- 1Institute of Clinical Medicine, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2Department of Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 3Division of Hematology, Department of Internal Medicine, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 4Center for Cell Therapy, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening disorder caused by severe ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13) deficiency (activity <10%). Urgent intervention based on the timely evaluation of ADAMTS13 level is crucial to guide optimal therapy. The recently developed PLASMIC score based on seven items allows the rapid identification of patients at high risk for TTP due to severe ADAMTS13 deficiency. This retrospective study included 31 hospitalized patients with suspicious thrombotic microangiopathy in National Cheng Kung University Hospital from December 2016 to July 2021. Data on ADAMTS13 activity and medical and laboratory information were retrieved from medical records. The PLASMIC score could be calculated in 24 of the 31 patients with available data, and the final cohort was stratified according to the 7-point PLASMIC score. All patients with high PLASMIC score (6–7) exhibited severe ADAMTS13 deficiency (activity ≤10%). One patient with a brain tumor and a PLASMIC score of 6 did not have severe ADAMTS13 activity of ≤10%. The patients in the intermediate- and low risk groups (PLASMIC scores of 5 and 0–4, respectively) exhibited ADAMTS13 activities of above 10%. Given the role of prompt diagnosis in the timely delivery of appropriate therapy, these findings confirm and strengthen the predictive value of the PLASMIC score in patients at high risk for TTP due to severe ADAMTS13 deficiency.
Introduction
Thrombotic thrombocytopenic purpura (TTP), first described by Moschcowitz in 1924 (1), is a life-threatening disease with a mortality rate of 10–20% despite appropriate therapeutic management (2). TTP is a rare form of thrombotic microangiopathy (TMA) characterized by microangiopathic hemolytic anemia (MAHA) accompanied with severe thrombocytopenia and organ ischemia secondary to disseminated microvascular platelet-rich thrombi. For a particular underlying cause, the clinical features observed in patients with TMA are neither sensitive nor specific. Therefore, rigorously derived and easily deployable clinical diagnostic tools that can identify individuals with TTP are critical.
In 1998, deficiency of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13), a von Willebrand factor-cleaving protease, has been recognized as the cause of TTP (3–5). Since then, several studies in patients with TMA have demonstrated that an ADAMTS13 activity below 10% is a specific feature of TTP. The diagnosis of TTP requires prompt attention given that it is a fatal condition that requires urgent plasmapheresis. However, testing for ADAMTS13 activity is not widely available and has long turnaround times.
In the last two decades, major advances have facilitated our understanding of TTP and ADAMTS13. Several clinical scoring systems have been developed for the rapid identification of patients who are most likely to have severe ADAMTS13 deficiency. These diagnostic scores have been evaluated for their ability to improve diagnostic accuracy and to guide early treatment in patients with TTP (6–9). In 2010, Bentley et al. published the first clinical diagnostic tool for TTP. Total of five parameters (platelet count, D-dimer, reticulocytes, creatinine, and indirect bilirubin) were found to be predictive of severe ADAMTS13 deficiency (10). The French score with three-components, platelet count, creatinine and antinuclear antibody, described by Coppo et al. in 2010, is an alternative scoring system used to identify TTP (11). Encouragingly, the PLASMIC score, which was derived based on the data of 214 patients in the multi-institutional Harvard TMA Research Collaborative registry, was able to predict severe ADAMTS13 deficiency. The PLASMIC score is used to stratify patients with TMA according to their risk of severe ADAMTS13 deficiency based on the following seven items: platelet count <30 × 109/L, hemolysis variable (elevated reticulocyte count, undetectable haptoglobin, or indirect bilirubin >2.0 mg/dL), no active cancer, no history of solid organ or stem cell transplant, mean corpuscular volume (MCV) <90 fL, international normalized ratio (INR) <1.5, and creatinine <2.0 mg/dL (7, 12, 13).
Therapeutic plasmapheresis (TPE) remains the cornerstone of TTP management (14, 15). Plasmapheresis, which usually starts as a 1.5-fold plasma volume exchange followed by a 1.0-fold plasma volume exchange thereafter, should be commenced immediately in patients undergoing diagnostic workup for suspicious TTP. Plasmapheresis is performed daily until the resolution of clinical manifestations related to organ involvement, stable recovery of platelet counts, and cessation of hemolysis (16).
Thrombotic thrombocytopenic purpura is a medical emergency, and its prompt recognition is imperative because of the high mortality rates in untreated or mismanaged patients. Disparities in early diagnosis and timely treatment remain a clinical unmet need.
