
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 13 June 2022
Sec. Dermatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.898790
This article is part of the Research Topic Stevens Johnson Syndrome: Past, Present, and Future Directions View all 11 articles
Immune checkpoint blockade (ICB) improves survival in many types of cancers including melanoma, non-small cell lung, renal cell, breast, and cervical cancers. However, many of these therapies are also associated with high grade dermatologic adverse events (DAEs), including Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), SJS/TEN-like reactions, high grade maculopapular and psoriasiform rashes, autoimmune bullous eruptions, drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP), which may limit their tolerability and use. It is important to properly identify and treat DAEs to ICB because these DAEs may be associated with positive anti-tumor response and patients may have limited options for alternative anti-cancer therapeutics. In this review, we describe high grade DAEs to increasingly used ICB agents, which target CTLA-4 and PD-1 or its ligand, PD-L1 and enable the immune system to target cancer cells. We further differentiate life-threatening adverse reactions from mimickers and report cases of serious DAEs which have been recorded in association with ICB through the FDA Adverse Events Reporting System (FAERS), which is an archive of adverse events associated with various drugs and therapeutic biologic products reported voluntarily by consumers and healthcare professionals as well as mandatorily by manufacturers. Lastly, we summarize management recommendations for these adverse events and discuss knowledge and evidence gaps in this area.
Immune checkpoint blockade (ICB) improves survival in many cancers including melanoma, non-small cell lung, renal cell, breast, and cervical cancers (1–5). However, it is also associated with dermatologic adverse events (DAEs), which may limit its tolerability and use. Although most DAEs are mild or moderate, others may be systemic and even life-threatening. High grade [Common Terminology Criteria for Adverse Events (CTCAE) grade ≥3, see Table 1] (6) DAEs cover a spectrum of entities, including Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), SJS/TEN-like reactions, bullous eruptions, drug reaction with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis (AGEP) (7). It is important to properly identify and treat DAEs to ICB because patients often have limited options for alternative anti-cancer therapeutics. In this review, we describe high grade DAEs to ICB, differentiating life-threatening DAEs from mimickers. We also report cases of serious DAEs which have been recorded in association with ICB through the FDA Adverse Events Reporting System (FAERS), which is an archive of adverse events associated with various drugs and therapeutic biologic products reported voluntarily by consumers and healthcare professionals as well as mandatorily by manufacturers.
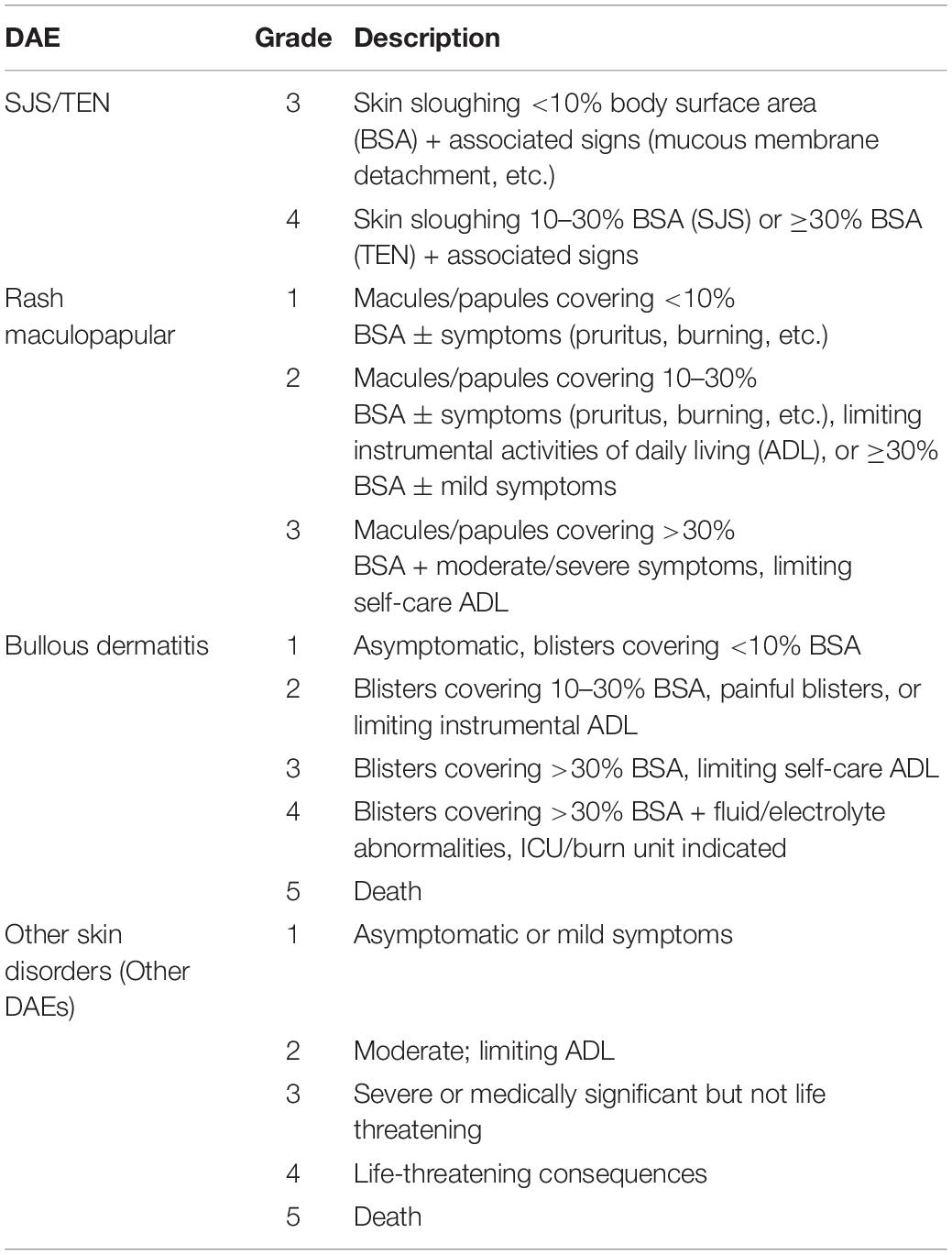
Table 1. DAE grading (Adapted from the CTCAE Version 5.0) (6).
