
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 27 April 2022
Sec. Infectious Diseases – Surveillance, Prevention and Treatment
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.892962
This article is part of the Research Topic Learning to Live With a Persistent Pandemic: Challenges of Balancing Resources between COVID-19 and Non-COVID-19 Patients In a New Era of Healthcare View all 9 articles
 Rebecca De Lorenzo1,2†
Rebecca De Lorenzo1,2† Marco Montagna1,2†
Marco Montagna1,2† Eleonora Bossi3
Eleonora Bossi3 Giordano Vitali2
Giordano Vitali2 Alba Taino4
Alba Taino4 Marta Cilla2
Marta Cilla2 Giulia Pata1,2
Giulia Pata1,2 Ludmilla Lazorova2
Ludmilla Lazorova2 Riccardo Pesenti1
Riccardo Pesenti1 Chiara Pomaranzi1,2
Chiara Pomaranzi1,2 Cecilia Bussolari1,2
Cecilia Bussolari1,2 Sabina Martinenghi2
Sabina Martinenghi2 Nicoletta Bordonaro2
Nicoletta Bordonaro2 Davide Di Napoli3
Davide Di Napoli3 Giuliano Rizzardini5
Giuliano Rizzardini5 Chiara Cogliati4
Chiara Cogliati4 Nuccia Morici6
Nuccia Morici6 Patrizia Rovere-Querini1,2*
Patrizia Rovere-Querini1,2*
Objective: To report a preliminary experience of outpatient management of patients with Coronavirus disease 2019 (COVID-19) through an innovative approach of healthcare delivery.
Patients and Methods: Patients evaluated at the Mild-to-Moderate COVID-19 Outpatient clinics (MMCOs) of San Raffaele University Hospital and Luigi Sacco University Hospital in Milan, Italy, from 1 October 2020 to 31 October 2021 were included. Patients were referred by general practitioners (GPs), Emergency Department (ED) physicians or hospital specialists (HS) in case of moderate COVID-19. A classification and regression tree (CART) model predicting ED referral by MMCO physicians was developed to aid GPs identify those deserving immediate ED admission. Cost-effectiveness analysis was also performed.
Results: A total of 660 patients were included. The majority (70%) was referred by GPs, 21% by the ED and 9% by HS. Patients referred by GPs had more severe disease as assessed by peripheral oxygen saturation (SpO2), ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2), C-reactive protein (CRP) levels and interstitial involvement at lung ultrasound. Among them, 18% were addressed to the ED following MMCO assessment. CART analysis identified three independent predictors, namely home-measured SpO2, age and body mass index (BMI), that robustly divide patients into risk groups of COVID-19 severity. Home-measured SpO2 < 95% and BMI ≥ 33 Kg/m2 defined the high-risk group. The model yielded an accuracy (95% CI) of 83 (77–88)%. Outpatient management of COVID-19 patients allowed the national healthcare system to spare 1,490,422.05 € when compared with inpatient care.
Conclusion: Mild-to-moderate COVID-19 outpatient clinics were effective and sustainable in managing COVID-19 patients and allowed to alleviate pressure on EDs and hospital wards, favoring effort redirection toward non-COVID-19 patients.
Coronavirus disease 2019 (COVID-19) pandemic has posed significant challenges on healthcare systems worldwide due to an overwhelming surge of patients simultaneously seeking medical care (1, 2). Emergency departments (ED) probably suffered the most, being the bottleneck of patients with acute disease, often regardless of symptom severity. In fact, a proportion of patients presenting to the ED had mild to moderate clinical features not requiring urgent care or hospital admission (3, 4).
While patients with mild disease and no risk factors for progression may benefit from medical assistance by general practitioners (GPs), those with moderate COVID-19 or harboring risk factors for adverse outcomes reside in a gray area between in-hospital and home management (5). Within the latter patient category, GPs may not have the tools to discriminate nor handle patients deserving more attentive monitoring. On the other hand, unfiltered hospital referral of these patients may cause unjustified ED overcrowding and saturation of hospital beds. Accurate patient evaluation in a hospital-based outpatient setting by expert physicians may fill this gap, allowing for timely risk classification and informed management decision-making.
On the heels of the first pandemic wave and with the belief that some measure had to be taken to avoid system collapse, health policymakers of Lombardy region in Italy at the beginning of the second wave designed an integrated approach of healthcare delivery, called “Hot Spot” or “Mild-to-moderate COVID-19 outpatient clinic” (MMCO), based on the strict, bidirectional collaboration with GPs and the ED (5). One year after the introduction of this novel service, here we describe our preliminary experience of patient management at two MMCOs of the metropolitan city of Milan, specifically those of San Raffaele University Hospital and Luigi Sacco University Hospital. Moreover, we provide an evidence-based tool for patient classification into risk groups by the GP beforehand, to identify patients deserving early ED referral with the aims of optimizing patient management and spare resources.
Mild-to-moderate COVID-19 outpatient clinic organization and the process of patient flow from referral to discharge is described in Figure 1. MMCOs are located within hospitals, in a strategic location that is both easy-to-reach by patients and in direct connection with the ED. This innovative healthcare service is addressed to two different categories of patients with nasopharyngeal swab-confirmed infection: (i) patients with moderate COVID-19 and (ii) patients at increased risk of adverse outcome due to pre-existing risk factors independent of COVID-19 severity. Both categories may need active surveillance and management by physicians with an established expertise in treating COVID-19 and its complications. Patients can be referred to the MMCO by GPs, ED physicians or hospital specialists (HS) through direct telephone call to the MMCO physician at a dedicated mobile number, which is active 12 h per day, 7 days per week.
