
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 13 July 2022
Sec. Intensive Care Medicine and Anesthesiology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.893459
This article is part of the Research Topic Perioperative Hemodynamic Monitoring and Management View all 10 articles
 Kristina E. Fuest1†
Kristina E. Fuest1† Ariane Servatius1
Ariane Servatius1 Bernhard Ulm1†
Bernhard Ulm1† Stefan J. Schaller1,2†
Stefan J. Schaller1,2† Bettina Jungwirth1,3†
Bettina Jungwirth1,3† Manfred Blobner1,3†
Manfred Blobner1,3† Sebastian Schmid1,3*†
Sebastian Schmid1,3*†Background: Post-operative delirium is common in elderly patients and associated with increased morbidity and mortality. We evaluated in this pilot study whether a perioperative goal-directed hemodynamic optimization algorithm improves cerebral oxygenation and can reduce the incidence of delirium.
Materials and Methods: Patients older than 70 years with high risk for post-operative delirium undergoing elective non-cardiac surgery were randomized to an intervention or control group. Patients in the intervention group received a perioperative hemodynamic optimization protocol based on uncalibrated pulse-contour analysis. Patients in the control group were managed according to usual standard of care. Incidence of delirium until day seven was assessed with confusion assessment method (CAM) and chart review. Cerebral oxygenation was measured with near-infrared spectroscopy.
Results: Delirium was present in 13 of 85 (15%) patients in the intervention group and 18 of 87 (21%) in the control group [risk difference −5.4%; 95% confidence interval, −16.8 to 6.1%; P = 0.47]. Intervention did not influence length of stay in hospital or in-hospital mortality. Amounts of fluids and vasopressors applied, mean arterial pressure, cardiac index, and near-infrared spectroscopy values were comparable between groups.
Conclusion: The hemodynamic algorithm applied in high-risk non-cardiac surgery patients did not change hemodynamic interventions, did not improve patient hemodynamics, and failed to increase cerebral oxygenation. An effect on the incidence of post-operative delirium could not be observed.
Clinical Trial Registration: [Clinicaltrials.gov], identifier [NCT01827501].
Delirium is a common post-operative complication in the elderly (1). It is defined as an acute neuropsychiatric disorder characterized by fluctuations in attention, awareness, and cognition. The incidence depends on several factors like age, number of comorbidities, pre-operative cognitive or functional impairment, and type of surgery (2–4). Particularly in patients admitted to the intensive care unit (ICU) the incidence is between 50 and 80% (5) and their length of stay in ICU and hospital is prolonged. As a consequence, delirium is both a huge burden on a patient’s wellbeing and on the healthcare system overall (6, 7). To prevent delirium multimodal and multidisciplinary interventions should be implemented during hospital stay and particularly in the perioperative course (8).
Since maintaining a sufficient perfusion is a general principle in anesthesia, the patient’s hemodynamic status could be one target point of intervention. Sufficient perfusion and oxygen delivery are essential in order to avoid impairment of the brain (9). In sepsis-associated delirium a correlation with cerebral perfusion pressure has been demonstrated as one of many contributing factors (10). In addition, the correlation between intraoperative hypotension and post-operative delirium has been shown in a recent clinical trial not exclusively for sepsis but also involving surgical patients (11). Furthermore, poorer cerebral perfusion pressure was associated with a higher risk of post-operative delirium as well as longer duration and higher severity of delirium, independent of demographic and medical predictors in a cohort of lung-transplant recipients (12). This indicates that individual adjustment of cerebral perfusion in terms of goal-directed hemodynamic optimization could be an approach to reduce the incidence of delirium via improvement of cerebral oxygenation especially in elderly patients (13). Uncalibrated pulse contour analysis requires only an arterial line and cardiac index is calculated with an algorithm using bodyweight and height of the patient. It allows efficient, continuous monitoring, targeting of optimal cardiac output and facilitates management of vasopressors and fluid administration during high-risk surgery (14, 15). As a consequence, intraoperative cerebral perfusion and oxygenation might be optimized by being able to target cardiac output measures. The hypothesis of our study is, that goal-directed hemodynamic optimization will improve cerebral perfusion and consequently cerebral oxygenation and thereby reduce the incidence of delirium in a high-risk population compared with standard therapy.
We performed a prospective, randomized, single-center study at a university hospital in Munich, Germany. Patients older than 70 years with a high risk of developing post-operative delirium were included and randomized into two treatments arms: intervention and control group.
The study was approved by the ethics committee of the Technical University of Munich (Ethikkommission der Technischen Universität München, Ismaninger Straße 22, 81675 München; Approval Number: 5687/13 S on February 28th, 2013 and October 24th, 2018; Chairperson Prof. Dr. G. Schmidt) and prospectively registered at Clinical Trials (April 2013; NCT01827501). There was one amendment to the study in 2018, when a new German law for data protection regulation has come into force and the patient information sheet had to be updated. The study was conducted in accordance with the Declaration of Helsinki.