Materials and Methods
Study Cohort
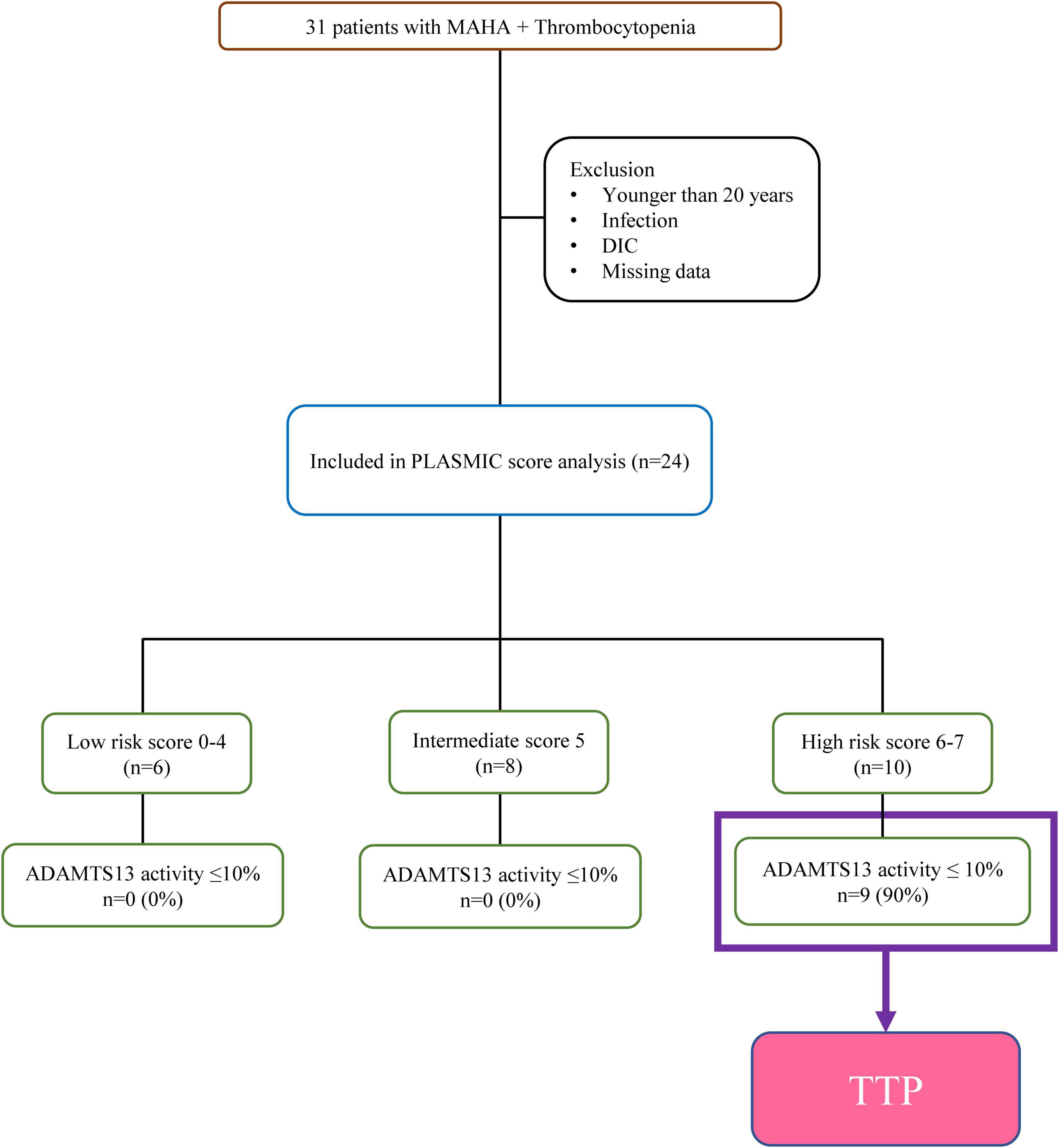
This retrospective cohort included adult patients aged ≥20 years who were evaluated for TMA at National Cheng Kung University Hospital in Taiwan between December 2016 and July 2021. The diagnosis of TMA was based on the presence of thrombocytopenia (platelet count <150 × 109/L) and MAHA (hemoglobin <10 g/dL in the presence of schistocytes). Patients with documented infection or disseminated intravascular coagulation were excluded. Patients with no laboratory data required before plasmapheresis and those with missing data were also excluded (Figure 1).