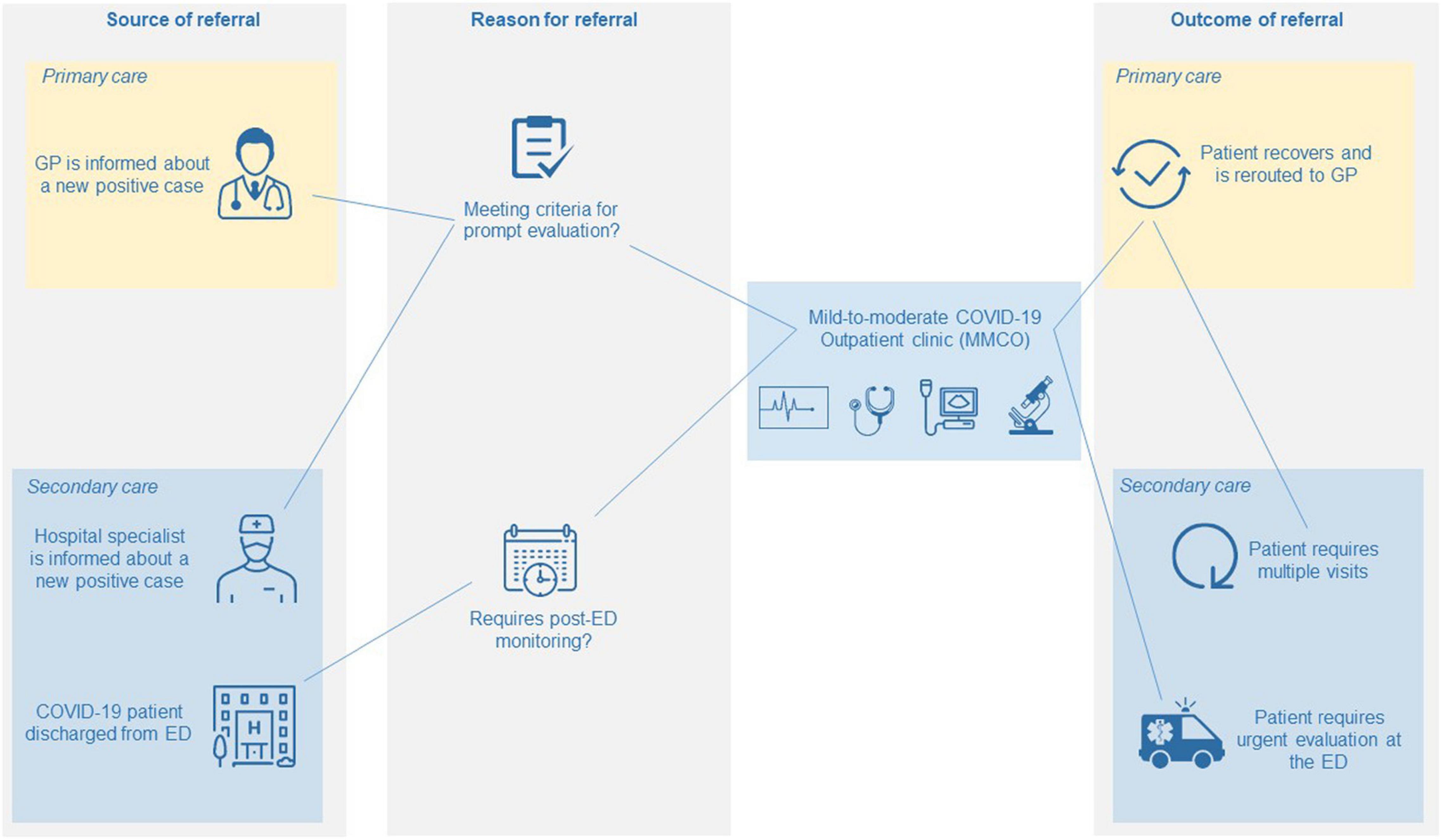
Figure 1. Care pathway of patients at MMCOs from referral to discharge. GP, general practitioner; ED, emergency department.
Mild-to-moderate COVID-19 outpatient clinic physicians are internal medicine doctors. Criteria for referral to MMCOs of patients with moderate COVID-19 by GPs are derived from official regional regulations (5, 6). Prior to patient evaluation at the MMCO, the GP provides the MMCO physician with a comprehensive report, in the form of a standardized questionnaire (Supplementary Figure 1), on the patient’s past medical history, COVID-19-related symptoms, time from symptom onset, peripheral oxygen saturation (SpO2), body mass index (BMI), chronic therapies and COVID-19-specific treatments. Criteria for referral of include: (i) age ≥65 years in the presence of body temperature ≥38°C and at least two comorbidities among obesity, active cancer, chronic kidney disease (CKD), chronic respiratory disease, immunosuppression, ischemic heart disease (IHD), diabetes mellitus (DM), coagulopathy, history of immunosuppression or organ transplant, HIV infection and cerebrovascular disease (CVD); (ii) body temperature ≥38°C for longer than 72 h; (iii) SpO2 between 90 and 94% (or between 88 and 90% in case of history of chronic obstructive pulmonary disease).
Emergency department physicians may refer patients who may benefit from prolonged monitoring in a hospital-based setting following clinical stabilization, with the dual purpose of relieving the ED from excessive burden and limiting hospitalization rates, while feeling at ease discharging patients with a non-negligible risk of disease evolution.
Hospital specialists, usually hematologists or oncologists, may especially benefit from extending referral to asymptomatic or mild COVID-19 patients in case of pre-existing risk factors for poor clinical outcome (i.e., cancer or other frailty conditions).
Following the first evaluation, patients may either be discharged from the MMCO and redirected to GP care, or be addressed to the ED in case of severe COVID-19 requiring more intense care, or be scheduled for further visits for a prolonged monitoring at the MMCO. Specifically, active surveillance at the MMCO consists of serial visits, at varying time intervals depending on disease severity, until disease stabilization or complete recovery.
The first visit at MMCO comprises a comprehensive physical examination with vital sign assessment (SpO2, heart and respiratory rates, blood pressure, body temperature, blood glucose) and measurement of anthropometric parameters including weight, height and waist circumference. Data about past and COVID-19-related medical history are accurately collected, integrating patient interview with the GP’s questionnaire and available medical records. Lung assessment relies on lung ultrasound (LUS) imaging. In addition to being easy and rapid to perform, LUS has higher sensitivity and specificity for lung parenchymal abnormalities than chest X-rays (7–9). Moreover, it can be performed at bedside and bears no radiological hazard (8). Through LUS, signs of interstitial lung disease including white lung pattern suggestive of more severe involvement and parenchymal consolidations may be detected. Also, LUS allows to calculate the Lung UltraSound Score (LUSS), a semi-quantitative score of lung aeration loss (10, 11), which has been associated with disease severity and mortality in COVID-19 (12, 13). Arterial blood gas analysis parallels LUS in the evaluation of pulmonary dysfunction, and the ratio of arterial oxygen partial pressure (PaO2, in mmHg) to fractional inspired oxygen (FiO2, in mmHg), expressed as a fraction, is used as a quantitative marker of respiratory insufficiency. Electrocardiography at rest and blood exams including complete blood count, C reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, ferritin and creatinine are also performed.
At the following visits, the abovementioned procedures may be repeated in varying combinations to allow for an individualized and attentive disease monitoring.
All patients aged 18 years or older, evaluated at the MMCOs of San Raffaele University Hospital and Luigi Sacco University Hospital in Milan, Italy, from 1 October 2020 to 31 October 2021 were included in the present study.