The anti-CTLA-4 monoclonal antibody ipilimumab, anti-PD-1 monoclonal antibodies cemiplimab, nivolumab, and pembrolizumab, and anti-PD-L1 monoclonal antibodies atezolizumab, avelumab, and durvalumab overcome immune checkpoints, allowing the immune system to target cancer cells. These agents are associated with many immune-related DAEs (irDAEs), which tend to develop earlier than non-cutaneous immune-related adverse events (irAEs) (8, 9). Although the most common irDAEs to ICB, such as maculopapular rash, pruritus, and lichenoid dermatoses, may be controlled with topical corticosteroids and oral anti-pruritics, high grade irDAEs may require prolonged systemic therapy and/or discontinuation of the culprit immunotherapy (10). Importantly, the development of irDAEs has been associated with better overall survival in patients treated with ICB (11).
True SJS/TEN has been described in association with ICB, with classic rapid onset and progression and high mortality rates ranging from 10% for SJS to 50% for TEN (10, 12). As of March 2022, 255 cases of SJS/TEN had been reported through FAERS with pembrolizumab, 102 with ipilimumab, 224 with nivolumab, 55 with atezolizumab, 3 with avelumab, 21 with durvalumab, and 4 with cemiplimab. Diagnosis of true SJS/TEN is based on mucocutaneous involvement with supportive histopathological findings. Irregularly shaped dark, dusky macules may spread from the trunk and proximal extremities to the rest of the body. Patients may first present with a prodrome of malaise, followed by mucocutaneous pain as mucosal membranes and skin undergo necrolysis, upper respiratory symptoms, and fever, later developing systemic involvement of the liver, lungs, or gastrointestinal tract (13). Biopsy typically reveals full thickness epidermal necrosis with vacuolar interface changes, cleavage along the dermal epidermal junction, and subepidermal lymphocytes (14).
When SJS/TEN is suspected, urgent dermatologic evaluation is necessary and inpatient admission should be considered and ICB as well as other potential culprit medications should be held (15). Those with widespread mucocutaneous desquamation or life-threatening complications should be admitted to the intensive care or burn unit (16). Skin biopsies should be assessed for full-thickness epidermal necrosis, which is seen in true SJS/TEN and SJS/TEN-like reactions. Management of true SJS/TEN in patients on ICB must include supportive care and ophthalmologic, gynecologic and/or urologic consultations depending on extent and location of mucosal involvement. ICB must be discontinued once true ICB-associated SJS/TEN diagnosis is confirmed. National Comprehensive Cancer Network (NCCN) Guidelines for Management of Immunotherapy-Related Toxicities (version 1.2022) (15) provide recommendations for SJS/TEN management (without differentiating the treatment for both true SJS/TEN and SJS/TEN-like rashes) with prednisone or methylprednisolone 1–2 mg/kg/day and intravenous immune globulin (IVIg) 1 g/kg/day and/or other immunosuppressive therapies, including etanercept and cyclosporine can be considered for true SJS/TEN (15).
Incidence of SJS/TEN-like reactions is not known. Because cases of SJS/TEN in the FAERS database are voluntarily reported and unverified, they likely include SJS/TEN-like reactions, which mimic SJS/TEN but vary in severity and clinical course. While ipilimumab has not independently been associated with SJS/TEN-like reactions, emerging evidence suggests anti-PD-1/PD-L1 therapies are associated more frequently with SJS/TEN-like reactions (Figures 1A–C) than true SJS/TEN (17, 18). Unlike true SJS/TEN, which presents acutely, some SJS/TEN-like reactions to anti-PD-1/PD-L1 blockade progress from mild DAEs over a few to several weeks. Initially, patients may present with a morbilliform eruption, which then turn into targetoid patches and epidermal detachment with associated mucositis. Alternatively, other SJS/TEN-like reactions occur de novo late in the course of treatment with anti-PD-1/PD-L1 therapy. In one series of 18 patients, 2 developed SJS/TEN-like reactions de novo without preceding rash more than 6 weeks after initiating treatment with anti-PD-1/PD-L1 blockade (19). These reactions develop weeks to months after initiating treatment (median: 52 days, range: 3–420 days) (14, 20). SJS/TEN-like reactions due to pembrolizumab typically occur later with a median onset of 11 weeks and average of 12.8 weeks after initiation (19). SJS/TEN-like reactions present with a more benign clinical course and favorable treatment response when compared to true SJS/TEN (18). However, concurrent use of multiple ICB agents such as ipilimumab with nivolumab can lead to earlier and more severe DAEs, as seen in one analysis of pooled safety data from 1,551 patients with advanced melanoma (21). SJS/TEN-like reactions may occur concurrently with extra-cutaneous irAEs. In a pooled analysis of three trials of 448 patients with advanced melanoma who received ipilimumab/nivolumab, the most frequently reported irAEs involved skin (64.3%) and GI (46.7%). Thirty percent of patients developed grade 2–4 irAEs in more than one organ system (22).