Patients were categorized as those with TTP and those with other forms of TMA. The diagnosis of TTP was based on a documented ADAMTS13 activity of ≤10%. All patients who did not meet the diagnostic criteria for TTP were classified as those with other forms of TMA, including atypical hemolytic uremic syndrome, systemic lupus erythematosus (SLE) associated TMA, drug-induced TMA, transplant-associated TMA, malignant hypertension-associated TMA, malignancy-associated TMA, and unexplained TMA. Relapsed TTP that was defined as disease recurrence after ≥30 days since TPE discontinuation (17). In patients who were refractory, it was defined as a failure of platelet response after 4–7 days of TPE or a clinical deterioration in a patient receiving standard therapy (18).
The ADAMTS13 activity was performed using a chromogenic enzyme-linked immunosorbent assay (TECHNOZYM® ADAMTS-13 activity ELISA; Technoclone). In the study institution, a hematology consult was required in cases where TMA was considered and ADAMTS13 activity could be determined only in patients with suspicious TMA.
Data Collection and Risk Stratification
The clinical and laboratory information were retrieved from the medical records. Data included age, sex, initial presentation, comorbidities, and treatment course. The study patients were stratified according to the 7-point PLASMIC score, which included platelet count <30 × 109/L, hemolysis variable (elevated reticulocyte count, undetectable haptoglobin, or indirect bilirubin >2.0 mg/dL), no active cancer, no history of solid organ or stem cell transplant, MCV <90 fL, INR <1.5, and creatinine <2.0 mg/dL. The presence of each item is worth 1 point, with a maximum PLASMIC score of 7. The PLASMIC scores of 0–4, 5, and 6–7 indicate low, intermediate, and high risk for severe ADAMTS13 deficiency, respectively (7). The level of agreement between the PLASMIC score and ADAMTS13 activity was evaluated. To verify the predictive value of severe acquired ADAMTS13 deficiency at the time of diagnosis, the French score, which involves three items (platelet count ≤30 × 109/L, serum creatinine ≤2.26 mg/dL, and antinuclear antibody positivity), was also calculated. The presence of each item in the French score is worth 1 point, and French scores of 0, 1, and 2–3 indicate low, intermediate, and high risk of severe ADAMTS13 deficiency, respectively (11). The study was approved by the Institutional Review Board at the National Cheng Kung University Hospital (IRB no., B-ER-110-256).
Statistical Analysis
GraphPad Prism statistical software (version 9; GraphPad, San Diego, CA, United States) was used for all statistical analyses. Continuous data were presented as means ± SD or medians (interquartile range) depending on the distribution. Categorical data were presented as numbers and percentages. Comparisons of normally and abnormally distributed continuous variables were performed using Student’s t and the Mann–Whitney U tests, respectively.
Results
During the study period, 24 of the 31 consecutive patients who were evaluated fulfilled the criteria for TMA (Figure 1). The ADAMTS13 activity levels were ≤10 and >10% in 9 and 15 patients with TMA, respectively. The PLASMIC score could be determined in all 24 patients (100%) who had data available for all seven items, and nine of the 24 patients with TMA were diagnosed with TTP according to the ADAMTS13 activity results.