Data were retrospectively collected as part of the retroPAUCI protocol (N. 140/INT/2021), approved by the Hospital Ethics Committees, in conformity to the declaration of Helsinki. Written informed consent was obtained by all patients.
Age, sex, past medical history (i.e., obesity, active cancer, CKD, chronic respiratory disease, immunosuppression, IHD, DM, coagulopathy, history of immunosuppression or organ transplant, HIV infection, CVD), BMI, COVID-19-related history including time of symptom onset, COVID-19-related symptoms (i.e., dyspnea, cough, taste and smell disturbances, pharyngodynia, myalgias, arthralgias, asthenia, diarrhea, nausea or vomiting, headache, syncope), home-measured SpO2 at time of MMCO referral and presence of fever for ≥72 h were collected for all patients. Recorded data on patient evaluation during the first MMCO visit comprised blood pressure, heart rate, SpO2, respiratory rate (RR), PaO2/FiO2 at arterial blood gas analysis, blood exams (i.e., absolute neutrophil and lymphocyte counts, neutrophil to lymphocyte ratio [NLR], LDH, CRP, creatinine, ferritin and D-dimer), as well as LUSS, the presence of white lung pattern or parenchymal consolidations at LUS. Moreover, rates of ED referral following MMCO evaluation and of hospitalization after ED admission, observation time (i.e., time interval from the first MMCO visit to MMCO discharge), and the number of visits at MMCO prior to discharge were also registered.
Prior to analysis, data were cross-checked with medical charts and verified by data managers and clinicians for accuracy.
To investigate which patients are at increased risk of adverse outcome, ED referral following MMCO evaluation was used as primary outcome.
We investigated whether managing patients at MMCO was economically convenient for the hospital compared with inpatient care. We considered that patients who received ≥2 MMCO visits would have otherwise been hospitalized due to the need of active surveillance. Therefore, for the purpose of the analysis, the number of patients who performed ≥2 MMCO visits was used to define the number of spared hospitalizations. The cost of one hospital stay for COVID-19 was computed as the weighted mean of the hospitalization costs for all COVID-19 patients hospitalized during the same time interval (i.e., October 2020–October 2021), not transferred to the intensive care unit. Specifically, the cost of each hospitalization was estimated based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes for diagnoses and procedures linked to COVID-19, according to the updated guidelines of Regional Health Authorities (14, 15). On the other hand, the overall cost for 1-year activity at the MMCO was calculated taking into account: (i) cost of the personnel (two medical doctors, one nurse, one clerk), (ii) cost of consumables (personal protective equipment, sanitary ware, stationery), (iii) cost of general utilities, building and instrument (i.e., electrocardiography, ultrasound and arterial blood gas analysis machinery) maintenance, etc., (iv) indirect hospital-related costs. Supplementary Table 1 describes in detail how the total cost of activity at MMCO during the study time was calculated.
Descriptive statistics were performed for all variables. Dichotomous variables were expressed as absolute counts (percentage), and continuous variables as medians (interquartile range, IQR) unless differently specified. χ2 test and Kruskal–Wallis test were used to perform group comparisons for categorical and continuous variables, respectively.
To identify early predictors of adverse outcome (i.e., ED referral following MMCO evaluation) and provide GPs with a tool for early risk classification, we employed a classification and regression tree (CART) algorithm within the cohort of patients referred to the MMCO by GPs. CART analysis relies on recursive partitioning to sequentially split a cluster of patients into homogeneous sub-groups based on independent variables, determining the hierarchy of prognostic factors and associated cut-points that best subdivides the initial population to obtain faithful risk groups (16, 17). Demographical data, comorbidities, BMI, and parameters that GPs can easily obtain through patient interview, including home-measured SpO2, the presence of fever for ≥72 h, COVID-19-related symptoms and time from symptom onset were included as predictors in the CART. The results of the analysis were graphically represented. The area under the receiver operating characteristic (ROC) curve (AUC) was used as a quality metric of the CART.
Missing data was not imputed.
All statistical analyses were performed using R statistical package (version 4.0.0, R Foundation for Statistical Computing, Vienna, Austria), with a two-sided significance level set at p < 0.05.
From 1 October 2020 to 31 October 2021, a total of 660 patients were evaluated at the MMCOs of San Raffaele University Hospital and Luigi Sacco University Hospital. The total number of visits was 1101 and their distribution within the study time interval is depicted in Supplementary Figure 2. Baseline patient characteristics and indicators of COVID-19 severity at MMCO evaluation are reported in Tables 1, 2, respectively. The source of MMCO referral was known for 572 patients. Of these, 400 (70%) were referred by GPs, 119 (21%) by the ED and 53 (9%) by HS.
