sensitivity in diagnosing clinical KCN, but six of them (ISV, IVA, KISA, PRC, RMS-HOA, and Back Kmax) demonstrate excellent utility in case-finding and screening of sKCN. Despite the fact that Back Kmax as a single diagnostic parameter is not sufficient, it does suggest to be very effective in distinguishing sKCN from normal corneas and KCN from normal corneas in association with other parameters.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The study involved human participants and was reviewed and approved by the Ethical Committee of the Oculens Clinic. Written informed consent for participation was not required for this study in accordance with the National Legislation and the Institutional requirements.
Author Contributions
CN, DN, and AB: study conception and design. CN, DN, KH, and SB: wrote the first draft of the manuscript. AB and AN: data collection. SB: data curation and analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rabinowitz YS, Nesburn AB, McDonnell PJ. Videokeratography of the fellow eye in unilateral keratoconus. Ophthalmology. (1993) 100:181–6. doi: 10.1016/S0161-6420(93)31673-8
2. Munir SZ, Munir WM, Albrecht J. Estimated prevalence of keratoconus in the United States from a large vision insurance database. Eye Contact Lens. (2021) 47:505–10. doi: 10.1097/ICL.0000000000000812
3. Hashemi H, Heydarian S, Yekta A, Ostadimoghaddam H, Aghamirsalim M, Derakhshan A, et al. High prevalence and familial aggregation of keratoconus in an Iranian rural population: a population-based study. Ophthalmic Physiol Opt. (2018) 38:447–55. doi: 10.1111/opo.12448
4. Hwang S, Lim DH, Chung TY. Prevalence and incidence of keratoconus in South Korea: a nationwide population-based study. Am J Ophthalmol. (2018) 192:56–64. doi: 10.1016/j.ajo.2018.04.027
5. Kristianslund O, Hagem AM, Thorsrud A, Drolsum L. Prevalence, and incidence of keratoconus in Norway: a nationwide register study. Acta Ophthalmol. (2021) 99:e694–9. doi: 10.1111/aos.14668
6. Heydarian S, Hashemi H, Yekta A, Ostadimoghaddam H, Derakhshan A, Aghamirsalim M, et al. Heritability of corneal curvature and pentacam topometric indices: a population-based study. Eye Contact Lens. (2019) 45:365–71. doi: 10.1097/ICL.0000000000000589
7. Wojcik K, Kaminska A. BlasiakJ, Szaflik J, Szaflik JP. Oxidative stress in the Pathogenesis of keratoconus and Fuchs Endothelial Corneal Dystrophy. Int J Mol Sci. (2013) 14:19294–308. 140919294 doi: 10.3390/ijms140919294
8. Kenney MC, Brown DJ. The cascade hypothesis of keratoconus. Cont Lens Anterior Eye. (2003) 26:139–46. doi: 10.1016/S1367-0484(03)00022-5
9. Chwa M, Atilano SR, Hertzog D, Zheng H, Langberg J, Kim DW, et al. Hypersensitive response to oxidative stress in keratoconus corneal fibroblasts. Invest Ophthalmol Vis Sci. (2008) 49:4361–9. doi: 10.1167/iovs.08-1969
10. Sahebjada S, Al-Mahrouqi HH, Moshegov S, Panchatcharam SM, Chan E, Daniell M, et al. Eye rubbing in the aetiology of keratoconus: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. (2021) 259:2057–67. doi: 10.1007/s00417-021-05081-8
11. Nakao G, Koh S, Inoue R, Maeno S, Maeda N, Nishida K. The characteristics and risk factors of very asymmetric keratoconus. Eye Contact Lens. (2021) 47:511–4. doi: 10.1097/ICL.0000000000000830
12. Jun AS, Cope L. SpeckC, Feng X, Lee S, Meng H, Hamad A, Chakravarti S. Subnormal cytokine profile in the tear fluid of keratoconus patients. PloS ONE. (2011) 6:e16437. doi: 10.1371/journal.pone.0016437