Patients were screened for eligibility during the pre-anesthesia visit. Inclusion criteria were age above 70 years, major elective non-cardiac surgery (defined by a scheduled surgery time ≥90 min) and a high risk for delirium (screening score ≥6 points; see below). Surgical procedures included all types of surgery except cardiac, major aortic, and neurosurgery. Patients with emergency procedures and patients who had general anesthesia within the last 30 days were excluded. Further exclusion criteria were valvular disorders grade II or higher as well as history of major aortic surgery, as these factors are known to distort the uncalibrated pulse contour analysis. A detailed interview with the patient and/or his caregivers was conducted by a research team member during the pre-anesthesia visit to assess the patient’s risk for delirium. Several predisposing and precipitating factors were identified and scored with one or two points according to Table 1. The scoring system is a modification of the risk score described by Marcantonio based on the work of Inouye (16, 17). We included only patients with a score of ≥6 points in the study as these patients have a high risk of at least 30% for delirium (18).
A research team member evaluated the patients’ eligibility, informed the patient in detail about the study, and obtained written informed consent. He enrolled the patient and assigned him to intervention or control group in a 1:1 ratio. The randomization list was generated by a study team member using a random generator without blocks (Microsoft Excel for Mac 14.0). For each randomization number we prepared a paper-based folder with all required materials including the group assignment. Only the folder with the lowest number was accessible to the study team member responsible for the allocation.
A Mini-Mental-State exam (MMSE) was performed to detect dementia or cognitive impairment (MMSE ≤ 24: mild cognitive impairment; MMSE > 20 and < 24: dementia) and the Confusion assessment method (CAM-) Score was obtained (19, 20). Patients with present delirium were excluded. Above that, activities of daily life were assessed to determine the presence of frailty according to the Clinical Frailty Scale (CFS) (21). As already determined in large multicentric international trials, patients with a CFS 5–8 were considered as frail (22). Above that, functional disability is present, when sensory or visionary aids are necessary, walking sticks, rollators or wheelchairs are required or patients need feeding, e.g., by a percutaneous endoscopic gastrostomy. To determine high medical comorbidities and cardiovascular risk factors medical records including clinical charts and nursing records were reviewed. Data collection included patient biometrics, comorbidities, clinical parameters and laboratory findings. The risk of the surgical-procedures was determined according to the German Society of Anesthesia (23).
After transfer to the operating theater, an arterial line was introduced via Seldinger technique in the radial (3 French) or femoral (4 French) artery under local anesthesia before induction of anesthesia. Following the induction of general anesthesia with sufentanil, propofol and rocuronium the patient was intubated. Anesthesia was maintained with sufentanil, rocuronium and sevoflurane. Depth of anesthesia was recorded using entropy and was kept between 40 and 60. A central venous catheter was placed when necessary, according to the attending specialist.