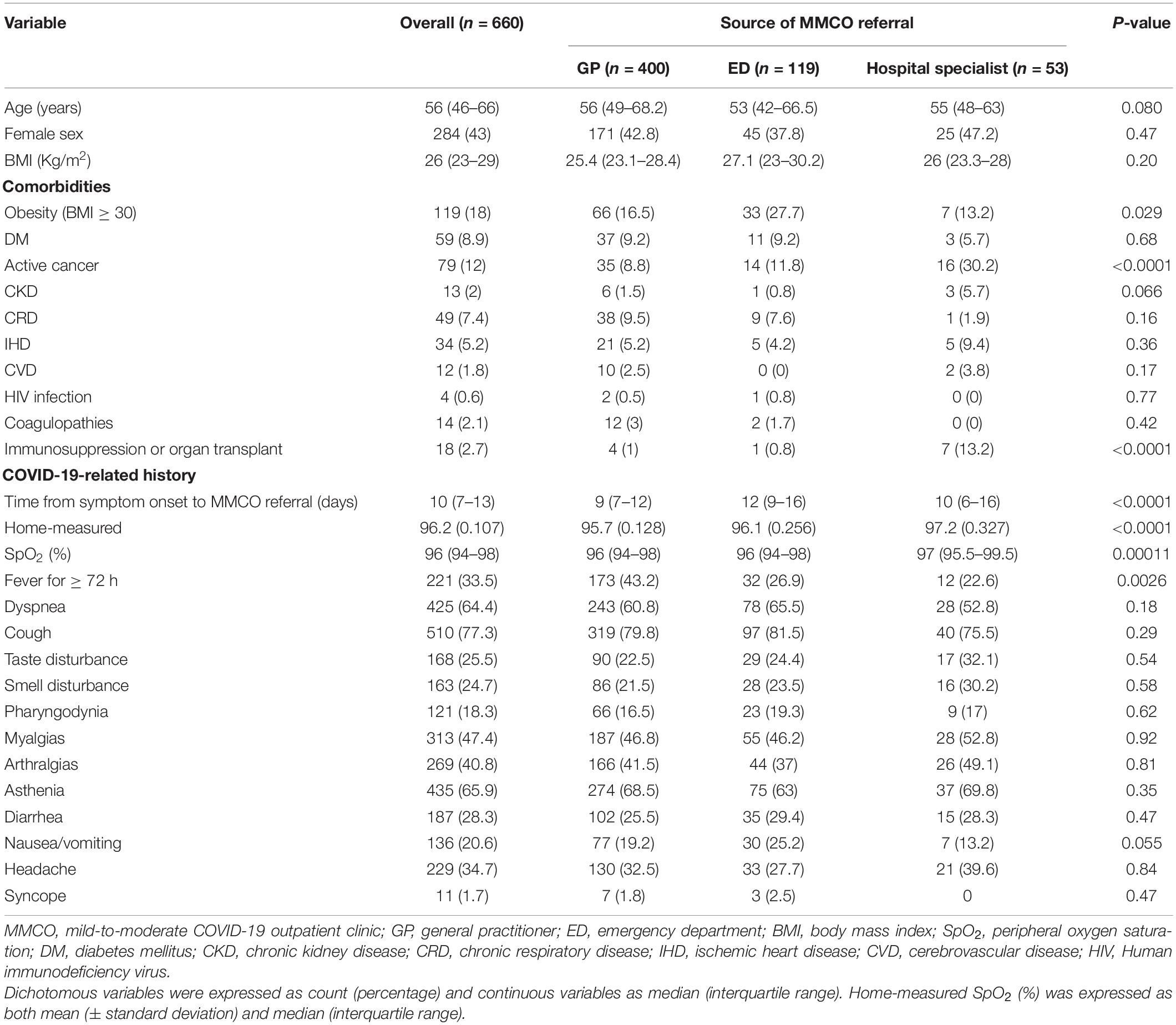
Table 1. General characteristics of COVID-19 patients evaluated at the mild-to-moderate COVID-19 outpatient clinic.
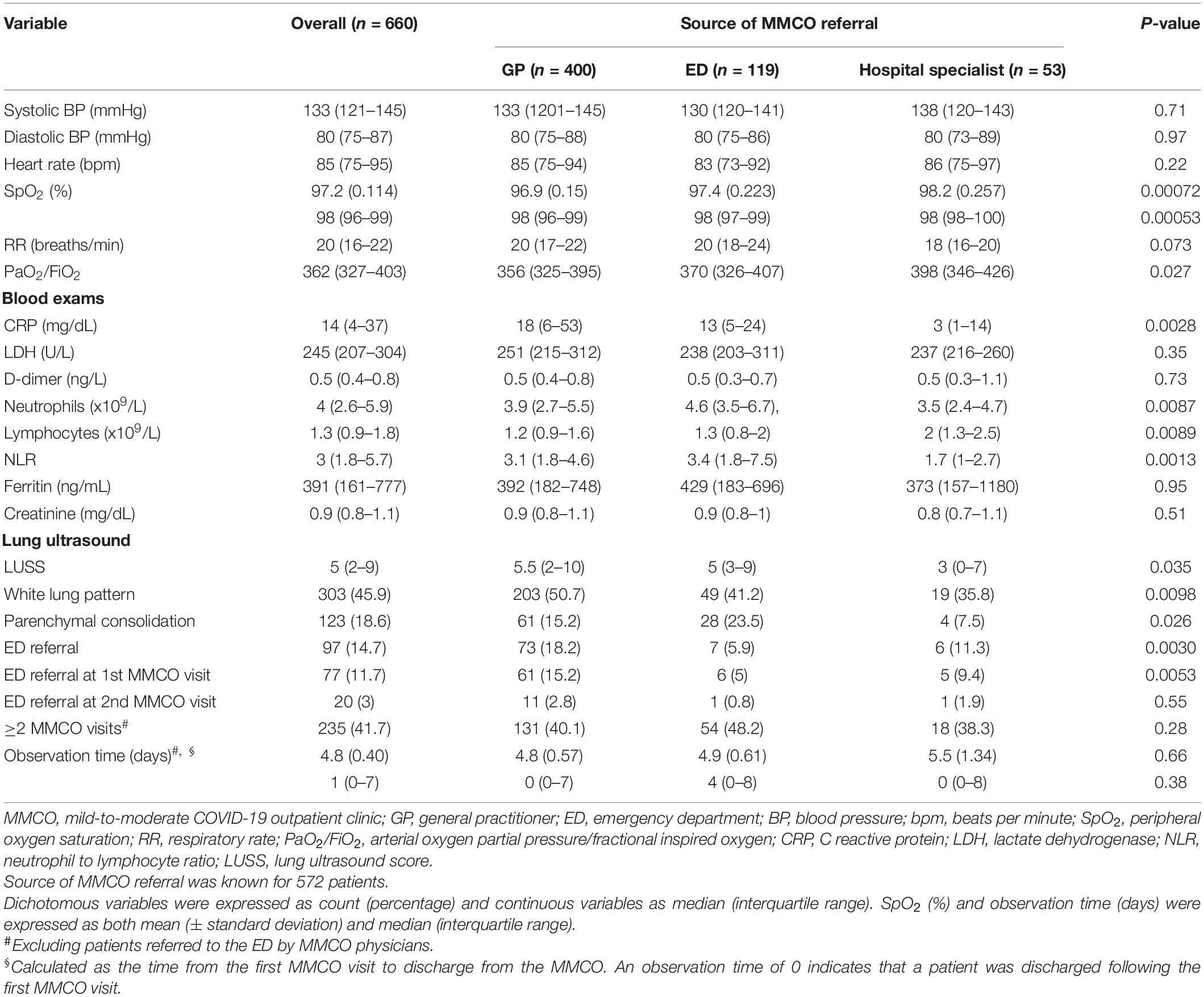
Table 2. Health status and indicators of disease severity in COVID-19 patients evaluated at the mild-to-moderate COVID-19 outpatient clinic.
Most patients were male and median age was 56 (46–66) years. Median BMI was in the overweight range, 18% of patients being obese. Obesity was more common in patients discharged from the ED (27.7 vs. 16.5% in patients referred by GPs and 13.2% in those referred by HS, p 0.029). Except for active cancer and immunosuppression or history of organ transplant, which were, as expected, significantly more common in patients referred by HS (both p < 0.0001), no difference among the three groups was recorded in terms of other comorbidities (Table 1).