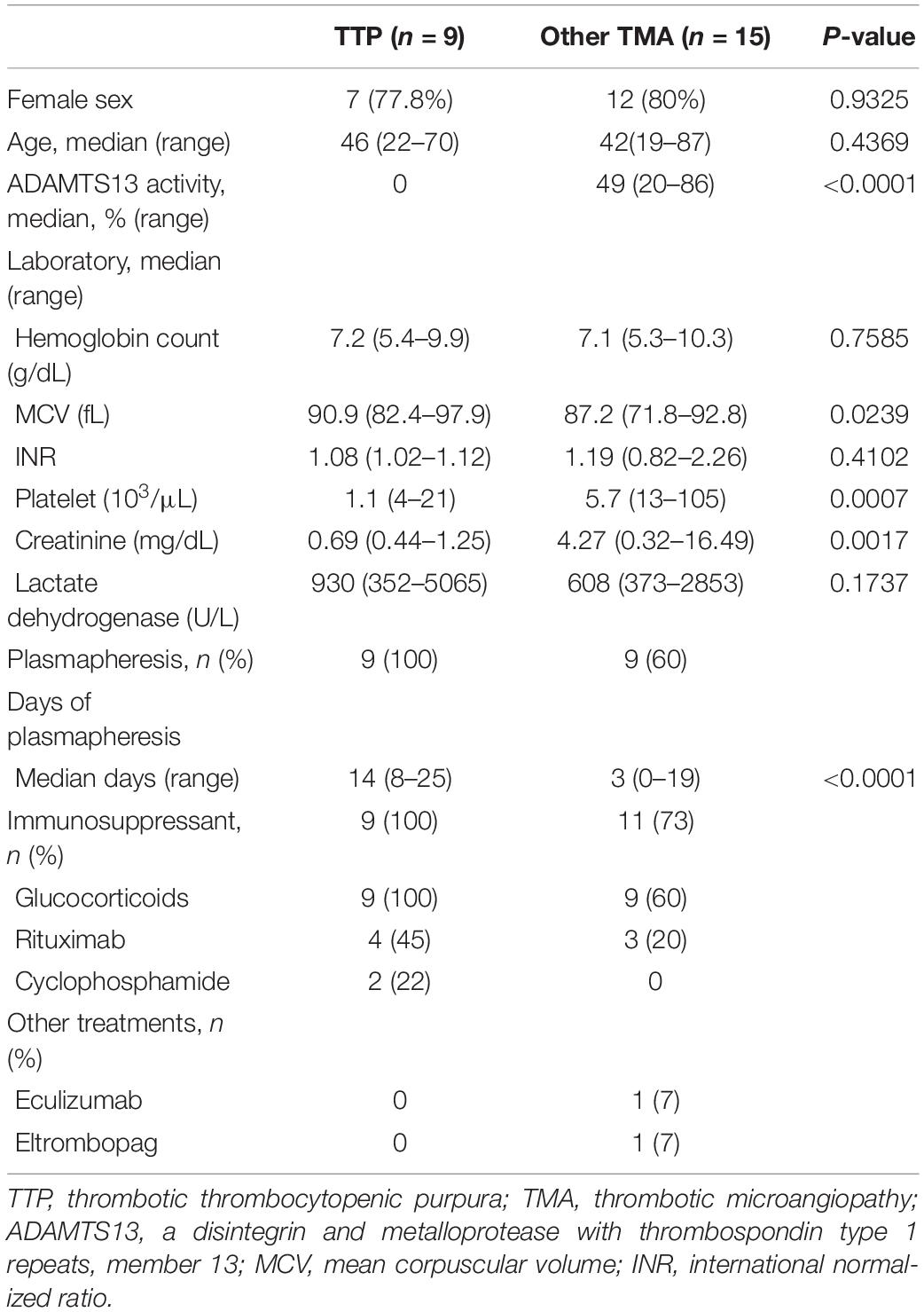
Table 1 summarizes the clinical and laboratory characteristics and the treatment course of the patients with TTP and those with other forms of TMA (non-TTP TMA). Briefly, sex (P = 0.9325), age (P = 0.4369), hemoglobin (P = 0.7585), INR (P = 0.4102), and lactate dehydrogenase (P = 0.1737) were not significantly different between the patients with TTP and those with other TMA. Conversely, the patients with TTP had significantly higher MCV (P = 0.0239), lower platelet count (P = 0.0007), and lower creatinine (P = 0.0017) compared with other TMA. Because of the severity of symptoms and the need for plasmapheresis, all patients with TTP underwent more cycles of plasmapheresis than those with other TMA (median, 14 versus 3 days; P < 0.0001) (Table 1).
The PLASMIC score was used to stratify the entire study cohort (n = 24) into three risk categories (Table 2). The diagnosis of TTP based on an ADAMTS13 activity of ≤10% was confirmed in nine of the ten patients stratified into the high risk group (PLASMIC score, 6 or 7). The positive predictive value of the PLASMIC score was 90% (95% confidence interval, 57.53–98.35%). The intermediate risk group (PLASMIC score, 5) included one patient with atypical hemolytic uremic syndrome, four patients with SLE-associated TMA, one patient with malignant hypertension, and two patients with unexplained TMA. The low risk group (PLASMIC score, 0–4) included one patient with SLE-associated TMA, two patients with drug-induced TMA, and two patients with transplant-associated TMA. The negative predictive value of the PLASMIC score was 100% (Supplementary Table 1).
In the present study cohort, the positive predictive value of the French score, a simpler ADAMTS13 deficiency risk prediction tool, was 64.3%, which was lower than that of the PLASMIC score (Supplementary Table 2).