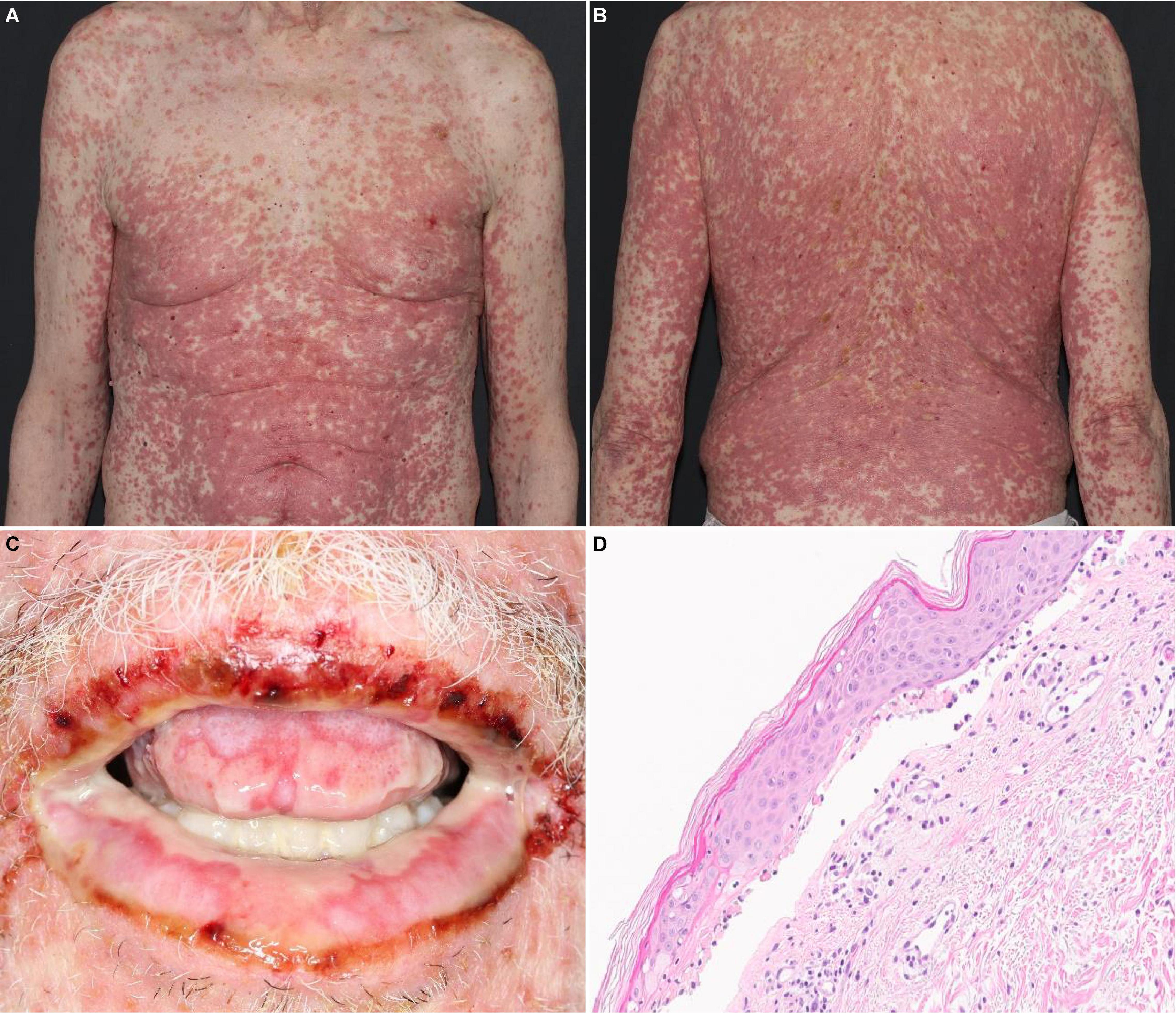
Figure 1. SJS/TEN-like reaction with erythematous macules and papules with dusky, purpuric centers covering about 80% BSA on the (A) trunk and (B) back. (C) Desquamation and hemorrhagic crusts on the oral mucosa. (D) Histology revealing lichenoid and subepidermal vesicular dermatitis with epidermal necrosis.
Antibiotic use may precipitate SJS/TEN and SJS/TEN-like reactions to ICB. A large retrospective study of 767 patients treated with ICB at a single institution and analysis of 38,705 safety reports of patients receiving anti-PD-1/PD-L1 from FAERS found that irAE potential risks including SJS/SJS-like development was higher in patients who used antibiotics during ICB therapy compared to those who did not (23). ICB may also increase a patient’s risk of developing SJS/TEN and SJS/TEN-like reactions to other agents. One series of seven patients who developed SJS-like reactions after anti-PD-1/PD-L1 with or without anti-CTLA-4 blockade found that all patients had received newly initiated drugs such as trimethoprim-sulfamethoxazole and allopurinol before DAE onset. A 2-hit hypothesis may play a part in the explanation for this association: ICB may first modulate the immune system to heighten drug sensitivity and then addition of a second drug/agent can then trigger an SJS-like reaction (18). Therefore, it is important to carefully identify the culprit agent and to differentiate SJS/TEN-like reactions from true SJS/TEN to potentially allow patients to continue therapy with ICB. Interestingly, even after discontinuation of ICB, patients are still at risk for SJS/TEN-like reactions (24, 25). This may be due to the long half-life of ICB and persistent immune activation in the setting of prolonged tumor responses, which has been observed with both anti-PD-1 and anti-CTLA-4 therapy (26).
Although SJS/TEN-like reactions resemble true SJS/TEN on histopathology (Figure 1D), with characteristic findings such as full-thickness epidermal necrolysis, subepidermal clefting, and interface dermatitis, severe clinical symptoms such as fever, ocular involvement, and maximal detachment are much rarer and seen in as few as 8% of patients (17).
In the setting of SJS/TEN-like reactions, ICB should initially be held along with other potential culprit medications. Wound care, topical emollients and high-strength topical steroids can be started (27). NCCN Guidelines for Management of Immunotherapy-Related Toxicities (version 1.2022) for SJS/TEN management (without differentiating the treatment for both true SJS/TEN and SJS/TEN-like rashes) with prednisone or methylprednisolone 1–2 mg/kg/day and IVIg 1 g/kg/day and/or other immunosuppressive therapies, including etanercept and cyclosporine can be considered for true SJS/TEN (15). While etanercept, cyclosporine, and/or IVIg are preferred for true SJS/TEN, topical and systemic steroids are typically used as first-line for SJS/TEN-like eruptions; use of cyclosporine, IVIg, and/or targeted therapies including etanercept, infliximab, tocilizumab, dupilumab may also be considered (27–29). For SJS/TEN-like eruptions, rechallenge of ICB may be considered once all skin and extracutaneous involvement resolves to grade ≤1, following a multidisciplinary discussion taking into consideration DAE severity, any required concurrent immunosuppressant for DAE management, prior cancer response to ICB, and alternative anti-cancer therapies (18, 30).
Pruritic, maculopapular rashes are among the most frequent DAEs associated with ICB (10). High grade (grade 3) maculopapular rashes covering >30% of total body surface area, which develop a median of 3.6 weeks after initiation of anti-CTLA-4 blockade, have been observed in up to 4% of patients (31). There are 1,190 reported cases of serious maculopapular rashes with pembrolizumab, 1,340 with ipilimumab, 1,934 with nivolumab, 385 with atezolizumab, 32 with avelumab, 122 with durvalumab, and 36 with cemiplimab recorded in FAERS. These maculopapular rashes typically present with numerous coalescing macules and papules and most often affects the trunk and extremities (10). Biopsy reveals interface and perivascular/periadnexal lymphocytic dermatitis with or without eosinophils (32). For high grade maculopapular rashes, NCCN guidelines recommend initial management with holding ICB and applying high potency topical steroids to affected areas. Patients can be given prednisone 0.5–1 mg/kg/day and up to 2 mg/kg/day if there is no improvement. After the rash resolves to grade 1 or 0, prednisone should be tapered over 4–6 weeks and ICB may be re-challenged (15). As a targeted, steroid-sparing agent, tocilizumab, an anti-IL-6R monoclonal antibody that limits Th17 differentiation and pro-inflammatory response, may be considered for persistent maculopapular rashes (32). Dupilumab, an anti-IL-4Rα monoclonal antibody that blocks signaling in Th2 pathways implicated in eczema and itch, may be considered for eczematous DAEs and for pruritus (30, 32). Omalizumab has also been shown to relieve pruritus with increased IgE (33). Per NCCN Guidelines for Management of Immunotherapy-Related Toxicities (version 1.2022), gabapentinoids, aprepitant, and narrow-band UVB phototherapy may also be considered for persistent and severe pruritus (15).
