
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 15 June 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.901239
This article is part of the Research TopicMechanism and Therapy of Autoimmune Skin DiseasesView all 8 articles
Pemphigus is a chronic and severe autoimmune bullous disease caused by autoantibodies targeting adhesion molecules between keratinocytes. It requires 2–3 years on average to manage the disease. To date, although Rituximab combined with short-term systemic glucocorticoids was accepted as first-line therapy, systemic glucocorticoids remain the primary therapeutic option for pemphigus patients, successfully decreasing morbidity and mortality from pemphigus. However, novel therapeutic strategies are desirable due to the low efficacy in some subset of patients and the long-term severe adverse effects of traditional therapies. Recently, immunotherapy has proved to be encouraging for disease control or cure. Based on the current understanding of the immune mechanisms of pemphigus, we review the immune targets and corresponding agents applied in practice or under clinical trials. The goals of the novel treatments are to improve the quality of life of pemphigus patients by improving efficacy and safety, minimizing side effects, achieving fast disease control, or curing the disease.
Pemphigus is an autoimmune and organ-specific bullous disease, with flaccid blisters and superficial erosions on the skin and mucous membrane of the patients. Two primary types are pemphigus vulgaris (PV) and pemphigus foliaceus (PF), of which PV is more common than PF (1). Diagnosis is based on the intraepidermal blister and acantholysis by histology, IgG deposition between acanthocytes by direct immunofluorescence study (DIF), and positive serologic IgG by indirect immunofluorescence study (IIF) or anti-Desmoglein 3 or 1 (Dsg3 or 1) autoantibodies by ELISA (2). The current mainstay therapy for pemphigus is systemic glucocorticoids, as administered in most other autoimmune diseases (3, 4). However, prolonged application of glucocorticoids often leads to many adverse effects, such as Cushing's syndrome, infectious complications, dysregulation of the hypothalamic-pituitary-adrenal axis, hypertension, hyperglycemia and osteoporosis (4, 5). Consequently, topical treatment with strong glucocorticoids is often chosen in clinical practice to minimize the side effects caused by systemic application (6, 7). Additionally, other immunosuppressants such as azathioprine (AZA), methotrexate (MTX), cyclosporine A (CSA), mycophenolate mofetil, and cyclophosphamide (CTX) were also standard options for the treatment of pemphigus patients (8, 9). However, severe side effects such as infertility, increased risk of cancer, genitourinary complications, hypertension, lymphopenia, teratogenic effects, and infection have limited its use (10–15). In the past decade, a series of studies have helped better understand the immune mechanisms of pemphigus. A milestone work has been the successful application of CD20 monoclonal antibody (mAb) (16–18). Recently, a few novel targets for immunotherapy have been identified, and the biological and immunologic agents developed specifically against these targets could provide more effective therapies for pemphigus patients.
Pemphigus is a life-threatening autoimmune bullous disease, and the patients have autoantibodies targeting the adhesion proteins (Dsg1 or 3) among keratinocytes, leading to acantholysis of skin and mucous membrane. The autoantibodies disrupt desmosomal Dsgs through steric hindrance, activation of transmembrane signaling, internalization, and intracellular degradation that down-regulates cell-cell adhesion (19–23). Current evidence has supported that autoreactive T cells, B cells, and the cytokines regulating their function are critical in developing autoimmunity and production of autoantibodies in pemphigus.
B cells have assumed a prominent position in producing pathogenic autoantibodies and contributing to antigen presentation and immune co-stimulation, suggesting that depleting B cells may be a practical approach for pemphigus therapy (24). Several novel therapeutic strategies targeting B cells have been in investigational or clinical trials for the treatment of pemphigus, and those included anti-CD20 antibodies and Bruton's tyrosine kinase inhibitors (BTKI) targeting B cell receptor signaling (25).
Several studies have indicated the importance of T cells in pemphigus (26, 27) and the role of Dsg3-specific CD4+ T cells has been elegantly demonstrated in an animal model by inducing a phenotype of interface dermatitis and PV (28), and defective regulatory T (Treg) cells may play a role in the onset of pemphigus by modulating the production of anti-Dsg3 autoantibodies (29). Tsunoda et al. have demonstrated that the interaction between autoreactive T cells and B cells was the key event for humoral autoimmunity targeting Dsg3 by transferring Dsg3-specific T cells or B cells into Dsg3+/+ Rag2−/− mice (30). Therefore, the T and B immune axis involved in the pemphigus immune mechanisms may serve as primary therapeutic targets for patients with pemphigus.
In addition to the immune cells, cytokines, a group of low molecular weight proteins produced during immune responses, act as a signaling mediator that allows complex interactions between lymphocytes. By binding to specific receptors in the target cells, they initiate a cascade of intracellular signaling leading to the regulation of important biological functions, such as the growth, activation, differentiation, survival and death of the cells (31, 32). Numerous factors promoting B-cell differentiation, function and survival have been identified, including TNF-α, IL-1, IL-2, IL-4, IL-6, and IL-10 (33). B-Lymphocyte Stimulator (BLyS, also called B-cell Activating Factor, BAFF) and APRIL (A Proliferation-Inducing Ligand) are members of the TNF superfamily that play an essential role in B-cell survival and proliferation (34). Thus, targeting these cytokines to inhibit the proliferation and activation of B cells may represent a new approach to disease therapy.