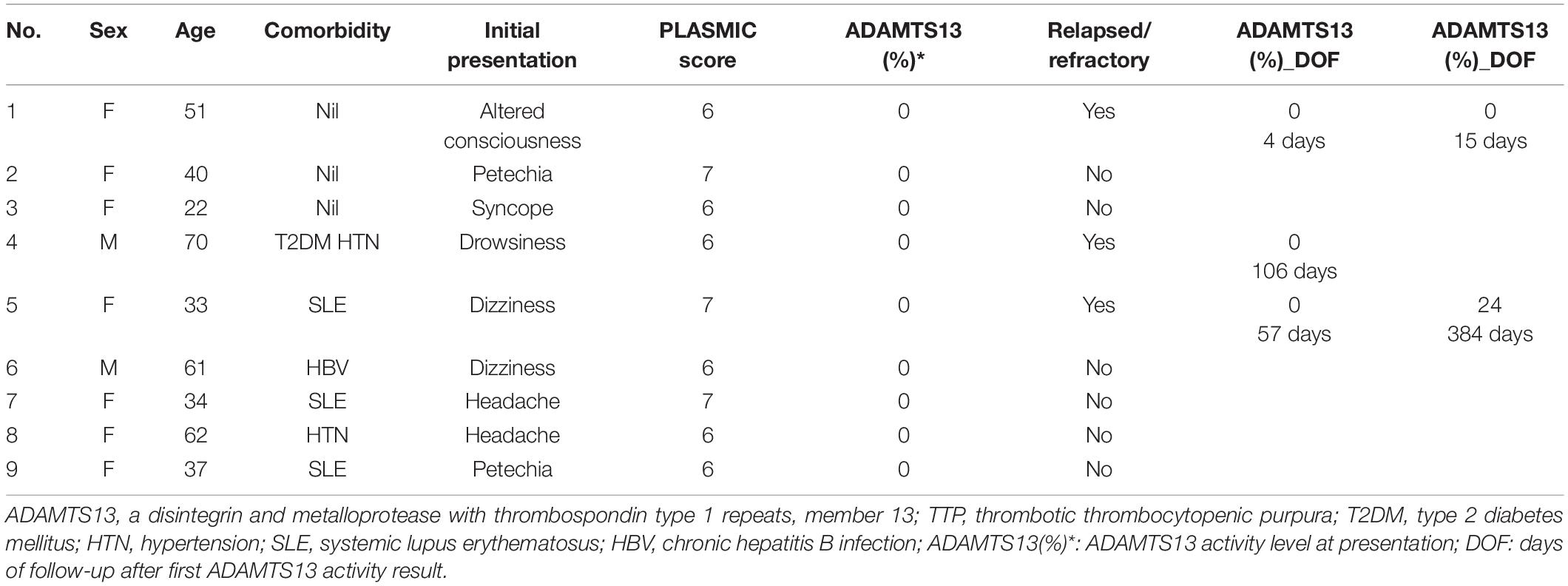
The median follow-up period was 18.5 months (range, 1–44 months). There was no mortality during the acute phase after TMA presentation in the TTP group. Nevertheless, three patients (patient nos. 1, 4, and 5) developed relapsed or refractory TTP (Table 3). These patients initially presented with neurological deficits, and patient no. 4 recovered after another cycle of consecutive plasmapheresis. Patient nos. 1 and 5 had a refractory disease and received anti-CD20 monoclonal antibody (rituximab) and other immunosuppressants in addition to the therapeutic plasma exchange.
In the high risk group, one of the 10 patients, a 38-year-old woman with ADAMTS13 activity level of >10%, initially presented with intermittent headache; the image showed brain tumor with hemorrhage. She exhibited an immediate drop in platelet count with MAHA, during the hospitalization. The clinical course was fulminant, and she eventually succumbed to septic shock.
Discussion
Timely identification of patients with TMA who require urgent treatment is a key unmet clinical need. The retrospective analysis of the current cohort of 24 patients based on risk stratification using the 7-point PLASMIC score confirmed the role of clinical assessment in the timely detection of severe ADAMTS13 deficiency in adult patients with TMA. In agreement with previous studies (8, 19, 20), none of the patients with low risk had severe ADAMTS13 deficiency. Additionally, 90% of the high risk patients with severe ADAMTS13 deficiency benefited from prompt therapeutic plasmapheresis. These patients received plasmapheresis and glucocorticoids treatment immediately once clinical judgment supports the diagnosis of TTP.