While classic DRESS is rare to ICB, patients commonly present with generalized maculopapular rash, fever, and concurrent extracutaneous irAEs (transaminitis, azotemia, and colitis) mimicking classic DRESS. While rarely reported in literature (34–37), FAERS has records of 24 reported cases of DRESS with pembrolizumab, 46 with ipilimumab, 89 with nivolumab, 6 with atezolizumab, 1 with avelumab, 3 with durvalumab, and 1 with cemiplimab. In classic DRESS, grade 2 eosinophilia (≥1,500 μL–1) is present in up to 81% of cases and grade 1 eosinophilia (700–1,499 μL–1) in 14% of cases (38); however, eosinophilia is less frequently observed in irDAEs, in about 51% (32). Histopathology of the morbilliform eruption of DRESS is often non-specific and may demonstrate features such as interface dermatitis that is present in various dermatoses (16). To manage ICB-DRESS, the culprit ICB should be held initially. Due to systemic involvement, high-dose and prolonged courses of corticosteroids may be required, with a slow 6- to 8-week taper after ICB-DRESS resolution. Anti-TNF-α, tocilizumab, and dupilumab may be considered as a steroid-sparing, precision medicine approach (16, 30, 37).
Acute generalized exanthematous pustulosis (AGEP) is an extremely rare DAE to ICB, characterized by small sterile pustules and edematous erythema. FAERS includes 11 reported cases of AGEP with pembrolizumab, 4 with ipilimumab, 6 with nivolumab, and 8 with atezolizumab. No cases of AGEP have been recorded with avelumab, durvalumab, or cemiplimab. Diagnosis is based on clinical and histopathological findings. AGEP has an acute onset, typically within 48 h of starting a new drug, and may have spontaneous rapid resolution (16, 39, 40). Biopsy reveals subcorneal pustules and subepidermal mixed cellular infiltrates with eosinophils (39, 41). Management of AGEP includes holding ICB and a combination of topical and systemic corticosteroids (oral prednisone 0.5–1 mg/kg/day (7, 16). After multi-disciplinary discussion, ICB may be resumed once AGEP has resolved to grade ≤1.
Although rare with anti-CTLA-4 blockade, bullous disorders secondary to anti-PD-1/PD-L1 therapies have been reported with increasing frequency and may become severe. Through FAERS, 204 cases of bullous dermatitis, autoimmune blistering disease, pemphigoid, or generalized bullous fixed drug eruption have been reported with pembrolizumab. Eighty-nine cases have been reported with ipilimumab, 479 with nivolumab, 41 with atezolizumab, 5 with avelumab, 44 with durvalumab, and 16 with cemiplimab.
Bullous pemphigoid (BP) (Figure 2) is the most frequently reported bullous disorder relating to anti-PD-1/PD-L1 blockade, and often presents with prodromal or concurrent pruritus. BP commonly develops as a delayed DAE, appearing >4 months after starting anti-PD-1/PD-L1 blockade (10). BP associated with anti-PD-1/PD-L1 blockade appears to present in younger patients (median age 74 years, range: 50-93 years) and affects the mucosal membranes more frequently (in 38.1% of patients on anti-PD-1/PD-L1 blockade) than idiopathic BP (42). In one study, subepidermal blisters were seen in 81% and eosinophilic infiltrate in 82% of anti-PD-1/PD-L1 blockade associated BP cases on histopathology. Direct immunofluorescence was positive in 79% of cases for IgG deposition and 80% for C3 deposition at the basement membrane zone of the dermal-epidermal junction. BP180 and BP230 antibodies were elevated on serology in 61 and 13% of cases, respectively (42).
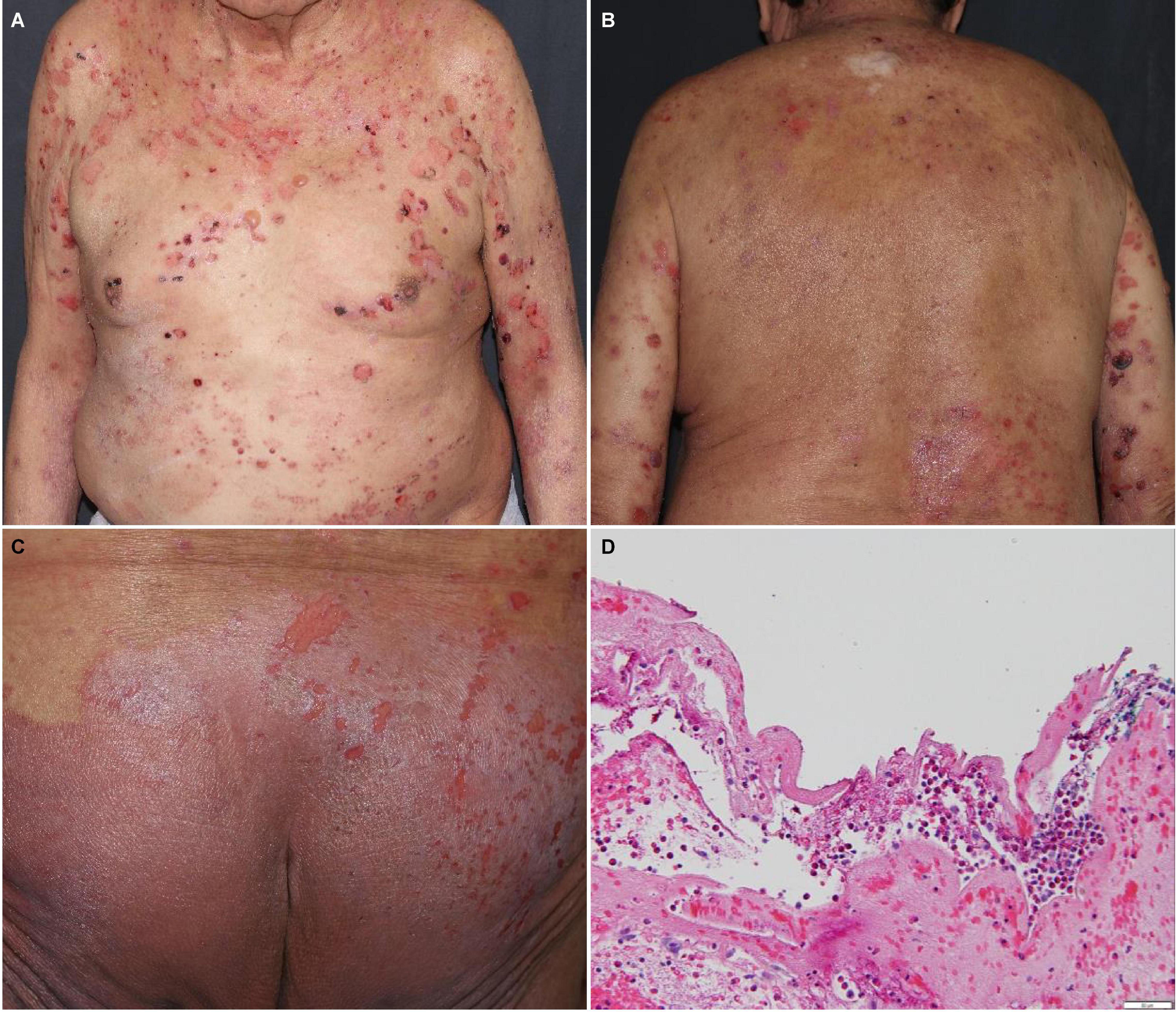
Figure 2. Bullous pemphigoid with tense bullae and erosions on the (A) trunk, (B) back, and (C) buttocks. (D) Subepidermal vesicular dermatitis with abundant eosinophils and fibrin. Direct immunofluorescence studies revealed linear deposits of IgG, IgG4 and C3 at the basement membrane zone of the dermal-epidermal junction, focal deposits of fibrin in the reticular dermis and deposits of fibrin in the debris within the cleft.