With regard to COVID-19-related history, time from symptom onset to MMCO was shorter in patients referred by GPs (9 [7–12] vs. 12 [9–16] in patients discharged from the ED and 10 [6–16] in those referred by HS, p < 0.0001). No difference was observed in terms of COVID-19-related symptoms, the most common complaint being cough in all groups. Home-measured SpO2 was significantly lower in these patients (mean [standard deviation, SD] 95.7 [0.128]) compared to patients referred by the ED (96.1 [0.256]) or by HS (97.2 [0.327], p < 0.0001). Patients referred by GPs also more frequently reported fever for ≥72 h (43.2 vs. 26.9% in patients referred by the ED and 22.6% in those referred by HS, p 0.0026, Table 1).
Overall, patients referred by GPs had more severe COVID-19 clinical features at MMCO evaluation than the other two groups. Specifically, both SpO2 and PaO2/FiO2 were significantly reduced in these patients (both p < 0.05), while those referred by HS registered the highest values in line with their expected milder clinical features. Similarly, CRP levels were significantly increased in patients referred by GPs (18 [6–53] vs. 13 [5–24] in patients discharged from the ED and 3 [1–14] in those referred by HS, p 0.0028). At LUS, more patients in the group referred by GPs had white lung pattern (50.7 vs. 41.2% in patients discharged from the ED and 35.8% in those referred by HS, p0.0098) and median LUSS was higher in this group (5.5 [2–10], p 0.035). Parenchymal consolidation was instead a more common finding in patients discharged from the ED (23.5 vs. 15.2% in patients referred by GPs and 7.5% in those referred by HS, p 0.026, Table 2).
Following patient assessment at the MMCO, 97 out of 660 patients (15%) were referred to the ED for an urgent shift toward more intense care. Specifically, 77 (79%) patients were addressed to the ED soon after the first MMCO visit, while 20 (21%) following the second visit. Of the 400 patients referred to MMCO by GPs, 73 (18%) were addressed to the ED, compared to 11% (6 of 53) of those referred by HS. A minority of patients discharged by the ED (7 of 119 [6%]) were redirected to the ED following MMCO evaluation. Rates of hospitalization following ED admission were 66% (48 out of 73), 71% (5 out of 7) and 67% (4 out of 6) in patients initially referred to the MMCO by GPs, ED, and HS, respectively.
Excluding patients addressed to the ED following MMCO visit, 235 out of 563 patients (42%) were scheduled for at least one additional MMCO visit due to the need of continued hospital-based monitoring, while 328 (58%) were discharged after the first evaluation and redirected to GP care due to mild COVID-19.
In light of the observation that patients addressed to the MMCO by GPs had overall more severe COVID-19 at MMCO evaluation, we hypothesized that some of these patients might benefit from early ED referral directly by the GP, prior to MMCO visit. Therefore, we aimed at providing GPs with an evidence-based tool able to identify high-risk patients prior to MMCO evaluation, avoiding unnecessary time lags.
As mentioned above, among the totality of patients referred by GPs (n = 400), 18% were addressed to the ED by the MMCO physician due to the need of more intense hospital-based assistance. We used CART analysis to build an easy-to-use algorithm that exploits parameters obtainable by simple patient interview. Among demographics, comorbidities, BMI, home-measured SpO2, the presence of fever for ≥72 h, COVID-19-related symptoms and time from symptom onset, CART analysis selected three variables, namely home-measured SpO2, age and BMI, able to robustly classify patients into risk groups for the early need of intense care. Moreover, for each of these variables, it identified the thresholds that maximized the segregation among the resulting patient clusters (Figure 2). Three risk groups were obtained: (i) low risk (home-measured SpO2 ≥ 95% and age < 71 years), (ii) moderate risk (home-measured SpO2 ≥ 95% and age ≥ 71 years or home-measured SpO2 < 95% and BMI < 33 Kg/m2), and (iii) high risk (home-measured SpO2 < 95% and BMI ≥ 33 Kg/m2). The AUC (95% confidence interval, CI) of the ROC for the CART (Figure 3) was 0.83 (0.77–0.88).
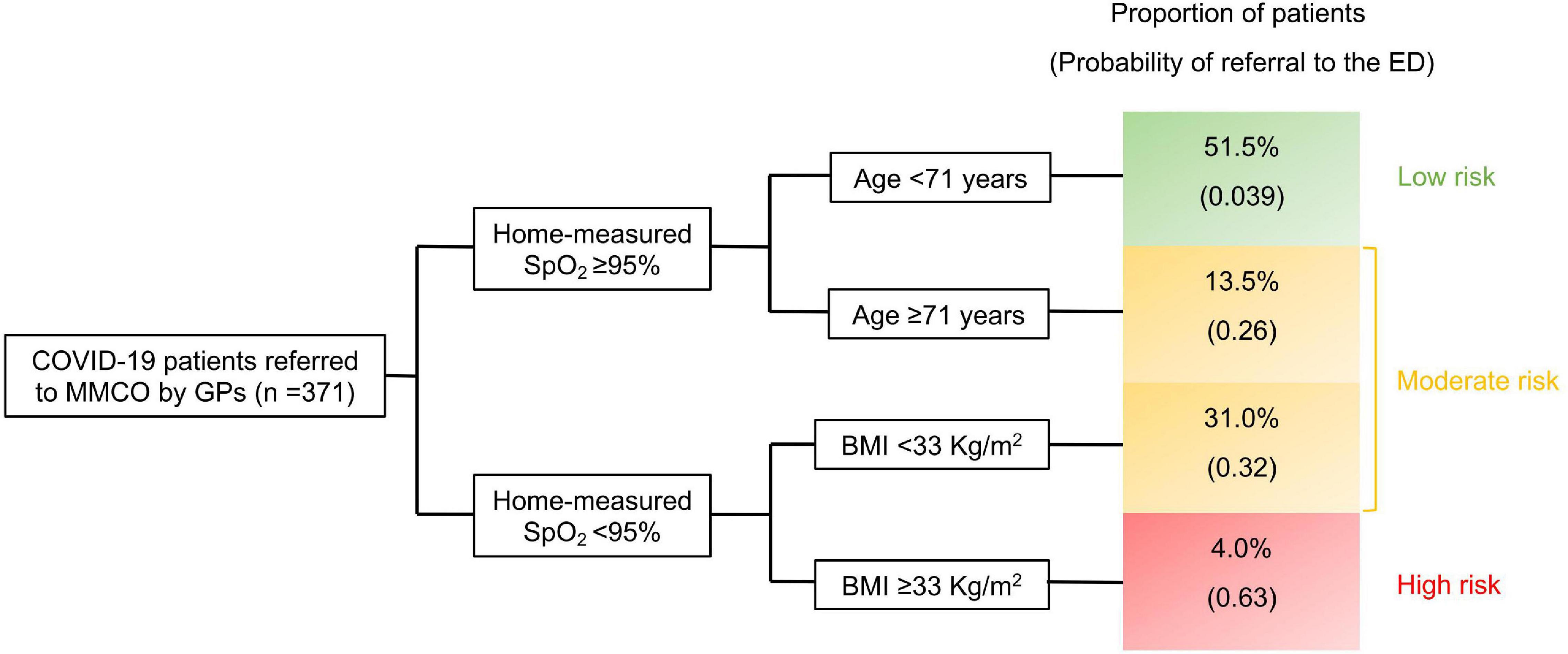
Figure 2. Classification and regression tree (CART) model predicting emergency department referral by the physician of the mild-to-moderate COVID-19 outpatient clinic (MMCO). MMCO, mild-to-moderate COVID-19 outpatient clinic; GP, general practitioner; SpO2, peripheral oxygen saturation; BMI, body mass index; ED, emergency department.