The French score, described by Coppo et al., is an alternative scoring system used to identify TTP (11). In the present study, the PLASMIC score with internal and external validation by the Harvard TMA registry, exhibiting superior performance in the prediction of ADAMTS13-deficient TTP compared with the French score (Supplementary Tables 1, 2). Data on antinuclear antibody levels were not collected at the initial presentation in most cases. Moreover, differences were observed in the TMA patient selection between the French and PLASMIC scores. The French Score relies on an increased level of clinical judgment from the provider to employ, whereas the PLASMIC score is intended for all-comers even when TTP does not have as high a degree of suspicion that the French score assumes at baseline. A recent brief report demonstrated a moderate correlation among three clinical TMA diagnostic scoring systems (PLASMIC, French, Bentley) and ADAMTS13 levels (21). Considering these instruments, two important caveats are identified: the exact definition of severe ADAMTS13 deficiency remains unclear, which may be assay dependent, and the cutoffs applied to generate these score systems varied across studies; no prospective research has been conducted on these prediction tools (22).
Acquired TTP, which is immunologically mediated, is associated with several autoimmune disorders such as SLE and Hashimoto’s thyroiditis. The diagnosis of TTP in the setting of SLE may be difficult because of overlapping clinical symptoms. Likewise, the laboratory criteria of MAHA, which include the presence of schistocytes in the peripheral blood smear, are extremely subjective and rely on experienced laboratorians and hematologists. In the present study, 3 of the 10 patients (30%) in the high risk group were definitively diagnosed with ADAMTS13-deficient TTP associated with SLE. Patients with SLE are at a higher risk for SLE-associated TTP-like MAHA. The underlying pathophysiology is frequently heterogeneous and can be secondary to antiphospholipid syndrome or vasculitis (23). Five of the patients with TMA in the intermediate- and low risk groups had concurrent SLE. In a study of 1,203 SLE cases in Korea, Kwok et al. reported that 2.2% of the patients presented with the clinical features of TTP at the time of diagnosis (24). In two other studies including 40 and 105 patients, concurrent SLE was present in 12 and 45% of the patients with TTP, respectively (25, 26). Moreover, a review of the literature encompassing the period from 1968 to 2002 reported that the mortality was higher among 56 patients with concurrent SLE and TTP compared with those with idiopathic TTP despite optimal treatment (27).
Relapsed TTP is defined as disease recurrence after ≥30 days since TPE discontinuation (17). Refractory TTP is defined as a failure of platelet response after 4–7 days of plasmapheresis or a clinical deterioration despite standard therapy (18). In patients with relapsed or refractory TTP despite treatment with ADAMTS13 inhibitors, patient reevaluation is important to identify other potential etiologies of MAHA and thrombocytopenia. In the present cohort, three of the nine patients with TTP (34%) experienced relapsed or refractory TTP and one of these patients received 100 mg rituximab once and exhibited a good response. Emerging evidence suggests that weekly pulse rituximab can achieve a good response in patients with relapsed or refractory TTP (28–30).
The study cohort utilized for the derivation of the PLASMIC score was from the Harvard TMA registry, representing mostly Western ethnic groups (7). In their study, Tiscia et al. demonstrated the good diagnostic performance of the PLASMIC score in a cohort from Southern Italy (19). Another study of a small Chinese cohort reported that a modified PLASMIC score, which included lactate dehydrogenase in addition to the original seven items of the PLASMIC scoring system, might be more suitable for identifying patients with ADAMTS13 deficiency (31). To the best of our knowledge, few studies with larger cohorts have validated the PLASMIC score as a new prediction tool and this is the first study to verify the ability of the PLASMIC score to predict TTP risk in an East Asian cohort.
The present study has some limitations that should be acknowledged. First, this was a retrospective study; however, this approach was unavoidable because of the rarity of TMA with severe ADAMTS13 deficiency. Second, the cohort size was small because of the low rate of TTP.
Albeit rare, acquired TTP is associated with high mortality because of an aggressive clinical course in the setting of delayed diagnosis without optimal treatment. The present study results support the utility of the PLASMIC score as a rapid, feasible, and reliable clinical assessment tool to predict severe ADAMTS13 deficiency in adult patients with TMA.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
C-HL designed and conducted the study, collected the data, performed the statistical analyses, and interpreted and wrote the manuscript. Y-CH conducted the study and collected the data. S-SL, Y-TH, and Y-PC conducted the study. T-YC designed the study, analyzed and interpreted the data, and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Wu-Chou Su (Chief of Department of Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University), Wei-Pang Chung and Yu-Min Yeh (Department of Oncology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University) for helpful advice and to thank medical technician Chia-Ling Wu in performing ADAMTS13 activity assay.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.893273/full#supplementary-material
References
1. Moschcowitz E. Hyaline thrombosis of the ternimal arterioles and capillaries: a hitherto undescribed disease. Proc NY Pathol Soc. (1924) 24:21–4.