In the goal-directed hemodynamic optimization group (group intervention) hemodynamic management was performed according to a previously published algorithm obtained by pulse contour analysis using the PulsioFlex® device (PULSION Medical Systems SE; Feldkirchen; Germany) (see Figure 1) (24). Evaluation of the algorithm was started before induction of anesthesia and continued until discharge from the post-anesthesia care unit (PACU).
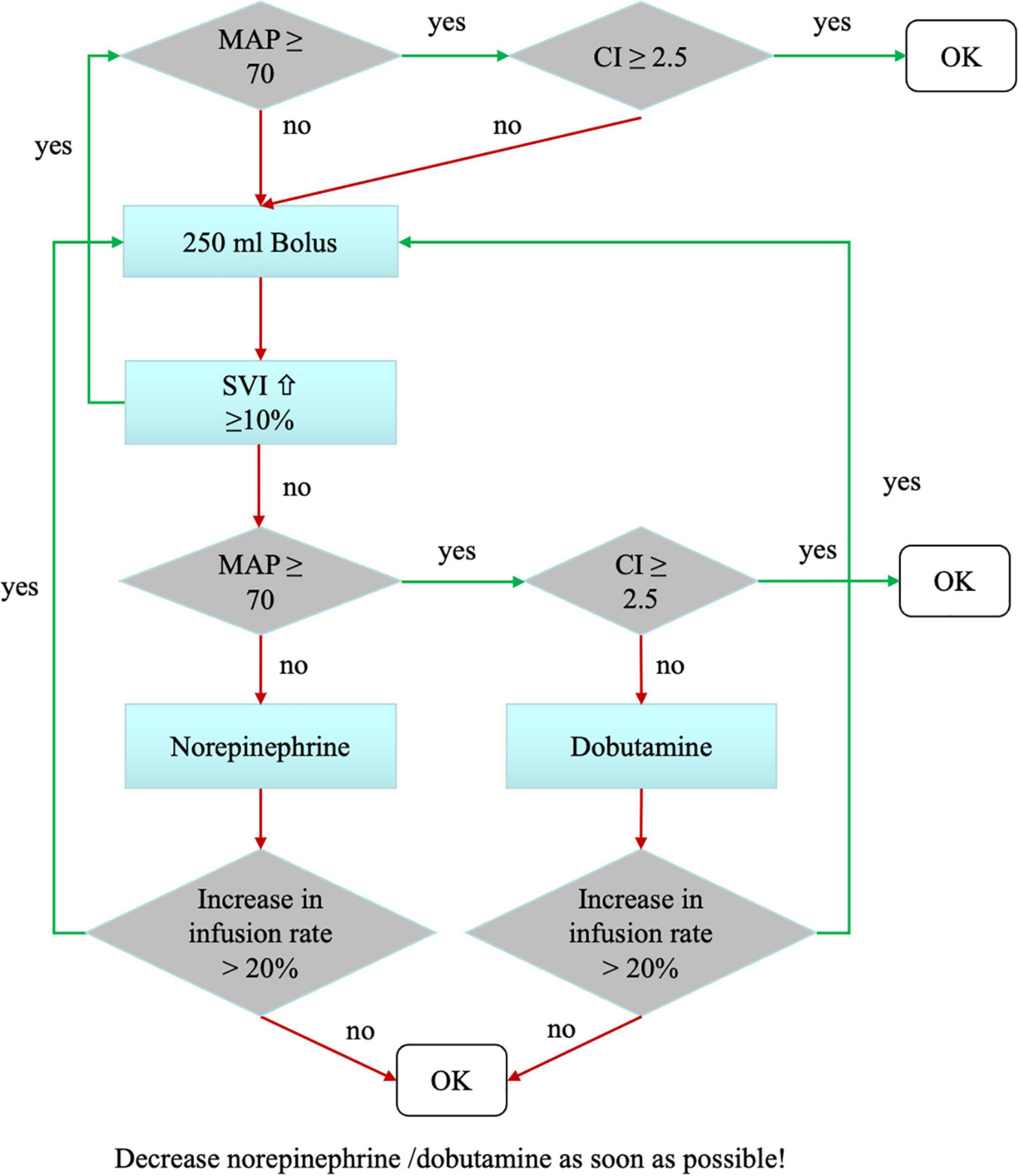
Figure 1. Hemodynamic treatment algorithm in the intervention group. The hemodynamic goals were mean arterial pressure (MAP) >70 mmHg and a cardiac index (CI) >2.5 L/kg/m2. Hemodynamics were evaluated routinely every 30 min, as well as at times of hemodynamic instability. We tested fluid responsiveness using a volume challenge of 250 mL Ringer’s acetate. Depending on changes in the stroke volume index (SVI), the patient received either volume or catecholamines in a titrated manner.
For volume therapy Ringer’s acetate was used. Every patient received a basal infusion with a dosage of 1 ml/kg per hour according to our standard care. The algorithm was based on the two factors mean arterial blood pressure and cardiac index. If both factors were in a sufficient range (MAP > 70 mmHg, cardiac index > 2.5 L/kg/m2), no intervention was necessary. In case of insufficient mean arterial pressure or cardiac index, the patient received a fluid bolus of 250 ml Ringer’s acetate in 5–10 min followed by another assessment of the algorithm. If after a fluid bolus the stroke volume index did not increase, drug therapy was initiated. Norepinephrine was used as vasopressor, Dobutamine as inotropic medication.
The goal-directed optimization was terminated, when the patient fulfilled the standard criteria for discharge from recovery room: the pain level was ≤3 according to the numeric rating scale (NRS), the hemodynamic situation was stable without catecholamines, the pulmonary situation was stable, and the patient fully awake and compliant.
In the control group an arterial line was placed as well and hemodynamic management was performed according to heart rate and blood pressure without using extended monitoring like pulse contour analysis or any other goal-directed hemodynamic monitoring. Ringer’s acetate was used for fluid replacement at the attending anesthetist’s discretion. Here, the responsible anesthetist was blinded to the results of the goal-directed hemodynamic monitoring which was obscured by the study team.
In both groups other medication like antibiotics, anticoagulants, or pain medication was administered according to the intraoperative standard operating procedure protocol of the department of anesthesia and intensive care medicine. Patients in all groups received red blood cell transfusion, when hemoglobin value dropped below 8 mg/dl or in case of cardiac impairment such as ST-depression. Coagulation factors and fresh frozen plasma were substituted according to the coagulation status assessed with rotational thromboelastometry (ROTEM™).
To test our hypothesis, that goal-directed hemodynamic optimization reduces delirium by improving perfusion and consequently enhancing oxygenation, it was necessary to measure the oxygen concentration of the tissue. In the last years NIRS has been introduced in daily clinical practice (25). The device is safe, non-invasive and was used in our patients to assess the oxygen concentration in the brain. In this study, the INVOS™ (Medtronic GmbH, Earl-Bakken-Platz 1, 40670 Meerbusch, Germany) cerebral somatic oximeter with two adult sensors placed on the left and right side of the forehead was used.