Rituximab is a monoclonal IgG1 antibody against CD20+ B cells (35). This antibody was studied in a prospective, multicenter, parallel-group, and open-label randomized trial and was granted a Breakthrough Therapy Designation by the US FDA for the initial treatment of PV. Subsequently, rituximab was accepted as a first-line therapeutic option when combined with short-term systemic corticosteroids (36–39). Additionally, high-dose rituximab was associated with a longer duration of complete clinical remission than low-dose rituximab (40). Long-term analysis of patients with pemphigus who received rituximab have shown that relapse was linked to the same anti-Dsg B cells observed during active disease, supporting that relapse resulted from the incomplete depletion of the autoreactive B cells clones (41). In addition to relapse, resistance to rituximab therapy could emerge during treatment, which could occur due to either genetic polymorphisms or the development of human anti-chimeric antibodies against the murine fragment of rituximab, preventing the drug from binding to B cells (42). Rituximab therapy also showed a risk of developing serious adverse events such as infection and hypogammaglobulinaemia (43). To improve the effectiveness and tolerability, new immunotherapy agents are currently under investigational trials.
Anti-CD20 antibodies are diverse and could be categorized as type I and type II according to the cellular response upon binding. Type I mAbs localize CD20 into lipid rafts on the plasma membrane, leading to clustering of CD20 that enhances the recruitment and activation of complement (44, 45). In contrast, Type II mAbs exhibit stronger homotypic adhesion and more direct induction of cell death than type I mAbs, albeit with a minimal complement-dependent cytotoxic (CDC) response.
Veltuzumab is so far the only next-generation anti-CD20 mAb that has been reported in the treatment of refractory PV patients. This antibody is a type I, humanized anti-CD20 mAb with framework regions of epratuzumab, a humanized anti-CD22 antibody. It's significant advantage over rituximab is that it can be administered subcutaneously in low doses, making it more convenient to be applied on patients (46).
Ofatumumab is a type I, fully human, anti-CD20 monoclonal antibody, which targets an epitope of CD20 different from the rituximab binding site and has been proved to be safe and effective for the treatment of lymphoproliferative and other autoimmune disorders (47). A phase III randomized placebo-controlled trial of subcutaneous ofatumumab in pemphigus was recently terminated in 2018 (NCT01920477), and the results of this study remain to be reported (48).
Additionally, ocrelizumab, obinutuzumab/GA-101, ocaratuzumab (AME-133v), and PRO131921, which are the third-generation anti-CD20 mAbs used for treating relapsing multiple sclerosis (49, 50) and chronic lymphocytic leukemia (CLL) with coexisting conditions (51, 52), representing promising therapeutic options for pemphigus in the future (38). In addition, the monoclonal antibody against CD19, inebilizumab, is considered an effective treatment for pemphigus patients who showed resistance to rituximab treatment due to the expression of CD19 on both B cells and plasmablasts (25, 53).
Bruton tyrosine kinase (BTK) is an enzyme that plays a vital role in the signaling transduction in most white blood cells other than T cells and plasma cells. BTK inhibitors (BTKI) are small molecules downregulating various B-cell activities, including cell proliferation, differentiation, maturation, and survival. Thus, BTKI are capable of suppressing the production of pemphigus autoantibodies (54). Among them, PRN1008 (rilzabrutinib) is a BTK inhibitor that was safe and well-tolerated following oral administration, and the report of a phase I study treated with PRN1008 demonstrated that PRN1008 could be effective on pemphigus. Moreover, PRN1008 has been granted Orphan Drug Designation by the United States FDA for PV therapy (55). Phase II trial of rilzabrutinib has been completed, and the result showed that rilzabrutinib alone or with low doses of corticosteroid was safe with rapid clinical activity in pemphigus vulgaris patients (56). Additionally, Jun Yamagami et al. investigated the efficacy and safety of tirabrutinib, another BTK inhibitor, in patients with refractory pemphigus in a multicenter, open-label, uncontrolled, single-arm phase II study. They reported that treatment with tirabrutinib enabled remission and reduced oral corticosteroids over time without significant safety concerns in patients with refractory pemphigus (57). Interestingly, another BTK inhibitor (PRN473) has been reported with a good response in canine pemphigus foliaceus (PF) (58).
The importance of T cells in orchestrating autoimmune reactions and efficient autoantibody production has been highlighted. Daclizumab and basiliximab (mAbs against CD25), have been developed as immunosuppressive drugs for patients after transplantation (59). It was used to successfully treat a PV patient who responded favorably to daclizumab in combination with prednisolone and azathioprine after a combination of conventional therapies failed (60). These data suggest that daclizumab and other anti-CD25 antibodies could provide an alternative treatment for recalcitrant pemphigus.
Additionally, CD40/CD154 and ICOS/ICOS-L interaction, altered peptide ligands (APLs), and p38 mitogen-activated protein kinase (p38MAPK) signaling are believed to play differential roles in activating adaptive immune responses (26, 61, 62) or blister formation in the pathogenesis of pemphigus (63), potentially providing new targets for the treatment of pemphigus.
As the concentration of TNF-α in the local skin lesions is elevated (64, 65), inhibition of TNF-α by infliximab or etanercept could be a successful treatment for pemphigus vulgaris in a few studies (66, 67). However, disease relapse has been reported in PV patients co-administrated with prednisone and infliximab after prednisone was tapered (68).
A recent case report showed the effectiveness of tocilizumab, a humanized mAb inhibiting IL-6 in the treatment of a patient with refractory PF and Behcet's disease, by blocking the IL-6 receptor binding site and regulating the immune responses (69, 70).
IL-4 is a key cytokine that is supposed to play a critical role in pemphigus. Dupilumab, a fully human mAb directed against the IL-4Rα blocking IL-4 related to IL-13 signaling (71), could be a therapeutic option for pemphigus (72).