13. Wollensak G, Aurich H, Wirbelauer C, Pham DT. Potential use of riboflavin/UVA cross-linking in bullous keratopathy. Ophthalmic Res. (2009) 41:114–7. doi: 10.1159/000187630
15. Ambrosio R Jr, Randleman JB. Screening for ectasia risk: what we are screening for and how should we screen for it? J Refract Surg. (2013) 29:230–2. doi: 10.3928/1081597X-20130318-01
16. Missoten L. Corneal topography. Curr Opin Ophthalmol. (1994) 5:68–74. doi: 10.1097/00055735-199408000-00010
17. Fan R, Tommy CY. Chan, Gaurav Prakash, Vishal Jhanji. Application of corneal topography and tomography: a review. Clin Exp Ophthalmol. (2018) 46:133–46. doi: 10.1111/ceo.13136
18. Maguire LJ, Bourne WM. Corneal topography of early keratoconus. Am J Ophthalmol. (1989) 108:107–12. doi: 10.1016/0002-9394(89)90001-9
19. de Sanctis U, Loiacono C, Richiardi L, Turco D, Mutani B, Grignolo FM. Sensitivity, and specificity of posterior elevation measured by Pentacam in discriminating keratoconus/ subclinical keratoconus. Ophthalmology. (2008) 115:1534–9. doi: 10.1016/j.ophtha.2008.02.020
20. Uçakhan ÖÖ, Cetinkor V, Özkan M, Kanpolat A. Evaluation of Scheimpflug imaging parameters in subclinical keratoconus, keratoconus, and normal eyes. J Cataract Refract Surg. (2011) 37:1116–24. doi: 10.1016/j.jcrs.2010.12.049
21. Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio R Jr, Guell JL, et al. Group of panelists for the global delphi panel of keratoconus and ectatic diseases. Global consensus on keratoconus and ectatic diseases. Cornea. (2015) 34:359–69. doi: 10.1097/ICO.0000000000000408
22. Belin MW, Duncan JK. Keratoconus: The ABCD grading system. Klin Monbl Augenheilkd. (2016) 233:701–7. doi: 10.1055/s-0042-100626
23. Rabinowitz YS, Rasheed K. KISA% index: a quantitative videokeratography algorithm embodying minimal topographic criteria for diagnosing keratoconus. J Cataract Refract Surg. (1999) 25:1327–35. errata (2000) 26:480. doi: 10.1016/S0886-3350(99)00195-9
24. Hashemi H, Beiranvand A, Yekta A, Maleki A, Yazdani N, Khabazkhoob M. Pentacam top indices for diagnosing subclinical and definite keratoconus. J Curr Ophthalmol. (2016) 28:21–6. doi: 10.1016/j.joco.2016.01.009
25. Bühren J, Kook D, Yoon G, Kohnen T. Detection of subclinical keratoconus by using corneal anterior and posterior surface aberrations and thickness spatial profiles. Invest Ophthalmol Vis Sci. (2010) 51:3424–32. doi: 10.1167/iovs.09-4960
26. Maeda N, Klyce SD, Smolek MK. Comparison of methods for detecting keratoconus using videokeratography. Arch Ophthalmol. (1995) 113:870–4. doi: 10.1001/archopht.1995.01100070044023
27. Rabinowitz YS. Videokeratographic indeces to aid in screening in keratoconus. J Refract Surg. (1995) 11:371–9. doi: 10.3928/1081-597X-19950901-14
28. de Sanctis U, Aragno V, Dalmasso P, Brusasco L, Grignolo F. Diagnosis of subclinical keratoconus using posterior elevation measured with 2 different methods. Cornea. (2013) 32:911–5. doi: 10.1097/ICO.0b013e3182854774
29. Ambrosió R Jr, Alonso RS, Luz A, Coca Velarde LG. Corneal-thickness spatial profile and corneal-volume distribution: tomographic indices to detect keratoconus. J Cataract Refract Surg. (2006) 32:1851–9. doi: 10.1016/j.jcrs.2006.06.025
30. Saad A, Gatinel D. Evaluation of total and corneal wavefront high order aberrations for the detection of forme fruste keratoconus. Invest Ophthalmol Vis Sci. (2012) 53:2978–92. doi: 10.1167/iovs.11-8803
31. Smolek MK, Klyce SD. Current keratoconus detection methods compared with a neural network approach. Invest Ophthalmol Vis Sci. (1997) 38:2290–9.