Compared to idiopathic BP, BP secondary to ICB may be more difficult to diagnose and manage (43). Serology with elevated BP180 antibodies and biopsy with direct immunofluorescence showing IgG and C3 deposition at the basement membrane zone of the dermal-epidermal junction are suggestive of BP (42). Unlike idiopathic BP which generally responds well to systemic steroid treatment, BP from ICB may be systemic steroid-refractory (44). CTCAE grade 1/2 BP in patients on ICB can be managed with high-dose topical steroids and low-dose systemic steroids. In more severe or refractory cases, systemic steroids can be increased to 0.5–1 mg/kg/day (28, 45). In one review, BP from ICB required discontinuation of ICB in 76% of cases (46). In lieu of continued systemic steroid use or for steroid-refractory cases, rituximab, intravenous immune globulin (IVIg), omalizumab, dapsone, dupilumab, or methotrexate can be considered (28, 47, 48).
Other high grade bullous disorders from ICB which have been less frequently observed include blistering lichenoid reactions, such as lichen planus pemphigoides and bullous lichen planus (49–51). Lichen planus pemphigoides presents with clinical features of both BP and lichen planus, with oral involvement in up to half of cases. Histopathological features can include lymphocyte-rich subepidermal bullae with margins exhibiting features of lichen planus including colloid bodies or focal vacuolar degeneration. As in BP, direct immunofluorescence can show IgG and C3 deposits along the basement membrane (49). In cases of bullous lichen planus associated with ICB, patients may present initially with lichenoid plaques that blister with onset time ranging from 3 to 8 months. Histopathology demonstrates lymphocytic infiltrate, as in lichen planus. Direct immunofluorescence may show non-linear IgM and C3 colloid bodies at the dermal-epidermal junction and BP180 antibodies are not expected to be elevated (51). Treatment of lichen planus pemphigoides in the setting of ICB can include topical steroids, systemic steroids, dupilumab, and rituximab, IVIg, as in BP (49). For CTCAE grade ≥3 lichenoid eruptions, biologics including infliximab and tocilizumab may be considered (10). For steroid-refractory bullous lichenoid DAEs, treatment with cyclosporine to inhibit T-cell activation may be used (52).
High grade psoriasiform DAEs to anti-CTLA-4 and anti-PD-1/PD-L1 blockade have been widely reported in the literature (53–56). In FAERS, 152 cases of psoriasiform DAEs have been recorded in association with pembrolizumab, 40 with ipilimumab, 243 with nivolumab, 57 with atezolizumab, 5 with avelumab, 24 with durvalumab, and 4 with cemiplimab. In one study of 21 patients, 72% had a pre-existing history of psoriasis (53). Psoriasiform DAEs subtypes included plaque (53.3%), scalp (20.0%), guttate (20.0%) psoriasis, or sebopsoriasis (6.8%) (53). Onset from ICB initiation to psoriasis development is 90.5 ± 77.7 days for new-onset psoriasis and 32.8 ± 21.8 days for flares of pre-existing psoriasis (53). In a multicenter study of 76 patients with pre-existing psoriasis and various malignancies treated with ICB, 43 (57%) patients had a psoriasis flare after a median of 44 days after ICB initiation. Seven patients experienced grade 3–4 psoriasiform DAEs and 16 (21%) required systemic therapy. Of the 15 patients with pre-existing psoriatic arthritis prior to ICB, 6 experienced arthritis flares (56). Notably, progression-free survival was significantly longer in patients who experienced a psoriasis flare compared to those who did not (39 vs. 8.7 months, p = 0.049) (56). When biopsied, psoriasiform DAEs show parakeratosis, diminished granular layers, and acanthosis, with varying concomitant spongiosis (14).
Psoriasiform DAEs are thought to develop due to upregulation of Th17 lymphocytes as a result of PD-1 blockade (28). Therefore, in addition to holding ICB and using topical steroids, targeted management for psoriasiform DAEs and psoriasis flares includes anti-IL-12/23, anti-IL-23, and anti-IL-17 inhibitors, or apremilast (28, 32). Table 2 summarizes management of the aforementioned high grade DAEs associated with ICB.