To evaluate the difference to pre-operative values, the monitoring was established before induction of anesthesia in both groups. In the intervention group values from NIRS monitoring were available to the responsible anesthetist. However, they were not included in the hemodynamic optimization algorithm. Thus, it was left to the treating anesthesiologist to react individually to possible insufficient NIRS values in the intervention group. Anesthesiologists in the control group were blinded to the NIRS monitoring.
Following data were recorded: demographics (age, sex, and co-morbidities) and information obtained during the pre-anesthesia visit including predisposing factors for delirium, surgical and anesthesiologic data extracted from the anesthesia and surgical protocol (including medication and administered fluids, goal-directed hemodynamic monitoring parameters as well as NIRS and entropy), parameters obtained during the post-operative visit that can be extracted from the clinical charts on the ward, length of stay in hospital, and follow-up data like mortality after 1 year.
For detection of delirium the CAM Score (19, 26, 27) was obtained from every patient once daily until day 7 after surgery. This was done by a member of the study team who had been thoroughly trained. If delirium was present, the severity was also assessed using CAM-S (28, 29). Delirium often occurs during the night. Since the visit by the study team took place during the day, we decided to improve detection of delirium by inspecting the patient files to review delirium associated medication like haloperidol and by exchanging information with the ward team. This ensured that even in the event of a poor handover of the night shift to the day shift, abnormalities during the night became apparent and could thus be evaluated with the treating team if necessary. Furthermore, inadequate qualification of the nursing staff (such as inexperienced in delirium symptoms and their clinical presentation) could thus be compensated for via the evaluation of the patient record. As delirium can be triggered by pain the NRS was registered daily. If the patient was admitted to ICU, the CAM was also obtained there. As in anesthetized and ventilated patients obtaining the CAM is not possible, we performed an additional sensitivity analysis and assigned these patients to the delirium group assuming worst outcome (worst-case imputation). Furthermore, a second MMS-Test was performed in every patient on day 7 or the day before discharge, whichever occurs first. Patients were subsequently followed for up to 1 year after surgery via telephone interview to assess mortality. In cases where we were not able to reach the patient, we reviewed the hospital record for information about survival during the last year.
Primary endpoint was the incidence of delirium until day 7. Duration of delirium as well as the day it first occurred were investigated as secondary endpoints. Further secondary endpoints were: length of stay in hospital, in-hospital mortality, and mortality after 1-year.
Patients were blinded to group allocation and intervention throughout the trial. Anesthesiologists treating the patient during surgery and in the PACU were not blinded but in the control group they were not able to assess the parameters of the goal-directed hemodynamic and NIRS monitoring. Nurses and physicians treating the patient on ICU or normal ward after surgery were blinded to group allocation. The outcome assessor was not blinded to the intervention.
Data analysis was performed with R version 4.1.0. Continuous variables are presented as median [interquartile range (IQR)]. Categorical variables are presented using absolute numbers and frequencies. Effect sizes were calculated using differences in median for continuous variables and risk difference (RD) for binary variables. In addition to effect sizes null hypothesis tests were conducted via Mann-Whitney U-tests for continuous variables and by χ2–tests for binary variables. To validate our results, we performed a sensitivity analysis using worst-case imputation. A two-sided P-value of less than 0.048 was considered statistically significant.
By using a screening score we intended to included patients with an expected delirium-incidence above 30%. As in most interventional studies the risk for post-operative delirium could be reduced by one third these figures could have been used for sample size calculation (4, 6). However, 6 or more points in the screening score correspond to a wide range of delirium-incidence between 30 and 50%. As the actual incidence had a significant impact on the number needed per group, we a priori planned an interim analysis after 100 included patients to assess the new sample size according to a modification of the O’Brian-Fleming technique (30). In the interim analysis the incidence of delirium was 3/47 (6%) in the intervention and 11/52 (21%) in the control group (P = 0.04; Fisher’s exact test; 1 patient excluded as pre-set surgery time was not adhered). As the difference of delirium between the two groups was not significant with a pre-defined α < 0.002 to finish the study, the sample size needed per group was adjusted. Thereafter, based on two-tailed χ2-test, assuming an α = 0.048 and a power of 80%, the analysis disclosed 86 patients per group. The calculation was performed via DataTab (URL).1 As a result, of the a priori planned interim analysis, the targeted sample size was now set to 172.
Between May 2013 and December 2019, 172 patients were included in the study. Follow-up was finished in February 2021. Figure 2 shows the CONSORT diagram of the study. Surgical procedures included all departments with abdominal surgery being the most frequent. 85 patients were randomized into the intervention group. 87 patients received standard care. Baseline characteristics were comparable between the two groups regarding screening score, frailty, and pre-medical condition (Table 2).