B-cell-activating factor (BAFF) is one of the TNF family members and an essential regulator of peripheral B-cell survival, maturation, antibody production, and class-switching (73, 74). A TNF receptor superfamily member 13C is expressed in most B cell subsets, promoting the survival of naive B cells and plasmablasts (75). The monoclonal antibody VAY736 targeting this receptor may have a broad range of effects on B cell depletion and plasmablast survival, and the phase II clinical study is under clinical trials to examine the efficacy in treating pemphigus (NCT01930175) (42).
In addition, a proliferation-inducing ligand (APRIL), another TNF superfamily ligand, is also implicated in B-cell ontogeny (76) and may become another target for pemphigus therapy. However, further studies are necessary to clarify the exact role of APRIL in this skin condition.
An experimental study showed that soluble Fas ligand, which is upregulated and released from keratinocytes, was believed to play a critical role in blistering in the pemphigus pathogenesis (77). In accordance with this observation, a novel anti-soluble Fas ligand human monoclonal antibody (PC111) has been tested for pemphigus therapy due to its low potential for immunogenicity, favorable chemical and physical stability, and high binding affinity (78).
Owing to the role of neonatal Fc receptor (FcRn) in autoantibody production, FcRn could be a promising therapeutic target for treating IgG-mediated autoimmune disorders by preventing the persistent autoantigen presentation and consequently inhibiting long-term autoantibody production (79, 80). SYNT001 (ALXN1830), a novel humanized IgG4 monoclonal antibody targeting FcRn at the immunoglobulin G (IgG) binding site, is considered another option for pemphigus therapy (81). More recently, the US Food and Drug Administration (FDA) has granted Orphan Drug Designation to SYNT001 to treat pemphigus in 2018 (38). Additionally, efgartigimod (ARGX-113) is an engineered Fc fragment derived from human IgG1 (82). A phase II, open-label study of efgartigimod in patients with pemphigus vulgaris and pemphigus foliaceus showed that efgartigimod induced early decrease of anti-desmoglein 1 and 3 autoantibodies in serum, representing a well-tolerated option of achieving early disease control and complete clinical remission of pemphigus while early corticosteroid tapering (83).
In order to eliminate the antigen-specific B cells that produce antibodies, Ellebreht et al. created a chimeric autoantibody receptor (CAAR), with the autoantigen Dsg3 as the CAAR extracellular domain, to engineer T cells to deplete the autoimmune memory B cells directly and Dsg3-specific short-lived plasma cells indirectly in PV patients (84). Dsg3-CAART therapy has been reported to lead to serological and histological improvements in experimental pemphigus mice without detectable off-target toxicity (85). However, the human study that assesses its efficacy and safety in humans is still needed. Nevertheless, the successful development of this strategy may lead to the generation of long-term memory CAAR-Tregs that could potentially cure the disease.
The immune system is a complex network and a large amount of evidence has verified the role of Tregs in regulating the immune system and preventing autoimmune diseases (86). There has been a clinical trial of Treg adoptive therapy treating graft vs. host diseases (GVHD) with expanded allogeneic Tregs (87), and another study demonstrated that the administration of autologous Tregs was safe and the disease activity of patients with insulin-dependent diabetes decreased (88). Additionally, a non-randomized, open-label, phase I clinical trial is under investigation (NCT03239470) to evaluate the effects of autologous expanded Tregs on the PV (48).
The efficiency of Autologous hematopoietic stem cell transplantation was reported in both PV and PF patients, together with a high risk of serious adverse events (89–92). Therefore, the effectiveness and safety of this strategy need to be further evaluated and verified by a long-term, large cohort study.
In summary, we have shown the current pemphigus immunotherapies, including biological agents and cell therapy strategies (Table 1) investigated for the clinical treatment of pemphigus and undergoing clinical trials (Figure 1). These therapies are primarily based on the current understanding of pemphigus disease pathology. Pemphigus disease is mainly mediated by circulating autoantibodies against Dsgs. These antibodies are expressed and secreted by Dsg3 autoreactive B cells that are activated presumably by the autoreactive T cells, in which cytokines could also play an essential role in pemphigus disease pathophysiology (Figure 2). The autoantibodies disrupt desmosomal Dsgs by the assembly and disassembly pathways (93). A few review papers have recently been published and described the potential therapies for pemphigus targeting these pathways (53, 94–97). The current review focuses on the immune mechanism-based therapies to target the Dsg3-specific B cells, T cells, and relevant cytokines. In future research, more efforts should be paid to minimize the adverse effects of conventional therapies and reduce the relapse frequency. The ultimate goal is to achieve rapid disease control, complete disease remission, and disease cure. With the accumulation of the knowledge of pemphigus pathogenesis, novel targets could be identified, and more therapeutic agents with improved efficacy will be developed and applied for PV management in clinical practice.