32. McMahon TT, Szczotka-Flynn L, Barr JT, A new method for grading the severity of keratoconus: the Keratoconus Severity Score (KSS). Cornea. (2006) 25:794–800. doi: 10.1097/01.ico.0000226359.26678.d1
33. Jiménez-García M, Kreps EO, Ní Dhubhghaill S, Koppen C, Rozema JJ. REDCAKE Study Group. Determining the Most Suitable Tomography-Based Parameters to Describe Progression in Keratoconus The Retrospective Digital Computer Analysis of Keratoconus Evolution Project. Eye Contact Lens. (2021) 47:486–93. doi: 10.1097/ICL.0000000000000800
34. Rabinovitz YS, Garbus J, Ms Donnell PJ. Computer-assisted corneal topography in family members of patients with keratoconus. Arch Ophthalmol. (1990) 108:365–71. doi: 10.1001/archopht.1990.01070050063032
35. Maguire LJ, Lowry JC. Identifying progression of subclinical keratoconus by serial topography analysis. Am J Ophthalmol. (1991) 112:41–5. doi: 10.1016/S0002-9394(14)76210-5
36. Li X, Rabinovitz YS, Rasheed K, Yang H. Longitudinal study of the normal eyes in unilateral keratoconus patients. Ophthalmology. (2004) 111:440–6. doi: 10.1016/j.ophtha.2003.06.020
37. Lopes BT, Ramos IC, Salomao MQ, Guerra FP, Schallhorn SC, Schallhorn JM, et al. Enhanced tomographic assesment to detect corneal ectasia based on artificial intelligence. Am J Ophthalmol. (2018) 195:223–32. doi: 10.1016/j.ajo.2018.08.005
38. Degirmenci C, Palamar M, Ismayilova N, Egrilmez S, Yagci A Topographic evaluation of unilateral keratoconus patients. Turk J Ophthalmol. (2019) 49:117–22. doi: 10.4274/tjo.galenos.2018.90958
39. Hashemi H, Pakzad R, Heydarian S, Yekta A, Ostadimoghaddam H, Mortazavi M, et al. Keratoconus indices and their determinants in healthy eyes of a rural population. J Curr Ophthalmol. (2020) 32:343–8. doi: 10.1016/j.joco.2019.10.003
40. Song P, Yang KL LI P, Liu Y, Liang DF, Ren SW, Zeng QY. Assessment of corneal pachymetry distribution and morphologic changes in subclinical keratoconus with normal biomechanics. Biomed Res Int. (2019) 2019:1748579. doi: 10.1155/2019/1748579
41. Sedghipour MR, Sadigh AL, Motlagh BF. Revisiting corneal topography for the diagnosis of keratoconus: use of Rabinowitz' KISA % index. Clin Ophthalmol. (2012) 6:181–4. doi: 10.2147/OPTH.S24219
42. Shetty R, Rao H, Khamar P, Sainani K, Vunnava K, Jayadev C, et al. Keratoconus screening indices and their diagnostic ability to distinquish normal from ectatic corneas. Am J Ophthalmol. (2017) 181:140–8. doi: 10.1016/j.ajo.2017.06.031
43. Heidari Z, Hashemi H, Mihammadpour M, Amanzaheb K, Fotouhi A. Evaluation of corneal topographic, tomographic, and biomechanical indices for detecting clinical and subclinical keratoconus: a comprehensive three device study. Int J Ophthalmol. (2021) 14:228–39. doi: 10.18240/ijo.2021.02.08
44. Raiskup F, Theuring A, Pillunat LE, Spoerl E. Corneal collagen crosslinking with riboflavin and ultraviolet-A light in progressive keratoconus: ten-year results. J Cataract Refract Surg. (2015) 41:41–6. doi: 10.1016/j.jcrs.2014.09.033
45. Caporossi A, Mazzotta C, Baiocchi S, Caporossi T. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Ophthalmol. (2010) 149:585–93. doi: 10.1016/j.ajo.2009.10.021