The number of crystalloids, colloids, blood products, and vasopressors infused was comparable between groups. Patients in the intervention group received more inotropes. Regarding hemodynamics patients in the control group had an increased MAP, whereas cardiac index was higher in the intervention group (Table 3). NIRS monitoring showed comparable cerebral oxygenation (median and delta from pre-induction) in both groups during surgery [median NIRS total: control 68 (IQR 78 to 96) vs. intervention 81 (75 to 88); median difference 2.8; 95% confidence interval (CI) −0.9 to 6.0; P = 0.09; Table 3] and in the PACU (Table 3).
Delirium was present in 13 patients in the intervention group (15%) and in 18 in the control group (21%) (RD, −5%; 95% CI, −16.8 to 6.1%; P = 0.47) (Table 4). The type of delirium assessment (CAM score or chart review) had no influence on the results (Table 4). In an additional sensitivity-analysis with anesthetized patients included in the group of delirium (worst-case imputation), there was no difference between groups (23 vs. 18%) (RD, −5%; 95% CI, −17 to 7%; P = 0.50). Length of delirium as well as severity of delirium was not significantly different between groups [2 (1 to 3) vs. 1 (1 to 2)], difference in medians, 1; 95% CI 1 to 2; P = 0.27).
There was no significant difference between groups regarding length of stay in hospital as well as in-hospital-mortality (Table 4). One-year mortality was reduced in the intervention group (12 vs. 24%) (RD, −14%; 95% CI, −25.4 to −1.5%; P = 0.03) (Table 4).
In this single-center, randomized-controlled pilot trial a goal-directed hemodynamic optimization algorithm did not lead to significantly different therapeutic interventions, and thus did not result in different hemodynamics or values of cerebral oxygenation. Consequently, the algorithm applied did not reduce post-operative delirium in elderly high-risk patients. There was no effect on secondary endpoints like length of stay in hospital as well as in-hospital-mortality. This monocentric trial does not support the use of this goal-directed hemodynamic optimization algorithm in the prevention of post-operative delirium.
The incidence of post-operative delirium with 21% in the control group and 15% in the intervention group (P = 0.47) was low compared to an anticipated occurrence of delirium of a least 30% in our high-risk population (3). There was a large difference in the incidence of delirium in the intervention group between the interim analysis (6%) and the final analysis (15%). Therefore, the intended level of power was not achieved. The reasons for this can only be speculated, as there were no changes to the study protocol or study team after the interim analysis. The missing effect of the intervention, nevertheless, is in line with the investigations from other authors. In a systematic review and meta-analysis multicomponent strategies were able to reduce the incidence of delirium in elderly patients with scheduled non-cardiac surgery. Strategies during the perioperative period (optimization of pain management or anesthesia) could only rarely improve the rate of delirium (4). However, in the special subgroup of patients in the prone position goal-directed fluid therapy improved hemodynamics and cerebral oxygenation and reduced the incidence of post-operative cognitive dysfunction (31, 32). Also, non-pharmacological multicomponent approaches were more effective. In our study pain management was sufficient in both groups and therefore no influencing factor for delirium. To this point monitoring depth of anesthesia is the best of the perioperative components to reduce delirium, especially to guide anesthetic titration during surgery and avoid long periods of burst suppression (33, 34). It must be emphasized that the evidence on this topic is still insufficient. Although delirium reduction of up to 30% was reported in the cited meta-analyses, the ENGAGES trial published in 2019 showed different results (35). Here, BIS-guided anesthesia was able to reduce the dosage of volatile anesthetics and subsequently the cumulative time with electroencephalography suppression, but not the delirium incidence within the first 5 days after major surgery. The authors attributed the differences from the meta-analyses to, among other things, older patients and compared major surgical procedures in their study. While the studies in the referred metanalyses used bispectral-index (BIS) entropy parameters in our study were comparable between groups and in the lower target range of around 40, indicating adequate depth of anesthesia.
Although in the intervention group fluid and catecholamine management was tightly controlled by the hemodynamic optimization protocol, both groups received equivalent amounts of fluids and vasopressors. This resulted in comparable intraoperative hemodynamic parameters, like sufficient mean arterial pressure and cardiac output. As cardiac output was only measured in the intervention group, this resulted in higher amounts of inotropes and therefore an increased cardiac index in this group. In the control group MAP was minimally elevated without clinical relevance. As hemodynamics did not differ between groups, not surprisingly NIRS values as surrogate parameters for cerebral perfusion and oxygenation were comparable between groups. Based on this no difference in post-operative delirium could be expected.
Although we did not see significant results in our primary endpoint, secondary analysis showed a difference in 1-year mortality between groups (P = 0.03). Since there was no influence on hemodynamics or cerebral oxygenation this result cannot be attributed to the intervention and we consider it as an epiphenomenon.