Figure 3. Receiver operating curve (ROC) of the classification and regression tree (CART) model predicting emergency department referral by the physician of the mild-to-moderate COVID-19 outpatient clinic (MMCO). AUC, area under the ROC curve.
The accuracy of the CART model was subsequently confirmed when comparing the identified risk groups in terms of indicators of disease severity assessed during MMCO evaluation. In fact, patients in the high-risk group had overall more severe COVID-19 than those in the moderate- and low-risk groups, differences being expectedly more pronounced compared with the low-risk group (Table 3). Specifically, SpO2 (%, 93 [91–94] in the high-risk group vs. 97 [95–98] in the moderate-risk and 98 [97–99] in the low-risk groups) and PaO2/FiO2 (314 [289–341] in the high-risk group vs. 348 [303–373] in the moderate-risk and 371 [339–409] in the low-risk groups) were significantly reduced in high-risk patients (both p < 0.0001), in parallel to a significant increase in RR (breaths/min, 22 [20–24] in the high-risk group vs. 20 [18–22] in the moderate-risk and 18 [16–21] in the low-risk groups, p < 0.0001). Similarly, blood levels of CRP and LDH, as well as NLR were significantly higher in patients in the high-risk group (all p < 0.05). Likewise, absolute lymphocyte count was significantly reduced in high-risk patients (p 0.0023), in line with a more severe disease. Ferritin showed a tendency toward being increased in the high-risk group (p 0.052). At LUS, white lung pattern was more common in patients in the moderate- and high-risk groups compared to the low-risk cluster, while no difference was observed in the rate of parenchymal consolidation. LUSS was significantly lower in the low-risk group compared to the other groups.
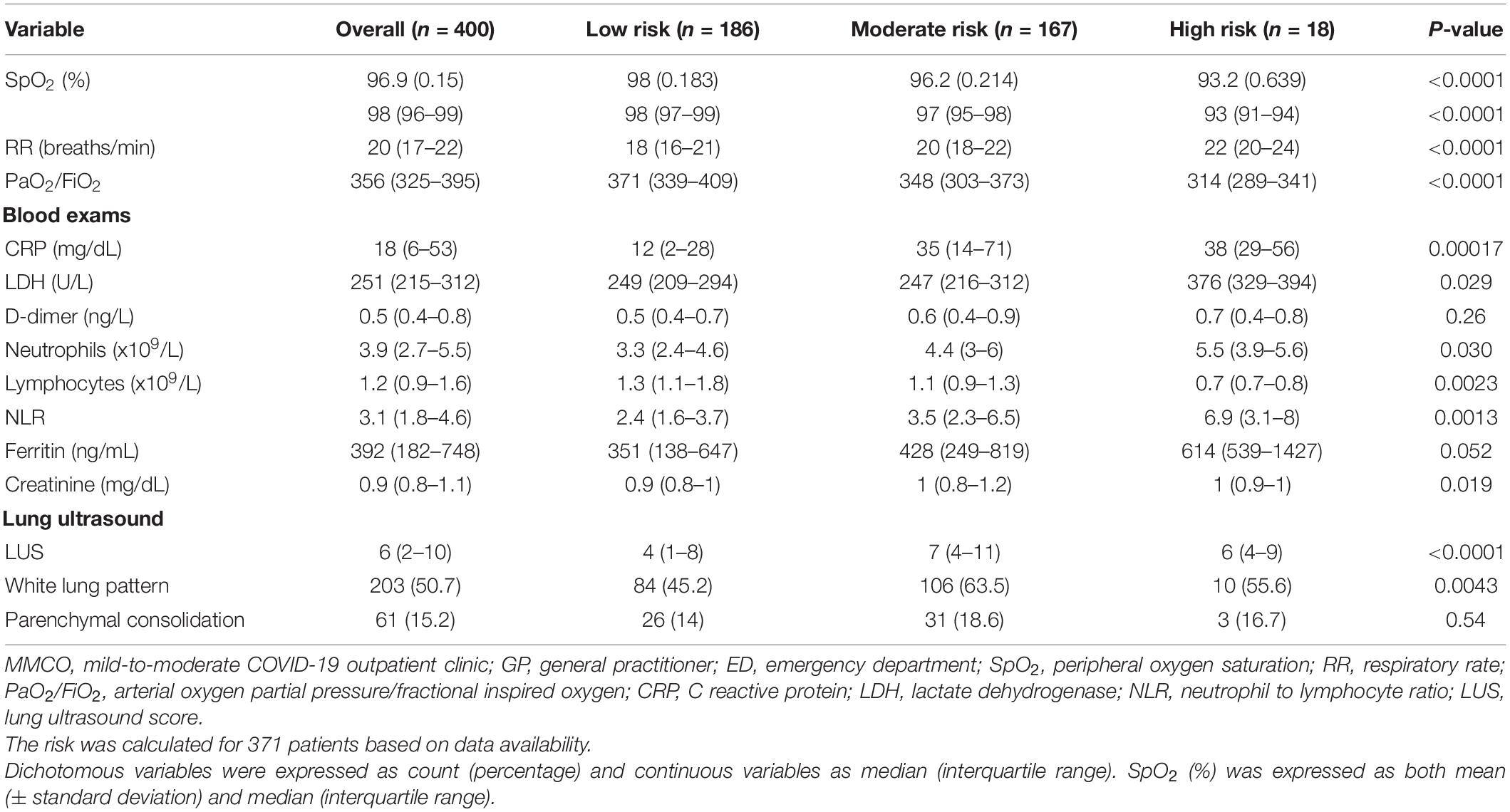
Table 3. Indicators of disease severity in COVID-19 patients referred to the mild-to-moderate COVID-19 outpatient clinic by GPs according to the risk of early ED referral.