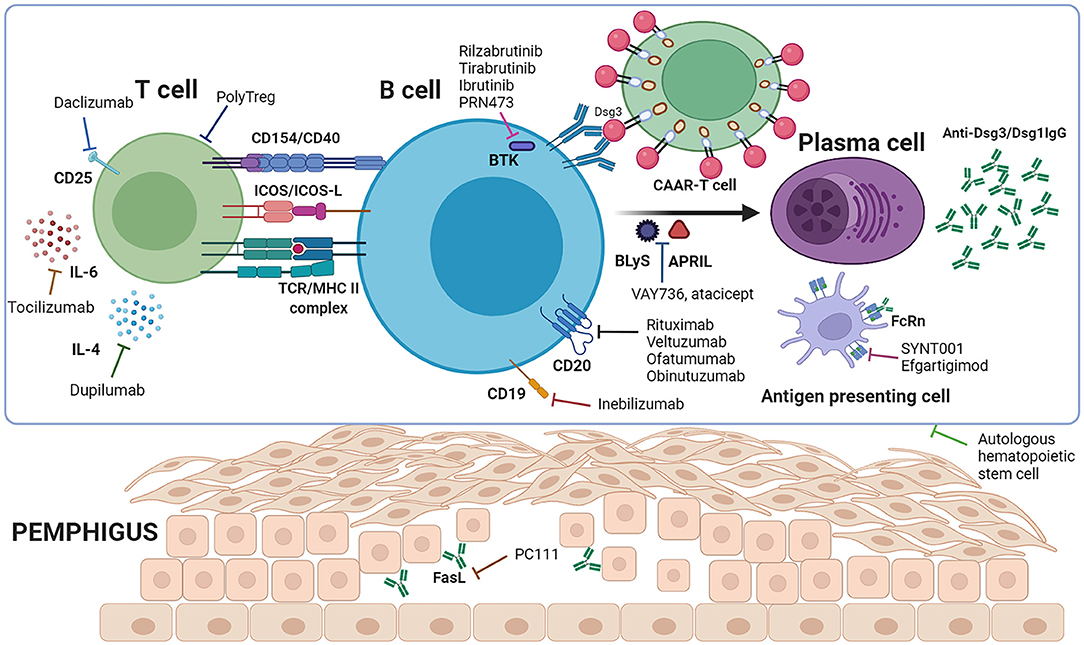
Figure 1. Immune mechanism of pemphigus and targeted therapeutic agents. T cells interact with B cells to provide co-stimulatory signals through CD154/CD40, ICOS/ICOS-L etc., leading to B cell activation, proliferation, and differentiation to plasma cells, and secretion of anti-Dsg3/Dsg1 autoantibodies. Binding of the antibodies to the target antigen among acanthocytes leads to the separation of keratinocytes and intraepidermal blister formation. Rituximab, veltuzumab, ofatumumab, obinutuzumab and inebilizumab deplete autoreactive B cells to prevent their differentiation to plasma cells. PolyTregs, daclizumab, tocilizumab and dupilumab act on T cells, while rilzabrutinib, tirabrutinib, ibrutinib, PRN473, VAY736 and atacicept target B cells, resulting in less activation of autoreactive B cells. CAAR-T cells work to eliminate Dsg3-specific B cells. SYNT001 and efgartigimod saturate FcRn to shorten the half-life of pathogenic IgG autoantibodies. Autologous hematopoietic stem cells function by eliminating autoreactive lymphocytes and re-establishing the immune system (Created with BioRender.com).
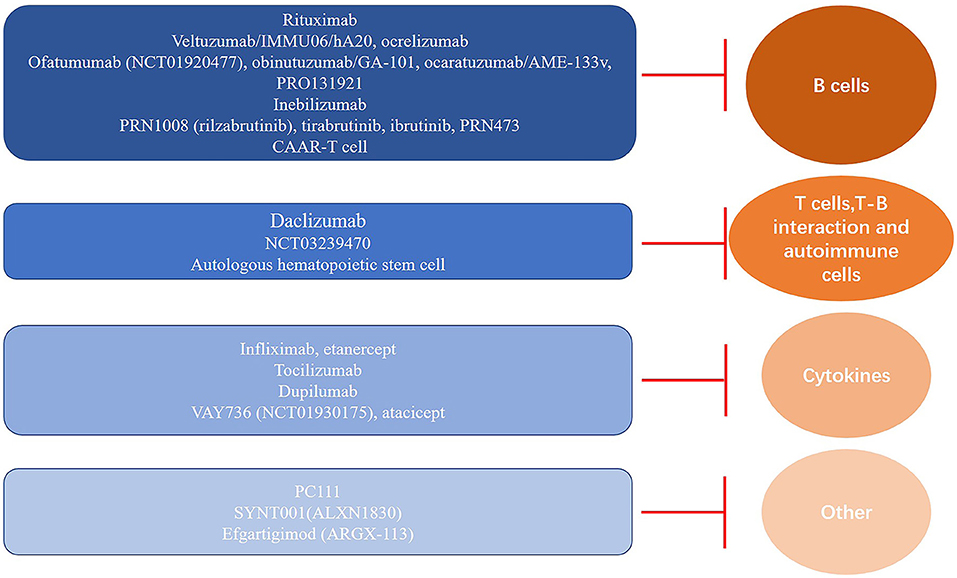
Figure 2. Immune pathomechanism-based targets and classification of therapeutic agents for pemphigus. Overview of the current and candidate agents for immunotherapy and their corresponding targets, including B cells, T cells, and cytokines, which are essential for pathogenic autoantibody production and secretion in pemphigus.