As a strength of our pilot study, using a screening tool for detection of patients with high risk for delirium made it possible to include a wide variety of patients with severe pre-existing conditions, extensive surgery, and an expected high risk of at least 30% for post-operative delirium. For identification of these high-risk patients, we used a modification of the risk-score published by Marcantonio (18). Several other scores have been introduced to stratify patients according to their individual risk for delirium. For example, Inouye identified five independent factors during hospitalization. However, these scores were mainly validated on general wards and the factors are not specific to surgical patients (17). In contrast, Marcantonio introduced a risk-score, that considers additional intraoperative and post-operative precipitating factors, like type of anesthesia and type of surgery. Daily post-operative visits for up to 7 days as well as inspection of the patient medical charts allowed for almost complete post-operative monitoring in order to detect all forms of delirium. The CAM-Score is the most reliable and validated score to detect delirium and is available in German. It has a sensitivity of 0.79 and a specificity of 0.97 (26).
However, there are limitations to our study: a single-center pilot trial only allows to assess the level-of-care provided in our hospital. This effect is emphasized by the already good standard hemodynamic management in the control group, that could not be further optimized by the algorithm. This lack of effect, especially in comparison with an already good control group, has already been shown, also in own work (36, 37). It might be explained by the fact that the algorithm used was based on absolute values of MAP and cardiac index. This may be a limitation of our study, as recent evidence suggests the use of individualized hemodynamic target parameters based on pre-operative measurements (38). In addition, the possibility of performance bias as reason for the missing effect of the intervention must also be considered. Above that, it must be further noted that the risk of delirium in our patient population may not have been severe enough in order for the intervention to achieve a difference.
Furthermore, hemodynamic variability must be taken into account, as it is possible that patients in the control group experienced periods of hypotension followed by periods of hypertension. However, the amount of time patients experienced intraoperative hypotension was not measured. Intervention was limited to the perioperative period as we did not provide guidelines for pre-operative optimization or therapy in the post-operative course. A further limitation is the missing information regarding post-operative inflammation as blood samples were not taken in a regular base.
Our intervention focused solely on hemodynamic optimization. In particular, no targeted intervention was provided for additional optimization of insufficient NIRS values. This is partly due to the fact that improved cardiac output was hypothesized to improve cerebral perfusion and subsequently oxygenation. Further research in this field should include management of pathological NIRS values in the algorithm, although there is only a weak association of low NIRS values and worse neurological outcome and the effect is most prominent in cardiac surgery (39, 40). An additional individualized, multi-component intervention strategy might have been more efficient in reducing delirium and should be investigated in a subsequent trial (4, 41). Lastly, the outcome-assessor was not blinded in this trial and outcome was assessed once daily, which can lead to bias. This effect might be somewhat mitigated by communicating with the team and assessing psychoactive medication utilization. However, the combination of assessment by a team member and chart review to a composite endpoint is also not ideal as it mixes different delirium screening methods. Nevertheless, because the incidences for both methods separately are comparable to the composite endpoint, the results are valid. There is a possibility that a significant amount of hypoactive delirium was missed and that the CAM-positive patients in the study had more severe delirium. Ideally, a more sensitive assessment method for delirium should be used in a follow-up trial and applied multiple times daily.
In conclusion, a goal-directed hemodynamic optimization protocol did not change hemodynamic interventions, did not improve the patients’ hemodynamics, and did not enhance cerebral oxygenation in old high-risk patients. The algorithm applied had no effect on the incidence of post-operative delirium.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethikkommission der Technischen Universität München, Ismaninger Straße 22, 81675 München. The patients/participants provided their written informed consent to participate in this study.
SS was the principal investigator and developed the protocol. BU was the study statistician. SS, BJ, and KEF were involved in the ethical approval. KEF, BU, AS, BJ, MB, SJS, and SS were involved in the analysis and interpretation of the data. KEF, SS, AS, and BJ were involved in the data acquisition and quality assurance. All authors critically revised the manuscript and approved its final version.
This work was supported by the institutional funds only.
BJ received honoraria for giving lectures from Pulsion Medical Systems SE (Feldkirchen, Germany). MB received research support from MSD (Haar, Germany) not related to this manuscript, received honoraria for giving lectures from GE Healthcare (Helsinki, Finland) and Grünenthal (Aachen, Germany). SJS reports grants from Reactive Robotics GmbH (Munich, Germany), grants and non-financial support from STIMIT AG (Biel, Switzerland), Liberate Medical LLC (Crestwood USA), ESICM (Geneva, Switzerland), grants, personal fees and non-financial support from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany), personal fees from Springer Verlag GmbH (Vienna, Austria) for educational purposes, non-financial support from Technical University of Munich (Munich, Germany) and from National and international societies (and their congress organizers) in the field of anesthesiology and intensive care medicine, outside the submitted work. SJS held stocks in small amounts from Rhön-Klinikum AG and holds stocks in small amounts from Alphabeth Inc., Bayer AG and Siemens AG; these holdings have not affected any decisions regarding his research or this study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hazzard WRBJ, Halter JB, Ouslander JG, Tinetti ME. Principles of Geriatric Medicine and Gerontology. 5th ed. New York, NY: McGraw-Hill (2003). XXVI, 1648S p.