True SJS/TEN due to ICB may be overdiagnosed (17) due to the similarity with and novelty of SJS/TEN-like reactions. Because SJS/TEN-like reactions to ICB present variably along a clinical spectrum, they have been described by various terms including: high grade lichenoid dermatosis or unclassified dermatosis (17), lichenoid mucocutaneous eruptions (57), and progressive immunotherapy-related mucocutaneous eruption (PIRME) further complicating definitive diagnosis of this pattern of reactions (18). Differentiating true SJS/TEN from DAE mimickers is integral to a patient’s cancer care, as emerging evidence suggests that although ICB challenge should not be attempted in cases of true SJS/TEN, it may be achievable after SJS/TEN-like reactions have improved (18).
Best management strategy for ICB-associated DAEs requires ongoing investigation. Evidence regarding the safety of systemic steroids for irDAE management is conflicting. Importantly, the risks and benefits of systemic corticosteroids for the management of high grade DAEs must be carefully weighed, as there is mixed evidence that systemic corticosteroid use may dampen the antitumor effects of ICB. Specifically, in patients treated with ipilimumab for melanoma, use of high-dose systemic corticosteroids was associated with significantly shorter overall survival and the time to treatment failure compared to use of low-dose corticosteroids (58). Similarly, in a study of patients treated with ICB for non-small cell lung cancer, use of systemic corticosteroids at the time of ICB initiation was significantly associated with decreased progression-free survival and overall survival (59). However, a pooled analysis of multiple phase III trials of nivolumab for advanced melanoma found no difference in objective response rates between patients who received systemic corticosteroids or other suppressive immune-modulating agents and those who did not (60).
Although steroids are currently the initial therapy for many cutaneous and extracutaneous ICB toxicities, there is increasing support for tailored approaches that account for clinical presentation and circulating biomarkers (61). In patients with DAEs associated with ICB, IL-6 has been found to be elevated in 52% of 65 patients, elafin in 30% of 43, IL-8 in 25% of 20, IgE in 24% of 101, and IFN-γ in 23% of 26 patients. Notably, serum IgE levels also correlate with DAE severity (32). In hospitalized cancer patients with high grade DAEs, elevated elafin, IL-6, and TNF-α were shown to be associated with higher all-cause mortality. As such, tocilizumab, an anti-IL-6 agent, was recently investigated and shown to be effective for management of ICB toxicities across various organ systems in 86% of 91 cancer patients without disease progression (62). Median resolution of ICB toxicity after tocilizumab initiation was 6.5 days (63). In patients with high grade DAEs associated with ICB and elevated IL-6, tocilizumab is a promising steroid-sparing agent (64). As precision medicine with targeted biologics continues to develop, future research is needed to determine its utility in the management of DAEs. In oncodermatology, continued research to explore cytokines associated with poor outcomes in cancer patients as potentially useful therapeutic targets is important (65).
Through data from the FAERS database, we show that high grade DAEs such as SJS/TEN to immunomodulatory agents are not uncommon. We expect that as these innovative anti-cancer therapies continue to be used and as new ones develop, more patients will develop high grade DAEs. Familiarization with high grade DAEs and understanding of how to manage these will result in better outcomes through prompt management of patients with life-threatening cutaneous adverse reactions such as SJS/TEN, DRESS, and AGEP, and ability to rechallenge and continue ICB in patients with mimickers of SJS/TEN such as SJS/TEN-like reactions, bullous pemphigoid, lichenoid planus pemphigoides, and bullous lichen planus. Further research must be done not only to better delineate the high grade DAEs associated with ICB use but also to identify effective management strategies via precision medicine that do not reduce ICB efficacy.
AK and AM contributed to all parts of the conception, design, and writing of the manuscript. Both authors contributed to the article and approved the submitted version.
We would like to thank NIH grant P30-CA008748.
AM received research funding from Incyte Corporation and Amryt Pharma, and royalties from UpToDate and sits on the advisory board for Alira Health, Blueprint Medicines, Janssen, and Protagonist therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rini BI, Plimack ER, Stus V, Gafanov R, Hawkins R, Nosov D, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. (2019) 380:1116–27.
2. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. (2019) 381:2020–31.
3. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2019) 381:1535–46. doi: 10.1056/NEJMoa1910836
4. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. (2022) 386:556–67. doi: 10.1056/NEJMoa2112651
5. Tewari KS, Monk BJ, Vergote I, Miller A, de Melo AC, Kim HS, et al. Survival with cemiplimab in recurrent cervical cancer. N Engl J Med. (2022) 386:544–55. doi: 10.1056/NEJMoa2112187
6. U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Washington, DC: U.S. Department of Health and Human Services (2017).
7. Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. (2017) 390:1996–2011.
8. Molina GE, Allen IM, Hughes MS, Zubiri L, Lee H, Mooradian MJ, et al. Prognostic implications of co-occurring dermatologic and gastrointestinal toxicity from immune checkpoint inhibition therapy for advanced malignancies: a retrospective cohort study. J Am Acad Dermatol. (2020) 82:743–6. doi: 10.1016/j.jaad.2019.07.049
9. Thompson LL, Krasnow NA, Chang MS, Yoon J, Li EB, Polyakov NJ, et al. Patterns of cutaneous and noncutaneous immune-related adverse events among patients with advanced cancer. JAMA Dermatol. (2021) 157:577–82. doi: 10.1001/jamadermatol.2021.0326
10. Geisler AN, Phillips GS, Barrios DM, Wu J, Leung DYM, Moy AP, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. (2020) 83:1255–68.
11. Bottlaender L, Amini-Adle M, Maucort-Boulch D, Robinson P, Thomas L, Dalle S. Cutaneous adverse events: a predictor of tumour response under anti-PD-1 therapy for metastatic melanoma, a cohort analysis of 189 patients. J Eur Acad Dermatol Venereol. (2020) 34:2096–105. doi: 10.1111/jdv.16311
12. Chen CB, Wu MY, Ng CY, Lu CW, Wu J, Kao PH, et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. Cancer Manag Res. (2018) 10:1259–73. doi: 10.2147/CMAR.S163391
13. Dodiuk-Gad RP, Chung WH, Valeyrie-Allanore L, Shear NH. Stevens-Johnson syndrome and toxic epidermal necrolysis: an update. Am J Clin Dermatol. (2015) 16:475–93.