HY organized the database and wrote the first draft of the manuscript. HY and XM contributed to the manuscript revision. All the authors contributed to the conception and design of the review, read, and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Nos. 81803128, 81730085, and 81974475) and the Shanghai Sailing Program (No. 18YF1414200).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hammers CM, Stanley JR. Recent advances in understanding pemphigus and bullous pemphigoid. J Invest Dermatol. (2020) 140:733–41. doi: 10.1016/j.jid.2019.11.005
2. Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. J Am Acad Dermatol. (1999) 40:167–70. doi: 10.1016/S0190-9622(99)70183-0
3. Ellebrecht CT, Payne AS. Setting the target for pemphigus vulgaris therapy. JCI Insight. (2017) 2:e92021. doi: 10.1172/jci.insight.92021
4. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. (2009) 103:975–94. doi: 10.1016/j.rmed.2009.01.003
5. Yanovsky RL, McLeod M, Ahmed AR. Treatment of pemphigus vulgaris: part 1 - current therapies. Expert Rev Clin Immunol. (2019) 15:1047–60. doi: 10.1080/1744666X.2020.1672535
6. Harman KE, Albert S, Black MMD. British Association of, Guidelines for the management of pemphigus vulgaris. Br J Dermatol. (2003) 149:926–37. doi: 10.1111/j.1365-2133.2003.05665.x
7. Yuan H, Zhou S, Liu Z, Cong W, Fei X, Zeng W, et al. Pivotal role of lesional and perilesional T/B lymphocytes in pemphigus pathogenesis. J Invest Dermatol. (2017) 137:2362–70. doi: 10.1016/j.jid.2017.05.032
8. Werth VP, Joly P, Mimouni D, Maverakis E, Caux F, Lehane P, et al. Rituximab versus mycophenolate mofetil in patients with pemphigus vulgaris. N Engl J Med. (2021) 384:2295–305. doi: 10.1056/NEJMoa2028564
9. Joly P, Horvath B, Patsatsi A, Uzun S, Bech R, Beissert S, et al. Updated S2K guidelines on the management of pemphigus vulgaris and foliaceus initiated by the european academy of dermatology and venereology (EADV). J Eur Acad Dermatol Venereol. (2020) 34:1900-13. doi: 10.1111/jdv.16752
10. Cholera M, Chainani-Wu N. Management of pemphigus vulgaris. Adv Ther. (2016) 33:910–58. doi: 10.1007/s12325-016-0343-4
11. Pasternak B, Svanstrom H, Schmiegelow K, Jess T, Hviid A. Use of azathioprine and the risk of cancer in inflammatory bowel disease. Am J Epidemiol. (2013) 177:1296–305. doi: 10.1093/aje/kws375
12. Fraser AG, Orchard TR, Robinson EM, Jewell DP. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. (2002) 16:1225–32. doi: 10.1046/j.1365-2036.2002.01297.x
13. Burton JL, Harman RR, Peachey RD, Warin RP. Azathioprine plus prednisone in treatment of pemphigoid. Br Med J. (1978) 2:1190–1. doi: 10.1136/bmj.2.6146.1190
14. Lannes G, Elias FR, Cunha B, Jesus N, Klumb EM, Albuquerque EM, et al. Successful pregnancy after cyclophosphamide therapy for lupus nephritis. Arch Gynecol Obstet. (2011) 283(Suppl. 1):61–5. doi: 10.1007/s00404-011-1859-0
15. Gregoriou S, Efthymiou O, Stefanaki C, Rigopoulos D. Management of pemphigus vulgaris: challenges and solutions. Clin Cosmet Investig Dermatol. (2015) 8:521–7. doi: 10.2147/CCID.S75908
16. Arin MJ, Engert A, Krieg T, Hunzelmann N. Anti-CD20 monoclonal antibody (rituximab) in the treatment of pemphigus. Br J Dermatol. (2005) 153:620–5. doi: 10.1111/j.1365-2133.2005.06651.x
17. Lunardon L, Tsai KJ, Propert KJ, Fett N, Stanley JR, Werth VP, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. (2012) 148:1031–6. doi: 10.1001/archdermatol.2012.1522
18. Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. (2008) 128:2850–8. doi: 10.1038/jid.2008.172
19. Seishima M, Esaki C, Osada K, Mori S, Hashimoto T, Kitajima Y. Pemphigus IgG, but not bullous pemphigoid IgG, causes a transient increase in intracellular calcium and inositol 1,4,5-triphosphate in DJM-1 cells, a squamous cell carcinoma line. J Invest Dermatol. (1995) 104:33–7. doi: 10.1111/1523-1747.ep12613469
20. Esaki SMC, Yamada T, Osada K, Kitajima Y. The pathophysiological significance of nondesmoglein targets of pemphigus autoimmunity. Arch Dermatol. (1998) 134:10. doi: 10.1001/archderm.134.8.971
21. Esaki C, Seishima M, Yamada T, Osada K, Kitajima Y. Pharmacologic evidence for involvement of phospholipase C in pemphigus IgG-induced inositol 1,4,5-trisphosphate generation, intracellular calcium increase, and plasminogen activator secretion in DJM-1 cells, a squamous cell carcinoma line. J Invest Dermatol. (1995) 105:329–33. doi: 10.1111/1523-1747.ep12319948
22. Osada K, Seishima M, Kitajima Y. Pemphigus IgG activates and translocates protein kinase C from the cytosol to the particulate/cytoskeleton fractions in human keratinocytes. J Investig Dermatol. (1997) 108:482–7. doi: 10.1111/1523-1747.ep12289726