2. Ellard L, Katznelson R, Wasowicz M, Ashworth A, Carroll J, Lindsay T, et al. Type of anesthesia and postoperative delirium after vascular surgery. J Cardiothorac Vasc Anesth. (2014) 28:458–61. doi: 10.1053/j.jvca.2013.12.003
3. Marcantonio ER, Flacker JM, Wright RJ, Resnick NM. Reducing delirium after hip fracture: a randomized trial. J Am Geriatr Soc. (2001) 49:516–22. doi: 10.1046/j.1532-5415.2001.49108.x
4. Janssen TL, Alberts AR, Hooft L, Mattace-Raso F, Mosk CA, van der Laan L. Prevention of postoperative delirium in elderly patients planned for elective surgery: systematic review and meta-analysis. Clin Interv Aging. (2019) 14:1095–117. doi: 10.2147/CIA.S201323
5. Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive care medicine. (2017) 43:1105–22. doi: 10.1007/s00134-017-4867-0
6. Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. J Am Geriatr Soc. (2011) 59(Suppl. 2):S241–3. doi: 10.1111/j.1532-5415.2011.03671.x
7. Inouye SK, Bogardus ST Jr., Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. (1999) 340:669–76. doi: 10.1056/NEJM199903043400901
8. Aldecoa C, Bettelli G, Bilotta F, Sanders RD, Audisio R, Borozdina A, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesth. (2017) 34:192–214. doi: 10.1097/EJA.0000000000000594
9. Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. (2021) 101:1487–559. doi: 10.1152/physrev.00022.2020
10. Atterton B, Paulino MC, Povoa P, Martin-Loeches I. Sepsis Associated Delirium. Medicina (Kaunas). (2020) 56:240. doi: 10.3390/medicina56050240
11. Maheshwari K, Ahuja S, Khanna AK, Mao G, Perez-Protto S, Farag E, et al. Association Between Perioperative Hypotension and Delirium in Postoperative Critically Ill Patients: A Retrospective Cohort Analysis. Anesth Anal. (2020) 130:636–43. doi: 10.1213/ANE.0000000000004517
12. Smith PJ, Blumenthal JA, Hoffman BM, Rivelli SK, Palmer SM, Davis RD, et al. Reduced Cerebral Perfusion Pressure during Lung Transplant Surgery Is Associated with Risk, Duration, and Severity of Postoperative Delirium. Ann Am Thorac Society. (2016) 13:180–7.
13. Burkhart CS, Rossi A, Dell-Kuster S, Gamberini M, Mockli A, Siegemund M, et al. Effect of age on intraoperative cerebrovascular autoregulation and near-infrared spectroscopy-derived cerebral oxygenation. Br J Anaesth. (2011) 107:742–8. doi: 10.1093/bja/aer252
14. Saugel B, Reuter DA. Perioperative Goal-Directed Therapy Using Invasive Uncalibrated Pulse Contour Analysis. Front Med (Lausanne). (2018) 5:12. doi: 10.3389/fmed.2018.00012
15. Saugel B, Kouz K, Scheeren TWL, Greiwe G, Hoppe P, Romagnoli S, et al. Cardiac output estimation using pulse wave analysis-physiology, algorithms, and technologies: a narrative review. Br J Anaesth. (2021) 126:67–76. doi: 10.1016/j.bja.2020.09.049
16. Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized elderly medical patients based on admission characteristics. Ann Intern Med. (1993) 119:474–81. doi: 10.7326/0003-4819-119-6-199309150-00005
17. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. (1996) 275:852–7. doi: 10.1001/jama.275.11.852
18. Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. (2012) 308:73–81. doi: 10.1001/jama.2012.6857
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. (1990) 113:941–8. doi: 10.7326/0003-4819-113-12-941
20. Kukull WA, Larson EB, Teri L, Bowen J, McCormick W, Pfanschmidt ML. The Mini-Mental State Examination score and the clinical diagnosis of dementia. J Clin Epidemiol. (1994) 47:1061–7.
21. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. (2005) 173:489–95.
22. Flaatten H, De Lange DW, Morandi A, Andersen FH, Artigas A, Bertolini G, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (>/= 80 years). Intens Care Med. (2017) 43:1820–8. doi: 10.1007/s00134-017-4940-8
23. Gemeinsame Empfehlung der Dgai DuD. Präoperative Evaluation erwachsener Patienten vor elektiven, nicht herz-thoraxchirurgischen Eingriffen. Anaästh Intens. (2017) 58:349–64.
24. Pearse R, Dawson D, Fawcett J, Rhodes A, Grounds RM, Bennett ED. Early goal-directed therapy after major surgery reduces complications and duration of hospital stay. A randomised, controlled trial [ISRCTN38797445]. Crit Care. (2005) 9:R687–93. doi: 10.1186/cc3887
25. Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. (2009) 103(Suppl. 1):i3–13.