46. Elbaz U, Shen C, Lichtinger A, Zauberman NA, Goldich Y. Chan CC, et al. Accelerated (9-mW/cm2) corneal collagen cross-linking for keratoconus-A 1-year follow-up. Cornea. (2014) 33:769–73. doi: 10.1097/ICO.0000000000000154
47. Chow V, Chan TCY Yu M, Wong V, Jhanji V. One-year outcomes of conventional and accelerated collagen cross-linking in progressive keratoconus. Sci Rep. (2015) 5:14425. doi: 10.1038/srep14425
48. Shetty R, Pahuja K, Nuijts R, Ajani A, Jayadev C, Sharma C, et al. Current protocols of corneal collagen cross-linking: visual, refractive, and tomographic outcomes. Am J Ophthalmol. (2015) 160:243–9. doi: 10.1016/j.ajo.2015.05.019
49. Mazzotta C, Baiocchi S, Bagaglia SA, Fruschelli M, Meduri A, Rechichi M. Accelerated 15 mW pulsed-light cross-linking to treat progressive keratoconus: Two-year clinical results. J Cataract Refract Surg. (2017) 43:1081–8. doi: 10.1016/j.jcrs.2017.05.030
50. Rechichi M, Mazzotta C, Daya S, Mencucci R, Lanza M, Meduri A. Intraoperative OCT pachymetry in patients undergoing dextran-free riboflavin UVA accelerated corneal collagen crosslinking. Curr Eye Res. (2016) 41:1310–5. doi: 10.3109/02713683.2015.1118130
51. Rechichi M, Daya S, Scorcia V, Meduri A, Scorcia G. Epithelial-disruption collagen cross-linking for keratoconus: one-year results. J Cataract Refract Surg. (2013) 39:1171–8. doi: 10.1016/j.jcrs.2013.05.022
52. Delgado LM, Bayon Y, Pandit A, Dimitrios Zeugolis DI. To Cross-Link or Not to Cross-Link? Cross-Linking Associated Foreign Body Response of Collagen-Based Devices. Tissue Eng Part B Rev. (2015) 21:298–313. doi: 10.1089/ten.teb.2014.0290
53. Rechichi M, Mazzotta C, Oliverio GW, Romano V, Borroni D, Ferrise M, et al. Selective transepithelial ablation with simultaneous accelerated corneal cross-linking for corneal regularization of keratoconus: STARE-X protocol. J Cataract Refract Surg. (2021) 47:1403–10. doi: 10.1097/j.jcrs.0000000000000640
54. Sakellaris D, Balidis M, Gorou O, Szentmary N, Alexoudis A, Grieshaber M, et al. Intracorneal Ring Segment Implantation in the Management of Keratoconus: An Evidence-Based Approach. Ophthalmol Ther. (2019) 8:5–14. doi: 10.1007/s40123-019-00211-2
55. Choi JA, Lee MA, Kim MS. Long-term outcomes of penetrating keratoplasty in keratoconus: analysis of the factors associated with final visual acuities. Int J Ophthalmol. (2014) 7:517–21. doi: 10.3980/j.issn.2222-3959.2014.03.24
56. Rabinowitz YS, Mc Donnell PJ. Computer-assisted corneal topography in keratoconus. Refract Corneal Surg. (1989) 5:400–8. doi: 10.3928/1081-597X-19891101-10
57. Muftuoglu O, Ayar O, Ozulken K, Ozyol E, Akinci A. Posterior corneal elevation and back difference corneal elevation in diagnosing forme fruste keratoconus in the fellow eyes of unilateral keratoconus patients. J Cataract Refract Surg. (2013) 39:1348–57. doi: 10.1016/j.jcrs.2013.03.023
58. Zadnik K, Barr JT, Edrington TB, Everett DF, Jameson M, McMahon TT, et al. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Baseline findings in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) study. Invest Ophthalmol Vis Sci. (1998) 39:2537–46.
59. Mothlagh MN, Moshirfar M, Murri MS, Skanchy DF, Momeni Moghaddam H, Ronquillo YC, et al. Pentacam corneal tomography for screening of refractive surgery candidates: a review of the literature, part 1. Med Hypothesis Discov Innov Ophthalmol. (2019) 8:177–203.