A total of 235 out of 660 (41.7%) patients performed ≥2 visits at MMCO and were thus included in the cost-effectiveness analysis. Based on the ICD-9-CM and the updated guidelines of Regional Health Authorities codes for diagnoses and procedures linked to COVID-19, the cost of one COVID-19-related hospitalization was estimated to be 3,275.86 €. According to the regional reform of 2021 on the increased pricing for the activities provided to COVID-19 patients, the cost of each hospitalization was increased by 3,713 € (14, 15). Therefore, the total mean cost of one hospitalization for COVID-19 was estimated as being 6,988.86 € (3,275.86 + 3,713 €). Considering that patients receiving ≥2 MMCO evaluations would have most likely been hospitalized to continue hospital-based active surveillance, we calculated the theoretical total cost of hospitalizations by multiplying 6,988.86 € times 235 patients, resulting in 1,642,382.10 €. On the other hand, considing the totality of visits performed at MMCOs (n = 1101), the overall cost of MMCO activity during the study period was estimated as being 151,960.05 € (Supplementary Table 1). Therefore, the total amount of euros spared through the management of COVID-19 patients at MMCOs rather than by hospital admission during the period October 2020-October 2021 was 1,490,422.05 (i.e., 1,642,382.10–151,960.05 €).
Here we describe an innovative healthcare strategy to optimize the management system of COVID-19 patients while sparing resources for the care of patients with non-COVID-19-related conditions. Similar models have previously been proposed (18, 19). MMCOs were designed to fill the gap of care delivery to COVID-19 patients with clinical features that are neither too mild to be managed by the GP in a home-based setting nor too severe to require ED admission or hospitalization. In our experience, these novel infrastructures allowed the achievement of the dual goal of chaperoning GPs in the management of COVID-19 patients and alleviating pressure on EDs and hospital wards, favoring effort redirection toward patients affected by other conditions. Indeed, the first wave of COVID-19 pandemic forced an extensive reduction of several non-COVID-19-related activities to the detriment of non-COVID-19 care (20–22). The success of this approach dwells in the high degree of inter-system coordination and commitment to the integration of hospital and primary care services.
In a timespan of 1 year, two MMCOs in Milan took care of hundreds of patients who would otherwise be directed straightforwardly to the ED due to the intrinsic difficulty of GPs to deliver optimal care in the absence of hospital equipment or, perhaps, COVID-19 expertise. Most of these patients were indeed managed at MMCOs for the entire course of their disease through serial visits, always in strict collaboration with GPs, until clinical recovery. Only a minority of patients, specifically less than 15%, were addressed to the ED for an urgent evaluation in an emergency setting due to severe disease or high risk of disease progression. Noteworthily, the majority of these patients (65%) required hospitalization following ED admission, pointing to the high level of appropriateness of clinical decisions by MMCO physicians.
Considering that GPs may dispose of insufficient instruments to discriminate patients at increased risk of adverse outcome (23), in light of our observation that a higher proportion of patients among those referred by GPs than those referred by ED or HS were addressed to the ED following MMCO visit, we speculated that the early identification by GPs of patients deserving direct ED admission might guarantee proper and timely intervention. Therefore, we developed an evidence-based, easy-to-use tool that GPs can employ during patient interview to identify patients at high risk of disease progression. CART analysis, through a machine-learning approach, selected three variables, namely home-measured SpO2, age and BMI as the independent predictors that most robustly divide patients into faithful risk groups for severe disease. The model yielded an AUC of 83%, far above the ideal accuracy threshold of 70%. The predictive strength of the model was confirmed by subsequent analysis showing that patients in the high-risk group were indeed those who exhibited the highest degree of respiratory insufficiency, as measured by SpO2, RR and PaO2/FiO2, and the worse laboratory findings. Also, LUS demonstrated a decreased rate of interstitial abnormalities in patients in the low-risk group.
In addition to the clinical efficacy of MMCO in terms of support to GPs and relief on ED and hospital wards, the cost-effectiveness analysis showed that the proposed model of COVID-19 outpatient management is also economically sustainable for the National Healthcare System. Caution is, however, warranted in interpreting economical results, given that many factors besides COVID-19 diagnosis may influence the decision of hospital admission and the length of hospital stay. Nonetheless, outpatient management of COVID-19 patients should be preferred when feasible.
Overall, the establishment of MMCOs proved to be a deal-breaker for the management of COVID-19 patients in a sustainable and efficient way. Ideally, MMCOs may also serve as safe environments where candidate patients might receive COVID-19-directed therapies such as anti-SARS-CoV-2 monoclonal antibodies, antiviral therapies, etc., under the expert monitoring of trained personnel. This patient-centered, sustainable and flexible approach would ensure continuity of care through a 360-degree assistance and possibly serve as a template beyond COVID-19 outbreak.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Comitato Etico Ospedale San Raffaele. The patients/participants provided their written informed consent to participate in this study.
RD and MM: conception and design, acquisition of data, analysis and interpretation of data, statistical analyses, and drafting of the manuscript. EB, GV, AT, MC, GP, LL, RP, CP, CB, SM, NB, DD, and GR: acquisition of data and critical revision of the manuscript. CC, NM, and PR-Q: conception and design, interpretation of data, drafting of the manuscript, and supervision. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the Ministero della Salute, Italy and COVID-19 donations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We wish to thank Dario La Fauci and the administrative offices of San Raffaele University Hospital for their support in the economic analyses.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.892962/full#supplementary-material
Supplementary Figure 1 | Form of patient referral to the Mild-to-Moderate COVID-19 Outpatient Clinic by General Practitioners. SpO2, peripheral oxygen saturation; BMI, body mass index; HIV, Human Immunodeficiency Virus.
Supplementary Figure 2 | Distribution over time of COVID-19 patient visits at Mild-to-Moderate COVID-19 Outpatient Clinics.