23. Reto Caldelari AdB, Baumann D, Suter M, Bierkamp C, Balmer V, Müller E. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J Cell Biol. (2001) 153:12. doi: 10.1083/jcb.153.4.823
24. Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med. (1993) 177:679–90. doi: 10.1084/jem.177.3.679
25. Musette P, Bouaziz JD. B cell modulation strategies in autoimmune diseases: new concepts. Front Immunol. (2018) 9:622. doi: 10.3389/fimmu.2018.00622
26. Kim AR, Han D, Choi JY, Seok J, Kim SE, Seo SH, et al. Targeting inducible costimulator expressed on CXCR5(+)PD-1(+) TH cells suppresses the progression of pemphigus vulgaris. J Allergy Clin Immunol. (2020) 146:1070–1079.e8. doi: 10.1016/j.jaci.2020.03.036
27. Holstein J, Solimani F, Baum C, Meier K, Pollmann R, Didona D, et al. Immunophenotyping in pemphigus reveals a TH17/TFH17 cell-dominated immune response promoting desmoglein1/3-specific autoantibody production. J Allergy Clin Immunol. (2021) 147:2358–69. doi: 10.1016/j.jaci.2020.11.008
28. Takahashi H, Kouno M, Nagao K, Wada N, Hata T, Nishimoto S, et al. Desmoglein 3-specific CD4+ T cells induce pemphigus vulgaris and interface dermatitis in mice. J Clin Invest. (2011) 121:3677–88. doi: 10.1172/JCI57379
29. Yokoyama T, Matsuda S, Takae Y, Wada N, Nishikawa T, Amagai M, et al. Antigen-independent development of Foxp3+ regulatory T cells suppressing autoantibody production in experimental pemphigus vulgaris. Int Immunol. (2011) 23:365–73. doi: 10.1093/intimm/dxr020
30. Tsunoda K, Ota T, Suzuki H, Ohyama M, Nagai T, Nishikawa T, et al. Pathogenic autoantibody production requires loss of tolerance against desmoglein 3 in both T and B cells in experimental pemphigus vulgaris. Eur J Immunol. (2002) 32:627–33. doi: 10.1002/1521-4141(200203)32:3<627::AID-IMMU627>3.0.CO;2-1
31. Zhang Y, Li J, Zhang YM, Zhang XM, Tao J. Effect of TACI signaling on humoral immunity and autoimmune diseases. J Immunol Res. (2015) 2015:247426. doi: 10.1155/2015/247426
32. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. (2006) 354:610–21. doi: 10.1056/NEJMra052723
33. Franciotta D, Salvetti M, Lolli F, Serafini B, Aloisi F. B cells and multiple sclerosis. Lancet Neurol. (2008) 7:852–8. doi: 10.1016/S1474-4422(08)70192-3
34. Cancro MP. The BLyS/BAFF family of ligands and receptors: key targets in the therapy and understanding of autoimmunity. Ann Rheum Dis. (2006) 65(Suppl. 3):iii34-6. doi: 10.1136/ard.2006.058412
35. Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. (1998) 92:1927–32.
36. Joly P, Maho-Vaillant M, Prost-Squarcioni C, Hebert V, Houivet E, Calbo S, et al. French study group on autoimmune bullous skin, First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. (2017) 389:2031–40. doi: 10.1016/S0140-6736(17)30070-3
37. Colliou N, Picard D, Caillot F, Calbo S, Le Corre S, Lim A, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. (2013) 5:175ra30. doi: 10.1126/scitranslmed.3005166
38. Temel B, Murrell DF. Pharmacological advances in pemphigus. Curr Opin Pharmacol. (2019) 46:44–49. doi: 10.1016/j.coph.2019.01.001
39. Amber KT, Hertl M. An assessment of treatment history and its association with clinical outcomes and relapse in 155 pemphigus patients with response to a single cycle of rituximab. J Eur Acad Dermatol Venereol. (2015) 29:777–82. doi: 10.1111/jdv.12678
40. Wang HH, Liu CW, Li YC, Huang YC. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm Venereol. (2015) 95:928–32. doi: 10.2340/00015555-2116
41. Hammers CM, Chen J, Lin C, Kacir S, Siegel DL, Payne AS, et al. Persistence of anti-desmoglein 3 IgG(+) B-cell clones in pemphigus patients over years. J Invest Dermatol. (2015) 135:742–9. doi: 10.1038/jid.2014.291
42. Kasperkiewicz M, Ellebrecht CT, Takahashi H, Yamagami J, Zillikens D, Payne AS, et al. Pemphigus. Nat Rev Dis Primers. (2017) 3:17026. doi: 10.1038/nrdp.2017.26
43. Feldman RJ, Ahmed AR. Relevance of rituximab therapy in pemphigus vulgaris: analysis of current data and the immunologic basis for its observed responses. Expert Rev Clin Immunol. (2011) 7:529–41. doi: 10.1586/eci.11.22
44. Klein C, Lammens A, Schafer W, Georges G, Schwaiger M, Mossner E, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. (2013) 5:22–33. doi: 10.4161/mabs.22771
45. Huang A, Madan RK, Levitt J. Future therapies for pemphigus vulgaris: Rituximab and beyond. J Am Acad Dermatol. (2016) 74:746–53. doi: 10.1016/j.jaad.2015.11.008