2. Sadler JE. What’s new in the diagnosis and pathophysiology of thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. (2015) 2015:631–6. doi: 10.1182/asheducation-2015.1.631
3. Furlan M, Robles R, Galbusera M, Remuzzi G, Kyrle PA, Brenner B, et al. Von willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med. (1998) 339:1578–84. doi: 10.1056/NEJM199811263392202
4. Furlan M, Lammle B. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von willebrand factor-cleaving protease. Best Pract Res Clin Haematol. (2001) 14:437–54. doi: 10.1053/beha.2001.0142
5. Chapman K, Seldon M, Richards R. Thrombotic microangiopathies, thrombotic thrombocytopenic purpura, and adamts-13. Semin Thromb Hemost. (2012) 38:47–54. doi: 10.1055/s-0031-1300951
6. Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, Peyvandi F, et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. (2012) 158:323–35. doi: 10.1111/j.1365-2141.2012.09167.x
7. Bendapudi PK, Hurwitz S, Fry A, Marques MB, Waldo SW, Li A, et al. Derivation and external validation of the plasmic score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. (2017) 4:e157–64. doi: 10.1016/s2352-3026(17)30026-1
8. Li A, Khalighi PR, Wu Q, Garcia DA. External validation of the plasmic score: a clinical prediction tool for thrombotic thrombocytopenic purpura diagnosis and treatment. J Thromb Haemost. (2018) 16:164–9. doi: 10.1111/jth.13882
9. Bentley MJ, Wilson AR, Rodgers GM. Performance of a clinical prediction score for thrombotic thrombocytopenic purpura in an independent cohort. Vox Sang. (2013) 105:313–8. doi: 10.1111/vox.12050
10. Bentley MJ, Lehman CM, Blaylock RC, Wilson AR, Rodgers GM. The utility of patient characteristics in predicting severe ADAMTS13 deficiency and response to plasma exchange. Transfusion. (2010) 50:1654–64. doi: 10.1111/j.1537-2995.2010.02653.x
11. Coppo P, Schwarzinger M, Buffet M, Wynckel A, Clabault K, Presne C, et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One. (2010) 5:e10208. doi: 10.1371/journal.pone.0010208
12. Jamme M, Rondeau E. The plasmic score for thrombotic thrombocytopenic purpura. Lancet Haematol. (2017) 4:e148–9. doi: 10.1016/s2352-3026(17)30024-8
13. Oliveira DS, Lima TG, Benevides FLN, Barbosa SAT, Oliveira MA, Boris NP, et al. Plasmic score applicability for the diagnosis of thrombotic microangiopathy associated with ADAMTS13-acquired deficiency in a developing country. Hematol Transfus Cell Ther. (2019) 41:119–24. doi: 10.1016/j.htct.2018.10.002
14. Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. (2004) 103:4043–9. doi: 10.1182/blood-2003-11-4035
15. Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian apheresis study group. N Engl J Med. (1991) 325:393–7. doi: 10.1056/NEJM199108083250604
16. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. (2017) 129:2836–46. doi: 10.1182/blood-2016-10-709857
17. Zheng XL, Vesely SK, Cataland SR, Coppo P, Geldziler B, Iorio A, et al. Isth guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. (2020) 18:2496–502. doi: 10.1111/jth.15010
18. Sayani FA, Abrams CS. How I treat refractory thrombotic thrombocytopenic purpura. Blood. (2015) 125:3860–7. doi: 10.1182/blood-2014-11-551580
19. Tiscia GL, Ostuni A, Cascavilla N, Cappucci F, Scalzulli P, Battista C, et al. Validation of plasmic score and follow-up data in a cohort of patients with suspected microangiopathies from Southern Italy. J Thromb Thrombolysis. (2018) 46:174–9. doi: 10.1007/s11239-018-1674-6
20. Paydary K, Banwell E, Tong J, Chen Y, Cuker A. Diagnostic accuracy of the plasmic score in patients with suspected thrombotic thrombocytopenic purpura: a systematic review and meta-analysis. Transfusion. (2020) 60:2047–57. doi: 10.1111/trf.15954