14. Ellis SR, Vierra AT, Millsop JW, Lacouture ME, Kiuru M. Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol. (2020) 83:1130–43. doi: 10.1016/j.jaad.2020.04.105
15. Thompson JA, Schneider BJ, Brahmer J, Achufusi A, Armand P, Berkenstock MK, et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2022) 20:387–405. doi: 10.6004/jnccn.2022.0020
16. Coleman EL, Olamiju B, Leventhal JS. The life-threatening eruptions of immune checkpoint inhibitor therapy. Clin Dermatol. (2020) 38:94–104. doi: 10.1016/j.clindermatol.2019.10.015
17. Ingen-Housz-Oro S, Milpied B, Badrignans M, Carrera C, Elshot YS, Bensaid B, et al. Severe blistering eruptions induced by immune checkpoint inhibitors: a multicentre international study of 32 cases. Melanoma Res. (2022) 32:205–10. doi: 10.1097/CMR.0000000000000819
18. Molina GE, Yu Z, Foreman RK, Reynolds KL, Chen ST. Generalized bullous mucocutaneous eruption mimicking Stevens-Johnson syndrome in the setting of immune checkpoint inhibition: a multicenter case series. J Am Acad Dermatol. (2020) 83:1475–7. doi: 10.1016/j.jaad.2020.03.029
19. Maloney NJ, Ravi V, Cheng K, Bach DQ, Worswick S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol. (2020) 59:e183–8. doi: 10.1111/ijd.14811
20. Oro S, Milpied B, Carrera C, Bensaid B, Segura S, Apalla Z, et al. Severe Blistering Drug Reactions Induced by Immune Checkpoint Inhibitors: a Retrospective International Case Series of 29 Patients. Poster Presentation EADV. (2021). Avaliable online at: https://www.em-consulte.com/article/1484980/article/toxidermies-bulleuses-graves-induites-par-les-inhi
21. Hassel JC, Heinzerling L, Aberle J, Bahr O, Eigentler TK, Grimm MO, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. (2017) 57:36–49. doi: 10.1016/j.ctrv.2017.05.003
22. Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol. (2017) 35:3815–22. doi: 10.1200/JCO.2016.72.1167
23. Jing Y, Chen X, Li K, Liu Y, Zhang Z, Chen Y, et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J Immunother Cancer. (2022) 10:e003779. doi: 10.1136/jitc-2021-003779
24. Komatsu-Fujii T, Ogawa M, Nonoyama S, Fukumoto T, Tanabe H. Recurrence of nivolumab-induced Stevens-Johnson syndrome due to tegafur/gimeracil/oteracil (TS-1(R)) after nivolumab discontinuation. Eur J Dermatol. (2021) 31:98–9. doi: 10.1684/ejd.2020.3957
25. Watanabe Y, Yamaguchi Y, Takamura N, Takahashi Y, Aihara M. Toxic epidermal necrolysis accompanied by several immune-related adverse events developed after discontinuation of nivolumab. Eur J Cancer. (2020) 131:1–4. doi: 10.1016/j.ejca.2020.02.044
26. Wang LL, Patel G, Chiesa-Fuxench ZC, McGettigan S, Schuchter L, Mitchell TC, et al. Timing of onset of adverse cutaneous reactions associated with programmed cell death protein 1 inhibitor therapy. JAMA Dermatol. (2018) 154:1057–61. doi: 10.1001/jamadermatol.2018.1912
27. Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol. (2018) 36:1714–68.
28. Muntyanu A, Netchiporouk E, Gerstein W, Gniadecki R, Litvinov IV. Cutaneous immune-related adverse events (irAEs) to immune checkpoint inhibitors: a dermatology perspective on management [formula: see text]. J Cutan Med Surg. (2021) 25:59–76. doi: 10.1177/1203475420943260
29. Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: a single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol. (2019) 80:990–7. doi: 10.1016/j.jaad.2018.10.062
30. Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for immunotherapy of cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J Immunother Cancer. (2021) 9:e002435. doi: 10.1136/jitc-2021-002435
31. Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. (2012) 30:2691–7. doi: 10.1200/JCO.2012.41.6750
32. Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A, et al. Treatment outcomes of immune-related cutaneous adverse events. J Clin Oncol. (2019) 37:2746–58.
33. Barrios DM, Phillips GS, Geisler AN, Trelles SR, Markova A, Noor SJ, et al. IgE blockade with omalizumab reduces pruritus related to immune checkpoint inhibitors and anti-HER2 therapies. Ann Oncol. (2021) 32:736–45. doi: 10.1016/j.annonc.2021.02.016
34. Mirza S, Hill E, Ludlow SP, Nanjappa S. Checkpoint inhibitor-associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res. (2017) 27:271–3. doi: 10.1097/CMR.0000000000000326
35. Lu J, Thuraisingam T, Chergui M, Nguyen K. Nivolumab-associated DRESS syndrome: a case report. JAAD Case Rep. (2019) 5:216–8. doi: 10.1016/j.jdcr.2018.11.017
36. Ai L, Gao J, Zhao S, Li Q, Cui YH, Liu Q, et al. Nivolumab-associated DRESS in a genetic susceptible individual. J Immunother Cancer. (2021) 9:e002879. doi: 10.1136/jitc-2021-002879
37. Quach HT, Johnson DB, LeBoeuf NR, Zwerner JP, Dewan AK. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. (2021) 85:956–66. doi: 10.1016/j.jaad.2020.09.054
38. Kardaun SH, Sekula P, Valeyrie-Allanore L, Liss Y, Chu CY, Creamer D, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. (2013) 169:1071–80. doi: 10.1111/bjd.12501
39. Page B, Borradori L, Beltraminelli H, Yawalkar N, Hunger RE. Acute generalized exanthematous pustulosis associated with ipilimumab and nivolumab. J Eur Acad Dermatol Venereol. (2018) 32:e256–7. doi: 10.1111/jdv.14282
40. Matsubara T, Uchi H, Haratake N, Takamori S, Toyozawa R, Miura N, et al. Acute generalized exanthematous pustulosis caused by the combination of pembrolizumab plus chemotherapy in a patient with squamous-cell carcinoma. Clin Lung Cancer. (2020) 21:e54–6. doi: 10.1016/j.cllc.2019.11.009
41. Hwang A, Iskandar A, Dasanu CA. Stevens-Johnson syndrome manifesting late in the course of pembrolizumab therapy. J Oncol Pharm Pract. (2019) 25:1520–2. doi: 10.1177/1078155218791314
42. Juzot C, Sibaud V, Amatore F, Mansard S, Seta V, Jeudy G, et al. Clinical, biological and histological characteristics of bullous pemphigoid associated with anti-PD-1/PD-L1 therapy: a national retrospective study. J Eur Acad Dermatol Venereol. (2021) 35:e511–4. doi: 10.1111/jdv.17253
43. Molina GE, Reynolds KL, Chen ST. Diagnostic and therapeutic differences between immune checkpoint inhibitor-induced and idiopathic bullous pemphigoid: a cross-sectional study. Br J Dermatol. (2020) 183:1126–8. doi: 10.1111/bjd.19313
44. Povilaityte E, Gellrich FF, Beissert S, Abraham S, Meier F, Gunther C. Treatment-resistant bullous pemphigoid developing during therapy with immune checkpoint inhibitors. J Eur Acad Dermatol Venereol. (2021) 35:e591–3. doi: 10.1111/jdv.17321
45. Apalla Z, Lallas A, Delli F, Lazaridou E, Papalampou S, Apostolidou S, et al. Management of immune checkpoint inhibitor-induced bullous pemphigoid. J Am Acad Dermatol. (2021) 84:540–3. doi: 10.1016/j.jaad.2020.05.045
46. Lopez AT, Khanna T, Antonov N, Audrey-Bayan C, Geskin L. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. (2018) 57:664–9. doi: 10.1111/ijd.13984
47. Velin M, Dugourd PM, Sanchez A, Bahadoran P, Montaudie H, Passeron T. Efficacy and safety of methotrexate, omalizumab and dupilumab for bullous pemphigoid in patients resistant or contraindicated to oral steroids. A monocentric real-life study. J Eur Acad Dermatol Venereol. (2022). doi: 10.1111/jdv.17999 [Epub ahead of print].