46. Ellebrecht CT, Choi EJ, Allman DM, Tsai DE, Wegener WA, Goldenberg DM, et al. Subcutaneous veltuzumab, a humanized anti-CD20 antibody, in the treatment of refractory pemphigus vulgaris. JAMA Dermatol. (2014) 150:1331–5. doi: 10.1001/jamadermatol.2014.1939
47. Castillo J, Milani C, Mendez-Allwood D. Ofatumumab, a second-generation anti-CD20 monoclonal antibody, for the treatment of lymphoproliferative and autoimmune disorders. Expert Opin Investig Drugs. (2009) 18:491–500. doi: 10.1517/13543780902832679
48. Izumi K, Bieber K, Ludwig RJ. Current clinical trials in pemphigus and pemphigoid. Front Immunol. (2019) 10:978. doi: 10.3389/fimmu.2019.00978
49. Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. (2017) 376:221–34. doi: 10.1056/NEJMoa1601277
50. Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or AG. Comi de Seze J, et al. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med. (2017) 376:209–20. doi: 10.1056/NEJMoa1606468
51. Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. (2014) 370:1101–10. doi: 10.1056/NEJMoa1313984
52. Czuczman MS, Gregory SA. The future of CD20 monoclonal antibody therapy in B-cell malignancies. Leuk Lymphoma. (2010) 51:983–94. doi: 10.3109/10428191003717746
53. Didona D, Maglie R, Eming R, Hertl M. Pemphigus: current and future therapeutic strategies. Front Immunol. (2019) 10:1418. doi: 10.3389/fimmu.2019.01418
54. Weber ANR, Bittner Z, Liu X, Dang TM, Radsak MP, Brunner C. Bruton's tyrosine kinase: an emerging key player in innate immunity. Front Immunol. (2017) 8:1454. doi: 10.3389/fimmu.2017.01454
55. Smith PF, Krishnarajah J, Nunn PA, Hill RJ, Karr D, Tam D, et al. A phase I trial of PRN1008, a novel reversible covalent inhibitor of Bruton's tyrosine kinase, in healthy volunteers. Br J Clin Pharmacol. (2017) 83:2367–76. doi: 10.1111/bcp.13351
56. Murrell DF, Patsatsi A, Stavropoulos P, Baum S, Zeeli T, Kern JS, et al. Proof of concept for the clinical effects of oral rilzabrutinib, the first Bruton tyrosine kinase inhibitor for pemphigus vulgaris: the phase II BELIEVE study. Br J Dermatol. (2021) 185:745–55. doi: 10.1111/bjd.20431
57. Yamagami J, Ujiie H, Aoyama Y, Ishii N, Tateishi C, Ishiko A, et al. A multicenter, open-label, uncontrolled, single-arm phase 2 study of tirabrutinib, an oral Bruton's tyrosine kinase inhibitor, in pemphigus. J Dermatol Sci. (2021) 103:135–42. doi: 10.1016/j.jdermsci.2021.07.002
58. Hertl M, Jedlickova H, Karpati S, Marinovic B, Uzun S, Yayli S, et al. Pemphigus. S2 Guideline for diagnosis and treatment–guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). J Eur Acad Dermatol Venereol. (2015) 29:405–14. doi: 10.1111/jdv.12772
59. Nashan B, Moore R, Amlot P, Schmidt AG, Abeywickrama K, Soulillou JP. Randomised trial of basiliximab versus placebo for control of acute cellular rejection in renal allograft recipients. CHIB 201 International Study Group Lancet. (1997) 350:1193–8. doi: 10.1016/S0140-6736(97)09278-7
60. Renkl A, Mockenhaupt M, Technau K, Herouy Y, Norgauer J. A novel therapeutic option in pemphigus vulgaris: humanized monoclonal anti-CD25 antibody. Br J Dermatol. (2004) 150:1220–2. doi: 10.1111/j.1365-2133.2004.05977.x
61. Aoki-Ota M, Kinoshita M, Ota T, Tsunoda K, Iwasaki T, Tanaka S, et al. Tolerance induction by the blockade of CD40/CD154 interaction in pemphigus vulgaris mouse model. J Invest Dermatol. (2006) 126:105–13. doi: 10.1038/sj.jid.5700016
62. Kridin K. Emerging treatment options for the management of pemphigus vulgaris. Ther Clin Risk Manag. (2018) 14:757–78. doi: 10.2147/TCRM.S142471
63. Mao X, Sano Y, Park JM, Payne AS. p38 MAPK activation is downstream of the loss of intercellular adhesion in pemphigus vulgaris. J Biol Chem. (2011) 286:1283–91. doi: 10.1074/jbc.M110.172874
64. Feliciani C, Toto P, Amerio P, Pour SM, Coscione G, Shivji G, et al. In vitro and in vivo expression of interleukin-1alpha and tumor necrosis factor-alpha mRNA in pemphigus vulgaris: interleukin-1alpha and tumor necrosis factor-alpha are involved in acantholysis. J Invest Dermatol. (2000) 114:71–7. doi: 10.1046/j.1523-1747.2000.00835.x
65. Tavakolpour S. Current and future treatment options for pemphigus: Is it time to move towards more effective treatments? Int Immunopharmacol. (2017) 53:133–42. doi: 10.1016/j.intimp.2017.10.027
66. Jacobi A, Shuler G, Hertl M. Rapid control of therapy-refractory pemphigus vulgaris by treatment with the tumour necrosis factor-alpha inhibitor infliximab. Br J Dermatol. (2005) 153:448–9. doi: 10.1111/j.1365-2133.2005.06744.x
67. Berookhim B, Fischer HD, Weinberg JM. Treatment of recalcitrant pemphigus vulgaris with the tumor necrosis factor alpha antagonist etanercept. Cutis. (2004) 74:245−7.