26. Luetz A, Heymann A, Radtke FM, Chenitir C, Neuhaus U, Nachtigall I, et al. Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med. (2010) 38:409–18.
27. Krewulak KD, Rosgen BK, Ely EW, Stelfox HT, Fiest KM. The CAM-ICU-7 and ICDSC as measures of delirium severity in critically ill adult patients. PLoS One. (2020) 15:e0242378. doi: 10.1371/journal.pone.0242378
28. Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. (2014) 160:526–33. doi: 10.7326/M13-1927
29. Vasunilashorn SM, Fong TG, Albuquerque A, Marcantonio ER, Schmitt EM, Tommet D, et al. Delirium severity post-surgery and its relationship with long-term cognitive decline in a cohort of patients without dementia. J Alzheimers Dis. (2018) 61:347–58.
30. O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. (1979) 35:549–56.
31. Wang DD, Li Y, Hu XW, Zhang MC, Xu XM, Tang J. Comparison of restrictive fluid therapy with goal-directed fluid therapy for postoperative delirium in patients undergoing spine surgery: a randomized controlled trial. Perioper Med (Lond). (2021) 10:48. doi: 10.1186/s13741-021-00220-5
32. Zhang N, Liang M, Zhang DD, Xiao YR, Li YZ, Gao YG, et al. Effect of goal-directed fluid therapy on early cognitive function in elderly patients with spinal stenosis: A Case-Control Study. Int J Surg. (2018) 54(Pt A):201–5. doi: 10.1016/j.ijsu.2018.04.007
33. Chan MT, Cheng BC, Lee TM, Gin T. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol. (2013) 25:33–42. doi: 10.1097/ANA.0b013e3182712fba
34. Radtke FM, Franck M, Lendner J, Krüger S, Wernecke KD, Spies CD. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. (2013) 110(Suppl. 1):i98–105. doi: 10.1093/bja/aet055
35. Wildes TS, Mickle AM, Ben Abdallah A, Maybrier HR, Oberhaus J, Budelier TP, et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: The ENGAGES randomized clinical trial. JAMA. (2019) 321:473–83. doi: 10.1001/jama.2018.22005
36. Schmid S, Kapfer B, Heim M, Bogdanski R, Anetsberger A, Blobner M, et al. Algorithm-guided goal-directed haemodynamic therapy does not improve renal function after major abdominal surgery compared to good standard clinical care: a prospective randomised trial. Crit Care. (2016) 20:50.
37. Bartha E, Arfwedson C, Imnell A, Fernlund ME, Andersson LE, Kalman S. Randomized controlled trial of goal-directed haemodynamic treatment in patients with proximal femoral fracture. Br J Anaesth. (2013) 110:545–53. doi: 10.1093/bja/aes468
38. Saugel B, Vincent JL, Wagner JY. Personalized hemodynamic management. Curr Opin Crit Care. (2017) 23:334–41.
39. Bendahan N, Neal O, Ross-White A, Muscedere J, Boyd JG. Relationship between near-infrared spectroscopy-derived cerebral oxygenation and delirium in critically Ill patients: a systematic review. J Intens Care Med. (2019) 34:514–20. doi: 10.1177/0885066618807399
40. Ortega-Loubon C, Herrera-Gómez F, Bernuy-Guevara C, Jorge-Monjas P, Ochoa-Sangrador C, Bustamante-Munguira J, et al. Near-infrared spectroscopy monitoring in cardiac and noncardiac surgery: pairwise and network meta-analyses. J Clin Med. (2019) 8:2208. doi: 10.3390/jcm8122208
Keywords: outcome, post-operative delirium, goal-directed hemodynamic monitoring, goal-directed therapy, frailty
Citation: Fuest KE, Servatius A, Ulm B, Schaller SJ, Jungwirth B, Blobner M and Schmid S (2022) Perioperative Hemodynamic Optimization in Patients at Risk for Delirium – A Randomized-Controlled Trial. Front. Med. 9:893459. doi: 10.3389/fmed.2022.893459
Received: 10 March 2022; Accepted: 15 June 2022;
Published: 13 July 2022.
Edited by:
Guo-wei Tu, Fudan University, ChinaReviewed by:
Konlawij Trongtrakul, Chiang Mai University, ThailandCopyright © 2022 Fuest, Servatius, Ulm, Schaller, Jungwirth, Blobner and Schmid. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Schmid, cy5zY2htaWRAdW5pLXVsbS5kZQ==
†ORCID: Kristina E. Fuest, orcid.org/0000-0002-6988-038X; Bernhard Ulm, orcid.org/0000-0002-9396-2510; Stefan J. Schaller, orcid.org/0000-0002-6683-9584; Bettina Jungwirth, orcid.org/0000-0001-9749-7460; Manfred Blobner, orcid.org/0000-0002-0370-5247; Sebastian Schmid, orcid.org/0000-0002-1232-5350
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.