48. Zhang Y, Xu Q, Chen L, Chen J, Zhang J, Zou Y, et al. Efficacy and safety of dupilumab in moderate-to-severe bullous pemphigoid. Front Immunol. (2021) 12:738907. doi: 10.3389/fimmu.2021.738907
49. Boyle MM, Ashi S, Puiu T, Reimer D, Sokumbi O, Soltani K, et al. Lichen planus pemphigoides associated with PD-1 and PD-L1 inhibitors: a case series and review of the literature. Am J Dermatopathol. (2022) 44:360–7. doi: 10.1097/DAD.0000000000002139
50. Biolo G, Caroppo F, Salmaso R, Alaibac M. Linear bullous lichen planus associated with nivolumab. Clin Exp Dermatol. (2019) 44:67–8. doi: 10.1111/ced.13700
51. de Lorenzi C, Andre R, Vuilleumier A, Kaya G, Abosaleh M. Bullous lichen planus and anti-programmed cell death-1 therapy: case report and literature review. Ann Dermatol Venereol. (2020) 147:221–7. doi: 10.1016/j.annder.2019.07.008
52. Reschke R, Mockenhaupt M, Simon JC, Ziemer M. Severe bullous skin eruptions on checkpoint inhibitor therapy – in most cases severe bullous lichenoid drug eruptions. J Dtsch Dermatol Ges. (2019) 17:942–8. doi: 10.1111/ddg.13876
53. Bonigen J, Raynaud-Donzel C, Hureaux J, Kramkimel N, Blom A, Jeudy G, et al. Anti-PD1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol. (2017) 31:e254–7. doi: 10.1111/jdv.14011
54. Cutroneo P, Ingrasciotta Y, Isgro V, Rullo EV, Berretta M, Fiorica F, et al. Psoriasis and psoriasiform reactions secondary to immune checkpoint inhibitors. Dermatol Ther. (2021) 34:e14830. doi: 10.1111/dth.14830
55. Voudouri D, Nikolaou V, Laschos K, Charpidou A, Soupos N, Triantafyllopoulou I, et al. Anti-PD1/PDL1 induced psoriasis. Curr Probl Cancer. (2017) 41:407–12. doi: 10.1016/j.currproblcancer.2017.10.003
56. Halle BR, Betof Warner A, Zaman FY, Haydon A, Bhave P, Dewan AK, et al. Immune checkpoint inhibitors in patients with pre-existing psoriasis: safety and efficacy. J Immunother Cancer. (2021) 9:e003066. doi: 10.1136/jitc-2021-003066
57. Shi VJ, Rodic N, Gettinger S, Leventhal JS, Neckman JP, Girardi M, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. (2016) 152:1128–36. doi: 10.1001/jamadermatol.2016.2226
58. Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. (2018) 124:3706–14. doi: 10.1002/cncr.31629
59. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. (2018) 36:2872–8.
60. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. (2017) 35:785–92. doi: 10.1200/JCO.2015.66.1389
61. Ferrara R, Campochiaro C, Garassino MC. Shifting from a “one size fits all” to a tailored approach for immune-related adverse events. J Thorac Oncol. (2021) 16:183–6. doi: 10.1016/j.jtho.2020.11.029
62. Campochiaro C, Farina N, Tomelleri A, Ferrara R, Lazzari C, De Luca G, et al. Tocilizumab for the treatment of immune-related adverse events: a systematic literature review and a multicentre case series. Eur J Intern Med. (2021) 93:87–94. doi: 10.1016/j.ejim.2021.07.016
63. Dimitriou F, Hogan S, Menzies AM, Dummer R, Long GV. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer. (2021) 157:214–24. doi: 10.1016/j.ejca.2021.08.031
64. Hibler BP, Markova A. Treatment of severe cutaneous adverse reaction with tocilizumab. Br J Dermatol. (2020) 183:785–7. doi: 10.1111/bjd.19129
65. Mori S, Hickey A, Dusza SW, Lacouture ME, Markova A. Markers of systemic involvement and death in hospitalized cancer patients with severe cutaneous adverse reactions. J Am Acad Dermatol. (2019) 80:608–16. doi: 10.1016/j.jaad.2018.10.039
Keywords: toxic epidermal necrolysis (TEN), Stevens-Johnson syndrome (SJS), immune checkpoint blockade (ICB), acute generalized exanthematous pustulosis (AGEP), drug reaction with eosinophilia and systemic symptoms (DRESS), bullous pemphigoid (BP), rash, dermatologic adverse events (DAEs)
Citation: Kuo AM and Markova A (2022) High Grade Dermatologic Adverse Events Associated With Immune Checkpoint Blockade for Cancer. Front. Med. 9:898790. doi: 10.3389/fmed.2022.898790
Received: 17 March 2022; Accepted: 25 May 2022;
Published: 13 June 2022.
Edited by:
Elizabeth Phillips, Vanderbilt University, United StatesReviewed by:
Sherrie Divito, Brigham and Women’s Hospital and Harvard Medical School, United StatesCopyright © 2022 Kuo and Markova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alina Markova, markovaa@mskcc.org
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.