60. Bolboacă SD. Medical diagnostic tests: a review of test anatomy, phases, and statistical treatment of data. Comput Math Methods Med. (2019) 2019:1891569. doi: 10.1155/2019/1891569
61. Solis-Vivanco A, Hernandez-Quintela E, Fromow-Guerra JJ, Cruz-Sanchez A. Ponce-de-Leon S, Naranjo-Tackman R. Corneal Topography as a Screening Method for Keratoconus Suspects. Invest Ophthalmol Vis Sci. (2002) 43:172.
62. Claude S, Verdier R, Arnaud B, Schmitt-Bernard CF. [Accuracy of videokeratographic quantitative criteria for detection of keratoconus suspects in families with keratoconus]. J Fr Ophtalmol. (2004) 27:773–8. doi: 10.1016/S0181-5512(04)96212-2
63. Arbalaez MC, Versaci F, Vestri G, Barboni P, Savini G. Use of a support vector machine for keratoconus and subclinical keratoconus detection by topographic and tomographic data. Ophthalmology. (2012) 119:2231–8. doi: 10.1016/j.ophtha.2012.06.005
64. Ambrosio R. Jr, Faria-Correia F, Ramos I. Enhaced screening for ectasia susceptibility among refractive candidates: the role of corneal tomography and biomechanics. Curr Ophthalmol Rep. (2013) 1:28–38. doi: 10.1007/s40135-012-0003-z
65. Correia FF, Ramos I, Lopes B, Salomão MQ, Luz A, Correa RO, et al. Topometric and tomographic indices for the diagnosis of keratoconus. Int J Keratoconus Ectatic Corneal Dis. (2012) 1:92–9. doi: 10.5005/jp-journals-10025-1018
66. Gordon-Shaag A, Millodot M, Ifrah R, Shneor E. Aberrations and topography in normal, keratoconus-suspect, and keratoconic eyes. Optom Vis Sci. (2012) 89:411–8. doi: 10.1097/OPX.0b013e318249d727
67. Muftuoglu O, Ayar O, Hurmeric V, Orucoglu F, Kilic I. Comparison of multimetric D index with keratometric, pachymetric, and posterior elevation parameters in diagnosing subclinical keratoconus in fellow eyes of asymmetric keratoconus patients. J Cataract Refract Surg. (2015) 41:557–65. doi: 10.1016/j.jcrs.2014.05.052
68. Sedahagat MR, Momeni-Moghaddam H, Ambrozio R Jr, Heidari HR, Maddah N, Danesh Z, et al. Diagnostic ability of corneal shape and biomechanical parameters for detecting frank keratoconus. Cornea. (2018) 37:1025–34. doi: 10.1097/ICO.0000000000001639
69. Bae GH, Kim JR, Lim DH, Chung ES, Chung TY. Corneal topographic and tomographic analysis of fellow eyes in unilateral keratoconus patients using Pentacam. Am J Ophthalmol. (2014) 157:103–109.e1. doi: 10.1016/j.ajo.2013.08.014
70. Du XL, Chen M, Xie LX. Correlation of basic indicators with stages of keratoconus assesd by Pentacam tomography. Int J Ophthalmol. (2015) 8:1136–40. doi: 10.3980/j.issn.2222-3959.2015.06.10
Keywords: keratoconus, subclinical keratoconus, corneal topography, corneal tomography, clinical utility
Citation: Nicula CA, Bulboacă AE, Nicula D, Nicula AP, Horvath KU and Bolboacă SD (2022) Performances of Corneal Topography and Tomography in the Diagnosis of Subclinical and Clinical Keratoconus. Front. Med. 9:904604. doi: 10.3389/fmed.2022.904604
Received: 25 March 2022; Accepted: 28 April 2022;
Published: 26 May 2022.
Edited by:
Alessandro Meduri, University of Messina, ItalyReviewed by:
Francesco Gazia, University of Messina, ItalyLaura De Luca, Policlinico Universitario G. Martino Oftalmologia, Italy
Copyright © 2022 Nicula, Bulboacă, Nicula, Nicula, Horvath and Bolboacă. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sorana D. Bolboacă, c2JvbGJvYWNhQHVtZmNsdWoucm8=
 Cristina Ariadna Nicula
Cristina Ariadna Nicula Adriana Elena Bulboacă
Adriana Elena Bulboacă Dorin Nicula2
Dorin Nicula2 Sorana D. Bolboacă
Sorana D. Bolboacă