1. Armocida B, Formenti B, Ussai S, Palestra F, Missoni E. The Italian health system and the COVID-19 challenge. Lancet Public Health. (2020) 5:e253. doi: 10.1016/S2468-2667(20)30074-8
2. Miller IF, Becker AD, Grenfell BT, Metcalf CJE. Disease and healthcare burden of COVID-19 in the United States. Nat Med. (2020) 26:1212–7. doi: 10.1038/s41591-020-0952-y
3. Schreyer KE, del Portal DA, King LJL, Blome A, DeAngelis M, Stauffer K, et al. Emergency department management of the covid-19 pandemic. J Emerg Med. (2020) 59:946–51. doi: 10.1016/J.JEMERMED.2020.07.022
4. Mitchell R, Banks C. Emergency departments and the COVID-19 pandemic: making the most of limited resources. Emerg Med J. (2020) 37:258–9. doi: 10.1136/EMERMED-2020-209660
5. Morici N, Puoti M, Zocchi MT, Brambilla C, Mangiagalli A, Savonitto S. Home-based COVID 19 management: a consensus document from Italian general medical practitioners and hospital consultants in the Lombardy region (Italy). Eur J Intern Med. (2021) 84:94–6. doi: 10.1016/j.ejim.2020.11.025
6. Regione L. la Giunta: Deliberazione N° XI / 3876. Determinazioni per la Gestione Integrata Ospedale-Territorio Per L’assistenza dei Pazienti Affetti da COVID 19 o Sospetti. Milano: Gazzetta Ufficiale. (2020).
7. Ticinesi A, Lauretani F, Nouvenne A, Mori G, Chiussi G, Maggio M, et al. Lung ultrasound and chest x-ray for detecting pneumonia in an acute geriatric ward. Medicine (Baltimore). (2016) 95:e4153. doi: 10.1097/MD.0000000000004153
8. Amatya Y, Rupp J, Russell FM, Saunders J, Bales B, House DR. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int J Emerg Med. (2018) 11:1–5. doi: 10.1186/S12245-018-0170-2/TABLES/2
9. Martínez Redondo J, Comas Rodríguez C, Pujol Salud J, Crespo Pons M, García Serrano C, Ortega Bravo M, et al. Higher accuracy of lung ultrasound over chest X-ray for early diagnosis of COVID-19 pneumonia. Int J Environ Res Public Health. (2021) 18:3481. doi: 10.3390/IJERPH18073481
10. Allinovi M, Parise A, Giacalone M, Amerio A, Delsante M, Odone A, et al. Lung ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med Biol. (2020) 46:2908–17. doi: 10.1016/J.ULTRASMEDBIO.2020.07.018
11. Volpicelli G, Lamorte A, Villén T. What’s new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med. (2020) 46:1445–8. doi: 10.1007/S00134-020-06048-9
12. Yin W, Zou T, Qin Y, Yang J, Li Y, Zeng X, et al. Poor lung ultrasound score in shock patients admitted to the ICU is associated with worse outcome. BMC Pulm Med. (2019) 19:1. doi: 10.1186/S12890-018-0755-9/TABLES/6
13. Song G, Qiao W, Wang X, Yu X. Association of lung ultrasound score with mortality and severity of COVID-19: a meta-analysis and trial sequential analysis. Int J Infect Dis. (2021) 108:603–9. doi: 10.1016/J.IJID.2021.06.026
14. Circolare Aiop. COVID-19 – Funzione Assistenziale e Incremento Tariffario per le Attività Rese a Pazienti Affetti da COVID-19 – Art.4 D.L 34/2020 – Decreto Interministeriale. (2021). Rome: Gazzetta Ufficiale.
15. Decreto interministeriale. Remunerazione di Una Funzione Assistenziale e di un Incremento Tariffario per le Attività rese a Pazienti Affetti da COVID-19. (2021). Rome: Gazzetta Ufficiale.
16. De Lorenzo R, Conte C, Lanzani C, Benedetti F, Roveri L, Mazza MG, et al. Residual clinical damage after COVID-19: a retrospective and prospective observational cohort study. PLoS One. (2020) 15:e0239570. doi: 10.1371/journal.pone.0239570
17. Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and regression trees. Classif Regres Trees. (2017) 1–358.
18. De Lorenzo R, Magnaghi C, Cinel E, Vitali G, Martinenghi S, Mazza MG, et al. A nomogram-based model to predict respiratory dysfunction at 6 months in non-critical COVID-19 survivors. Front Med. (2022) 9:781410. doi: 10.3389/FMED.2022.781410
19. Quiroz-Juárez MA, Torres-Gómez A, Hoyo-Ulloa I, de León-Montiel RDJ, U’Ren AB. Identification of high-risk COVID-19 patients using machine learning. PLoS One. (2021) 16:e0257234. doi: 10.1371/journal.pone.0257234
20. Indini A, Aschele C, Cavanna L, Clerico M, Daniele B, Fiorentini G, et al. Reorganisation of medical oncology departments during the novel coronavirus disease-19 pandemic: a nationwide Italian survey. Eur J Cancer. (2020) 132:17–23. doi: 10.1016/J.EJCA.2020.03.024
21. Danese S, Ran ZH, Repici A, Tong J, Omodei P, Aghemo A, et al. Gastroenterology department operational reorganisation at the time of covid-19 outbreak: an Italian and Chinese experience. Gut. (2020) 69:981–3. doi: 10.1136/gutjnl-2020-321143
22. Jin P, Park H, Jung S, Kim J. Challenges in urology during the COVID-19 pandemic. Urol Int. (2021) 105:3–16. doi: 10.1159/000512880
Keywords: COVID-19, outpatient clinic, healthcare, cost-effectiveness, prediction model, care pathway
Citation: De Lorenzo R, Montagna M, Bossi E, Vitali G, Taino A, Cilla M, Pata G, Lazorova L, Pesenti R, Pomaranzi C, Bussolari C, Martinenghi S, Bordonaro N, Di Napoli D, Rizzardini G, Cogliati C, Morici N and Rovere-Querini P (2022) A Pilot Study of the Efficacy and Economical Sustainability of Acute Coronavirus Disease 2019 Patient Management in an Outpatient Setting. Front. Med. 9:892962. doi: 10.3389/fmed.2022.892962
Received: 09 March 2022; Accepted: 05 April 2022;
Published: 27 April 2022.
Edited by:
Zhimin Tao, Jiangsu University, ChinaReviewed by:
Zhang Jianguo, Jiangsu University, ChinaCopyright © 2022 De Lorenzo, Montagna, Bossi, Vitali, Taino, Cilla, Pata, Lazorova, Pesenti, Pomaranzi, Bussolari, Martinenghi, Bordonaro, Di Napoli, Rizzardini, Cogliati, Morici and Rovere-Querini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrizia Rovere-Querini, cm92ZXJlLnBhdHJpemlhQGhzci5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.