68. Garcia-Rabasco A, Alsina-Gibert M, Pau-Charles I, Iranzo P. Infliximab therapy failure in two patients with pemphigus vulgaris. J Am Acad Dermatol. (2012) 67:e196–7. doi: 10.1016/j.jaad.2011.05.029
69. Caso F, Iaccarino L, Bettio S, Ometto F, Costa L, Punzi L, et al. Refractory pemphigus foliaceus and Behcet's disease successfully treated with tocilizumab. Immunol Res. (2013) 56:390–7. doi: 10.1007/s12026-013-8411-1
70. Fonseca JE, Santos MJ, Canhao H, Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev. (2009) 8:538–42. doi: 10.1016/j.autrev.2009.01.012
71. Tavakolpour S, Tavakolpour V. Interleukin 4 inhibition as a potential therapeutic in pemphigus. Cytokine. (2016) 77:189–95. doi: 10.1016/j.cyto.2015.09.017
72. Tavakolpour S. Dupilumab: a revolutionary emerging drug in atopic dermatitis and its possible role in pemphigus. Dermatol Ther. (2016) 29:299. doi: 10.1111/dth.12327
73. Lied GA, Berstad A. Functional and clinical aspects of the B-cell-activating factor (BAFF): a narrative review. Scand J Immunol. (2011) 73:1–7. doi: 10.1111/j.1365-3083.2010.02470.x
74. Mackay F, Browning JL. BAFF a fundamental survival factor for B cells. Nat Rev Immunol. (2002) 2:465–75. doi: 10.1038/nri844
75. Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. (2003) 112:286–97. doi: 10.1172/JCI18025
76. Hahne M, Kataoka T, Schroter M, Hofmann K, Irmler M, Bodmer JL, et al. APRIL a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med. (1998) 188:1185–90. doi: 10.1084/jem.188.6.1185
77. Lotti R, Shu E, Petrachi T, Marconi A, Palazzo E, Quadri M, et al. Soluble fas ligand is essential for blister formation in pemphigus. Front Immunol. (2018) 9:370. doi: 10.3389/fimmu.2018.00370
78. Kridin K, Ahn C, Huang WC, Ansari A, Sami N. Treatment update of autoimmune blistering diseases. Dermatol Clin. (2019) 37:215–28. doi: 10.1016/j.det.2018.12.003
79. Baker K, Rath T, Pyzik M, Blumberg RS. The role of FcRn in antigen presentation. Front Immunol. (2014) 5:408. doi: 10.3389/fimmu.2014.00408
80. Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. (2005) 115:3440–50. doi: 10.1172/JCI24394
81. Nixon AE, Chen J, Sexton DJ, Muruganandam A, Bitonti AJ, Dumont J, et al. Fully human monoclonal antibody inhibitors of the neonatal fc receptor reduce circulating IgG in non-human primates. Front Immunol. (2015) 6:176. doi: 10.3389/fimmu.2015.00176
82. Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. (2005) 23:1283–8. doi: 10.1038/nbt1143
83. Goebeler M, Bata-Csorgo Z, De Simone C, Didona B, Remenyik E, Reznichenko N, et al. Treatment of pemphigus vulgaris and foliaceus with efgartigimod, a neonatal Fc receptor inhibitor: a phase II multicentre, open-label feasibility trial. Br J Dermatol. (2022) 186:429–39. doi: 10.1111/bjd.20782
84. Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. (2016) 353:179–84. doi: 10.1126/science.aaf6756
85. Lee J, Lundgren DK, Mao X, Manfredo-Vieira S, Nunez-Cruz S, Williams EF, et al. Antigen-specific B cell depletion for precision therapy of mucosal pemphigus vulgaris. J Clin Invest. (2020) 130:6317–24. doi: 10.1172/JCI138416
86. Yang M, Wu H, Zhao M, Chang C, Lu Q. The pathogenesis of bullous skin diseases. J Transl Autoimmun. (2019) 2:100014. doi: 10.1016/j.jtauto.2019.100014
87. Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, et al. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. (2009) 133:22–6. doi: 10.1016/j.clim.2009.06.001
88. Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. (2015) 7:315ra189. doi: 10.1126/scitranslmed.aad4134
89. Oyama Y, Parker ER, Brieva J, Guitart J, Statkute L, Verda L, et al. High-dose immune suppression and autologous hematopoietic stem cell transplantation in refractory pemphigus foliaceus. Bone Marrow Transplant. (2004) 34:1097–8. doi: 10.1038/sj.bmt.1704679
90. Suslova IM, Theodoropoulos DS, Cullen NA, Tetarnikova MK, Tetarnikov AS, Kolchak NA. Pemphigus vulgaris treated with allogeneic hematopoietic stem cell transplantation following non-myeloablative conditioning. Eur Rev Med Pharmacol Sci. (2010) 14:785−8.
91. Wang M, Cao C, Sun J, Peng X, Liu Q, Huang L, et al. Application of autologous hematopoietic stem cell transplantation for pemphigus. Int J Dermatol. (2017) 56:296–301. doi: 10.1111/ijd.13461
92. Vanikar AV, Trivedi HL, Patel RD, Kanodia KV, Modi PR, Shah VR. Allogenic hematopoietic stem cell transplantation in pemphigus vulgaris: a single-center experience. Indian J Dermatol. (2012) 57:9–11. doi: 10.4103/0019-5154.92667
93. Berkowitz P, Hu P, Liu Z, Diaz LA, Enghild JJ, Chua MP, et al. Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG-induced cytoskeleton reorganization. J Biol Chem. (2005) 280:23778–84. doi: 10.1074/jbc.M501365200
94. Ellebrecht CT, Maseda D, Payne AS. Pemphigus and pemphigoid: from disease mechanisms to druggable pathways. J Invest Dermatol. (2022) 142:907–14. doi: 10.1016/j.jid.2021.04.040
95. Didona D, Paolino G, Di Zenzo G, Didona B, Pampena R, Di Nicola MR, et al. Pemphigus vulgaris: present and future therapeutic strategies. Dermatol Pract Concept. (2022) 12:e2022037. doi: 10.5826/dpc.1201a37
96. Zhao W, Wang J, Zhu H, Pan M. Comparison of guidelines for management of pemphigus: a review of systemic corticosteroids, rituximab, and other immunosuppressive therapies. Clin Rev Allergy Immunol. (2021) 61:351–62. doi: 10.1007/s12016-021-08882-1
Keywords: pemphigus, improved efficacy, immunotarget, novel therapy, clinical trial
Citation: Yuan H, Pan M, Chen H and Mao X (2022) Immunotherapy for Pemphigus: Present and Future. Front. Med. 9:901239. doi: 10.3389/fmed.2022.901239
Received: 21 March 2022; Accepted: 09 May 2022;
Published: 15 June 2022.
Edited by:
Ralf J. Ludwig, University of Lübeck, GermanyReviewed by:
Jong Hoon Kim, Yonsei University, South KoreaCopyright © 2022 Yuan, Pan, Chen and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuming Mao, bWFveEBtYWlsLm1lZC51cGVubi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.