21. Baysal M, Umit E, Kirkizlar HO, Demir AM. Comparison of clinical scoring systems in the management of patients with microangiopathic hemolytic anemia and thrombocytopenia. Turk J Haematol. (2021) 38:64–8. doi: 10.4274/tjh.galenos.2020.2020.0348
22. Bendapudi PK, Upadhyay V, Sun L, Marques MB, Makar RS. Clinical scoring systems in thrombotic microangiopathies. Semin Thromb Hemost. (2017) 43:540–8. doi: 10.1055/s-0037-1603100
23. Matsuyama T, Kuwana M, Matsumoto M, Isonishi A, Inokuma S, Fujimura Y. heterogeneous pathogenic processes of thrombotic microangiopathies in patients with connective tissue diseases. Thromb Haemost. (2009) 102:371–8. doi: 10.1160/TH08-12-0825
24. Kwok SK, Ju JH, Cho CS, Kim HY, Park SH. Thrombotic thrombocytopenic purpura in systemic lupus erythematosus: risk factors and clinical outcome: a single centre study. Lupus. (2009) 18:16–21. doi: 10.1177/0961203308094360
25. Musio F, Bohen EM, Yuan CM, Welch PG. Review of thrombotic thrombocytopenic purpura in the setting of systemic lupus erythematosus. Semin Arthritis Rheum. (1998) 28:1–19. doi: 10.1016/s0049-0172(98)80023-1
26. Jiang H, An X, Li Y, Sun Y, Shen G, Tu Y, et al. Clinical features and prognostic factors of thrombotic thrombocytopenic purpura associated with systemic lupus erythematosus: a literature review of 105 cases from 1999 to 2011. Clin Rheumatol. (2014) 33:419–27. doi: 10.1007/s10067-013-2312-5
27. Hamasaki K, Mimura T, Kanda H, Kubo K, Setoguchi K, Satoh T, et al. Systemic lupus erythematosus and thrombotic thrombocytopenic purpura: a case report and literature review. Clin Rheumatol. (2003) 22:355–8. doi: 10.1007/s10067-003-0742-1
28. Fakhouri F, Vernant JP, Veyradier A, Wolf M, Kaplanski G, Binaut R, et al. efficiency of curative and prophylactic treatment with rituximab in ADAMTS13-deficient thrombotic thrombocytopenic purpura: a study of 11 cases. Blood. (2005) 106:1932–7. doi: 10.1182/blood-2005-03-0848
29. Dane K, Chaturvedi S. Beyond plasma exchange: novel therapies for thrombotic thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. (2018) 2018:539–47. doi: 10.1182/asheducation-2018.1.539
30. Zwicker JI, Muia J, Dolatshahi L, Westfield LA, Nieters P, Rodrigues A, et al. Adjuvant low-dose rituximab and plasma exchange for acquired TTP. Blood. (2019) 134:1106–9. doi: 10.1182/blood.2019000795
31. Zhao N, Zhou L, Hu X, Sun G, Chen C, Fan X, et al. A modified plasmic score including the lactate dehydrogenase/the upper limit of normal ratio more accurately identifies Chinese thrombotic thrombocytopenic purpura patients than the original plasmic score. J Clin Apher. (2020) 35:79–85. doi: 10.1002/jca.21760
Keywords: thrombotic thrombocytopenic purpura, thrombotic microangiopathy, ADAMTS13 (a disintegrin-like and metalloprotease with thrombospondin type 1 repeats), PLASMIC score, relapsed, refractory, systemic lupus erythematosus
Citation: Lee C-H, Huang Y-C, Li S-S, Hsu Y-T, Chen Y-P and Chen T-Y (2022) Application of PLASMIC Score in Risk Prediction of Thrombotic Thrombocytopenic Purpura: Real-World Experience From a Tertiary Medical Center in Taiwan. Front. Med. 9:893273. doi: 10.3389/fmed.2022.893273
Received: 10 March 2022; Accepted: 07 April 2022;
Published: 09 May 2022.
Edited by:
Eleni Gavriilaki, G. Papanikolaou General Hospital, GreeceReviewed by:
Senthil Sukumar, The Ohio State University, United StatesInés Gómez, La Fe Hospital, Spain
Copyright © 2022 Lee, Huang, Li, Hsu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tsai-Yun Chen, dGVyZXNhQG1haWwubmNrdS5lZHUudHc=
 Chun-Hui Lee
Chun-Hui Lee Yi-Ching Huang
Yi-Ching Huang Sin-Syue Li1,3
Sin-Syue Li1,3 Ya-Ting Hsu
Ya-Ting Hsu Ya-Ping Chen
Ya-Ping Chen